Abstract
Recent studies from our laboratory indicate that chlorthalidone triggers persistent activation of the sympathetic nervous system and promotes insulin resistance in hypertensive patients, independent of serum potassium. Mechanisms underlying these adverse effects of chlorthalidone remain unknown but increasing evidence in rodents suggests the role of angiotensin and aldosterone excess in inducing both sympathetic overactivity and insulin resistance. Accordingly, we conducted studies in 17 subjects with untreated stage I hypertension, measuring sympathetic nerve activity (SNA) at baseline, after 12 weeks of chlorthalidone alone (25 mg/day), chlorthalidone plus spironolactone, and chlorthalidone plus irbesartan, using randomized crossover design. We found that chlorthalidone alone decreased 24-hour ambulatory BP (ABP) from 135±3/84±2 to 124±2/78±2 mm Hg and significantly increased SNA from baseline (from 41±3 vs 49±4 bursts/min, p < 0.01). Addition of spironolactone to chlorthalidone returned SNA value to baseline (42±3 bursts/min, p = NS) while addition of irbesartan failed to alter the SNA response to chlorthalidone in the same subjects (52±2 bursts/min, p < 0.01) despite similar reduction in ABP (121±2/75±2 and 121±2/75±2 mmHg, respectively). Chlorthalidone alone also increased indices of insulin resistance, which was not observed when used in combination with spironolactone. In conclusion, our study demonstrates beneficial effects of spironolactone in attenuating both chlorthalidone-induced sympathetic activation and insulin resistance in humans, independent of BP reduction. Because sympathetic overactivity and insulin resistance contributes to the poor prognosis in patients with cardiovascular disease, combination therapy of chlorthalidone with mineralocorticoid receptor antagonists may constitute a preferable regimen than chlorthalidone alone in hypertensive patients.
Keywords: diuretics, sympathetic nervous system, insulin resistance, hypertension
Introduction
Chlorthalidone has been proposed as the preferred diuretic for treatment of hypertension, given its superiority in reducing BP and hypertensive target organ complications when compared to other thiazide-type diuretics 1. Previous studies demonstrated that chlorthalidone-induced BP reduction was accompanied by reflex sympathetic activation 2–4 and detrimental indices of insulin resistance in hypertensive patients 2, 5. Furthermore, the magnitude of chlorthalidone-induced increase in insulin resistance was found to be correlated with the increase in sympathetic nerve activity (SNA) and independent of serum potassium in our previous study 2. Despite increasing popularity of chlorthalidone use in the United States 6, mechanisms underlying chlorthalidone-induced sympathetic excitation and insulin resistance remain unknown and effective therapy in preventing these adverse effects of chlorthalidone has not been identified.
Chlorthalidone is known to induce a sustained activation of the renin angiotensin aldosterone system (RAAS) in hypertensive patients 2–4. In animal experimental models, both angiotensin II (Ang II) and aldosterone cross the blood brain barrier and to directly stimulate central sympathetic outflow to the heart and peripheral circulation 7–9. In addition, Ang II further triggers aldosterone synthesis in the brain, thereby amplifying central neuronal activation and hypertension even with a small elevation in circulating Ang II 9. Both Ang II and aldosterone have also been implicated in the pathogenesis of insulin resistance by inhibiting insulin signaling pathway in the adipocytes and skeletal muscle 10, 11, resulting in impaired insulin mediated glucose uptake 12, 13. Whether addition of angiotensin receptor blockers (ARB) or mineralocorticoid receptor (MR) antagonists prevents chlorthalidone-induced sympathetic activation and insulin resistance has not been previously investigated.
Accordingly, the goal of the present investigation is to determine if addition of MR antagonists or ARBs constitutes an effective strategy in reducing both chlorthalidone-induced sympathetic activation and insulin resistance in hypertensive patients. In untreated hypertensive patients, we performed a randomized crossover trial in which we recorded postganglionic sympathetic action potentials with intraneural microelectrodes and assessed indices of insulin resistance at baseline, after chlorthalidone alone, after chlorthalidone plus spironolactone and after chlorthalidone plus ARB irbesartan. Because Ang II and aldosterone have been shown to impair baroreflex function, which exerts inhibitory influence on the SNA, we also assess baroreflex control of SNA and HR during each treatment arm.
Methods
Seventeen patients with untreated stage 1 hypertension participated in the study after providing written informed consent. The study was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center at Dallas. All subjects had BP between 140-159/90-99 mmHg on 3 determinations by oscillometric technique in the seated position. The subjects had no history of heart disease, diabetes mellitus, or evidence of target organ damage such as left ventricular hypertrophy by electrocardiography or chronic kidney disease. The patients had not received antihypertensive drugs for at least 4 weeks before the study.
Experimental protocols
All subjects were randomized to receive 12 weeks of chlorthalidone, 25 mg/day, alone; chlorthalidone, 25 mg daily, plus spironolactone 25 mg daily, and chlorthalidone 25 mg daily plus irbesartan of 150 mg daily, using a single-blind crossover design without washout between treatments. Each subject was followed every 4 week for measurement of serum potassium (K). All subjects were given oral KCl supplementation to maintain serum K between 4.0–4.5 mmol/L. Ambulatory BP (ABP) monitoring was performed at baseline and after 12 weeks of each treatment phase. Following completion of ABP monitoring, measurement of SNA by peroneal microneurography, casual BP, arterial baroreflex sensitivity, fasting plasma glucose, insulin, plasma renin activity (PRA), and serum aldosterone were performed in the supine position during the morning hours between 8–11 AM (see method detail in the supplement). All subjects were instructed to take study drug 1–2 hours before microneurographic study. Analysis of these variables was performed without the knowledge of treatment each subject had received.
