Abstract
Neisseria gonorrhoeae is the second most common sexually transmitted bacterial pathogen worldwide. Diseases associated with N. gonorrhoeae cause localized inflammation of the urethra and cervix. Despite this inflammatory response, infected individuals do not develop protective adaptive immune responses to N. gonorrhoeae. N. gonorrhoeae is a highly adapted pathogen that has acquired multiple mechanisms to evade its host's immune system, including the ability to manipulate multiple immune signaling pathways. N. gonorrhoeae has previously been shown to engage immunosuppressive signaling pathways in B and T lymphocytes. We have now found that N. gonorrhoeae also suppresses adaptive immune responses through effects on antigen presenting cells. Using primary, murine bone marrow-derived dendritic cells and lymphocytes, we show that N. gonorrhoeae-exposed dendritic cells fail to elicit antigen-induced CD4+ T lymphocyte proliferation. N. gonorrhoeae exposure leads to upregulation of a number of secreted and dendritic cell surface proteins with immunosuppressive properties, particularly Interleukin 10 (IL-10) and Programmed Death Ligand 1 (PD-L1). We also show that N. gonorrhoeae is able to inhibit dendritic cell- induced proliferation of human T-cells and that human dendritic cells upregulate similar immunosuppressive molecules. Our data suggest that, in addition to being able to directly influence host lymphocytes, N. gonorrhoeae also suppresses development of adaptive immune responses through interactions with host antigen presenting cells. These findings suggest that gonococcal factors involved in host immune suppression may be useful targets in developing vaccines that induce protective adaptive immune responses to this pathogen.
Introduction
There are approximately 60 million cases of N. gonorrhoeae infection each year worldwide [1]. N. gonorrhoeae generally infects the female cervix or male urethra, where the local inflammatory response to mucosal invasion by the organism leads to symptoms of urethritis or cervicitis. Additionally, asymptomatic infection or colonization of mucosal surfaces with minimal inflammatory response occurs in approximately half of all infected individuals [2], [3]. N. gonorrhoeae infections significantly impact female reproductive health, as ascending infections of fallopian tubes are associated with infertility and perinatal infection can be transmitted to the neonate during birth. Furthermore, infection with N. gonorrhoeae is associated with increased risk of HIV transmission through effects on both HIV-infected and HIV-uninfected individuals. HIV-infected individuals with N. gonorrhoeae co-infection have increased levels of HIV virus in their blood, genital secretions, and semen [4], [5]. HIV-uninfected individuals with gonorrhea have increased numbers of inflammatory cells in their genital mucosa, some of which are susceptible to HIV infection, thereby increasing the risk that N. gonorrhoeae-infected individuals will acquire HIV from HIV-infected partners. Thus, prevention of N. gonorrhoeae infection is an important public health issue.
Despite experiencing localized inflammatory responses to N. gonorrhoeae, which can be very robust, most infected individuals do not develop protective adaptive immune responses to N. gonorrhoeae. This is clearly demonstrated by a high-frequency of recurrent infections caused by the same strain of N. gonorrhoeae in STD clinic patients [6]. Additionally, titers of anti-gonococcal antibodies are low and transient in patients with uncomplicated natural N. gonorrhoeae infection [7], [8] as well as experimentally-induced gonococcal urethritis [7], [8]. Mechanisms leading to this ineffective adaptive immune response are likely multifactorial including both antigenic variation of major surface molecules and active suppression of host immune signaling by this highly adapted human pathogen.
Neisseria species are known to induce inflammatory signaling in host cells through activation of innate pattern receptor molecules, including Toll-like receptors (TLR), TLR2 and TLR4, as well as C-lectin receptors, including dendritic-cell-specific ICAM-3 grabbing non-integrin [9], [10], [11]. N. gonorrhoeae also engages immunosuppressive signaling pathways in mammalian cells including B and T lymphocytes [12], [13]. A number of outer membrane adhesin proteins encoded by opa genes from N. gonorrheoae and N. meningitidis have been shown to engage host surface receptors known as carcinoembryonic antigen-related cellular adhesion molecules (CEACAMs) [14]. Ligation of CEACAM1 and CEACAM3 on human B and T cells by N. gonorrhoeae Opa proteins inhibits antibody production and cellular proliferation and can induce apoptosis [12], [15], [16], [17]. At high bacteria to T cell ratios, Opa-expressing Neisseria do not inhibit human T cell proliferation induced by mitogen exposure in vitro [18]. The gonococcal type IV pilus has also been implicated in T cell interaction. The gonococcal pilus has been shown to interact with CD46 and induce production of IL-10, an immunoregulatory cytokine. Piliated N. gonorrhoeae and isolated pilin also induce T cell proliferation [19]. The significance of direct effects of N. gonorrhoeae on T cells, through Opa-CEACAM and pilus-CD46 interactions, and the physiologic consequences of gonococcal engagement of this signaling system on infection, pathogenesis and immunity remain to be fully determined.
Recent studies have also demonstrated that immunologic response to N. gonorrhoeae is not only blunted but also skewed towards non-protective responses. In mice, infection with N. gonorrheoae induces differentiation of IL-17-producing CD4+ lymphocytes known as TH17 cells [20]. IL-17 levels are increased in both mice and humans infected with N. gonorrheoae [20], [21]. TH17 activation drives induction of localized inflammation including recruitment of host neutrophils, which is relatively ineffective in protecting against N. gonorrhoeae infection because the bacteria are relatively resistant to neutrophil mediated killing [22], [23]. Liu and Russell also demonstrated that blockade of host TGF-β during infection inhibits TH17 skewing and promotes the development of protective immune responses to N gonorrhoeae, suggesting that both inhibition and skewing of immunologic responses likely contribute to the host's inability to mount effective immunologic responses to this organism [24].
Prior to this report, the potential for N. gonorrhoeae to manipulate host immunologic response through antigen presenting cells, which act as the bridge between the innate and adaptive immune systems, was largely unexplored. We now show that N. gonorrhoeae potently inhibits the ability of antigen-primed dendritic cells to trigger T cell proliferation by inducing expression of both immunosuppressive cytokines and tolerance-inducing cell surface proteins.
Results
N. gonorrhoeae inhibits DC antigen-induced T cell proliferation
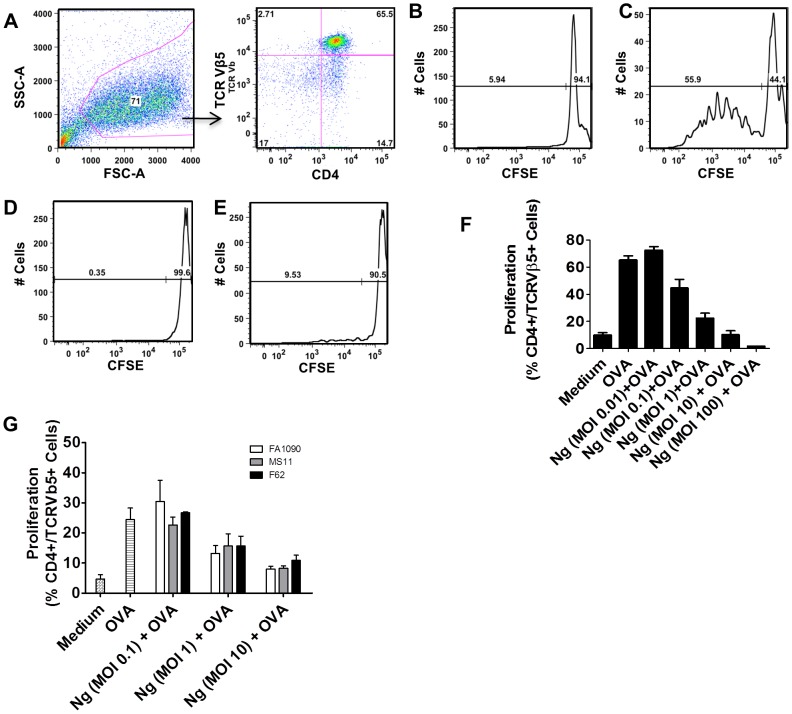
We sought to assess the effects of N. gonorrhoeae on antigen presenting cell-directed T cell proliferation. Because there is no known immunologic correlate of protection for gonorrhea, and the immunologic response to N. gonorrhoeae is weak in the human host, we sought to study the effect of gonococci on dendritic cell-T cell interactions in a system with a defined MHC class II-antigen-T-cell receptor combination. We co-cultured ovalbumin-treated murine C57BL6 bone marrow-derived dendritic cells (BMDC) with lymphocytes from OT-II mice. OT-II mice express a transgenic recombinant T cell receptor that recognizes amino acids 322 to 339 of the ovalbumin protein in the context of the MHC Class II I-A2 allele [25]. After seven days of differentiation, BMDCs were pulsed with ovalbumin, N. gonorrhoeae (strain FA1090), or the combination of both. To eliminate the possibility that N. gonorrhoeae might exert direct effects on the cultured T cells, extracellular N. gonorrhoeae in the culture medium were killed by addition of gentamicin after four hours, and gentamicin was left in the culture medium overnight. Quantitative culture of both medium and lysed dendritic cells confirmed that no viable N. gonorrhoeae were present 24 hours after antibiotic exposure (data not shown). Survival of dendritic cell-associated N. gonorrhoeae was measured after the initial four-hour incubation of N. gonorrhoeae and murine dendritic cells. At multiplicities of infection (MOI) of 1 or 10 cfu/dendritic cell, less than 1% of the initial N. gonorrhoeae inoculum was associated with dendritic cells (Figure S1). In dendritic cells exposed to N. gonorrhoeae at an MOI of ∼1, intracellular bacteria were not detectable even one hour after administration of extracellular gentamicin. In dendritic cells exposed to a higher inoculum, more than 99% of intracellular N. gonorrhoeae were eliminated by 1 hour, and no intracellular N. gonorrhoeae were detectable at 24 hours, even when extracellular gentamicin was removed from the culture (Figure S1). After 24 hours of exposure to ovalbumin or ovalbumin with N. gonorrhoeae, dendritic cells were washed to remove excess bacteria and bacterial products, and these cells were then co-cultured with carboxyfluorescein succinimidyl ester (CFSE)-labeled, enriched T lymphocytes from OT-II mice. Following seven days of co-culture with ovalbumin (OVA)-pulsed dendritic cells, OT-II transgenic T cells (CD4+,Vβ5+) showed significant proliferation, which was demonstrated by antigen-dependent dilution of CFSE-fluorescence in these cells (Figure 1 C) compared to cells exposed to dendritic cells treated with medium or N. gonorrhoeae in the absence of OVA (Figure 1 B and D). The T cell proliferative response to OVA-pulsed dendritic cells was essentially ablated by exposure of the DCs to N. gonorrhoeae (Figure 1 E). The inhibition of T cell proliferation was studied across a range of bacterial concentrations (MOI from 0.1 to 100 cfu/dendritic cell) and was found to be quite potent, with a noticeable effect often seen with a ratio of bacteria to dendritic cells as low as 1 to 10 (MOI 0.1). Nearly complete inhibition was usually noted at MOI of 1.0 (Figure 1 E). Similar inhibitory effects were noted when dendritic cells were treated with N. gonorrhoeae strain MS11 or F62 (Figure 1G). A similar lack of proliferative response to antigen was noted when an ovalbumin peptide was expressed in a surface–exposed loop of the N. gonorrhoeae outer-membrane protein OpaB, demonstrating that inhibition was not an artifact of co-administration of exogenous antigen and bacteria (Figure S2) [26]. These results suggest that N. gonorrhoeae exerts an immunosuppressive effect on CD4 T cells through antigen presenting cells that are exposed to the bacteria.
