Abstract
Islet transplantation provides an approach to compensate for loss of insulin-producing cells in patients with type 1 diabetes. However, the intraportal route of transplantation is associated with instant inflammatory reactions to the graft and subsequent islet destruction as well. Although matrix metalloprotease (MMP)-2 and -9 are involved in both remodeling of extracellular matrix and leukocyte migration, their influence on the outcome of islet transplantation has not been characterized. We observed comparable MMP-2 mRNA expressions in control and transplanted groups of mice, whereas MMP-9 mRNA and protein expression levels increased after islet transplantation. Immunostaining for CD11b (Mac-1)-expressing leukocytes (macrophage, neutrophils) and Ly6G (neutrophils) revealed substantially reduced inflammatory cell migration into islet-transplanted liver in MMP-9 knockout recipients. Moreover, gelatinase inhibition resulted in a significant increase in the insulin content of transplanted pancreatic islets and reduced macrophage and neutrophil influx compared with the control group. These results indicate that the increase of MMP-9 expression and activity after islet transplantation is directly related to enhanced leukocyte migration and that early islet graft survival can be improved by inhibiting MMP-9 (gelatinase B) activity.
Type 1 diabetes is defined as an autoimmune disease that results in the permanent destruction of insulin-producing β-cells of the pancreas. In a therapeutic procedure, pancreatic islets are injected into the liver via the portal vein, where they take up residence and start producing insulin (1,2). However, the success of isolated islet transplantation is limited because of inflammatory, apoptotic, and coagulation processes that occur in the hepatic environment and that seem to be responsible for the failure of islet transplantation (3–5).
Extracellular cell matrix (ECM) interactions have been shown to influence islet integrity, proliferation, differentiation, and insulin secretion (6–8). In particular, matrix metalloproteases (MMPs) are a family of zinc-dependent endopeptidases, which are involved in the turnover of the ECM in several conditions, including embryonic development, inflammatory cell invasion, or wound healing (9–12). The majority of MMPs are secreted as proenzymes, and their proteolytic activation occurs in the pericellular and extracellular space. In two of the secreted MMPs also designated gelatinases, MMP-2 (gelatinase A) and MMP-9 (gelatinase B), the catalytic zinc-carrying domain contains a structural module of three fibronectin-like repeats interacting with elastin and types I, III, and IV gelatins when activated and facilitating their degradation within connective tissue matrices.
Both neutrophils and macrophages express and secrete gelatinases (10,11,13). Previous studies have shown that such cells contribute to the first line of defense following islet transplantation (3,4). Moreover, elevation of MMP-9 plasma levels was observed in diabetic patients (14), whereas in mice with acute pancreatitis, trypsin induced the activation of MMP-2 and -9. When activated by endogeneous trypsin, MMP-9 was diabetogenic, as it cleaved secreted insulin (15,16). Tissue inhibitor of metalloproteinase-1 protects against apoptosis and restores glucose-stimulated insulin release of islets in the presence of cytokines (17). In addition, inhibition of pancreatic MMP-9 activity suppressed leukocyte migration and inflammation in type 1 diabetes (18).
Basement membrane–degrading gelatinases, such as MMP-9, play an important role in immune-mediated tissue destruction (10,11). Thus, MMP-9 may serve as candidate target for therapeutic intervention, as gelatinase activity could change the ECM composition of transplanted islets and, at the same time, promote leukocyte migration into the graft.
The current study therefore was undertaken to investigate the role of gelatinases in islet transplantation. Using in situ zymography, gel-gelatin zymography, immunostaining, and RT-PCR, we were able to show increased MMP-9 activity after islet transplantation into recipient liver. Furthermore, a pharmacological approach with synthetic MMP inhibitors and a genetic approach using MMP-9 knockout mice demonstrated a critical role for MMP-9 in the recruitment of inflammatory cells and islet graft loss.
RESEARCH DESIGN AND METHODS
Pig islet isolation.
Pig islets were isolated using previously described techniques of collagenase digestion and Ficoll purification (19). In brief, islets from a single pig pancreas were isolated after a vascular flush with University of Wisconsin solution (Du Pont Critical Care, Waukegan, IL). The quality of islet isolation was evaluated by trypan blue exclusion, dithizone staining, and glucose-stimulated insulin secretion to check viability, purity, and function. Animal research was approved by the Regional Commission Giessen (Germany) under the code number GI20/11-Nr.15/2006. Animal husbandry was performed according to the German Animal Welfare Law, as published in the latest version under http://bundesrecht.juris.de/tierschg.
Culture condition.
Pig islets were cultured in non-CO2 air in CMRL-1066 (PAA, Pasching, Austria) supplemented with 25 mmol/L HEPES, 20% heat-inactivated pig serum, 100 units/mL penicillin (Biochrom, Berlin, Germany), 100 μg/mL streptomycin, and 2 mg/mL glucose.
Islet transplantation.
Diabetes was induced in C57BL/6 recipients (12 weeks old, male) by a single injection of 200 mg/kg i.p. streptozotocin (Sigma, Munich, Germany), and blood glucose levels were monitored by the Elite glucometer (Bayer, Leverkusen, Germany). Mice with a nonfasting blood glucose concentration of >16.7 mmol/L for 2 consecutive days were selected for transplantation. Recipients were anesthetized with avertine and maintained with isoflurane. A total of 2,000 islet equivalent porcine islets washed with PBS were transplanted into the liver via the portal vein of mice with a 27-gauge needle using a previously described method with modifications (19). MMP-9 knockout and respective control mice (aged 6–7 weeks) were a gift from Dr. Eva Korpos (Westfälische Wilhelms-Universität, Münster, Germany). They originally were generated at the University of Leuven, Belgium (20), and backcrossed with C57BL/6 mice for >10 generations. Mice were characterized for the absence or presence of MMP-9 by using both RT-PCR and gel-gelatin zymography.
RT-PCR.
Following islet transplantation, animals were killed after 4 h and total RNA was prepared from liver using TRIzol reagent (Invitrogen, Darmstadt, Germany), according to the manufacturer’s instructions, to analyze mRNA expression of MMP-2 and MMP-9. To amplify the MMP-9, MMP-2, and glyceraldehyde-3-phosphate dehydrogenase complementary DNA fragments,the sequences of PCR primers were as follows: MMP-2 forward primer 5′-AGATCTTCTTCTTCAAGGACCGGTT-3′, reverse primer 5′-GGCTGGTCAGTGGCTTGGGGTA-3′, 225 bp; MMP-9 forward primer 5′-GTTTTTGATGCTATTGCTGAGATCCA-3′, reverse primer 5′-CCCACATTTGACGTCCAGAGAAGAA-3′, 208 bp; and glyceraldehyde-3-phosphate dehydrogenase forward primer 5′-GGAGCGAGACCCCACTAACAT-3′, reverse primer 5′-GCGGAGATGATGACCCTTTT-3′, 135 bp.
