Abstract
Miliary tuberculosis (TB) is a potentially lethal disease if not diagnosed and treated early. Diagnosing miliary TB can be a challenge that can perplex even the most experienced clinicians. Clinical manifestations are nonspecific, typical chest radiograph findings may not be evident till late in the disease, high resolution computed tomography (HRCT) shows randomly distributed miliary nodules and is relatively more sensitive. Ultrasonography, CT and magnetic resonance imaging (MRI) are useful in discerning the extent of organ involvement by lesions of miliary TB in extra-pulmonary locations. Fundus examination for choroid tubercles, histopathological examination of tissue biopsy specimens, conventional and rapid culture methods for isolation of Mycobacterium tuberculosis, drug-susceptibility testing, along with use of molecular biology tools in sputum, body fluids, other body tissues are useful in confirming the diagnosis. Although several prognostic markers have been described which predict mortality, yet untreated miliary TB has a fatal outcome within one year. A high index of clinical suspicion and early diagnosis and timely institution of anti-tuberculosis treatment can be life-saving. Response to first-line anti-tuberculosis drugs is good but drug-induced hepatotoxicity and drug-drug interactions in human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) patients pose significant problems during treatment. However, sparse data are available from randomized controlled trials to define the optimum regimen and duration of treatment in patients with drug-sensitive as well as drug-resistant miliary TB, including those with HIV/AIDS.
Keywords: Complications, diagnosis, human immunodeficiency virus, miliary tuberculosis, treatment
Introduction
In 1700, John Jacob Manget1 described a form of disseminated tuberculosis (TB) and likened the tiny tubercles evident on gross pathological examination to that of innumerable millet seeds in size and appearance. He coined the term miliary TB (derived from the Latin word miliarius, meaning related to millet seed) to denote this fatal form of disseminated TB. Miliary TB results from a massive lymphohaematogeneous dissemination from a Mycobacterium tuberculosis-laden focus2–4 (Fig. 1).
Fig. 1.
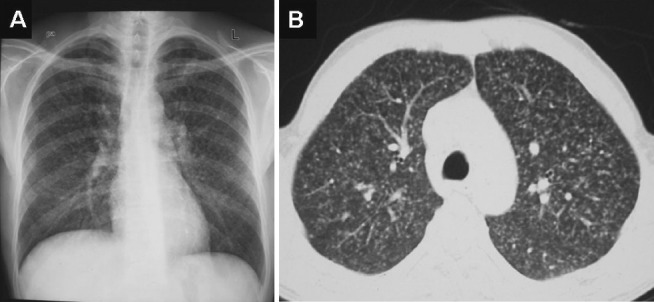
Chest radiograph (postero-anterior view) (A) and chest CT (lung window) (B) showing classical miliary pattern.
Miliary TB still remains a perplexing disease that continues to elude the most erudite and experienced clinicians and is a diagnostic and therapeutic challenge. Mortality from this disease has remained high despite effective therapy being available. The myriad clinical manifestations, atypical radiographic findings and difficulties in establishing TB as the aetiological diagnosis, among others, are challenges in diagnosis and treatment of miliary TB (Table I).
Table I.
Why miliary TB is a challenge for diagnosis and treatment?

In this review, we first provide an overview regarding the epidemiology, current understanding of key pathogenetic mechanisms, molecular basis of dissemination, predisposing and associated conditions, the varied clinical manifestations that have been documented in miliary TB, and then the challenges in the diagnosis and treatment of miliary TB are addressed.
Burden of the problem
Mortality from this disease has remained high despite effective therapy being available. For a long time, miliary TB has been considered to be a childhood disease. However, during the last three decades, it is increasingly being recognized in adults as well. Several reasons are thought to be responsible for this changing epidemiological trend. These include: human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS), ever increasing list of causes of immunosuppression, such as use of biologicals and immunosuppressive drugs for treatment of various medical disorders, increasing occurrence of organ transplantation, chronic haemodialysis programme, among others.
Interpretation of published epidemiological data on miliary TB is hampered by certain methodological issues. Even after giving allowance for non-availability of community based data on the prevalence, different denominators used, lack of a “gold standard” for the diagnosis and variation in the nature of invasive methods used for securing tissue to confirm the diagnosis, sparse autopsy data regarding miliary TB in children certain conclusions can be drawn regarding the epidemiology of miliary TB. Among immunocompetent adults, miliary TB accounts for less than 2 per cent of all cases of TB and up to 20 per cent of all extra-pulmonary TB (EPTB) cases in various clinical studies5–12. In late HIV infection, EPTB accounts for more than 50 per cent of all cases of TB4. In autopsy studies13–19, the corresponding figures have been higher; miliary TB accounts for 0.3 to 13.3 per cent of all autopsies and 11.9 to 40.5 per cent of all cases of TB. In the pre-antibiotic era, miliary TB was predominantly a disease of infants and children20,21. Currently, two peaks are evident- one involving adolescents and young adults and another later in life among elderly persons4,9,11,22–44. Males seem to be more frequently affected by miliary TB in paediatric as well as adult series4,9,11,22–44. A few recent adult series on miliary TB9,19,29,33 describe a female preponderance probably reflecting increased awareness and utilization of health services by women. In USA, a higher incidence of miliary TB has been described in African Americans in some of the earlier publications though such a trend is not evident from recent data4,24,34.
Predisposing, associated conditions
Several predisposing or associated conditions have been described in patients with miliary TB. These include childhood infections, malnutrition, HIV/AIDS, alcoholism, diabetes mellitus, chronic kidney disease, dialysis, post-gastrectomy, organ transplantation, connective tissue disorders, pregnancy, postpartum, presence of an underlying malignancy, and silicosis4. However, their pathogenetic significance is not clear.
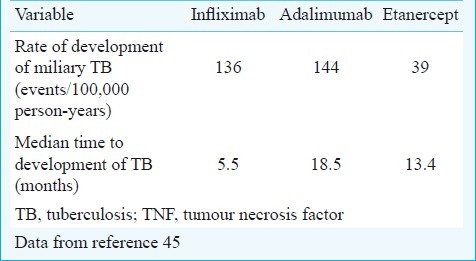
In addition to corticosteroids, immunosuppressive and cytotoxic drugs are known to predispose to the development of miliary TB, use of immunomodulator drugs (biologicals) has been documented to cause fatal TB including miliary TB in rheumatoid arthritis45–48. These include anti-tumour necrosis factor (TNF) agents infliximab48, etanercept47, and adalimumab46. In a recent prospective study among patients who received anti-TNF therapy45, EPTB constituted 62 per cent of all cases of TB; disseminated and miliary TB accounted for 27.5 per cent of all TB cases, 44 per cent of extra-pulmonary TB. The rate of development of TB was higher for adalimumab and infliximab than for etanercept. The median time to development of TB was lowest for infliximab compared with etanercept and adalimumab (Table II). Patients with non-white ethnicity had a 6-fold higher risk of TB compared with white patients45.
Table II.
Risk of developing miliary TB with the use of anti-TNF agents in patients with rheumatoid arthritis
Several procedures and interventions have been implicated in the causation of miliary TB. These include ureteral catheterization, extracorporeal shockwave lithotripsy, laser lithotripsy, cardiac valve homograft replacement, intravesical bacille Calmette-Guerin (BCG) therapy for urinary bladder carcinoma49–53.
Immunopathogenesis
The inadequacy of effector T-cell response in containment of M. tuberculosis is thought to be responsible for the development of miliary TB54–57. The abundance of Th1 and Th2 polarized effector T (Teff) cells in the peripheral blood and local disease site(s) among patients with miliary TB suggest that miliary TB probably represents the Th2 end of the spectrum56,57. Interleukin-4 (IL-4), with its ability to downregulate inducible nitric oxide synthase (iNOS), toll-like receptor 2 (TLR2) and macrophage activation, may play an important role in the events that determine whether the infection becomes latent or progressive4,54,55. M. tuberculosis can either fail to evoke the protective response or can drive the protective mechanisms and then deliberately ‘sabotage’ them leading to progressive disease55–57. In miliary TB, frequency of regulatory T (Treg) cells (CD4+CD25+FoxP3+) and higher levels of FoxP3 mRNA were significantly increased in local disease site specimens57. Further, FoxP3+ Treg cells obtained from the bronchoalveolar lavage (BAL) fluid of patients with miliary TB predominantly produced interleukin-10 (IL-10) and could suppress the autologous T-cell proliferation in response to M. tuberculosis antigen56. In miliary TB, the attempt by the host to selectively recruit the Teff cells at the pathologic site, however, fails to provide an adequate level of effector immunity at the disease site due to efficient and comparable homing of Treg cells (FoxP3+), which inhibit the function of the Teff cells that have infiltrated the disease site. It has been postulated that when the balance of homing of Treg and Teff cells shifts toward the former, there is a state of local immunosuppression leading to disease dissemination4,56,57.
Observations regarding the cellular characteristics of BAL fluid in patients with miliary TB have yielded conflicting results58–60. Though the diagnostic significance of these findings is not clear, these may facilitate the understanding of the pathogenesis of miliary TB. The proportion and absolute number of lymphocytes are substantially increased in BAL fluid. A raised CD4+/CD8+ T-lymphocyte ratio and B-lymphocytes as well as a decrease in CD4+/CD8+ T-lymphocyte ratio have earlier been reported in BAL fluid61,62. Polyclonal hypergammaglobulinaemia with increase in immunoglobulin (Ig) G, IgA, and IgM was observed in peripheral blood and BAL fluid61. These findings probably result from increased local synthesis by activated B-lymphocytes. Increased BAL fluid fibronectin and serum C3 levels reflect an acute phase response to ongoing inflammation61,62. Lymphocytic alveolitis and increased IgG and IgA levels have persisted following antituberculosis treatment61.
Molecular basis of dissemination
Several molecular mechanisms have been implicated in the development of miliary TB. These include impaired expansion of γ/δ T-cells63, failure to generate adequate cell-mediated immunity64, presence of HLA-Bw1565, HLA-DRB1*15/16, DRB1*13, and DQB1*060266, absence of HLA-Cw6, HLA-DRB1*10, and DQB1*050166, impaired MHC class II restricted target cell lysis, and over-exuberant lysis of target cell macrophages67 and LTA+368 G/A polymorphisms68.
Clinical manifestations
The clinical manifestations of miliary TB in adults are protean, non-specific and can be obscure till late in the disease (Fig. 2).
Fig. 2.

Median prevalence of symptoms and signs at initial presentation in adult patients with miliary tuberculosis. Data from references 9,18,24–27,29,31–34,36,37,39–42,44.
Constitutional symptoms: Patients with miliary TB classically present with fever with evening rise of temperature of several weeks duration, anorexia, weight loss, weakness and cough3,4. Occurrence of daily morning temperature spikes69 is reported to be characteristic of miliary TB. However, fever may be absent and the patients may present with progressive wasting strongly mimicking a metastatic carcinoma (cryptic miliary TB)70. Since its initial description, cryptic miliary TB is increasingly being reported in the elderly population20,21.
Chills and rigors, described in patients with malaria, or, sepsis and bacteraemia, have often been described in adult patients with miliary TB3,4. Night sweats are common. A “damp shadow” sign (where sweat engraved the patient's silhouette on the bed, closely resembling a body's shadow) has also been described in miliary TB71.
Systemic involvement: Since miliary TB can involve many organs, patients present with symptoms and signs referred to various organ systems (Fig. 2). Dry cough and dyspnoea are often present. Sputum may be scanty. Haemoptysis can occur rarely. Cutaneous lesions may offer a valuable clue to the diagnosis of miliary TB (Fig. 3). These include erythematous macules and papules (tuberculosis miliaria cutis)4.
Fig. 3.
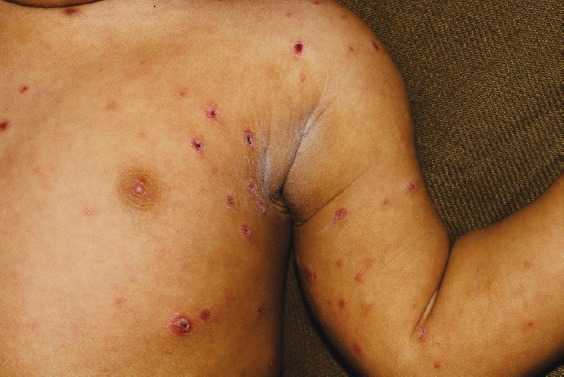
Clinical photograph of a child showing cutaneous lesions of miliary tuberculosis (Kind courtesy: Dr M. Ramam, Department of Dermatology, All India Institute of Medical Sciences, New Delhi, India).
Choroidal tubercles when present in an appropriate clinical setting, provide a valuable clue to the diagnosis of miliary TB. The presence of choroidal tubercles is considered to be pathognomonic of miliary TB3,4,39. Choroidal tubercles are bilateral, pale, gray-white or yellowish lesions usually less than one quarter of the size of the optic disc and are located within 2 cm of the optic nerve. These are more commonly seen in children. Therefore, a systematic ophthalmoscopic examination is recommended after mydriatric administration in all patients with suspected miliary TB (Fig. 4).
Fig. 4.
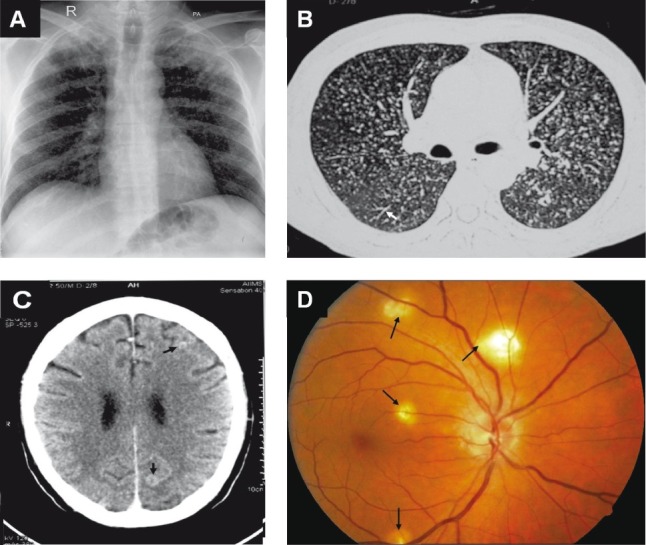
Chest radiograph (postero-anterior view) (A) and chest CT (lung window) (B) showing classical miliary pattern, tree-in-bud appearance (B) (arrow). The patient also had cerebral tuberculomas (arrows) and TB meningitis (C). Choroid tubercles, located in the posterior pole of the orbit (D) (arrows) offered an early valuable clue to the diagnosis. The present case illustrates the importance of documenting the presence of neurological involvement, particularly, TB meningitis in patients with miliary TB and thereby ensuring adequate duration of anti-tuberculosis treatment and need for corticosteroid treatment.
TB meningitis (TBM) has been described in 10 to 30 per cent adult patients with miliary TB9,18,22–27,29,30,32–39,41,42; conversely, about one-third of patients presenting with TB meningitis have underlying miliary TB72. In a recently published study73, the spectrum of neurological involvement in adult patients with miliary TB included TB meningitis with and without tuberculoma; and thoracic transverse myelopathy.