Figure 1. N. gonorrhoeae inhibits BMDC antigen-induced T cell proliferation.
BMDCs were exposed to N. gonorrhoeae at different MOIs with or without OVA for 24 hours and then co-cultured with CFSE-loaded OT-II T cells for seven days. T cell proliferation to OVA was assessed by flow cytometric analysis. A) Representative gating strategy of CD4+ Vβ5+ OT-II T cells. B) Representative T cell proliferation following co-culture with medium only-treated BMDCs. C) Representative T cell proliferation following co-culture with OVA (100 µg/mL) pulsed BMDCs. D) Representative T cell proliferation profile following co-culture with N. gonorrhoeae (MOI = 1) exposed BMDCs. E) Representative T cell proliferation following co-culture with N. gonorrhoeae (MOI = 1) plus OVA (100 µg/mL) pulsed BMDCs. F) Percentage of OT-II T cell OVA-induced proliferation with a dose range of N. gonorrhoeae (0.01–10 MOI)-exposed BMDCs. Data are mean ± standard deviation (N = 8–32). G. OVA (100 µg/mL) pulsed BMDC were treated with different N. gonorrhoeae strains (White bars: FA1090; Gray bars: MS11; Black bars: F62) at the indicated doses (MOI 0.1–10). Antigen-induced T cell proliferation was assessed after co-culture of the N. gonorrhoeae and OVA treated BMDC with CFSE-loaded OT-II T cells for seven days as noted above. The percentages of proliferated T cells are plotted. Data are mean ± standard deviation (N = 3).
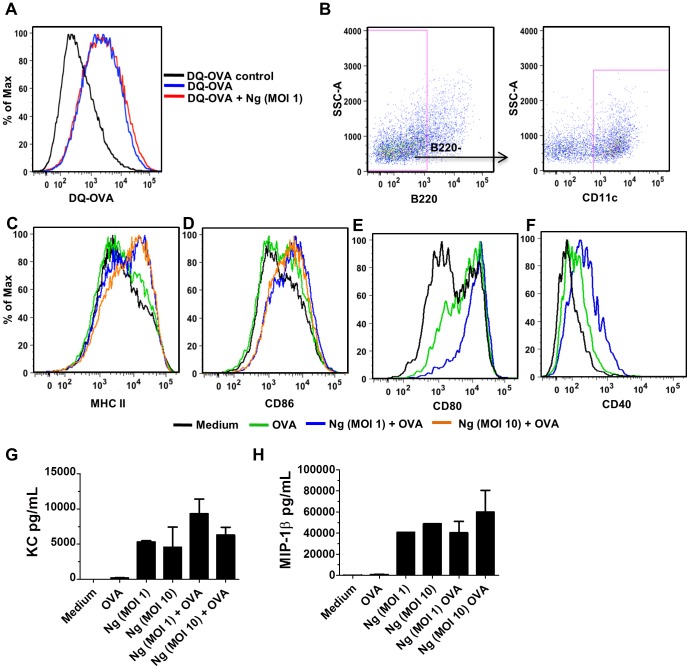
Several lines of investigation were pursued to better define the mechanism by which N. gonorrhoeae exerted this anti-proliferative effect on T cells cultured with antigen-pulsed DCs. To determine whether N. gonorrhoeae was simply blocking the uptake or processing of ovalbumin antigen in the murine DCs, DCs were incubated with ovalbumin covalently linked to both a fluorescent reporter and a fluorescent quenching molecule (DQ-OVA). When DCs take up and proteolytically process DQ-OVA, they exhibit a characteristic shift in fluorescence intensity (Figure 2 A). When N. gonorrhoeae was added to the culture medium with DQ-OVA, uptake and processing of DC-OVA by the dendritic cells, measured by flow cytometry, was not altered (Figure 2 A). Dendritic cells were clearly recognizing N. gonorrhoeae, as they robustly secreted proinflammatory cytokines and chemokines including KC (the murine equivalent of IL-8) and MIP1β after treatment with N. gonorrhoeae for 24 hours at MOI of 1 or 10. Antigen presentation and induction of T cell proliferation by DCs requires expression of both MHC class II and co-stimulatory surface molecules that interact with receptors on T cells, including CD80, CD86, and CD40 [27]. DCs that were pulsed with ovalbumin and N. gonorrhoeae upregulated expression of each of these co-stimulatory molecules equal to or greater than upregulation seen after exposure to ovalbumin alone. Additionally, expression of both MHC class II and CD86 increased with increasing doses of N. gonorrhoeae while the ability of the dendritic cells to stimulate T-cell proliferation was decreased (Figure 2 C–F).
Figure 2. N. gonorrhoeae does not impact OVA uptake and processing by BMDCs, but does induce maturation and co-stimulatory molecule expression and inflammatory cytokine/chemokine production on BMDCs.
A) Flow cytometric analysis of DQ-OVA endocytosis by BMDCs exposed to N. gonorrhoeae versus control (MOI = 1). B) Gating strategy of BMDCs used for surface marker expression analysis (B220− CD11c+). C–F) Representative histograms from 3–4 independent experiments showing BMDC expression of CD80, CD86, CD40 and MHC class II 24 hours post stimulation with medium only, OVA (100 µg/mL), or N. gonorrhoeae (MOI = 1, 10) with OVA (100 µg/mL). BMDCs were exposed to N. gonorrhoeae at MOI of 1 or 10, alone or with OVA and cytokine and chemokine secretion was determined using multiplex bead-based assay analysis of the culture supernatants as described in the experimental procedures. G) KC, H) MIP-1β Data shown are mean ± standard deviation (N = 4).
Overall, these data indicate that N. gonorrhoeae does not prevent antigen-pulsed DCs-induced T cell proliferation by blockade of DC maturation, expression of MHC class II or co-stimulatory molecules. IL-2 is an important cytokine in T cell proliferation that is initially expressed by DCs and later by stimulated T cells [27]. Despite dramatic reductions in proliferating T cells, the levels of IL-2 in culture supernatants from DC-T cell co-cultures were equivalent to levels from co-cultures with DCs pulsed with ovalbumin or ovalbumin with N. gonorrhoeae (Figure S3).
N. gonorrhoeae inhibits DC-mediated, antigen-induced T cell proliferation through multiple mechanisms
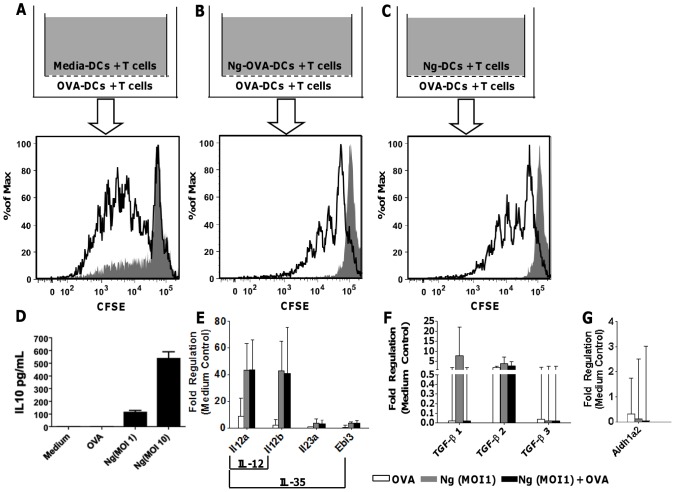
We next determined whether N. gonorrhoeae-treated DCs inhibited T cell proliferation through secretion of soluble factors. DCs pulsed with ovalbumin and/or N. gonorrhoeae were co-cultured with OT-II lymphocytes in transwells, with a pore size of 0.4 µm, to allow diffusion of soluble factors while preventing translocation of dendritic cells between cultures (Figure 3 A–C). Whereas co-incubation with N. gonorrhoeae completely abrogated T cell proliferation in wells occupied by N. gonorrhoeae-treated DCs, there was only partial inhibition of ovalbumin-pulsed DC induced-T cell proliferation in the inserted transwell. These data suggest that soluble factors produced by N. gonorrhoeae-treated dendritic cell-T cell co-culture contributed to inhibition of T cell proliferation, but these soluble factors were insufficient to recapitulate the entire inhibitory effect.
Figure 3. Soluble factors in BMDC/T cell co-culture partially inhibit OVA-induced T cell proliferation.
A–C) CFSE proliferation profiles for OT-II T cells co-cultured with BMDCs under indicated conditions. Representative CFSE profiles for T cells from transwell insert (gray) and transwell itself (open) are shown (from three independent experiments). D) IL-10 protein production by BMDCs cultured with Medium, OVA, N. gonorrhoeae (MOI = 1, 1). Mean pg/mL ± SD, N = 3. E) Il12a, Il12b, Il23a and Ebi3 mRNA steady-state expression in BMDCs cultured with OVA, N. gonorrhoeae (MOI = 1), or N. gonorrhoeae (MOI = 1) with OVA. Mean fold regulation ± SD, N = 3. F) Steady-state expression of mRNA encoding TGF-β 1, 2 and 3 in BMDCs cultured with OVA, N. gonorrhoeae (MOI = 1), or N. gonorrhoeae (MOI = 1) plus OVA. Mean fold regulation ± SD, N = 3. G) Aldh1a2 mRNA steady-state expression in BMDCs cultured with OVA, N. gonorrhoeae (MOI = 1), or N. gonorrhoeae (MOI = 1) with OVA. Mean fold regulation (decrease) ± SD, N = 3.
