Elizabeth blackburn, jack szostak, and carol greider were recently awarded the Nobel Prize in Physiology or Medicine for their elucidation of the structure and maintenance of telomeres (the tips of chromosomes). These investigators discovered that telomeres are DNA sequences with a structure that protects chromosomes from erosion and that a specific enzyme, telomerase, is involved in their repair after mitosis.1,2 In this review, we discuss the medical implications of these discoveries.
Telomeres were causally connected to human disease when mutations in the DKC1 gene were detected in a rare inherited form of bone marrow failure.3 DKC1 encodes a protein of the telomerase complex.4 Telomeres are short in many patients with inherited or apparently acquired aplastic anemia, and mutations affecting telomerase have been identified in these forms of aplastic anemia; telomerase mutations also have been associated with fibrosis of the lungs and the liver. Moreover, the telomerase gene is a susceptibility locus for cancer, and short telomeres may be risk factors for cardiovascular disease. Thus, a common molecular mechanism appears to underlie a range of clinical entities. An understanding of the role of telomeres in disease has important implications for diagnosis, genetic counseling, clinical management, and therapy.
TELOMERES AND TELOMER ASE
Telomeres and telomerase provide protection against threats to the genome that arise from a difficulty inherent in the asymmetric replication of DNA. Without telomeres, genetic material would be lost every time a cell divides. DNA polymerase requires an RNA primer with a 3′ hydroxyl donor group to initiate DNA replication, during which the “end-replication problem” arises.5 The primer dissociates as the DNA polymerase moves along the template strand, leaving behind a gap at the ends of chromosomes. As a result, the newly synthesized DNA strand is shorter than the original template. Telomeres and telomerase ameliorate this problem by providing a repetitive template for enzymatic repair of the ends of chromosomes, thereby avoiding the loss of genetically encoded information during mitosis.
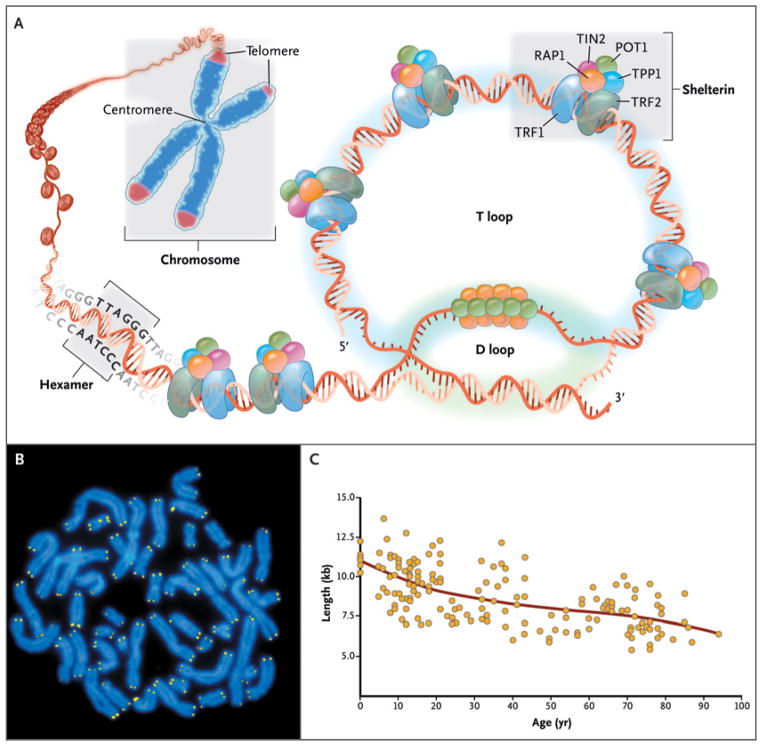
Telomeres consist of repetitive DNA sequences coated by capping proteins (shelterin) at the ends of linear chromosomes (Fig. 1). In human cells, telomeres consist of hundreds to thousands of TTAGGG tandem repeats in the leading strand.7 A single-stranded 3′-hydroxyl overhang is generated by the catalytic addition of telomeric repeats to the 3′ end and by postreplicative processing of the lagging strand. Shelterin proteins, which coat the telomeric DNA sequence,8 serve as a molecular signal to prevent the cellular DNA repair machinery from mistaking telomeres for double-stranded DNA breaks.
Figure 1. Telomere Structure.
As shown in Panel A, telomeres are located at the ends of linear chromosomes; they are composed of hundreds to thousands of tandem DNA repeat sequences: hexameric TTAGGG in the leading strand and CCCTAA in the lagging strand in humans. Protective proteins associated with telomere DNA are collectively termed shelterin (TRF1, TRF2, TIN2, POT1, TPP1, and RAP1). The 3′ end of the telomeric leading strand terminates as a single-stranded overhang, which folds back and invades the double-stranded telomeric helix, forming the T loop. As shown in Panel B, telomeres can be directly visualized under the microscope at the ends of metaphase chromosomes (four telomere signals per chromosome) by fluorescence in situ hybridization (FISH). (Image provided by Peter Lansdorp, M.D., Ph.D.) Average telomere length can be measured by several methods: a technique that combines flow cytometry and FISH (flow-FISH), Southern blotting, and a quantitative polymerase-chain-reaction (qPCR) assay. Flow-FISH can measure the telomere length in different cell subgroups, such as granulocytes or CD4+ T lymphocytes; Southern blotting reveals length and length heterogeneity; and qPCR is a rapid assay that requires very small amounts of DNA. As shown in Panel C, the average length of telomeres in human leukocytes varies, ranging from approximately 11 kb at birth (in umbilical-cord blood) to 6 kb at 90 years of age. Telomere loss is most rapid early in life, and over a life span it is not linear but follows a third-order polynomial. Data are from Yamaguchi et al.6
When they are too short, telomeres signal the arrest of cell proliferation, senescence, and apoptosis. This process explains the interruption of proliferation in cultured human cells — the “Hayflick limit” (Fig. 2).9 If protective mechanisms, such as the TP53 tumor-suppressor gene, are inactive, thus allowing continued proliferation, telomeres become extremely short and dysfunctional; end-to-end fusions ultimately cause chromosomal instability. Conversely, cells transfected with the telomerase gene can proliferate indefinitely.10
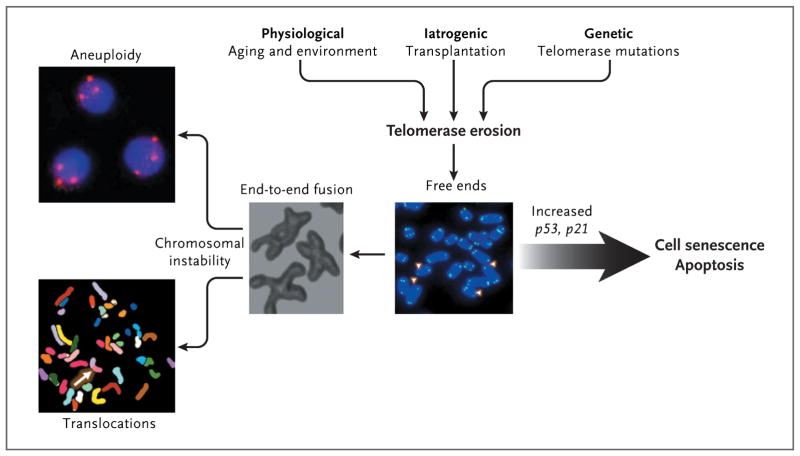
Figure 2. Consequences of Telomere Erosion in the Cell.
