Abstract
Dehydroleucodine (DhL) is a sesquiterpene lactone of the guaianolide group with gastric citoprotective activity. Recent studies have also demonstrated that DhL inhibits the proliferation of vascular smooth muscle cells. In this study we examined the effect of DhL in the differentiation 3T3-L1 preadipocytes. The addition of DhL significantly inhibited the differentiation 3T3-L1 preadipocytes along with significant decrease in the accumulation of lipid content by a dramatic down regulation of the expression of adipogenic-specific transcriptional factors PPARγ and C-EBPα. However, phosphorylation of AMPKα, Erk1/2 and Akt1 was not inhibited by DhL treatment. Interestingly, we also found that 11,13-dihydro-dehydroleucodine, a derivative of DhL with inactivated α-methylene-γ-lactone function, also inhibited the differentiation 3T3-L1 preadipocytes. Taken together, these data suggest DhL has an important inhibitory effect in cellular pathways regulating adipocyte differentiation by modulating the PPARγ expression, which is known to play a pivotal role during adipogenesis.
Keywords: Dehydroleucodine, adipocytes, α-methylene-γ-lactone function, transcriptional factors
1. Introduction
Obesity is a prevalent health risk in industrialized countries and is associated with multiple pathological disorders, including diabetes (Sartipy and Loskutoff, 2003), hypertension (Pi-Sunyer, 2002), cancer (Lagra et al., 2004), gallbladder disease (Galal, 2003), and aterosclerosis (Wofford et al., 1999). Obesity has also been associated with cancer and cardiovascular disease, which have reached epidemic proportions worldwide (Roberts et al., 2010; Yun, 2010). It has been reported that weight loss reduces lipid levels, blood pressure, and the incidence of type 2 diabetes mellitus (Sheard, 1993).
Animal models as well as in vitro systems have been used extensively in diabetes research. 3T3-L1 preadipocytes have been a useful in vitro model for the study of obesity, due to an observable accumulation of triglycerides upon differentiating in culture (Rosen and Spiegelman, 2000). Adipocyte differentiation is induced by the expression and/or phosphorylation of specific genes, such as AMPK, Akt1, Erk1/2, (Kemp et al., 2003; Rosen and Spiegelman, 2000; Rosen et al., 2000; Spiegelman et al., 1993), PPARγ and C-EBPα (Bost et al., 2005). It is anticipated that compounds, which inhibit adipocyte differentiation could beneficially prevent and/or treat obesity. Thus, natural products such as crude aqueous and chloroform plant extracts as well as other pure active compounds that specifically target and inhibit adipogenesis have been considered potentially promising treatments of obesity (Roberts et al., 2010).
Sesquiterpene lactones are a large and structurally diverse group of plant second metabolites (Heinrich et al., 1998) with distinctive biological activities, including gastric cytoprotector effects (Penissi et al., 1998), anti-migraine (Beekman et al., 1997), antiviral and antimicrobial activities (Hayashi et al., 1996; Perry and Foster, 1995), anti-tumor (Robles et al., 1995) and neurotoxic effect (Cheng et al., 1992).
Sesquiterpene lactones are also blockers of smooth muscle contractility (Hay et al., 1994) aromatase activity (Blanco et al., 1997) and NF-kappa B activation (Hehner et al., 1998; Lyss et al., 1998). Also, it has been found that sesquiterpene lactones inhibit the activation of cyclooxygenase and proinflammatory cytokines in macrophages (Hwang et al., 1996). Within the group of sesquiterpene lactones, helenalin, which occurs in the aerial portion of Arnica Montana L., was also found to block the hormonally induced Sky2 mRNA and Akt phosphorylation during early stages of adipocyte differentiation (Auld et al., 2006). Inhibitory activities have been principally linked to the α-methylene-γ-lactone function (Heinrich et al., 1998). However, the reduction of the α-methylene-γ-lactone limited its cytotoxicity effect without affecting the anti-proliferative and anti-aromatase activity (Blanco et al., 1997).
Dehydroleucodine (DhL) is a sesquiterpene lactone of the guaianolide group, which also contains a α-methylene-γ-lactone ring in its molecule. It was first isolated from Lidbeckia pectinata (Bohlmann and Zdero, 1972). The aerial parts of Artemisia douglasiana Besser are also rich in DhL (Giordano et al., 1990). Chloroform extracts of the air-dried aerial parts of Artemisia douglasiana showed significant gastric cytoprotective activity (Giordano et al., 1992). In addition, DhL inhibited cell proliferation (Polo et al., 2007) and growth of Trypanosome cruzi in culture (Brengio et al., 2000). In this study, we investigated the effects of both DhL and 11,13-dihydro-dehydroleucodine (DH-DhL) on the differentiation of 3T3-L1 preadipocytes, and its mechanism of action at the cellular and molecular levels.
2. Material and Methods
2.1. Materials
DhL was isolated from Artemisia douglasiana as previously described (Giordano et al., 1990) and DH-DhL was obtained for DhL reduction as previously described (Giordano et al., 1992). 3T3-L1 cells were purchased from American Type Culture Collection (ATCC, Manassas, VA). Dulbecco's modified Eagle's medium high glucose (DMEM), penicillin/streptomycin and L-glutamine were purchased from Mediatech, Inc. (Manassas, VA). C-EBPα antibodies were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). PPARγ, total (T)-Erk, phospho (P)-Erk, T-Akt, P-Akt, T-AMPKα and P-AMPKα antibodies were from Cell Signaling Technology (Boston, MA). Tubulin antibodies were from Sigma-Aldrich (St. Louis, MO). All secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). All other chemicals were obtained from Sigma-Aldrich unless otherwise stated.
2.2. Cell culture and differentiation
3T3-L1 cells were grown to confluence in Dulbecco's modified Eagle's medium (DMEM) high glucose supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA), 1% penicillin/ streptomycin and 1% L-glutamine (growth media) in a humidified atmosphere of 5% CO2 at 37°C. Culture was fed every 48 hours, both for cell growth and differentiation. To trigger differentiation cells were exposed to induction medium (IM) (growth media supplemented with 670nM insulin, 65nM dexamethasone and 0.5mM 3-isobutyl-1-methylxanthine [IBMX]) for the first two days, then fed with post-differentiation media (DMEM high glucose supplemented with 5% fetal bovine serum, 1% penicillin/streptomycin and 1% L-glutamine) for the next seven days. Cells were exposed to DhL throughout the entire differentiation process, unless otherwise indicated.
2.3. Cell viability assay
Cells were cultured on 6-well plates and treated with DhL as indicated for each experiment. Cell viability was measured using the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide) colorimetric assay following the manufacturer's instructions (ATCC). In brief, MTT reagent was added to cells at a final concentration of 10μl/ml. Then cells were incubated as indicated in the Fig. 1 at 37°C and 5% CO2. After incubation, the medium was removed, and formazan crystals were dissolved in detergent reagent. Optical density for each condition was determined at 570 nm.