Statistical Analysis
Mixed linear models were used to conduct the repeated measures analysis to assess differences between baseline period, chlorthalidone alone, chlorthalidone plus spironolactone phases and chlorthalidone plus irbesartan. Contrasts from these models were used for pair-wise comparisons. Treatment order was also assessed in the models and no effect of treatment order on any outcome variables was found. Because data sets for insulin, PRA, HOMA-IR, and HOMA-βF data are not normally distributed, the data were analyzed after a natural logarithmic transformation. The 0.05 level of significance was used for model main effects and the 0.02 level of significance was used for pair-wise tests to adjust for multiple testing. Pearson correlation coefficient was used to assess the association between changes in SNA with changes in indices of insulin resistance and other non-metabolic variables. Levels of insulin, PRA, HOMA-IR, and HOMA-βF are expressed as median and the inter-quartile range. Other variables are expressed as mean and SEM. Statistical analysis was performed with SAS version 9.2 (SAS Institute Inc., Cary, NC).
Results
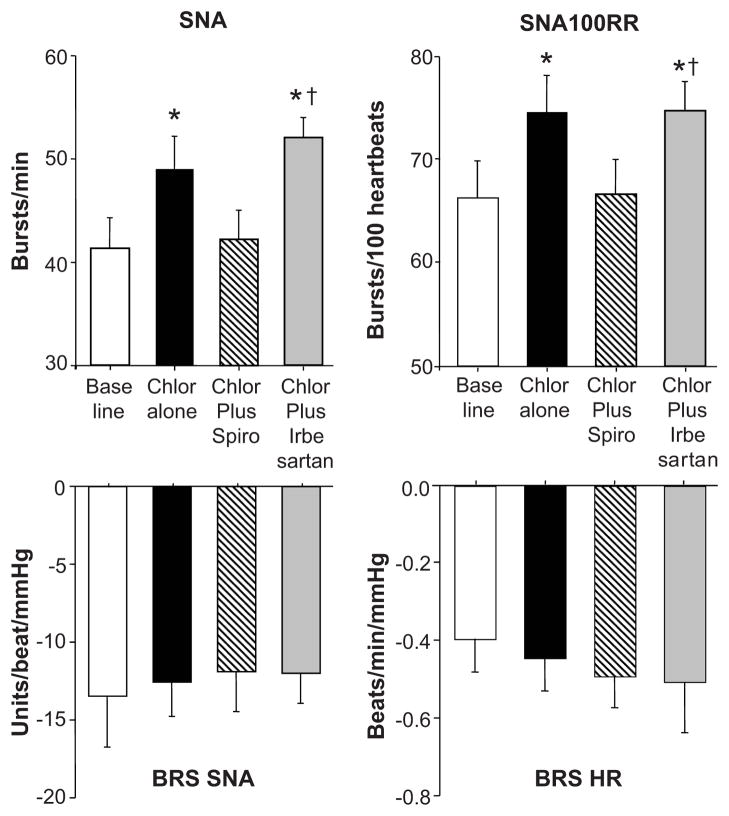
Baseline characteristics of subjects participated in the study are shown in table 1. Chlorthalidone alone caused significant reduction in 24-hr ambulatory BP without affecting 24-hr heart rate (HR, table 1). When spironolactone or irbesartan was added to chlorthalidone, there was a small reduction in ambulatory and casual BP but the reduction did not reach statistical significance when compared to chlorthalidone alone (table 1). Treatment with chlorthalidone plus irbesartan, however, significantly increased the nighttime HR compared to baseline (p = 0.01). Chlorthalidone alone caused a significant increase in plasma renin activity and serum aldosterone levels. Addition of spironolactone to chlorthalidone did not alter increase in PRA and serum aldosterone induced by chlorthalidone alone. Addition of irbesartan, however, caused a further increase in PRA compared with chlorthalidone alone without affecting aldosterone responses (table 1). Chlorthalidone alone significantly increased SNA and SNA per 100 RR intervals from baseline (from 41±3 10 49±4 bursts/min and 66±4 to 74±4 bursts/100 RR, p < 0.01 vs baseline, fig 1 and 2). Addition of spironolactone to chlorthalidone returned SNA value to baseline (42±3 bursts/min and 67±3 bursts/100 RR, p = NS vs baseline, fig 1 and 2) while addition of irbesartan failed to alter SNA response to chlorthalidone in the same subjects (52±2 bursts/min and 75±3 bursts/100 RR, p < 0.01 vs baseline and vs. chlorthalidone plus spironolactone) despite similar reduction in 24-hour ABP (121±2/75±2 and 121±2/75±2 mmHg, respectively, table 1). Baroreflex control of SNA and HR remained unchanged during all phases of treatment (fig 2).