We examined DC production of known immunomodulatory cytokines IL-10, IL-35, and TGF-β after treatment with N. gonorrhoeae. Secreted IL-10 protein was detected in supernatants of dendritic cell cultures treated with N. gonorrhoeae or N. gonorrhoeae with ovalbumin (Figure 3 D). IL-35 protein was undetectable by ELISA in dendritic cell culture supernatants (data not shown). IL-35 is comprised of two subunits, the α chain of IL-12 (encoded by Il12a) and the Epstein Barr virus induced gene-3 (Ebi3). While mRNA for Il12a was greatly upregulated by N. gonorrhoeae treatment, Ebi3 upregulation was modest at the steady-state mRNA level (Figure 3 E). mRNA encoding TGF-β1, TGF-β2, and TGF-β3 were not induced in N. gonorrhoeae-treated dendritic cells relative to ovalbumin treated dendritic cells (Figure 3 F). Because TGF β exerts immunomodulatory effects in the murine gonococcal infection model [24], these results suggest that TGF-β is either being produced in response to N. gonorrhoeae by other cell types in the genital tract or that it is produced constitutively in the antigen presenting cells, and other factors induced by N. gonorrhoeae exposure work in conjunction with TGF-β to suppress immune responses to this pathogen.
In addition to secreted cytokines, dendritic cells are also known to produce small molecules (e.g. retinoic acid) that promote immunosuppressive T regulatory cell production. The synthesis of retinoic acid is regulated by expression of the retinaldehyde dehydrogenase enzyme (RALHD2, encoded by the Aldh1a2 gene) [28]. N. gonorrhoeae-treated dendritic cells did not show any significant change in Aldh1a2 mRNA (Figure 3 G).
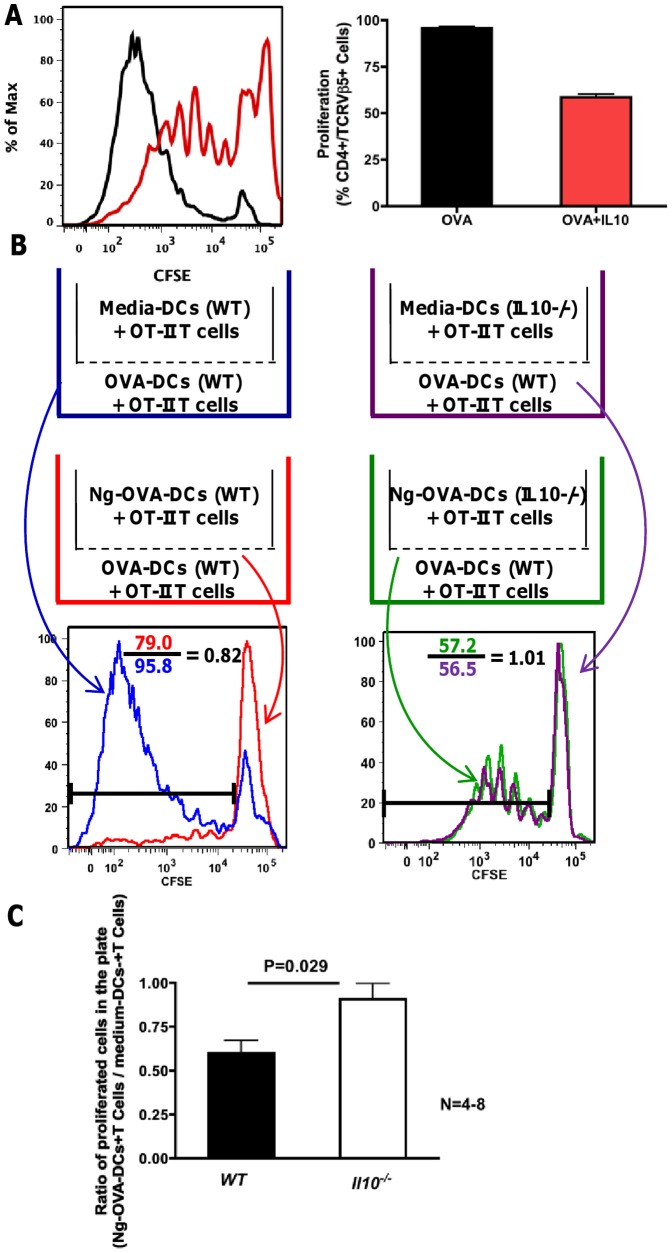
We sought to further characterize the role of IL-10 secretion in the N. gonorrhoeae-induced suppression of dendritic cell-mediated T cell proliferation. First, purified, recombinant IL-10 was added to OVA-pulsed dendritic cells during antigen exposure as well as co-culture with OT-II T cells. This treatment did reduce T cell proliferation, indicating that IL-10 was capable of the suppressive effect observed in these cultures (Figure 4A). Second, dendritic cells derived from mice with a genetically disrupted Il10 gene (Il10−/ −) were treated with N. gonorrhoeae and cultured in transwells adjacent to OVA-pulsed dendritic cell/OT-II T cell co-cultures, as described in figure 4B. N. gonorrhoeae-treated dendritic cells from Il10−/− mice did not inhibit T cell proliferation in adjacent transwells as well as wild type control dendritic cells treated with N. gonorrhoeae (Figure 4 B and C). Overall, these results indicate that IL-10 is upregulated in dendritic cells after N. gonorrhoeae exposure, and that IL-10 released by N. gonorrhoeae-treated dendritic cells is one of the soluble factors that inhibits T cell proliferation. Interestingly, OVA-induced proliferation was equally inhibited by N. gonorrhoeae treatment of wild-type C57/BL6 and Il10−/− BMDCs (Figure S4). These data, combined with the fact that transwell experiments demonstrate that dendritic cell-derived soluble factors alone are insufficient to fully mediate N. gonorrhoeae-induced suppression of T cell proliferation in this co-culture system, indicate that surface inhibitory factors, and possibly other soluble inhibitory factors, can compensate for the loss of BMDC IL-10. Therefore, we sought to determine whether N. gonorrhoeae induced expression of known inhibitory cell surface molecules on dendritic cells.
Figure 4. IL-10 inhibits OVA-DC-induced T cell proliferation.
OVA-pulsed dendritic cells were co-cultured with CFSE-loaded OT-II T cells with or without IL-10 for seven days. A) Representative histogram overlay and bar graph show T cell proliferation profiles following culture with OVA-pulsed DCs (black) or OVA-pulsed DCs+IL-10 (red). The bar graph shows the proliferation of OT-II T cell in the presence of OVA-pulsed DCs with and without exogenous IL-10 from three independent experiments. Data are mean ± standard deviation (N = 3). B) Transwell experiment scheme. WT OVA-DC with OT-II T cell co-culture was placed in all transwell plates. In the insert medium treated-DCs or N. gonorrhoeae-treated DCs from wild type or Il10−/− were co-cultured with OT-II T cells as indicated. T cell proliferation from the transwell plate is shown in the histogram overlays. OVA-induced T cell proliferation in the plate was inhibited by N. gonorrhoeae-treated wild type DCs in the insert (red) but not by wild type medium treated-DCs in the insert (blue). OVA-induced T cell proliferation in the plate was the same for N. gonorrhoeae-treated Il10−/− DCs in the insert (green) and medium treated Il10−/− DCs in the insert (purple). C) Ratio of proliferated T cells from transwell plates with inserts supplying N. gonorrhoeae-OVA-DCs or medium-DCs. Ratio of T cell proliferation in the plate was obtained by dividing the N. gonorrhoeae-OVA-DCs insert by medium-DCs insert. The black bars represent proliferation ratio from transwell plate supplied with wild type BMDCs in insert (N = 8), the open bars represent proliferation ratio from transwell plate supplied with Il10−/− BMDCs in insert (N = 4).
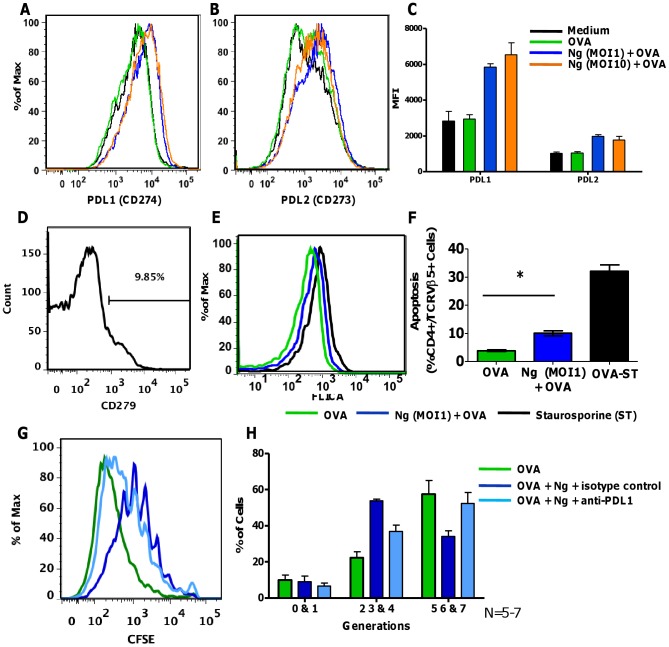
Expression of several DC surface proteins known to induce T cell anergy or tolerance was determined after treatment with N. gonorrhoeae. Some tolerogenic DCs express Programmed Death Ligand 1 and 2 (PD-L1 and PD-L2), which can activate the receptor, Programmed Death-1 (PD1), found on activated T cells to promote differentiation of immunosuppressive T regulatory cells [29]. DCs treated with N. gonorrhoeae with or without ovalbumin upregulated surface expression of both PD-L1 and PD-L2 (Figure 5 A–C). Using flow cytometry, PD1 was detected on the surface of about 10% (10.11+/−2.4%, N = 3) of CD4+ T cells used in the co-culture system (Figure 5 D). Activation of PD1 by PD-L1 and PD-L2 can induce apoptosis of activated T cells as well as promote anergy [30]. Consistent with this mechanism of action, increased activation of the apoptotic proteinase Caspase-3 was observed in T cells incubated with N. gonorrhoeae-treated DCs (Figure 5 E and Figure S5). Inhibition of T cell proliferation by N. gonorrhoeae was tested in the presence of anti-PD-L1 neutralizing antibodies or an isotype antibody control. Addition of PD-L1 neutralizing antibody to N. gonorrhoeae-treated, OVA-pulsed dendritic cells partially restored their ability to stimulate T cell proliferation (Figure 5 G and H). Other surface molecules that have been implicated in tolerogenic responses include ILT3/4 and ICOSL. Steady-state levels of mRNA encoding ILT3 and ICOSL were not modulated by exposure to N. gonorrhoeae (data not shown) [31], [32]. These data indicate that N. gonorrhoeae promoted up-regulation of at least two immunomodulatory surface proteins (PD-L1 and PD-L2). Further, N. gonorrhoeae-induced expression of PD-L1 is capable of attenuating antigen-specific CD4 T cell responses. In total, it appears that N. gonorrhoeae blocks DC induction of T cell proliferation through multiple mechanisms. This may represent functional redundancy or may indicate that significant suppression of the immune system requires cumulative effects on several regulatory pathways.