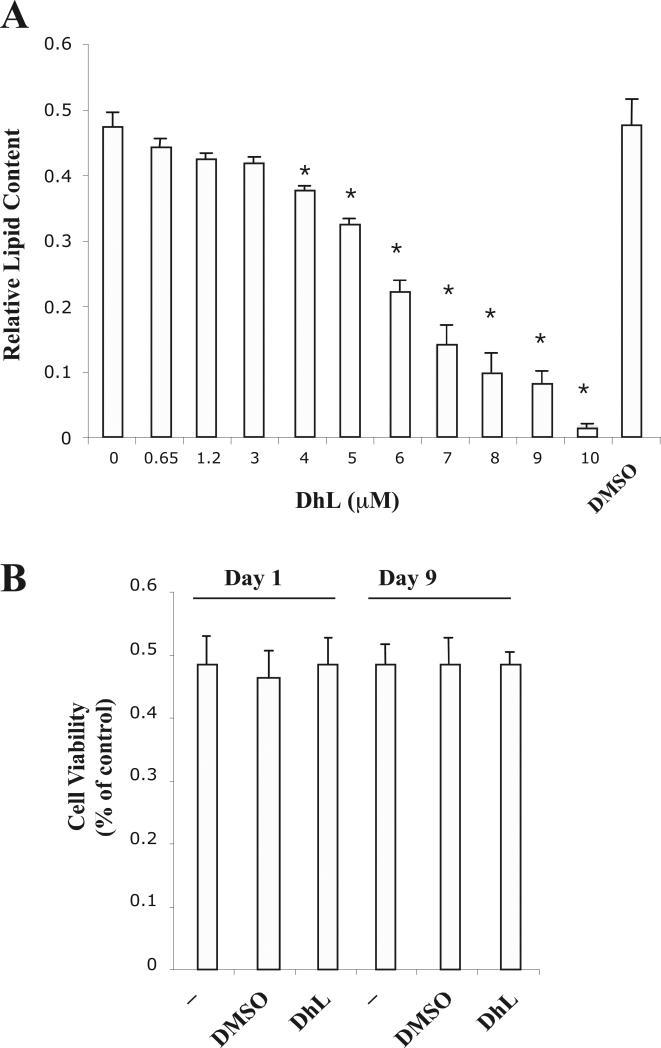
Fig. 1. Dehydroleucodine inhibited adipogenesis of 3T3-L1 preadipocytes without reducing cell viability.
(A) 3T3-L1 preadipocytes were differentiated into adipocytes in the absence or in the presence of various amounts of DhL (0.65 to 10 μM) as described in Material and Methods. Results were represented as relative lipid contents. Data represent the mean ± S.E.M. of three independent experiments. *P < 0.05 by Student's t-test compared to DMSO and only induction media-treated cells. (B) Cells were treated with 8 μM DhL for either 24hrs (Day 1) or for 9 days (Day 9). Cell viability was measured using the MTT assay as described in Material and Methods. Data represent the mean ± S.E.M. of three independent experiments.
2.4. Oil Red O staining and microscopy
On the ninth day cells were fixed with 10% formalin (Thermo Fisher Scientific. Inc., Pittsburgh, PA) in phosphate buffer saline (PBS) 1 hour at 4°C. Oil Red O (Allied Chemical, Morristown, NJ) stock solution (0.6g in 100ml of isopropanol) was diluted with 0.6 parts of water, filtered and added to the fixed cells for 15 minutes at room temperature. Cells were then washed with water and analyzed in a Leica DM IRB inverted microscope. Photos of lipid droplets were taken using digital Leica DC 500 camera. To quantify the lipid droplets, Oil Red O was eluted with 100% isopropanol for 10 minutes at 37°C, collected, and its optical density was measured at 540 nm.
2.5. Triglyceride assay
Nine days 3T3-L1 adipocytes were washed with PBS, scraped and centrifuged. Pellets were resuspended in PBS and then homogenized by sonication and the cell suspension was assayed for total triglyceride (Triglyceride assay kit; Cayman Chemical Company, Ann Arbor, MI) according to the method of (Mendez et al., 1986). Results were expressed as total triglyceride per cellular protein (DC protein assay; Bio-Rad, Hercules, CA).
2.6. Western blot analysis
To prepare cell lysates, cell monolayers where washed with ice-cold lysis buffer (20mM Tris-HCL pH7.5, 150mM NaCl, 1mM Na2 EDTA, 10% NP40 and 10% Na Deoxycholate) containing protease and phosphatase inhibitors. Lysates were collected by centrifugation and protein concentrations were estimated by using BCA protein assay (Thermo Fisher Scientific, Inc., Pittsburgh, PA) following the manufacturer's instructions. Proteins were resolved by SDS-PAGE and transferred to nitrocellulose membranes, blocked, probed with the specific antibodies and then visualized by Western analysis. Relative levels of Erk1/2 and Akt1 proteins were determined by densitometry using the ratio of P-Erk1/2 to T-Erk1/2 and P-Akt1 to T-Akt1, respectively. Relative levels of PPARγ and C-EBPα were determined using the ratio of PPARγ to tubulin and C-EBPα to tubulin, respectively.
2.7. High-performance liquid chromatography analysis
The high-performance liquid chromatography (HPLC) equipment consisted of a SpectraSystem SMC1000 solvent delivery system, vacuum membrane degasser, P4000 gradient pumps and AS3000 autosampler (Thermo Electro Corporation, San Jose, CA). Column effluent was monitored at 254 nm with Spectra System UV6000LP variable wavelength PDA detector and ChromQuest 4.1 software. DhL, (11S)DH-DhL and (11R)DH-DhL were separated using a C18 YMC column (A-302, 150 × 4.3 mm i.d., S-5 μm, 12 nm; Waters) and the following solvents: A. acetonitrile; B. 0.1 % TFA in water. System 1: linear gradient 10% to 100 % A in 120 min; flow rate 1 ml/min. Preparative HPLC was performed in the above equipment with a XTerra Prep MS C18 OBD column, 15 μm, 19 × 50 mm (Waters) and the solvent system 25 % acetonitrile – 75 % 0.1 % TFA in water (isocratic); flow rate 3 ml/min.
2.8. Gas chromatography (GC)/flame ionization detector (FID) and GC/mass spectrometry (MS) analysis
GC/FID analyses were performed on a Trace GC Ultra apparatus (Thermo Electro Corporation, San Jose, CA) equipped with a flame ionization detector. The output was recorded using a ChromQuest version 4.1 data system. A DB-5MS capillary column (0.25 mm i.d. × 30 m; film thickness 0.25 μm; J & W Scientific, Folsom, CA) was employed. The temperature was programmed 105 to 240°C at 3°C/min (linear increase) and then the temperature was held at 240°C for 10 min. The injector temperature was 250°C with a split ratio of 1/20. The detector temperature was 270°C. Helium was used as gas carrier at 1 ml/min. DHL, (11S)DH-DHL and (11R)DH-DhL were dissolved in ethyl acetate and two μl of the solution were injected. GC/MS determinations were carried out in a Hewlett Packard model 6890 instrument coupled to a Q-Mass 910 quadrupole selective detector at 70 eV and equipped with a DB-5MS capillary column. Temperature program and other conditions were as indicated above.