Telomeres inexorably shorten with every cell division, and telomere attrition is an inevitable physiological consequence of aging. Telomere shortening also may be iatrogenic; for example, telomere shortening occurs after bone marrow transplantation, in which highly proliferative hematopoietic stem cells and progenitor cells reconstitute hematopoiesis. Environmental factors also may accelerate telomere loss. In addition, telomere attrition may be genetic; there may be an inherited inability to elongate telomeres as a result of mutations in components of the telomerase complex. When telomeres become critically short, inappropriately capped chromosomes or telomere-free ends emerge, which lead to cell senescence or apoptosis. If the cell overrides senescence and continues to proliferate (e.g., because of inactive p53), uncapped telomeres may cause end-to-end fusion of chromosomes, breakage-fusion-bridge cycles, aneuploidy, and chromosomal translocations.
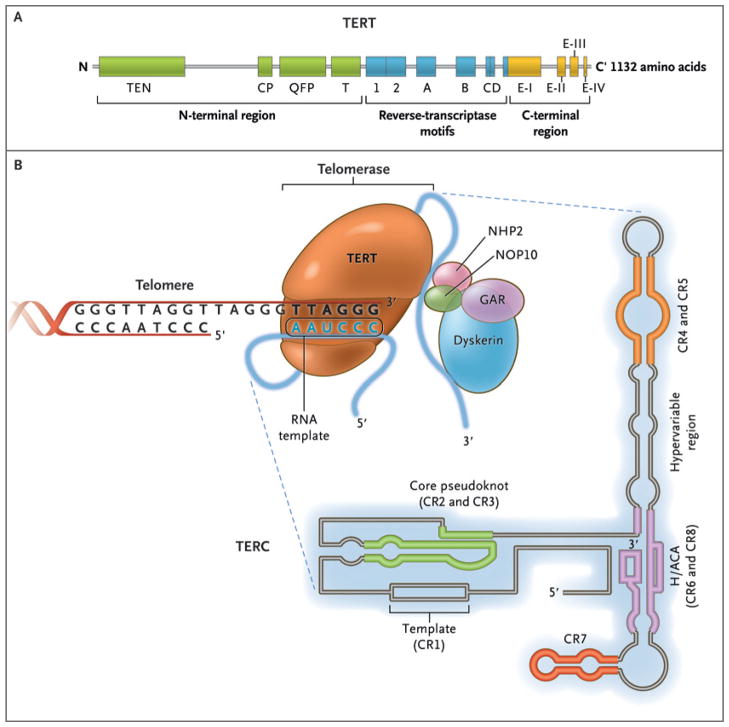
To avoid the attrition of telomeres, germ-line cells and some somatic cells produce telomerase, an enzyme that catalyzes DNA synthesis to maintain telomere length. Telomerase reverse transcriptase (TERT) uses the telomerase RNA component (TERC) as a template to synthesize telomere DNA (Fig. 3). The catalytic unit of telomerase contains two copies each of TERT, TERC, and dyskerin (encoded by the DKC1 gene), and proteins that stabilize the complex.
Figure 3. The Telomerase Complex and Its Components.
The enzyme telomerase reverse transcriptase (TERT), its RNA component (TERC), the protein dyskerin, and other associated proteins (NHP2, NOP10, and GAR1) are shown. Telomerase catalytically adds TTAGGG hexameric nucleotide repeats to the 3′-hydroxyl end of the telomeric leading strand, using a specific sequence in the RNA component as the template. TERT contains three major domains: the N-terminal region, the reverse-transcriptase motifs, and the C-terminal region, all containing evolutionarily conserved motifs. TERC contains 451 nucleotides in seven conserved regions (CR1 through CR7), including the template (CR1), and an H/ACA box, a hairpin nucleotide sequence characteristic of a class of small nucleolar RNAs involved in RNA processing.
Telomerase has functions other than elongating telomeres.11 For example, telomerase over-expression in adult mice mobilizes stem cells and induces stem-cell proliferation in the absence of telomere elongation by modulation of the wingless in drosophila (Wnt)–β-catenin signaling pathway.12
Mice in which telomerase genes have been knocked out have been used to model the role of these genes in higher organisms. However, differences in telomere biology between mice and humans preclude ideal modeling of human biology in the mouse system. Moreover, in contrast to mice in the wild, laboratory strains have very long telomeres, and the first generation of telomerase-knockout mice does not show critical telomere shortening or a phenotype. Tissue abnormalities usually appear after the fifth generation; by the sixth generation, mice are infertile and hematopoietic progenitor function is defective.
DISEASES OF TELOMERES
BONE MARROW FAILURE
Hematopoietic dysfunction caused by defective telomere structure and repair has a broad clinical spectrum. The manifestations may occur from birth to late adulthood, they may range in severity from no abnormalities or mild hematologic abnormalities to extreme pancytopenia, and they may be associated with anomalies, as in dyskeratosis congenita. Telomere mutations are inherited, but penetrance can vary, even within pedigrees.
DYSKERATOSIS CONGENITA
A form of ectodermal dysplasia, dyskeratosis congenita, is characterized by a triad of signs: dystrophic nails, patchy skin hyperpigmentation, and oral leukoplakia (Fig. 4). Mucocutaneous findings are present in infancy, and bone marrow failure follows in the first or second decade; aplastic anemia is usually fatal. There is variation in the clinical presentation, other organ systems may be involved, and pulmonary disease can be lethal.14 A particularly severe variant of dyskeratosis congenita is the Hoyeraal–Hreidarsson syndrome of progressive pancytopenia, neurologic manifestations of microcephaly, ataxia, and growth retardation in young children. Dyskeratosis congenita may occasionally be first diagnosed in midlife, with only minimally abnormal blood counts.15
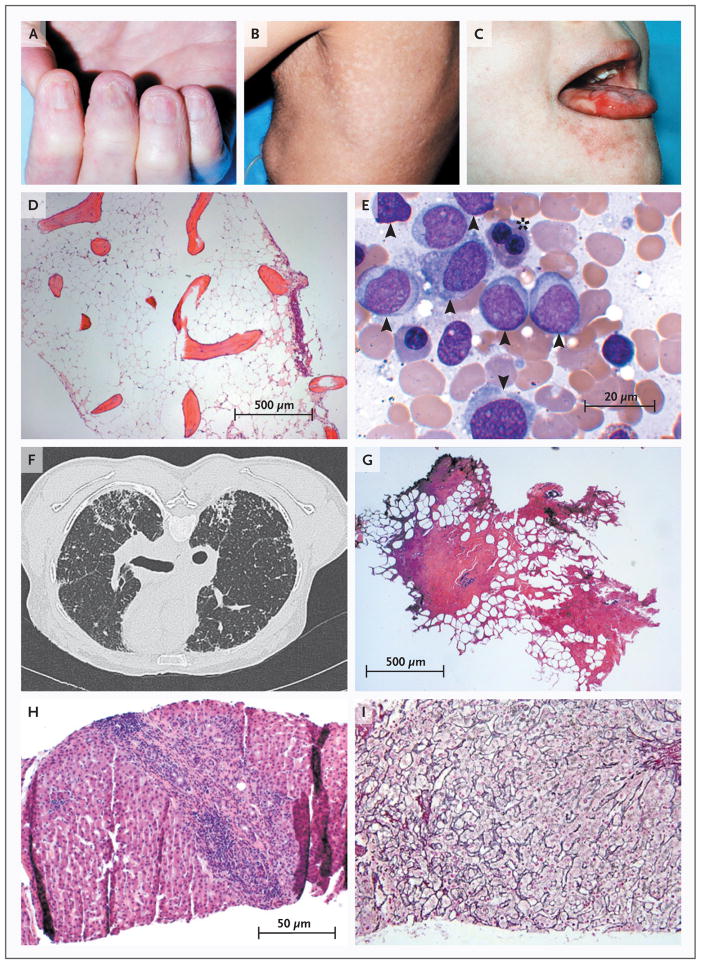
Figure 4. Pathologic Consequences of Telomere Erosion in Organs and Tissues.