Before the advent of modern imaging modalities, such as CT, MRI and echocardiography, clinically evident cardiac or renal involvement was seldom documented in patients with miliary TB. Overt adrenal insufficiency manifesting as Addison's disease at initial presentation, or during anti-tuberculosis treatment has also been described in miliary TB74,75.
Children
There are only a few published series on childhood miliary TB6–8,22–38. Miliary TB develops less often in children who have received the BCG vaccination4. Compared with adults, chills and night sweats, haemoptysis, productive cough are less common; peripheral lymphadenopathy and hepatosplenomegaly are more frequent in children with miliary TB. A larger proportion of children with miliary TB (20-40%) suffer from TBM6–8,22–38 compared to adults (15-30%)9,18,22–27,29,31–34,36–42,44.
Miliary tuberculosis in immunosuppressed individuals
The prevalence of miliary TB in persons with early HIV infection (CD4+ cell counts >200 cells/mm3) is similar to that observed in immunocompetent individuals. With progression of immunosuppression, in late, advanced stage of HIV infection (CD4+ cell counts <200 cells/mm3), miliary TB is seen more often4,58,76. Table III shows a comparison of various aspects of miliary TB in late advanced stage of HIV infection and in immunocompetent individuals, early HIV infection4,41,58,76–82. Cutaneous involvement, a rare clinical manifestation in HIV-seronegative patients with miliary TB, is more commonly seen in late HIV infection with severe immunosuppression4,58,76–82. The skin manifestations include tiny papules or vesiculopapules, (tuberculosis cutis miliaris disseminata, tuberculosis cutis acuta generalisita), and disseminated tuberculosis of the skin (Fig. 3). Sometimes, macular, pustular, or purpuric lesions, indurated ulcerating plaques, and subcutaneous abscesses have been reported83.
Table III.
Clinical presentation of miliary TB in adult patients with and without HIV co-infection
In miliary TB patients co-infected with HIV, especially in those with profound immunosuppression, intrathoracic lymphadenopathy and tuberculin anergy are more common; sputum smears are seldom positive and blood culture may grow M. tuberculosis4,58,76–82 (Table III). These observations seem to be applicable to other causes of immunosuppression as well84.
Uncommon clinical manifestations and complications
Several uncommon clinical manifestations and complications have been observed in patients with miliary TB (Table IV). In some patients, complications like ARDS or myocarditis may in fact be the initial presentation. Atypical clinical presentation often delays the diagnosis and treatment and miliary TB is often a “missed diagnosis”.
Table IV.
Uncommon clinical manifestations and complications in miliary tuberculosis
Acute respiratory distress syndrome
Although ARDS may develop anytime during the course of miliary TB, it is usually seen at the time of initial presentation (Fig. 5); ARDS may develop as a component of the multiorgan dysfunction syndrome (MODS) due to TB or as a manifestation of immune reconstitution inflammatory syndrome (IRIS)85–89. In a study from two large teaching hospitals at New Delhi and Tirupati in India89, among patients with TB, prolonged illness, miliary TB, absolute lymphocytopaenia and elevated alanine aminotransferase (ALT) were found to be independently associated with the development of ARDS. In another study90 from Korea, higher C-reactive protein levels and an increasing nutritional risk score were found to be independent risk factors for the development of ARDS in patients with miliary TB.
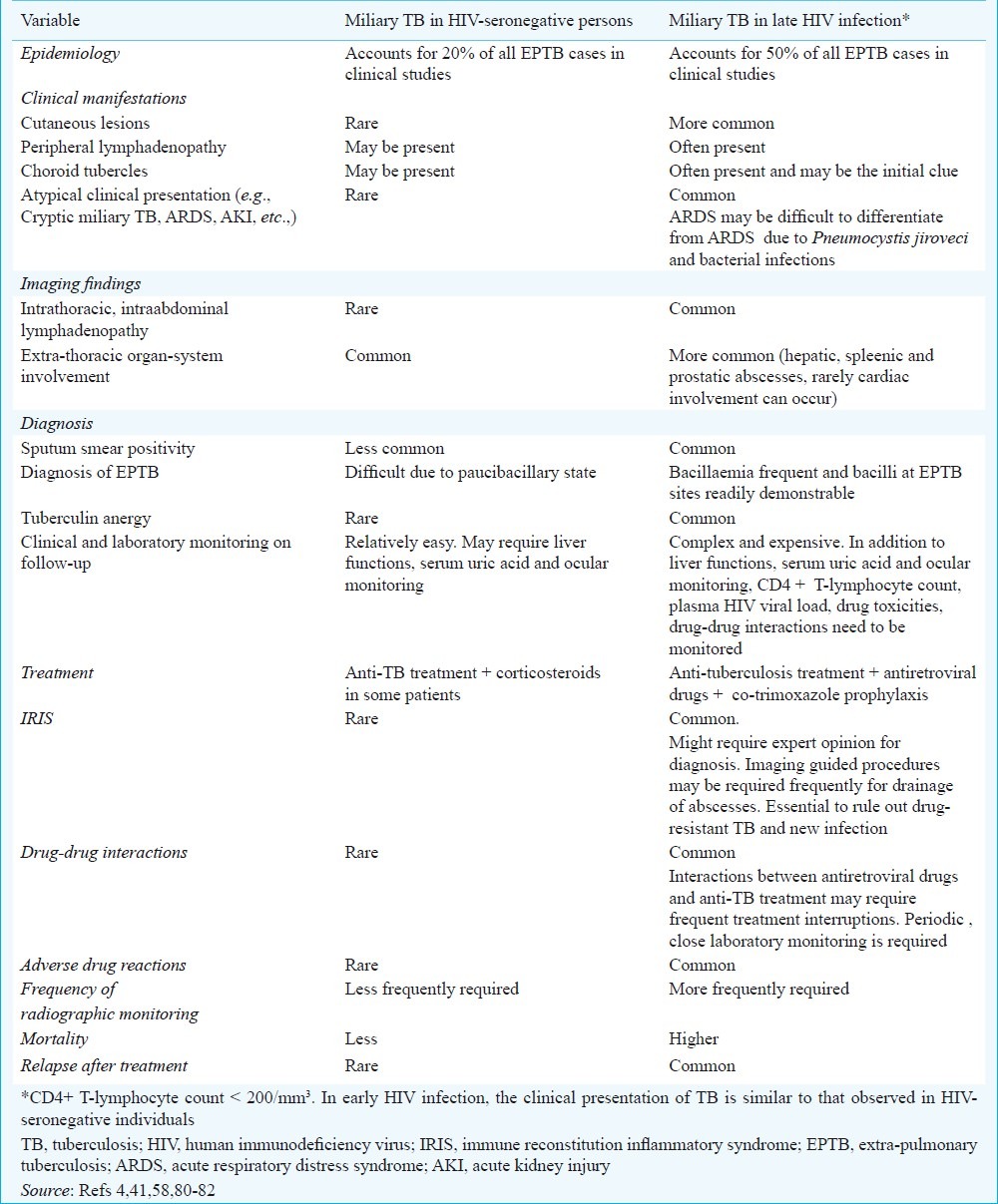
Fig. 5.
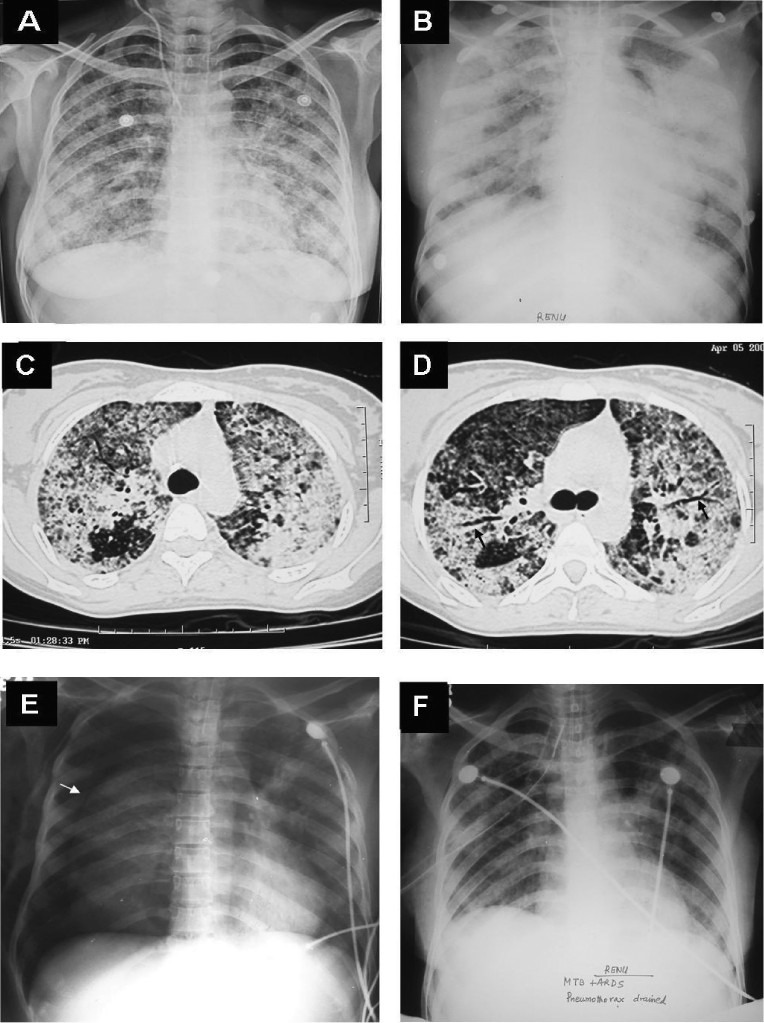
Chest radiograph (postero-anterior view) of a pregnant woman who presented with prolonged pyrexia showing a classical miliary pattern (A). Fundus examination following mydriatic administration in both the eyes revealed choroid tubercles and had raised the suspicion of miliary TB. The patient developed ARDS during the course of her illness. Chest radiograph (antero-posterior view), obtained with a portable X-ray machine, bed-side showing bilateral frontal opacities suggestive of ARDS (B). CT chest obtained at the same time reveals air-space consolidation (C and D); air-bronchogram (arrow) (D). While assisted ventilation was being administered, the patient developed pneumothorax (asterisk) on the right side; collapsed lung border is also evident (arrow) (E). The patient required tube thoracostomy and underwater seal drainage. Eventually the patient was weaned off the ventilator and the intercostal tube was removed following resolution of the pneumothorax. The chest radiograph obtained thereafter shows significant improvement in the lesions (F). The patient survived the turbulent in-hospital course, went on to complete full-term of pregnancy and was successfully delivered a live baby. ARDS, acute respiratory distress syndrome; CT, computed tomography; TB, tuberculosis.
Air-leak syndromes
Pneumothorax, which may sometimes be bilateral, may be the presenting feature or may sometimes develop while the patient is receiving anti-tuberculosis treatment3,4,91,92. Classical miliary shadows may not be discernible initially and may become apparent once lung expands. Intrapulmonary rupture of alveoli and consequent air-leak that traverses into the mediastinum after spreading along the vascular sheath can result in pneumomediastinum with subcutaneous emphysema which may be fatal93. Rarely, pneumothorax may develop as a complication in TB-ARDS patients receiving mechanical ventilation (Fig. 5E).
Acute kidney injury
In addition to being a part of MODS, acute kidney injury (AKI) may occur due to direct renal parenchymal involvement in patients with miliary TB4,94. In HIV co-infected patients with miliary TB, AKI can also develop as a manifestation of IRIS95. Uncommonly, renal failure can develop as a consequence of obstructive uropathy caused by the disease process58.
Hepatic and gastrointestinal manifestations
Fulminant hepatic failure may rarely be the presenting manifestation in miliary TB. In some of these patients the characteristic pulmonary lesions that constitute the hall mark of miliary TB are absent96,97. This could probably be the result of extrapulmonary focus discharging the tubercle bacilli into the portal circulation, resulting in hepatic miliary TB. Peritoneal involvement may be evident by the presence of ascites. Some patients may manifest diarrhoea or altered bowel habit suggestive of intestinal involvement. Small intestinal perforations at the site of granulomatous involvement have been described in some patients while on treatment98.
Lesions located elsewhere in the body
Thoracic and abdominal lymphadenopathy: Patients with miliary TB, especially those co-infected with HIV manifest associated intrathoracic lymphadenopathy. Sometimes, intra-abdominal lymphadenopathy involving portahepatis, pre- and para-aortic and mesenteric lymph nodes; retroperitoneal lymphadenopathy may be present.
Pott's spine: Pott's spine with or without myelopathy may also be present in patients with miliary TB.
Cold abscesses: Cold abscesses in association with peripheral lymphadenopathy in the cervical or axillary regions, Pott's spine with paraspinal cold abscess may also be evident in patients with miliary TB.
Genitourinary lesions: Women with miliary TB can manifest pelvic ascites, tubo-ovarian masses, pyosalpinx and should be carefully evaluated for the same.
Cardiovascular manifestations
In patients with miliary TB, life-threatening complications such as myocarditis99, congestive heart failure99, native100–102 and prosthetic valve103 endocarditis, pericarditis, intracardiac mass99, mycotic aneurysm104, infection of a pacemaker pulse-generator pocket105, infection of ventriculoatrial shunt causing miliary TB106 and sudden cardiac death107–109 have been described.
Immune reconstitution inflammatory syndrome (IRIS)
The IRIS, occasionally described in HIV-negative individuals with TB, has been reported to occur in about one-third of patients co-infected with HIV and TB within days to weeks of the initiation of highly active antiretroviral therapy (HAART)110,111. The IRIS can be brief or prolonged with multiple recurrences. Manifestations of IRIS range from isolated instances of fever to increased or initial appearance of lymphadenopathy, new or worsening pulmonary infiltrates, serositis, cutaneous lesions, and new or expanding central nervous system mass lesions76,110,111 ; AKI95,112 or ARDS85 can develop during the course of IRIS.
Differential diagnosis
Radiologically, the miliary pattern has been defined as “a collection of tiny discrete pulmonary opacities that are generally uniform in size and widespread in distribution, each of which measures 2 mm or less in diameter”113. Many conditions can present with a miliary pattern on the chest radiograph or CT (Fig. 6) and are listed in Table V. These conditions must be differentiated from miliary TB by detailed diagnostic work-up.
Fig. 6.
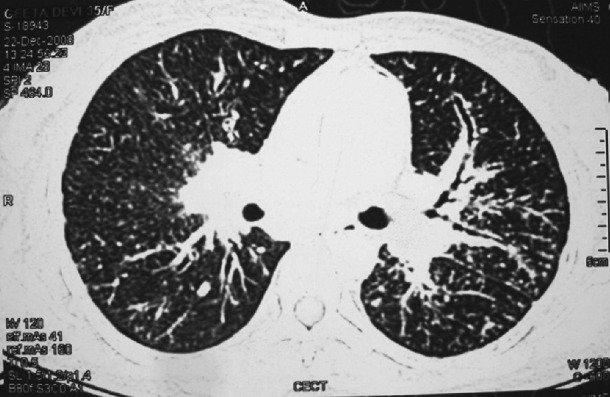
CT of the chest showing miliary sarcoidosis. While the lesions of miliary TB (Fig. 1B) are randomly distributed, the lesions of miliary sarcoidosis are distributed along the bronchovascular bundle (lymphangitic distribution). Thus transbronchial lung biopsy gives a higher diagnostic yield in miliary sarcoidosis.