2.9. Compound identification
Dehydroleucodine {(1S,6S,2R)-9,13-dimethyl-5-methylene-3 oxatricyclo[8.3.0.0<2,6>]trideca-9,12-diene-4,11-dione, IUPAC nomenclature}. DhL (compound 1): HPLC (System 1), Rt 27.74 min; GC/MS, Rt 22.72 min; UV/PDA λmax 256 nm; MS m/z (rel. int.), 244 (100) M+, 173 (18.2), 145 (19.8), 129 (18.9), 115 (18.5), 105 (18.3), 91 (62.9), 79 (17.7), 77 (28.4), 65 (21.6), 53 (30.8). (1S,2S,5S,6S)-5,9,13-trimethyl-3-oxatricyclo[8.3.0.0<2,6>]trideca-9,12-diene-4,11-dione;11,13-dihydro-dehydroleucodine (IUPAC nomenclature), (11S)DH-DhL (compound 2): HPLC (System 1), Rt 28.16 min; GC/MS, Rt 21.87; UV/PDA λmax 257 nm; MS, m/z (rel. int.): 246 (100) M+, 217 (26.7), 173 (37.6), 172 (31.2), 145 (26.2), 105 (23.2), 91 (60.7), 77 (26.2), 55 (24.3). (1S,2S,6S,5R)-5,9,13-trimethyl-3-oxatricyclo[8.3.0.0<2,6>]trideca-9,12-diene-4,11-dione, 11,13-dihydro-dehydroleucodine (IUPAC nomenclature), (11R)DH-DhL (compound 3): HPLC (System 1), Rt 26.23 min; GC/MS, Rt 22.84; UV/PDA λmax 257 nm; MS, m/z (rel. int.): 246 (100) M+, 217 (31.5), 173 (35.5), 172 (33.1), 145 (27.5), 105 (26.1), 91 (67.1), 77 (29.0), 55 (25.8).
2.10. Statistical analysis
All experiments were done in duplicates and they were repeated at least three times. Values are represented as the standard error of the mean (S.E.M.) of triplicates and the statistical significance was analyzed by One-way ANOVA or Student's test. Results with *P <0.05 and **P < 0.01 were considered as statistically significant.
3. Results
3.1. DhL inhibits differentiation of preadipocytes
To examine the potential role of DhL on the differentiation of preadipocytes, cells were incubated with induction media in the presence of various concentration of DhL. In Fig. 1A, we show that the addition of DhL inhibited the lipid content in a dose-dependent manner with an IC50 of 6 μM. Furthermore, our experimental conditions demonstrate that the addition of DMSO (0.2%) exclusively does not hinder 3T3-L1 preadipocytes differentiation (Fig. 1A).
We were further interested in examining the effect of DhL on the viability of 3T3-L1 preadipocytes. Cells were incubated with induction media in the presence of 8 μM DhL for 24hrs (Day 1) or throughout the entire 9 days of differentiation (Day 9). Cell viability was measured using the MTT colorimetric assay as described in Material and Methods. In Fig. 1A, we show that the addition of DhL blocked the differentiation of 3T3-L1 preadipocytes whereas cell viability was not affected as compared with DMSO or untreated cells (Fig. 1B). However, at higher DhL concentrations (>10μM), 3T3-L1 preadipocytes detached from the plate, which was coupled with a significant reduction in the MTT assay (data not shown). These results suggest that, up to a maximum concentration of 10 μM, DhL has a strong inhibitory activity of 3T3-L1 preadipocytes differentiation without significant effect on cell viability.
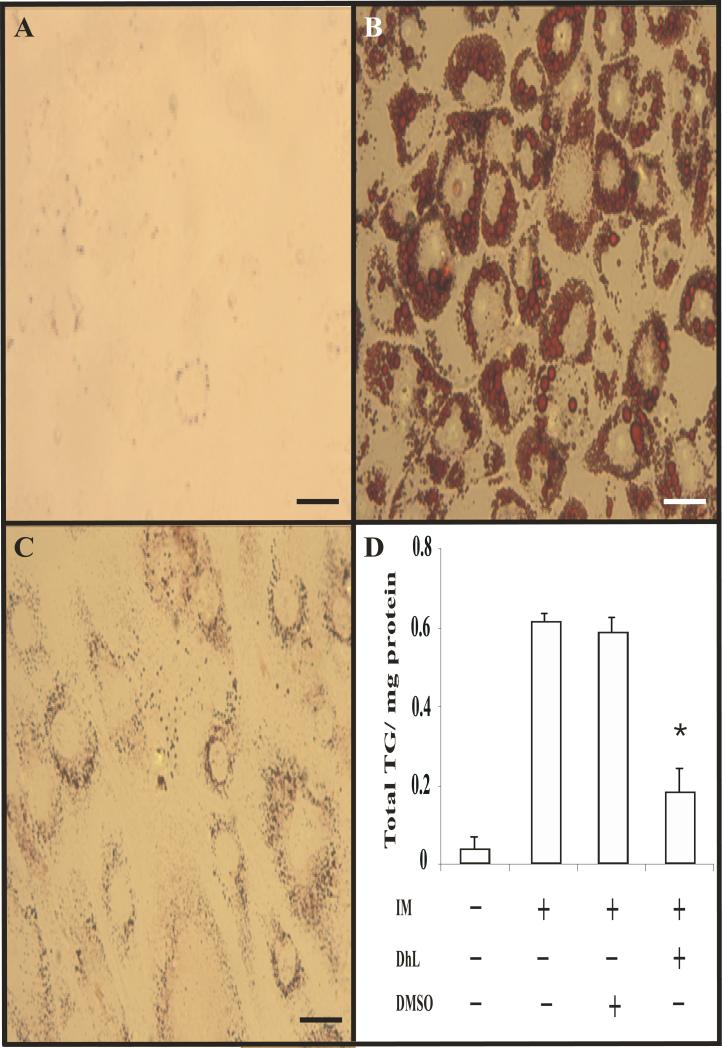
Adipocyte differentiation can be also monitored by formation of intracellular lipid droplets (Rosen and Spiegelman, 2000). As described above, 3T3-L1 preadipocytes were cultivated and induced to differentiate into adipocytes with induction media in the absence or presence of 8 μM DhL. At day 9, Oil Red O staining showed that an abundant number of lipid droplets suggesting a significant lipid accumulation in untreated differentiated cells. However, lipid droplets were not observed in untreated non-differentiated cells (compare Fig. 2A and B). More importantly, formation of lipid droplets was inhibited by 8 μM DhL treatment (Fig. 2C). These observations were further supported with the quantitative measurement of lipid content by determining the absorbance at 540 nm (Fig. 1A). In addition, DhL treatment significantly inhibited adipogenic morphology (i.e., transition from a fibroblast-like shape to an increasingly rounded-up appearance with an accumulation of cytoplasmic lipid droplets; compare Fig. 2, B and C).
Fig. 2. Dehydroleucodine blocked the formation of lipid droplet by induction media in 3T3-L1 cells.