Table 1.
| Variables | Normal Range | Baseline | Chlorthalidone alone | Chlorthalidone plus Spironolactone | Chlorthalidone plus Irbesartan | ANOVA p |
|---|---|---|---|---|---|---|
| Age, years | 50.6±2.3 | |||||
| BMI, kg/m2 | 30.1 ± 1.9 | |||||
| Female, % | 35% | |||||
| African Americans, % | 35% | |||||
| 24-hr SBP, mmHg | < 130 | 135.2±2.6 | 123.8±2.1* | 120.9±2.4* | 120.6±2.4* | 0.001 |
| 24-hr DBP, mmHg | < 80 | 83.5±2.1 | 77.6±2.1† | 75.3±2.0* | 74.9±2.1* | 0.003 |
| 24-hr HR, beats/min | 60–100 | 75±2 | 76±2 | 76±2 | 80±2 | NS |
| Day SBP, mmHg | < 135 | 138.5±2.3 | 126.5±2.5* | 124.9±2.9* | 124.1±2.7* | 0.002 |
| Day DBP, mmHg | < 85 | 86.6±2.1 | 79.9±1.7* | 78.1±1.5* | 76.8±1.9* | 0.004 |
| Day HR, beats/min | 60–100 | 77±2 | 77±2 | 78±2 | 81±2 | NS |
| Night SBP, mmHg | < 120 | 131.1±2.6 | 119.7±2.1* | 117.2±2.4* | 114.1±2.4* | 0.0003 |
| Night DBP, mmHg | < 80 | 79.5±2.1 | 74.1±2.1† | 71.7±2.0* | 68.7±2.1* | 0.0007 |
| Night HR, beats/min | 60–100 | 70±2 | 71±2 | 71±3 | 75±2† | 0.049 |
| Casual SBP, mmHg | < 140 | 149.5±4.2 | 131.7± 2.3* | 132.9± 2.1* | 128.9± 3.2* | < 0.0001 |
| Casual DBP, mmHg | < 90 | 88.0±2.1 | 80.9±1.3* | 81.3±2.1* | 80.5±1.8* | 0.0014 |
| Casual HR, beats/min | 60–100 | 64±2 | 64±2 | 70±2 | 71±2 | NS |
| PRA, ng/ml/hr | 0.05–3.3 | 0.44 | 3.27* | 5.01* | 13.62*§ | <0.0001 |
| interquartile range | 0.05–1.16 | 0.84–7.93 | 2.39–11.50 | 5.73–17.67 | ||
| Aldosterone (ng/dL) | < 21 | 5.3±1.5 | 9.2±1.4* | 10.8±2.2* | 13.3±5.1* | 0.013 |
| Insulin, mU/L, median | < 12 | 3.9 | 7.6* | 4.87‡ | 6.8 | 0.024 |
| interquartile range | 3.00–6.05 | 4.08–19.7 | 3.50–6.78 | 3.28–10.25 | ||
| QUICKI | > 0.31 | 0.40±0.02 | 0.35±0.01* | 0.39±0.02‡ | 0.37±0.01 | 0.023 |
p < 0.01 vs baseline
p < 0.05 vs baseline
p = 0.02 vs chlorthalidone alone
p < 0.01 vs chlorthalidone alone
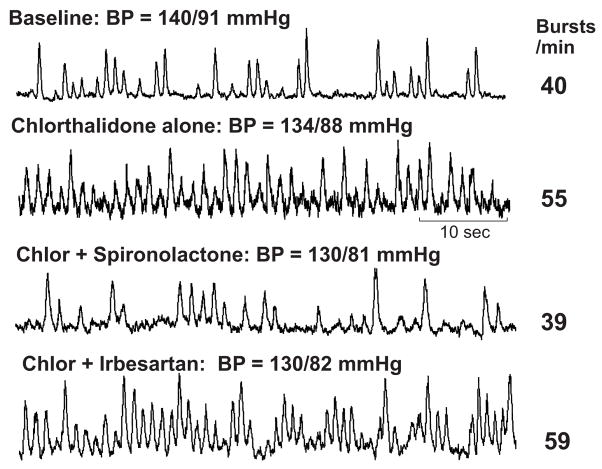
Figure 1.
Original recordings of SNA at baseline, after 12 weeks of chlorthalidone alone, after 12 weeks of chlorthalidone plus spironolactone, and after 12 weeks of chlorthalidone plus irbesartan in one hypertensive subject. In this subject, SNA increased from 40 to 55 bursts/min with chlorthalidone alone but returned to baseline (39 bursts/min) with combination of chlorthalidone plus spironolactone. In contrast, addition of irbesartan failed to alter SNA response to chlorthalidone (59 bursts/min) despite similar reduction in BP.
Figure 2.
Summary data showing changes in SNA (top left) and SNA per 100 RR intervals (top right), baroreflex control of SNA (bottom left) and baroreflex control of HR (bottom right) in all subjects after 12 weeks of chlorthalidone (Chlor) alone (solid bars), chlorthalidone plus spironolactone (Spiro, hatched bars), and chlorthalidone plus irbesartan (gray bars) compared with baseline (white bars). Data are mean ± SE. * p < 0.01 vs baseline, † p < 0.01 vs chlorthalidone plus spironolactone.