Table V.
Some conditions presenting with a miliary pattern on the chest radiograph
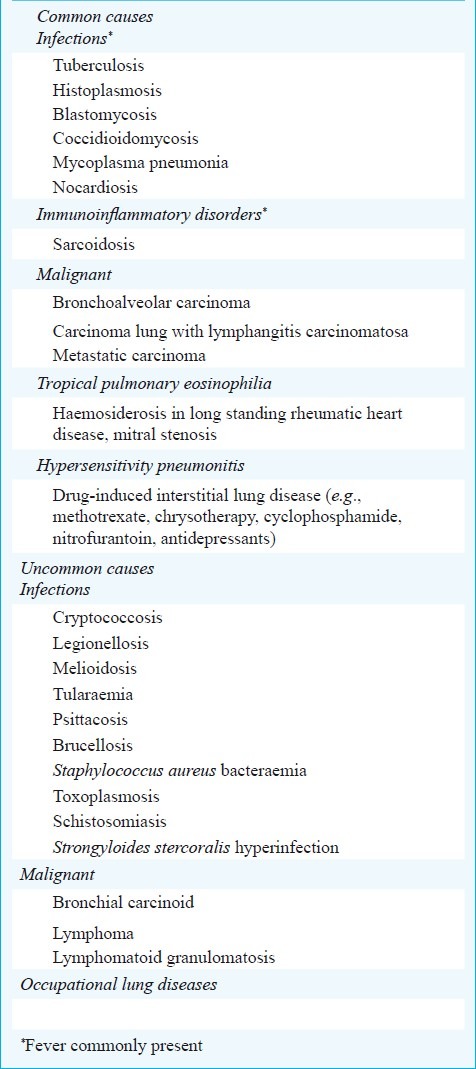
Diagnosis
The diagnosis of miliary TB can be difficult as the clinical manifestations are non-specific, the chest radiographs do not always reveal the classical miliary changes and patients may present with complications thus distracting the clinicians. Therefore, a high index of clinical suspicion and a systematic approach to diagnostic testing is required to establish the diagnosis of miliary TB.
Following criteria are useful for the diagnosis of miliary TB: (i) clinical presentation consistent with a diagnosis of tuberculosis such as, pyrexia with evening rise of temperature, weight loss, anorexia, tachycardia and night sweats of greater than six weeks duration responding to antituberculosis treatment; (ii) classical miliary pattern on chest radiograph; (iii) bilateral diffuse reticulonodular lung lesions on a background of miliary shadows demonstrable either on plain chest radiograph or HRCT; and (iv) microbiological and/or histopathological evidence of TB39. The diagnostic approach to a patient with suspected miliary TB is shown in Fig. 7.
Fig. 7.
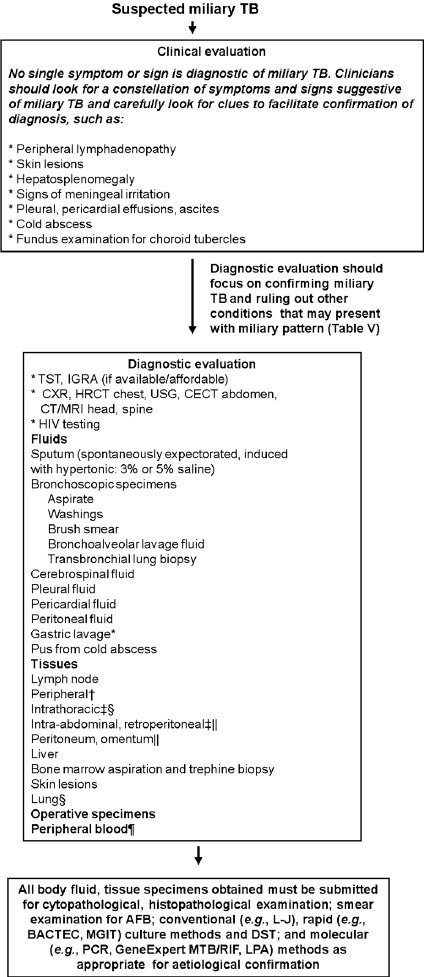
Algorithm for the diagnostic work-up of a patient with suspected miliary TB. The clinical and imaging diagnostic work-up should also aim at accurately assessing the extent of extrapulmonary involvement to facilitate monitoring and ensure adequate duration of treatment. All laboratory testing, especially, antituberculosis drug-susceptibility testing must be carried out in quality assured, periodically accredited laboratories. *Often used in children; †FNAC/excision biopsy; ‡ Radiologically guided FNAC/biopsy; §Mediastinoscopic/video-assisted thoracoscopic surgery, biopsy; ||Laparoscopic biopsy; ¶Useful in advanced HIV infection. TB, tuberculosis; TST, tuberculin skin test; IGRA, interferon-γ release assays; HRCT, high resolution computed tomography; CECT, contrast enhanced computed tomography; MRI, magnetic resonance imaging; FNAC, fine needle aspiration cytology; HIV, human immunodeficiency virus; AFB, acid-fast bacilli; L-J, Lowenstein-Jensen medium; DST, drug-susceptibility testing; MGIT, mycobacterial growth inhibitor tube; BACTEC, radiometric culture method; PCR, polymerase chain reaction; GeneExpert MTB/RIF, GeneXpert MTB/RIF assay (Cepheid, Sunnyvale, CA); LPA, line probe assay.
Tuberculin skin test
Tuberculin anergy is more common in miliary TB than in pulmonary and ep0 TB; tuberculin skin test
(TST) conversion may occur following successful treatment. In various published paediatric6–8,22,38 and adult series24–27,31,32–34,36,37,114 tuberculin anergy has ranged from 35 to 74 per cent and 20 to 70 per cent, respectively. However, a positive TST only indicates infection with M. tuberculosis and does not always indicate active disease. A positive reaction with necrosis often (but not always) indicates active disease.
Interferon-gamma release assays
Newer in vitro T-cell based interferon-gamma release assays (IGRAs) available in the enzyme linked immunosorbent assay (ELISA) and enzyme linked immunospot (ELISPOT) formats appear to be promising in detecting latent TB infection (LTBI) and have several advantages over the TST. These tests may be particularly useful in children, BCG vaccinated individuals and in HIV infection and AIDS115,116. As with TST, a positive IGRA test result, however, does not distinguish between LTBI and active disease, but a negative IGRA result may be helpful in ruling-out a diagnosis of TB116. These tests are costly and on the basis of available evidence their routine use is not indicated.
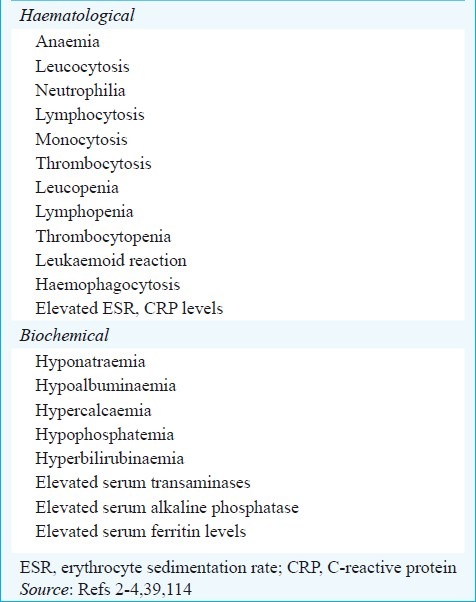
Haematological and biochemical abnormalities
A number of haematological and biochemical abnormalities are known to occur in miliary TB (Table VI)6–9,18,22,23,24–27,29,31,32–36–42,44 but their significance is controversial. Disseminated intravascular coagulation (DIC)86,89 has been described in patients with miliary TB in the setting of ARDS and MODS and is associated with a high mortality. Immune mechanisms have been implicated to cause bone marrow suppression and miliary TB has also been implicated as a cause of pancytopenia, hypoplastic anaemia4,117. Hypercalcaemia has been documented in miliary TB, but is uncommon118.
Table VI.
Laboratory abnormalities in miliary TB
Hyponatraemia in miliary TB can occur due to an acquired disturbance of neurohypophyseal function resulting in unregulated antidiuretic hormone (ADH) release; an antidiuretic principle in the lung tissue affected by TB that may either produce ADH or absorb an inappropriately released hormone from the posterior pituitary119–121. Hyponatraemia may indicate the presence of TB meningitis34 and may also be a predictor of mortality39 in patients with miliary TB. Rifampicin-induced adrenal crisis in a patient with miliary TB and Addison's disease who developed generalized malaise and hyponatraemia while initiated on antituberculosis treatment has also been described75.
Imaging studies
Miliary pattern on the chest radiograph is often the first clue suggestive of miliary TB. Several other imaging modalities, such as, ultrasonography, CT, MRI, positron emission tomography (PET) help to assess the extent of organ involvement and are also useful in evaluating response to treatment (Figs. 8 and 9).
Fig. 8.
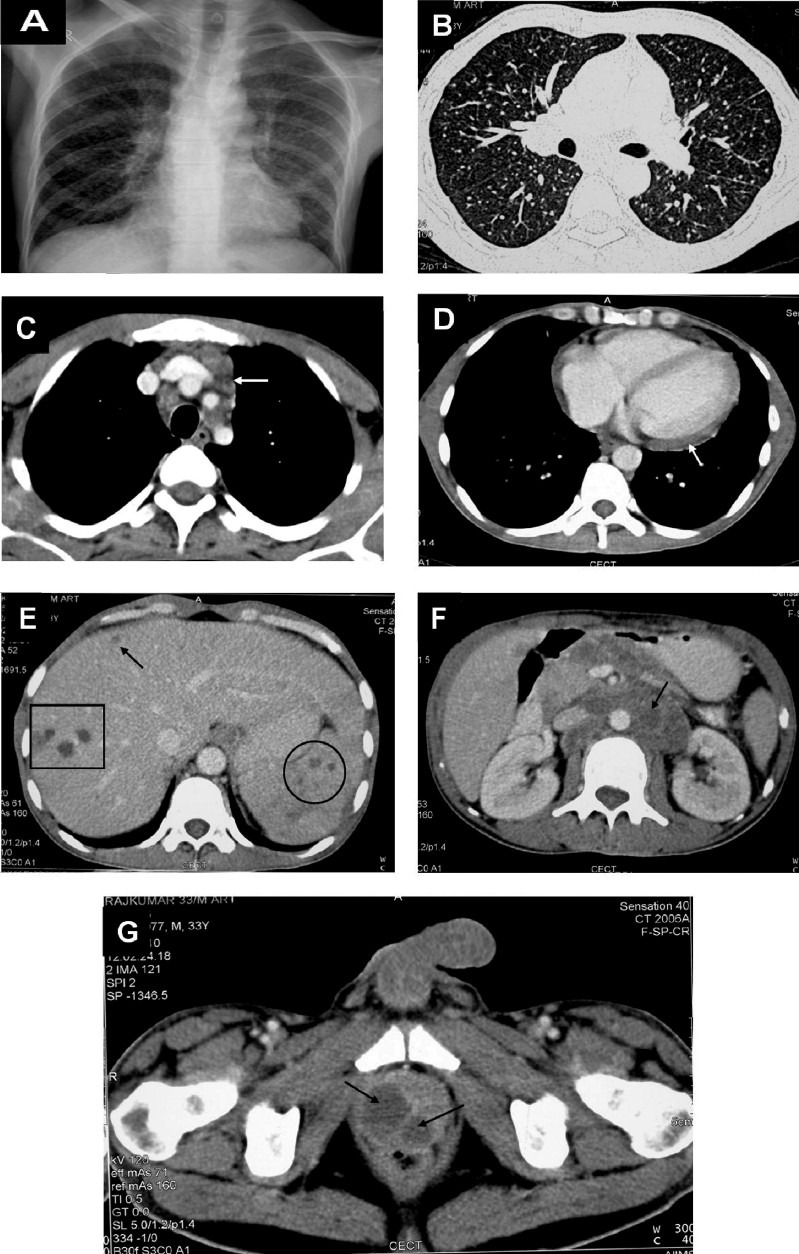
Chest radiograph in a patient with HIV/AIDS (postero-anterior view) (A) and chest CT (lung window) (B) showing classical miliary pattern. The CECT chest (mediastinal window) also reveals intrathoracic lymphadenopathy (arrow) (C) and pericardial thickening and effusion (D). The CECT of the abdomen of the same patient reveals focal miliary lesions in the liver (square, arrow) and spleen (circle) (E) and retroperitoneal lymphadenopathy (arrow) (F); pelvic CECT shows a prostatic abscess (arrows) (G). Ultrasound guided trans-rectal prostatic aspirate smear and culture examination confirmed the diagnosis of miliary TB. The diagnostic evaluation of this patient illustrates the judicious use of imaging modalities to define the extent of organ system involvement and procuring tissue for diagnostic confirmation. Such extensive involvement usually occurs in HIV/AIDS with miliary TB. HIV, human immunodeficiency virus; AIDS, acquired immunodeficiency syndrome; CT, computed tomography; CECT, contrast enhanced computed tomography; TB, tuberculosis.
Fig. 9.
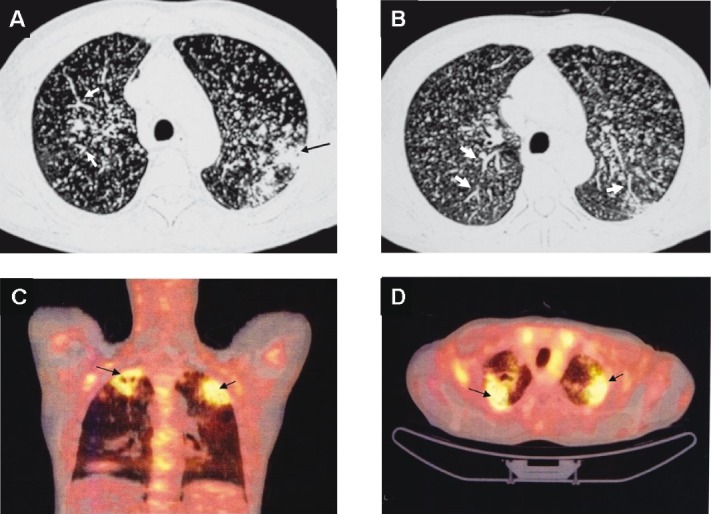
Chest CT (lung window) of the same patient as in Fig. 4 showing pulmonary parenchymal lesions (black arrow) (A). In addition to the miliary pattern, well-defined, linear, branching opacities (tree-in-bud appearance) (thick white arrows) (A and B) are also seen. This pattern is evident when centrilobular bronchioles are dilated, or, are filled with mucus, fluid or, pus and represents endobronchial spreading of infection. 18FDG-PET CT of the same patient showing increased activity in the pulmonary parenchymal lesions (arrows) but not in the miliary lesions (C and D). The 18FDG-PET has potential to further understanding the clinico-radiographic-functional correlation in miliary tuberculosis and merits further study. However, it may not be useful in intracranial TB. CT, computed tomography; 18FDG-PET CT, 18F labelled 2-deoxy-D-glucose positron emission tomography-computed tomography; TB, tuberculosis.