Reverse transcription was performed for 15 min at 70°C followed by a 2-min incubation at 95°C for denaturation. Amplification started by 15 s at 94°C, 20 s at 60°C, and 10 s at 72°C for 39 cycles. RT-PCR products were loaded on 2% agarose gels and analyzed after staining with Syber green (Invitrogen) using the imaging system BIO 1D (Vilber Lourmat, Eberhardzell, Germany).
Immunohistochemistry and in situ gelatin zymography.
Cryostat sections (7 μm) of pancreas and islet-transplanted liver were fixed in precooled acetone for 10 min. The sections were washed thrice with PBS. Following blocking with 3% donkey serum, sections were incubated with polyclonal guinea pig anti-swine insulin primary antibody (Dako, Glostrup, Denmark) for 2 h. After using a donkey anti-guinea pig secondary antibody (RhodRedx; Jackson ImmunoResearch, Hamburg, Germany), in situ zymography was performed. DQ gelatin (10%; Invitrogen) that was dissolved in 1% low-melting multi-purpose agarose (Roche, Grenzach, Germany) containing 10 μmol/L ZnCl2, 1 mmol/L CaCl2, and 1 µL DAPI was put on top of the coverslip to cover each tissue. Slides were kept in a moist chamber at 37°C, and at time 0 (immediately after placing sections on the slides) as well as after 1 h sections were viewed in a fluorescent microscope (Leica DMLB; Leica Microsysteme Vertrieb, Bensheim, Germany) and processed using the Leica application suite. Gelatin gel with captopril (10 mmol/L; Sigma) was taken as negative control. The presence of autofluorescence was excluded by incubating sections in agarose-containing medium without DQ gelatin.
Gel-gelatin zymography.
Wild-type and islet-transplanted liver tissue was homogenized by using lysis buffer (50 mmol/L Tris-HCl, pH 7.6; 150 mmol/L NaCl; 0.1% SDS; and 0.1% Na-deoxycholate). The homogenate was centrifuged for 20 min at 4°C at 9,000g. Protein concentration was measured using a bicinchoninic protein assay (Pierce), and an equivalent amount (90 μg) of protein was electrophoresized on nonreducing SDS-PAGE–containing gelatin (0.2%) as substrate, which is readily cleaved by MMPs. After electrophoretic separation of proteins, gels were washed thrice, 15 min each, in 2.5% Triton X-100. Gels then were treated with incubation buffer (50 mmol/L Tris-HCl, pH 7.6; 0.1% NaN3; 1% Triton X-100; 10 mmol/L CaCl2; and 1 mol/L ZnCl2) for 24 h at 37°C and then stained with Coomassie Brilliant Blue R-250. After destaining the gel, clear bands indicated gelatinase activity. Gels incubated with incubation buffer containing 5 mmol/L EDTA were used as the negative control.
Immunohistochemistry.
Pig islets were transplanted into mice liver, and after 4 h islet-transplanted livers were embedded in TissueTek OCT compound and snap frozen in liquid nitrogen. Frozen sections (7 μm) were prepared using a cryostat and were stored at −20°C until they were used for staining. Sections were fixed in 10% acetone at −20°C and blocked with 10% donkey serum. After washing with PBS, the sections were incubated overnight at 4°C with rat anti-mouse Ly6G (Gr1, clone RB6-8C5; eBioscience, Frankfurt, Germany) and rat anti-mouse CD11b (Mac-1; ImmunoTools, Friesoythe, Germany) in a humidifier. The sections were washed in PBS and incubated with secondary antibodies fluorescein isothiocyanate–donkey anti-rat for CD11b and Ly6G (all from Jackson ImmunoResearch) for 1 h at 22°C. Sections were washed again with PBS and counterstained with DAPI while being inspected under the fluorescent microscope. The same method was used for MMP-9 staining in control and transplanted liver, whereby rabbit anti–MMP-9 polyclonal antibody (Millipore) was used as the primary and RhodRedx-donkey anti-rabbit as the secondary antibody. For insulin staining, guinea pig anti-insulin primary antibody and fluorescein isothiocyanate–donkey anti-guinea pig (Jackson ImmunoResearch) secondary antibody were used. In addition, some sections were incubated with rat anti-mouse F4/80 (AbD Serotec, Dusseldorf, Germany) primary antibody and RhodRedx-donkey anti-rat secondary antibody. F4/80-positive cells were counted (×200 magnification) in fields of liver sections without islet (Supplementary Fig. 1D) or in the proximity of transplanted islets at different time points (Supplementary Fig. 1E).
Liver immune cell preparation.
Islet-transplanted livers were perfused through the vena cava with 20 mL PBS, followed by the removal of the gallbladder and careful excision of the liver from the abdomen. Isolation of single cells by mechanical disruption in RPMI (RPMI without phenol red containing 10% FCS and 25 mmol/L HEPES) with surgical scissors was performed. This preparation was dissolved in RPMI containing collagenase and DNase (RPMI without phenol red containing 10% FCS, 25 mmol/L HEPES, 0.5 mg/mL collagenase D, and 0.025 mg/mL DNaseI) and kept for 45 min at 37°C with shaking (1 cycle/s). The reaction was stopped by the addition of 20 mmol/L EDTA. After passing the content through a 100-μm cell strainer, RPMI containing 5% FCS was added, followed by centrifugation at 1,250 rpm for 15 min at 4°C. The cell pellet was mixed with 40% Percoll and then poured on top of 70% Percoll. After centrifugation at 2,000 rpm for 20 min at 20°C, immune cells were removed from the interphase of the gradient, washed with RPMI, and used for analysis by FACSCalibur (Becton Dickinson, Heidelberg, Germany).
Antibodies and flow cytometric analysis.
One million cells were suspended in PBS containing 0.3% bovine serum albumin and 0.1% sodium azide. For inhibiting nonspecific binding of antibodies, Fc-receptors were blocked by preincubating cells with monoclonal antibody directed against the FcR III/II CD16/CD32 (BD Biosciences). Cells were incubated with 1 μg/106 cells of the CD11b antibody (phycoerythrin-conjugated anti-CD11b; BD-bioscience) for 45 min at 4°C and washed twice. They were analyzed by using Cell Quest software for FACSCalibur (Becton Dickinson).
Peritoneal macrophage isolation.
