Abstract
Enteroviruses of the human enterovirus B species (HEV-Bs) (e.g., coxsackie B viruses [CVBs] and echoviruses) have been implicated as environmental factors that trigger/accelerate type 1 diabetes, but the underlying mechanism remains elusive. The aim of this study was to gain insight into the cytokines and chemokines that are produced by human pancreatic islets upon infection with CVBs. To this end, we studied the response of human islets of Langerhans upon mock or CVB3 infection. Using quantitative PCR, we showed that upon CVB3 infection, transcription of interferon (IFN), IFN-stimulated genes, and inflammatory genes was induced. Analysis of secreted cytokines and chemokines by Luminex technology confirmed production and secretion of proinflammatory cytokines (e.g., interleukin [IL]-6 and tumor necrosis factor-α) as well as various chemotactic proteins, such as IFN-γ–induced protein 10, macrophage inflammatory protein (MIP)-1α, MIP-1β, and IL-8. Infection with other HEV-Bs induced similar responses, yet their extent depended on replication efficiency. Ultra violet–inactivated CVB3 did not induce any response, suggesting that virus replication is a prerequisite for antiviral responses. Our data represent the first comprehensive overview of inflammatory mediators that are secreted by human islets of Langerhans upon CVB infection and may shed light on the role of enteroviruses in type 1 diabetes pathogenesis.
Type 1 diabetes is a chronic inflammatory disorder of which the etiology is not fully understood. It is known, however, that both genetic and environmental factors are involved. Enteroviruses, particularly human enterovirus B species (HEV-Bs), such as coxsackie B viruses (CVBs) and echoviruses (EVs), are implicated as environmental factors, and a recent meta-analysis confirms that there is a clinically significant association between enterovirus infection and autoimmunity/type 1 diabetes (1). Enterovirus infections are common and usually mild, yet severe infections where virus spreads to pancreas, brain, and heart do occur. Several groups have detected HEV-Bs in the pancreatic islets of type 1 diabetic patients at autopsy, providing evidence that these viruses are able to infect β-cells in vivo (2–4). Consistently, human pancreatic islets can be infected with several different species of enteroviruses in vitro (5,6). Cytokines and chemokines that are induced upon HEV-B infection of pancreatic islets, and the resulting inflammation, may be critical during the pathogenesis of type 1 diabetes.
Microarray studies previously have been performed on CVB-infected human islets of Langerhans (7,8), yet a detailed analysis of cytokine production and secretion upon enterovirus infection in human islets is lacking. Detailed insight into the inflammatory mediators that are induced upon HEV-B infection is critical to better understand potential HEV-B–mediated type 1 diabetes pathogenesis. In this study, we set out to investigate production and secretion of a broad range of cytokines and chemokines to gain further insight into the inflammatory processes that are initiated in human islets of Langerhans upon HEV-B infection. We show that various inflammatory mediators are produced and secreted upon infection with CVBs, which may be detrimental for immune homeostasis in the islets of Langerhans and may be critical during type 1 diabetes pathogenesis.
RESEARCH DESIGN AND METHODS
Virus stocks and purification.
CVB3 Nancy (CVB3) and CVB4 Edwards2 were provided by R. Kandolf (University of Tübingen, Germany) and J.W. Yoon (University of Calgary, Canada), respectively. EV1 and CVB4 Tilo were obtained from the National Institute for Public Health and the Environment (RIVM), the Netherlands. Production of virus stocks was performed as described (6).
Human islet culture and infection.
Human pancreatic islets were isolated from deceased organ donors at the University of Pittsburgh with consent as described (6,9). Islet batches used in this study were obtained from 15 pancreatic organs, cultured in CMRL-1066 medium containing 10% FCS, 2 mmol/L l-glutamine, 100 units/mL penicillin, and 0.1 mg/mL streptomycin (Complete CMRL). After culture for 3–6 days, islets were sent to Nijmegen as free-floating islets and cultured in Complete CMRL or RPMI 1640 supplemented with 10% FCS, 2 mmol/L l-glutamine, 100 units/mL penicillin, 0.1 mg/mL streptomycin, and 1 g/L glucose (Complete RPMI) in ultra-low attachment culture plates (Corning). Islets were cultured in Nijmegen for a maximum of 2 days before start of the experiments. Four experiments were performed in Complete RPMI medium; the remaining 11 experiments were performed in Complete CMRL. Infection of human pancreatic islets was performed as described (6). Islets from one additional donor were isolated at the Leiden University Medical Center as described (10), sent to Nijmegen as free-floating islets, and cultured in Complete CMRL. Observed cytokine/chemokine inductions upon CVB3 infection were similar in Complete RPMI and Complete CMRL media; however, islets cultured in Complete RPMI showed higher basal and higher virus-induced cytokine levels compared with islets cultured in Complete CMRL (data not shown).