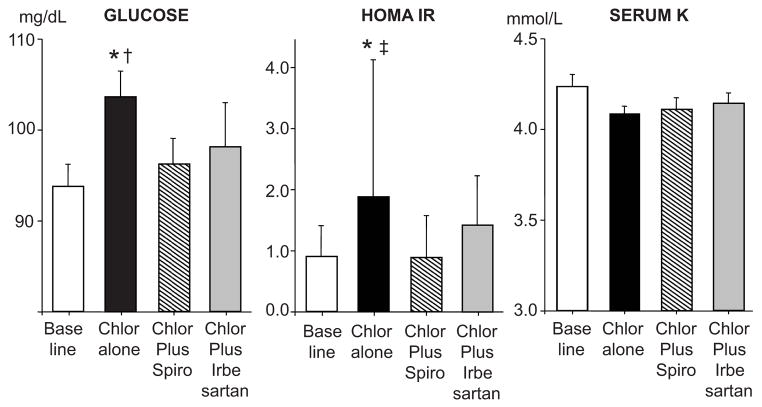
Chlorthalidone alone significantly increased fasting plasma glucose, insulin, and HOMA-IR and reduced QUICKI from baseline (table 1 and fig 3). In contrast, addition of spironolactone returned HOMA-IR and serum insulin levels to baseline in the same subjects, to values significantly lower than chlorthalidone alone (fig 3). HOMA-IR and insulin levels during irbesartan plus chlorthalidone were not significantly different from during chlorthalidone alone or baseline period. Neither chlorthalidone nor combination of chlorthalidone with spironolactone or irbesartan had any detectable effect on HOMA-βF compared to baseline (111±24 vs 68±19 vs 91±26, vs 72±19%, respectively, p > 0.05). With K supplementation, there were no changes in serum K after 12-week of chlorthalidone from baseline (figure 3) or during any treatment period. Percent changes in fasting plasma glucose during chlorthalidone treatment alone from baseline was significantly correlated with percent changes in SNA in both bursts/min (r = 0.64, p = 0.01) and bursts/ 100 RR interval (r = 0.76, p = 0.001) which was not observed when chlorthalidone was combined with spironolactone or irbesartan (p > 0.05). There were no correlation between changes in insulin, HOMA-IR, QUICKI, serum aldosterone, or PRA with changes in SNA during all treatment phases (data not shown).
Figure 3.
Summary data showing changes in fasting plasma glucose (left), HOMA-IR (middle), and serum potassium (K), after 12 weeks of chlorthalidone (Chlor) alone (solid bars), chlorthalidone plus spironolactone (Spiro, hatched bars), and chlorthalidone plus irbesartan (gray bars) compared with baseline (white bars). Data are mean ± SE. * p < 0.01 vs baseline, † p < 0.05 vs chlorthalidone plus spironolactone, ‡ p < 0.01 vs chlorthalidone plus spironolactone.
Discussion
There are two major new findings of this study. First, addition of spironolactone to chlorthalidone, prevents adverse effects of chlorthalidone on both the sympathetic nervous system and insulin sensitivity in hypertensive patients. Second, these beneficial effects of spironolactone were not observed with the ARB irbesartan despite similar reductions in BP.
Previous work from our laboratory has indicated that chlorthalidone triggers sustained sympathetic activation in hypertensive patients, but the underlying mechanism(s) of this potentially detrimental response remain unknown 2. Activation of angiotensin receptor subtype I (AT1R) by Ang II in the central nervous system (CNS) following chlorthalidone-induced volume depletion might be one mechanism. The effects of ARBs on SNA in humans, however, are inconsistent, and studies have found SNA to be unchanged, increased, or decreased after ARB treatment 14, 15. An increasing body of evidence in rodents suggests that circulating aldosterone penetrates the blood brain barrier and directly stimulates central sympathetic outflow via activation of central mineralocorticoid receptors (MRs) 7, 8, 16. This central sympathoexcitatory and pressor action of aldosterone is attenuated by intracerebroventricular (ICV) infusion of MR antagonists at doses that had no systemic spillover 17, 18. Furthermore, aldosterone can be produced locally in the brain upon stimulation by circulating Ang II 9, 19, which may contribute to chlorthalidone-induced sympathetic activation. Inhibition of aldosterone synthesis in the CNS with ICV infusion of aldosterone synthase inhibitor FAD286 has been shown to prevent central neuronal activation and pressor action of Ang II in rats, independent of circulating aldosterone levels 9.
In humans, recent study from our laboratory provided additional support for the animal data as patients with primary aldosteronism (PA) from aldosterone-producing adenomas were found to have sympathetic overactivity which was reversible after surgical resection of the tumor 20. In contrast, 2 previous studies failed to show elevated SNA in the PA subjects 21, 22. The difference in the results may be related to more rigorous selection criteria for our subjects which requires demonstration of sustained elevation in plasma aldosterone level or adrenal aldosterone production despite increased sodium intake according to the current Endocrine Society guidelines 20 while the PA subjects in 2 previous studies were identified by only elevated random levels of aldosterone 21, 22.
Despite variability in the SNA data among these cross-sectional studies comparing PA subjects to normal controls, our data from the randomized crossover study provide more definitive evidence for sympathoinhibitory effect of spironolactone when added to chlorthalidone treatment. In our previous study, spironolactone alone was not shown to alter SNA in patients with essential hypertension 2, suggesting that the sympathoinhibitory action of MR antagonists is more apparent in the setting of renin-angiotensin-aldosterone excess. Furthermore, suppression in SNA during chlorthalidone-spironolactone therapy occurred without any detectable changes in baroreceptor control of SNA or heart rate, suggesting direct effects of spironolactone on the central sympathetic outflow.