Four percent brewer’s thioglycollate medium (Becton Dickinson) was injected into the peritoneal cavity of each mouse. Peritoneal fluid was obtained 72 h after injection by flushing the peritoneal cavity with ice-cold serum-free RPMI-1640 medium using a 22G needle. Fluid was centrifuged in a 15-mL conical centrifuge tube at 1,200 rpm for 5 min at 4°C, and the pellet was resuspended in RPMI-1640 medium supplemented with 10% heat-inactivated FCS, 15 mmol/L HEPES buffer, and 1% penicillin/streptomycin. Erythrocytes were lysed and the remaining cells were counted and checked for viability by the trypan blue dye exclusion method. 6 × 106 Cells were cultured in a 70-mm dish and incubated at 3 h at 37°C for adherence. Nonadherent cells were removed by washing with serum-free RPMI-1640 medium.
Cell migration/invasion assay.
Under sterile conditions 12-well transwell plates (Greiner Bio One, Frickenhausen, Germany) with 8-μm–pore size matrigel-coated filters (BD Matrigel) were used. Lower wells contained 600 μL of islet supernatant, whereby the upper wells contained a final volume of 250 μL after the addition of cells. To begin the assay, 0.5 × 106 cells in medium were added to the upper wells in the absence and presence of MMP inhibitors (5 mmol/L captopril or 10 μmol/L GM6001) and were allowed to migrate for 48 h at 37°C in a 5% CO2 humidified atmosphere. After completion of migration, cells were labeled with 450 μL of calcein AM (Sigma) that was added to the lower chamber at an 8 μmol/L final concentration. Cells migrating to the underside of the filter were removed by using trypsin EDTA and detected using a fluorescence plate reader (Mithras LB940; Berthold Technologies, Bad Herrenalb, Germany).
Islet transplantation with captopril.
Captopril was dissolved in normal saline and injected subcutaneously for 7 days into mice (0.5 mg/kg, n = 10, C57BL/6) starting from the day of transplantation. Two thousand islet equivalents were transplanted into the portal vein of diabetic mice. Recipients were anesthetized with 2.5% avertine (Sigma). Fourteen days after transplantation, grafted livers were retrieved for insulin extraction, as described previously (19). The transplants were homogenized mechanically and dissolved in acid ethanol, and the supernatants were collected for quantification of insulin by enzyme-linked immunosorbent assay (DRG Instruments, Marburg, Germany).
Islet transplantation with MMP-2/-9 inhibitor.
A hydrophobic cyclic peptide that acts as an inhibitor of MMP-2 and -9 (10 mmol/L IC50) was used to confirm results obtained from captopril treatment (MMP-2/-9 inhibitor III; Calbiochem-Merck, Nottingham, U.K.). Pig islets were transplanted to mice liver and MMP-2/-9 inhibitor was injected intravenously for 7 days into mice (15 mg/kg, n = 4, C57BL/6), as described previously (21). Two thousand islet equivalents were transplanted into the portal vein of diabetic mice. Recipients were anesthetized with 2.5% avertine (Sigma).
Statistics.
The Student t test and one-way ANOVA were applied for the analysis of the data. A P value of <0.05 was considered significant (*P < 0.05; **P < 0.001; ***P < 0.0001).
RESULTS
Gelatinase activity in liver after islet transplantation.
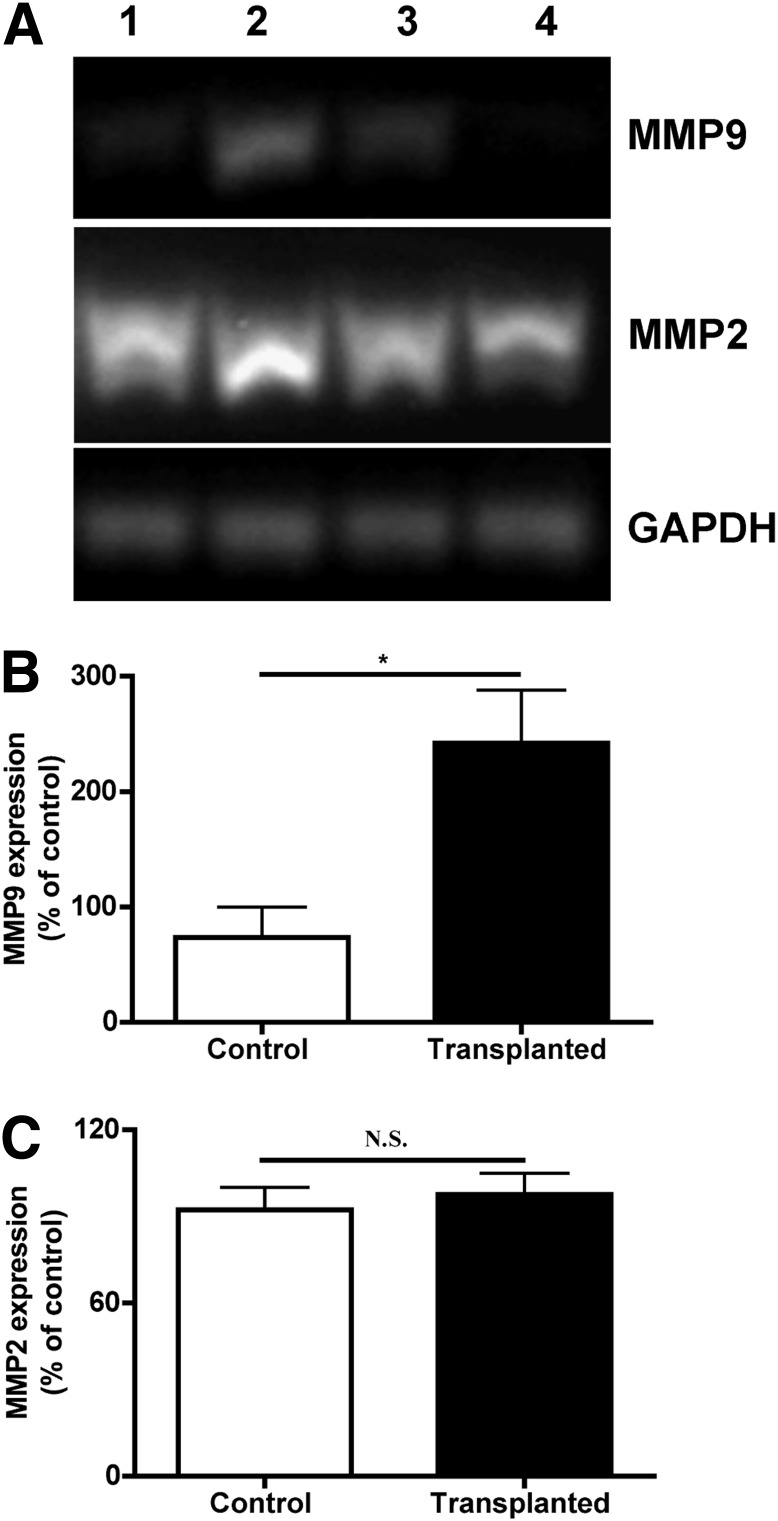
To verify gelatinase expression in normal and islet-transplanted liver, RT-PCR, in situ gelatin zymography, and immunostaining were performed. RT-PCR showed higher expression of MMP-9 in transplanted compared with control mice (Fig. 1A). Quantification of band intensity indicated significant increases of MMP-9 mRNA (Fig. 1B) in transplanted mice compared with control mice, whereas MMP-2 mRNA levels did not change after transplantation (Fig. 1C).