RNA isolation and quantitative PCR.
RNA isolation and quantitative (q)PCR were performed as described (11). In brief, RNA was isolated from islets, treated with DNase I (amplification grade; Invitrogen), and reverse transcribed into cDNA using random hexamers and Moloney murine leukemia virus reverse transcriptase (Invitrogen). To exclude genomic DNA contamination, we included an “-RT” control in which the reverse transcriptase was replaced with RNase-free water. mRNA levels for the genes of interest were determined by qPCR with a Bio-Rad CFX apparatus with SYBR Green (Applied Biosystems). Analysis was done using Bio-Rad CFX-1.6 software, and expression levels were determined relative to glyceraldehyde-3-phosphate dehydrogenase expression. mRNA expression of mock-infected islets is set to 1 to determine relative expression levels.
Cytokine detection.
Supernatant of islet cultures was harvested at indicated times postinfection (pi) and stored at −20°C. Prior to cytokine measurements, supernatants were filtered using Microcon Centrifugal filter devices with a cutoff value of 100 kDa (Millipore) to remove infectious virus. Cytokine levels were measured on a Luminex-100 System (Luminex) using a 27-plex Bio-Plex Kit (Bio-Rad Laboratories) or 22-plex Milliplex Kit (Merck Millipore). Data analysis was performed with Bio-Plex Manager software (Bio-Rad Laboratories).
Statistical analysis.
Statistical analysis was performed using paired Student t-test (two-tailed distribution). P < 0.05 was considered significantly different.
RESULTS
Inflammatory gene expression in human pancreatic islets upon enterovirus infection.
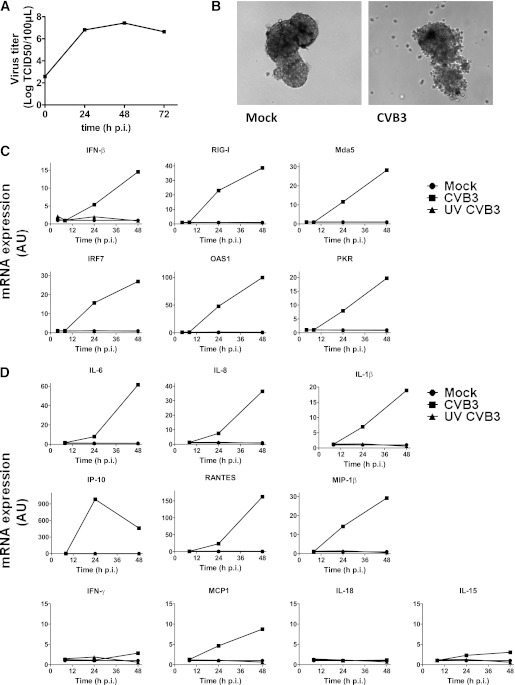
First, we infected islets with CVB3 and determined expression of interferon (IFN)-β and IFN-stimulated genes (ISGs). Replication of CVB3 was very efficient, reaching >4 logs increase of virus over time (Fig. 1A) and inducing clear cytopathic effects (Fig. 1B). IFN-β mRNA expression was induced 24 h pi and further increased over time (Fig. 1C). All ISGs tested were induced, with the highest induction observed for OAS1 at 48 h pi (Fig. 1C) in five out of six donors tested. Although ISGs were induced upon infection in all experiments, the magnitude of the response varied greatly between individual donors.
FIG. 1.
mRNA induction of antiviral genes, cytokines, and chemokines in human pancreatic islets upon CVB3 infection. A: Human islets were infected at a multiplicity of infection (MOI) of 10, and virus titers were determined by end point titration at the indicated times pi. B: Images of islets taken 48 h pi. C: Human islets were infected with CVB3 or UV-inactivated CVB3 (MOI 10) or were mock infected, and at indicated times, mRNA induction of indicated genes was determined using qPCR. D: mRNA induction of indicated genes was determined by qPCR in islets infected as in C. Data are representative examples of at least three experiments using different donors. AU, arbitrary unit.
