Abstract
A series of pyridylpiperazines was synthesized and analyzed for sigma receptor binding affinity to determine the optimal pyridyl nitrogen position and chain length for the σ1 and σ2 receptor recognition. The (3-pyridyl)piperazines and (4-pyridyl)piperazines favor σ1 receptors, while previously studied (2-pyridyl)piperazines favor σ2 receptors.
The continued growth in the abuse of methamphetamine necessitates the urgent development of pharmacotherapies. No pharmacotherapies for methamphetamine abuse currently exist and efforts have mainly focused on the development of therapies for the dopaminergic systems.1–4 Our studies have utilized the fact that methamphetamine interacts with sigma receptors5, 6 and sigma antagonists attenuate both the stimulant and neurotoxic effects of methamphetamine. Although sigma receptors were first thought to be a subtype of opioid receptors, they are now considered to be a unique class of receptors7 comprised of two subtypes, σ1 and σ2.8 σ1 Receptors have been cloned9, 10 and are involved in intracellular signaling, synaptic transmission, modulation of inositol phosphates, protein kinases, and calcium.11–15 In addition, σ1 antagonists reduce the convulsive, lethal, locomotor stimulatory and rewarding actions of cocaine in mice.16–20 σ2 Receptors have not yet been cloned; however they appear to be comprised of heterodimers and are smaller in size compared to σ1.21–23 Further studies have demonstrated that σ1 selective antagonists reduce the stimulant effects of methamphetamine, while AC927 (N-phenethylpiperidine), a mixed σ1 and σ2 antagonist, attenuates the locomotor stimulant and neurotoxic effects of methamphetamine in mice.6, 24 A selective σ2 antagonist is therefore urgently required to further study the relationship between σ2 antagonism and methamphetamine neurotoxicity.
Truly selective σ2 antagonists continue to be the goal of several research groups.25–27 One of the major disadvantages of the current σ2 antagonists is their ability to bind to the dopamine receptors, opioid receptors, and N-methyl-D-aspartate (NMDA) receptors.28 Recent studies showed that CM156 (3-(4-(4-cyclohexylpiperazin-1-yl)butyl)benzo[d]thiazole-2(3H)-thione) exhibits better affinity for the sigma receptor however, it has poor metabolic stability.25 Studies performed previously by our laboratory have showed that N-(2-pyridyl)piperazines not only have the tendency to favor σ2 receptors but they also favor sigma receptors over opioid and NMDA receptors with low affinity for the dopamine receptor.29, 30 Specifically, compound 5, 1-(2-Phenylethyl)-4-(2-pyridyl)piperazine, produced protective actions against cocaine induced convulsions which provides evidence that compound 5 is an antagonist.29, 31 Moreover, 1-(3-phenylpropyl)-4-(2-pyridyl)piperazine, 6, has 17-fold preference for the σ2 receptor, over σ1.30 In an effort to design a pharmacophore for selective σ2 antagonism in this series, we have investigated the effect of pyridyl nitrogen position and chain length in the phenylalkylpiperazinepyridine series.
Compounds 1–4 (Figure 1) were prepared by the alkylation of the corresponding halogenated alkyl phenyls with the appropriate pyridinylpiperazine in the presence of K2CO3 in DMF at room temperature. and purified as oxalate salts from methanol.30 All salt targets were characterized using NMR and MS and all elemental analyses of salts were within ±0.4%.
Figure 1.
