Abstract
Endothelial dysfunction can develop at an early age in children with risk factors for cardiovascular disease. A clear understanding of the nature of this dysfunction and how it can worsen over time requires detailed information on the normal growth-related changes in endothelial function on which the pathological changes are superimposed. This review summarizes our current understanding of these normal changes, as derived from studies in four different mammalian species. Although the endothelium plays an important role in controlling vascular tone from birth onward, the vasoactive molecules that mediate this control often change during postnatal or juvenile growth. The specifics of this transition to an adult endothelial cell phenotype can vary depending on the vascular bed. During growth, the contribution of nitric oxide to endothelium-dependent dilation generally increases in the lung, cerebral cortex and skeletal muscle, but decreases in the intestine. Endothelial capacity for release of other vasoactive factors (e.g., cyclooxygenase products, hydrogen peroxide, carbon monoxide) can also increase or decrease during growth. Although these changes have been well documented, there is less information on their underlying cellular or molecular events. Further research is required to clarify these mechanisms, and to evaluate the functional significance of such shifts in endothelial phenotype.
Keywords: postnatal growth, maturation, vascular function, endothelium
INTRODUCTION
Over the past few decades, the incidence of cardiovascular disease in children has been increasing due to rising obesity rates and other risk factors [25,90]. The vascular deficits that develop in these children often involve severe endothelial dysfunction [1,30,39,89,94]. Numerous other conditions can lead to endothelial dysfunction in the newborn [65,91]. To clearly define the mechanisms underlying the progression of this dysfunction, it is first necessary to understand the changes in endothelial phenotype that normally take place soon after birth and during subsequent growth. The rapid postnatal enlargement of many organs is accompanied by extensive growth of their arteriolar, capillary and venular networks [54,83,86,95,98] as well as increases in microvascular wall mass [16,98]. These structural changes are associated with progressive increases in microvascular pressure and network blood flow, often with an attendant increase or decrease in hemodynamic shear stress [55,56,98,110] (Figure 1). Because such alterations in the local environment of endothelial cells can modify many aspects of their function [27,46,84], it is not surprising that growth is accompanied by marked changes in the endothelium-dependent regulation of arteriolar diameter and blood flow. In some vascular beds, a maturational shift in tissue metabolic requirements may also contribute to changes in local flow regulation over this period [42,67].
Figure 1.
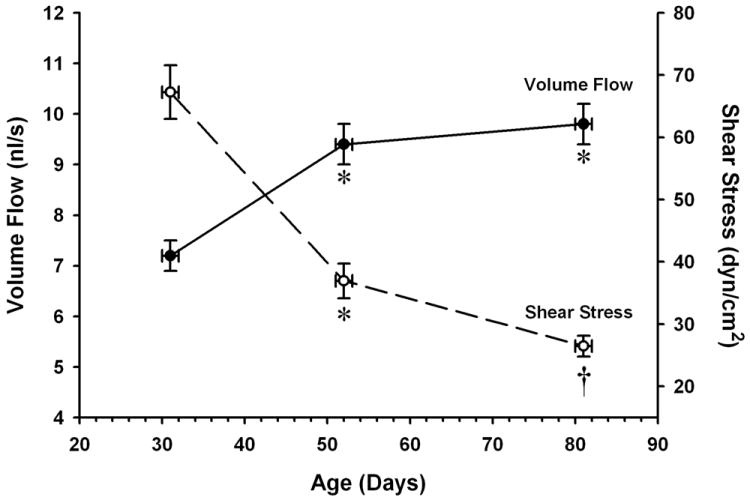
Effect of age on volume flow and wall shear stress in proximal arterioles of the rat spinotrapezius muscle. Wall shear stress is calculated using in vivo measurements of vessel diameters and flow velocities, assuming a parabolic flow profile and an average blood viscosity of 3.5 centipoise. Values are means ± SEM. *p <0.05 vs. 31-day value. † p < 0.05 vs. 52-day value. Data from Linderman and Boegehold, 1999 (Ref. 56).
Differences in endothelium-dependent dilation between young and adult animals were first demonstrated in large arteries [24,43,44], but more information has since become available on growth-related changes in smaller resistance arteries and microvessels. The intent of this review is to summarize the current state of our knowledge of changes in endothelial function that occur in both arteries and resistance vessels during rapid postnatal or juvenile growth, and where possible, to address the impact of these changes on local blood flow control. To illustrate the consequences of deviation from these normal growth-related changes in humans, the role of endothelial dysfunction in the pathogenesis of newborn pulmonary hypertension and in necrotizing colitis is also briefly explored.
To date, most of the information on changes in endothelial function during growth has come from studies on the pulmonary, intestinal, cerebral and skeletal muscle vascular beds in different species.
PULMONARY CIRCULATION
At birth, the transition of gas exchange from the placenta to the lungs requires a large decrease in pulmonary vascular resistance and an 8- to10-fold increase in pulmonary blood flow [38,85]. These changes are due to a number of physical and chemical stimuli that interact in a complex and incompletely understood manner. In addition to an increase in arterial blood oxygen content, which directly relaxes pulmonary vascular smooth muscle and can also stimulate endothelial nitric oxide (NO) release [22], pulmonary vasodilation at birth is partially due to vasoactive molecules released in response to lung expansion, such as prostacyclin (PGI2) and mast cell-derived prostaglandin D2 (PGD2) or histamine [22,47]. Some of these factors contribute not only to the initial fall in pulmonary vascular resistance at birth, but also to the maintenance of this lower resistance over the following days and weeks. If pulmonary vascular resistance is not appropriately reduced throughout this early postnatal period, this will give rise to persistent pulmonary hypertension of the newborn (PPHN), a condition associated with significant morbidity and mortality [91] (see below). Consequently, there has been much interest in the mechanisms that underlie pulmonary vascular control during the first 2-3 weeks of extrauterine life. Studies to clarify these mechanisms have been carried out in least three different species.
In the pig, pulmonary arteries do not dilate when challenged with endothelium-dependent agonists immediately after birth, but then begin to exhibit endothelium-dependent dilation within 2-3 days [4,5,14,57], with the magnitude of these responses generally increasing over the next 7-10 days [108] (Figure 2). This is partly attributable to a progressive increase in vascular smooth muscle responsiveness to NO, as judged by relaxation to NO donors such as sodium nitroprusside (SNP) [57,60,76,104,108], which in turn correlates with increased expression of soluble guanylyl cyclase (sGC) [60]. However, a recent study also suggests that NO production may actually be absent in these vessels at birth, with endothelial capacity for basal and stimulated NO release emerging in the first postnatal week largely because eNOS begins to interact with heat shock protein 90 (Hsp90), a key chaperone protein that facilitates eNOS phosphorylation and activation [5].
Figure 2.
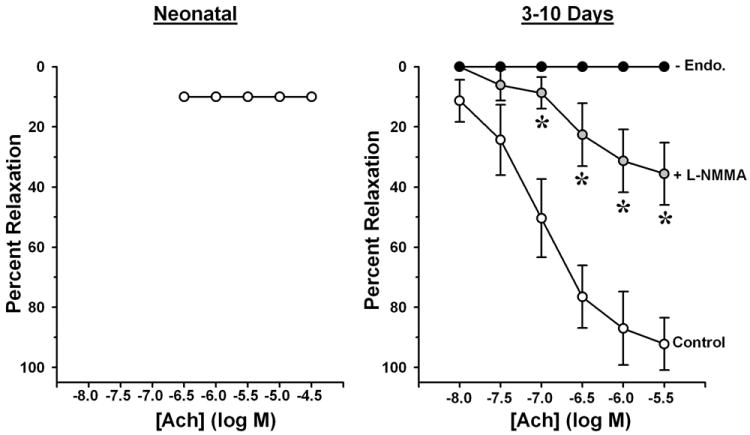
Effects of NG-monomethyl L-arginine (L-NMMA) and endothelial removal (-Endo.) on acetylcholine (ACh)-induced relaxation of pulmonary artery rings from pigs aged 5 min.-2 hr. (Neonatal) and 3-10 days. Rings were pre-contracted with prostaglandin F2α. Values are means ± SEM. *p <0.05, L-NMMA vs. Control. Redrawn from Liu et al., 1992 (Ref. 57).