We also examined expression of a selection of cytokines and chemokines and found that mRNA expression of several genes was induced (Fig. 1D). IFN-γ–induced protein (IP)-10 induction was most pronounced in all donors; increases of up to 1,000-fold were observed compared with mock-infected islets. As with the ISGs, variations between donors were considerable. IP-10 mRNA was maximally induced at 24 h pi, and in two of three donors, the third donor tested had equal IP-10 induction at 24 and 48 h pi. Furthermore, induction of RANTES (Regulated upon Activation Normal T-cell Expressed and Secreted), interleukin (IL)-6, IL-8, macrophage inflammatory protein (MIP)-1β, and IL-1β mRNA was detected. Other cytokine and chemokine mRNAs tested (IFN-γ, IL-15, IL-18, and monocyte chemoattractant protein [MCP]1) were not induced, were induced at low levels only, or were induced in one or two out of six donors only. No responses were observed when using ultraviolet (UV)-inactivated virus (Fig. 1C and D).
Several cytokines and chemokines are produced upon infection with CVB3.
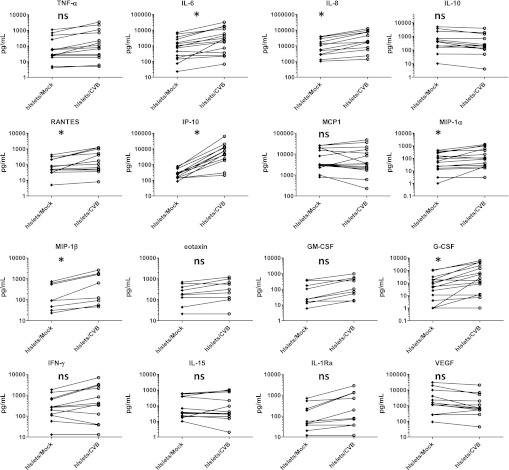
Next we investigated the magnitude of secreted cytokine and chemokine proteins in supernatant of mock- or CVB3-infected islet cultures. We studied a broad range of cytokines and chemokines using 27-plex or 22-plex arrays at 48 h pi. Mock-infected islets produced relatively high basal levels (>1 ng/mL) of IL-8. Furthermore, IL-6, MCP1, and vascular endothelial growth factor (VEGF) were substantially produced by most donors (Fig. 2). The high steady-state levels of IL-6, IL-8, and MCP1 may be partially due to stress generated during the islet isolation procedure (9), whereas VEGF may be induced by damaged epithelium to revascularize the islets.
FIG. 2.
Human islets (hIslets) produce various cytokines and chemokines upon CVB3 infection. Human islets were mock or CVB3 infected at a multiplicity of infection of 10; at 48 h pi, supernatant was harvested and analyte levels were determined as described. Data shown are from 15 different experiments using different donors. ns, not significant; *P < 0.05. Each connected set of diamonds/circles represents one donor. GM-CSF, granulocyte macrophage colony-stimulating factor.
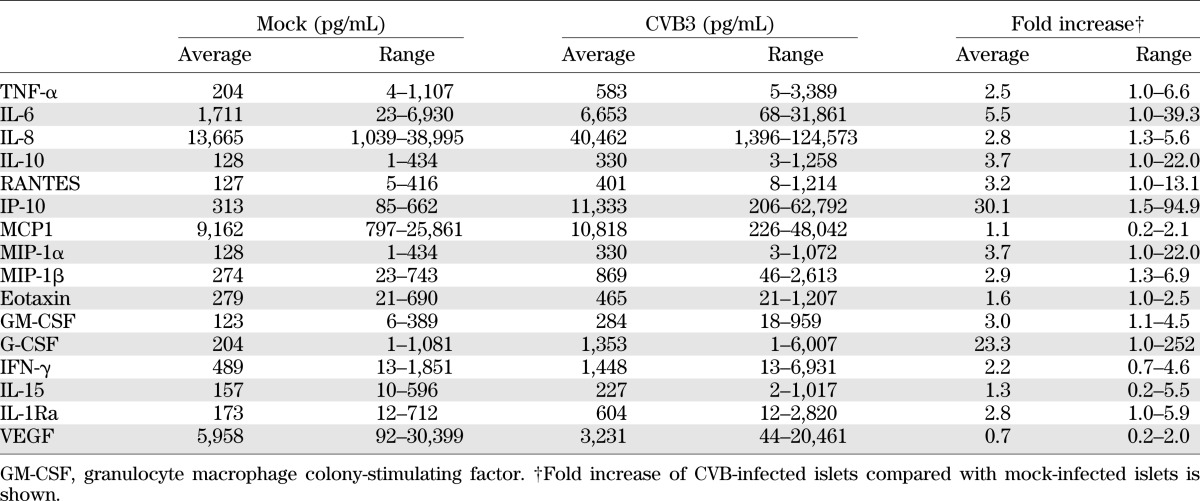