FIG. 1.
MMP-9 expression in transplanted liver. A: mRNA levels of MMP-2 and -9 were analyzed by RT-PCR in total RNA extracts from control liver (lanes 1 and 4) and islet-transplanted liver (lanes 2 and 3). GAPDH, glyceraldehyde-3-phosphate dehydrogenase. B: MMP-9 expressions in transplanted mice were significantly higher in comparison with control mice (*P < 0.05, n = 3). C: MMP-2 mRNA level was not significantly different in control and islet-transplanted liver (P > 0.05, n = 3).
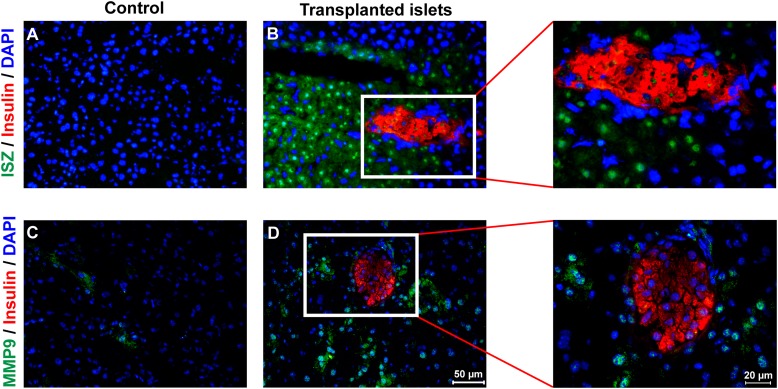
Analysis of gelatinase activity by in situ zymography provided information about the precise localization of MMP-2 and MMP-9 activity in liver sections by dye-quenched DQ gelatin labeling using fluorescein isothiocyanate. The basal MMP activity was low in untreated animals (Fig. 2A), whereas in islet-transplanted liver it was increased (Fig. 2B). Next, we examined MMP-9 immunostaining within control (Fig. 2C) and islet-transplanted liver (Fig. 2D) and found a similar pattern. Hepatic MMP-9 activity increased in the vicinity of the transplanted islets (Fig. 2B and D) and also was observed within them (Fig. 3A and B).
FIG. 2.
Gelatinase activity in islet-transplanted liver. Control liver sections (A and C) and islet-transplanted liver sections (B and D) were analyzed by in situ zymography (ISZ) for gelatinase activity (A and B, green) as well as stained with specific antibody for MMP-9 immunoreactivity (C and D, green). The figures are representative of four independent experiments. (Magnification ×200, inserts ×400). Insulin (red) and DAPI (blue) were stained for localization of islet and nucleus, respectively. (A high-quality digital representation of this figure is available in the online issue.)
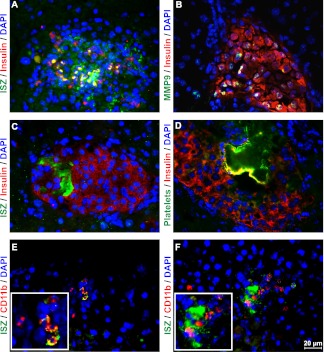
FIG. 3.
Gelatinase activity associated with islet transplant. Following transplantation of islet grafts into liver for 4 h, in situ zymography (ISZ; green) for gelatinase activity (A) and immunostaining for MMP-9 (B, green) was performed, which showed increased gelatinase activity within a transplanted islet. In situ gelatin activity (C, green) also was seen next to thrombus material, which was confirmed by staining platelet aggregates (D, green) with GbIIb/IIIa antibody. Gelatinase activity (E and F, green) was costained with CD11b-positive cells (red) in islet-transplanted liver. The figures are representative of three independent experiments. Magnification ×400. (A high-quality digital representation of this figure is available in the online issue.)
Gelatinase activity was detected in association with platelets and thrombus-like material (Fig. 3C), as indicated by staining with an antibody against GPIIb/IIIa (Fig. 3D). Increased gelatinase activity also was observed next to CD11b-positive cells (Fig. 3E and F) that occur within liver tissue after islet transplantation.
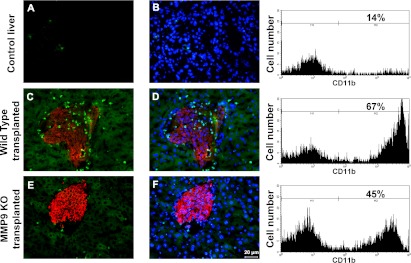
MMP-9 knockout mice and migration of CD11b-positive cells.
To confirm the absence of MMP-9 activity in MMP-9 knockout mice liver after islet transplantation substrate, zymography was performed (Supplementary Fig. 2). Gel zymography is based on degradation of gelatin, giving precise information about MMP-2 and MMP-9 levels with their pro and active forms. We did not observe MMP-9 in MMP-9 knockout islet-transplanted liver, although MMP-9 was present in wild-type islet-transplanted liver. Because MMP-9 secretion was inducible in the islet-transplanted liver but not in control liver, the role of MMP-9 after transplantation was further investigated by analyzing the distribution of CD11b-positive (neutrophils and macrophages) cells using MMP-9 knockout and respective control mice as recipients. It is known from previous studies that MMP-9 is actively involved in recruitment of CD11b-positive cells driving inflammatory processes, such as in postischemic liver and neutrophil migration (21,22). Thus, we examined the involvement of MMP-9 in the migration of CD11b-positive cells to the pancreatic islet transplants. Only very few leukocytes were observed in nontransplanted control liver (Fig. 4A and B). Their number was increased in sections of wild-type recipient mice with hepatic islet grafts (Fig. 4C and D) compared with MMP-9 knockout recipients (Fig. 4E and F). These results were confirmed by fluorescence-activated cell sorter analysis of liver immune cells, where we found 67% of CD11b-positive cells in wild-type islet-transplanted liver as opposed to 45% in MMP-9 knockout mice.
FIG. 4.
MMP-9 in CD11b-positive cell migration. Compared with control (A and B), CD11b-positive cells (green) in association with insulin-positive islet (red) transplant are indicated in wild-type mouse liver (C and D) as well as in MMP-9 knockout (KO) liver (E and F) with transplanted islets (red). More CD11b-positive cells migrated into wild-type mouse liver (C and D) with transplanted islets compared with MMP-9 knockout liver (E and F) with transplanted islets. B, D, and F: Costaining of nucleus with DAPI (blue) is added. Magnification ×200 (n = 3). Fluorescence-activated cell sorter data are placed next to respective immunostaining figures. There were a total of 67% of CD11b-positive cells in wild-type transplanted mice compared with 45% in MMP-9 knockout transplanted mice. All figures are representative of three independent experiments. (A high-quality digital representation of this figure is available in the online issue.)