In sheep, acetylcholine (ACh) triggers more cGMP production and endothelium-dependent relaxation in pulmonary arteries from adults than in those from newborns, and as in the pig, at least part of this difference is due to greater smooth muscle sGC expression in the adults [45]. Steinhorn et al. [92] found no change in the responsiveness of pulmonary artery smooth muscle to NO between birth and 5-6 weeks of age (juvenile sheep), suggesting that any increase in smooth muscle sGC expression or activity, and therefore in the overall influence of NO on vascular tone, must occur between 6 weeks of age and adulthood. Findings from this latter study also indicate that the dilation of these vessels to ACh is mediated entirely by endothelial prostaglandin release in newborns, with NO assuming an equal role in this dilation by 5-6 weeks. However, there are contradictory findings from other laboratories. O’Donnell et al. [72] reported that treatment with an L-arginine analog attenuates endothelium-dependent relaxation to ACh and bradykinin (BK) in pulmonary arteries from newborn (2 day-old) sheep but not in those from 1-month-old sheep, suggesting that the contribution of NO to these responses declines with age. Furthermore, in contrast to the studies cited above, these investigators found less relaxation to SNP in vessels from1-month-old sheep than in those from newborns [72].
In the rat, the expression of eNOS in pulmonary resistance vessels does not change between 7 and 14 days of age, but over this period there is a marked increase in the modulating effect of NO on the constriction of these vessels [18]. This change is attributable to both an increase in vascular NO production (via increased eNOS activity) and an increase in vascular smooth muscle responsiveness to NO (via increased sGC expression).
Taken in aggregate, the above findings offer some insight into how the endothelium-dependent control of pulmonary vascular tone can change during postnatal growth. Although not a consensus, the majority of studies suggest that across different species, the days and weeks immediately following birth are characterized by a general increase in the influence of NO on vascular tone due to an increase in smooth muscle responsiveness to NO, often coupled with an increase in endothelial NO production.
Persistent pulmonary hypertension of the newborn
Severe PPHN occurs in approximately 0.2% of live-born term infants [91]. This condition is multifactorial in origin, but there is evidence that disruption of the endothelial NO-cGMP pathway contributes to the pulmonary vasoconstriction that underlies PPHN in some cases [91,106]. Reduced expression and activity of eNOS has been found in pulmonary arteries of lambs with experimentally-induced PPHN [88], and eNOS expression is reduced in umbilical vein endothelial cells from infants with PPHN [96]. Reduced NO availability in these infants may also result from the postnatal persistence of relatively high circulating levels of the endogenous NOS inhibitor asymmetric dimethylarginine (ADMA). These levels are relatively high in the fetal blood, but then normally drop to undetectable levels soon after birth [106]. However, abnormally high levels of ADMA have been found in the urine of infants with PPHN [75]. A relative deficiency of the NOS substrate L-arginine may also occur with PPHN [97].
Exogenous NO inhalation has been studied as a standard therapy for infants with PPHN, and has proved beneficial by increasing oxygenation in some infants [19,68]. Because there is also evidence that reduced expression and activity of vascular smooth muscle sGC can contribute to PPHN, compounds that inhibit cGMP-metabolizing phosphodiesterases are also being explored as possible treatments [10]
INTESTINAL CIRCULATION
Like the lungs, the intestine is for the most part functionally dormant during fetal life. Birth is marked by a transition from reliance on the placenta to reliance on the gastrointestinal tract for nutrition. This leads to a large increase in intestinal oxygen uptake, presumably reflecting the increase in metabolic activity required to support postprandial nutrient absorption [15]. Not surprisingly, this transition is also accompanied by a marked decrease in intestinal vascular resistance and an increase in tissue perfusion [64,79].
Although there are profound increases in both pulmonary and intestinal blood flow at birth, the changes in intestinal vascular control that take place over the ensuing days and weeks are notably different from those in the pulmonary circulation. More specifically, studies conducted in pig intestine indicate that the role of endothelial NO in regulation of vascular tone declines during postnatal growth. Intestinal vascular resistance is at a minimum between 3 and 12 days of age, then progressively increases until it reaches a higher plateau at around 30 days of age (after weaning) [64]. The relatively low resistance in the 3 day-old pigs is due in part to a large influence of basally released NO on resting vascular tone, with this influence becoming negligible by 35 days of age [63]. Consistent with this observation, increased shear stress stimulates endothelial NO release and completely overrides the myogenic constriction of mesenteric resistance vessels from 3 day-old pigs, but does not stimulate NO release and exerts no modulating effect on the diameter of vessels from 35 day-old pigs [63,78,79] (Figure 3). Reber et al. [79] have estimated that under basal flow conditions, the rate of constitutive NO production is approximately 3-fold greater in the mesenteric resistance vessels of the younger animals than in the older animals. The endothelium-dependent agonists ACh and Substance P also elicit greater NO-mediated dilation in the younger vessels than in the older vessels [63], suggesting that the general capacity for endothelial NO release is greater in the younger animals. This conclusion is supported by subsequent findings that expression of eNOS protein in the vascular wall is significantly greater in 1- and 3-day-old animals than in 30-day-old animals [80].
Figure 3.
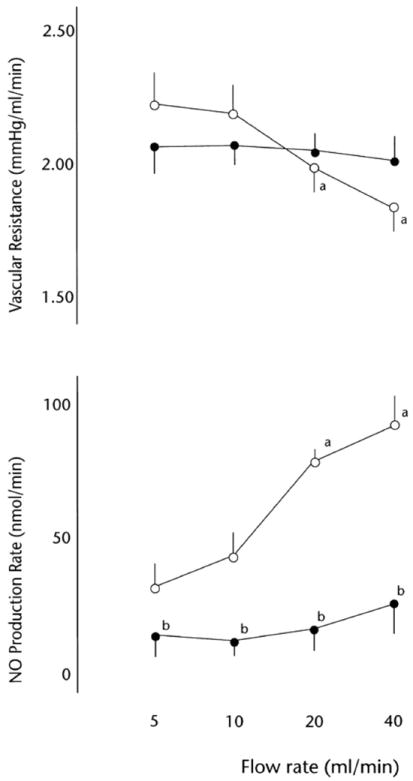
Relationship between flow rate, nitric oxide (NO) production rate and vascular resistance in the pump-perfused mesenteric vascular bed from pigs at age 3 days (○) and 35 days (●). Values are means ± SD; n = 5. a P < 0.01 vs. 5 ml/min flow rate; b P < 0.01 vs. 3-day-old group. From Reber et al., 2001 (Ref. 79), used with permission.
Necrotizing enterocolitis
The vasoconstrictor peptide endothelin-1 (ET-1) also exerts a major influence in the intestinal circulation of newborns, such that a dynamic balance between the effects of ET-1 and those of NO exists at this early stage [65]. In premature human infants, a shift in this balance away from NO and towards ET-1 contributes to the ischemic injury associated with necrotizing enterocolitis (NEC) [69,70]. This shift may be due to an increase in ET-1 expression coupled with decreased NO production due to low L-arginine levels and/or increased ADMA levels [12,69,82,107]. Administration of L-arginine to premature infants has been found to decreases the incidence of NEC [2, 87], and ET-1 receptor antagonists may also prove to be useful therapy for NEC [69].
CEREBRAL CIRCULATION
In the pig, the dilation of cerebral arterioles to a variety of stimuli (including BK) and hypercapnia) is largely mediated by the release of endothelial prostanoids in newborns (age 1-3 days), with endothelial NO assuming a progressively greater role in these responses during subsequent growth to the juvenile stage (age 3-4 months) [3,102,103,109] (Figure 4). This is consistent with a report that cerebral microvessels display a 2-3 fold increase in both the expression and activity of eNOS over this period, with no corresponding increase in the expression or activity of cyclooxygenase (COX) [73]. In newborn pigs, carbon monoxide (CO) generated in the endothelium and/or vascular smooth muscle also plays an important role in regulating cerebral arteriolar diameter. This CO has been found to modulate constrictor responses to hypocapnia and hypertension [105], and to contribute to the dilation induced by hypoxia, excitatory amino acids, and a reduction in cerebral perfusion pressure [23,41,50]. Endothelium-derived NO and PGI2 can play permissive roles in these responses by activating intracellular pathways that allow CO to increase smooth muscle KCa channel activity [11,48]. Recent findings suggest this influence of CO may also change during growth; juvenile maturation in the pig is accompanied by a reduction in arteriolar smooth muscle responsiveness to CO and loss of the permissive effect of endogenous NO on CO-induced dilation [37].