Chest radiograph: The chest radiographic abnormalities in miliary TB are depicted in Table VII 4,114. Miliary pattern on chest radiograph3,4,113 is the hall mark of miliary TB and is evident in a majority of patients . Classically, subtle miliary lesions are best delineated in slightly underpenetrated films especially when the diamond shaped areas of the lung in between the ribs are carefully scrutinized using bright light122,123. However, in 10 per cent of the cases, the nodules may be greater than 3 mm in diameter124. Before the advent of CT, it was observed that classical miliary pattern would not be evident in the chest radiograph in up to 50 per cent of the patients and would be detected only at the time of autopsy14,17,18,24,34,124.
Table VII.
Chest radiographic abnormalities in miliary TB
When caseous material, collagen or both are present in the tubercles, these became visible on the chest radiograph122. Classical miliary pattern on the chest radiograph represents summation of densities of the tubercles that are perfectly aligned and imperfectly aligned tubercles result in curvilinear densities and a reticulonodular pattern125. Rarely, lymphatic obstruction or infiltration can result in ground glass appearance126. In some patients, predominance of lesions on one side may be evident (Fig. 10). Some patients may have normal chest radiographs initially and the typical miliary pattern may evolve over the course of disease. This is particularly evident in ARDS due to miliary TB where the chest radiograph findings may be identical to that seen in ARDS due to other causes86,89. One of the patients seen by the authors39 had undergone tonsillectomy and the histopathological diagnosis was reported as miliary TB. On further diagnostic testing, a repeat chest radiograph revealed classical miliary pattern that was not discernible in the earlier chest radiographs, thus, emphasizing the importance of periodic repeat chest radiographic examination in patients with suspected miliary TB4,39.
Fig. 10.
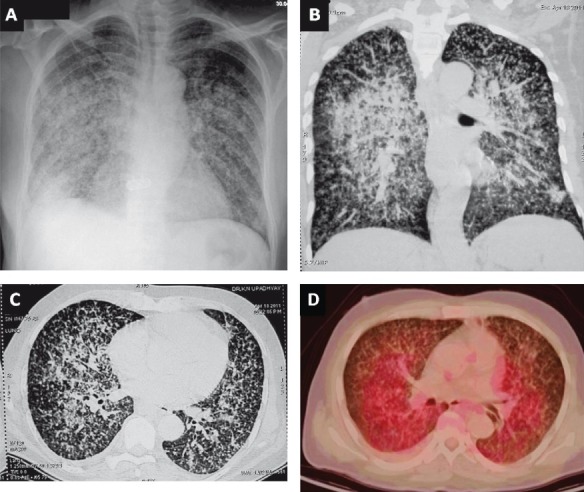
Chest radiograph (poster-anterior view) (A) and chest CT (lung window) (B and C) showing predominance of miliary lesions on the right side. 18FDG-PET CT of the same patient (D) showing increased activity in the coalesced pulmonary lesions, which is evident more prominently on the right side. CT, computed tomography; 18FDG-PET CT, 18F labelled 2-deoxy-D-glucose positron emission tomography-computed tomography.
Ultrasonography: Ultrasonography helps in detecting ascites which may sometimes be loculated, focal hepatic and splenic lesions and cold abscesses, intra-abdominal lymphadenopathy, involvement of other abdominal organs and pleural effusion(s). Ultrasonography guidance also facilitates diagnostic thoracic or abdominal paracentesis to procure pleural or peritoneal fluid for diagnostic testing especially if the fluid is loculated.
Computed tomography (CT) and magnetic resonance imaging (MRI): In comparison with pre-CT era, high resolution CT (HRCT), thin-section multidetector row CT (MDCT) have facilitated the antemortem diagnosis of miliary TB. With the availability of these imaging modalities, cryptic miliary TB, that could previously be diagnosed only at autopsy, can now be diagnosed antemortem4,40.
The HRCT reveals a mixture of both sharply and poorly defined, less than 2 mm nodules that are widely disseminated throughout the lungs associated with diffuse reticulation127,128. Importantly, the HRCT may reveal classical miliary pattern even when the chest radiograph looks apparently normal4,39 and also facilitates identification of additional findings such as intrathoracic lymphadenopathy, calcification, pleural and pericardial lesions (Fig. 8D)129.
Air trapping has been described on HRCT both at presentation and during follow up period128. The clinical significance of these findings is unclear. Rupture of these areas of air trapping may perhaps be responsible for the development of air-leak syndromes in miliary TB. The interlobular septal thickening or intralobular fine networking seen on HRCT in miliary TB seems to be caused by the caseation necrosis in the alveolar walls and interlobular septa. Sometimes, in subjects with active post-primary disease, centrilobular nodules and branching linear structures giving a “tree-in-bud appearance” may be evident127,128. A higher prevalence of interlobular septal thickening, necrotic lymph nodes and extrathoracic involvement has been observed in HIV-seropositive patients with miliary TB80.
CT and MRI have been useful in identifying miliary lesions at extra-pulmonary sites. Abdominal CT has been useful in identifying lesions in the liver, spleen, intestine, mesentery, peritoneum, adrenals and lymph nodes, and also detects cold abscesses4. Unlike the CT of the chest where the classical less than 2 mm nodular lesions are evident, miliary lesions in the liver and spleen may appear as discrete hypodense lesions (Fig. 8E) a few of which may be confluent, sometimes with irregular peripheral rim enhancement130.
The MRI of brain and spine is very useful in the evaluation of patients with miliary TB and TBM, and spinal TB. The MRI is particularly helpful in identification and delineating the extent of tuberculomas and cold abscesses and monitoring the response to treatment4.
Pelvic evaluation with all imaging modalities should be routinely done in all female patients for defining the extent of involvement. Tubo-ovarian masses, hydro- and pyosalpinx, fluid collection in the pouch of Douglas may become obvious. Image guided radiological procedures such as fine needle aspiration for cytological examination (FNAC) and biopsy under CT or MRI guidance are useful for procuring tissue/body fluids for diagnostic testing.
Sputum examination
Though not all patients with miliary TB manifest productive cough, when available, sputum must be subjected to smear and mycobacterial culture examination. Sputum smear microscopy using Ziehl-Neelsen stain is useful in detecting acid-fast bacilli (AFB). Fluorescent staining may also facilitate rapid diagnosis. Sputum mycobacterial culture and drug susceptibility testing carried out in an accredited laboratory with external quality assurance can facilitate identification and appropriate management of drug-resistant TB.
Bronchoscopy
Fibreoptic bronchoscopy, BAL, bronchoscopic aspirate, brushings, washings, and transbronchial lung biopsy (TBLB) are useful in confirming the diagnosis of miliary TB. The cumulative diagnostic yield for various bronchoscopic specimens by smear and culture methods in published studies has been found to be 46.8 per cent23,31,32,37,39,131. In patients with dry cough BAL fluid obtained through fiberoptic bronchoscopy should be submitted for mycobacterial smear, culture and molecular methods (if facilities exist).
Laparascopy
Laparascopy provides an opportunity to visualise the lesions, facilitates biopsy from the liver, spleen, peritoneum, omentum, mesenteric lymph nodes for diagnostic confirmation132. When associated abdominal involvement is present, laparascopy should be considered for procuring tissue for diagnostic testing.
Body fluids and tissue examination
In patients with suspected miliary TB, depending on the extent of organ system involvement, appropriate tissue and body fluid samples must be obtained to confirm histopathological microbiological diagnosis. Elevated serum alkaline phosphatase levels indicate diffuse liver involvement; needle biopsy of the liver can be useful in confirming the diagnosis. Bone marrow aspiration and needle biopsy have also been found to be useful for the diagnosis of miliary TB. Pleural fluid, pericardial fluid, ascitic fluid, CSF, urine, bronchoscopic secretions, blood and tissue biopsy specimens have all been employed to confirm the diagnosis of disseminated and miliary TB. The diagnostic yield of various tissue and body fluid specimens has been variable6–9,18,22–27,29,31–34,36–41,42,44 (Fig. 11).
Fig. 11.
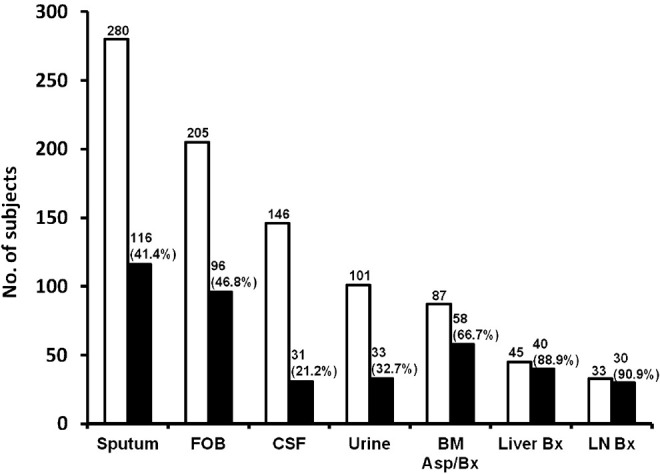
Cumulative diagnostic yield of various body fluids and tissues in the diagnosis of miliary TB. Cumulative diagnostic yield is expressed as percentage. The data are pooled for various specimen categories and may not be comparable across various series because different criteria were employed; however, these can be appropriately used in the individual patient to ascertain the diagnosis of miliary TB. FOB, fibreoptic bronchoscopy; CSF, cerebrospinal fluid; LN, lymph node; BM, bone marrow; Bx, biopsy. Source: Refs 9,18,24–27,29,31–34,36,37,39–42,44
When imaging studies reveal focal lesions in organs (e.g., liver, spleen prostate), intrathoracic or intra-abdominal lymphadenopathy or cold abscess, image-guided FNAC or tru-cut biopsy can be carried out and subjected to histopathological and microbiological evaluation to confirm the aetiological diagnosis.
Mycobacterial culture and drug-susceptibility testing (DST)
Mycobacterial culture and drug susceptibility testing of sputum, body fluids and tissue specimens carried out in an accredited laboratory with external quality assurance can facilitate identification and appropriate management of drug-resistant TB. Rapid culture methods such as the BACTEC-460 radiometric method, mycobacterial growth indicator tube (MGIT), and molecular methods may be useful for rapid drug-susceptibility testing4.
Serodiagnostic methods
The recently published World Health Organization (WHO) policy statement on the use of serodiagnostic tests strongly recommends that the currently available commercial serodiagnostic tests should not be used for the diagnosis of active pulmonary and extra-pulmonary TB disease including miliary TB133.
Adenosine deaminase
Adenosine deaminase (ADA) and interferon-gamma level estimation in ascitic fluid, pleural fluid can be helpful in the diagnosis of miliary TB134–139. A recent study139 has shown that that CSF-ADA is a more sensitive indicator than PCR for the diagnosis in patients with TB meningitis. However, another recent systematic review and meta-analysis140 has shown that CSF-ADA cannot distinguish between TB meningitis and bacterial meningitis. Since ADA estimation is a cheap, cost-effective test, utility of CSF-ADA estimation in the diagnosis of TB meningitis merits further study.
Molecular methods
Polymerase chain reaction (PCR) of CSF, tissue biopsy specimens and blood (especially in HIV-infected patients), may be useful for confirmation of diagnosis58. The PCR has been found to be most useful when applied to clean specimens such as CSF where its sensitivity and specificity have been reported to be 0.5 to 0.9 and 1.0, respectively71.
In patients with suspected miliary TB, wherever possible, automated molecular tests for M. tuberculosis detection and drug-resistance testing may be used for early confirmation of diagnosis. Based on currently available evidence and expert opinion, molecular assays to detect gene mutations that signal drug resistance have been endorsed by the WHO as being most suited for rapid diagnosis141. The GeneXpert MTB/RIF assay (Cepheid, Sunnyvale, CA) that uses heminested real-time PCR assay to amplify M. tuberculosis -specific sequence of the rpoB gene which is then probed with molecular beacons for mutations within the rifampicin-resistance determining region can facilitate rapid diagnosis from clinical specimens, such as, sputum in about 2 h142,143. Line probe assays (LPAs), such as, the INNO-LiPA® Rif. TB kit (Innogenetics NV, Gent, Belgium) and the GenoType® MTBDRplus assay (Hain Lifescience GmbH, Nehren, Germany) have been found to be useful for rapid screening of patients at risk for multidrug-resistant TB (MDR-TB)144.
Positron emission tomography
Positron emission tomography CT (PET-CT) using the radiopharmaceutical 18F labelled 2-deoxy-D-glucose (FDG) is useful to assess activity of various infectious lesions including TB (Fig. 9)145,146. The PET-CT is ideally suited to define the extent of disease at the time of initial presentation. The utility of PET-CT in assessing the activity of lesions that might persist following antituberculosis treatment in miliary TB also merits further study.
Pulmonary function, gas exchange abnormalities and cardiopulmonary exercise testing
Miliary TB is associated with abnormalities of pulmonary function typical of interstitial lung disease. Impairment of diffusion is the most common abnormality and may sometimes be severe59,147. Other abnormalities include, a mild reduction in flow rates suggestive of peripheral airways involvement59. During the acute stage, arterial hypoxaemia due to widening of the alveolar-arterial oxygen gradient and hypocapnia due to tachypnoea are also observed148. Often, the pulmonary function and gas exchange abnormalities may be of a greater magnitude than might be expected from the chest radiograph61,148–150.
Abnormal cardiopulmonary exercise performance has been described in patients with miliary TB. The salient abnormalities included lower maximum oxygen consumption, maximal work rate, anaerobic threshold, peak minute ventilation, breathing reserve, and low maximal heart rate4,114,148,150. Other abnormalities included higher respiratory frequency, peak minute ventilation at submaximal work, and high physiological dead space/tidal volume. A demonstrable fall in oxygen saturation (to 4% or more) with exercise was observed. Following successful anti-tuberculosis treatment, these abnormalities reversed in a majority of the patients148,150.
Treatment
Patients with miliary TB must be promptly treated with standard anti-tuberculosis treatment as the disease is uniformly fatal if not treated3,4. However, there is no consensus regarding the optimum duration of treatment. There are no published randomized controlled trials assessing the efficacy of the standard WHO treatment regimens that have been widely used in national tuberculosis control programmes worldwide151.
The American Thoracic Society (ATS), the Centers for Disease Control and Prevention (CDC), the Infectious Disease Society of America (IDSA)152 and National Institute for Health and Clinical Excellence (NICE)153 guidelines from UK state that six months of treatment (2-month intensive phase with isoniazid, rifampicin, pyrazinamide, and ethambutol or streptomycin, followed by a 4-month continuation phase with isoniazid and rifampicin) , whereas the American Academy of Pediatrics (AAP)154 advocates nine months of treatment for newly diagnosed cases of miliary TB without meningeal involvement. When TB meningitis is present, it is suggested that the treatment be extended for 12 months136–138. In several parts of the world, patients with miliary TB get treated under national TB control programmes, with DOTS using short-course intermittent, thrice-weekly treatment151.