Figure 5. PD-L1 and PD-L2 are induced on N. gonorrhoeae exposed BMDCs.
BMDCs treated for 24 hours with medium only, OVA, N. gonorrhoeae (MOI = 1,10) with OVA were immunostained for flow cytometric analysis of CD273 and CD274 on DCs (B220−, CD11c+). Representative overlay histograms of: A) CD274 (PD-L1) and B) CD273 (PD-L2). C) Median fluorescence intensity (MFI) of PD-L1 and PD-L2 expression on BMDCs treated as indicated. D) Histogram of PD1 (CD279) expression on CD4+ Vβ5+ OT-II T cells prior to co-culture with BMDCs. E–F) Caspase 3&7 activity (FLICA) form CD4+ Vβ5+ OT-II T cells following co-culture with OVA or N. gonorrhoeae (MOI = 1) plus OVA (100 µg/mL) pulsed BMDCs. E) Representative overlay histograms of Caspase 3&7 activity (FLICA) from CD4+ Vβ5+ OT-II T cells following co-cultured with BMDCs for 24 hours. F) Percentage of apoptotic CD4+ Vβ5+ OT-II T cells following co-cultured with BMDCs for 24 hours. Data are mean ± standard deviation (N = 4 replicates). T cells treated with 1 µM staurosporine (ST) for 3 hours was used as positive control. G) Representative overlay histograms of OT-II T cell proliferation induced by BMDCs treated with OVA (green) versus N. gonorrhoeae (MOI = 0.1) with OVA plus anti-PD-L1 (1∶10 dilute, light blue), N. gonorrhoeae (MOI = 0.1) with OVA plus isotype control (1∶10 dilute, dark blue). H) Mean % ± SD of OT-II T cells proliferated through generations 0–1, 2–4, 5–7 following indicated culture conditions, N = 5–7.
N. gonorrhoeae induces tolerogenic responses in human DC
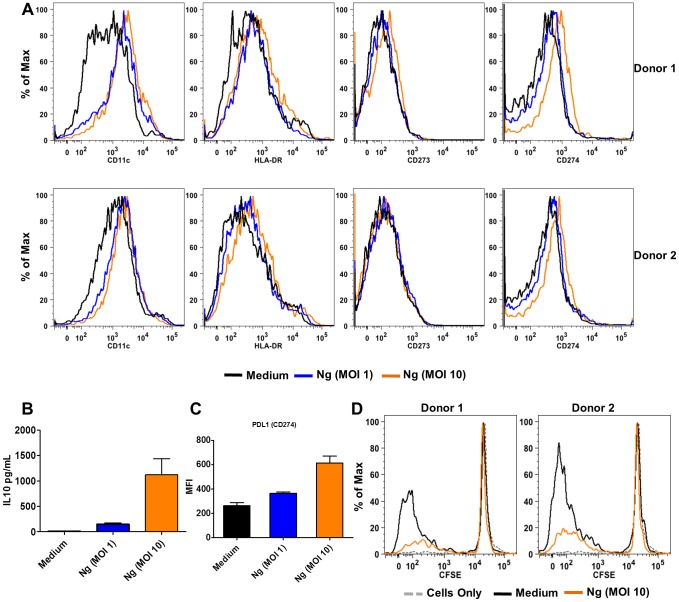
Because mice are not natural hosts of N. gonorrhoeae, we sought to determine whether N. gonorrhoeae could also induce PD-L1 expression and IL-10 secretion in human dendritic cells. Primary human dendritic cells were generated by in vitro differentiation of CD34+ cells from peripheral blood for 14 days. These cultured cells upregulated HLA-DR, CD11c in response to N. gonorrhoeae treatment (Figure 6 A), just as we observed in murine BMDC. Under these conditions, primary human dendritic cells also secreted IL-10 and upregulated surface PD-L1 expression (Figure 6 B and C). N. gonorrhoeae-treated human DC were co-cultured with heterologous CFSE labeled lymphocytes and other non-adherent cells. As observed in the murine co-culture system, N. gonorrhoeae-treatment inhibited the ability of dendritic cells to stimulate CD4+ T cell proliferation (Figure 6 D). Thus, N. gonorrhoeae inhibits murine and human dendritic cell-induced T cell proliferation.
Figure 6. N. gonorrhoeae inhibits dendritic cell-induced T cell proliferation in human primary immune cells.
A) Representative histograms from 2 donors showing unregulated expression of CD11c, HLA-DR, CD274 and CD273 at 24 hours post stimulation with N. gonorrhoeae (MOI = 1, 10). B) IL-10 protein production by human DCs treated with N. gonorrhoeae (MOI = 1,10). C) MFI of PD-L1 expression on human DCs treated with N. gonorrhoeae (MOI = 1,10). D) N. gonorrhoeae inhibits human DCs induced allogeneic T cell proliferation in the Mixed Lymphocyte Reaction (MLR). CFSE proliferation profiles of CD4+ cells after non-adherent cells (NAD) co-cultured with human DCs treated with medium or N. gonorrhoeae (MOI = 10) for 7 days at the ratio of 10∶1.
Discussion
It has long been recognized that N. gonorrhoeae has the capacity to modulate host immunologic responses to prevent protective adaptive immunity. Though T and B lymphocyte populations are generally responsible for mediating adaptive immunity, dendritic cells serve as the primary host cell involved in presenting pathogen-derived molecules to lymphocytes in order to generate adaptive immune responses. Both stimulatory and inhibitory effects have been observed in T and B lymphocyte function following exposure to N. gonorrhoeae. However, the effects of N. gonorrhoeae on lymphocyte function through antigen presenting cells, dendritic cells in particular, has not previously been reported. Recent studies by Russell and colleagues demonstrate that N. gonorrhoeae drives CD4+ T cell differentiation in vivo and in vitro towards the TH17 lineage [20], [24]. This process is dependent on host TGF-β, and inhibition of this cytokine permits the in vivo development of protective TH1 and TH2 immunologic responses in a murine model of N. gonorrhoeae [24]. Here we showed that, in addition to skewing the CD4+ T helper cell phenotype in a TGF-β dependent fashion, N. gonorrhoeae can also inhibit antigen specific CD4+ T cell proliferation through effects on host dendritic cells. The majority of experimental evidence is from murine immune cells because of the immunologic tools available for studying antigen specific stimulation and homogeneity of the host cells from inbred mouse strains. In addition, we showed that N. gonorrhoeae also mediates similar effects in human cells, highlighting the likely clinical relevance of these findings. The effects of N. gonorrhoeae on antigen-dependent T cell proliferation are not unlike those reported for Lactobacillus, a commensal organism of the lower female genital tract [33]. N. gonorrhoeae closely resembles other commensal Neisseria species in morphology and genetic make-up [34]. Interestingly, the gonococcus actually often appears to behave as a commensal in the setting of asymptomatic colonization of the vaginal mucosa, which occurs in over half of female patients with gonorrhea [35]. It is therefore not surprising that N. gonorrhoeae suppresses host adaptive immune responses that might aid the host in clearing bacteria through interactions with antigen presenting cells.
We found that N. gonorrhoeae inhibition of antigen-induced proliferations appears to result from modulation of multiple host factors. N. gonorrhoeae induced production of cell surface molecules on DCs that block T cell proliferation including PD-L1 and PD-L2. PD-L1 is known to play a role in reproductive tract immune tolerance, where its expression is critical to maternal fetal tolerance [36]. Further, PD-L1-mediated immunosuppression plays a role in response to other bacteria in the genital tract. Lactococcus lactis, a vaginal commensal bacterium, also activates tolerogenic, PD-L1-expressing dendritic cells [37]. Similarly, PD-L1 expression actually protects the upper genital tract from inflammatory damage in murine models of chlamydial infection [38]. We have now found that blockade of PD-L1 partially reverses gonococcal inhibition of T cell proliferation, confirming the notion that PD-L1 is involved in N. gonorrhoeae-induced immunomodulation. However, the partial reversal suggests contributions by other surface and secreted molecules from N. gonorrhoeae exposed dendritic cells in suppressing antigen induced T cell proliferation.
In transwell experiments, we showed that N. gonorrhoeae also suppressed T cell proliferation through DC secretion of soluble factors. IL-10 was upregulated and was required for full suppressive effect of N. gonorrhoeae in transwell-based experiments. Commensal bacteria-induced IL-10 secretion from antigen presenting cells is important in preventing uncontrolled colonic inflammation. Secreted IL-10 likely plays a role in preventing uncontrolled inflammation at other mucosal surfaces with high levels of commensal bacteria, like the vagina [39], [40]. Polymorphisms in the human IL10 gene are associated with diminished T cell proliferation in response to chlamydial infection and with increased risk of tubal damage and infertility after chlamydial infection [41]. We showed that N. gonorrhoeae induces IL-10-mediated tolerance, paralleling this mechanism of immune suppression used by Chlamydia trachomatis [42]. IL-10 is elevated in cervical secretions of women infected with C. trachomatis and N. gonorrhoeae, suggesting this response occurs in clinical disease [43], [44]. Despite very different life cycles, these pathogens, both of which are highly adapted to the human lower genital tract, seem to have exploited similar host mechanisms in order to prevent effective adaptive immune responses.
PD-L1+, IL-10 expressing macrophages are responsible for initiating antigen tolerance in an autoimmune encephalitis model [45]. Both IL-10 and PD-L1 have been implicated in the development of a suppressive CD4 T cell population known as Treg. Interestingly, Treg cells are abundant in vaginal tissues of N. gonorrhoeae-infected mice [46], [47], [48]. Our data suggest that N. gonorrhoeae may prevent robust protective immune responses by programming host antigen presenting cells to induce tolerogenic responses, including Treg cells, directed towards gonococcal antigens. However, further research is needed to confirm that Treg polarization occurs in response to N. gonorrhoeae treated dendritic cells.
There are likely other host molecules involved in this pathogen-manipulated immunologic response. For example, we examined the expression of an array of genes during activation and antigen presentation by DCs using real time qRT-PCR (Figure S7 and Table 1). Culture with N. gonorrhoeae and ovalbumin stimulated greater than four-fold induction (compared to ovalbumin treatment alone) of 26 genes and resulted in a four-fold reduction in six genes. Seventeen of the 26 induced genes encode secreted cytokines or chemokines that are not known to suppress T cell proliferation. Steady-state mRNA encoding inhibin A was strongly upregulated (∼18-fold). Inhibin A (Inhba) induces tolerogenic signaling from DCs and is believed to be involved in immune tolerance during pregnancy [27]. Additional molecular tools including neutralizing antibodies, genetic inactivation, or siRNA-mediated silencing will be required to further investigate the role of inhibin A in N. gonorrheoae-induced immune tolerance. It is possible that non-biased gene expression profiling may reveal additional candidate genes whose expression is regulated in dendritic cells by exposure to N. gonorrhoeae.