Figure 4.
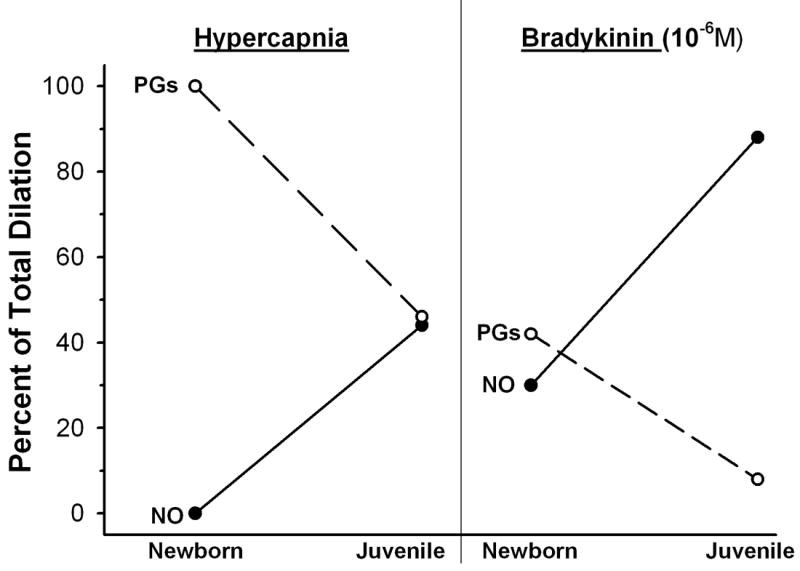
Percent of dilator responses to hypercapnia and bradykinin attributable to nitric oxide (NO) and prostaglandins (PGs) in cerebral arterioles of pigs at age 1-3 days (Newborn) and 3-5 months (Juvenile). Derived from data reported by Willis and Leffler, 1999 (Ref. 102) and Willis and Leffler, 2001 (Ref. 103).
In contrast to the pig, the endothelium-dependent dilation of cerebral arteries in newborn (age 0-7 days) sheep is mediated almost exclusively by NO, not COX metabolites [100,101]. However, the capacity of these vessels for NO release continues to increase during growth, despite a modest decrease in eNOS expression [101]. This could be explained by a growth-related increase in eNOS activity, as has been suggested for the sheep carotid artery based on responses to shear stress [99]. There is some evidence that differences in the contribution of NO to endothelium-dependent dilation in newborns between these studies and those discussed above are related to species differences (pig vs. sheep) rather than differences between cerebral arterioles (in pig) and the larger upstream arteries (in sheep). For example, the influence of endothelial NO on myogenic tone and vascular wall Ca2+ levels in smaller (~ 150 μm) cerebral arteries is greater in near-term fetal sheep than in adult sheep [29].
In cerebral microvessels of the mouse, the modulation of myogenic tone by endogenous NO and vasodilator prostanoids is almost non-existent at 4-8 days of age but considerably greater at 42-56 days of age [28], although the mechanisms that underlie these changes have not been explored.
The above findings indicate that the extent to which NO contributes to the endothelium-dependent dilation of cerebral vessels soon after birth can vary widely depending on the species. However, regardless of whether this early contribution is large or small, there is in all species a further increase in the size of this NO component during subsequent growth due to an increase in either eNOS expression or activity.
SKELETAL MUSCLE CIRCULATION
Measurements in rat spinotrapezius muscle indicate that between 31 days of age (1 wk post weaning) and 52 days of age (early adulthood) there is a 25% increase in total network blood flow but more than a doubling of muscle mass, such that blood flow per gram of tissue decreases by approximately 40% over this period [56]. This is consistent with evidence that tissue metabolic demand peaks in early life, but then begins to decline as juvenile growth continues [42,67]. There is little or no change in the resting tone of spinotrapezius muscle arterioles over this period [55,56], which agrees with findings in the cremaster muscle of growing hamsters and rats [77,98]. However, the capacity for active adjustments in arteriolar tone, and the specific mechanisms responsible for these adjustments, can change profoundly during juvenile growth.
Arterioles from the gracilis muscle of 25-28 day-old rats exhibit robust endothelium-dependent dilation to receptor-dependent agonists (ACh, simvastatin, VEGF) as well as the Ca2+ ionophore A23187 [6,7,9]. However, these vessels lack the general capacity for endothelial NO production, as judged by the ineffectiveness of the NOS inhibitor Nω-nitro-L-arginine methyl ester (L-NAME) in reducing the responses [6]. In contrast, L-NAME greatly reduces the responses in vessels from 42-46 day-old rats, suggesting there is a large contribution of NO to the dilation by this age [6] (Figure 5). We have verified this age-related difference in capacity for NO production through direct measurements of NO with polarographic sensors [40]. In that study, we also found no difference between age groups in arteriolar wall eNOS expression, implying that there must be a change in the regulation of eNOS activity sometime between 28 and 42 days of age. The details of this transition are not yet clear, but our earlier findings in rat spinotrapezius muscle suggest this is a complex process. In those studies, ACh or A23187 could stimulate arteriolar NO release at 32 days of age, but other stimuli (shear stress, VEGF, simvastatin) could not stimulate NO release until approximately 1 week later [56,71]. We have found no differences between 25-28 and 42-46 day-old rats in arteriolar wall tetrahydrobiopterin (BH4) or L-arginine (L-Arg) levels, ruling out the possibility of an age-related increase in the availability of eNOS substrate or cofactors [40]. There is little information on how other determinants of eNOS activity may change over this period, but among the many possibilities are a change in the interaction of eNOS with key regulatory proteins (e.g., Hsp 90 [5]) or in the pattern of eNOS phosphorylation [61]. Another interesting possibility is that circulating levels of the endogenous NOS inhibitor asymmetric dimethylarginine (ADMA) could be relatively high at an early age and then drop during subsequent growth, as recently reported in humans [33,59].
Figure 5.
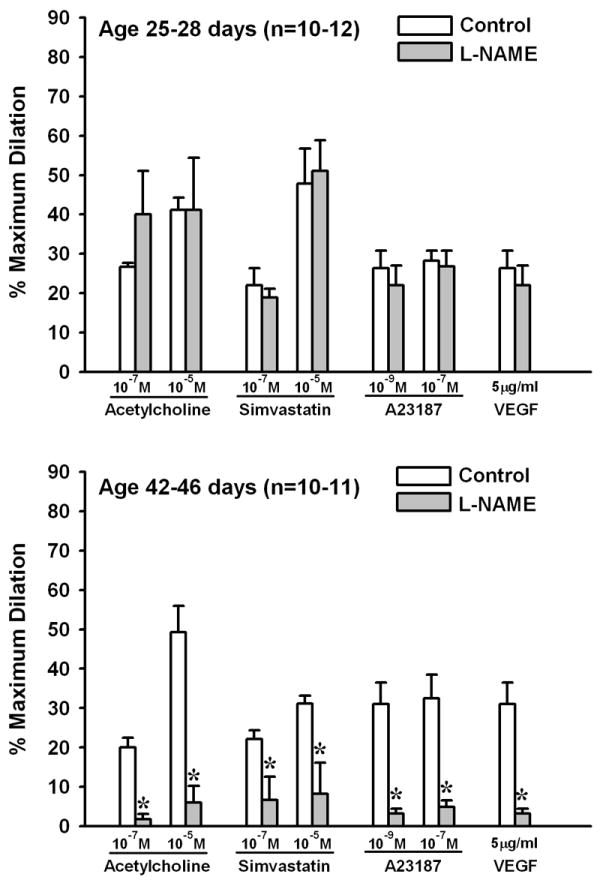
Endothelium-dependent dilation of rat gracilis muscle arterioles in response to receptor-dependent and -independent agonists before and then after exposure to Nω-nitro-L-arginine methyl ester (L-NAME). Top panel: arterioles from 25-28 day-old rats. Bottom panel: arterioles from 42-46 day-old rats. Values are means ± SEM. n = number of vessels. *p <0.05 vs. Control. Data derived from Balch-Samora et al., 2007 (Ref. 6).