In the WHO guidelines for the treatment of TB155, patients are categorized as “new patients” or “previously treated patients”. In these guidelines, miliary TB is classified as pulmonary TB because there are lesions in the lungs. New patients with miliary TB receive 6 months of daily or intermittent treatment as described above. The guidelines mention that some experts recommend 9 to 12 months of treatment when TB meningitis is present given the serious risk of disability and mortality; and 9 months of treatment when bone and joint TB is also present.
For previously treated patients, the guidelines advocate that specimens for culture and DST should be obtained at or before the start of treatment. The DST should be performed for at least isoniazid and rifampicin and in settings where rapid molecular-based DST results are available, the results should guide the choice of regimen.
These observations highlight the importance of accurately assessing the extent of involvement clinically and radiologically. Thus, if underlying TB meningitis remains undiagnosed in a patient with miliary TB, such a patient has a risk of receiving anti-tuberculosis treatment only for 6 months which may be sub-optimal. Therefore, though the standard duration of treatment may be sufficient for many, each patient needs to be assessed individually, and wherever indicated, treatment duration may have to be extended.
Other issues such as quality of anti-tuberculosis drugs and their bio-availability are important in resource-poor nations. Especially in HIV-seropositive patients, consideration must also be given to inadequate plasma levels of anti-tuberculosis drugs in spite of regular intake in adequate dosage due to malabsorption problem.
Monitoring for adverse drug reactions
Patients with miliary TB receiving anti-tuberculosis treatment should be carefully monitored for adverse drug reactions, especially, anti-tuberculosis drug-induced hepatotoxicity (DIH). Asymptomatic rise in hepatic transaminases is common in patients with miliary TB and unless definitive evidence of DIH is present156–159, anti-tuberculosis treatment should not be withheld on this evidence alone. In this scenario, liver functions should be periodically monitored. In tropical countries, acute viral hepatitis must be ruled out in patients who develop antituberculosis DIH160,161.
When patients with miliary TB develop anti-tuberculosis DIH, the potentially hepatotoxic drugs (rifampicin, isoniazid and pyrazinamide) should be stopped158. These patients should be treated with non-hepatotoxic anti-tuberculosis drugs, such as ethambutol, streptomycin and a fluoroquinolone, till the liver functions normalize. In patients who have developed anti-tuberculosis DIH, it is usually possible to reintroduce the same hepatotoxic drugs that have been implicated in the causation of DIH once the liver functions normalize. However, till recently, there has been a lack of consensus regarding the optimal sequence and dosage schedule for reintroduction. A recently published prospective randomized controlled study162, provides useful data on this topic. In this study from India, 175 patients with a diagnosis of antituberculosis DIH were randomized to receive one of three different pre-defined reintroduction regimens of anti-tuberculosis drugs and were evaluated prospectively. The recurrence rate of DIH was not significantly different between the groups receiving isoniazid, rifampicin, and pyrazinamide administered simultaneously at full dosage from day 1; and the groups receiving reintroduction regimens based on the ATS158 and British Thoracic Society guidelines163. Further studies are required to clarify the issue.
Corticosteroids
Only limited published data are available specifically evaluating the role of adjunct corticosteroid treatment in patients with miliary TB and the results have been conflicting. While a beneficial response was observed in some studies164, such a benefit could not be observed in others165. Associated adrenal insufficiency is an absolute indication for the administration of adjunctive corticosteroid treatment. However, in patients with miliary TB, adjunctive corticosteroid treatment is considered to be beneficial with TB meningitis, large pericardial effusion, IRIS, ARDS, immune complex nephritis, and histiocyticphagocytosis syndrome4,85,166.
Antiretroviral drugs
The efficacy of standard anti-tuberculosis treatment regimens in the treatment of HIV, miliary TB co-infection has not been studied in detail in the field setting under programme conditions. Treatment of miliary TB in patients co-infected with HIV requires careful consideration of drug-drug interactions between anti-tuberculois and anti-retroviral drugs167,168. Co-administration of rifampicin, by inducing the hepatic cytochrome P450 pathway, may result in dangerously low levels of anti-retroviral agents. Rifabutin is preferred over rifampicin especially when protease inhibitors are used but is costly. Efavirenz is preferred over nevirapine but should be avoided during pregnancy. Pregnancy test needs to be done while female patients are on efavirenz. A close monitoring of laboratory parameters is required to detect drug-drug interaction when patients are receiving both treatments. Recently, there has been a change in the WHO revised recommendations167 based on Grading of Recommendations Assessment, Development and Evaluation (GRADE) system169 regarding the time of starting anti-retroviral drugs, the choice of drugs and the time of initiation in relation to institution of anti-tuberculosis treatment. The algorithm for treatment and monitoring of patients with miliary TB is shown in Figs. 12A and B.
Fig. 12A.
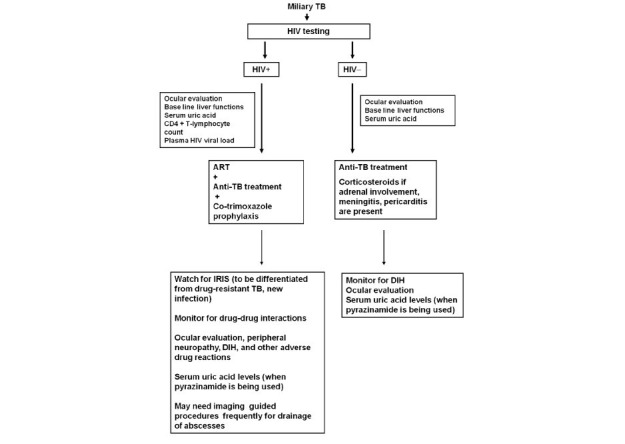
Algorithm for treatment of miliary TB patients with and without HIV co-infection.TB, tuberculosis; HIV, human immunodeficiency virus; +, seropositive; -, seronegative; ART, anti-retroviral treatment; IRIS, immune reconstitution inflammatory syndrome; DIH, anti-tuberculosis drug induced hepatotoxicity
Fig. 12B.
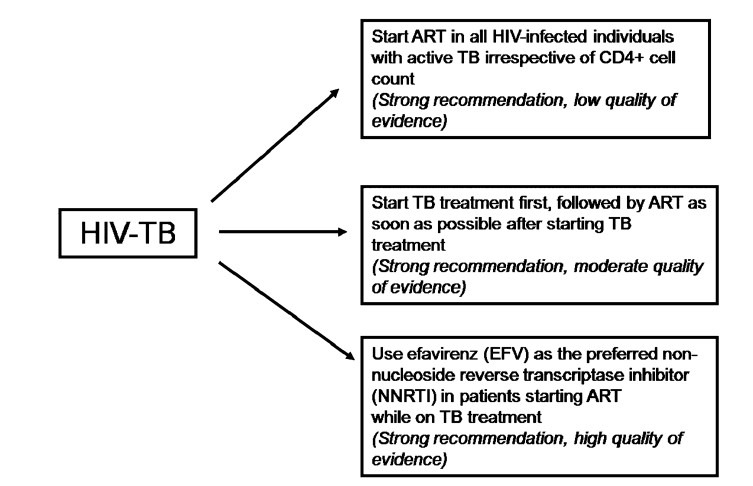
Guidelines on timing of antiretroviral treatment in patients with HIV-tuberculosis co-infection. HIV, human immunodeficiency virus; TB, tuberculosis; ART, antiretroviral therapy
Source: Ref. 167
In peripheral hospitals in endemic areas where HIV and TB are common, quality assured laboratory facilities for HIV ELISA, CD4+ T-lymphocyte counts and plasma HIV viral load estimation may not be available. Timing of initiation and antiretroviral treatment (ART), choice of ART and antituberculosis treatment regimens, drug-drug interactions, all require careful consideration. A high degree of suspicion and appropriate training of the staff is required to identify IRIS, recognize adverse drug reactions and drug toxicities, drug adherence issues.
Mechanical ventilation
Intensive care, assisted mechanical ventilation and other interventions may be required for the management of patients with miliary TB-ARDS86,89. Patients with miliary TB receiving assisted mechanical ventilation should be carefully watched for complications such as pneumothorax (Fig. 5F)
Interventional radiology
Image-guided pigtail catheter drainage for draining psoas abscess, coil or gel foam embolization for treating haemoptysis, among others are useful adjuncts for the management of complications in patients with miliary TB.
Surgery
Surgery is often required to procure specimens for diagnostic testing and to ameliorate complications, such as small bowel perforation where it may be lifesaving. Surgery may be indicated when patients fail to respond to chemotherapy with evidence of ongoing infection and for relief of spinal cord compression with persistence or recurrence of neurological deficits, or instability of the spine.
Mortality
The mortality related to miliary TB is about 15 to 20 per cent in children and is slightly higher in adults (25 to 30%)24–27,31–34,36,37. Delay in diagnosis and consequently, delayed initiation of specific anti-tuberculosis treatment appears to be the most important factor responsible for mortality in miliary TB.
Prognostic factors
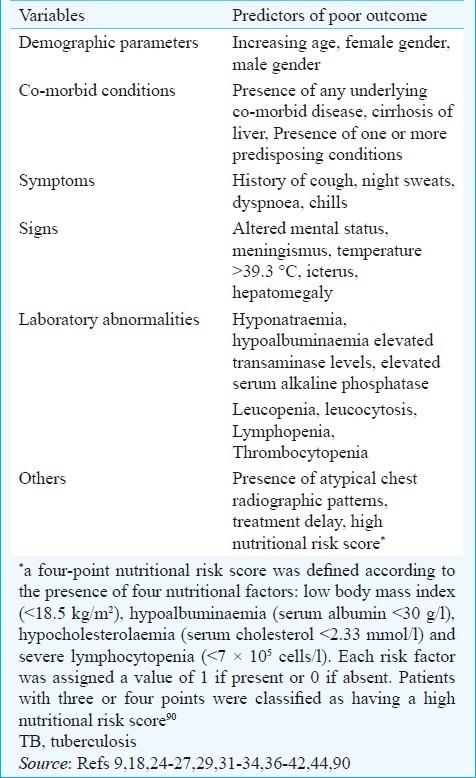
Several factors have been identified as predictors of poor outcome in patients with miliary TB (Table VIII). In patients with miliary TB-ARDS89, acute physiological and chronic health evaluation (APACHE II) score >18; APACHE II score ≤18 in the presence of hyponatraemia and arterial oxygen tension to fraction of inspired oxygen (PaO2/FIO2) ratio ≤108.5 have been identified to be predictors of death. Identification of these factors can alert the clinicians managing patients with miliary TB.
Table VIII.
Predictors of poor outcome in patients with miliary TB
Prevention
The BCG vaccination is effective in reducing the incidence of miliary TB, especially in children170. However, it is not effective in individuals who have latent TB infection and should not be administered to immunosuppressed hosts, such as, HIV infected infants. Targeted tuberculin testing is practised in countries with low prevalence of TB such as the USA152,171, but anti-TB drug-induced hepatotoxicity is a potential risk with this intervention. Ongoing research172,173 is likely to provide a more effective vaccine than BCG.
Lessons learnt
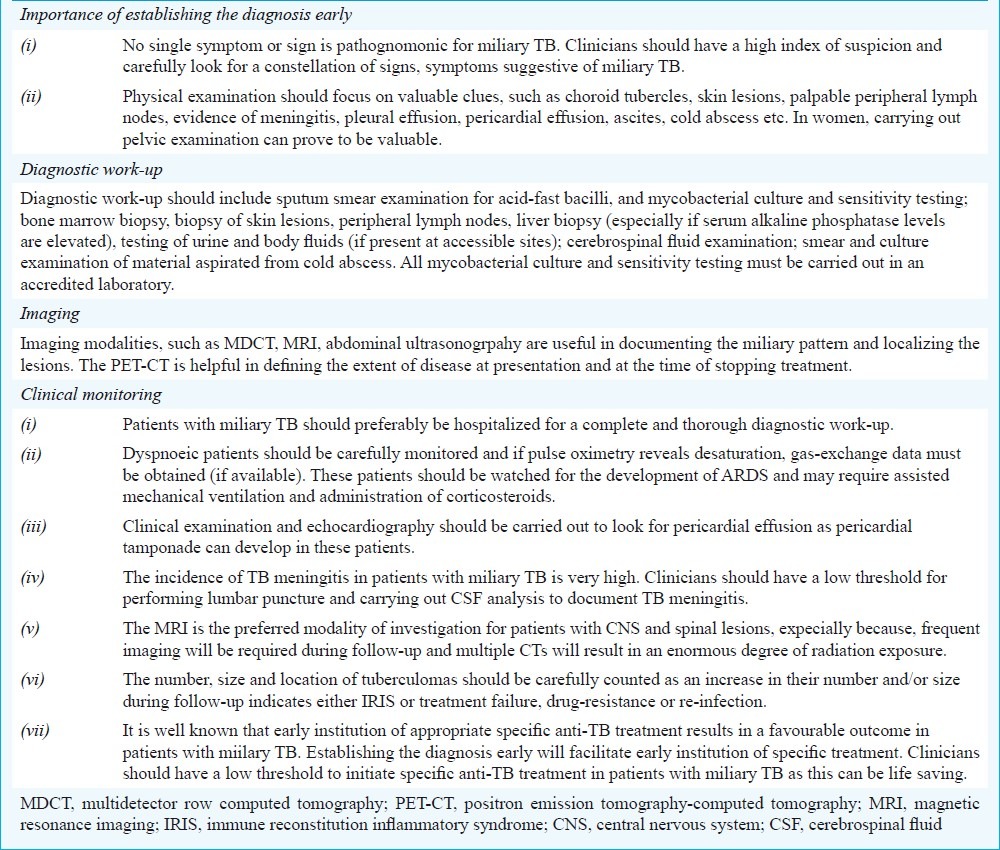
Miliary TB remains an elusive diagnosis even in areas where TB is highly endemic. The key practical issues in the diagnosis and management of miliary TB are listed in Table IX. As the clinical symptomatology and physical signs are non-specific, clinicians should have a low threshold for suspecting miliary TB. Careful physical examination for diagnostic clues such as peripheral lymphadenopathy, cold abscess, pleural effusion, ascites, among others will help in procuring tissue and body fluids for confirming the diagnosis. Fundus examination for detecting choroid tubercles must be done in all patients with suspected miliary TB as their presence is pathognomonic of miliary TB. Specific efforts should also be directed at documenting the presence of TB meningitis as this has therapeutic significance.
Table IX.
Key practical issues in the diagnosis and management of miliary TB
Imaging modalities such as CT and MRI are useful to establish the miliary pattern. In conjunction with ultrasonography, CT, MRI and PET can help in establishing the extent of organ system involvement in miliary TB. Image guided FNAC or biopsy from various organ sites, needle biopsy of the liver, bone marrow aspiration and biopsy, sputum smear and culture examination, including drug-susceptibility testing (if access to an accredited laboratory is available) must be carried out in all patients.