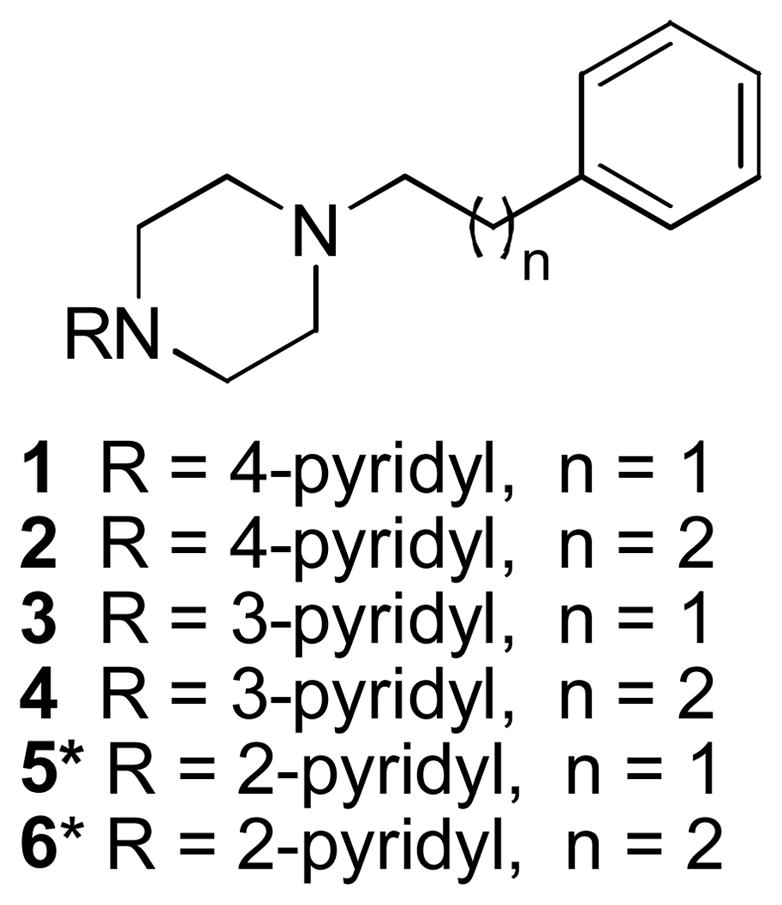
Phenylalkylpiperazinepyridines
*Reported in reference ref. 30
In vitro competition binding assays were preformed as follows. Preparation of rat brain membrane and binding assays for the σ1 and σ2 receptor were performed as previously described in detail.32, 33 In brief, σ1 receptors were labeled with 5 nM [3H](+)-pentazocine. The σ2 receptors were labeled with 3 nM [3H]di-o-tolylguanidine (DTG) in the presence of 300 nM (+)-pentazocine to block σ1 receptors. Nonspecific binding was determined in the presence of 10 μM haloperidol. Ten concentrations of each sigma compound (0.1–10,000 nM) were used in the assays. The compounds were incubated for 120 min at 25°C to measure their ability to displace the radioligands from their binding sites. Termination of the reaction was achieved through rapid vacuum filtration over glass fiber filters which were previously soaked in 1% polyethyleneimine for at least 45 min. Ki values were calculated using the Cheng-Prusoff equation.34
All compounds possessed affinity at both σ1 and σ2 receptors (Table 1). As shown previously, (2-pyridyl)piperazines (5,6) favored σ2 receptors,30 while (3-pyridyl)piperazines (3,4) and (4-pyridyl)piperazines (1,2) showed preference for σ1 receptors. Similar binding affinities were achieved by the (4-pyridyl)piperazine compounds (1,2) independent of the chain length, whereas the phenylpropyl linker in both (3-pyridyl)piperazine and (2-pyridyl)piperazine resulted in higher affinity for both σ1 and σ2 receptors. All new compounds showed significantly lower affinity for σ2 receptors than our lead compound 6.
Table 1.