In rat gracilis muscle arterioles, the change in endothelial function between 28 and 42 days of age encompasses more than simply a change in NO production. For example, the COX inhibitor indomethacin has no effect on dilation to A23187, VEGF or simvastatin in 25-28 day-old arterioles, but greatly reduces these responses in 42-46 day-old arterioles [6]. This suggests that COX metabolites initially do not contribute to endothelium-dependent dilation, but then emerge as important contributors to these responses over the same period as NO. We also found no evidence for a contribution of hydrogen peroxide (H2O2) to endothelium-dependent dilation of the younger arterioles. However, as with NO and COX metabolites, a role for H2O2 in mediating these responses emerges by 42-46 days of age [7].
After ruling out NO, prostanoids and H2O2 as possible mediators of endothelium-dependent dilation in the arterioles of young rats, we did find that these responses were reduced by the heme oxygenase inhibitor chromium (III) mesoporphyrin IX, implicating CO as an important endothelial vasoactive factor in the younger vessels [9]. This is similar to the above-mentioned findings in the cerebral circulation of young pigs [23,41,50]. It is clear that other endothelium-derived molecules also contribute to regulation of arteriolar tone in young animals, but these have not yet been identified.
The above findings indicate that during juvenile growth, the endothelium of skeletal muscle arterioles develops the capacity for generation of NO, prostanoids and H2O2 in response to various stimuli. This transition of the endothelium to an adult phenotype occurs over a relatively brief time span (2 weeks), and may have important functional consequences (see below). The mechanism(s) that underlie this transition are presently unclear.
IMPACT OF GROWTH-RELATED CHANGES IN ENDOTHELIAL FUNCTION
As mentioned above, endothelium-derived PGI2 and/or NO contribute importantly to the decreases in pulmonary and intestinal vascular resistance that occur at or immediately following birth, as well as the maintenance of low resistance in these organs during the first few weeks of extrauterine life. Because the specific changes in endothelial phenotype that accompany growth can vary widely from one vascular bed to another, it is likely that there is also some variability in the functional impact of these changes among vascular beds that do not undergo a large perinatal flow increase. Insight into such variability can be gained from in vivo studies in which the integrated behavior of arterioles is assessed at different time points during rapid growth. To date, most of these studies have been focused on the microcirculation in skeletal muscle.
In addition to the negligible amount of agonist-induced NO release in skeletal muscle arterioles from young rats in vitro [6,40], there also appears to be no basal NO release from these vessels in vivo. In rat spinotrapezius muscle, the NOS inhibitor NG-monomethyl L-arginine (L-NMMA) has no effect on resting arteriolar diameters or network blood flow at 31-32 days of age but causes arteriolar constriction and reduced blood flow at 52-55 days of age [55,56,71]. The responsiveness of arteriolar smooth muscle to NO does not change over this period [56], implicating an absence of endothelial NO release as the reason for the lack of effect of L-NMMA on arterioles in young rats. Since flow-related shear stress is the main stimulus for basal NO release in these vessels [26], this is consistent with a report that the arteriolar endothelium is insensitive to shear stress at this age [56]. Basally released NO normally serves to temper arteriolar responses to increased sympathetic nerve activity in a number of vascular beds [34,66]. We have confirmed that this moderating effect of NO is present in spinotrapezius muscle arterioles at 52-55 days of age but not at 31-32 days of age [55]. Therefore, the skeletal muscle microcirculation in young rats lacks this potential mechanism for preventing an excessive reduction in local blood flow during periods of elevated sympathoadrenal activity, such as might occur with hemorrhage or stress.
Because NO can contribute importantly to arteriolar dilation and active hyperemia in contracting muscles of adult rats [21,36,62], low arteriolar NO production in skeletal muscle of young rats could also lead to less effective coupling between tissue metabolism and blood flow in these animals. Consistent with this possibility are findings that the magnitude of vascular smooth muscle relaxation during muscle contraction is reduced in mice with targeted disruption of the eNOS gene (eNOS-/- mice) [32]. If this scenario is correct, then the emergence of NO as an important endothelial factor during growth would improve the overall capacity of the resistance vasculature for increasing blood flow and oxygen delivery during periods of increased metabolic demand. Alternatively, this capacity may not be compromised in young animals if the actions of some other locally-produced vasodilator(s) are sufficient to ensure appropriate arteriolar dilation during contraction in the absence of NO release. This possibility is consistent with a report that oxygen delivery in the contracting cremaster muscle of young hamsters is actually superior to that in adult hamsters, although this could simply reflect shorter intercapillary distances in the young animals [77]. However, another study in this muscle indicates that despite a progressive reduction in some key determinants of oxygen delivery (capillary erythrocyte content and erythrocyte flux), the overall capacity for microvascular oxygen delivery remains constant during maturation, possibly due to a greater capacity for network recruitment [13]. Additional studies are clearly needed to rigorously investigate the impact of growth-related changes in arteriolar NO production on active hyperemia in skeletal muscle.
GROWTH-RELATED CHANGES IN VASCULAR SMOOTH MUSCLE FUNCTION
For any vascular bed, the physiological impact of growth-related changes in endothelial function can be difficult to predict because these changes are sometimes superimposed on changes in vascular smooth muscle as well. As with the endothelium, these changes can be highly species- or organ-specific. For example, juvenile growth in the rat is accompanied by an increase in the myogenic responsiveness of skeletal muscle arterioles [8], but a decrease in the myogenic responsiveness of cerebral arteries (due to a reduction in stretch-induced Ca2+ influx across the smooth muscle membrane) [17]. In the pig, the myogenic behavior of mesenteric resistance vessels is greatest at 1 day of age, declines between 3 and 10 days of age, and is completely absent by 35 days of age [64,78,93]. This eventual loss of myogenic activity may be related to a progressive decrease in the contribution of the α and ε isoforms of protein kinase C to myogenic signaling [93].
During myogenic constriction, activation of smooth muscle Ca2+-activated K+ (KCa) channels and membrane hyperpolarization represents an important negative feedback mechanism for modulating myogenic behavior [35]. In newborn rat and pig, this mechanism is sometimes absent in cerebral arteries due to a low frequency of the Ca2+ sparks that normally activate KCa channels [30] or an insensitivity of these channels to Ca2+ sparks [51]. However, in sheep cerebral arteries, KCa channel activity is greater at birth than in adults due to a shift in the pattern of channel phosphorylation, leading to a lower Ca2+ setpoint [52,53,58].
Other growth-related changes in K+ channel activity can also occur in vascular smooth muscle. In small cerebral arteries of sheep (125-200 μm i.d.), ATP-sensitive (KATP) channels are less sensitive to activation in newborns than in adults [74]. The responsiveness of sheep pulmonary arteries to acute hypoxia increases with maturation due to an increase in the activity of voltage-gated K+ (Kv) channels in the vascular smooth muscle membrane [20]. Furthermore, the predominant K+ channel for regulation of resting membrane potential in these arteries apparently changes from the Kv channel to the KCa channel during postnatal growth [81].
CONCLUSION
Studies on vessels from different vascular beds have provided us with some understanding of the extent to which endothelium-dependent control of vascular tone can change during postnatal or juvenile growth. These changes often involve a shift in the relative influence of different vasoactive molecules produced by the endothelium in response to physical and chemical stimuli. With respect to NO, findings from different species indicate that this molecule becomes increasingly more important as a contributor to endothelium-dependent dilation in the pulmonary, cerebral and skeletal muscle vascular beds. In contrast, the contribution of NO to endothelium-dependent dilation decreases with growth in the intestinal vascular bed. Other endothelial factors that become either more or less important for vascular control during growth include COX metabolites, H2O2 and CO. Further investigation of the cellular and molecular underpinnings of these changes is necessary to provide true mechanistic insight into this transition of the endothelium from an immature to an adult phenotype. By serving as a point of reference, this basic information will allow for a more precise identification of the abnormalities in endothelial phenotype that can arise in the early stages of cardiovascular disease.
Acknowledgments
This work was funded by grants from the American Heart Association (# 0755264B and 09GRNT2250298) and the National Institutes of Health (HL44012).