Adipocyte differentiation was induced by treating confluent 3T3-L1 preadipocytes with induction media in the absence or presence of 8 μM DhL. Morphological changes of 3T3-L1 preadipocytes were monitored by microscope and photographed after 9 days from the onset of differentiation. (A) Vehicle only, (B) cells treated induction media in the presence DMSO, or (C) in the presence of DhL. (D) Nine days after induction of differentiation, cells were lysed for triglyceride and protein assays as described in Material and Methods. Vehicle only (line 1), cells treated with induction media alone (line 2), cells treated with induction media in the presence DMSO (line 3), and cells treated with induction media in the presence of 8 μM DhL (line 4). Bars=10μm. Data represent the mean ± S.E.M. of three independent experiments. *P < 0.05 by Student's t-test compared to DMSO-treated cells and only induction media-treated cells.
Given that DhL inhibited differentiation to 3T3-L1 preadipocytes, we next considered whether DhL would inhibit triglyceride accumulation. Cells treated with induction medium in the presence of 8 μM of DhL accumulated roughly 31±5% of the intracellular triglyceride contained in controls (Fig. 2D). As expected, DMSO-treated cells did not affect the formation of triglyceride accumulation as compared with cells incubated with induction media alone (Fig. 2D).
3.2. DhL inhibits key adipogenic transcriptional factors
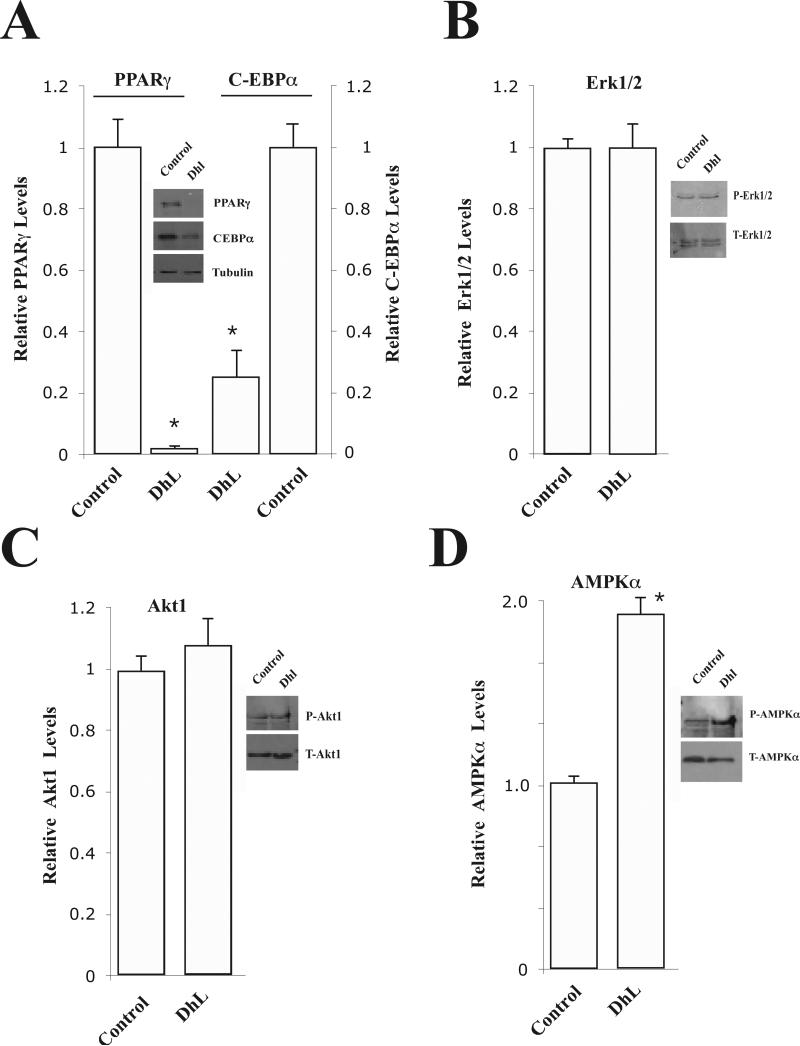
Adipogenesis is a highly regulated process requiring coordinated expression and activation of key transcriptional factors and signaling molecules (Rosen and Spiegelman, 2000) To investigate whether DhL affects the expression of PPARγ and C-EBPα, 3T3-L1 preadipocytes were incubated with induction media in the absence or presence of 8 μM DhL as described in Fig. 1, and then harvested at day 9 for Western blot analysis using anti-PPARγ and C-EBPα antibodies. In Fig. 3A, we show that the addition of DhL clearly attenuated the expression of PPARγ and C-EBPα. We also examined the effect of DhL on the phosphorylation of Akt1 and Erk1/2 proteins. To our surprise we found that the addition of DhL did not attenuate phosphorylation of Erk1/2 and Akt1 (Fig. 3B and C). Furthermore, we tested whether the addition of DhL affected the phosphorylation of AMPKα during 3T3-L1 preadipocytes differentiation. This result shows that phosphorylation of AMPKα (P-AMPKα) was not inhibited by the addition of DhL (Fig. 3D). In contrast, DhL further enhanced phosphorylation of AMPKα (Fig. 3D). Furthermore, we also found that the level of total AMPKα appeared relatively constant in the presence or absence of DhL. Therefore, these results indicated that the level of phosphorylated AMPKα increased in the presence of DhL. Interestingly, both PPARγ and C-EBPα are selectively expressed during the differentiation of 3T3-L1 preadipocytes. The levels of expression of these transcription factors is undetectable in preadipocytes; however, expression increases 2 days after induction and they are expressed 5 days after the induction of differentiation (Rosen and Spiegelman, 2000). Consistent with these observations, we also observed that the expression of PPARγ and C-EBPα was significant increased with the progression of the differentiation of 3T3-L1 preadipocyte (supplementary Fig 3A and B). However, DhL treatment at the concentration of 8μM significantly blocked the expression of PPARγ and C-EBPα (supplementary Fig. 3A and B). In addition, the differentiation of 3T3-L1 preadipocyte progressed with an increase of the phosphorylation status of AMPKα (supplementary Fig. 3C). Treatment with DhL further significantly increased the phospho-status of AMPKα, suggesting that DhL also induced the activation of AMPKα (supplementary Fig. 3C). Taken together, these data suggest that DhL selectively blocks the expression of PPARγ and C-EBPα and also increases the phosphorylation of AMPKα during the differentiation of 3T3-L1 preadipocytes.
Fig. 3. Dehydroleucodine attenuated the expression of PPARγ during 3T3-L1 preadipocyte differentiation.
3T3-L1 preadipocytes were induced to differentiate by induction media into adipocytes in the absence (insert: –DhL, control) or in the presence (insert: +DhL) of 8 μM DhL. Total protein extracts were prepared at day 9 from each sample. The proteins were subset to 12% SDS-PAGE electrophoresis, blotted to a nitrocellulose membrane, and probed with anti-bodies specific to (A) PPARγ, C-EBPα and tubulin, (B) P-Erk1/2, T-Erk1/2, (C) P-Akt1, T-Akt1, and (D) P-AMPKα and T-AMPKα respectively. Relative levels of proteins were determined by densitometry as describe in Material and Methods. Data represent the mean ± S.E.M. of three independent experiments. *P < 0.05 by Student's t-test compared to DMSO induction media-treated cells (control).