Binding affinities of phenylalkylpiperazinepyridines 1–6 at sigma receptors.
| Ki (nM)±SEM | Selectivity | ||
|---|---|---|---|
|
| |||
| Cmpds | σ1a | σ2b | σ1/σ2 |
| 1 | 41.8 ± 5.9 | 69.7 ± 6.3 | 0.60 |
| 2 | 34.2 ± 2.8 | 84.0 ± 5.9 | 0.41 |
| 3 | 97.2 ± 6.9 | 440 ± 20 | 0.22 |
| 4 | 21.2 ± 2.3 | 110.0 ± 8.6 | 0.19 |
| 5* | 326 ± 41.2 | 119 ± 3.8 | 2.7 |
| 6* | 82.9 ± 0.21 | 4.91 ± 0.77 | 16.9 |
Citations reference previously known compounds and results ref. 30
Displacement of [3H](+)-pentazocine
Displacement of [3H]DTG in presence of (+)-pentazocine
In summary, binding affinity studies showed that the (3-pyridyl)piperazines and (4-pyridyl)piperazines have lower affinity for σ2 receptors, than the previously reported lead compound 6. Moreover, both new series lost σ2 selectivity, indicating that (2-pyridyl)piperazines are optimal for the development of highly selective σ2 ligands.
Acknowledgments
This work was supported in part by the National Institute on Drug Abuse, National Institutes of Health (NIDA, NIH) (DA013978) and Independent Scientist Award (K02) to AC (DA-19634).
References and notes
- 1.Bastianetto S, Rouquier L, Perrault G, Sanger DJ. Neuropharmacology. 1995;34:281. doi: 10.1016/0028-3908(94)00156-m. [DOI] [PubMed] [Google Scholar]
- 2.Booth RG, Baldessarini RJ. Brain Res. 1991;557:349. doi: 10.1016/0006-8993(91)90159-s. [DOI] [PubMed] [Google Scholar]
- 3.Gundlach AL, Largent BL, Snyder SH. J Neurosci. 1986;6:1757. doi: 10.1523/JNEUROSCI.06-06-01757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiser SD, Patrick SL, Mascarella SW, Downing-Park J, Bai X, Carroll FI, Walker JM, Patrick RL. Eur J Pharmacol. 1995;275:1. doi: 10.1016/0014-2999(94)00718-m. [DOI] [PubMed] [Google Scholar]
- 5.Itzhak Y. Eur J Pharmacol. 1993;230:243. doi: 10.1016/0014-2999(93)90810-5. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen EC, McCracken KA, Liu Y, Pouw B, Matsumoto RR. Neuropharmacology. 2005;49:638. doi: 10.1016/j.neuropharm.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 7.Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. J Pharmacol Exp Ther. 1976;197:517. [PubMed] [Google Scholar]
- 8.Quirion R, Bowen WD, Itzhak Y, Junien JL, Musacchio JM, Rothman RB, Su TP, Tam SW, Taylor DP. Trends Pharmacol Sci. 1992;13:85. doi: 10.1016/0165-6147(92)90030-a. [DOI] [PubMed] [Google Scholar]
- 9.Prasad PD, Li HW, Fei YJ, Ganapathy ME, Fujita T, Plumley LH, Yang-Feng TL, Leibach FH, Ganapathy V. J Neurochem. 1998;70:443. doi: 10.1046/j.1471-4159.1998.70020443.x. [DOI] [PubMed] [Google Scholar]
- 10.Mei J, Pasternak GW. Biochem Pharmacol. 2001;62:349. doi: 10.1016/s0006-2952(01)00666-9. [DOI] [PubMed] [Google Scholar]
- 11.Bermack JE, Debonnel G. J Pharmacol Sci. 2005;97:317. doi: 10.1254/jphs.crj04005x. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi T, Maurice T, Su TP. J Pharmacol Exp Ther. 2000;293:788. [PubMed] [Google Scholar]