References
- 1.Aggoun Y, Farpour-Lambert NJ, Marchand LM, Golay E, Maggio AB, Beghetti M. Impaired endothelial and smooth muscle functions and arterial stiffness appear before puberty in obese children and are associated with elevated ambulatory blood pressure. Eur Heart J. 2008;29:792–799. doi: 10.1093/eurheartj/ehm633. [DOI] [PubMed] [Google Scholar]
- 2.Amin HJ, Zamora SA, McMillan DD, Fick GH, Butzner JD, Parsons HG, Scott RB. Arginine supplementation prevents necrotizing enterocolitis in the premature infant. J Pediatr. 2002;140:425–431. doi: 10.1067/mpd.2002.123289. [DOI] [PubMed] [Google Scholar]
- 3.Armstead WM, Zuckerman SL, Shibata M, Parfenova H, Leffler CW. Different pial arteriolar responses to acetylcholine in the newborn and juvenile pig. J Cereb Blood Flow Metab. 1994;14:1088–1095. doi: 10.1038/jcbfm.1994.142. [DOI] [PubMed] [Google Scholar]
- 4.Aschner JL, Smith TK, Kovacs N, Pinheiro JMB, Fuloria M. Mechanisms of bradykinin-mediated dilation in newborn piglet pulmonary conducting and resistance vessels. Am J Physiol Lung Cell Mol Physiol. 2002;283:L373–L382. doi: 10.1152/ajplung.00032.2002. [DOI] [PubMed] [Google Scholar]
- 5.Aschner JL, Zeng H, Kaplowitz MR, Zhang Y, Slaughter JC, Fike CD. Heat shock protein 90-eNOS interactions mature with postnatal age in the pulmonary circulation of the piglet. Am J Physiol Lung Cell Mol Physiol. 2009;296:L555–L564. doi: 10.1152/ajplung.90456.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balch Samora J, Frisbee JC, Boegehold MA. Growth-dependent changes in endothelial factors regulating arteriolar tone. Am J Physiol Heart Circ Physiol. 2007;292:H207–H214. doi: 10.1152/ajpheart.00677.2006. [DOI] [PubMed] [Google Scholar]
- 7.Balch Samora J, Frisbee JC, Boegehold MA. Hydrogen peroxide emerges as a regulator of tone in skeletal muscle arterioles during juvenile growth. Microcirculation. 2008;15:151–161. doi: 10.1080/10739680701508497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balch Samora J, Frisbee JC, Boegehold MA. Increased myogenic responsiveness of skeletal muscle arterioles with juvenile growth. Am J Physiol Heart Circ Physiol. 2008;294:H2344–H2351. doi: 10.1152/ajpheart.00053.2008. [DOI] [PubMed] [Google Scholar]
- 9.Balch Samora J, Goodwill AG, Frisbee JC, Boegehold MA. Growth-dependent changes in the contribution of carbon monoxide to arteriolar function. J Vasc Res. 2010;47:23–34. doi: 10.1159/000231718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baquero H, Soliz A, Neira F, Venegas ME, Sola A. Oral sildenafil in infants with persistent pulmonary hypertension of the newborn: a pilot randomized blinded study. Pediatrics. 2006;117:1077–1083. doi: 10.1542/peds.2005-0523. [DOI] [PubMed] [Google Scholar]
- 11.Barkoudah E, Jagger JH, Leffler CW. The permissive role of endothelial NO in CO-induced cerebrovascular dilation. Am J Physiol Heart Circ Physiol. 2004;287:H1459–H1465. doi: 10.1152/ajpheart.00369.2004. [DOI] [PubMed] [Google Scholar]
- 12.Becker RM, Wu G, Galanko JA, Chen W, Maynor AR, Bose CL, Rhoads JM. Reduced serum amino acid concentrations in infants with necrotizing enterocolitis. J Pediatr. 2000;137:785–793. doi: 10.1067/mpd.2000.109145. [DOI] [PubMed] [Google Scholar]
- 13.Berg BR, Sarelius IH. Erythrocyte flux in capillary networks during maturation: implications for oxygen delivery. Am J Physiol Heart Circ Physiol. 1996;271:H2263–H2273. doi: 10.1152/ajpheart.1996.271.6.H2263. [DOI] [PubMed] [Google Scholar]
- 14.Boels PJ, Deutsch J, Gao B, Haworth SG. Maturation of the response to bradykinin in resistance and conduit pulmonary arteries. Cardiovasc Res. 1999;44:416–428. doi: 10.1016/s0008-6363(99)00217-5. [DOI] [PubMed] [Google Scholar]
- 15.Bohlen HG. Integration of intestinal structure, function, and microvascular regulation. Microcirculation. 1998;5:27–37. [PubMed] [Google Scholar]
- 16.Bohlen HG, Lobach D. In vivo study of microvascular wall characteristics and resting control in young and mature spontaneously hypertensive rats. Blood Vessels. 1978;15:322–330. doi: 10.1159/000158177. [DOI] [PubMed] [Google Scholar]
- 17.Charles SM, Zhang L, Longo LD, Buchholz JN, Pearce WJ. Postnatal maturation attenuates pressure-evoked myogenic tone and stretch-induced increases in Ca2+ in rat cerebral arteries. Am J Physiol Reg Int Comp Physiol. 2007;293:R737–R744. doi: 10.1152/ajpregu.00869.2006. [DOI] [PubMed] [Google Scholar]
- 18.Chicoine LG, Paffett ML, Girton MR, Metropoulus MJ, Joshi MS, Bauer JA, Nelin LD, Resta TC, Walker BR. Maturational changes in the regulation of pulmonary vascular tone by nitric oxide in neonatal rats. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1261–L1270. doi: 10.1152/ajplung.00235.2006. [DOI] [PubMed] [Google Scholar]
- 19.Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA, Roy BJ, Keszler M, Kinsella JP. Low dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. N Engl J Med. 2000;342:469–474. doi: 10.1056/NEJM200002173420704. [DOI] [PubMed] [Google Scholar]
- 20.Cornfield DN, Saqueton CB, Porter VA, Herron J, Resnik E, Haddad IY, Reeve HL. Voltage-gated K+-channel activity in ovine pulmonary vasculature is developmentally regulated. Am J Physiol Lung Cell Mol Physiol. 2000;278:L1297–L1304. doi: 10.1152/ajplung.2000.278.6.L1297. [DOI] [PubMed] [Google Scholar]
- 21.Dua AK, Dua N, Murrant CL. Skeletal muscle contraction-induced vasodilator complement production is dependent on stimulus and contraction frequency. Am J Physiol Heart Circ Physiol. 2009;297:H433–H442. doi: 10.1152/ajpheart.00216.2009. [DOI] [PubMed] [Google Scholar]
- 22.Fineman JR, Soifer SJ, Heymann MA. Regulation of pulmonary vascular tone in the perinatal period. Annu Rev Physiol. 1995;57:115–134. doi: 10.1146/annurev.ph.57.030195.000555. [DOI] [PubMed] [Google Scholar]
- 23.Fiumana E, Parfenova H, Jaggar JH, Leffler CW. Carbon monoxide mediates vasodilator effects of glutamate in isolated pressurized cerebral arterioles of newborn pigs. Am J Physiol Heart Circ Physiol. 2003;284:H1073–H1079. doi: 10.1152/ajpheart.00881.2002. [DOI] [PubMed] [Google Scholar]
- 24.Fleisch JH. Age-related changes in the sensitivity of blood vessels to drugs. Pharmac Ther. 1980;8:477–487. doi: 10.1016/0163-7258(80)90055-8. [DOI] [PubMed] [Google Scholar]
- 25.Flynn JT. Pediatric hypertension: Recent trends and accomplishments, future challenges. Am J Hyperten. 2008;21:605–612. doi: 10.1038/ajh.2008.159. [DOI] [PubMed] [Google Scholar]
- 26.Friebel M, Klotz KF, Ley K, Gaehtgens P, Pries AR. Flow-dependent regulation of arteriolar diameter in rat skeletal muscle in situ: Role of endothelium-derived relaxing factor and prostanoids. J Physiol. 1995;483:715–726. doi: 10.1113/jphysiol.1995.sp020616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garlanda C, Dejana E. Heterogeneity of endothelial cells. Specific markers. Arterioscler Thromb Vasc Biol. 1997;17:1193–1202. doi: 10.1161/01.atv.17.7.1193. [DOI] [PubMed] [Google Scholar]