Treatment of miliary TB should be started at the earliest as per the standard WHO guidelines. Adjunct corticosteroid treatment is helpful when there is adrenal insufficiency, with TB meningitis, large pericardial or pleural effusion, dyspnoea and/or disabling chest pain, IRIS, ARDS, immune complex nephritis, and histiocyticphagocytosis syndrome. In patients co-infected with HIV, careful consideration must be given for drug-drug interactions between anti-tuberculosis and anti-retroviral drugs. Patients receiving anti-tuberculosis drugs must be carefully monitored for adverse drug reactions, especially DIH and other complications of miliary TB. In new patients with miliary TB without TB meningitis, nine months of anti-tuberculosis treatment should be adequate. When TB meningitis is present, 12 months of antituberculosis treatment may be required. However, the duration of treatment may have to be prolonged based on individual requirements.
In light of the continuing HIV/AIDS epidemic and increasing use of immunosuppressive and cytotoxic drugs, the burden of miliary TB will continue to rise. The utility of newer diagnostic methods such as IGRAs needs to be clarified. Even though clinical trials of a few new anti-tuberculosis drugs are ongoing, no other new anti-tuberculosis drugs appear to be on the threshold of being introduced into practice. Therefore, judicious use of available drugs to ensure regular, complete and adequate treatment is imperative. The role of adjunctive corticosteroid therapy needs to be elucidated in future studies. The scope and utility of PET-CT in assessing the activity of lesions that might persist following anti-tuberculosis treatment in miliary TB needs to be studied further. The quest for a better vaccine than BCG is still on and more data on the candidate vaccines that are currently being evaluated is likely to emerge.
References
- 1.Manget JJ. Sepulchretum sive anatomica practica. Vol. 1. London: Cramer and Perachon; 1700. Observatio XLVII (3 vols) [Google Scholar]
- 2.Sahn SA, Neff TA. Miliary tuberculosis. Am J Med. 1974;56:494–505. doi: 10.1016/0002-9343(74)90482-3. [DOI] [PubMed] [Google Scholar]
- 3.Sharma SK, Mohan A. Miliary tuberculosis. In: Schlossberg D, editor. Tuberculosis and nontuberculous mycobacterial infections. 6th ed. Washington: American Society for Microbiology Press; 2011. pp. 415–35. [Google Scholar]
- 4.Sharma SK, Mohan A, Sharma A, Mitra DK. Miliary tuberculosis: new insights into an old disease. Lancet Infect Dis. 2005;5:415–30. doi: 10.1016/S1473-3099(05)70163-8. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez S, McCabe WR. Extrapulmonary tuberculosis revisited: a review of experience at Boston City and other hospitals. Medicine (Baltimore) 1984;63:25–55. [PubMed] [Google Scholar]
- 6.Gurkan F, Bosnak M, Dikici B, Bosnak V, Yaramis A, Tas MA, et al. Miliary tuberculosis in children: a clinical review. Scand J Infect Dis. 1998;30:359–62. doi: 10.1080/00365549850160648. [DOI] [PubMed] [Google Scholar]
- 7.Hussey G, Chisholm T, Kibel M. Miliary tuberculosis in children: a review of 94 cases. Pediatr Infect Dis J. 1991;10:832–6. doi: 10.1097/00006454-199111000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Kim PK, Lee JS, Yun DJ. Clinical review of miliary tuberculosis in Korean children.84 cases and review of the literature. Yonsei Med J. 1969;10:146–52. doi: 10.3349/ymj.1969.10.2.146. [DOI] [PubMed] [Google Scholar]
- 9.Long R, O’Connor R, Palayew M, Hershfield E, Manfreda J. Disseminated tuberculosis with and without a miliary pattern on chest radiograph: a clinical-pathologic-radiologic correlation. Int J Tuberc Lung Dis. 1997;1:52–8. [PubMed] [Google Scholar]
- 10.Alsoub H, Al Alousi FS. Miliary tuberculosis in Qatar: a review of 32 adult cases. Ann Saudi Med. 2001;21:16–20. doi: 10.5144/0256-4947.2001.16. [DOI] [PubMed] [Google Scholar]
- 11.Somu N, Vijayasekaran D, Ravikumar T, Balachandran A, Subramanyam L, Chandrabhushanam A. Tuberculous disease in a pediatric referral centre: 16 years experience. Indian Pediatr. 1994;31:1245–9. [PubMed] [Google Scholar]
- 12.Udani PM, Bhat US, Bhave SK, Ezuthachan SG, Shetty VV. Problem of tuberculosis in children in India: epidemiology, morbidity, mortality and control programme. Indian Pediatr. 1976;13:881–90. [PubMed] [Google Scholar]
- 13.Ansari NA, Kombe AH, Kenyon TA, Hone NM, Tappero JW, Nyirenda ST, et al. Pathology and causes of death in a group of 128 predominantly HIV-positive patients in Botswana, 1997-1998. Int J Tuberc Lung Dis. 2002;6:55–63. [PubMed] [Google Scholar]
- 14.Chapman CB, Whorton CM. Acute generalised miliary tuberculosis in adults.A clinicopathological study based on sixty three cases diagnosed at autopsy. N Engl J Med. 1946;235:239–48. doi: 10.1056/nejm194608222350801. [DOI] [PubMed] [Google Scholar]
- 15.Jacques J, Sloan TM. The changing pattern of miliary tuberculosis. Thorax. 1970;25:237–40. doi: 10.1136/thx.25.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jagirdar J, Zagzag D. Pathology and insights into pathogenesis of tuberculosis. In: Rom WN, Garay SM, editors. Tuberculosis. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 323–44. [Google Scholar]
- 17.Lewison M, Frelich EB, Ragins OB. Correlation of clinical diagnosis and pathological diagnosis with special reference to tuberculosis: analysis of autopsy findings in 893 cases. Am Rev Tuberc. 1931;24:152–71. [Google Scholar]
- 18.Slavin RE, Walsh TJ, Pollack AD. Late generalized tuberculosis: a clinical pathologic analysis and comparison of 100 cases in the preantibiotic and antibiotic eras. Medicine (Baltimore) 1980;59:352–66. [PubMed] [Google Scholar]
- 19.Vasankari T, Liippo K, Tala E. Overt and cryptic miliary tuberculosis misdiagnosed until autopsy. Scand J Infect Dis. 2003;35:794–6. doi: 10.1080/00365540310016961. [DOI] [PubMed] [Google Scholar]
- 20.Anonymous. Miliary tuberculosis: a changing pattern. Lancet. 1970;1:985–6. [PubMed] [Google Scholar]
- 21.Braun MM, Cote TR, Rabkin CS. Trends in death with tuberculosis during the AIDS era. JAMA. 1993;269:2865–8. [PubMed] [Google Scholar]
- 22.Aderele WI. Miliary tuberculosis in Nigerian children. East Afr Med J. 1978;55:166–71. [PubMed] [Google Scholar]
- 23.Al-Jahdali H, Al-Zahrani K, Amene P, Memish Z, Al-Shimemeri A, Moamary M, et al. Clinical aspects of miliary tuberculosis in Saudi adults. Int J Tuberc Lung Dis. 2000;4:252–5. [PubMed] [Google Scholar]
- 24.Biehl JP. Miliary tuberculosis; a review of sixty-eight adult patients admitted to a municipal general hospital. Am Rev Tuberc. 1958;77:605–22. doi: 10.1164/artpd.1958.77.4.605. [DOI] [PubMed] [Google Scholar]
- 25.Campbell IG. Miliary tuberculosis in British Columbia. Can Med Assoc J. 1973;108:1517–9. [PMC free article] [PubMed] [Google Scholar]
- 26.Gelb AF, Leffler C, Brewin A, Mascatello V, Lyons HA. Miliary tuberculosis. Am Rev Respir Dis. 1973;108:1327–33. doi: 10.1164/arrd.1973.108.6.1327. [DOI] [PubMed] [Google Scholar]
- 27.Grieco MH, Chmel H. Acute disseminated tuberculosis as a diagnostic problem.A clinical study based on twenty-eight cases. Am Rev Respir Dis. 1974;109:554–60. doi: 10.1164/arrd.1974.109.5.554. [DOI] [PubMed] [Google Scholar]
- 28.Jacob JT, Mehta AK, Leonard MK. Acute forms of tuberculosis in adults. Am J Med. 2009;122:12–7. doi: 10.1016/j.amjmed.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 29.Hussain SF, Irfan M, Abbasi M, Anwer SS, Davidson S, Haqqee R, et al. Clinical characteristics of 110 miliary tuberculosis patients from a low HIV prevalence country. Int J Tuberc Lung Dis. 2004;8:493–9. [PubMed] [Google Scholar]
- 30.Monie RD, Hunter AM, Rocchiccioli KM, White JP, Campbell IA, Kilpatrick GS. Retrospective survey of the management of miliary tuberculosis in South and West Wales, 1976-8. Thorax. 1983;38:369–72. doi: 10.1136/thx.38.5.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim JH, Langston AA, Gallis HA. Miliary tuberculosis: epidemiology, clinical manifestations, diagnosis, and outcome. Rev Infect Dis. 1990;12:583–90. doi: 10.1093/clinids/12.4.583. [DOI] [PubMed] [Google Scholar]
- 32.Maartens G, Willcox PA, Benatar SR. Miliary tuberculosis: rapid diagnosis, hematologic abnormalities, and outcome in 109 treated adults. Am J Med. 1990;89:291–6. doi: 10.1016/0002-9343(90)90340-j. [DOI] [PubMed] [Google Scholar]
- 33.Mert A, Bilir M, Tabak F, Ozaras R, Ozturk R, Senturk H, et al. Miliary tuberculosis: clinical manifestations, diagnosis and outcome in 38 adults. Respirology. 2001;6:217–24. doi: 10.1046/j.1440-1843.2001.00328.x. [DOI] [PubMed] [Google Scholar]
- 34.Munt PW. Miliary tuberculosis in the chemotherapy era: with a clinical review in 69 American adults. Medicine (Baltimore) 1972;51:139–55. doi: 10.1097/00005792-197203000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Noertjojo K, Tam CM, Chan SL, Chan-Yeung MM. Extrapulmonary and pulmonary tuberculosis in Hong Kong. Int J Tuberc Lung Dis. 2002;6:879–86. [PubMed] [Google Scholar]
- 36.Onadeko BO, Dickinson R, Sofowora EO. Miliary tuberculosis of the lung in Nigerian adults. East Afr Med J. 1975;52:390–5. [PubMed] [Google Scholar]
- 37.Prout S, Benatar SR. Disseminated tuberculosis.A study of 62 cases. S Afr Med J. 1980;58:835–42. [PubMed] [Google Scholar]
- 38.Rahajoe NN. Miliary tuberculosis in children.A clinical review. Paediatr Indones. 1990;30:233–40. [PubMed] [Google Scholar]
- 39.Sharma SK, Mohan A, Pande JN, Prasad KL, Gupta AK, Khilnani GC. Clinical profile, laboratory characteristics and outcome in miliary tuberculosis. Q J Med. 1995;88:29–37. [PubMed] [Google Scholar]
- 40.Federele MP, editor. The year book of diagnostic radiology. St. Louis: Mosby-Year Book, Inc; 1997. pp. 91–3. [Google Scholar]
- 41.Swaminathan S, Padmapriyadarsini C, Ponnuraja C, Sumathi CH, Rajasekaran S, Amerandran VA, et al. Miliary tuberculosis in human immunodeficiency virus infected patients not on antiretroviral therapy: clinical profile and response to short course chemotherapy. J Postgrad Med. 2007;53:228–31. doi: 10.4103/0022-3859.37509. [DOI] [PubMed] [Google Scholar]
- 42.Teklu B, Butler J, Ostrow JH. Miliary tuberculosis. A review of 83 cases treated between 1950 and 1968. Ethiop Med J. 1977;15:39–48. [PubMed] [Google Scholar]
- 43.Udani PM, Bhat US, Bhave SK, Ezuthachan SG, Shetty VV. Problem of tuberculosis in children in India: epidemiology, morbidity, mortality and control programme. Indian Pediatr. 1976;13:881–90. [PubMed] [Google Scholar]
- 44.El Shamy AS, Al Saidi F, Baidas G, Al Bader M, Sawy M, Hakkim R. Miliary tuberculosis in Kuwait: clinical presentation, diagnosis and treatment outcome. Kuwait Med J. 2008;40:288–92. [Google Scholar]
- 45.Dixon WG, Hyrich KL, Watson KD, Lunt M, Galloway J, Ustianowski A, Symmons DP BSR BR Control Centre Consortium, B.S.R. Biologics Register. Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: results from the British Society for Rheumatology Biologics Register (BSRBR) Ann Rheum Dis. 2010;69:552–8. doi: 10.1136/ard.2009.118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malipeddi AS, Rajendran R, Kallarackal G. Disseminated tuberculosis after anti-TNF alpha treatment. Lancet. 2007;369:162. doi: 10.1016/S0140-6736(07)60078-6. [DOI] [PubMed] [Google Scholar]
- 47.Toussirot E, Streit G, Wendling D. Infectious complications with anti-TNF alpha therapy in rheumatic diseases: a review. Recent Pat Inflamm Allergy Drug Discov. 2007;1:39–47. doi: 10.2174/187221307779815039. [DOI] [PubMed] [Google Scholar]
- 48.Uthman I, Kanj N, El-Sayad J, Bizri AR. Miliary tuberculosis after infliximab therapy in Lebanon. Clin Rheumatol. 2004;23:279–80. doi: 10.1007/s10067-004-0873-z. [DOI] [PubMed] [Google Scholar]
- 49.Anyanwu CH, Nassau E, Yacoub M. Miliary tuberculosis following homograft valve replacement. Thorax. 1976;31:101–6. doi: 10.1136/thx.31.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morano Amado LE, Amador Barciela L, Rodriguez Fernandez A, Martinez-Sapina Llamas I, Vazquez Alvarez O, Fernandez Martin J. Extracorporeal shock wave lithotripsy complicated with miliary tuberculosis. J Urol. 1993;149:1532–4. doi: 10.1016/s0022-5347(17)36437-6. [DOI] [PubMed] [Google Scholar]
- 51.Rabe J, Neff KW, Lehmann KJ, Mechtersheimer U, Georgi M. Miliary tuberculosis after intravesical bacille Calmette-Guerin immunotherapy for carcinoma of the bladder. AJR Am J Roentgenol. 1999;172:748–50. doi: 10.2214/ajr.172.3.10063874. [DOI] [PubMed] [Google Scholar]
- 52.Silverblatt A, DeSimone JA, Babinchak TJ. Acute miliary tuberculosis following laser lithotripsy. Infect Med. 2002;19:80–2. [Google Scholar]
- 53.Yekanath H, Gross PA, Vitenson JH. Miliary tuberculosis following ureteral catheterization. Urology. 1980;16:197–8. doi: 10.1016/0090-4295(80)90084-9. [DOI] [PubMed] [Google Scholar]