The childhood syndrome dyskeratosis congenita is characterized clinically by the mucocutaneous triad of nail dystrophy (Panel A), reticular skin hyperpigmentation or hypopigmentation (Panel B), and leukoplakia (Panel C) (reprinted from Alter,13 with the permission of the publisher). In the bone marrow, telomere shortening confers a predisposition to aplastic anemia (Panel D, Giemsa stain) and progression to myelodysplasia and acute myeloid leukemia (Panel E, Giemsa stain). Leukemic bone marrow is characterized by an increased number of myeloid blasts (arrowheads) and dysplasia or dyserythropoiesis (asterisk). In the lungs, telomere attrition can be clinically manifested as pulmonary fibrosis and radiologically characterized by diffuse fibrosis predominantly in the subpleural region (Panel F). Histologically, fibrotic zones alternate with less affected parenchyma (Panel G, hematoxylin and eosin). Telomere shortening in the liver has diverse histologic appearances, including cirrhosis with inflammation (Panel H, hematoxylin and eosin) and nodular regenerative hyperplasia (Panel I, reticulin stain).
Family studies led to the recognition of mutations affecting telomere maintenance. Most cases of dyskeratosis occur in boys. Genetic analysis of multiplex pedigrees linked the phenotype to the Xq28 region, and the gene was named DKC1.3 The encoded protein, dyskerin, is a small nucleolar protein that binds to RNA (including ribosomal RNA and TERC) and affects many cell functions.
After the discovery of DKC1 mutations, very short telomeres were detected in all patients with dyskeratosis congenita.16 Mutations were then sought in the RNA template gene in autosomal dominant pedigrees.4,17 Genetic screening revealed heterozygous mutations in TERC, homozygous mutations in NOP1018 and NHP219 (genes encoding proteins that, like dyskerin, associate with the complex), and in TERT. Recently, mutated TINF2 was detected in autosomal dominant dyskeratosis congenita20; loss of this protein in the shelterin complex causes extremely short telomeres.8 There is no clear relation between specific mutations and phenotype, but patients with the shortest telomeres (as in the Hoyeraal–Hreidarsson syndrome) tend to have the most severe disease. These data indicate that dyskeratosis congenita is the result of defective repair or protection of telomeres. An indication of the complexity of the pathways involved in the disease is the fact that a genetic defect has not yet been identified in most patients with this syndrome.
There is no agreed-on case definition of dyskeratosis congenita. As a result, there may be uncertainty regarding the characteristics of a patient with a mutation. We recommend reserving the diagnosis of dyskeratosis congenita for well-defined kindreds with abnormalities of the integument and an early onset of visceral-organ involvement. Laboratory testing to detect dyskeratosis congenita is also problematic. The presence of very short telomeres in lymphocytes, detected by means of flow cytometry and fluorescence in situ hybridization in commercial laboratories, appears to distinguish dyskeratosis congenita from other constitutional syndromes involving bone marrow failure.21
Telomere dysfunction has several peculiar and important clinical consequences. Some families show “anticipation,” or worsening manifestations of disease in succeeding generations as a consequence of inadequate repair of telomeres in germ-line cells.22 With the exception of DKC1 deficiency, which is complete because of X-linkage of the gene, dominance of the telomere repair defect as a result of heterozygous TERC and TERT mutations is due to haploinsufficiency.23 The remaining normal gene might be induced to compensate (e.g., by means of sex hormone therapy). Both male and female sex hormones up-regulate TERT expression and telomerase function in cultured hematopoi-etic cells.24 Within families, genetically normal members may inherit short telomeres from one parent with the mutation, but it is unclear whether, with their normal telomere repair capacity, they are at risk for disease. Pedigree interpretation occasionally can be complicated by mosaicism, and girls and women may have a mild phenotype with a single X-linked DKC1 mutation.14
The treatment of dyskeratosis congenita has not been studied systematically. Androgens improve blood counts in about 60% of patients.15 Experience with bone marrow transplantation in dyskeratosis congenita is limited: children have been cured, but multiorgan complications often occur.25,26 Regardless of the severity of bone marrow manifestations or therapy, lifelong monitoring for cancer is imperative.27,28
ACQUIRED APLASTIC ANEMIA
Measurements of telomere length in hematopoietic cells preceded the discovery of the mutations in dyskeratosis. Studies of clinical specimens were stimulated by the potential role of telomeres as “mitotic clocks,” or surrogates of the cell’s proliferative history.29 For example, telomeres of leukocytes are shorter in transplant recipients than in their donors, at least transiently.30,31 In studies that showed that telomere length was short in some patients with acquired aplastic anemia, it was assumed to be due to proliferative stress on limited numbers of hematopoietic stem cells.32,33 After the cause of dyskeratosis congenita was revealed, this explanation became suspect.
Systematic screening of patients with apparently acquired bone marrow failure showed a few patients with TERC mutations34; they were adults with a diagnosis of acquired disease and without the physical signs of dyskeratosis congenita.35 Hematologic abnormalities, if any, in other family members with TERC mutations were often mild and not progressive, but the bone marrow was very hypocellular, hematopoietic progenitors were diminished, and there were elevated levels of circulating hematopoietic growth factors. Bone marrow transplantation from a histocompatible sibling with an unrecognized TERC mutation culminated in early death from graft failure in one patient; this led to the selection of an unrelated donor rather than a sibling donor in a second patient.
Mutations in TERT were first discovered in patients with aplastic anemia.6,36 As with TERC, most patients were adults with a recent onset of bone marrow failure; in some patients, there was progression from moderate to severe pancytopenia, and in others, blood counts remained stable. Family histories were not obvious for blood disease, but phenotyping of pedigrees showed a range of blood counts in TERT mutation carriers. The failure of organs other than the bone marrow, including the liver and the lung, was also associated with TERT mutations.
Overall, mutations in telomerase genes (but not in DKC1) appear to explain the short telomeres detected in about 10% of patients with aplastic anemia. Mutations are associated with short telomere length (adjusted for the patient’s age) of blood leukocytes. The enzymatic activity of mutant telomerase is decreased. As in dyskeratosis congenita, heterozygosity causes disease by means of a dominant mechanism of haploinsufficiency.