Upon CVB3 infection, the increase in IP-10 protein was most pronounced in the majority of the donors, reaching an average of ∼30-fold relative to mock infection (Table 1). These data corroborated our qPCR findings, where IP-10 was also most substantially induced. Likewise, the cytokines IL-6 and RANTES; chemokines IL-8, MIP-1α, and MIP-1β; and the growth factor granulocyte colony-stimulating factor (G-CSF) were significantly increased upon infection (Fig. 2). The observed basal cytokine expression, as well as the CVB3-induced cytokine production, varied greatly between different donors (Table 1), as observed in previous studies using human islets (12). Levels of IL-1β, IL-4, IL-5, IL-13, platelet-derived growth factor, and MCP3 remained at undetectable or very low levels (<100 pg in all donors/stimuli) and were not significantly altered upon CVB3 infection (data not shown).
TABLE 1.
Production (average and range) of cytokines and chemokines upon CVB3 infection of human islets of Langerhans (48 h pi)
Similar cytokine/chemokine production upon infection with different HEV-Bs.
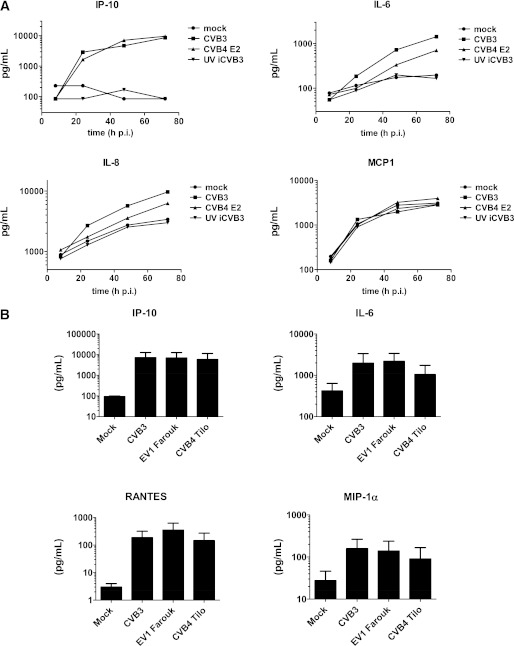
To investigate whether (major) differences exist between the response of human islets to CVB3 and other HEV-Bs, we compared secretion of a selected group of cytokines upon infection with the CVB4 E2 strain (often referred to as a diabetogenic CVB4), the CVB4 Tilo strain, and EV1. The CVB4 E2 strain showed an induction pattern similar to that of CVB3, with almost identical IP-10 kinetics and induction yet somewhat delayed IL-6 and IL-8 induction (Fig. 3A), which may reflect a small difference in titer of virus inoculums. EV1 yielded results similar to CVB3 (Fig. 3B). The response to CVB4 Tilo was somewhat less pronounced, which correlated with fewer virus-positive cells in the islets as determined by immunofluorescence, as well as with lower amounts of virus yield, compared with CVB3 and EV1. During enterovirus replication, a double-stranded RNA intermediate is formed that may trigger antiviral responses and cytokine/chemokine production. Using UV-inactivated CVB3, we showed that virus replication is required for induction of proinflammatory cytokines and chemokines (Fig. 3A).
FIG. 3.
Cytokine production upon infection with different HEV-Bs and UV-inactivated CVB3 (UV iCVB3). A: Human islets were infected with indicated viruses (multiplicity of infection 15) or mock infected. Supernatant was harvested at indicated times pi and analyte levels were determined. B: Islets were infected as in A; at 48 h pi, analytes were assessed. Shown is representative example (A) or mean + SEM (B) from two experiments using different donors.