Table 1. Fold change in genes over-expressed in BMDCs with N. gonorrhoeae (MOI = 1) plus OVA versus OVA only.
| Gene | Relative expression (Ng+OVA:OVA Only treatment) |
| Cxcl1 | 855.4809 |
| Il12a | 187.5184 |
| Cd40 | 161.802 |
| Cxcl2 | 125.5174 |
| Il12b | 125.1483 |
| Cxcl10 | 111.149 |
| Il6 | 87.5084 |
| Ccl17 | 26.7567 |
| Ccl5 | 22.3534 |
| Inhba | 18.3513 |
| Ifit3 | 16.9495 |
| Ccl7 | 12.9143 |
| Ccl3 | 11.6476 |
| Ccl2 | 11.4902 |
| Tnf | 11.077 |
| Erbb2 | 7.9394 |
| Cd80 | 7.197 |
| Cd1d2 | 7.0462 |
| Cd86 | 7.0154 |
| Ccl4 | 6.6678 |
| Tlr1 | 5.6101 |
| Cd4 | 5.2185 |
| Ifng | 5.0386 |
| Ccl8 | 4.7898 |
| Ccl19 | 4.5297 |
| Fas | 4.0752 |
| Relb | 3.9584 |
| Cd209a | 3.8636 |
| Icam1 | 3.7087 |
| Fcamr | 3.5618 |
| Il8ra | 3.416 |
| Ccl12 | 3.3347 |
| Nfkb2 | 2.9508 |
| Il2 | 2.9132 |
| Nfkb1 | 2.9025 |
| Ebi3 | 2.9012 |
| Fcer1g | 2.7914 |
| Ccl20 | 2.726 |
| Il23a | 2.4116 |
| Rela | 2.3402 |
| Tapbp | 2.1733 |
| B2m | 2.1432 |
| Cdkn1a | 2.0629 |
| Cxcl12 | 2.0003 |
Titration of N. gonorrhoeae demonstrated that at MOI<1, gonococci caused significant inhibition of dendritic cell-mediated antigen-induced T cell proliferation (Figure 2 F). This observation suggested that N. gonorrhoeae might exert an effect on dendritic cells in part through release of inhibitory factors into the culture medium. Prolific outer-membrane blebbing is a characteristic of pathogenic Neisseria species; however, the consequences of this process are not fully understood. Additionally, like Bordetella pertussis, N. gonorrhoeae releases high levels of anhydrous peptidoglycan monomers, also known as tracheal cytotoxin [49], [50], [51]. These released peptidoglycans are likely to be recognized by cellular peptidoglycan fragment sensing pattern recognition sensors, NOD1, NOD2, and TLR2. Recently, peptidoglycan-mediated activation of NOD2 and TLR2 signaling has been implicated in the upregulation of host PD-L1 and induction of immunologic tolerance [52]. Our data suggest that bleb-associated factors or peptidoglycan shedding may play an important role in manipulating the host immunologic response to N. gonorrheoae and possibly N. meningitidis.
The current findings indicate that N. gonorrhoeae likely suppresses protective host immune responses at the level of the antigen presenting cell, in addition to its direct effects on T and B lymphocytes, as previously reported [12], [13], [16], [19], [53]. We showed that N. gonorrhoeae suppresses dendritic cells' ability to induce CD4+ T cell proliferation in response to bacterially-expressed ovalbumin (Figure S2). Intracellular pathogens including Salmonella enterica, and Mycobacterium bovus and extracellular bacteria like E. coli have all previously been shown to induce specific proliferative responses when ovalbumin derived antigens are expressed by the bacteria [54], [55], [56]. Interestingly, N. gonorrhoeae-induced suppression can also be seen in trans, when antigens are co-delivered with the bacteria. This may have profound implications for STIs that are co-transmitted with N. gonorrhoeae, preventing the host's ability to mount immune responses to both N. gonorrhoeae and to other pathogens that are acquired at the same time. N. gonorrhoeae infection is associated with increased transmission of HIV, an effect likely mediated by the presence of increased viral burden in the semen of co-infected individuals as well as increased inflammation and HIV-susceptible cells at the sight of infection in HIV-negative individuals with gonorrhea [5]. It is certainly possible that the host immunologic response to HIV in the setting of N. gonorrhoeae infection may be hampered, leading to increased HIV acquisition rates in exposed individuals or impaired virologic control in those who are infected. Further studies into the mechanisms and gonococcal factors involved in this immuosuppressive effect may ultimately yield treatments or vaccine targets that will boost protective host responses to gonococcal antigens and possibly reduce the impact of N. gonorrhoeae infection on transmission of or host response to other sexually transmitted pathogens.
Materials and Methods
Ethics Statement
All protocols were conducted in accordance with National Institutes of Health guidelines for the care and use of laboratory animals and human subjects. The use of laboratory animals was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of North Carolina at Chapel Hill (UNC IACUC protocol # 09-229.0). Human dendritic cells were generated from patients enrolled in a study approved by UNC IRB (Study #05-2860) after providing informed consent. The cells were provided as de-identified samples prior to utilization in the described studies. The use of the de-identified samples was reviewed by the UNC Office of Human Research Ethics, which determined that the study (Study #12-0024) does not constitute human subjects research as defined under federal regulations [45 CFR 46.102 (d or f) and 21 CFR 56.102(c)(e)(l)] and does not require further IRB approval.
Preparation of N. gonorrhoeae
N. gonorrhoeae strain FA1090 was prepared as previously described [57]. Briefly, a predominantly Opa+ frozen stock of N. gonorrhoeae FA1090 was inoculated to GCB agar and grown overnight (16–18 hours) at 37°C and 5% CO2. The Opa protein expression was previously determined by whole cell immunoblotting 98 individual colonies and probing with a combination of five specific anti-Opa monoclonal antibodies, the greater than 80% of the colonies in the frozen population express at least one Opa with a predominance of OpaA, OpaD, and OpaI noted [57], [58]. Colonies were collected using a sterile cotton swab and inoculated to DMEM with 10% FCS. The bacterial density was estimated by measurement of OD600 and confirmed by plating serial dilutions. N. gonorrhoeae strains MS11 and F62 were prepared in a similar manner, though the specific Opa proteins expressed were not assessed for these two strains.
Construction of OpaB-ovalbumin fusion protein expressing N. gonorrhoeae
A gene encoding a fusion protein in which peptides derived from Gallus gallus ovalbumin (257–264 and 323–339) were incorporated into the hyper-variable region, HV1, of OpaB was generated using a hybrid synthetic oligo/PCR approach (Figure S6). OpaB was amplified as two gene fragments from N. gonorrhoeae strain FA1090opaA-K(B+), in which the expression of OpaB is phase locked on [59], and subcloned into the TOPO TA cloning vector (Invitrogen Corp., Carlsbad, CA). Nucleotides encoding an in-frame addition of ovalbumin amino acids 257–264 were incorporated into the oligonucleotide primer. These gene fragments were fused together at a synthetic SpeI site in pBluescript SK+ (Stratagene Cloning Systems). Synthetic DNA encoding OVA323–339 created from self-annealing oligos was inserted at the synthetic SpeI site creating a gene encoding the OpaB (OVA(257–264;323–339)) fusion protein. This gene was targeted to the opaB locus of gonococcal strain FA1090 using a two-step process. First, N. gonorrhoeae strain FA1090 was transformed with an opaB gene containing a cat/rpsL cassette within the HV-1 region and selected for chloramphenicol resistance and streptomycin sensitivity. Next, the opaB::cat/rpsL locus was replaced by homologous recombination with the opaB::OVA fusion gene and selected for streptomycin resistance and chloramphenicol sensitivity. OVA peptide expression in the gonococcus was confirmed by whole cell dot blot probed with a rabbit polyclonal anti-chicken ovalbumin sera (Bethyl Laboratories, Inc, Montgomery, TX), OpaB-specific monoclonal antibody H4, and porin P1B3-specific monoclonal antibody H5 (figure S2) [58].
Culture of murine bone marrow-derived dendritic cells
BMDCs were prepared from 9–12 week old C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) as modified from a previous method [60]. In brief, femurs and tibiae were removed and left in 70% ethanol for 2–5 minutes and then washed with 1× PBS. Both ends of the bones were cut and bone marrow precursors were harvested by flushing with RPMI 1640 medium (Invitrogen Corp., Carlsbad, CA) supplemented with 10% FBS (Thermo Scientific, Logan, UT) using a syringe with 27 gauge needle (BD Biosciences, Franklin Lakes, NJ). Clusters within the marrow suspension were disintegrated by 21 gauge needle. Cells were centrifuged at 450× g for 8 minutes and cell pellets were then treated with 1X RBC lysis buffer [150 mM NH4Cl, 10 mM KHCO3, and 0.1 mM Na2EDTA (pH 7.4)] for 5 minutes at room temperature. After two additional washes, cell pellets were resuspended in 10% FBS RPMI 1640 medium containing GM-CSF (25 ng/mL; Peprotech, Rocky Hill, NJ) and IL-4 (10 ng/mL; Peprotech, Rocky Hill, NJ) and seeded into 6-well tissue culture plates at a density of 0.5×106/mL with a total volume of 4 mL per well. Cultures were pulsed every 48 hours with fresh medium containing GM-CSF and IL-4. After 7 days in culture, immature DCs were harvested and used in T cell co-cultures as antigen presentation cells (APCs).
Infection and stimulation of BMDC
BMDCs were washed and resuspended in antibiotic-free medium at a density of 1×106/mL. Cells were infected with N. gonorrhoeae strain FA1090 with the indicated multiplicity of infection (MOI) in the absence or presence of soluble Ovalbumin (OVA; 100 µg/mL; Sigma-Aldrich, St. Louis, MO) [57]. BMDCs stimulated with OVA or medium only served as controls. Four hours post-infection or treatment, BMDC cultures were supplemented with 50 µg/mL Gentamicin (Invitrogen Corp., Carlsbad, CA) to kill extracellular bacteria. Cultures were returned to 37°C, 5% CO2 humidified incubators and harvested 24 hours post infection/treatment for co-culture with T cells or down-stream assays.
To determine the survival of N. gonorrhoeae within murine BMDC, Murine BMDCs were inoculated with either MOI 1 or MOI 10 N. gonorrhoeae and incubated at 37°C 5% CO2 in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum in 5 mL polystyrene tubes. After 4 h, cells were spun at 2500 rpm and washed 2× with 1 mL fresh medium (t = 0 h). Gentamicin was added to the cells to a concentration of 50 µg/mL gentamicin for 1 hour. Subsequently, cells were washed 2× with medium (t = 1 h) and allowed to grow for an additional 19 hours (t = 20 h) at 37°C 5% CO2. At each time point (t = 0 h, 1 h, 20 h) cell-associated bacteria were determined by adding saponin to 1% for 10 minutes followed by plating serial dilutions on GCB Agar plates in triplicate. After a 48 h growth period, colonies were counted using Synbiosis aCOLyte colony counter.