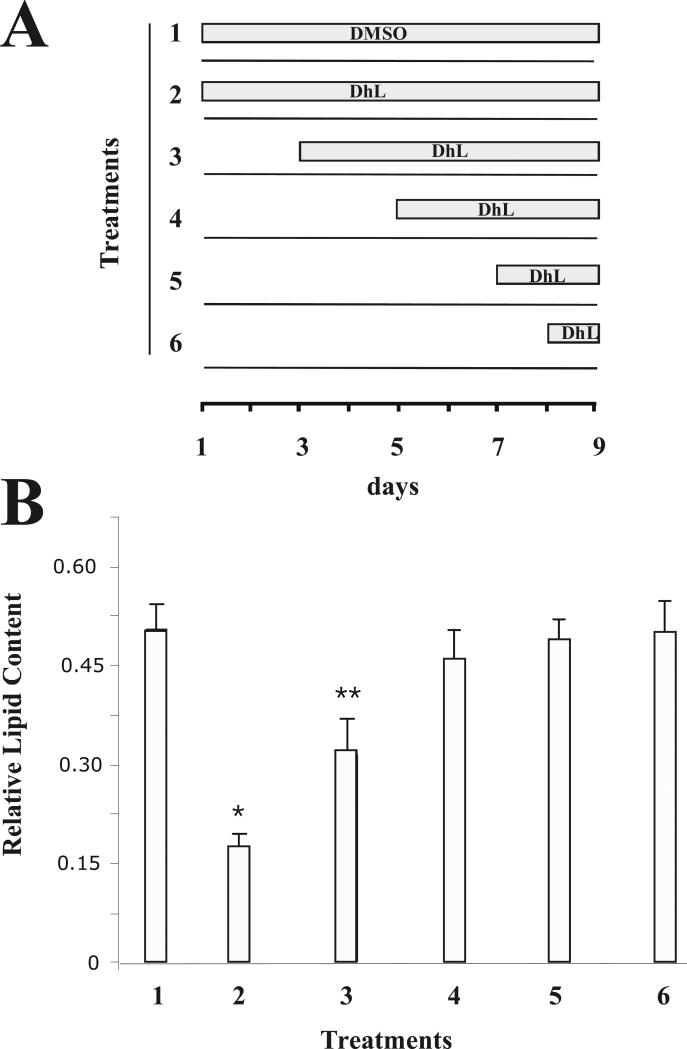
It is also possible that the extent of inhibition is dependent on the timing of DhL addition. For this purpose, 8 μM DhL was added in discrete periods during differentiation (Fig. 4A). We observed a significant reduction in the differentiation of 3T3-L1 preadipocytes when compared with DMSO-control cells upon early DhL addition, corresponding to days 1, or 3 of treatment (Fig. 4A and B). However, this inhibitory effect was not apparent when DhL was added on day 5, 7 or at day 8 post-induction. These results indicate that DhL may affect early adipocyte gene expression during in vitro differentiation.
Fig. 4. Dehydroleucodine blocked adipocyte differentiation in a time-dependent manner.
(A) 3T3-L1 preadipocyte cells were treated with 8 μM DhL for the time indicated in the schematic representation of the experiment. (B) In each treatment (1-6), the accumulation of lipid droplets was measured by the incorporation of Oil red O as described in Material and Methods. Data represent the mean ± S.E.M. of three independent experiments. *P < 0.05 and **P < 0.01 by Student's t-test compared to DMSO induction media-treated cells (control).
3.3. Effect of 11,13-dihydro-dehydroleucodine on the differentiation of 3T3-L1 preadipocytes
Our results clearly show that the addition of DhL inhibited differentiation of 3T3-L1 preadipocytes in a dose-dependent manner without a significant effect of cell toxicity (Fig. 1). However, it has been postulated that the non-saturated α-methylene-γ-lactone function of sesquiterpene lactones produces an unspecific toxic effect leading to cell death (Polo et al., 2007). Therefore, we then investigated the effect of DH-DhL, which is a derivative of DhL lacking alkylating function, on the differentiation of 3T3-L1 preadipocytes.
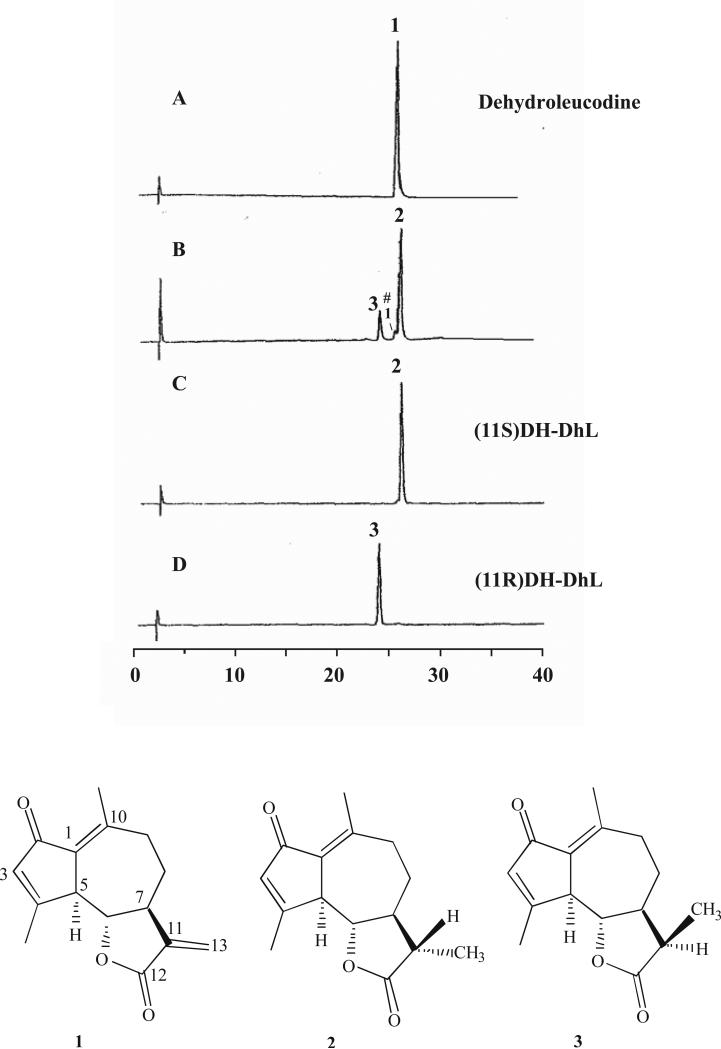
DhL (Fig. 5A, compound 1) can be gently reduced with sodium borohydride to give the corresponding 11,13-dihydro derivative (classical nomenclature; (Giordano et al., 1990). Analysis of the reaction product (DH-DhL) by GC show that two epimers (Fig. 5 B, compounds 2 and 3) are formed in different amounts (Fig. 5B) due to the generation of a chiral center at C-11 of the molecule during reduction. The 11S-epimer (compound 2) is the major reaction product (Giordano et al., 1992). It is accompanied by the minor 11R-epimer (compound 3), and by traces of unreacted DhL (compound 1) (Fig. 5B). In the following these epimers are denoted (11S)DH-DhL and (11R)DH-DhL, respectively, for simplicity. After separation by preparative HPLC, the epimers were obtained in pure form and confirmed by GC analysis (Fig. 5, C and D). They were studied, in parallel with DhL, for their effect on the differentiation of 3T3-L1 preadipocytes.