In contrast to spironolactone, addition of the ARB irbesartan failed to attenuate the increase in SNA in the same subject, suggesting that Ang II is less important than aldosterone in mediating chlorthalidone-induced increase in SNA. Alternatively, limited oral bioavailability of irbesartan may be responsible for failure of irbesartan to inhibit central AT1 receptors as suggested by a previous study by Leenen et al in the rat model of Ang II –induced hypertension 23. Thus, the results of our study may differ with higher dose of irbesartan or other ARBs. Incomplete AT1R blockade during ARB treatment or failure to suppress aldosterone release, i.e. aldosterone escape or breakthrough phenomenon is another potential mechanisms underlying failure of irbesartan to prevent chlorthalidone-induced increases in sympathetic nerve discharge 24. Nevertheless, the results of our study are consistent with previous study by Fu et al, which showed markedly increased SNA when hydrochlorothiazide (HCTZ) was administered in combination with losartan 14.
In addition to stimulation of sympathetic nervous system, chlorthalidone is well known to increase plasma glucose and the risk of progression to diabetes mellitus. Although hypokalemia is thought to be the main mechanism of thiazide diuretic-induced dysglycemia, possibly by impairing pancreatic release of insulin 25, previous studies from our group and others demonstrated a component of insulin resistance which is independent of serum potassium 2, 26. Activation of the renin-angiotensin system is thought to worsen insulin sensitivity, since Ang II has also been shown to both inhibit the insulin signaling pathways in the adipocyte and skeletal muscle and to impair pancreatic beta cell function 27. Treatment with angiotensin converting enzyme inhibitors (ACEIs) or ARBs has been shown to reduce the development of diabetes mellitus in patients with hypertension or impaired glucose tolerance 28. In contrast, previous studies have shown variable effects of ACEIs and ARBs on development of thiazide diuretic-induced insulin resistance. While the combination of losartan with HCTZ 29 and combination of captopril with bendrofluazide 30 failed to prevent the detrimental effect of thiazide diuretics on glucose homeostasis, combination of valsartan with HCTZ was shown to prevent the increase in fasting plasma glucose and to restore glucose-induced insulin secretion to pretreatment values in obese patients with hypertension 31. This variability in study results might derive from variability in the dose and potency of various diuretics, as well as the specific type of ACEIs or ARBs. Nevertheless, the ARB irbesartan failed to prevent the adverse metabolic effects of chlorthalidone in our study.
Like Ang II, aldosterone has been implicated in the pathogenesis of insulin resistance in large populational studies 32 and in patients with primary aldosteronism 33. In vitro studies demonstrate that aldosterone inhibits insulin signaling pathways both in adipocytes 10 and in vascular smooth muscle cells 11. In vivo studies have indicated that aldosterone induces dysglycemia in rodents by impairing glucose uptake in the skeletal muscle and liver via inhibition of GLUT2 and GLUT4 gene expression 13. Treatment with MR antagonists improves insulin sensitivity and skeletal muscle glucose transport in rodents with aldosterone excess 34; however, there are no previous studies that have addressed the impact of MR blockade during thiazide therapy to insulin sensitivity in humans. Thus, our study represents the first demonstration that spironolactone prevents the chlorthalidone-induced insulin resistance in hypertensive patients. Furthermore, this beneficial effect of spironolactone was not explained by changes in serum potassium, suggesting direct effects of MR blockade.
Our study is limited by lack of a placebo arm but at least the randomized crossover design allows us to compare SNA and indices of insulin resistance during each combination therapy to during chlorthalidone treatment alone in the same subjects. Our study is also limited by the small sample size, which may explain failure to detect significant reduction in the ambulatory or casual BP when irbesartan or spironolactone was added to chlorthalidone. However, the nighttime HR was found to be significantly increased during chlorthalidone plus irbesartan phase compared to baseline, consistent with increased sympathetic drive to the sinus node. Changes in insulin sensitivity were calculated from the HOMA-IR and QUICKI equations rather than directly obtained from the hyperinsulinemic-euglycemic clamp method. Both HOMA-IR and QUICKI have been validated against the hyperinsulinemic glucose clamp and shown to be reliable indices of insulin sensitivity, supporting our findings 35, 36. Only irbesartan was tested in this study and results may not be applicable for all ARBs. Only SNA targeted to the skeletal muscle vasculature was measured and our study results may not be applicable to other regional sympathetic outflow. Lastly, we cannot ascertain if the ability of spironolactone to improve insulin sensitivity in chlorthalidone-treated subjects is the cause or consequence of sympathoinhibitory effect of spironolactone. Exogenous infusion of insulin has been shown to acutely increase muscle SNA in normotensive subjects during a euglycemic clamp 37. Conversely, activation of the sympathetic nervous system has been directly implicated in the pathogenesis of insulin resistance by reducing skeletal muscle glucose uptake both by flow-dependent 38, 39 and flow-independent 40, 41 mechanisms. This latter hypothesis is supported by one recent study, wherein catheter-based renal sympathetic denervation improved insulin sensitivity in patients with resistant hypertension 42.