PULMONARY FIBROSIS
The characteristics of idiopathic pulmonary fibrosis are cough, dyspnea, impaired gas exchange, and reduced lung volume. Pathologically, there is patchy fibrosis of the lungs and interstitial inflammation, normal lung alternating with fibrosis, inflammation, and collagen deposition (Fig. 4F and 4G). In about 20% of patients with dyskeratosis congenita, lung complications ultimately diagnosed as pulmonary fibrosis develop15; there is often a family history of interstitial pneumonia.17,37,38 Respiratory failure is also a common fatal complication after hematopoietic stem-cell transplantation in patients with dyskeratosis.26,39 These associations led to the discovery of telomerase mutations in about 15% of patients with familial idiopathic pulmonary fibrosis.40–42 In these kindreds, there was also bone marrow failure or liver cirrhosis.41,43 Smoking is common among affected patients, suggesting a role of environmental factors in the development of the disease. Telomerase mutations are also sometimes present in patients with sporadic idiopathic pulmonary fibrosis.41 Many more patients with idiopathic pulmonary fibrosis have short telomeres than identified mutations, suggesting as-yet-unidentified genetic abnormalities in a higher proportion of patients.43
LIVER DISEASE
Some patients with dyskeratosis congenita have liver abnormalities or fatal liver complications after bone marrow transplantation.15,39 Patients with pulmonary fibrosis and short telomeres can also have cryptogenic hepatic cirrhosis; this implicates telomere loss in both fibrotic processes. We have observed that many relatives of patients with aplastic anemia and a telomerase mutation have liver disease.44 Liver diseases associated with TERT and TERC mutations are mainly fibrosis with inflammation and nodular regenerative hyperplasia, a leading cause of noncirrhotic portal hypertension (Fig. 4H and 4I). The genetic basis of the peculiar familial association of bone marrow, liver, and lung disease38,45,46 is telomere erosion and telomerase mutations.
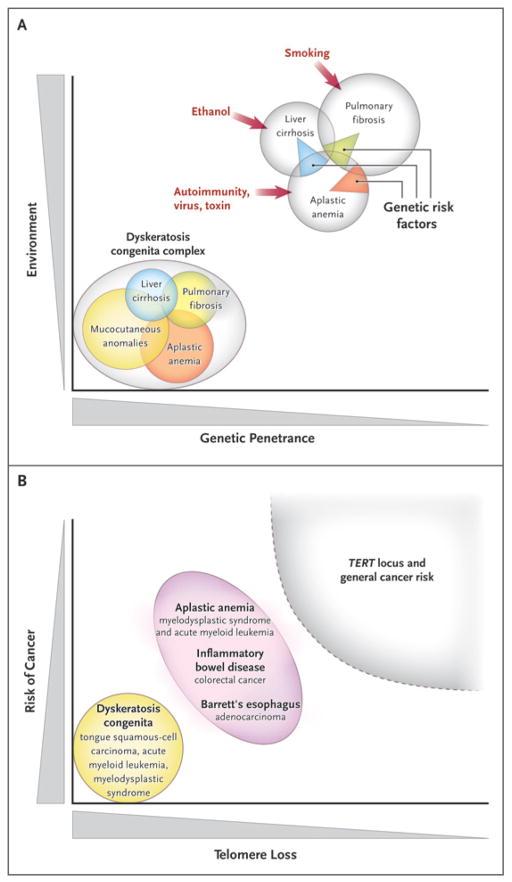
In summary, telomere diseases can be viewed as a spectrum, from genes acting as determinants of the stereotypical dyskeratosis congenita syndrome to genetic risk factors in specific types of organ failure and fibrosis (Fig. 5A).
Figure 5. Telomere Erosion and Human Diseases.
Panel A shows a Venn diagram of mutations of the telomerase complex and human telomere diseases. Dyskeratosis congenita is the most evident and severe manifestation of genetic lesions causing telomere diseases, with high genetic penetrance and congenital clinical manifestations. However, telomerase mutations may be less penetrant and induce single-organ damage in adults without suggestive family histories and the classic physical signs of dyskeratosis congenita. Thus, telomerase mutations represent risk factors rather than genetic determinants in aplastic anemia, pulmonary fibrosis, and liver cirrhosis. Environmental, epigenetic, and other genetic factors probably contribute to disease development in these patients. Panel B shows the relationship between telomere shortening and the risk of cancer. In dyskeratosis congenita, in which genetic penetrance is high, the risk of the development of cancer — particularly head and neck squamous-cell carcinoma and acute myeloid leukemia — also is elevated. In addition, patients with aplastic anemia are at risk for the development of clonal malignant disorders, but the risk is lower than that among patients with dyskeratosis congenita. Similarly, short telomeres appear to predict the progression of chronic inflammatory gastrointestinal states to adenocarcinoma. In multiple genomewide association studies, the TERT locus has appeared as a significant susceptibility locus for a variety of cancers, but at relatively low odds ratios. Shaded areas representing diseases and disease states are not drawn to scale.
TELOMERE ATTRITION AND CANCER
ANIMAL MODELS OF TELOMERE MAINTENANCE AND CHROMOSOMAL INSTABILITY
Chromosomal instability was originally postulated by Boveri in 1914 as a fundamental event in the origin of tumors.47 This inference from studies of sea urchins is applicable to the gross derangement in the number and structure of chromosomes in most cancers. Cell experiments and animal models led to the proposal that telomere attrition is a mechanism for the loss or gain of chromosomes.48–52 When telomere maintenance is disrupted in yeast, the few cells that escape senescence show chromosomal abnormalities; in the absence of telomerase, mutation rates increase as a result of terminal chromosome deletions and repeated cycles of break-fusion-bridge rearrangements.53 In late-generation Terc-knockout mice, short telomeres cause chromosomal instability through end-to-end fusions. Apoptosis removes most of these cells, but they can be rescued if DNA damage is not adequately monitored. Thus, in Terc−/− mice that are also deficient in p53, a variety of cancers develop in association with nonreciprocal translocations, mimicking human malignant conditions.54
ACCELERATED TELOMERE ATTRITION, INFLAMMATION, AND MALIGNANT TRANSFORMATION
In humans, studies are often limited by the necessity to measure telomeres in leukocytes, the uncertain significance of telomere length in tumor cells (in which up-regulated telomerase or the alternative pathway of telomere repair may allow evasion of cell senescence), and the difficulty of performing longitudinal assessments. Nevertheless, telomere length has been linked to several types of cancer. The finding of short telomeres in colorectal cancer suggested that telomere loss contributes to tumorigenesis and genetic instability of the malignant cell.55 Telomerase deficiency has been detected in the histologically normal mucosa of patients with inflammatory bowel disease (cancer cells expressed high telomerase activity).56 Losses of chromosomes in nondysplastic tissue in patients with ulcerative colitis were associated with telomere shortening and the appearance of anaphase bridges, especially in patients in whom cancer developed.57
The major risk factor for esophageal cancer is the chronic inflammation of Barrett’s esophagus. In one study, the telomere length in leukocytes at first presentation was inversely proportional to the risk of later esophageal cancer; possible explanations were a genetic predisposition to defective repair of DNA in mucosa or long-term exposure to oxidative stress that provokes cell proliferation.58 Short telomeres, visualized directly by means of fluorescence staining of biopsy specimens of Barrett’s esophagus, and chromosomal instability associated with dysplastic changes suggest that both are early events in the development of esophageal cancer.59 Telomeres of leukocytes that are short relative to the patient’s age have been implicated as a risk factor60,61 or biomarker for many solid tumors, but not for all of them; breast cancer is one exception.60–63
Hematologic and other cancers develop in patients with bone marrow failure. In a National Cancer Institute review of 50 cases of classic dyskeratosis congenita, the authors found markedly elevated risks of tumors (overall, about 11 times as high as in the general population), especially head and neck squamous-cell carcinomas, skin and anorectal cancers, and acute myeloid leukemia.64 In our study of acquired aplastic anemia, the telomere length of leukocytes was the major predictor of clonal evolution: almost all patients in whom monosomy 7 myelodysplastic syndrome and acute myeloid leukemia developed were in the lowest quartile of telomere length when they first presented with bone marrow failure.65 Because leukemia is linked to TERT and TERC mutations in some pedigrees, we screened multiple cohorts of patients with acute myeloid leukemia.66 Constitutional TERT mutations were detected in about 9% of these patients and were strongly associated with the risk of cytogenetic abnormalities. In small studies, telomere length has been associated with the risk of leukemic transformation from myelodysplasia after chemotherapy and autologous hematopoietic-cell transplantation,67–69 and short telomeres of blast cells have been correlated with chromosomal abnormalities.70,71
TERT AND THE RISK OF CANCER
Genomewide association studies have shown polymorphisms in the TERT gene at a higher frequency than normal in patients with cancer. However, the level of risk is much lower than in individual diseases or in patient populations that are assessed serially, for malignant conditions that develop in the setting of inflammation. For example, a massive genomewide scan of more than 30,000 European patients with cancer and 45,000 control DNA samples showed an association between the TERT locus and 5 of 16 cancers, including basal-cell cancer of the skin and cancers of the lung, bladder, prostate, and cervix (all tumors that are caused in part by environmental factors); the overall risks were relatively small (relative risk, 1.12 to 1.21) but consistent across diverse ethnic populations.72 Similar statistical associations have been reproducible for lung cancer in genomewide association studies involving large European populations73 and in Chinese patients, in whom risk was also linked to short telomeres.74 TERT-locus polymorphisms have been associated with susceptibility to gliomas73,75 and renal-cell carcinoma76; they have also been associated with relative resistance to melanoma77 and breast cancer.78
In summary, telomere shortening can be related to the risk of cancer, ranging from high rates of specific cancers in dyskeratosis congenita to modest contributions to oncogenesis in general. In some specific inflammatory and immune diseases, telomere attrition may be the critical factor in promoting the development of cancer (Fig. 5B).