Measurement of cytokines/chemokines, TLR2 activation and caspase-3 activation
Mouse KC, TNF-α, MIP-1β, RANTES, IL-2 and IL-10 levels in cell culture supernatants were determined by multiplex bead-based assays using Bio-Plex Pro™ Mouse Cytokine assays (BioRad, Hercules, CA) according to the manufacturer's protocol. Bead assays were quantified on the Bio-Plex protein array reader (BioRad, Hercules, CA) in the Duke Human Vaccine Institute Immune Reconstitution and Biomarker Facility.
DQ-OVA endocytosis assay
DQ-OVA (Invitrogen Corp., Carlsbad, CA) is a self-quenched conjugate of ovalbumin that exhibits bright green fluorescence upon proteolytic degradation and can be measured by flow cytometry. BMDCs were resuspended in antibiotic-free medium at a density of 1×106/mL and pulsed with DQ-OVA (10 µg/mL) with or without N. gonorrhoeae (MOI = 1). BMDCs were incubated at 37°C for 1, 4 or 24 hours. Gentamicin was added to kill extracellular N. gonorrhoeae at 1 or 4 hours after infection. BMDCs were collected at various time points, washed three times with cold 1× PBS and re-suspended in FACS buffer (1× PBS, 1% BSA, 0.1% NaN3). Cells were evaluated immediately via flow cytometry for DQ-OVA uptake and processing. Controls include cells with DQ-OVA at 4°C and cells incubated at 37°C without DQ-OVA.
BMDC-T Cell co-culture/proliferation assay
Spleen and lymph nodes were excised from OT-II mice (obtained from Jackson Laboratories, Bar Harbor, ME) and single-cell suspensions were prepared by dissociating cells through 70 µm cell strainers (BD, Bedford, MA) and removing red cells with 1X RBC lysis buffer. Splenocytes/LN cells were first separated by lymphocyte separation medium (LSM, Accurate Chemical & Scientific Corporation, Westbury, NY) and then passed over T cell enrichment columns (R&D Systems, Minneapolis, MN). For BMDC/T co-culture experiments, enriched T cells (0.5×106/mL) were labeled with carboxy fluoroscein succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) and mixed with 0.5×105/mL of pretreated BMDCs. Cells were cultured in RPMI 1640 medium containing 10% FBS in a total volume of 1 mL per well in 48-well tissue culture plates or in tissue culture plates containing transwell (0.4 µm) inserts (Costar, Corning, NY). After 7 days, BMDC/T cells were harvested and T cell proliferation was measured by flow cytometric analysis of CFSE dilution.
Generation of human dendritic cells and mixed lymphocyte reaction (MLR) assay
Primary human dendritic cells were generated by culture of CD34+ cells from peripheral blood in the presence of Stem Cell Factor (SCF 50 ng/mL) Flt3L (100 ng/mL), GM-CSF(800 U/mL) and IL-4(500 U/mL) in AIM V medium with 10% human AB serum for 14 days. Human peripheral blood mononuclear cells were depleted of antigen-presenting cells by adherence to T75 tissue culture flask supplied with 10% AB serum AIM V medium without agitation. Two hours later, non-adherent (NAD) cells were collected and incubated into another T75 tissue culture flask for another 2 hours incubation. The NAD cells were collected and pooled from 5 donors and used as responder cells and labeled with CFSE. These CFSE-labeled lymphocytes were co-cultured with medium or N. gonorrhoeae exposed human DCs (MOI 1, 10) in 96-well U-bottom plate. CFSE-labeled NAD cells (responder cells) were plated at 1×105 cells per well in a volume of 200 µL and co-cultured with human DCs (stimulator cells) at a ratio of 3∶1, 10∶1 and 30∶1. NAD cells alone were used as negative control. After co-cultured for 3 and 7 days, mixed lymphocyte cultures were harvested and stained with CD4-PE-Cy5, CD4+ T cell proliferation was then measured by flow cytometric analysis of CFSE dilution.
Immunophenotyping and flow cytometry
Polychromatic immunophenotyping was performed using peridinin chlorophyll protein conjugated to the cyanine dye 5.5 (PerCP-Cy5.5), phycoerythrin conjugated to the cyanine dye 5 (PE-Cy5), phycoerythrin conjugated to the cyanine dye 7 (PE-Cy7), allophycocyanin (APC), allophycocyanin conjugated to the cyanine dye 7 (APC-Cy7) and Alexa Fluor 700 as fluorescent dyes. Directly-conjugated anti-mouse monoclonal antibodies were used against: CD3-APC, CD3-APC-Cy7, CD3-PE-Cy7, CD4-APC-Cy7, CD8-PE-Cy7, CD80-PE, CD86-PE-Cy5, CD45R/B220-PE-Cy7, CD11c-APC-Cy7, CD273-APC and CD274-PE (BD Biosciences, San Jose, CA and eBioscience, San Diego, CA). Biotin anti-mouse V beta 5.1/5.2, Biotin anti-mouse I-A [b] and APC Streptavidin were also used (BD Biosciences, San Jose, CA). Cell viability was assessed with AnexinV-PE and 7AAD (BD Biosciences, San Jose, CA), and Caspase-3 was detected with FLICA and propidium iodide (PI) (Immunochemistry Technologies, Bloomington, MN).
Saturating amounts of antibody were used to stain approximately 1×106 cells in FACS Buffer (1× PBS, 1% BSA, 0.1% NaN3) (final volume of 100 µL) at 25°C for 1 hour. All samples were washed with 3 mL FACS Buffer and resuspended in 200 µL of FACS Buffer with 0.4% (w/v) paraformaldehyde. Stained samples were analyzed either on a BD-FACS Canto or a BD LSRII-SOS (BD Biosciences, Palo Alto, CA) in the Duke Human Vaccine Institute Flow Cytometry Facility. For each sample, forward and side angle light scatter profiles were used to acquire 10,000–100,000 events. Data were saved as FCS 3.0 and analyzed with FlowJo software (Tree Star, Inc. Ashland, OR). When necessary, the gating strategies are introduced in the respective figures as a representative scatter plot. In some experiments, the comparison of a single fluorescent parameter between two experimental groups is performed using overlayed histograms in which the Y values are normalized to the peak value in that sample in order to facilitate the comparison of the distribution of fluorescence in each population. In these cases, the Y-axis is labeled as % Max.
Real-time reverse-transcription PCR arrays
Quantitative real-time RT-PCR was used to profile expression of a panel of genes involved in antigen presentation (Table 1) and the additional genes in BMDC cultures. Total RNA was extracted from cell pellets using the RNeasy Mini Kit (Qiagen, Valencia, CA) per manufacturer's instructions, and quantified on a Nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE). Isolated RNA (500 ng) was reverse transcribed, and real-time PCR performed, following the provided protocols with RT2 First-Strand cDNA Synthesis Kit and Mouse Dendritic and Antigen Presenting Cell or Custom Mouse PCR Array, respectively (SA Biosciences, Frederick, MD). Relative gene expression was quantified using the comparative CT method (ΔΔCT) via the PCR Array Data Analysis Web Portal at http://www.sabiosciences.com/pcrarraydataanalysis.php.
Supporting Information
N. gonorrhoeae does not survive intracellularly in murine BMDC. Murine BMDCs were incubated with the indicated dose (MOI 1 & MOI 10) of N. gonorrhoeae for 4 hours. Extracellular bacteria were removed from the culture by washing, followed by 1-hour treatment with gentamicin and subsequent culture of the BMDC for an additional 19 hours. The quantity of BMDC-associated N. gonorrhoeae was assessed by lysing washed BMDC with saponin and plating serial dilutions at the following time points: after 4 h incubation with mouse BMDCs (0 h); after an additional 1 h incubation with gentamicin (1 h); and following an additional 19 h of growth (20 h). Mean colony forming units (cfu) +/− S.E.M. from triplicate plates are plotted. Asterix (*) indicates below the detectable limit (6 cfu).
(TIF)
OVA-expressing N. gonorrhoeae inhibits BMDCs antigen-induced T cell proliferation. A) The predicted membrane topology of the OpaB (OVA(257–264;323–339)) fusion protein is shown in two dimensions. The hypervariable (HV) regions are indicated by hashed lines and the insertion of OVA(257–264;323–339) into hyper variable region-1 (HV1) is indicated. B) The indicated strains of N. gonorrhoeae strains were grown for 18 hours, harvested, and resuspended. The resuspended bacteria (100 µL, 0.2 OD600) or isolated OVA (1.0 µg) were spotted to nitrocellulose and probed with the indicated antibodies as described in the materials and methods. C–G) BMDCs were exposed to OVA-expressing N. gonorrhoeae (MOI = 1) with or without OVA for 24 hours and then co-cultured with CFSE-loaded OT-II T cells for 7 days. OT-II T cell proliferation to OVA was assessed by flow cytometric analysis (CFSE dilution). C) Representative gating strategy of CD4+ Vβ5+ OT-II T cells. Representative OT-II T cell proliferation profile following co-culture with BMDCs treated with D) medium only, E) OVA (100 µg/mL), F) OVA-expressing N. gonorrhoeae (MOI = 1).
(TIFF)
N. gonorrhoeae does not impact OVA induced IL-2 production in T-cell/BMDC co-culture. BMDCs were exposed to N. gonorrhoeae at MOI of 1 with or without OVA for 24 hours and then co-cultured with OT-II T cells for seven days as described in Figure 2. Secreted IL-2 levels in culture supernatant were measured using a multiplex bead assay in seven-day DC-T cell co-culture supernatant N = 4, ND = Not Detectable).
(TIF)
N. gonorrhoeae -treated BMDCs from Il10−/− mice demonstrate similar inhibition on T cell proliferation as seen with WT BMDCs. BMDCs were exposed to N. gonorrhoeae at different MOIs with or without OVA for 24 hours and then co-cultured with CFSE-loaded OT-II T cells for seven days. T cell proliferation to OVA was assessed by flow cytometric analysis. Percent proliferation of T cells normalized to OVA-DC-induced T cell proliferation. Data are mean ± standard deviation (N = 3).
(TIF)
CD4+ T cell apoptosis is unchanged by BMDC exposure to N. gonorrhoeae in the absence of antigen. Caspase 3&7 activity (FLICA) form CD4+ Vβ5+ OT-II T cells following co-culture with medium or N. gonorrhoeae (MOI = 1) pulsed BMDCs. Percentage of apoptotic CD4+ Vβ5+ OT-II T cells following co-cultured with BMDCs for 24 hours. Data are mean ± standard deviation (N = 4 replicates).