Perspectives
Regardless of the mechanisms underlying the beneficial effects of spironolactone on glucose metabolism and SNA, it is known that both insulin resistance and sympathetic overactivity contribute to the poor prognosis of patients with cardiovascular diseases 43, 44. Thus, addition of spironolactone might maximize the long-term cardiovascular benefit of chlorthalidone therapy in hypertensive patients by reducing the adverse metabolic consequences and neurohormonal activation. Additional large clinical trials are needed to determine if the combination of chlorthalidone with spironolactone is superior to chlorthalidone alone or other combination therapy in reducing cardiovascular outcomes in hypertensive patients.
What Is New?
Chlorthalidone, a thiazide-like diuretic, is known to cause insulin resistance and activation of sympathetic nervous system in hypertensive patients but effective measures to prevent these side effects have not been identified.
The present study demonstrates that spironolactone, another diuretic which reduces BP by blocking actions of aldosterone hormone, prevents chlorthalidone-induced insulin resistance and sympathetic overactivity.
What Is Relevant?
Chlorthalidone is widely accepted to be the preferred diuretic for treatment of hypertension but many associated metabolic side effects, particularly increased risk of diabetes mellitus, limits its use in clinical practice.
Summary - of the conclusions of the study
Addition of spironolactone to chlorthalidone may allow hypertensive patients to receive BP lowering benefit from chlorthalidone with minimal metabolic side effects.
Acknowledgments
We gratefully acknowledge Dr. Norman Kaplan for invaluable advice regarding the critical review of the manuscript; and Drs. J. Brian Byrd, Jennifer Yoshimoto, and Jiayan Liu for performing the PRA assays
Sources of funding: This work was supported by NIH grant HL-078782 (WV). The project was also supported by the Donald W. Reynolds Cardiovascular Clinical Research Center (WV), and the O'Brien Kidney Center (WV).
Footnotes
Disclosure Statement: Dr. Vongpatanasin is supported by research grant from the National Institute of Health (RO1HL-078782). Other authors have nothing to declare.
References
- 1.Ernst ME, Carter BL, Goerdt CJ, Steffensmeier JJ, Phillips BB, Zimmerman MB, Bergus GR. Comparative antihypertensive effects of hydrochlorothiazide and chlorthalidone on ambulatory and office blood pressure. Hypertension. 2006;47:352–358. doi: 10.1161/01.HYP.0000203309.07140.d3. [DOI] [PubMed] [Google Scholar]
- 2.Menon DV, Arbique D, Wang Z, Adams-Huet B, Auchus RJ, Vongpatanasin W. Differential effects of chlorthalidone versus spironolactone on muscle sympathetic nerve activity in hypertensive patients. J Clin Endocrinol Metab. 2009;94:1361–1366. doi: 10.1210/jc.2008-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weidmann P, Beretta-Piccoli C, Meier A, Keusch G, Gluck Z, Ziegler WH. Antihypertensive mechanism of diuretic treatment with chlorthalidone. Complementary roles of sympathetic axis and sodium. Kidney Int. 1983;23:320–326. doi: 10.1038/ki.1983.22. [DOI] [PubMed] [Google Scholar]
- 4.Lawton WJ, Fitz A, Grant C, Witte DL. Dopamine beta-hydroxylase and plasma renin activity in patients with low-, normal-, and high-renin essential hypertension. Circulation. 1979;59:1063–1069. doi: 10.1161/01.cir.59.5.1063. [DOI] [PubMed] [Google Scholar]
- 5.The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Major Outcomes in High-Risk Hypertensive Patients Randomized to Angiotensin-Converting Enzyme Inhibitor or Calcium Channel Blocker vs Diuretic. JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 6.Ernst ME, Lund BC. Renewed interest in chlorthalidone: evidence from the Veterans Health Administration. J Clin Hypertens (Greenwich) 2010;12:927–934. doi: 10.1111/j.1751-7176.2010.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felder RB. Mineralocorticoid receptors, inflammation and sympathetic drive in a rat model of systolic heart failure. Exp Physiol. 2010;95:19–25. doi: 10.1113/expphysiol.2008.045948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez-Sanchez EP. The mammalian mineralocorticoid receptor: tying down a promiscuous receptor. Exp Physiol. 2010;95:13–18. doi: 10.1113/expphysiol.2008.045914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang BS, Ahmadi S, Ahmad M, White RA, Leenen FH. Central neuronal activation and pressor responses induced by circulating ANG II: role of the brain aldosterone-"ouabain" pathway. Am J Physiol Heart Circ Physiol. 2010;299:H422–430. doi: 10.1152/ajpheart.00256.2010. [DOI] [PubMed] [Google Scholar]