- 54.Collins HL, Kaufmann SH. The many faces of host responses to tuberculosis. Immunology. 2001;103:1–9. doi: 10.1046/j.1365-2567.2001.01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rook GA, Hernandez-Pando R, Dheda K, Teng Seah G. IL-4 in tuberculosis: implications for vaccine design. Trends Immunol. 2004;25:483–8. doi: 10.1016/j.it.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 56.Sharma PK, Saha PK, Singh A, Sharma SK, Ghosh B, Mitra DK. FoxP3+ regulatory T cells suppress effector T-cell function at pathologic site in miliary tuberculosis. Am J Respir Crit Care Med. 2009;179:1061–70. doi: 10.1164/rccm.200804-529OC. [DOI] [PubMed] [Google Scholar]
- 57.Sharma SK, Mitra DK, Balamurugan A, Pandey RM, Mehra NK. Cytokine polarization in miliary and pleural tuberculosis. J Clin Immunol. 2002;22:345–52. doi: 10.1023/a:1020604331886. [DOI] [PubMed] [Google Scholar]
- 58.Sharma SK, Mohan A. Extrapulmonary tuberculosis. Indian J Med Res. 2004;120:316–53. [PubMed] [Google Scholar]
- 59.Ainslie GM, Solomon JA, Bateman ED. Lymphocyte and lymphocyte subset numbers in blood and in bronchoalveolar lavage and pleural fluid in various forms of pulmonary tuberculosis at presentation and during recovery. Thorax. 1992;47:513–8. doi: 10.1136/thx.47.7.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharma SK, Pande JN, Verma K. Bronchoalveolar lavage (BAL) in miliary tuberculosis. Tubercle. 1988;69:175–8. doi: 10.1016/0041-3879(88)90018-9. [DOI] [PubMed] [Google Scholar]
- 61.Sharma SK, Pande JN, Singh YN, Verma K, Kathait SS, Khare SD, et al. Pulmonary function and immunologic abnormalities in miliary tuberculosis. Am Rev Respir Dis. 1992;145:1167–71. doi: 10.1164/ajrccm/145.5.1167. [DOI] [PubMed] [Google Scholar]
- 62.Prabhakaran D, Sharma SK, Verma K, Pande JN. Estimation of fibronectin in bronchoalveolar lavage fluid in various diffuse interstitial lung diseases. Am Rev Respir Dis. 1990;141:A51. [Google Scholar]
- 63.Barnes PF, Grisso CL, Abrams JS, Band H, Rea TH, Modlin RL. Gamma delta T lymphocytes in human tuberculosis. J Infect Dis. 1992;165:506–12. doi: 10.1093/infdis/165.3.506. [DOI] [PubMed] [Google Scholar]
- 64.Ellner JJ. The immune response in human tuberculosis-implications for tuberculosis control. J Infect Dis. 1997;176:1351–9. doi: 10.1086/514132. [DOI] [PubMed] [Google Scholar]
- 65.Al-Arif LI, Goldstein RA, Affronti LF, Janicki BW. HLA-Bw15 and tuberculosis in a North American black population. Am Rev Respir Dis. 1979;120:1275–8. doi: 10.1164/arrd.1979.120.6.1275. [DOI] [PubMed] [Google Scholar]
- 66.Balamurugan A. HLA-DR restriction of Th1/Th2 cytokine profile in tuberculosis: impact of genetic diversity. Ph.D. Thesis. New Delhi: All India Institute of Medical Sciences; 2002. [Google Scholar]
- 67.Kumararatne DS, Pithie AS, Drysdale P, Gaston JS, Kiessling R, Iles PB, et al. Specific lysis of mycobacterial antigen-bearing macrophages by class II MHC-restricted polyclonal T cell lines in healthy donors or patients with tuberculosis. Clin Exp Immunol. 1990;80:314–23. doi: 10.1111/j.1365-2249.1990.tb03287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taype CA, Shamsuzzaman S, Accinelli RA, Espinoza JR, Shaw MA. Genetic susceptibility to different clinical forms of tuberculosis in the Peruvian population. Infect Genet Evol. 2010;10:495–504. doi: 10.1016/j.meegid.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 69.Cunha BA, Krakakis J, McDermott BP. Fever of unknown origin (FUO) caused by miliary tuberculosis: diagnostic significance of morning temperature spikes. Heart Lung. 2009;38:77–82. doi: 10.1016/j.hrtlng.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 70.Proudfoot AT, Akhtar AJ, Douglas AC, Horne NW. Miliary tuberculosis in adults. BMJ. 1969;2:273–6. doi: 10.1136/bmj.2.5652.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Flores-Franco RA, Ríos-Ortiz LA. The “damp shadow” sign: another clinical indicator of miliary tuberculosis. Heart Lung. 2010;39:87–8. doi: 10.1016/j.hrtlng.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 72.Thwaites GE, Nguyen DB, Nguyen HD, Hoang TQ, Do TT, Nguyen TC, et al. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351:1741–51. doi: 10.1056/NEJMoa040573. [DOI] [PubMed] [Google Scholar]
- 73.Garg RK, Sharma R, Kar AM, Kushwaha RA, Singh MK, Shukla R, et al. Neurological complications of miliary tuberculosis. Clin Neurol Neurosurg. 2010;112:188–92. doi: 10.1016/j.clineuro.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 74.Braidy J, Pothel C, Amra S. Miliary tuberculosis presenting as adrenal failure. J Can Med Assoc. 1981;82:254–6. [PMC free article] [PubMed] [Google Scholar]
- 75.Yokoyama T, Toda R, Kimura Y, Mikagi M, Aizawa H. Addison's disease induced by miliary tuberculosis and the administration of rifampicin. Intern Med. 2009;48:1297–300. doi: 10.2169/internalmedicine.48.1974. [DOI] [PubMed] [Google Scholar]
- 76.Sharma SK, Mohan A, Kadhiravan T. HIV-TB co-infection: epidemiology, diagnosis and management. Indian J Med Res. 2005;121:550–67. [PubMed] [Google Scholar]
- 77.Sharma SK, Mohan A. Co-infection of human immunodeficiency virus (HIV) and tuberculosis: Indian perspective. Indian J Tuberc. 2004;51:5–16. [Google Scholar]
- 78.Sharma SK, Mohan A, Gupta R, Kumar A, Gupta AK, Singhal VK, et al. Clinical presentation of tuberculosis in patients with AIDS: an Indian experience. Indian J Chest Dis Allied Sci. 1997;39:213–20. [PubMed] [Google Scholar]
- 79.Lado Lado FL, Barrio Gomez E, Carballo Arceo E, Cabarcos Ortiz de Barron A. Clinical presentation of tuberculosis and the degree of immunodeficiency in patients with HIV infection. Scand J Infect Dis. 1999;31:387–91. doi: 10.1080/00365549950163842. [DOI] [PubMed] [Google Scholar]
- 80.Kim JY, Jeong YJ, Kim KI, Lee IS, Park HK, Kim YD, et al. Miliary tuberculosis: a comparison of CT findings in HIV-seropositive and HIV-seronegative patients. Br J Radiol. 2010;83:206–11. doi: 10.1259/bjr/95169618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zumla A, Malon P, Henderson J, Grange JM. Impact of HIV infection on tuberculosis. Postgrad Med J. 2000;76:259–68. doi: 10.1136/pmj.76.895.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Harries A, Maher D, Graham S. TB/HIV: a clinical manual. 2nd ed. Geneva: World Health Organization; 2004. p. 329. WHO/HTM/TB/2004. [Google Scholar]
- 83.del Giudice P, Bernard E, Perrin C, Bernardin G, Fouché R, Boissy C, et al. Unusual cutaneous manifestations of miliary tuberculosis. Clin Infect Dis. 2000;30:201–4. doi: 10.1086/313587. [DOI] [PubMed] [Google Scholar]
- 84.Shao C, Qu J, He L. A comparative study of clinical manifestations caused by tuberculosis in immunocompromised and non-immunocompromised patients. Chin Med J (Engl) 2003;116:1717–22. [PubMed] [Google Scholar]
- 85.Goldsack NR, Allen S, Lipman MC. Adult respiratory distress syndrome as a severe immune reconstitution disease following the commencement of highly active antiretroviral therapy. Sex Transm Infect. 2003;79:337–8. doi: 10.1136/sti.79.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mohan A, Sharma SK, Pande JN. Acute respiratory distress syndrome in miliary tuberculosis: a 12-year experience. Indian J Chest Dis Allied Sci. 1996;38:147–52. [PubMed] [Google Scholar]
- 87.Kim JY, Park YB, Kim YS, Kang SB, Shin JW, Park IW, et al. Miliary tuberculosis and acute respiratory distress syndrome. Int J Tuberc Lung Dis. 2003;7:359–64. [PubMed] [Google Scholar]
- 88.Penner C, Roberts D, Kunimoto D, Manfreda J, Long R. Tuberculosis as a primary cause of respiratory failure requiring mechanical ventilation. Am J Respir Crit Care Med. 1995;151:867–72. doi: 10.1164/ajrccm/151.3_Pt_1.867. [DOI] [PubMed] [Google Scholar]
- 89.Sharma SK, Mohan A, Banga A, Saha PK, Guntupalli KK. Predictors of development and outcome in patients with acute respiratory distress syndrome due to tuberculosis. Int J Tuberc Lung Dis. 2006;10:429–35. [PubMed] [Google Scholar]
- 90.Kim DK, Kim HJ, Kwon SY, Yoon HI, Lee CT, Kim YW, et al. Nutritional deficit as a negative prognostic factor in patients with miliary tuberculosis. Eur Respir J. 2008;32:1031–6. doi: 10.1183/09031936.00174907. [DOI] [PubMed] [Google Scholar]
- 91.Gupta PP, Mehta D, Agarwal D, Chand T. Recurrent pneumothorax developing during chemotherapy in a patient with miliary tuberculosis. Ann Thorac Med. 2007;2:173–5. doi: 10.4103/1817-1737.36555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lakin BD, Riordan FA, John CM. Air leak in miliary tuberculosis. Am J Trop Med Hyg. 2009;80:325. [PubMed] [Google Scholar]
- 93.Krishnaswami KV. Mediastinal emphysema in miliary tuberculosis. JAMA. 1977;69:227–9. [PubMed] [Google Scholar]
- 94.Mallinson WJ, Fuller RW, Levison DA, Baker LR, Cattell WR. Diffuse interstitial renal tuberculosis - an unusual cause of renal failure. Q J Med. 1981;50:137–48. [PubMed] [Google Scholar]
- 95.Salliot C, Guichard I, Daugas E, Lagrange M, Verine J, Molina JM. Acute kidney disease due to immune reconstitution inflammatory syndrome in an HIV-infected patient with tuberculosis. J Int Assoc Physicians AIDS Care (Chic Ill) 2008;7:178–81. doi: 10.1177/1545109708320683. [DOI] [PubMed] [Google Scholar]
- 96.Asada Y, Hayashi T, Sumiyoshi A, Aburaya M, Shishime E. Miliary tuberculosis presenting as fever and jaundice with hepatic failure. Hum Pathol. 1991;22:92–4. doi: 10.1016/0046-8177(91)90068-z. [DOI] [PubMed] [Google Scholar]
- 97.Hussain W, Mutimer D, Harrison R, Hubscher S, Neuberger J. Fulminant hepatic failure caused by tuberculosis. Gut. 1995;36:792–4. doi: 10.1136/gut.36.5.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Seabra J, Coelho H, Barros H, Alves JO, Goncalves V, Rocha-Marques A. Acute tuberculous perforation of the small bowel during antituberculosis therapy. J Clin Gastroenterol. 1993;16:320–2. doi: 10.1097/00004836-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 99.Rose AG. Cardiac tuberculosis. A study of 19 patients. Arch Pathol Lab Med. 1987;111:422–6. [PubMed] [Google Scholar]
- 100.Theuer CP, Hopewell PC, Elias D, Schecter GF, Rutherford GW, Chaisson RE. Human immunodeficiency virus infection in tuberculosis patients. J Infect Dis. 1990;162:8–12. doi: 10.1093/infdis/162.1.8. [DOI] [PubMed] [Google Scholar]
- 101.Cope AP, Heber M, Wilkins EG. Valvular tuberculous endocarditis: a case report and review of the literature. J Infect. 1990;21:293–6. doi: 10.1016/0163-4453(90)94029-y. [DOI] [PubMed] [Google Scholar]
- 102.Wainwright J. Tuberculous endocarditis: a report of 2 cases. S Afr Med J. 1979;56:731–3. [PubMed] [Google Scholar]
- 103.Yamane H, Fujiwara T, Doko S, Inada H, Nogami A, Masaki H, et al. Two cases of miliary tuberculosis following prosthetic valve replacement. Kokyu To Junkan. 1989;37:803–5. [PubMed] [Google Scholar]
- 104.Felson B, Akers PV, Hall GS, Schreiber JT, Greene RE, Pedrosa CS. Mycotic tuberculous aneurysm of the thoracic aorta. JAMA. 1977;237:1104–8. [PubMed] [Google Scholar]
- 105.Doherty JG, Rankin R, Kerr F. Miliary tuberculosis presenting as infection of a pacemaker pulse-generator pocket. Scott Med J. 1996;41:20–1. doi: 10.1177/003693309604100108. [DOI] [PubMed] [Google Scholar]
- 106.Shibolet S, Dan M, Jedwab M, Goldhammer Y, Baum GL. Recurrent miliary tuberculosis secondary to infected ventriculoatrial shunt. Chest. 1979;76:328–30. doi: 10.1378/chest.76.3.328. [DOI] [PubMed] [Google Scholar]
- 107.Tseng HL, Roulet F. Cardiac involvement in miliary tuberculosis. Am Rev Tuberc. 1953;68:771–4. doi: 10.1164/art.1953.68.5.771. [DOI] [PubMed] [Google Scholar]
- 108.Biedrzycki OJ, Baithun SI. TB-related sudden death (TBRSD) due to myocarditis complicating miliary TB: a case report and review of the literature. Am J Forensic Med Pathol. 2006;27:335–6. doi: 10.1097/01.paf.0000233633.16185.32. [DOI] [PubMed] [Google Scholar]
- 109.Wallis PJ, Branfoot AC, Emerson PA. Sudden death due to myocardial tuberculosis. Thorax. 1984;39:155–6. doi: 10.1136/thx.39.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Meintjes G, Lawn SD, Scano F, Maartens G, French MA, Worodria W, et al. International Network for the Study of HIV-associated IRIS. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8:516–3. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sharma SK, Dhooria S, Barwad P, Kadhiravan T, Ranjan S, Miglani S, et al. A study of TB-associated immune reconstitution inflammatory syndrome using the consensus case-definition. Indian J Med Res. 2010;131:804–8. [PubMed] [Google Scholar]
- 112.Jehle AW, Khanna N, Sigle JP, Glatz-Krieger K, Battegay M, Steiger J, et al. Acute renal failure on immune reconstitution in an HIV-positive patient with miliary tuberculosis. Clin Infect Dis. 2004;38:e32–5. doi: 10.1086/381441. [DOI] [PubMed] [Google Scholar]
- 113.Tuddenham WJ. Glossary of terms for thoracic radiology: recommendations of the Nomenclature Committee of the Fleischner Society. AJR Am J Roentgenol. 1984;143:509–17. doi: 10.2214/ajr.143.3.509. [DOI] [PubMed] [Google Scholar]
- 114.Sharma SK, Mohan A. Disseminated and miliary tuberculosis. In: Sharma SK, Mohan A, editors. Tuberculosis. 2nd ed. New Delhi: Jaypee Brothers Medical Publishers; 2009. pp. 493–518. [Google Scholar]