(TIFF)
Construction of OpaB(OVA(257–264;323–339)) -expressing N. gonorrhoeae FA1090 strain. N. gonorrhoeae OpaB containing intermediate plasmid constructs used to generate an OpaB (OVA(257–264;323–339))-expressing N. gonorrhoeae strain are shown (designated pJAD). Oligonucleotides used to amplify segments of OpaB or insert sequences encoding amino acids 323 to 339 of G. gallus ovalbumin are designated OVA-1 to OVA-6.
(TIF)
Expression of inflammatory genes was upregulated in BMDCs 24 hours post N. gonorrhoeae exposure. Representative scatter plots of gene expression (qRT-PCR arrays) from A) medium only versus OVA-pulsed BMDCs and B) OVA-pulsed BMDCs versus N. gonorrhoeae (MOI = 1) with OVA, N = 3.
(TIFF)
Acknowledgments
Antibodies to OpaB and FA1090 porB were kindly provided by Dr. Janne Cannon. Antisera from mice immunized with cyclic porB-derived peptides were provided by Dr. Ann Jerse. The cat/rpsL cassette was provided by Dr. Christopher E. Thomas. Shannon Reisdorf provided technical assistance with human dendritic cells. Flow cytometry was performed in the Duke Human Vaccine Institute Research Flow Cytometry Shared Resource Facility (Durham, NC) under the direction of Dr. John F. Whitesides. qRT-PCR and multiplex cytokine profiling were performed in the Duke Human Vaccine Institute Immune Reconstitution & Biomarker Shared Resource Facility (Durham, NC) under the supervision of Mr. Jeffrey Hale and Ms. Kristina Riebe.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The work reported in this publication was supported by the Burroughs Wellcome Fund Career Awards for Medical Scientists awarded to J.A.D. and the following NIH grants and contracts: U19-AI031496 (Southeastern Sexually Transmitted Infection Cooperative Research Center) to J.A.D., M.M.H., and G.D.S. and UC6-AI058607 (Regional Biocontainment Laboratory at Duke University Medical Center) awarded to G.D.S et al. K.J.K. is an awardee of the UNC STD and HIV Training Program (T32-AI007001). L.M.O. received support from the American Society for Pharmacology and Experimental Therapeutics through a grant to the Carolina Summer Fellowship Program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Tapsall JW. Antibiotic resistance in Neisseria gonorrhoeae. Clin Infect Dis. 2005;41:S263–268. doi: 10.1086/430787. [DOI] [PubMed] [Google Scholar]
- 2.Handsfield HH, Lipman TO, Harnisch JP, Tronca E, Holmes KK. Asymptomatic gonorrhea in men. Diagnosis, natural course, prevalence and significance. The New England journal of medicine. 1974;290:117–123. doi: 10.1056/NEJM197401172900301. [DOI] [PubMed] [Google Scholar]
- 3.Korenromp EL, Sudaryo MK, de Vlas SJ, Gray RH, Sewankambo NK, et al. What proportion of episodes of gonorrhoea and chlamydia becomes symptomatic? International journal of STD & AIDS. 2002;13:91–101. doi: 10.1258/0956462021924712. [DOI] [PubMed] [Google Scholar]
- 4.Cohen MS, Hoffman IF, Royce RA, Kazembe P, Dyer JR, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet. 1997;349:1868–1873. doi: 10.1016/s0140-6736(97)02190-9. [DOI] [PubMed] [Google Scholar]
- 5.Ghys PD, Fransen K, Diallo MO, Ettiegne-Traore V, Coulibaly IM, et al. The associations between cervicovaginal HIV shedding, sexually transmitted diseases and immunosuppression in female sex workers in Abidjan, Cote d'Ivoire. Aids. 1997;11:F85–93. doi: 10.1097/00002030-199712000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Fox KK, Thomas JC, Weiner DH, Davis RH, Sparling PF, et al. Longitudinal evaluation of serovar-specific immunity to Neisseria gonorrhoeae. Am J Epidemiol. 1999;149:353–358. doi: 10.1093/oxfordjournals.aje.a009820. [DOI] [PubMed] [Google Scholar]
- 7.Cohen IR. Natural and immune human antibodies reactive with antigens of virulent Neisseria gonorrhoeae: immunoglobulins G, M, And A. Journal of bacteriology. 1967;94:141–148. doi: 10.1128/jb.94.1.141-148.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tapchaisri P, Sirisinha S. Serum and secretory antibody responses to Neisseria gonorrhoeae in patients with gonococcal infections. The British journal of venereal diseases. 1976;52:374–380. doi: 10.1136/sti.52.6.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massari P, Henneke P, Ho Y, Latz E, Golenbock DT, et al. Cutting edge: Immune stimulation by neisserial porins is toll-like receptor 2 and MyD88 dependent. Journal of immunology. 2002;168:1533–1537. doi: 10.4049/jimmunol.168.4.1533. [DOI] [PubMed] [Google Scholar]
- 10.Pridmore AC, Jarvis GA, John CM, Jack DL, Dower SK, et al. Activation of toll-like receptor 2 (TLR2) and TLR4/MD2 by Neisseria is independent of capsule and lipooligosaccharide (LOS) sialylation but varies widely among LOS from different strains. Infection and Immunity. 2003;71:3901–3908. doi: 10.1128/IAI.71.7.3901-3908.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steeghs L, van Vliet SJ, Uronen-Hansson H, van Mourik A, Engering A, et al. Neisseria meningitidis expressing lgtB lipopolysaccharide targets DC-SIGN and modulates dendritic cell function. Cellular microbiology. 2006;8:316–325. doi: 10.1111/j.1462-5822.2005.00623.x. [DOI] [PubMed] [Google Scholar]
- 12.Pantelic M, Kim YJ, Bolland S, Chen I, Shively J, et al. Neisseria gonorrhoeae kills carcinoembryonic antigen-related cellular adhesion molecule 1 (CD66a)-expressing human B cells and inhibits antibody production. Infect Immun. 2005;73:4171–4179. doi: 10.1128/IAI.73.7.4171-4179.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boulton IC, Gray-Owen SD. Neisserial binding to CEACAM1 arrests the activation and proliferation of CD4+ T lymphocytes. Nat Immunol. 2002;3:229–236. doi: 10.1038/ni769. [DOI] [PubMed] [Google Scholar]
- 14.Chen T, Grunert F, Medina-Marino A, Gotschlich EC. Several carcinoembryonic antigens (CD66) serve as receptors for gonococcal opacity proteins. The Journal of experimental medicine. 1997;185:1557–1564. doi: 10.1084/jem.185.9.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen T, Gotschlich EC. CGM1a antigen of neutrophils, a receptor of gonococcal opacity proteins. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:14851–14856. doi: 10.1073/pnas.93.25.14851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee HS, Ostrowski MA, Gray-Owen SD. CEACAM1 dynamics during neisseria gonorrhoeae suppression of CD4+ T lymphocyte activation. Journal of immunology. 2008;180:6827–6835. doi: 10.4049/jimmunol.180.10.6827. [DOI] [PubMed] [Google Scholar]
- 17.McCaw SE, Liao EH, Gray-Owen SD. Engulfment of Neisseria gonorrhoeae: revealing distinct processes of bacterial entry by individual carcinoembryonic antigen-related cellular adhesion molecule family receptors. Infect Immun. 2004;72:2742–2752. doi: 10.1128/IAI.72.5.2742-2752.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Youssef AR, van der Flier M, Estevao S, Hartwig NG, van der Ley P, et al. Opa+ and Opa− isolates of Neisseria meningitidis and Neisseria gonorrhoeae induce sustained proliferative responses in human CD4+ T cells. Infection and Immunity. 2009;77:5170–5180. doi: 10.1128/IAI.00355-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plant LJ, Jonsson AB. Type IV pili of Neisseria gonorrhoeae influence the activation of human CD4+ T cells. Infect Immun. 2006;74:442–448. doi: 10.1128/IAI.74.1.442-448.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feinen B, Jerse AE, Gaffen SL, Russell MW. Critical role of Th17 responses in a murine model of Neisseria gonorrhoeae genital infection. Mucosal immunology. 2010;3:312–321. doi: 10.1038/mi.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gagliardi MC, Starnino S, Teloni R, Mariotti S, Dal Conte I, et al. Circulating levels of interleukin-17A and interleukin-23 are increased in patients with gonococcal infection. FEMS immunology and medical microbiology. 2011;61:129–132. doi: 10.1111/j.1574-695X.2010.00759.x. [DOI] [PubMed] [Google Scholar]
- 22.Criss AK, Katz BZ, Seifert HS. Resistance of Neisseria gonorrhoeae to non-oxidative killing by adherent human polymorphonuclear leucocytes. Cellular microbiology. 2009;11:1074–1087. doi: 10.1111/j.1462-5822.2009.01308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Witt K, Veale DR, Smith H. Resistance of Neisseria gonorrhoeae to ingestion and digestion by phagocytes of human buffy coat. Journal of medical microbiology. 1976;9:1–12. doi: 10.1099/00222615-9-1-1. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Russell MW. Diversion of the Immune Response to Neisseria gonorrhoeae from Th17 to Th1/Th2 by Treatment with Anti-Transforming Growth Factor {beta} Antibody Generates Immunological Memory and Protective Immunity. mBio 2. 2011. [DOI] [PMC free article] [PubMed]
- 25.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunology and cell biology. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 26.Malorny B, Morelli G, Kusecek B, Kolberg J, Achtman M. Sequence diversity, predicted two-dimensional protein structure, and epitope mapping of neisserial Opa proteins. Journal of bacteriology. 1998;180:1323–1330. doi: 10.1128/jb.180.5.1323-1330.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mondino A, Jenkins MK. Surface proteins involved in T cell costimulation. Journal of leukocyte biology. 1994;55:805–815. doi: 10.1002/jlb.55.6.805. [DOI] [PubMed] [Google Scholar]
- 28.Manicassamy S, Ravindran R, Deng J, Oluoch H, Denning TL, et al. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nature medicine. 2009;15:401–409. doi: 10.1038/nm.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Chung Y, Bishop C, Daugherty B, Chute H, et al. Regulation of T cell activation and tolerance by PD-L2. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:11695–11700. doi: 10.1073/pnas.0601347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nature medicine. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 31.Chang CC, Ciubotariu R, Manavalan JS, Yuan J, Colovai AI, et al. Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nature immunology. 2002;3:237–243. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- 32.Vlad G, Chang CC, Colovai AI, Vasilescu ER, Cortesini R, et al. Membrane and soluble ILT3 are critical to the generation of T suppressor cells and induction of immunological tolerance. International reviews of immunology. 2010;29:119–132. doi: 10.3109/08830180903281185. [DOI] [PubMed] [Google Scholar]