- 10.Wada T, Ohshima S, Fujisawa E, Koya D, Tsuneki H, Sasaoka T. Aldosterone inhibits insulin-induced glucose uptake by degradation of insulin receptor substrate (IRS) 1 and IRS2 via a reactive oxygen species-mediated pathway in 3T3-L1 adipocytes. Endocrinology. 2009;150:1662–1669. doi: 10.1210/en.2008-1018. [DOI] [PubMed] [Google Scholar]
- 11.Hitomi H, Kiyomoto H, Nishiyama A, Hara T, Moriwaki K, Kaifu K, Ihara G, Fujita Y, Ugawa T, Kohno M. Aldosterone suppresses insulin signaling via the downregulation of insulin receptor substrate-1 in vascular smooth muscle cells. Hypertension. 2007;50:750–755. doi: 10.1161/HYPERTENSIONAHA.107.093955. [DOI] [PubMed] [Google Scholar]
- 12.Luther JM, Luo P, Kreger MT, Brissova M, Dai C, Whitfield TT, Kim HS, Wasserman DH, Powers AC, Brown NJ. Aldosterone decreases glucose-stimulated insulin secretion in vivo in mice and in murine islets. Diabetologia. 2011;54:2152–2163. doi: 10.1007/s00125-011-2158-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selvaraj J, Muthusamy T, Srinivasan C, Balasubramanian K. Impact of excess aldosterone on glucose homeostasis in adult male rat. Clin Chim Acta. 2009;407:51–57. doi: 10.1016/j.cca.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 14.Fu Q, Zhang R, Witkowski S, Arbab-Zadeh A, Prasad A, Okazaki K, Levine BD. Persistent sympathetic activation during chronic antihypertensive therapy: a potential mechanism for long term morbidity? Hypertension. 2005;45:513–521. doi: 10.1161/01.HYP.0000158312.63381.c1. [DOI] [PubMed] [Google Scholar]
- 15.Krum H, Lambert E, Windebank E, Campbell DJ, Esler M. Effect of angiotensin II receptor blockade on autonomic nervous system function in patients with essential hypertension. Am J Physiol Heart Circ Physiol. 2006;290:H1706–1712. doi: 10.1152/ajpheart.00885.2005. [DOI] [PubMed] [Google Scholar]
- 16.de Kloet ER, Van Acker SA, Sibug RM, Oitzl MS, Meijer OC, Rahmouni K, de Jong W. Brain mineralocorticoid receptors and centrally regulated functions. Kidney Int. 2000;57:1329–1336. doi: 10.1046/j.1523-1755.2000.00971.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang ZH, Yu Y, Kang YM, Wei SG, Felder RB. Aldosterone acts centrally to increase brain renin-angiotensin system activity and oxidative stress in normal rats. Am J Physiol Heart Circ Physiol. 2008;294:H1067–1074. doi: 10.1152/ajpheart.01131.2007. [DOI] [PubMed] [Google Scholar]
- 18.Gomez-Sanchez EP. Intracerebroventricular infusion of aldosterone induces hypertension in rats. Endocrinology. 1986;118:819–823. doi: 10.1210/endo-118-2-819. [DOI] [PubMed] [Google Scholar]
- 19.Gomez-Sanchez EP, Ahmad N, Romero DG, Gomez-Sanchez CE. Is aldosterone synthesized within the rat brain? Am J Physiol Endocrinol Metab. 2005;288:E342–346. doi: 10.1152/ajpendo.00355.2004. [DOI] [PubMed] [Google Scholar]
- 20.Kontak AC, Wang Z, Arbique D, Adams-Huet B, Auchus RJ, Nesbitt SD, Victor RG, Vongpatanasin W. Reversible Sympathetic Overactivity in Hypertensive Patients with Primary Aldosteronism. J Clin Endocrinol Metab. 2010;95:4756–4761. doi: 10.1210/jc.2010-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyajima E, Yamada Y, Yoshida Y, Matsukawa T, Shionoiri H, Tochikubo O, Ishii M. Muscle sympathetic nerve activity in renovascular hypertension and primary aldosteronism. Hypertension. 1991;17:1057–1062. doi: 10.1161/01.hyp.17.6.1057. [DOI] [PubMed] [Google Scholar]
- 22.Matsukawa T, Miyamoto T. Does infusion of ANG II increase muscle sympathetic nerve activity in patients with primary aldosteronism? Am J Physiol Regul Integr Comp Physiol. 2008;294:R1873–1879. doi: 10.1152/ajpregu.00471.2007. [DOI] [PubMed] [Google Scholar]
- 23.Leenen FH, Yuan B. Prevention of hypertension by irbesartan in Dahl S rats relates to central angiotensin II type 1 receptor blockade. Hypertension. 2001;37:981–984. doi: 10.1161/01.hyp.37.3.981. [DOI] [PubMed] [Google Scholar]
- 24.Bomback AS, Klemmer PJ. The incidence and implications of aldosterone breakthrough. Nat Clin Pract Nephrol. 2007;3:486–492. doi: 10.1038/ncpneph0575. [DOI] [PubMed] [Google Scholar]
- 25.Carter BL, Einhorn PT, Brands M, He J, Cutler JA, Whelton PK, Bakris GL, Brancati FL, Cushman WC, Oparil S, Wright JT., Jr Thiazide-induced dysglycemia: call for research from a working group from the national heart, lung, and blood institute. Hypertension. 2008;52:30–36. doi: 10.1161/HYPERTENSIONAHA.108.114389. [DOI] [PubMed] [Google Scholar]