Inhibition of gelatinase activity by captopril.
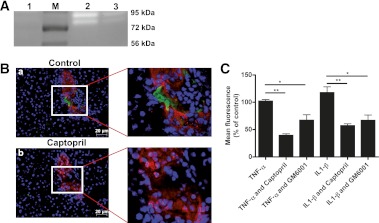
Next we examined the effect of captopril on gelatinase inhibition. Islets were transplanted into C57BL/6 mice liver in the absence or presence of captopril. Livers were removed after 4 h of transplantation and explored for gelatinase activity by substrate zymography (Fig. 5A). Increase in MMP-9 activity (pro ~92 KDa and active ~86 KDa) was observed in wild-type islet-transplanted liver, which was inhibited in captopril-treated mice. There was no detectable MMP-2 activity in islet-transplanted liver. Captopril was used to inhibit gelatinase activity, and its effect was confirmed by in situ zymography. Administration of 10 mmol/L captopril completely abrogated enzymatic activity on tissue sections (Fig. 5B).
FIG. 5.
Captopril inhibits gelatinase activity and macrophage migration. A: Islet-transplanted liver isolated from mice injected with captopril was used for gel-gelatin zymography. Gelatinase activity was not present in control liver (lane 1), although it was increased 4 h after islet transplantation (lane 2) and was inhibited by captopril injection (lane 3). M, marker. B: In situ gelatin zymography (green) was performed without (a) or with (b) addition of captopril in islet-transplanted liver sections. Captopril inhibited MMP activity (green) in islet (red) surroundings (b). The nucleus was stained with DAPI. C: Murine peritoneal macrophages were allowed to migrate with or without MMP inhibitors. Numbers are expressed as the percentage of macrophages that migrated toward islet-conditioned medium without inhibitor. Macrophage migration was significantly inhibited by captopril in tumor necrosis factor-α (TNF-α) (**P < 0.001, n = 3) and interleukin-1-β (IL1-β)-treated cell-conditioned medium (**P < 0.001, n = 3). The effect also was significant when macrophages were treated with GM6001 (*P < 0.05, *P < 0.05, n = 3). (A high-quality digital representation of this figure is available in the online issue.)
Macrophage invasion in the presence of MMP inhibitors.
Macrophages are known to migrate toward transplanted islets (23). We observed that there was a significant increase in migration of CD11b- and F4/80-positive cells toward islet grafts after transplantation (Fig. 4C and D and Supplementary Fig. 1E). Because all hepatic Kupffer cells do not express CD11b, anti-F4/80 antibody was used to detect them. To determine the specificity of captopril treatment, macrophage migration was measured in the absence or presence of the specific gelatinase inhibitor GM6001 (10 μmol/L) and captopril (5 mmol/L). As shown in Fig. 5, significant inhibition of macrophage migration toward tumor necrosis factor-α–treated islet cell–conditioned medium occurred in the presence of captopril or GM6001 (Fig. 5C). A similar effect was observed when macrophages were subjected to interleukin-1-β–treated islet cell–conditioned medium in the presence of captopril or GM6001.
Neutrophil and macrophage migration after MMP-9 inhibition in vivo.
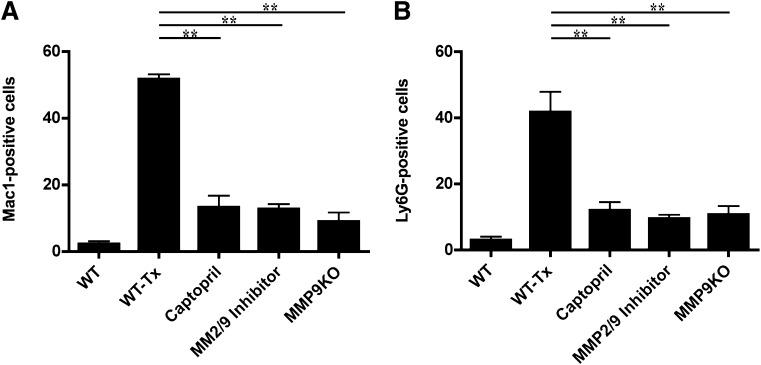
The influence of MMP-9 inhibition on in vivo macrophage and neutrophil migration was studied. Following islet transplantation, immunostaining for CD11b and Ly6G was performed (Fig. 6). CD11b- and Ly6G-positive cell numbers were increased in wild-type transplanted mice as opposed to those animals treated with captopril or MMP-2/-9 inhibitor and in transplanted MMP-9 knockout mice, altogether demonstrating that this effect was mediated mainly by inhibition of MMP-9.
FIG. 6.
MMP-9 inhibition and reduction of macrophages and neutrophils. A: Leukocyte migration was quantitated in islet-transplanted liver sections with and without in vivo MMP-9 inhibition. CD11b (Mac-1) staining revealed significant inhibition of CD11b-positive cell migration toward islet-transplanted liver in captopril or MMP-2/-9 inhibitor–treated and MMP-9 knockout (KO) mice compared with wild-type islet-transplanted mice (WT-Tx) (**P < 0.001, n = 3 mice). B: Number of Ly6G-positive cells also was reduced in captopril, MMP-2/-9 inhibitor–treated, and MMP-9 knockout animals compared with wild-type islet-transplanted mice. (**P < 0.001, n = 3, three fields per section).
Islet transplantation in vivo.
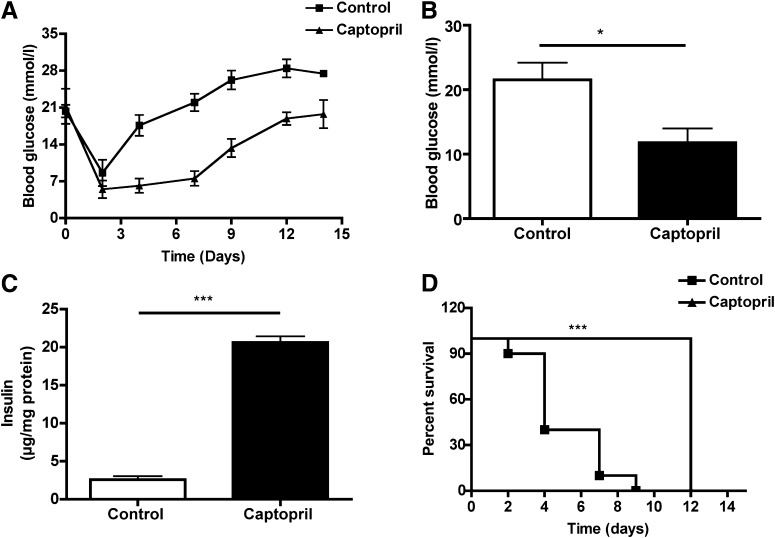
When pig islets were transplanted to C57BL/6 recipients with streptozotocin-induced diabetes, blood glucose was initially normalized, but diabetes recurred within 5 days as expected. The rejection was delayed in the captopril-treated group compared with the control group, and mean blood glucose levels in the captopril-treated group were lower (Fig. 7A and B). At the end of the experiment, insulin contents of the grafts retrieved from recipients treated with captopril were significantly increased compared with controls (Fig. 7C). Log-rank analysis of the graft survival curve revealed that treatment with captopril significantly extended survival times compared with controls (Fig. 7D). These data indicated that gelatinase inhibition is beneficial for early islet engraftment.