DISCUSSION
Enterovirus infections are associated with the development of type 1 diabetes, yet the underlying mechanisms that result in β-cell destruction remain elusive. Here we report that upon CVB infection, a broad antiviral response is generated in primary human islets, which encompasses the production of a variety of IFNs, ISGs, cytokines, and chemokines. Our study shows that these cytokines and chemokines are not only expressed at the mRNA level but also translated and secreted. The observed CVB-induced responses may initiate the influx of additional immune cells that further aggravate the inflammatory processes in the pancreas that may contribute to (autoimmune-mediated) β-cell destruction and type 1 diabetes.
Previous microarray studies by Ylipaasto et al. (7) and Olsson et al. (8) reveal that upon CVB5 and CVB4 infection, respectively, many antiviral genes, as well as various cytokines and chemokines, were induced at the mRNA level. Here we confirm and expand those data. Upon CVB3 infection, highest induction was found for IP-10, consistent with earlier studies (7,12). Recent reports show that IP-10 is detected in islets of type 1 diabetic patients and that CXCR3+ T cells are found as well (13,14). This implies an important role for (locally produced) IP-10 in disease development. Mouse studies support this concept: β-cells secreting IP-10 in response to inflammation mediate the chemoattraction of T cells (15), while neutralization of IP-10 has been shown to decrease diabetes in mice (16,17).
Besides IP-10, several other proinflammatory cytokines and chemokines were produced upon infection, such as IL-8, IL-6, RANTES, MIP-1α, and MIP-1β. These can have adverse effects on islet function and may induce endoplasmic reticulum stress and β-cell death (reviewed by Eizirik et al.) (18). Furthermore, T cells, monocytes, NK cells, and other immune cells may be attracted to the site of infection and activated by the proinflammatory environment. Our findings suggest that a full-blown inflammatory response is ongoing in infected islets after CVB infection. Feedback mechanisms should restrict further inflammation when the infection resolves. However, these feedback mechanisms may be differently regulated in different individuals, and unrestrained inflammation may result in type 1 diabetes (18). The underlying mechanism for sustained inflammation in type 1 diabetes is unknown but could relate to delayed or ineffective clearance of virus infection and/or the genetic makeup of these individuals. For example, single nucleotide polymorphisms that are associated with type 1 diabetes are found in inflammatory mediators, such as RANTES, tumor necrosis factor (TNF)-α, and others (19,20). Furthermore, single nucleotide polymorphisms in Mda5, which is the viral sensor for enteroviruses, have been associated with type 1 diabetes (21,22).
A question that remains concerns which cells produce the IFN-α/β and cytokines/chemokines. Islets consist of several types of endocrine cells, endothelium, and resident immune cells, such as macrophages and dendritic cells. It has been reported that the β-cells themselves can produce cytokines (e.g., IP-10 and IFN-α) (13,14,23); thus, they may be responsible for the observed responses. To identify the cell type responsible for production of cytokines and chemokines, further investigation is required. It is notable that in vitro culture of mouse islets revealed that dendritic cells gradually disappear in cultured islets (24). Although this has not been studied for human islets, it suggests that endocrine cells may be responsible for the observed responses, since our experiments were performed in islets cultured for a minimum of 3 days before start of the experiments.
Our analysis of cytokines secreted upon infection with CVB3 largely confirmed our qPCR data. One exception was IFN-β, which was highly induced on mRNA but was not detected in the supernatant (<31 pg/mL). We did observe production of many IFN-α/β–induced genes, which suggests that secreted IFN-β may have been consumed rapidly by neighboring islet cells. Another exception was the IL-1β gene, which was induced upon CVB infection in six out of seven donors on mRNA level (48 h pi) but was low (<100 pg/mL or undetectable) in all donors when measuring secreted cytokine and did not increase upon CVB infection. Conflicting data exists in the literature regarding the question whether β-cells are capable of producing IL-1β. Arnush et al. (25) showed that macrophages are the source for IL-1β production in islets upon stimulation with TNF-α/lipopolysaccharide/IFN-γ. However, two other studies show that β-cells are capable of producing IL-1β upon stimulation with polyinosinic:polycytidylic acid/IFN-γ or high glucose, respectively (26,27). IL-1β is produced as a precursor protein that needs to be cleaved by caspase-1 to generate bioactive IL-1β. The lack of bioactive IL-1β production upon CVB infection suggests that this cleavage event may not occur in CVB-infected islets of Langerhans under the conditions tested. Another explanation could be that only very low levels of IL-1β are produced, which rapidly bind to neighboring cells and, thus, are not detected in the supernatant. Furthermore, interplay of infected islets with other cells, such as duct cells and/or immune cells attracted by inflammation, may be a source for IL-1β in vivo.