- 13.Hayashi T, Su TP. Proc Natl Acad Sci U S A. 2001;98:491. doi: 10.1073/pnas.98.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morin-Surun MP, Collin T, Denavit-Saubie M, Baulieu EE, Monnet FP. Proc Natl Acad Sci U S A. 1999;96:8196. doi: 10.1073/pnas.96.14.8196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guitart X, Codony X, Monroy X. Psychopharmacology (Berl) 2004;174:301. doi: 10.1007/s00213-004-1920-9. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto RR, McCracken KA, Pouw B, Zhang Y, Bowen WD. Neuropharmacology. 2002;42:1043. doi: 10.1016/s0028-3908(02)00056-4. [DOI] [PubMed] [Google Scholar]
- 17.Romieu P, Martin-Fardon R, Maurice T. Neuroreport. 2000;11:2885. doi: 10.1097/00001756-200009110-00011. [DOI] [PubMed] [Google Scholar]
- 18.Romieu P, Meunier J, Garcia D, Zozime N, Martin-Fardon R, Bowen WD, Maurice T. Psychopharmacology (Berl) 2004;175:154. doi: 10.1007/s00213-004-1814-x. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto RR, McCracken KA, Pouw B, Miller J, Bowen WD, Williams W, De Costa BR. Eur J Pharmacol. 2001;411:261. doi: 10.1016/s0014-2999(00)00917-1. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto RR, Mack AL. Eur J Pharmacol. 2001;417:R1. doi: 10.1016/s0014-2999(01)00891-3. [DOI] [PubMed] [Google Scholar]
- 21.Hellewell SB, Bowen WD. Brain Res. 1990;527:244. doi: 10.1016/0006-8993(90)91143-5. [DOI] [PubMed] [Google Scholar]
- 22.Hellewell SB, Bruce A, Feinstein G, Orringer J, Williams W, Bowen WD. Eur J Pharmacol. 1994;268:9. doi: 10.1016/0922-4106(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 23.Moebius FF, Burrows GG, Striessnig J, Glossmann H. Mol Pharmacol. 1993;43:139. [PubMed] [Google Scholar]
- 24.Matsumoto RR, Shaikh J, Wilson LL, Wang J, Coop A. FASEB J. 2007;21:A777. [Google Scholar]
- 25.Xu YT, Kaushal N, Shaikh J, Wilson LL, Mesangeau C, McCurdy CR, Matsumoto RR. J Pharmacol Exp Ther. doi: 10.1124/jpet.109.161398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mesangeau C, Narayanan S, Green AM, Shaikh J, Kaushal N, Viard E, Xu YT, Fishback JA, Poupaert JH, Matsumoto RR, McCurdy CR. J Med Chem. 2008;51:1482. doi: 10.1021/jm701357m. [DOI] [PubMed] [Google Scholar]
- 27.Chu W, Xu J, Zhou D, Zhang F, Jones LA, Wheeler KT, Mach RH. Bioorg Med Chem. 2009;17:1222. doi: 10.1016/j.bmc.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsumoto RR. Sigma receptors: Historical perspective and background, in Sigma Receptors: Chemistry, Cell Biology and Clinical Implications. New York: Springer; 2007. [Google Scholar]
- 29.Matsumoto RR, Pouw B, Mack AL, Daniels A, Coop A. Pharmacol Biochem Behav. 2007;86:86. doi: 10.1016/j.pbb.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maeda DY, Williams W, Kim WE, Thatcher LN, Bowen WD, Coop A. Bioorg Med Chem Lett. 2002;12:497. doi: 10.1016/s0960-894x(01)00788-0. [DOI] [PubMed] [Google Scholar]
- 31.Matsumoto RR, Liu Y, Lerner M, Howard EW, Brackett DJ. Eur J Pharmacol. 2003;469:1. doi: 10.1016/s0014-2999(03)01723-0. [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto RR, Bowen WD, Tom MA, Vo VN, Truong DD, De Costa BR. Eur J Pharmacol. 1995;280:301. doi: 10.1016/0014-2999(95)00208-3. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto RR, Shaikh J, Wilson LL, Vedam S, Coop A. Eur Neuropsychopharmacol. 2008;18:871. doi: 10.1016/j.euroneuro.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng Y, Prusoff WH. Biochem Pharmacol. 1973;22:3099. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]