- 28.Geary GG, Buchholz JN, Pearce WJ. Maturation depresses mouse cerebrovascular tone through endothelium-dependent mechanisms. Am J Physiol Reg Int Comp Physiol. 2003;284:R734–R741. doi: 10.1152/ajpregu.00510.2002. [DOI] [PubMed] [Google Scholar]
- 29.Geary GG, Osol GJ, Longo LD. Development affects in vitro vascular tone and calcium sensitivity in ovine cerebral arteries. J Physiol. 2004;558:883–96. doi: 10.1113/jphysiol.2003.056945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Głowińska-Olszewska B, Tołwińska J, Urban M. Relationship between endothelial dysfunction, carotid artery intima media thickness and circulating markers of vascular inflammation in obese hypertensive children and adolescents. J Pediatr Endocrinol Metab. 2007;20:1125–1136. [PubMed] [Google Scholar]
- 31.Gollasch M, Wellman GC, Knot HJ, Jaggar JH, Damon DH, Bonev AD, Nelson MT. Ontogeny of local sarcoplasmic reticulum Ca2+ signals in cerebral arteries: Ca2+ sparks as elementary physiological events. Circ Res. 1998;83:1104–1114. doi: 10.1161/01.res.83.11.1104. [DOI] [PubMed] [Google Scholar]
- 32.Grange RW, Isotani E, Lau KS, Kamm KE, Huang PL, Stull JT. Nitric oxide contributes to vascular smooth muscle relaxation in contracting fast-twitch muscles. Physiol Genomics. 2001;5:35–44. doi: 10.1152/physiolgenomics.2001.5.1.35. [DOI] [PubMed] [Google Scholar]
- 33.Gruber HJ, Mayer C, Meinitzer A, Almer G, Horejsi R, Möller R, Pilz S, März W, Gasser R, Truschnig-Wilders M, Mangge H. Asymmetric dimethylarginine (ADMA) is tightly correlated with growth in juveniles without correlations to obesity related disorders. Exp Clin Endocrinol Diabetes. 2008;116:520–524. doi: 10.1055/s-2008-1062712. [DOI] [PubMed] [Google Scholar]
- 34.Häbler H-J, Wasner G, Jänig W. Attenuation of neurogenic vasoconstriction by nitric oxide in hindlimb microvascular beds of the rat in vivo. Hypertension. 1997;30:957–961. doi: 10.1161/01.hyp.30.4.957. [DOI] [PubMed] [Google Scholar]
- 35.Hill MA, Zou H, Potocnik SJ, Meininger GA, Davis MJ. Invited review: arteriolar smooth muscle mechanotransduction: Ca2+ signaling pathways underlying myogenic reactivity. J Appl Physiol. 2001;91:973–983. doi: 10.1152/jappl.2001.91.2.973. [DOI] [PubMed] [Google Scholar]
- 36.Hirai T, Visneski MD, Kearns KJ, Zelis R, Musch TI. Effects of NO synthase inhibition on the muscular blood flow response to treadmill exercise in rats. J Appl Physiol. 1994;77:1288–1293. doi: 10.1152/jappl.1994.77.3.1288. [DOI] [PubMed] [Google Scholar]
- 37.Holt DC, Fedinec AL, Vaughn AN, Leffler CW. Age and species dependence of pial arteriolar responses to topical carbon monoxide in vivo. Exp Biol Med. 2007;232:1465–1469. doi: 10.3181/0705-BC-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iwamoto HS, Teitel D, Rudolph AM. Effects of birth-related events on blood flow distribution. Pediatr Res. 1987;22:634–640. doi: 10.1203/00006450-198712000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Järvisalo MJ, Raitakari M, Toikka JO, Putto-Laurila A, Rontu R, Laine S, Lehtimäki T, Rönnemaa T, Viikari J, Raitakari OT. Endothelial dysfunction and increased arterial intima-media thickness in children with type 1 diabetes. Circulation. 2004;109:1750–1755. doi: 10.1161/01.CIR.0000124725.46165.2C. [DOI] [PubMed] [Google Scholar]
- 40.Kang LS, Nurkiewicz TR, Wu G, Boegehold MA. Effect of juvenile growth on arteriolar eNOS phosphorylation. FASEB J. 2010 doi: 10.1152/ajpheart.00277.2011. In Press. (Abstr) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanu A, Whitfield J, Leffler CW. Carbon monoxide contributes to hypotension-induced cerebrovascular vasodilation in piglets. Am J Physiol Heart Circ Physiol. 2006;91:H2409–H2414. doi: 10.1152/ajpheart.01368.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelly AM, Rosser BWC, Hoffman R, Panettieri RA, Schiaffino S, Rubinstein NA, Nemeth PM. Metabolic and contractile protein expression in developing rat diaphragm muscle. J Neurosci. 1991;11:1231–1242. doi: 10.1523/JNEUROSCI.11-05-01231.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koga T, Takata Y, Kobayashi K, Fujii K, Nagao T, Fujishima M. Age-related changes in P2-purinergic receptors on vascular smooth muscle and endothelium. Hypertension. 1992;19:286–289. doi: 10.1161/01.hyp.19.3.286. [DOI] [PubMed] [Google Scholar]
- 44.Koga T, Takata Y, Kobayashi K, Takishita S, Yamashita Y, Fujishima M. Age and hypertension promote endothelium-dependent contractions to acetylcholine in the aorta of the rat. Hypertension. 1989;14:542–548. doi: 10.1161/01.hyp.14.5.542. [DOI] [PubMed] [Google Scholar]
- 45.Kolber KA, Gao Y, Raj JU. Maturational changes in endothelium-derived nitric oxide-mediated relaxation of ovine pulmonary arteries. Biol Neonate. 2000;77:123–130. doi: 10.1159/000014206. [DOI] [PubMed] [Google Scholar]
- 46.Laughlin MH, Newcomer SC, Bender SB. Importance of hemodynamic forces as signals for exercise-induced changes in endothelial cell phenotype. J Appl Physiol. 2008;104:588–600. doi: 10.1152/japplphysiol.01096.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leffler CW, Hessler JR, Green RS. The onset of breathing at birth stimulates pulmonary vascular prostacyclin synthesis. Pediatr Res. 1984;18:938–942. doi: 10.1203/00006450-198410000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Leffler CW, Fedinec AL, Parfenova H, Jagger JH. Permissive contributions of NO and prostacyclin in CO-induced cerebrovascular dilation in piglets. Am J Physiol Heart Circ Physiol. 2005;289:H432–H438. doi: 10.1152/ajpheart.01195.2004. [DOI] [PubMed] [Google Scholar]
- 49.Leffler CW, Balabanova L, Fedinec AL, Parfenova H. Nitric oxide increases carbon monoxide production by piglet cerebral microvessels. Am J Physiol Heart Circ Physiol. 2005;289:H1442–H1447. doi: 10.1152/ajpheart.00464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leffler CW, Nasjletti A, Yu C, Johnson RA, Fedinec AL, Walker N. Carbon monoxide and cerebral microvascular tone in newborn pigs. Am J Physiol Heart Circ Physiol. 1999;276:H1641–H1646. doi: 10.1152/ajpheart.1999.276.5.H1641. [DOI] [PubMed] [Google Scholar]
- 51.Li A, Adebiyi A, Leffler CW, Jaggar JH. KCa channel insensitivity to Ca2+ sparks underlies fractional uncoupling in newborn cerebral artery smooth muscle cells. Am J Physiol Heart Circ Physiol. 2006;291:H1118–H1125. doi: 10.1152/ajpheart.01308.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin MT, Hessinger DA, Pearce WJ, Longo LD. Developmental differences in Ca2+-activated K+ channel activity in ovine basilar artery. Am J Physiol Heart Circ Physiol. 2003;285:H701–H709. doi: 10.1152/ajpheart.00138.2003. [DOI] [PubMed] [Google Scholar]
- 53.Lin MT, Longo LD, Pearce WJ, Hessinger DA. Ca2+-activated K+ channel-associated phosphatase and kinase activities during development. Am J Physiol Heart Circ Physiol. 2005;289:H414–H425. doi: 10.1152/ajpheart.01079.2004. [DOI] [PubMed] [Google Scholar]
- 54.Linderman JR, Boegehold MA. Arteriolar network growth in rat striated muscle during juvenile maturation. Int J Microcirc Clin Exp. 1996;16:232–239. doi: 10.1159/000179179. [DOI] [PubMed] [Google Scholar]