FIG. 7.
Captopril and graft function. Captopril was injected subcutaneously for 7 days into mice transplanted with pig islets (n = 10 in each group). A: Mean blood glucose levels in the captopril-treated group (▲) were lower than in the control group (■). B: Statistical analysis showed significant difference in mean blood glucose value (*P < 0.05). C: Insulin content of liver grafts retrieved from recipients after 14 days of transplantation in the captopril-treated group was increased compared with the untreated liver grafts (***P < 0.0001). D: Log-rank analysis of the graft survival curve determined from daily blood glucose levels showed significant difference (***P < 0.0001) of groups of transplanted mice treated with captopril (▲) or with solvent (■). The day of graft loss was defined as the last one of successive levels of >16.7 mmol/L glucose.
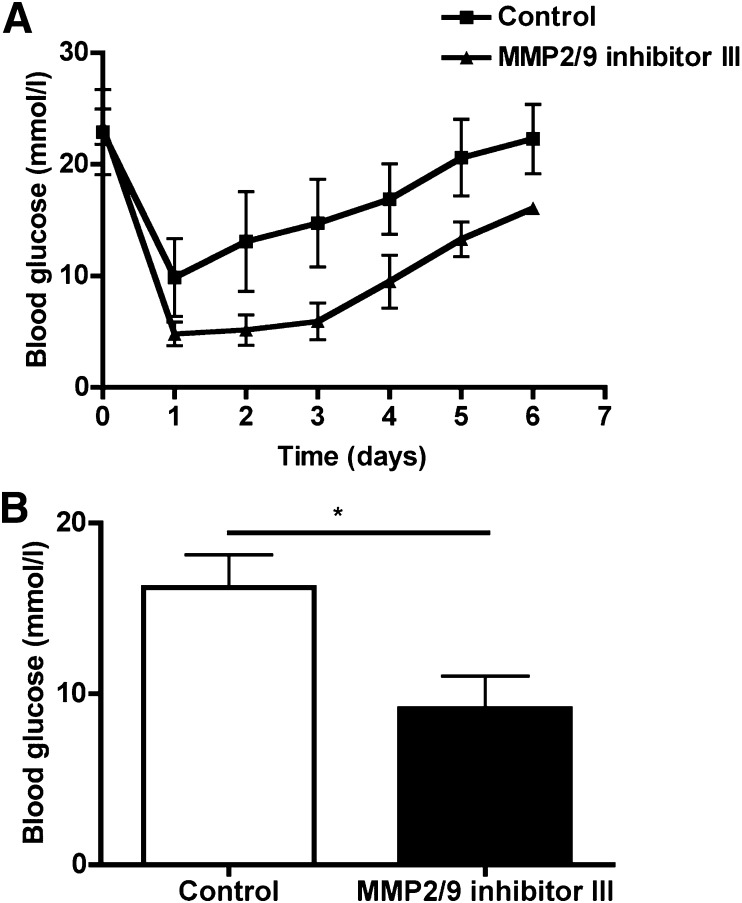
Improved early islet graft function after captopril treatment was compared with the specific gelatinase inhibitor MMP-2/-9 III. We observed decreased blood glucose levels in MMP-2/-9 inhibitor–treated mice compared with islet-transplanted mice without MMP inhibitor treatment. The transplantation experiments confirm that inhibition of gelatinases is an effective treatment in early islet transplantation (Fig. 8A and B).
FIG. 8.
MMP-9 inhibitor peptide and graft function. MMP-2/-9 inhibitor (15 mg/kg) was injected intravenously for 7 days to mice transplanted with pig islets (n = 4 in each group). A: Mean blood glucose levels in the group treated with the inhibitor (▲) were lower compared with the control group (■). B: Statistical analysis showed a significant difference in mean blood glucose value (*P < 0.05). The day of graft loss was defined as the last one of successive levels of >16.7 mmol/L glucose.
DISCUSSION
The aim of the present work was to investigate the role of gelatinases in the transplantation of islets and to provide measures for the protection of pancreatic islets against the infiltration of immune cells. Gelatinases have long been known to enhance inflammatory cell migration or to generate the biologically active forms of cytokines (10,11,24). Islet infusion into the portal vein was assumed to induce protease activity in the context of an instant inflammatory or procoagulant response triggered by tissue factor or blood constituents (25,26), and this response potentially may be amplified by drainage of endotoxins from the intestine (27). MMP-9 was shown to play a role in diabetes as well as in chronic pancreatitis (14–18). Gelatinases were studied in models of acute and chronic allograft rejection in the posttransplant period and ameliorated graft failure by reduction of profibrogenic factors (28).
Neutrophils are a key component of the inflammatory response. They can generate chemotactic signals and thereby attract dendritic cells and macrophages (29,30). Because neutrophil chemotaxis has been reported to occur in islet grafts (3), the influence of pharmaceutical MMP inhibition on the migration of Ly6G-positive cells toward transplanted islets has been investigated. Previous reports showed that neutrophils play a major role in early graft loss by increasing local interferon-γ production (4). We also confirmed that CD11b- and Ly6G-positive cells were the main mediators of the instant inflammatory reaction following islet transplantation. Moreover, by therapeutically targeting these cells the functional damage to the graft observed during the very first hours was significantly reduced. No significant enhancement of F4/80-positive cells (Supplementary Fig. 1D) was noted in complete liver section 24 h after islet transplantation compared with nontransplanted liver sections. We observed a slight increase of the number of F4/80-positive cells in the surroundings of transplanted islets at 4 and 24 h as opposed to 30 min posttransplantation (Supplementary Fig. 1A–C and E). There was no difference in the composition of CD4- and CD8-positive cells in wild-type and islet-transplanted mouse liver (data not shown). However, by 4 h after transplantation, macrophage and neutrophil accumulation was enhanced in islet-transplanted liver.