- 26.Amery A, Birkenhager W, Brixko P, Bulpitt C, Clement D, Deruyttere M, De Schaepdryver A, Fagard R, Forette F, Forte J, Williams B. Glucose intolerance during diuretic therapy in elderly hypertensive patients. A second report from the European Working Party on high blood pressure in the elderly (EWPHE) Postgrad Med J. 1986;62:919–924. doi: 10.1136/pgmj.62.732.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luther JM, Brown NJ. The renin-angiotensin-aldosterone system and glucose homeostasis. Trends Pharmacol Sci. 2011;32:734–739. doi: 10.1016/j.tips.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta-analysis. Lancet. 2007;369:201–207. doi: 10.1016/S0140-6736(07)60108-1. [DOI] [PubMed] [Google Scholar]
- 29.Bakris G, Molitch M, Hewkin A, Kipnes M, Sarafidis P, Fakouhi K, Bacher P, Sowers J. Differences in glucose tolerance between fixed-dose antihypertensive drug combinations in people with metabolic syndrome. Diabetes Care. 2006;29:2592–2597. doi: 10.2337/dc06-1373. [DOI] [PubMed] [Google Scholar]
- 30.Hunter SJ, Harper R, Ennis CN, Crothers E, Sheridan B, Johnston GD, Atkinson AB, Bell PM. Effects of combination therapy with an angiotensin converting enzyme inhibitor and thiazide diuretic on insulin action in essential hypertension. J Hypertens. 1998;16:103–109. doi: 10.1097/00004872-199816010-00015. [DOI] [PubMed] [Google Scholar]
- 31.Sowers JR, Raij L, Jialal I, Egan BM, Ofili EO, Samuel R, Zappe DH, Purkayastha D, Deedwania PC. Angiotensin receptor blocker/diuretic combination preserves insulin responses in obese hypertensives. J Hypertens. 2010;28:1761–1769. doi: 10.1097/HJH.0b013e32833af380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumagai E, Adachi H, Jacobs DR, Jr, Hirai Y, Enomoto M, Fukami A, Otsuka M, Kumagae S, Nanjo Y, Yoshikawa K, Esaki E, Yokoi K, Ogata K, Kasahara A, Tsukagawa E, Ohbu-Murayama K, Imaizumi T. Plasma aldosterone levels and development of insulin resistance: prospective study in a general population. Hypertension. 2011;58:1043–1048. doi: 10.1161/HYPERTENSIONAHA.111.180521. [DOI] [PubMed] [Google Scholar]
- 33.Giacchetti G, Ronconi V, Turchi F, Agostinelli L, Mantero F, Rilli S, Boscaro M. Aldosterone as a key mediator of the cardiometabolic syndrome in primary aldosteronism: an observational study. J Hypertens. 2007;25:177–186. doi: 10.1097/HJH.0b013e3280108e6f. [DOI] [PubMed] [Google Scholar]
- 34.Lastra G, Whaley-Connell A, Manrique C, Habibi J, Gutweiler AA, Appesh L, Hayden MR, Wei Y, Ferrario C, Sowers JR. Low-dose spironolactone reduces reactive oxygen species generation and improves insulin-stimulated glucose transport in skeletal muscle in the TG(mRen2)27 rat. Am J Physiol Endocrinol Metab. 2008;295:E110–116. doi: 10.1152/ajpendo.00258.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 36.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 37.Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest. 1991;87:2246–2252. doi: 10.1172/JCI115260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jamerson KA, Julius S, Gudbrandsson T, Andersson O, Brant DO. Reflex sympathetic activation induces acute insulin resistance in the human forearm. Hypertension. 1993;21:618–623. doi: 10.1161/01.hyp.21.5.618. [DOI] [PubMed] [Google Scholar]
- 39.Sartori C, Trueb L, Nicod P, Scherrer U. Effects of sympathectomy and nitric oxide synthase inhibition on vascular actions of insulin in humans. Hypertension. 1999;34:586–589. doi: 10.1161/01.hyp.34.4.586. [DOI] [PubMed] [Google Scholar]
- 40.Lembo G, Capaldo B, Rendina V, Iaccarino G, Napoli R, Guida R, Trimarco B, Sacca L. Acute noradrenergic activation induces insulin resistance in human skeletal muscle. Am J Physiol. 1994;266:E242–247. doi: 10.1152/ajpendo.1994.266.2.E242. [DOI] [PubMed] [Google Scholar]
- 41.Navegantes LC, Sjostrand M, Gudbjornsdottir S, Strindberg L, Elam M, Lonnroth P. Regulation and counterregulation of lipolysis in vivo: different roles of sympathetic activation and insulin. J Clin Endocrinol Metab. 2003;88:5515–5520. doi: 10.1210/jc.2003-030445. [DOI] [PubMed] [Google Scholar]
- 42.Mahfoud F, Schlaich M, Kindermann I, Ukena C, Cremers B, Brandt MC, Hoppe UC, Vonend O, Rump LC, Sobotka PA, Krum H, Esler M, Bohm M. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation. 2011;123:1940–1946. doi: 10.1161/CIRCULATIONAHA.110.991869. [DOI] [PubMed] [Google Scholar]
- 43.Kaye DM, Lefkovits J, Jennings GL, Bergin P, Broughton A, Esler MD. Adverse consequences of high sympathetic nervous activity in the failing human heart. J Am Coll Cardiol. 1995;26:1257–1263. doi: 10.1016/0735-1097(95)00332-0. [DOI] [PubMed] [Google Scholar]
- 44.Pyorala M, Miettinen H, Halonen P, Laakso M, Pyorala K. Insulin resistance syndrome predicts the risk of coronary heart disease and stroke in healthy middle-aged men: the 22-year follow-up results of the Helsinki Policemen Study. Arterioscler Thromb Vasc Biol. 2000;20:538–544. doi: 10.1161/01.atv.20.2.538. [DOI] [PubMed] [Google Scholar]