TELOMERES, DEGENERATIVE DISEASES, AND AGING
TELOMERES AND HEART DISEASE
Telomere length has been connected with cardiovascular complications, but the associations have varied across studies, exploratory epidemiologic surveys often do not correct for multiple variables, and there is no accepted pathophysiological link.79–84 In one study, endothelial progenitor-cell telomeres were shorter in patients with coronary artery disease than in healthy persons, and intensive lipid-lowering therapy both reduced oxidative DNA damage and prevented further telomere attrition.85 In one of many epidemiologic surveys, people with short leukocyte telomeres were at risk for coronary disease, which appeared to be attenuated by statin therapy.86 In the Cardiovascular Health Study, shortened telomeres corresponded with a risk of myocardial infarction among younger patients that was three times as high as the risk among older patients,87 and in the Heart and Soul Study, shorter-than-normal telomeres were a biomarker for the risk of death in patients with stable coronary artery disease.88 Telomere length was short in a study involving British patients with premature myocardial infarction.89 In the Framingham Heart Study, shortened telomere length correlated with carotid-artery intimal thickening.90
TELOMERES AND AGING
Does telomere biology explain physiologic aging in humans?52,91 Telomere attrition leads to cell senescence and the Hayflick phenomenon. Telomerase defects affect yeast viability and replication and account for the shortened life span of knockout mice. In addition, under specific conditions, telomere length is a “mitotic clock” for a cell’s proliferative history, and telomere loss is linked to DNA damage by reactive oxygen species, which accumulates over time. Proteins that are released from dysfunctional cells because of telomere shortening have been proposed as biomarkers of aging and age-dependent degenerative diseases.92
Dyskeratosis congenita is sometimes misclassified with aging syndromes such as Hutchinson–Gilford progeria and Werner’s syndrome, but typical patients with this condition do not appear old, nor do they prematurely have atherosclerosis, Alzheimer’s disease, or other classic characteristics of aging such as osteopenia or type 2 diabetes. Many people with TERT and TERC mutations have normal life expectancies. Telomere shortening is not uniform among tissues (e.g., the brain and heart show little shortening) or among the various cells within a tissue. It is not known whether the decline with age in bone marrow cellularity and lung compliance, as well as nodular regeneration in autopsies of the elderly, is due to physiologic telomere attrition. Inbred mice, despite their long telomeres, do age. Decreases in telomere length over a human life span93 do not establish telomere shortening as the cause of aging.
MODULATION OF TELOMERASE ACTIVITY
Because telomerase is activated in leukemia and solid tumors, the repair complex has been targeted in drug therapy for malignant conditions.79 Since telomere shortening is a risk factor for cancer in patients with dyskeratosis congenita and those with immune-mediated or inflammatory diseases such as aplastic anemia, ulcerative colitis, and Barrett’s esophagus, and since telomere attrition may underlie many cancers and degenerative diseases of aging, strategies to maintain or delay telomere attrition may be useful. Prevention of accelerated telomere attrition also may be necessary for effective stem-cell therapies.80
Telomere maintenance and telomerase activation are highly regulated. Although twin studies show telomere length to be largely genetically determined,81,82 some modulation is environmental.83 In tissue culture, exposure to reactive oxygen species appears to accelerate telomere shortening.84 In vitro, hormones and growth factors affect telomerase activity,94 including hematopoietic growth factors.95,96 TERT can be directly activated by the tumor-suppressor protein c-Myc,97 and other components of the repair complex are influenced by specific ubiquitin ligases and protein kinases. Smoking status, diet, socioeconomic status, stress level, and lifestyle might influence telomere dynamics.62,98–100 The sex hormones directly increase TERT transcription and telomerase activity in human cells.24,101 Natural and synthetic androgens can restore telomerase activity to normal levels in cells in patients with TERT and TERC mutations24; this probably explains the benefit of these agents in syndromes involving hematopoietic failure. Sex hormones might be used in the treatment of other telomere diseases, such as pulmonary fibrosis and hepatic cirrhosis, for which we now have no effective therapies. Sex-hormone replacement and the use of sex hormones in pharmacologic doses could also be therapeutic in patients with accelerated telomere attrition and a known risk of secondary malignant conditions (e.g., after intensive chemotherapy or hematopoietic stem-cell transplantation). The theoretical desirability of hormone replacement in older healthy persons to “stabilize” telomere loss would need to be balanced against the risks of undesirable effects on secondary sex organs and known associated malignant conditions.