- 55.Linderman JR, Boegehold MA. Modulation of arteriolar sympathetic constriction by local nitric oxide: onset during rapid juvenile growth. Microvasc Res. 1998;56:192–202. doi: 10.1006/mvre.1998.2096. [DOI] [PubMed] [Google Scholar]
- 56.Linderman JR, Boegehold MA. Growth-related changes in the influence of nitric oxide on arteriolar tone. Am J Physiol Heart Circ Physiol. 1999;277:H1570–H1578. doi: 10.1152/ajpheart.1999.277.4.H1570. [DOI] [PubMed] [Google Scholar]
- 57.Liu SF, Hislop AA, Haworth SG, Barnes PJ. Developmental changes in endothelium-dependent pulmonary vasodilation in pigs. Br J Pharmacol. 1992;106:324–330. doi: 10.1111/j.1476-5381.1992.tb14335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Long W, Zhang L, Longo LD. Cerebral artery KATP- and KCa-channel activity and contractility: changes with development. Am J Physiol Reg Int Comp Physiol. 2000;279:R2004–R2014. doi: 10.1152/ajpregu.2000.279.6.R2004. [DOI] [PubMed] [Google Scholar]
- 59.Lücke T, Kanzelmeyer N, Kemper MJ, Tsikas D, Das AM. Developmental changes in the L-arginine/nitric oxide pathway from infancy to adulthood: plasma asymmetric dimethylarginine levels decrease with age. Clin Chem Lab Med. 2007;45:1525–1530. doi: 10.1515/CCLM.2007.300. [DOI] [PubMed] [Google Scholar]
- 60.Moreno L, Gonzalez-Luis G, Cogolludo A, Lodi F, Lopez-Farre A, Tamargo J, Villamor E, Perez-Vizcaino F. Soluble guanylyl cyclase during postnatal porcine pulmonary maturation. Am J Physiol Lung Cell Mol Physiol. 2005;288:L125–L130. doi: 10.1152/ajplung.00244.2004. [DOI] [PubMed] [Google Scholar]
- 61.Mount PF, Kemp BE, Power DA. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol. 2007;42:271–279. doi: 10.1016/j.yjmcc.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 62.Murrant CL, Sarelius IH. Multiple dilator pathways in skeletal muscle contraction-induced arteriolar dilations. Am J Physiol Reg Int Comp Physiol. 2002;282:R969–R978. doi: 10.1152/ajpregu.00405.2001. [DOI] [PubMed] [Google Scholar]
- 63.Nankervis CA, Nowicki PT. Role of nitric oxide in regulation of vascular resistance in postnatal intestine. Am J Physiol Gastrointest Liver Physiol. 1995;268:G949–G958. doi: 10.1152/ajpgi.1995.268.6.G949. [DOI] [PubMed] [Google Scholar]
- 64.Nankervis CA, Reber KM, Nowicki PT. Age-dependent changes in the postnatal intestinal microcirculation. Microcirculation. 2001;8:377–387. doi: 10.1038/sj/mn/7800110. [DOI] [PubMed] [Google Scholar]
- 65.Nankervis CA, Giannone PJ, Reber KM. The neonatal intestinal vasculature: contributing factors to necrotizing enterocolitis. Semin Perinatol. 2008;32:83–91. doi: 10.1053/j.semperi.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 66.Nase GP, Boegehold MA. Endothelium-derived nitric oxide limits sympathetic neurogenic constriction in intestinal microcirculation. Am J Physiol Heart Circ Physio. 1997;273:H426–433. doi: 10.1152/ajpheart.1997.273.1.H426. [DOI] [PubMed] [Google Scholar]
- 67.Nemeth PM, Norris BJ, Solanki L, Kelly AM. Metabolic specialization in fast and slow muscle fibers of the developing rat. J Neurosci. 1989;9:2336–2343. doi: 10.1523/JNEUROSCI.09-07-02336.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Neonatal Inhaled Nitric Oxide Study Group. Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. New Engl J Med. 1997;336:597–604. doi: 10.1056/NEJM199702273360901. [DOI] [PubMed] [Google Scholar]
- 69.Nowicki PT, Dunaway DJ, Nankervis CA, Giannone RJ, Reber KM, Hammond SB, Besner GE, Caniano DA. Endothelin-1 in human intestine resected for necrotizing enterocolitis. J Pediatr. 2005;146:805–810. doi: 10.1016/j.jpeds.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 70.Nowicki PT, Caniano DA, Hammond SB, Giannone RJ, Besner GE, Reber KM, Nankervis CA, Hammond SB. Endothelial nitric oxide synthase in human intestine resected for necrotizing enterocolitis. J Pediatr. 2007;150:40–45. doi: 10.1016/j.jpeds.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 71.Nurkiewicz TR, Boegehold MA. Calcium-independent release of endothelial nitric oxide in the arteriolar network: onset during rapid juvenile growth. Microcirculation. 2004;11:453–462. doi: 10.1080/10739680490475999. [DOI] [PubMed] [Google Scholar]
- 72.O’Donnell DC, Tod ML, Gordon JB. Developmental changes in endothelium-dependent relaxation of pulmonary arteries: role of EDNO and prostanoids. J Appl Physiol. 1996;81:2013–2019. doi: 10.1152/jappl.1996.81.5.2013. [DOI] [PubMed] [Google Scholar]
- 73.Parfenova H, Massie V, Leffler CW. Developmental changes in endothelium-derived vasorelaxant factors in cerebral circulation. Am J Physiol Heart Circ Physiol. 2000;278:H780–788. doi: 10.1152/ajpheart.2000.278.3.H780. [DOI] [PubMed] [Google Scholar]
- 74.Pearce WJ, Elliott SR. Maturation enhances the sensitivity of ovine cerebral arteries to the ATP-sensitive potassium channel activator lemakalim. Pediatric Res. 1994;35:729–732. doi: 10.1203/00006450-199406000-00021. [DOI] [PubMed] [Google Scholar]
- 75.Pierce CM, Krywawych S, Petros AJ. Asymmetric dimethyl arginine and symmetric dimethyl arginine levels in infants with persistent pulmonary hypertension of the newborn. Pediatr Crit Care Med. 2004;5:517–520. doi: 10.1097/01.PCC.0000144715.03515.55. [DOI] [PubMed] [Google Scholar]
- 76.Pérez-Vizcaíno F, López-López JG, Santiago R, Cogolludo A, Zaragozá-Arnáez F, Moreno L, Alonso MJ, Salaices M, Tamargo J. Postnatal maturation in nitric oxide-induced pulmonary artery relaxation involving cyclooxygenase-1 activity. Am J Physiol Lung Cell Mol Physiol. 2002;283:L839–L848. doi: 10.1152/ajplung.00293.2001. [DOI] [PubMed] [Google Scholar]
- 77.Proctor KG, Damon DN, Duling BR. Tissue PO2 and arteriolar responses to metabolic stimuli during maturation of striated muscle. Am J Physiol Heart Circ Physiol. 1981;241:H325–H331. doi: 10.1152/ajpheart.1981.241.3.H325. [DOI] [PubMed] [Google Scholar]
- 78.Reber KM, Nowicki PT. Pressure and flow characteristics of terminal mesenteric arteries in postnatal intestine. Am J Physiol Gastrointest Liver Physiol. 1998;274:G290–G298. doi: 10.1152/ajpgi.1998.274.2.G290. [DOI] [PubMed] [Google Scholar]
- 79.Reber KM, Mager GM, Miller CE, Nowicki PT. Relationship between flow rate and NO production in postnatal mesenteric arteries. Am J Physiol Gastrointest Liver Physiol. 2001;280:G43–G50. doi: 10.1152/ajpgi.2001.280.1.G43. [DOI] [PubMed] [Google Scholar]
- 80.Reber KM, Su BY, Clark KR, Pohlman DL, Miller CE, Nowicki PT. Developmental expression of eNOS in postnatal swine mesenteric artery. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1328–G1335. doi: 10.1152/ajpgi.00067.2002. [DOI] [PubMed] [Google Scholar]
- 81.Reeve HL, Weir EK, Archer SL, Cornfield DN. A maturational shift in pulmonary K+ channels, from Ca2+ sensitive to voltage dependent. Am J Physiol Lung Cell Mol Physiol. 1998;275:L1019–1025. doi: 10.1152/ajplung.1998.275.6.L1019. [DOI] [PubMed] [Google Scholar]