Gelatinase activity in association with transplanted islets was significant in surrounding tissue but was minor within islet grafts. We observed a preferential increase of its activity in nonendocrine cells, such as in neutrophils, platelets, and hepatocytes. Accordingly, no morphological reorganization of transplanted islets was observed, such as aggregation or rearrangement of endocrine cells. In addition, elevated levels of MMP-9 mRNA and protein were found in the recipient liver, whereas MMP-2 mRNA expression was essentially the same in control or transplanted mice liver and not detectable at the protein level. These data indicate that increased MMP-9 expression and activity provides a likely candidate responsible for early islet graft loss.
We used two approaches, namely transplantation into MMP-9 knockout mice and application of the MMP inhibitors captopril and MMP-2/-9 inhibitor III to influence loss of islet grafts and to challenge the increased survival after islet transplantation. In the first approach, accumulation of CD11b-positive cells in the islet-transplanted liver was reduced, indicating that MMP-9 supports early islet graft loss by increasing immune cell infiltration to the site of transplantation. From these data we conclude that although MMP-9 is required for revascularization following islet transplantation (31), the recipient tissue devoid of MMP-9 is apparently protected against inflammatory cell infiltration toward grafted islets, and, thus, graft survival is increased.
In a second approach, in vitro and in vivo functional studies were performed with captopril and MMP-2/-9 inhibitor III as prominent gelatinase inhibitors. Captopril forms complexes with the catalytically functional zinc ion present at the active site of ACE and gelatinase (32–35). To dissect which of the two zinc-dependent proteases is more prominent in pancreatic islet transplantation, we compared captopril with losartan (data not shown). The latter is a selective antagonist to the angiotensin receptor but without affinity to zinc (36,37). Captopril amplified the glucose-lowering function of transplanted islets more effectively than losartan, indicating that metalloprotease activated minutes after intraportal islet transplantation is activated by mechanisms other than the angiotensin receptor.
Not only was the drug able to inhibit macrophage migration/invasion toward islet supernatant in vitro, similar to the action of the MMP inhibitor GM6001, but captopril treatment was found to increase insulin content and reduce blood glucose levels when compared with untreated mice. MMP-2/-9 inhibitor III also improved blood glucose level in transplanted diabetic mice and, in addition, reduced neutrophil and macrophage accumulation in islet-transplanted liver to the same extent as found in captopril treated or MMP-9 knockout recipient mice. Captopril and MMP-2/-9 inhibitor III can both reduce gelatinase activity, but because only MMP-9 protein level was increased after islet transplantation to liver, it was assumed that the observed effect was predominantly specific for MMP-9. Together, these data demonstrate that MMP-9 inhibition has an important positive influence on islet graft survival. Thus, inhibition of gelatinase B activity in early pancreatic islet grafts serves as a protective treatment for the reduction of leukocyte infiltration and for the restoration of inflammation and graft function.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants from the Deutsche Forschungsgemeinschaft (Bonn, Germany) by the Excellence Cluster Cardiopulmonary System (to K.T.P.), Behring-Roentgen-Stiftung (to T.L.), and Research Ministry of the Federal State of Hessia, LOEWE-MIBIE program (to T.L.).
No potential conflicts of interest relevant to this article were reported.
N.L. designed the research, researched the data, and wrote the manuscript. M.P., B.S., and R.G.B. reviewed the manuscript. K.T.P. designed parts of the study and reviewed and edited the manuscript. T.L. designed the research and wrote the manuscript. T.L. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Doris Erb, Gundula Hertl, Birte Hussmann (Third Medical Department, Giessen, Germany), Dr. Uslu Özgür (Radiology Department, Homburg/Saar), and Dr. Karin Hersemeyer (Institute for Biochemistry, Giessen, Germany) for skillful technical assistance. The authors also thank Andreas O. Schultz (Third Medical Department, Giessen, Germany) for preparing the figures for the final version for publication.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-1143/-/DC1.
REFERENCES
- 1.Sutherland DE, Matas AJ, Goetz FC, Najarian JS. Transplantation of dispersed pancreatic islet tissue in humans: autografts and allografts. Diabetes 1980;29(Suppl. 1):31–44 [DOI] [PubMed] [Google Scholar]
- 2.Brandhorst H, Brandhorst D, Hering BJ, Bretzel RG. Significant progress in porcine islet mass isolation utilizing liberase HI for enzymatic low-temperature pancreas digestion. Transplantation 1999;68:355–361 [DOI] [PubMed] [Google Scholar]
- 3.Moberg L, Korsgren O, Nilsson B. Neutrophilic granulocytes are the predominant cell type infiltrating pancreatic islets in contact with ABO-compatible blood. Clin Exp Immunol 2005;142:125–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yasunami Y, Kojo S, Kitamura H, et al. Valpha14 NK T cell-triggered IFN-gamma production by Gr-1+CD11b+ cells mediates early graft loss of syngeneic transplanted islets. J Exp Med 2005;202:913–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim HI, Yu JE, Lee SY, et al. The effect of composite pig islet-human endothelial cell grafts on the instant blood-mediated inflammatory reaction. Cell Transplant 2009;18:31–37 [DOI] [PubMed] [Google Scholar]
- 6.Hammar E, Parnaud G, Bosco D, et al. Extracellular matrix protects pancreatic β-cells against apoptosis: role of short- and long-term signaling pathways. Diabetes 2004;53:2034–2041 [DOI] [PubMed] [Google Scholar]
- 7.Kaido T, Yebra M, Cirulli V, Rhodes C, Diaferia G, Montgomery AM. Impact of defined matrix interactions on insulin production by cultured human β-cells: effect on insulin content, secretion, and gene transcription. Diabetes 2006;55:2723–2729 [DOI] [PubMed] [Google Scholar]