Acknowledgments
We thank Drs. Stephen Chanock, Cynthia Dunbar, Eva Hell-ström-Lindberg, Richard Hodes, Dan Longo, Joel Moss, and David Nathan for their careful reading of an earlier version of the manuscript and insightful comments.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Szostak JW, Blackburn EH. Cloning yeast telomeres on linear plasmid vectors. Cell. 1982;29:245–55. doi: 10.1016/0092-8674(82)90109-x. [DOI] [PubMed] [Google Scholar]
- 2.Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405–13. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- 3.Heiss NS, Knight SW, Vulliamy TJ, et al. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat Genet. 1998;19:32–8. doi: 10.1038/ng0598-32. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell JR, Wood E, Collins K. A telo-merase component is defective in the human disease dyskeratosis congenita. Nature. 1999;402:551–5. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- 5.Olovnikov AM. Principle of marginotomy in template synthesis of polynucleotides. Dokl Akad Nauk SSSR. 1971;201:1496–9. (In Russian.) [PubMed] [Google Scholar]
- 6.Yamaguchi H, Calado RT, Ly H, et al. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med. 2005;352:1413–24. doi: 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]
- 7.Moyzis RK, Buckingham JM, Cram LS, et al. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci U S A. 1988;85:6622–6. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–10. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 9.Blasco MA. Telomere length, stem cells and aging. Nat Chem Biol. 2007;3:640–9. doi: 10.1038/nchembio.2007.38. [DOI] [PubMed] [Google Scholar]
- 10.Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumour cells with defined genetic elements. Nature. 1999;400:464–8. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 11.Sarin KY, Cheung P, Gilisonee E, et al. Conditional telomerase induction causes proliferation of hair follicle stem cells. Nature. 2005;436:1048–52. doi: 10.1038/nature03836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi J, Southworth LK, Sarin KY, et al. TERT promotes epithelial proliferation through transcriptional control of a Myc- and Wnt-related developmental program. PLoS Genet. 2008;4(1):e10. doi: 10.1371/journal.pgen.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alter BP. Inherited bone marrow failure syndromes. In: Nathan DG, Orkin SH, Look AT, Ginsburg D, editors. Nathan and Oski’s hematology of infancy and childhood. 6. Philadelphia: W.B. Saunders; 2003. pp. 280–365. [Google Scholar]
- 14.Knight S, Vulliamy T, Copplestone A, Gluckman E, Mason P, Dokal I. Dyskeratosis Congenita (DC) Registry: identification of new features of DC. Br J Haematol. 1998;103:990–6. doi: 10.1046/j.1365-2141.1998.01103.x. [DOI] [PubMed] [Google Scholar]
- 15.Dokal I. Dyskeratosis congenita in all its forms. Br J Haematol. 2000;110:768–79. doi: 10.1046/j.1365-2141.2000.02109.x. [DOI] [PubMed] [Google Scholar]
- 16.Vulliamy TJ, Knight SW, Mason PJ, Dokal I. Very short telomeres in the peripheral blood of patients with X-linked and autosomal dyskeratosis congenita. Blood Cells Mol Dis. 2001;27:353–7. doi: 10.1006/bcmd.2001.0389. [DOI] [PubMed] [Google Scholar]
- 17.Vulliamy T, Marrone A, Goldman F, et al. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001;413:432–5. doi: 10.1038/35096585. [DOI] [PubMed] [Google Scholar]
- 18.Walne AJ, Vulliamy T, Marrone A, et al. Genetic heterogeneity in autosomal recessive dyskeratosis congenita with one subtype due to mutations in the telomerase-associated protein NOP10. Hum Mol Genet. 2007;16:1619–29. doi: 10.1093/hmg/ddm111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vulliamy T, Beswick R, Kirwan M, et al. Mutations in the telomerase component NHP2 cause the premature ageing syndrome dyskeratosis congenita. Proc Natl Acad Sci U S A. 2008;105:8073–8. doi: 10.1073/pnas.0800042105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savage SA, Giri N, Baerlocher GM, Orr N, Lansdorp PM, Alter BP. TINF2, a component of the shelterin telomere protection complex, is mutated in dyskeratosis congenita. Am J Hum Genet. 2008;82:501–9. doi: 10.1016/j.ajhg.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alter BP, Baerlocher GM, Savage SA, et al. Very short telomere length by flow fluorescence in situ hybridization identifies patients with dyskeratosis congenita. Blood. 2007;110:1439–47. doi: 10.1182/blood-2007-02-075598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vulliamy T, Marrone A, Szydlo R, Walne A, Mason PJ, Dokal I. Disease anticipation is associated with progressive telomere shortening in families with dyskeratosis congenita due to mutations in TERC. Nat Genet. 2004;36:447–9. doi: 10.1038/ng1346. [DOI] [PubMed] [Google Scholar]
- 23.Marrone A, Stevens D, Vulliamy T, Dokal I, Mason PJ. Heterozygous telomerase RNA mutations found in dyskeratosis congenita and aplastic anemia reduce telomerase activity via haploinsufficiency. Blood. 2004;104:3936–40. doi: 10.1182/blood-2004-05-1829. [DOI] [PubMed] [Google Scholar]
- 24.Calado RT, Yewdell WT, Wilkerson KL, et al. Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood. 2009;114:2236–43. doi: 10.1182/blood-2008-09-178871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips RJ, Judge M, Webb D, Harper JI. Dyskeratosis congenita: delay in diagnosis and successful treatment of pancytopenia by bone marrow transplantation. Br J Dermatol. 1992;127:278–80. doi: 10.1111/j.1365-2133.1992.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 26.Langston AA, Sanders JE, Deeg HJ, et al. Allogeneic marrow transplantation for aplastic anaemia associated with dyskeratosis congenita. Br J Haematol. 1996;92:758–65. doi: 10.1046/j.1365-2141.1996.424984.x. [DOI] [PubMed] [Google Scholar]
- 27.Baykal C, Kavak A, Gulcan P, Büyükbabani N. Dyskeratosis congenita associated with three malignancies. J Eur Acad Dermatol Venereol. 2003;17:216–8. doi: 10.1046/j.1468-3083.2003.00585.x. [DOI] [PubMed] [Google Scholar]
- 28.Savage SA, Dokal I, Armanios M, et al. Dyskeratosis congenita: the first NIH clinical research workshop. Pediatr Blood Cancer. 2009;53:520–3. doi: 10.1002/pbc.22061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaziri H, Dragowska W, Allsopp RC, Thomas TE, Harley CB, Lansdorp PM. Evidence for a mitotic clock in human hematopoietic stem cells: loss of telomeric DNA with age. Proc Natl Acad Sci U S A. 1994;91:9857–60. doi: 10.1073/pnas.91.21.9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wynn RF, Cross MA, Hatton C, et al. Accelerated telomere shortening in recipients of allogeneic bone marrow transplants. Lancet. 1998;351:178–81. doi: 10.1016/S0140-6736(97)08256-1. [DOI] [PubMed] [Google Scholar]
- 31.Notaro R, Cimmino A, Tabarini D, Rotoli B, Luzzatto L. In vivo telomere dynamics of human hematopoietic stem cells. Proc Natl Acad Sci U S A. 1997;94:13782–5. doi: 10.1073/pnas.94.25.13782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ball SE, Gibson FM, Rizzo S, Tooze JA, Marsh JCW, Gordon-Smith EC. Progressive telomere shortening in aplastic anemia. Blood. 1998;91:3582–92. [PubMed] [Google Scholar]
- 33.Brümmendorf TH, Maciejewski JP, Mak J, Young NS, Lansdorp PL. Telomere length in leukocyte subpopulations of patients with aplastic anemia. Blood. 2001;97:895–900. doi: 10.1182/blood.v97.4.895. [DOI] [PubMed] [Google Scholar]
- 34.Yamaguchi H, Baerlocher GM, Lansdorp PM, et al. Mutations of the human telomerase RNA gene (TERC) in aplastic anemia and myelodysplastic syndrome. Blood. 2003;102:916–8. doi: 10.1182/blood-2003-01-0335. [DOI] [PubMed] [Google Scholar]