Fig. 5. GC analysis of dehydroleucodine and 11,13-dihydro-dehydroleucodine epimers.
A) DhL isolated from Artemisia douglassiana; (B) Mixture DH-DhL epimers as obtained by reduction of DhL, (#) denotes small amount of DhL after the reduction reaction; (C) DH-DhL epimer S, and, (D) DH-DhL epimer R after separation from the mixture. Chemical structures of DhL (compound 1), DH-DhL epimer 11S (compound 2) and DH-DhL epimer 11R (compound 3) (numbering according to classical nomenclature).
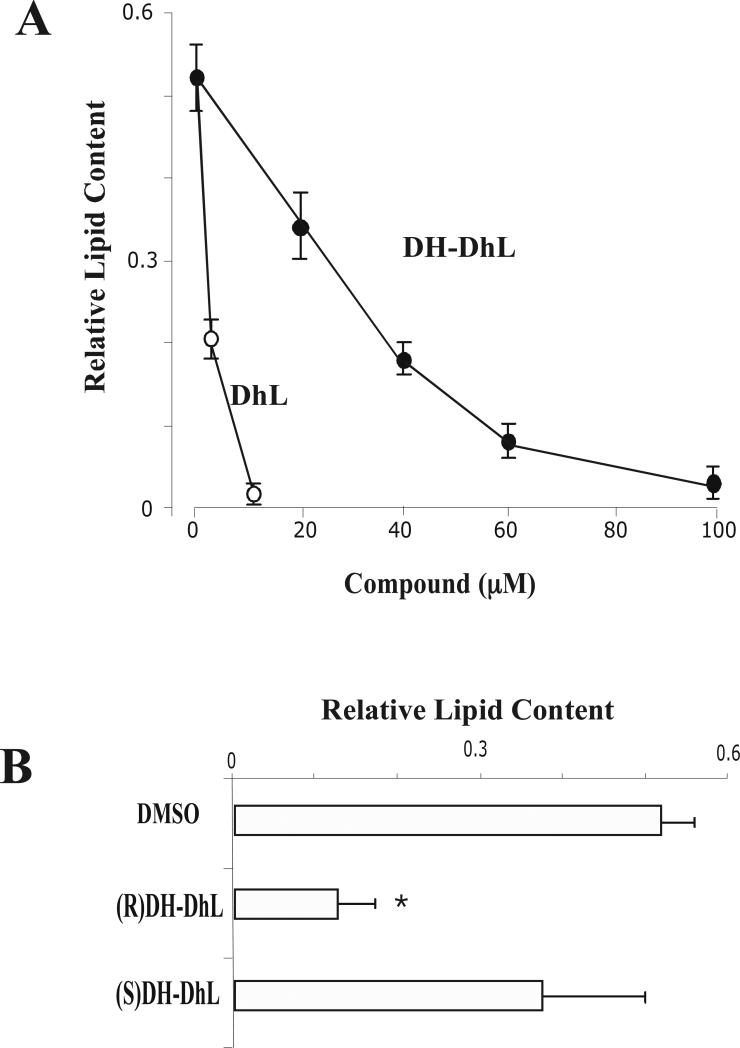
In Fig. 6A, we show that the addition of DH-DhL inhibited the differentiation of 3T3-L1 preadipocytes. This inhibition was dose-dependent and required a higher concentration of DH-DhL to produce a similar inhibitory effect as DhL. Thus, it required 10 times the DH-DhL concentration to achieve the same effect as DhL (Fig. 6A). Furthermore, we also found that 100 μM of DH-DhL did not affect cell viability (Control cells: 98±2% of viability vs. 80 μM DH-DhL treated cells: 97±2% of viability). These results suggest that the reduction of the α-methylene-γ-lactone group of the DhL was not required to block the differentiation 3T3-L1 preadipocytes.
Fig. 6. 11,13-dihydro-dehydroleucodine inhibited 3T3-L1 preadipocyte differentiation.
(A) 3T3-L1 preadipocytes were differentiated into adipocytes in the absence or in the presence of either DHL or DH-DhL. Results were represented as relative lipid content. Data represent the mean ± S.E.M. of three independent experiments. (B) 3T3-L1 preadipocyte cells were incubated with induction media supplemented with either DMSO, 80 μM DH-DhL epimer S or 80 μM DH-DhL epimer R and the incorporation of Oil Red O was measured by as described in Material and Methods. Results were represented as relative lipid content. Data represent the mean ± S.E.M. of three independent experiments. **P < 0.01 by Student's t-test compared to DMSO-treated cells.
To further investigate the role of these epimers of DH-DhL on the differentiation 3T3-L1 preadipocytes, the two methyl epimers at C-11 produced during the in vitro reduction of DhL were separated by preparative HPLC, concentrated, confirmed by GC analysis and then their effect on adipogenesis was examined. For this, 3T3-L1 preadipocytes were incubated with 80 μM of either (11S)DH-DhL or (11R)DH-DhL during the entire differentiation process. We found that the addition of (11R)DH-DhL epimer, but not (11S)DH-DhL epimer, inhibited the differentiation 3T3-L1 preadipocytes (Fig. 6B). These results suggest that (11R)DH-DhL epimer may be responsible in inhibiting adipocyte differentiation.
4. Discussion
In this study we investigated the effect of DhL in 3T3-L1 preadipocytes differentiation. The addition of DhL to the medium inhibited significantly the accumulation of lipid droplets in a dose-dependent manner. DhL attenuated dramatically the production of adipogenic transcriptional factors PPARγ and C-EBPα during adipogenesis without altering the activation of Erk1/2 and Akt1. In contrast, DhL increased the phosphorylation of AMPKα proteins. In addition, we also found that DH-DhL, a derivative of DhL with inactivated α-methylene-γ-lactone function, also inhibited the formation of adipocytes. However, it required ten times the DH-DhL concentration to achieve the same effect as DhL. More importantly, we have identified a specific DH-DhL epimer [i.e., (11R)DH-DhL], which may be responsible for such inhibitory activity.
Based on our observations, it is clear that in the adipocyte differentiation model, the α-methylene-γ-lactone moiety causes a significant decrease of 3T3-L1 pre-adipocytes differentiation at concentrations ≤10 μM without altering cell viability as demonstrated by the MTT assay. However, the addition of >10 μM DhL (i.e., 12 μM) caused cell toxicity as evidenced either by the incorporation of trypan blue dye or by the induction of detachment of cells from the tissue culture plate. Thus, the inhibition of 3T3-L1 differentiation by DhL and (11R)DH-DhL epimer may be induced by a process distinct from cell death induced by toxicity of these compounds.