- 115.Mori T. Usefulness of interferon-gamma release assays for diagnosing TB infection and problems with these assays. J Infect Chemother. 2009;15:143–5. doi: 10.1007/s10156-009-0686-8. [DOI] [PubMed] [Google Scholar]
- 116.Pai M, Joshi R, Kalantri SP. Diagnosis of latent tuberculosis infection: recent advances and future directions. In: Sharma SK, Mohan A, editors. Tuberculosis. 2nd ed. New Delhi: Jaypee Brothers Medical Publishers; 2009. pp. 186–99. [Google Scholar]
- 117.Singh KJ, Ahluwalia G, Sharma SK, Saxena R, Chaudhary VP, Anant M. Significance of haematological manifestations in patients with tuberculosis. J Assoc Physicians India. 2001;49:790–4. [PubMed] [Google Scholar]
- 118.Chan CH, Chan TY, Shek AC, Mak TW, Lui SF, Lai KN. Severe hypercalcaemia associated with miliary tuberculosis. J Trop Med Hyg. 1994;97:180–2. [PubMed] [Google Scholar]
- 119.Shalhoub RJ, Antoniou LD. The mechanism of hyponatremia in pulmonary tuberculosis. Ann Intern Med. 1969;70:943–62. doi: 10.7326/0003-4819-70-5-943. [DOI] [PubMed] [Google Scholar]
- 120.Vorherr H, Massry SG, Fallet R, Kaplan L, Kleeman CR. Antidiuretic principle in tuberculous lung tissue of a patient with pulmonary tuberculosis and hyponatremia. Ann Intern Med. 1970;72:383–7. doi: 10.7326/0003-4819-72-3-383. [DOI] [PubMed] [Google Scholar]
- 121.Winkler AW, Crankshaw DF. Chloride depletion in conditions other than Addison's disease. J Clin Invest. 1938;17:1–6. doi: 10.1172/JCI100920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Steiner PE. The histopathological basis for the X-ray diagnosability of pulmonary miliary tuberculosis. Am Rev Tuberc. 1937;36:692–705. [Google Scholar]
- 123.Kwong JS, Carignan S, Kang EY, Muller NL, Fitzgerald JM. Miliary tuberculosis. Diagnostic accuracy of chest radiography. Chest. 1996;110:339–42. doi: 10.1378/chest.110.2.339. [DOI] [PubMed] [Google Scholar]
- 124.Lee KS, Kim TS, Han J, Hwang JH, Yoon JH, Kim Y, et al. Diffuse micronodular lung disease: HRCT and pathologic findings. J Comput Assist Tomogr. 1999;23:99–106. doi: 10.1097/00004728-199901000-00022. [DOI] [PubMed] [Google Scholar]
- 125.Jamieson DH, Cremin BJ. High resolution CT of the lungs in acute disseminated tuberculosis and a pediatric radiology perspective of the term “miliary”. Pediatr Radiol. 1993;23:380–3. doi: 10.1007/BF02011965. [DOI] [PubMed] [Google Scholar]
- 126.Price M. Lymphangitis reticularis tuberculosa. Tubercle. 1968;49:377–84. doi: 10.1016/s0041-3879(68)80018-2. [DOI] [PubMed] [Google Scholar]
- 127.McGuinness G, Naidich DP, Jagirdar J, Leitman B, McCauley DI. High resolution CT findings in miliary lung disease. J Comput Assist Tomogr. 1992;16:384–90. doi: 10.1097/00004728-199205000-00009. [DOI] [PubMed] [Google Scholar]
- 128.Sharma SK, Mukhopadhyay S, Arora R, Verma K, Pande JN, Khilnani GC. Computed tomography in miliary tuberculosis: comparison with plain films, bronchoalveolar lavage, pulmonary functions and gas exchange. Australasian Radiol. 1996;40:113–8. doi: 10.1111/j.1440-1673.1996.tb00363.x. [DOI] [PubMed] [Google Scholar]
- 129.Pipavath SN, Sharma SK, Sinha S, Mukhopadhyay S, Gulati MS. High resolution CT (HRCT) in miliary tuberculosis (MTB) of the lung: correlation with pulmonary function tests & gas exchange parameters in north Indian patients. Indian J Med Res. 2007;126:193–8. [PubMed] [Google Scholar]
- 130.Yu RS, Zhang SZ, Wu JJ, Li RF. Imaging diagnosis of 12 patients with hepatic tuberculosis. World J Gastroenterol. 2004;10:1639–42. doi: 10.3748/wjg.v10.i11.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Willcox PA, Potgieter PD, Bateman ED, Benatar SR. Rapid diagnosis of sputum negative miliary tuberculosis using the flexible fibreoptic bronchoscope. Thorax. 1986;41:681–4. doi: 10.1136/thx.41.9.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ibrarullah M, Mohan A, Sarkari A, Srinivas M, Mishra A, Sundar TS. Abdominal tuberculosis: diagnosis by laparoscopy and colonoscopy. Trop Gastroenterol. 2002;23:150–3. [PubMed] [Google Scholar]
- 133.Commercial serodiagnostic tests for diagnosis of tuberculosis: policy statement. WHO/HTM/TB/2011.5. Geneva: World Health Organization; 2011. World Health Organization. [PubMed] [Google Scholar]
- 134.Riquelme A, Calvo M, Salech F, Valderrama S, Pattillo A, Arellano M, et al. Value of adenosine deaminase (ADA) in ascetic fluid for the diagnosis of tuberculous peritonitis: a meta-analysis. J Clin Gastroenterol. 2006;40:705–10. doi: 10.1097/00004836-200609000-00009. [DOI] [PubMed] [Google Scholar]
- 135.Sharma SK, Suresh V, Mohan A, Kaur P, Saha P, Kumar A, et al. A prospective study of sensitivity and specificity of adenosine deaminase estimation in the diagnosis of tuberculosis pleural effusion. Indian J Chest Dis Allied Sci. 2001;43:149–55. [PubMed] [Google Scholar]
- 136.Sharma SK, Banga A. Diagnostic utility of pleural fluid IFN gamma in tuberculosis pleural effusion. J Interferon Cytokine Res. 2004;24:213–7. doi: 10.1089/107999004323034088. [DOI] [PubMed] [Google Scholar]
- 137.Sharma SK, Banga A. Pleural fluid interferon-gamma and adenosine deaminase levels in tuberculosis pleural effusion: a cost-effectiveness analysis. J Clin Lab Anal. 2005;19:40–6. doi: 10.1002/jcla.20054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sharma SK, Tahir M, Mohan A, Smith-Rohrberg D, Mishra HK, Pandey RM. Diagnostic accuracy of ascitic fluid IFN-gamma and adenosine deaminase assays in the diagnosis of tuberculous ascites. J Interferon Cytokine Res. 2006;26:484–8. doi: 10.1089/jir.2006.26.484. [DOI] [PubMed] [Google Scholar]
- 139.Rana SV, Chacko F, Lal V, Arora SK, Parbhakar S, Sharma SK, et al. To compare CSF adenosine deaminase levels and CSF-PCR for tuberculous meningitis. Clin Neurol Neurosurg. 2010;112:424–30. doi: 10.1016/j.clineuro.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 140.Tuon FF, Higashino HR, Lopes MI, Litvoc MN, Atomiya AN, Antonangelo L, et al. Adenosine deaminase and tuberculous meningitis-a systematic review with meta-analysis. Scand J Infect Dis. 2010;42:198–207. doi: 10.3109/00365540903428158. [DOI] [PubMed] [Google Scholar]
- 141.World Health Organization. Policy statement. Molecular line probe assays for rapid screening of patients at risk of multidrug-resistant tuberculosis (MDR-TB) 2008. Jun 27, [accessed on April 19, 2012]. Available from: http://www.who.int/tb/features_archive/policy.pdf .
- 142.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–15. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rapid implementation of the Xpert MTB/RIF diagnostic test: technical and operational ‘How-to’; practical considerations. Geneva: World Health Organization; 2011. World Health Organization. WHO/HTM/TB/2011.2. [Google Scholar]
- 144.Ling DI, Zwerling AA, Pai M. Rapid diagnosis of drug-resistant TB using line probe assays: from evidence to policy. Expert Rev Respir Med. 2008;2:583–8. doi: 10.1586/17476348.2.5.583. [DOI] [PubMed] [Google Scholar]
- 145.Goo JM, Im JG, Do KH, Yeo JS, Seo JB, Kim HY, et al. Pulmonary tuberculoma evaluated by means of FDG PET: findings in 10 cases. Radiology. 2000;216:117–21. doi: 10.1148/radiology.216.1.r00jl19117. [DOI] [PubMed] [Google Scholar]
- 146.Ichiya Y, Kuwabara Y, Sasaki M, Yoshida T, Akashi Y, Murayama S, et al. FDG-PET in infectious lesions: The detection and assessment of lesion activity. Ann Nucl Med. 1996;10:185–91. doi: 10.1007/BF03165391. [DOI] [PubMed] [Google Scholar]
- 147.Williams NH, Jr, Kane C, Yoo OH. Pulmonary function in miliary tuberculosis. Am Rev Respir Dis. 1973;107:858–60. doi: 10.1164/arrd.1973.107.5.858. [DOI] [PubMed] [Google Scholar]
- 148.Sharma SK, Ahluwalia G. Effect of antituberculosis treatment on cardiopulmonary responses to exercise in miliary tuberculosis. Indian J Med Res. 2006;124:411–8. [PubMed] [Google Scholar]
- 149.McClement JH, Renzetti AD, Jr, Carroll D, Himmelstein A, Cournand A. Cardiopulmonary function in hematogenous pulmonary tuberculosis in patients with streptomycin therapy. Am Rev Tuberc. 1951;64:588–601. doi: 10.1164/art.1951.64.6.583. [DOI] [PubMed] [Google Scholar]
- 150.Sharma SK, Ahluwalia G. Exercise testing in miliary tuberculosis–some facts. Indian J Med Res. 2007;125:182–3. [PubMed] [Google Scholar]
- 151.Treatment of tuberculosis: Guidelines for National Programmes. 3rd ed. Geneva: World Health Organization; 2003. World Health Organization. WHO/CDS/TB/2003.313. [Google Scholar]
- 152.Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America. Treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603–62. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 153.Tuberculosis: clinical diagnosis and management of tuberculosis, and measures for its prevention and control. London: Royal College of Physicians; 2006. National Institute for Health and Clinical Excellence, National Collaborating Centre for Chronic Conditions. Management of non-respiratory tuberculosis; pp. 63–76. [Google Scholar]
- 154.American Academy of Pediatrics Committee on Infectious Diseases. Chemotherapy for tuberculosis in infants and children. Pediatrics. 1992;89:161–5. [PubMed] [Google Scholar]
- 155.Treatment of tuberculosis. Guidelines. 4th ed. Geneva: World Health Organization; 2009. World Health Organization. WHO/HTM/TB/2009.420. [PubMed] [Google Scholar]
- 156.Sharma SK, Mohan A. Antituberculosis treatment induced hepatotoxicity: from bench to bed-side. In: Gupta SB, editor. Medicine update. Mumbai: Association of Physicians of India; 2005. pp. 479–84. [Google Scholar]
- 157.Sharma SK. Antituberculosis drugs and hepatotoxicity. Infect Genet Evol. 2004;4:167–70. doi: 10.1016/j.meegid.2003.01.001. [DOI] [PubMed] [Google Scholar]
- 158.Saukkonen JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM, et al. An official ATS statement: Hepatotoxicity of anti tuberculosis therapy. Am J Respir Crit Care Med. 2006;174:935–52. doi: 10.1164/rccm.200510-1666ST. [DOI] [PubMed] [Google Scholar]
- 159.Singla R, Sharma SK, Mohan A, Makharia G, Sreenivas V, Jha B, et al. Evaluation of risk factors for antituberculosis treatment induced hepatotoxicity. Indian J Med Res. 2010;132:81–6. [PubMed] [Google Scholar]
- 160.Sarda P, Sharma SK, Mohan A, Makharia G, Jayaswal A, Pandey RM. Role of acute viral hepatitis as a confounding factor in antituberculosis treatment induced hepatotoxicity. Indian J Med Res. 2009;129:64–7. [PubMed] [Google Scholar]
- 161.Sharma SK, Singla R, Sreenivas V, Kumar S, Jha B, Rathored J, et al. Acute viral hepatitis as a confounding factor in patients with antituberculosis treatment induced hepatotoxicity. Indian J Med Res. 2009;130:200–1. [PubMed] [Google Scholar]
- 162.Sharma SK, Singla R, Sarda P, Mohan A, Makharia G, Jayaswal A, et al. Safety of 3 different reintroduction regimens of antituberculosis drugs after development of antituberculosis treatment-induced hepatotoxicity. Clin Infect Dis. 2010;50:833–9. doi: 10.1086/650576. [DOI] [PubMed] [Google Scholar]
- 163.Joint Tuberculosis Committee of the British Thoracic Society. Chemotherapy and management of tuberculosis in the United Kingdom: recommendations. Thorax. 1998;53:536–48. [PMC free article] [PubMed] [Google Scholar]
- 164.Sun TN, Yang JY, Zheng LY, Deng WW, Sui ZY. Chemotherapy and its combination with corticosteroids in acute miliary tuberculosis in adolescents and adults: analysis of 55 cases. Chin Med J (Engl) 1981;94:309–14. [PubMed] [Google Scholar]
- 165.Massaro D, Katz S, Sachs M. Choroidal tubercles. A clue to hematogenous tuberculosis. Ann Intern Med. 1964;60:231–41. doi: 10.7326/0003-4819-60-2-231. [DOI] [PubMed] [Google Scholar]
- 166.Marais S, Wilkinson RJ, Pepper DJ, Meintjes G. Management of patients with the immune reconstitution inflammatory syndrome. Curr HIV/AIDS Rep. 2009;6:162–71. doi: 10.1007/s11904-009-0022-z. [DOI] [PubMed] [Google Scholar]
- 167.Rapid advice. Antiretroviral therapy for HIV infection in adults and adolescents. Geneva: World Health Organization; 2009. World Health Organization. [Google Scholar]
- 168.Pozniak AL, Coyne KM, Miller RL, Lipman MCI, Freedman AR, Ormerod LP, et al. BHIVA Guidelines Subcommittee. British HIV Association guidelines for the treatment of TB/HIV coinfection 2011. HIV Med. 2011;12:517–24. doi: 10.1111/j.1468-1293.2011.00954.x. [DOI] [PubMed] [Google Scholar]
- 169.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–80. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 171.American Thoracic Society. Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR Recomm Rep. 2000;49:1–51. [PubMed] [Google Scholar]
- 172.Kaufmann SH, Hussey G, Lambert PH. New vaccines for tuberculosis. Lancet. 2010;375:2110–9. doi: 10.1016/S0140-6736(10)60393-5. [DOI] [PubMed] [Google Scholar]
- 173.Parida SK, Kaufmann SH. Novel tuberculosis vaccines on the horizon. Curr Opin Immunol. 2010;22:374–84. doi: 10.1016/j.coi.2010.04.006. [DOI] [PubMed] [Google Scholar]