- 35.Fogarty PF, Yamaguchi H, Wiestner A, et al. Late presentation of dyskeratosis congenita as apparently acquired aplastic anaemia due to mutations in telomerase RNA. Lancet. 2003;362:1628–30. doi: 10.1016/S0140-6736(03)14797-6. [DOI] [PubMed] [Google Scholar]
- 36.Calado RT, Young NS. Telomere maintenance and human bone marrow failure. Blood. 2008;111:4446–55. doi: 10.1182/blood-2007-08-019729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Armanios M, Chen J-L, Chang Y-PC, et al. Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita. Proc Natl Acad Sci U S A. 2005;102:15960–4. doi: 10.1073/pnas.0508124102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qazilbash MH, Liu JM, Vlachos A, et al. A new syndrome of familial aplastic anemia and chronic liver disease. Acta Haematol. 1997;97:164–7. doi: 10.1159/000203674. [DOI] [PubMed] [Google Scholar]
- 39.Rocha V, Devergie A, Socié G, et al. Unusual complications after bone marrow transplantation for dyskeratosis congenita. Br J Haematol. 1998;103:243–8. doi: 10.1046/j.1365-2141.1998.00949.x. [DOI] [PubMed] [Google Scholar]
- 40.Armanios MY, Chen JJ, Cogan JD, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356:1317–26. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 41.Tsakiri KD, Cronkhite JT, Kuan PJ, et al. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci U S A. 2007;104:7552–7. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mushiroda T, Wattanapokayakit S, Takahashi A, et al. A genome-wide association study identifies an association of a common variant in TERT with susceptibility to idiopathic pulmonary fibrosis. J Med Genet. 2008;45:654–6. doi: 10.1136/jmg.2008.057356. [DOI] [PubMed] [Google Scholar]
- 43.Alder JK, Chen JJ, Lancaster L, et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci U S A. 2008;105:13051–6. doi: 10.1073/pnas.0804280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Calado RT, Regal JA, Kleiner DE, et al. A spectrum of severe liver and blood disorders associated with telomerase mutations. PLoS One. 2009;4(11):e7926. doi: 10.1371/journal.pone.0007926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Talbot-Smith A, Syn WK, MacQuillan G, Neil D, Elias E, Ryan P. Familial idiopathic pulmonary fibrosis in association with bone marrow hypoplasia and hepatic nodular regenerative hyperplasia: a new “trimorphic” syndrome. Thorax. 2009;64:440–3. doi: 10.1136/thx.2008.099796. [DOI] [PubMed] [Google Scholar]
- 46.González-Huezo MS, Villela LM, Zepeda-Florencio MC, Carrillo-Ponce CS, Mondragón-Sánchez RJ. Nodular regenerative hyperplasia associated to aplastic anemia: a case report and literature review. Ann Hepatol. 2006;5:166–9. [PubMed] [Google Scholar]
- 47.Boveri T. Concerning the origin of malignant tumours. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press and The Company of Biologists; 2008. [Google Scholar]
- 48.Hackett JA, Greider CW. Balancing instability: dual roles for telomerase and telomere dysfunction in tumorigenesis. Oncogene. 2002;21:619–26. doi: 10.1038/sj.onc.1205061. [DOI] [PubMed] [Google Scholar]
- 49.Sharpless NE, DePinho RA. Telomeres, stem cells, senescence, and cancer. J Clin Invest. 2004;113:160–8. doi: 10.1172/JCI20761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Finkel T, Serrano M, Blasco MA. The common biology of cancer and ageing. Nature. 2007;448:767–74. doi: 10.1038/nature05985. [DOI] [PubMed] [Google Scholar]
- 51.de Lange T. Telomere-related genome instability in cancer. Cold Spring Harb Symp Quant Biol. 2005;70:197–204. doi: 10.1101/sqb.2005.70.032. [DOI] [PubMed] [Google Scholar]
- 52.Blackburn EH, Greider CW, Szostak JW. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med. 2006;12:1133–8. doi: 10.1038/nm1006-1133. [DOI] [PubMed] [Google Scholar]
- 53.Hackett JA, Feldser DM, Greider CW. Telomere dysfunction increases mutation rate and genomic instability. Cell. 2001;106:275–86. doi: 10.1016/s0092-8674(01)00457-3. [DOI] [PubMed] [Google Scholar]
- 54.Artandi SE, Chang S, Lee S-L, et al. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature. 2000;406:641–5. doi: 10.1038/35020592. [DOI] [PubMed] [Google Scholar]
- 55.Hastie ND, Dempster M, Dunlop MG, Thompson AM, Green DK, Allshire RC. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990;346:866–8. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- 56.Usselmann B, Newbold M, Morris AG, Nwokolo CU. Deficiency of colonic telomerase in ulcerative colitis. Am J Gastroenterol. 2001;96:1106–12. doi: 10.1111/j.1572-0241.2001.03752.x. [DOI] [PubMed] [Google Scholar]
- 57.O’Sullivan JN, Bronner MP, Brentnall TA, et al. Chromosomal instability in ulcerative colitis is related to telomere shortening. Nat Genet. 2002;32:280–4. doi: 10.1038/ng989. [DOI] [PubMed] [Google Scholar]
- 58.Risques RA, Vaughan TL, Li X, et al. Leukocyte telomere length predicts cancer risk in Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev. 2007;16:2649–55. doi: 10.1158/1055-9965.EPI-07-0624. [DOI] [PubMed] [Google Scholar]
- 59.Finley JC, Reid BJ, Odze RD, et al. Chromosomal instability in Barrett’s esophagus is related to telomere shortening. Cancer Epidemiol Biomarkers Prev. 2006;15:1451–7. doi: 10.1158/1055-9965.EPI-05-0837. [DOI] [PubMed] [Google Scholar]
- 60.Liu X, Bao G, Huo T, Wang Z, He X, Dong G. Constitutive telomere length and gastric cancer risk: case-control analysis in Chinese Han population. Cancer Sci. 2009;100:1300–5. doi: 10.1111/j.1349-7006.2009.01169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jang JS, Choi YY, Lee WK, et al. Telomere length and the risk of lung cancer. Cancer Sci. 2008;99:1385–9. doi: 10.1111/j.1349-7006.2008.00831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mirabello L, Huang W-Y, Wong JY, et al. The association between leukocyte telomere length and cigarette smoking, dietary and physical variables, and risk of prostate cancer. Aging Cell. 2009;8:405–13. doi: 10.1111/j.1474-9726.2009.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gebre-Medhin S, Broberg K, Jonson T, et al. Telomeric associations correlate with telomere length reduction and clonal chromosome aberrations in giant cell tumor of bone. Cytogenet Genome Res. 2009;124:121–7. doi: 10.1159/000207516. [DOI] [PubMed] [Google Scholar]
- 64.Alter BP, Giri N, Savage SA, Rosen-berg PS. Cancer in dyskeratosis congenita. Blood. 2009;113:6549–57. doi: 10.1182/blood-2008-12-192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cooper JN, Calado R, Wu C, Scheinberg P, Young N. Telomere length of peripheral blood leukocytes predicts relapse and clonal evolution after immunosuppressive therapy in severe aplastic anemia. Presented at the 50th annual meeting of the American Society of Hematology; San Francisco. December 6–9, 2008; abstract. [Google Scholar]
- 66.Calado RT, Regal JA, Hills M, et al. Constitutional hypomorphic telomerase mutations in patients with acute myeloid leukemia. Proc Natl Acad Sci U S A. 2009;106:1187–92. doi: 10.1073/pnas.0807057106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Engelhardt M, Ozkaynak MF, Drullinsky P, et al. Telomerase activity and telomere length in pediatric patients with malignancies undergoing chemotherapy. Leukemia. 1998;12:13–24. doi: 10.1038/sj.leu.2400889. [DOI] [PubMed] [Google Scholar]
- 68.Ohyashiki JH, Ohyashiki K, Fujimura T, et al. Telomere shortening associated with disease evolution patterns in myelodysplastic syndromes. Cancer Res. 1994;54:3557–60. [PubMed] [Google Scholar]