Adipocyte differentiation involves a complex progression of changes in morphology, hormone sensitivity, and gene expression (Rosen and Spiegelman, 2000). 3T3L1 preadipocytes transform into adipocytes through growth arrest, clonal selection and expression of adipocyte selective markers following hormonal induction (Rosen et al., 2000). The addition of induction media containing IBMX, dexamethasone, and insulin activated several transcriptional factors, including PPARγ, C-EBPα, C-EBPβ, C-EBPδ, which in turn regulate a number of genes required during adipocyte differentiation (Spiegelman et al., 1993; Tanaka et al., 1997). Insulin is also required to ensure complete conversion of preadipocytes into adipocytes, but the precise role that it plays in this process remains unclear. The insulin receptor shows tyrosine kinase activity, and during its activation can further activate a series of signaling pathways including Erk1/2 and Akt1 activities. Several laboratories have investigated the role of Erk1/2 in adipogenesis regulation, but conclusions are somewhat controversial (Rosen and Spiegelman, 2000). Nevertheless, the role of Akt in adipogenesis is far more clearly understood since several lines of evidence have indicated the important function of Akt1 signaling cascade during adipogenesis (Koppen and Kalkhoven, 2008; Rosen et al., 2000). In addition, AMPK is serine/ threonine known to play a major role in energy homeostasis (Kemp et al., 2003) and it is regulated by adenosine monophosphate (Shaw et al., 2004). AMPK cascades have also emerged as novel targets for the treatment of obesity (Meisse et al., 2002; Song et al., 2002). Thus, it is possible to assume that DhL may selectively block pathways regulating adipocytic differentiation containing the adipocyte-specific factors.
Treatment of 3T3-L1 preadipocytes with DhL dramatically reduced protein expression of PPARγ and C-EBPα, which is strictly concordant with the appearance of cytoplasmic lipid droplets. These data suggest that DhL may target specific pathways related to the expression of PPARγ and in minor extension to the expression C-EBPα, because of the strong inhibition of PPARγ upon addition of DhL. We also observed a significant increase in the phosphorylation of AMPKα during 3T3-L1 differentiation. However, the level of phosphorylated AMPKα was further enhanced by the presence of DhL during 3T3-L1 differentiation. Therefore, these results indicated that the addition of DhL significantly increased the phosphorylation of AMPKα during 3T3-L1 differentiation, which was detrimental to 3T3-L1 differentiation. In addition, our observations showed that the effect of DhL on adipocyte differentiation may be selective since both Akt1 and Erk1/2 activities, which are thought to be sensitive to insulin signal, were not affected by the addition of DhL. Thus, the inhibitory effect of DhL does not seem to be related to these two signaling molecules.
In summary, this report suggests that DhL inhibits the adipogenic differentiation process. The mechanisms by which DhL regulates adipogenesis include the inhibition of expression of the adipogenic transcription factors, PPARγ and C-EBPα. In addition, we also found that a DH-DhL epimer, lacking a highly reactive and nonspecific α-methylene-γ-lactone moiety, may improve its use without a cytotoxic effect. Therefore, it is anticipated that the inhibition of differentiation into adipocytes by DhL and its derivates may be beneficial for the prevention of obesity. For this reason, natural products that specifically inhibited adipogenesis might be considered with regard to their potential in treatment of obesity. However, it remains to be determined whether manipulating adipogenesis can be beneficial and/or healthy without leading to other metabolic diseases.
Supplementary Material
Acknowledgment
This work was partially supported by the National Institutes of Health grant SC1DK084343 (to MAB) and by SECyTP, UNCuyo 06 J 213 grant and ANPCYT PICT-R 2005 32850 grant (to LAL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Auld CA, Hopkins RG, Fernandes KM, Morrison RF. Novel effect of helenalin on Akt signaling and Skp2 expression in 3T3-L1 preadipocytes. Biochem. Biophys. Res. Commun. 2006;346:314–320. doi: 10.1016/j.bbrc.2006.05.117. [DOI] [PubMed] [Google Scholar]
- Beekman AC, Woerdenbag HJ, van Uden W, Pras N, Konings AW, Wikstrom HV, Schmidt TJ. Structure-cytotoxicity relationships of some helenanolide-type sesquiterpene lactones. J. Nat. Prod. 1997;60:252–257. doi: 10.1021/np960517h. [DOI] [PubMed] [Google Scholar]
- Blanco JG, Gil RR, Alvarez CI, Patrito LC, Genti-Raimondi S, Flury A. A novel activity for a group of sesquiterpene lactones: inhibition of aromatase. FEBS. Lett. 1997;409:396–400. doi: 10.1016/s0014-5793(97)00560-7. [DOI] [PubMed] [Google Scholar]
- Bohlmann F, Zdero C. Zwei neue Sesquiterpen-lactone aus Lidbeckia pectinata Berg. und Pentzia elegans DC. Tetrahedron Lett. 1972;13:621–624. [Google Scholar]
- Bost F, Aouadi M, Caron L, Binetruy B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie. 2005;87:51–56. doi: 10.1016/j.biochi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Brengio SD, Belmonte SA, Guerreiro E, Giordano OS, Pietrobon EO, Sosa MA. The sesquiterpene lactone dehydroleucodine (DhL) affects the growth of cultured epimastigotes of Trypanosoma cruzi. J. Parasitol. 2000;86:407–412. doi: 10.1645/0022-3395(2000)086[0407:TSLDDA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Cheng CH, Costall B, Hamburger M, Hostettmann K, Naylor RJ, Wang Y, Jenner P. Toxic effects of solstitialin A 13-acetate and cynaropicrin from Centaurea solstitialis L. (Asteraceae) in cell cultures of foetal rat brain. Neuropharmacology. 1992;31:271–277. doi: 10.1016/0028-3908(92)90177-q. [DOI] [PubMed] [Google Scholar]
- Galal O. Nutrition-related health patterns in the Middle East. Asia Pac. J. Clin. Nutr. 2003;12:337–343. [PubMed] [Google Scholar]
- Giordano OS, Guerreiro E, Pestchanker MJ, Guzman J, Pastor D, Guardia T. The gastric cytoprotective effect of several sesquiterpene lactones. J. Nat. Prod. 1990;53:803–809. doi: 10.1021/np50070a004. [DOI] [PubMed] [Google Scholar]
- Giordano OS, Pestchanker MJ, Guerreiro E, Saad JR, Enriz RD, Rodriguez AM, Jauregui EA, Guzman J, Maria AO, Wendel GH. Structure-activity relationship in the gastric cytoprotective effect of several sesquiterpene lactones. J. Med. Chem. 1992;35:2452–2458. doi: 10.1021/jm00091a013. [DOI] [PubMed] [Google Scholar]