When comparing the response of islets with different HEV-B strains, we found that all HEV-Bs tested induced a similar pattern of cytokines. The amount of cytokines/chemokines induced correlated with virus yield, as well as with the amount of virus-positive cells (data not shown). This suggests that in vivo, the extent to which inflammatory mediators are produced may correlate to the severity of the infection. This hypothesis is supported by a mouse study that showed that the amount of replicating virus present in the pancreas determines whether mice develop type 1 diabetes upon CVB infection (28). Thus, all HEV-B strains may be able to induce inflammatory responses, yet the extent to which this occurs depends on virus infectious dose and replication rate.
In conclusion, we report that a broad antiviral response is generated by human pancreatic islets upon CVB infection. This response is characterized by the induction of IFN-α/β and ISGs and further encompasses the secretion of several proinflammatory cytokines and chemokines. Virus replication is a prerequisite for these responses. Our data provide new insight into the antiviral responses of human pancreatic islets and a mechanism by which enteroviruses may induce/accelerate type 1 diabetes in susceptible individuals.
ACKNOWLEDGMENTS
This work was supported by grants from the Radboud University Nijmegen Medical Center (2005-8) to J.M.G. and F.J.M.v.K. and from the Juvenile Diabetes Research Foundation (24-2008-949) to G.J.A. and F.J.M.v.K.
No potential conflicts of interest relevant to this article were reported.
B.M.S. designed and performed research, analyzed data, and wrote the manuscript. K.H.W.L. and E.D.K.-R. performed research. J.D.P., R.B., M.T., T.R.D.J.R., M.A.E., E.J.P.d.K., and J.M.G. helped coordinate the studies and reviewed and edited the manuscript. R.J.F.H. performed research and analyzed data. G.J.A. and F.J.M.v.K. designed experiments, discussed data, and reviewed and edited the manuscript. B.M.S. and F.J.M.v.K. are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Yeung WC, Rawlinson WD, Craig ME. Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies. BMJ 2011;342:d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dotta F, Censini S, van Halteren AG, et al. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc Natl Acad Sci U S A 2007;104:5115–5120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ylipaasto P, Klingel K, Lindberg AM, et al. Enterovirus infection in human pancreatic islet cells, islet tropism in vivo and receptor involvement in cultured islet beta cells. Diabetologia 2004;47:225–239 [DOI] [PubMed] [Google Scholar]
- 4.Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia 2009;52:1143–1151 [DOI] [PubMed] [Google Scholar]
- 5.Roivainen M, Ylipaasto P, Savolainen C, Galama J, Hovi T, Otonkoski T. Functional impairment and killing of human beta cells by enteroviruses: the capacity is shared by a wide range of serotypes, but the extent is a characteristic of individual virus strains. Diabetologia 2002;45:693–702 [DOI] [PubMed] [Google Scholar]
- 6.Schulte BM, Kramer M, Ansems M, et al. Phagocytosis of enterovirus-infected pancreatic beta-cells triggers innate immune responses in human dendritic cells. Diabetes 2010;59:1182–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ylipaasto P, Kutlu B, Rasilainen S, et al. Global profiling of coxsackievirus- and cytokine-induced gene expression in human pancreatic islets. Diabetologia 2005;48:1510–1522 [DOI] [PubMed] [Google Scholar]
- 8.Olsson A, Johansson U, Korsgren O, Frisk G. Inflammatory gene expression in Coxsackievirus B-4-infected human islets of Langerhans. Biochem Biophys Res Commun 2005;330:571–576 [DOI] [PubMed] [Google Scholar]
- 9.Bottino R, Balamurugan AN, Tse H, et al. Response of human islets to isolation stress and the effect of antioxidant treatment. Diabetes 2004;53:2559–2568 [DOI] [PubMed] [Google Scholar]
- 10.Smelt MJ, Faas MM, de Haan BJ, et al. Susceptibility of human pancreatic β cells for cytomegalovirus infection and the effects on cellular immunogenicity. Pancreas 2012;41:39–49 [DOI] [PubMed] [Google Scholar]