- 69.Chakraborty S, Sun C-L, Francisco L, et al. Accelerated telomere shortening precedes development of therapy-related myelodysplasia or acute myelogenous leukemia after autologous transplantation for lymphoma. J Clin Oncol. 2009;27:791–8. doi: 10.1200/JCO.2008.17.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Swiggers SJJ, Kuijpers MA, de Cort MJM, Beverloo HB, Zijlmans MJM. Critically short telomeres in acute myeloid leukemia with loss or gain of parts of chromosomes. Genes Chromosomes Cancer. 2006;45:247–56. doi: 10.1002/gcc.20286. [DOI] [PubMed] [Google Scholar]
- 71.Sieglová Z, Zilovcová S, Cermák J, et al. Dynamics of telomere erosion and its association with genome instability in my-elodysplastic syndromes (MDS) and acute myelogenous leukemia arising from MDS: a marker of disease prognosis? Leuk Res. 2004;28:1013–21. doi: 10.1016/j.leukres.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 72.Rafnar T, Sulem P, Stacey SN, et al. Sequence variants at the TERT-CLPTM1L locus associate with many cancer types. Nat Genet. 2009;41:221–7. doi: 10.1038/ng.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McKay JD, Hung RJ, Gaborieau V, et al. Lung cancer susceptibility locus at 5p15. 33. Nat Genet. 2008;40:1404–6. doi: 10.1038/ng.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hosgood HD, III, Cawthon RM, He X, Chanock SJ, Lan Q. Genetic variation in telomere maintenance genes, telomere length, and lung cancer susceptibility. Lung Cancer. 2009 Mar 12; doi: 10.1016/j.lungcan.2009.02.005. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shete S, Hosking FJ, Robertson LB, et al. Genome-wide association study identifies five susceptibility loci for glioma. Nat Genet. 2009;41:899–904. doi: 10.1038/ng.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen M, Ye Y, Yang H, et al. Genome-wide profiling of chromosomal alterations in renal cell carcinoma using high-density single nucleotide polymorphism arrays. Int J Cancer. 2009;125:2342–8. doi: 10.1002/ijc.24642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stacey SN, Sulem P, Masson G, et al. New common variants affecting susceptibility to basal cell carcinoma. Nat Genet. 2009;41:909–14. doi: 10.1038/ng.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Savage SA, Chanock SJ, Lissowska J, et al. Genetic variation in five genes important in telomere biology and risk for breast cancer. Br J Cancer. 2007;97:832–6. doi: 10.1038/sj.bjc.6603934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Folini M, Gandellini P, Zaffaroni N. Targeting the telosome: therapeutic implications. Biochim Biophys Acta. 2009;1792:309–16. doi: 10.1016/j.bbadis.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 80.Armstrong L, Saretzki G, Peters H, et al. Overexpression of telomerase confers growth advantage, stress resistance, and enhanced differentiation of ESCs toward the hematopoietic lineage. Stem Cells. 2005;23:516–29. doi: 10.1634/stemcells.2004-0269. [DOI] [PubMed] [Google Scholar]
- 81.Slagboom PE, Droog S, Boomsma DL. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet. 1994;55:876–82. [PMC free article] [PubMed] [Google Scholar]
- 82.Graakjaer J, Pascoe L, Der-Sarkissian H, et al. The relative lengths of individual telomeres are defined in the zygote and strictly maintained during life. Aging Cell. 2004;3:97–102. doi: 10.1111/j.1474-9728.2004.00093.x. [DOI] [PubMed] [Google Scholar]
- 83.Huda N, Tanaka H, Herbert BS, Reed T, Gilley D. Shared environmental factors associated with telomere length maintenance in elderly male twins. Aging Cell. 2007;6:709–13. doi: 10.1111/j.1474-9726.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- 84.von Zglinicki T, Saretzki G, Ladhoff J, d’Adda di Fagagna F, Jackson SP. Human cell senescence as a DNA damage response. Mech Ageing Dev. 2005;126:111–7. doi: 10.1016/j.mad.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 85.Satoh M, Minami Y, Takahashi Y, Tabuchi T, Itoh T, Nakamura M. Effect of intensive lipid-lowering therapy on telomere erosion in endothelial progenitor cells obtained from patients with coronary artery disease. Clin Sci (Lond) 2009;116:827–35. doi: 10.1042/CS20080404. [DOI] [PubMed] [Google Scholar]
- 86.Brouilette SW, Moore JS, McMahon AD, et al. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007;369:107–14. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 87.Fitzpatrick AL, Kronmal RA, Gardner JP, et al. Leukocyte telomere length and cardiovascular disease in the Cardiovascular Health Study. Am J Epidemiol. 2007;165:14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 88.Farzaneh-Far R, Cawthon RM, Na B, Browner WS, Schiller NB, Whooley MA. Prognostic value of leukocyte telomere length in patients with stable coronary artery disease: data from the Heart and Soul Study. Arterioscler Thromb Vasc Biol. 2008;28:1379–84. doi: 10.1161/ATVBAHA.108.167049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ. White cell telomere length and risk of premature myo-cardial infarction. Arterioscler Thromb Vasc Biol. 2003;23:842–6. doi: 10.1161/01.ATV.0000067426.96344.32. [DOI] [PubMed] [Google Scholar]
- 90.O’Donnell CJ, Demissie S, Kimura M, et al. Leukocyte telomere length and carotid artery intimal medial thickness: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2008;28:1165–71. doi: 10.1161/ATVBAHA.107.154849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stewart SA, Weinberg RA. Telomeres: cancer to human aging. Annu Rev Cell Dev Biol. 2006;22:531–57. doi: 10.1146/annurev.cellbio.22.010305.104518. [DOI] [PubMed] [Google Scholar]
- 92.Jiang H, Schiffer E, Song Z, et al. Proteins induced by telomere dysfunction and DNA damage represent biomarkers of human aging and disease. Proc Natl Acad Sci U S A. 2008;105:11299–304. doi: 10.1073/pnas.0801457105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Frenck RW, Jr, Blackburn EH, Shannon KM. The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci U S A. 1998;95:5607–10. doi: 10.1073/pnas.95.10.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bayne S, Liu J-P. Hormones and growth factors regulate telomerase activity in age-ing and cancer. Mol Cell Endocrinol. 2005;240:11–22. doi: 10.1016/j.mce.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 95.Engelhardt M, Kumar R, Albanell J, Pettengel R, Han W, Moore MAS. Telomerase regulation, cell cycle, and telo-mere stability in primitive hematopoietic cells. Blood. 1997;90:182–93. [PubMed] [Google Scholar]
- 96.Beyne-Rauzy O, Prade-Houdellier N, Demur C, et al. Tumor necrosis factor-alpha inhibits hTERT gene expression in human myeloid normal and leukemic cells. Blood. 2005;106:3200–5. doi: 10.1182/blood-2005-04-1386. [DOI] [PubMed] [Google Scholar]
- 97.Wu K-J, Grandori C, Amacker M, et al. Direct activation of TERT transcription by c-MYC. Nat Genet. 1999;21:220–4. doi: 10.1038/6010. [DOI] [PubMed] [Google Scholar]
- 98.Woo J, Suen EW, Leung JC, Tang NL, Ebrahim S. Older men with higher self-rated socioeconomic status have shorter telomeres. Age Ageing. 2009;38:553–8. doi: 10.1093/ageing/afp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–5. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Getliffe KM, Al Dulaimi D, Martin-Ruiz C, et al. Lymphocyte telomere dynamics and telomerase activity in inflammatory bowel disease: effect of drugs and smoking. Aliment Pharmacol Ther. 2005;21:121–31. doi: 10.1111/j.1365-2036.2005.02311.x. [DOI] [PubMed] [Google Scholar]
- 101.Nanni S, Narducci M, Della Pietra L, et al. Signaling through estrogen receptors modulates telomerase activity in human prostate cancer. J Clin Invest. 2002;110:219–27. doi: 10.1172/JCI15552. [DOI] [PMC free article] [PubMed] [Google Scholar]