- Hay AJ, Hamburger M, Hostettmann K, Hoult JR. Toxic inhibition of smooth muscle contractility by plant-derived sesquiterpenes caused by their chemically reactive alpha-methylenebutyrolactone functions. Br. J. Pharmacol. 1994;112:9–12. doi: 10.1111/j.1476-5381.1994.tb13020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Hayashi T, Ujita K, Takaishi Y. Characterization of antiviral activity of a sesquiterpene, triptofordin C-2. J. Antimicrob. Chemother. 1996;37:759–768. doi: 10.1093/jac/37.4.759. [DOI] [PubMed] [Google Scholar]
- Hehner SP, Heinrich M, Bork PM, Vogt M, Ratter F, Lehmann V, Schulze-Osthoff K, Droge W, Schmitz ML. Sesquiterpene lactones specifically inhibit activation of NF-kappa B by preventing the degradation of I kappa B-alpha and I kappa B-beta. J. Biol. Chem. 1998;273:1288–1297. doi: 10.1074/jbc.273.3.1288. [DOI] [PubMed] [Google Scholar]
- Heinrich M, Robles M, West JE, Ortiz de Montellano BR, Rodriguez E. Ethnopharmacology of Mexican asteraceae (Compositae). Annu. Rev. Pharmacol. Toxicol. 1998;38:539–565. doi: 10.1146/annurev.pharmtox.38.1.539. [DOI] [PubMed] [Google Scholar]
- Hwang D, Fischer NH, Jang BC, Tak H, Kim JK, Lee W. Inhibition of the expression of inducible cyclooxygenase and proinflammatory cytokines by sesquiterpene lactones in macrophages correlates with the inhibition of MAP kinases. Biochem. Biophys. Res. Commun. 1996;226:810–818. doi: 10.1006/bbrc.1996.1433. [DOI] [PubMed] [Google Scholar]
- Kemp BE, Stapleton D, Campbell DJ, Chen ZP, Murthy S, Walter M, Gupta A, Adams JJ, Katsis F, van Denderen B, Jennings IG, Iseli T, Michell B,J, Witters LA. AMP-activated protein kinase, super metabolic regulator. Biochem. Soc. Trans. 2003;31:162–168. doi: 10.1042/bst0310162. [DOI] [PubMed] [Google Scholar]
- Koppen A, Kalkhoven E. Brown vs white adipocytes: the PPARgamma coregulator story. FEBS. Lett. 2008;584:3250–3259. doi: 10.1016/j.febslet.2010.06.035. [DOI] [PubMed] [Google Scholar]
- Lagra F, Karastergiou K, Delithanasis I, Koutsika E, Katsikas I, Papadopoulou-Zekeridou P. Obesity and colorectal cancer. Tech. Coloproctol. 2004;8(Suppl 1):s161–163. doi: 10.1007/s10151-004-0144-7. [DOI] [PubMed] [Google Scholar]
- Lyss G, Knorre A, Schmidt TJ, Pahl HL, Merfort I. The anti-inflammatory sesquiterpene lactone helenalin inhibits the transcription factor NF-kappaB by directly targeting p65. J. Biol. Chem. 1998;273:33508–33516. doi: 10.1074/jbc.273.50.33508. [DOI] [PubMed] [Google Scholar]
- Meisse D, Van de Casteele M, Beauloye C, Hainault I, Kefas BA, Rider MH, Foufelle F, Hue L. Sustained activation of AMP-activated protein kinase induces c-Jun N-terminal kinase activation and apoptosis in liver cells. FEBS. Lett. 2002;526:38–42. doi: 10.1016/s0014-5793(02)03110-1. [DOI] [PubMed] [Google Scholar]
- Mendez AJ, Cabeza C, S.L. H. a fluormetric metod for the determination of triglycerides in nanamolar quantities. Anal. Biochem. 1986;156:386–389. doi: 10.1016/0003-2697(86)90269-1. [DOI] [PubMed] [Google Scholar]
- Penissi AB, Fogal TH, Guzman JA, Piezzi RS. Gastroduodenal mucosal protection induced by dehydroleucodine: mucus secretion and role of monoamines. Dig. Dis. Sci. 1998;43:791–798. doi: 10.1023/a:1018822215956. [DOI] [PubMed] [Google Scholar]
- Perry NB, Foster LM. Sesquiterpene/quinol from a New Zealand liverwort, Riccardia crassa. J. Nat. Prod. 1995;58:1131–1135. doi: 10.1021/np50121a027. [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer FX. The obesity epidemic: pathophysiology and consequences of obesity. Obes. Res. 2002;10(Suppl 2):97S–104S. doi: 10.1038/oby.2002.202. [DOI] [PubMed] [Google Scholar]
- Polo LM, Castro CM, Cruzado MC, Collino CJ, Cuello-Carrion FD, Ciocca DR, Giordano OS, Ferrari M, Lopez LA. 11,13-dihydro-dehydroleucodine, a derivative of dehydroleucodine with an inactivated alkylating function conserves the anti-proliferative activity in G2 but does not cause cytotoxicity. Eur. J. Pharmacol. 2007;556:19–26. doi: 10.1016/j.ejphar.2006.10.049. [DOI] [PubMed] [Google Scholar]
- Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu. Rev. Med. 2010;61:301–316. doi: 10.1146/annurev.med.080708.082713. [DOI] [PubMed] [Google Scholar]
- Robles M, Aregullin M, West J, Rodriguez E. Recent studies on the zoopharmacognosy, pharmacology and neurotoxicology of sesquiterpene lactones. Planta. Med. 1995;61:199–203. doi: 10.1055/s-2006-958055. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. Molecular regulation of adipogenesis. Annu. Rev. Cell. Dev. Biol. 2000;16:145–171. doi: 10.1146/annurev.cellbio.16.1.145. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes. Dev. 2000;14:1293–1307. [PubMed] [Google Scholar]
- Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc. Natl. Acad. Sci. U S A. 2003;100:7265–7270. doi: 10.1073/pnas.1133870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMPactivated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. U S A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheard NF. Cow's milk, diabetes, and infant feeding. Nutr. Rev. 1993;51:79–81. doi: 10.1111/j.1753-4887.1993.tb03074.x. [DOI] [PubMed] [Google Scholar]
- Song XM, Fiedler M, Galuska D, Ryder JW, Fernstrom M, Chibalin AV, Wallberg-Henriksson H, Zierath JR. 5-Aminoimidazole-4-carboxamide ribonucleoside treatment improves glucose homeostasis in insulin-resistant diabetic (ob/ob) mice. Diabetologia. 2002;45:56–65. doi: 10.1007/s125-002-8245-8. [DOI] [PubMed] [Google Scholar]
- Spiegelman BM, Choy L, Hotamisligil GS, Graves RA, Tontonoz P. Regulation of adipocyte gene expression in differentiation and syndromes of obesity/diabetes. J. Biol. Chem. 1993;268:6823–6826. [PubMed] [Google Scholar]
- Tanaka T, Yoshida N, Kishimoto T, Akira S. Defective adipocyte differentiation in mice lacking the C/EBPbeta and/or C/EBPdelta gene. Embo. J. 1997;16:7432–7443. doi: 10.1093/emboj/16.24.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wofford MR, Andrew ME, Brown A, King D, Pickett RA, Stevens J, Wyatt S, Jones DW. Obesity Hypertension in the Atherosclerosis Risk in Communities Cohort: Implications of Obesity Guidelines. J. Clin. Hypertens. 1999;1:27–32. [PubMed] [Google Scholar]
- Yun JW. Possible anti-obesity therapeutics from nature--a review. Phytochemistry. 2010;71:1625–1641. doi: 10.1016/j.phytochem.2010.07.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.