- 82.Richir MC, Siroen MPC, van Elburg RM, Fetter WPF, Quik F, Nijveldt RJ, Heij HA, Smit BK, Teerlink T, van Leeuwen PAM. Low plasma concentrations of arginine and asymmetric dimethylarginine in premature infants with necrotizing enterocolitis. Br J Nutr. 2007;97:906–911. doi: 10.1017/S0007114507669268. [DOI] [PubMed] [Google Scholar]
- 83.Ripoll E, Sillau AH, Banchero N. Changes in the capillarity of skeletal muscle in the growing rat. Pflugers Arch. 1979;380:153–158. doi: 10.1007/BF00582151. [DOI] [PubMed] [Google Scholar]
- 84.Risau W. Differentiation of endothelium. FASEB J. 1995;9:926–933. [PubMed] [Google Scholar]
- 85.Rudolph AM. Fetal and neonatal pulmonary circulation. Annu Rev Phys Chem. 1979;41:383–395. doi: 10.1146/annurev.ph.41.030179.002123. [DOI] [PubMed] [Google Scholar]
- 86.Sarelius IH, Damon DN, Duling BR. Microvascular adaptations during maturation of striated muscle. Am J Physiol Heart Circ Physiol. 1981;241:H317–H324. doi: 10.1152/ajpheart.1981.241.3.H317. [DOI] [PubMed] [Google Scholar]
- 87.Shah P, Shah V. Arginine supplementaiton for prevention of necrotising enterocolitis in preterm infants. Cochrane Database Syst Rev. 18 doi: 10.1002/14651858.CD004339.pub2. CD004339. [DOI] [PubMed] [Google Scholar]
- 88.Shaul PW, Yuhanna IS, German Z, Chen Z, Steinhorn RH, Morin FC., III Pulmonary endothelial NO synthase gene expression is decreased in fetal lambs with pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 1997;272:L1005–L1012. doi: 10.1152/ajplung.1997.272.5.L1005. [DOI] [PubMed] [Google Scholar]
- 89.Sorensen KE, Celermajer DS, Georgakopoulos D, Hatcher G, Betteridge DJ, Deanfield JE. Impairment of endothelium-dependent dilation is an early event in children with familial hypercholesterolemia and is related to the lipoprotein(a) level. J Clin Invest. 1994;93:50–55. doi: 10.1172/JCI116983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sorof J, Daniels S. Obesity hypertension in children. A problem of epidemic proportions. Hypertension. 2002;40:441–447. doi: 10.1161/01.hyp.0000032940.33466.12. [DOI] [PubMed] [Google Scholar]
- 91.Steinhorn RH. Nitric oxide and beyond: new insights and therapies for pulmonary hypertension. J Perinatol. 2008;28(Suppl 3):S67–S71. doi: 10.1038/jp.2008.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Steinhorn RH, Morin FC, III, Gugino SF, Giese EC, Russell JA. Developmental differences in endothelium-dependent responses in isolated pulmonary arteries and veins. Am J Physiol Heart Circ Physiol. 1993;264:H2162–H2167. doi: 10.1152/ajpheart.1993.264.6.H2162. [DOI] [PubMed] [Google Scholar]
- 93.Su BY, Reber KM, Nankervis CA, Nowicki PT. Development of the myogenic response in postnatal intestine: role of PKC. Am J Physiol Gastrointest Liver Physiol. 2003;284:G445–G452. doi: 10.1152/ajpgi.00259.2002. [DOI] [PubMed] [Google Scholar]
- 94.Tounian P, Aggoun Y, Dubern B, Varille V, Guy-Grand B, Sidi D, Girardet JP, Bonnet D. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet. 2001;358:1400–1404. doi: 10.1016/S0140-6736(01)06525-4. [DOI] [PubMed] [Google Scholar]
- 95.Unthank JL, Bohlen HG. Intestinal microvascular growth during maturation in diabetic juvenile rats. Circ Res. 1988;63:429–436. doi: 10.1161/01.res.63.2.429. [DOI] [PubMed] [Google Scholar]
- 96.Villaneuva ME, Zaher FM, Svinarich DM, Konduri GG. Decreased gene expression of endothelial nitric oxide synthase in newborns with persistent pulmonary hypertension. Pediatr Res. 1998;44:338–343. doi: 10.1203/00006450-199809000-00012. [DOI] [PubMed] [Google Scholar]
- 97.Vosatka RJ, Kashyap S, Trifiletti RR. Arginine deficiency accompanies persistent pulmonary hypertension of the newborn. Biol Neonate. 1994;66:65–70. doi: 10.1159/000244091. [DOI] [PubMed] [Google Scholar]
- 98.Wang DH, Prewitt RL. Microvascular development during normal growth and reduced blood flow: introduction of a new model. Am J Physiol Heart Circ Physiol. 1991;260:H1966–H1972. doi: 10.1152/ajpheart.1991.260.6.H1966. [DOI] [PubMed] [Google Scholar]
- 99.White CR, Hamade MW, Siami K, Chang MM, Mangalwadi A, Frangos JA, Pearce WJ. Maturation enhances fluid shear-induced activation of eNOS in perfused ovine carotid arteries. Am J Physiol Heart Circ Physiol. 2005;289:H2220–H2227. doi: 10.1152/ajpheart.01013.2004. [DOI] [PubMed] [Google Scholar]
- 100.Williams JM, Pearce WJ. Age-dependent modulation of endothelium-dependent vasodilation by chronic hypoxia in ovine cranial arteries. J Appl Physiol. 2006;100:225–232. doi: 10.1152/japplphysiol.00221.2005. [DOI] [PubMed] [Google Scholar]
- 101.Williams JM, Hull AD, Pearce WJ. Maturational modulation of endothelium-dependent vasodilation in ovine cerebral arteries. Am J Physiol Reg Int Comp Physiol. 2005;288:R149–R157. doi: 10.1152/ajpregu.00427.2004. [DOI] [PubMed] [Google Scholar]
- 102.Willis AP, Leffler CW. NO and prostanoids: age dependence of hypercapnia and histamine-induced dilations of pig pial arterioles. Am J Physiol Heart Circ Physiol. 1999;277:H299–H307. doi: 10.1152/ajpheart.1999.277.1.H299. [DOI] [PubMed] [Google Scholar]
- 103.Willis AP, Leffler CW. Endothelial NO and prostanoid involvement in newborn and juvenile pig pial arteriolar vasomotor responses. Am J Physiol Heart Circ Physiol. 2001;281:H2366–H2377. doi: 10.1152/ajpheart.2001.281.6.H2366. [DOI] [PubMed] [Google Scholar]
- 104.Wilson LE, Levy M, Stuart-Smith K, Haworth SG. Postnatal adrenoreceptor maturation in porcine intrapulmonary arteries. Pediatric Res. 1993;34:591–595. doi: 10.1203/00006450-199311000-00007. [DOI] [PubMed] [Google Scholar]
- 105.Winestone JS, Bonner C, Leffler CW. Carbon monoxide as an attenuator of vasoconstriction in piglet cerebral arterioles. Exp Biol Med. 2003;228:46–50. doi: 10.1177/153537020322800106. [DOI] [PubMed] [Google Scholar]
- 106.Wojciak-Stothard B, Haworth SG. Perinatal changes in pulmonary vascular endothelial function. Pharmacol Ther. 2006;109:78–91. doi: 10.1016/j.pharmthera.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 107.Zamora SA, Amin HJ, McMillan DD, Kubes P, Fick GH, Butzner JD, Parsons HG, Scott RB. Plasma L-arginine concentrations in premature infants with necrotizing enterocolitis. J Pediatr. 1997;131:226–232. doi: 10.1016/s0022-3476(97)70158-6. [DOI] [PubMed] [Google Scholar]
- 108.Zellers TM, Vanhoutte PM. Endothelium-dependent relaxations of piglet pulmonary arteries augment with maturation. Pediatric Res. 1991;30:176–180. doi: 10.1203/00006450-199108000-00011. [DOI] [PubMed] [Google Scholar]
- 109.Zuckerman SL, Armstead WM, Hsu P, Shibata M, Leffler CW. Age dependence of cerebrovascular response mechanisms in domestic pigs. Am J Physiol Heart Circ Physiol. 1996;271:H535–H540. doi: 10.1152/ajpheart.1996.271.2.H535. [DOI] [PubMed] [Google Scholar]
- 110.Zweifach BW, Kovalcheck S, DeLano F, Chen P. Micropressure-flow relationships in a skeletal muscle of spontaneously hypertensive rats. Hypertension. 1981;3:601–614. doi: 10.1161/01.hyp.3.5.601. [DOI] [PubMed] [Google Scholar]