- 8.Weber LM, Hayda KN, Anseth KS. Cell-matrix interactions improve beta-cell survival and insulin secretion in three-dimensional culture. Tissue Eng Part A 2008;14:1959–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rutges JP, Nikkels PG, Oner FC, et al. The presence of extracellular matrix degrading metalloproteinases during fetal development of the intervertebral disc. Eur Spine J 2010;19:1340–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong Y, Hart E, Shchurin A, Hoover-Plow J. Inflammatory macrophage migration requires MMP-9 activation by plasminogen in mice. J Clin Invest 2008;118:3012–3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khandoga A, Kessler JS, Hanschen M, et al. Matrix metalloproteinase-9 promotes neutrophil and T cell recruitment and migration in the postischemic liver. J Leukoc Biol 2006;79:1295–1305 [DOI] [PubMed] [Google Scholar]
- 12.Mirastschijski U, Schnabel R, Claes J, et al. Matrix metalloproteinase inhibition delays wound healing and blocks the latent transforming growth factor-beta1-promoted myofibroblast formation and function. Wound Repair Regen 2010;18:223–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parks WC, Wilson CL, López-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol 2004;4:617–629 [DOI] [PubMed] [Google Scholar]
- 14.Maxwell PR, Timms PM, Chandran S, Gordon D. Peripheral blood level alterations of TIMP-1, MMP-2 and MMP-9 in patients with type 1 diabetes. Diabet Med 2001;18:777–780 [DOI] [PubMed] [Google Scholar]
- 15.Descamps FJ, Van den Steen PE, Martens E, Ballaux F, Geboes K, Opdenakker G. Gelatinase B is diabetogenic in acute and chronic pancreatitis by cleaving insulin. FASEB J 2003;17:887–889 [DOI] [PubMed] [Google Scholar]
- 16.Descamps FJ, Martens E, Ballaux F, Geboes K, Opdenakker G. In vivo activation of gelatinase B/MMP-9 by trypsin in acute pancreatitis is a permissive factor in streptozotocin-induced diabetes. J Pathol 2004;204:555–561 [DOI] [PubMed] [Google Scholar]
- 17.Han X, Sun Y, Scott S, Bleich D. Tissue inhibitor of metalloproteinase-1 prevents cytokine-mediated dysfunction and cytotoxicity in pancreatic islets and beta-cells. Diabetes 2001;50:1047–1055 [DOI] [PubMed] [Google Scholar]
- 18.Kang S, Park EJ, Joe Y, et al. Systemic delivery of TNF-related apoptosis-inducing ligand (TRAIL) elevates levels of tissue inhibitor of metalloproteinase-1 (TIMP-1) and prevents type 1 diabetes in nonobese diabetic mice. Endocrinology 2010;151:5638–5646 [DOI] [PubMed] [Google Scholar]
- 19.Chen C, Kuehn C, Bretzel RG, Linn T. Anti-inflammatory thalidomide improves islet grafts survival and functions in a xenogenic environment. PLoS ONE 2009;4:e6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubois B, Masure S, Hurtenbach U, et al. Resistance of young gelatinase B-deficient mice to experimental autoimmune encephalomyelitis and necrotizing tail lesions. J Clin Invest 1999;104:1507–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamada T, Fondevila C, Busuttil RW, Coito AJ. Metalloproteinase-9 deficiency protects against hepatic ischemia/reperfusion injury. Hepatology 2008;47:186–198 [DOI] [PubMed] [Google Scholar]
- 22.D’Haese A, Wuyts A, Dillen C, et al. In vivo neutrophil recruitment by granulocyte chemotactic protein-2 is assisted by gelatinase B/MMP-9 in the mouse. J Interferon Cytokine Res 2000;20:667–674 [DOI] [PubMed] [Google Scholar]
- 23.Sigrist S, Oberholzer J, Bohbot A, et al. Activation of human macrophages by allogeneic islets preparations: inhibition by AOP-RANTES and heparinoids. Immunology 2004;111:416–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schönbeck U, Mach F, Libby P. Generation of biologically active IL-1 beta by matrix metalloproteinases: a novel caspase-1-independent pathway of IL-1 beta processing. J Immunol 1998;161:3340–3346 [PubMed] [Google Scholar]
- 25.Johansson H, Lukinius A, Moberg L, et al. Tissue factor produced by the endocrine cells of the islets of Langerhans is associated with a negative outcome of clinical islet transplantation. Diabetes 2005;54:1755–1762 [DOI] [PubMed] [Google Scholar]
- 26.Ozmen L, Ekdahl KN, Elgue G, Larsson R, Korsgren O, Nilsson B. Inhibition of thrombin abrogates the instant blood-mediated inflammatory reaction triggered by isolated human islets: possible application of the thrombin inhibitor melagatran in clinical islet transplantation. Diabetes 2002;51:1779–1784 [DOI] [PubMed] [Google Scholar]
- 27.Vargas F, Vives-Pi M, Somoza N, et al. Endotoxin contamination may be responsible for the unexplained failure of human pancreatic islet transplantation. Transplantation 1998;65:722–727 [DOI] [PubMed] [Google Scholar]
- 28.Lutz J, Yao Y, Song E, et al. Inhibition of matrix metalloproteinases during chronic allograft nephropathy in rats. Transplantation 2005;79:655–661 [DOI] [PubMed] [Google Scholar]
- 29.Bennouna S, Bliss SK, Curiel TJ, Denkers EY. Cross-talk in the innate immune system: neutrophils instruct recruitment and activation of dendritic cells during microbial infection. J Immunol 2003;171:6052–6058 [DOI] [PubMed] [Google Scholar]
- 30.Chertov O, Ueda H, Xu LL, et al. Identification of human neutrophil-derived cathepsin G and azurocidin/CAP37 as chemoattractants for mononuclear cells and neutrophils. J Exp Med 1997;186:739–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsson R, Maxhuni A, Carlsson PO. Revascularization of transplanted pancreatic islets following culture with stimulators of angiogenesis. Transplantation 2006;82:340–347 [DOI] [PubMed] [Google Scholar]
- 32.Brower GL, Levick SP, Janicki JS. Inhibition of matrix metalloproteinase activity by ACE inhibitors prevents left ventricular remodeling in a rat model of heart failure. Am J Physiol Heart Circ Physiol 2007;292:H3057–H3064 [DOI] [PubMed] [Google Scholar]
- 33.Williams RN, Parsons SL, Morris TM, Rowlands BJ, Watson SA. Inhibition of matrix metalloproteinase activity and growth of gastric adenocarcinoma cells by an angiotensin converting enzyme inhibitor in in vitro and murine models. Eur J Surg Oncol 2005;31:1042–1050 [DOI] [PubMed] [Google Scholar]
- 34.Bouloumié A, Sengenès C, Portolan G, Galitzky J, Lafontan M. Adipocyte produces matrix metalloproteinases 2 and 9: involvement in adipose differentiation. Diabetes 2001;50:2080–2086 [DOI] [PubMed] [Google Scholar]
- 35.Chen P, Yuan Y, Wang S, Zhan L, Xu J. Captopril, an angiotensin-converting enzyme inhibitor, attenuates the severity of acute pancreatitis in rats by reducing expression of matrix metalloproteinase 9. Tohoku J Exp Med 2006;209:99–107 [DOI] [PubMed] [Google Scholar]
- 36.Derosa G, Maffioli P, Ferrari I, et al. Different actions of losartan and ramipril on adipose tissue activity and vascular remodeling biomarkers in hypertensive patients. Hypertens Res 2011;34:145–151 [DOI] [PubMed] [Google Scholar]
- 37.Liang C, Wu ZG, Ding J, et al. Losartan inhibited expression of matrix metalloproteinases in rat atherosclerotic lesions and angiotensin II-stimulated macrophages. Acta Pharmacol Sin 2004;25:1426–1432 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.