- 11.Kramer M, Schulte BM, Toonen LW, et al. Phagocytosis of picornavirus-infected cells induces an RNA-dependent antiviral state in human dendritic cells. J Virol 2008;82:2930–2937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skog O, Korsgren O, Frisk G. Modulation of innate immunity in human pancreatic islets infected with enterovirus in vitro. J Med Virol 2011;83:658–664 [DOI] [PubMed] [Google Scholar]
- 13.Uno S, Imagawa A, Saisho K, et al. Expression of chemokines, CXC chemokine ligand 10 (CXCL10) and CXCR3 in the inflamed islets of patients with recent-onset autoimmune type 1 diabetes. Endocr J 2010;57:991–996 [DOI] [PubMed] [Google Scholar]
- 14.Tanaka S, Nishida Y, Aida K, et al. Enterovirus infection, CXC chemokine ligand 10 (CXCL10), and CXCR3 circuit: a mechanism of accelerated beta-cell failure in fulminant type 1 diabetes. Diabetes 2009;58:2285–2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frigerio S, Junt T, Lu B, et al. Beta cells are responsible for CXCR3-mediated T-cell infiltration in insulitis. Nat Med 2002;8:1414–1420 [DOI] [PubMed] [Google Scholar]
- 16.Morimoto J, Yoneyama H, Shimada A, et al. CXC chemokine ligand 10 neutralization suppresses the occurrence of diabetes in nonobese diabetic mice through enhanced beta cell proliferation without affecting insulitis. J Immunol 2004;173:7017–7024 [DOI] [PubMed] [Google Scholar]
- 17.Christen U, McGavern DB, Luster AD, von Herrath MG, Oldstone MB. Among CXCR3 chemokines, IFN-gamma-inducible protein of 10 kDa (CXC chemokine ligand (CXCL) 10) but not monokine induced by IFN-gamma (CXCL9) imprints a pattern for the subsequent development of autoimmune disease. J Immunol 2003;171:6838–6845 [DOI] [PubMed] [Google Scholar]
- 18.Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat Rev Endocrinol 2009;5:219–226 [DOI] [PubMed] [Google Scholar]
- 19.Zhernakova A, Alizadeh BZ, Eerligh P, et al. Genetic variants of RANTES are associated with serum RANTES level and protection for type 1 diabetes. Genes Immun 2006;7:544–549 [DOI] [PubMed] [Google Scholar]
- 20.Settin A, Ismail A, El-Magd MA, El-Baz R, Kazamel A. Gene polymorphisms of TNF-alpha-308 (G/A), IL-10(-1082) (G/A), IL-6(-174) (G/C) and IL-1Ra (VNTR) in Egyptian cases with type 1 diabetes mellitus. Autoimmunity 2009;42:50–55 [DOI] [PubMed] [Google Scholar]
- 21.Smyth DJ, Cooper JD, Bailey R, et al. A genome-wide association study of nonsynonymous SNPs identifies a type 1 diabetes locus in the interferon-induced helicase (IFIH1) region. Nat Genet 2006;38:617–619 [DOI] [PubMed] [Google Scholar]
- 22.Liu S, Wang H, Jin Y, et al. IFIH1 polymorphisms are significantly associated with type 1 diabetes and IFIH1 gene expression in peripheral blood mononuclear cells. Hum Mol Genet 2009;18:358–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foulis AK, Farquharson MA, Meager A. Immunoreactive alpha-interferon in insulin-secreting beta cells in type 1 diabetes mellitus. Lancet 1987;2:1423–1427 [DOI] [PubMed] [Google Scholar]
- 24.Rutzky LP, Bilinski S, Kloc M, et al. Microgravity culture condition reduces immunogenicity and improves function of pancreatic islets1. Transplantation 2002;74:13–21 [DOI] [PubMed] [Google Scholar]
- 25.Arnush M, Heitmeier MR, Scarim AL, Marino MH, Manning PT, Corbett JA. IL-1 produced and released endogenously within human islets inhibits beta cell function. J Clin Invest 1998;102:516–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heitmeier MR, Arnush M, Scarim AL, Corbett JA. Pancreatic beta-cell damage mediated by beta-cell production of interleukin-1. A novel mechanism for virus-induced diabetes. J Biol Chem 2001;276:11151–11158 [DOI] [PubMed] [Google Scholar]
- 27.Maedler K, Sergeev P, Ris F, et al. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest 2002;110:851–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanno T, Kim K, Kono K, Drescher KM, Chapman NM, Tracy S. Group B coxsackievirus diabetogenic phenotype correlates with replication efficiency. J Virol 2006;80:5637–5643 [DOI] [PMC free article] [PubMed] [Google Scholar]