- 33.Baba N, Samson S, Bourdet-Sicard R, Rubio M, Sarfati M. Commensal bacteria trigger a full dendritic cell maturation program that promotes the expansion of non-Tr1 suppressor T cells. Journal of leukocyte biology. 2008;84:468–476. doi: 10.1189/jlb.0108017. [DOI] [PubMed] [Google Scholar]
- 34.Marri PR, Paniscus M, Weyand NJ, Rendon MA, Calton CM, et al. Genome sequencing reveals widespread virulence gene exchange among human Neisseria species. PLoS One. 2010;5:e11835. doi: 10.1371/journal.pone.0011835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Detels R, Green AM, Klausner JD, Katzenstein D, Gaydos C, et al. The incidence and correlates of symptomatic and asymptomatic Chlamydia trachomatis and Neisseria gonorrhoeae infections in selected populations in five countries. Sexually transmitted diseases. 2011;38:503–509. [PMC free article] [PubMed] [Google Scholar]
- 36.Guleria I, Khosroshahi A, Ansari MJ, Habicht A, Azuma M, et al. A critical role for the programmed death ligand 1 in fetomaternal tolerance. The Journal of experimental medicine. 2005;202:231–237. doi: 10.1084/jem.20050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jounai K, Ikado K, Sugimura T, Ano Y, Braun J, et al. Spherical lactic acid bacteria activate plasmacytoid dendritic cells immunomodulatory function via TLR9-dependent crosstalk with myeloid dendritic cells. PLoS One. 2012;7:e32588. doi: 10.1371/journal.pone.0032588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng B, Lu C, Tang L, Yeh IT, He Z, et al. Enhanced upper genital tract pathologies by blocking Tim-3 and PD-L1 signaling pathways in mice intravaginally infected with Chlamydia muridarum. BMC infectious diseases. 2011;11:347. doi: 10.1186/1471-2334-11-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Albright CA, Sartor RB, Tonkonogy SL. Endogenous antigen presenting cell-derived IL-10 inhibits T lymphocyte responses to commensal enteric bacteria. Immunology letters. 2009;123:77–87. doi: 10.1016/j.imlet.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larsen JM, Steen-Jensen DB, Laursen JM, Sondergaard JN, Musavian HS, et al. Divergent pro-inflammatory profile of human dendritic cells in response to commensal and pathogenic bacteria associated with the airway microbiota. PLoS One. 2012;7:e31976. doi: 10.1371/journal.pone.0031976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohman H, Tiitinen A, Halttunen M, Lehtinen M, Paavonen J, et al. Cytokine polymorphisms and severity of tubal damage in women with Chlamydia-associated infertility. The Journal of Infectious Diseases. 2009;199:1353–1359. doi: 10.1086/597620. [DOI] [PubMed] [Google Scholar]
- 42.Marks E, Tam MA, Lycke NY. The female lower genital tract is a privileged compartment with IL-10 producing dendritic cells and poor Th1 immunity following Chlamydia trachomatis infection. PLoS pathogens. 2010;6:e1001179. doi: 10.1371/journal.ppat.1001179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen CR, Plummer FA, Mugo N, Maclean I, Shen C, et al. Increased interleukin-10 in the the endocervical secretions of women with non-ulcerative sexually transmitted diseases: a mechanism for enhanced HIV-1 transmission? Aids. 1999;13:327–332. doi: 10.1097/00002030-199902250-00004. [DOI] [PubMed] [Google Scholar]
- 44.Geisler WM, Wang C, Tang J, Wilson CM, Crowley-Nowick PA, et al. Immunogenetic correlates of Neisseria gonorrhoeae infection in adolescents. Sexually transmitted diseases. 2008;35:656–661. doi: 10.1097/OLQ.0b013e31816b6593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Getts DR, Turley DM, Smith CE, Harp CT, McCarthy D, et al. Tolerance induced by apoptotic antigen-coupled leukocytes is induced by PD-L1+ and IL-10−producing splenic macrophages and maintained by T regulatory cells. Journal of immunology. 2011;187:2405–2417. doi: 10.4049/jimmunol.1004175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bellinghausen I, Konig B, Bottcher I, Knop J, Saloga J. Inhibition of human allergic T-helper type 2 immune responses by induced regulatory T cells requires the combination of interleukin-10-treated dendritic cells and transforming growth factor-beta for their induction. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2006;36:1546–1555. doi: 10.1111/j.1365-2222.2006.02601.x. [DOI] [PubMed] [Google Scholar]
- 47.Wan YY, Flavell RA. TGF-beta and regulatory T cell in immunity and autoimmunity. Journal of clinical immunology. 2008;28:647–659. doi: 10.1007/s10875-008-9251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strauss L, Bergmann C, Szczepanski M, Gooding W, Johnson JT, et al. A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:4345–4354. doi: 10.1158/1078-0432.CCR-07-0472. [DOI] [PubMed] [Google Scholar]
- 49.Cookson BT, Tyler AN, Goldman WE. Primary structure of the peptidoglycan-derived tracheal cytotoxin of Bordetella pertussis. Biochemistry. 1989;28:1744–1749. doi: 10.1021/bi00430a048. [DOI] [PubMed] [Google Scholar]
- 50.Goldman WE, Herwaldt LA. Bordetella pertussis tracheal cytotoxin. Developments in biological standardization. 1985;61:103–111. [PubMed] [Google Scholar]
- 51.Sinha RK, Rosenthal RS. Release of soluble peptidoglycan from growing conococci: demonstration of anhydro-muramyl-containing fragments. Infection and Immunity. 1980;29:914–925. doi: 10.1128/iai.29.3.914-925.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hewitt RE, Pele LC, Tremelling M, Metz A, Parkes M, et al. Immuno-inhibitory PD-L1 can be induced by a Peptidoglycan/NOD2 mediated pathway in primary monocytic cells and is deficient in Crohn's patients with homozygous NOD2 mutations. Clinical immunology. 2012. [DOI] [PubMed]
- 53.van Vliet SJ, Steeghs L, Bruijns SC, Vaezirad MM, Snijders Blok C, et al. Variation of Neisseria gonorrhoeae lipooligosaccharide directs dendritic cell-induced T helper responses. PLoS pathogens. 2009;5:e1000625. doi: 10.1371/journal.ppat.1000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morel C, Badell E, Abadie V, Robledo M, Setterblad N, et al. Mycobacterium bovis BCG-infected neutrophils and dendritic cells cooperate to induce specific T cell responses in humans and mice. European journal of immunology. 2008;38:437–447. doi: 10.1002/eji.200737905. [DOI] [PubMed] [Google Scholar]
- 55.Svensson M, Johansson C, Wick MJ. Salmonella enterica serovar typhimurium-induced maturation of bone marrow-derived dendritic cells. Infection and Immunity. 2000;68:6311–6320. doi: 10.1128/iai.68.11.6311-6320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Serre K, Mohr E, Toellner KM, Cunningham AF, Granjeaud S, et al. Molecular differences between the divergent responses of ovalbumin-specific CD4 T cells to alum-precipitated ovalbumin compared to ovalbumin expressed by Salmonella. Molecular immunology. 2008;45:3558–3566. doi: 10.1016/j.molimm.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 57.Duncan JA, Gao X, Huang MT, O'Connor BP, Thomas CE, et al. Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome. J Immunol. 2009;182:6460–6469. doi: 10.4049/jimmunol.0802696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jerse AE, Cohen MS, Drown PM, Whicker LG, Isbey SF, et al. Multiple gonococcal opacity proteins are expressed during experimental urethral infection in the male. J Exp Med. 1994;179:911–920. doi: 10.1084/jem.179.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cole JG, Fulcher NB, Jerse AE. Opacity proteins increase Neisseria gonorrhoeae fitness in the female genital tract due to a factor under ovarian control. Infection and Immunity. 2010;78:1629–1641. doi: 10.1128/IAI.00996-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fields RC, Osterholzer JJ, Fuller JA, Thomas EK, Geraghty PJ, et al. Comparative analysis of murine dendritic cells derived from spleen and bone marrow. Journal of immunotherapy. 1998;21:323–339. doi: 10.1097/00002371-199809000-00001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
N. gonorrhoeae does not survive intracellularly in murine BMDC. Murine BMDCs were incubated with the indicated dose (MOI 1 & MOI 10) of N. gonorrhoeae for 4 hours. Extracellular bacteria were removed from the culture by washing, followed by 1-hour treatment with gentamicin and subsequent culture of the BMDC for an additional 19 hours. The quantity of BMDC-associated N. gonorrhoeae was assessed by lysing washed BMDC with saponin and plating serial dilutions at the following time points: after 4 h incubation with mouse BMDCs (0 h); after an additional 1 h incubation with gentamicin (1 h); and following an additional 19 h of growth (20 h). Mean colony forming units (cfu) +/− S.E.M. from triplicate plates are plotted. Asterix (*) indicates below the detectable limit (6 cfu).
(TIF)
OVA-expressing N. gonorrhoeae inhibits BMDCs antigen-induced T cell proliferation. A) The predicted membrane topology of the OpaB (OVA(257–264;323–339)) fusion protein is shown in two dimensions. The hypervariable (HV) regions are indicated by hashed lines and the insertion of OVA(257–264;323–339) into hyper variable region-1 (HV1) is indicated. B) The indicated strains of N. gonorrhoeae strains were grown for 18 hours, harvested, and resuspended. The resuspended bacteria (100 µL, 0.2 OD600) or isolated OVA (1.0 µg) were spotted to nitrocellulose and probed with the indicated antibodies as described in the materials and methods. C–G) BMDCs were exposed to OVA-expressing N. gonorrhoeae (MOI = 1) with or without OVA for 24 hours and then co-cultured with CFSE-loaded OT-II T cells for 7 days. OT-II T cell proliferation to OVA was assessed by flow cytometric analysis (CFSE dilution). C) Representative gating strategy of CD4+ Vβ5+ OT-II T cells. Representative OT-II T cell proliferation profile following co-culture with BMDCs treated with D) medium only, E) OVA (100 µg/mL), F) OVA-expressing N. gonorrhoeae (MOI = 1).
(TIFF)
N. gonorrhoeae does not impact OVA induced IL-2 production in T-cell/BMDC co-culture. BMDCs were exposed to N. gonorrhoeae at MOI of 1 with or without OVA for 24 hours and then co-cultured with OT-II T cells for seven days as described in Figure 2. Secreted IL-2 levels in culture supernatant were measured using a multiplex bead assay in seven-day DC-T cell co-culture supernatant N = 4, ND = Not Detectable).
(TIF)
N. gonorrhoeae -treated BMDCs from Il10−/− mice demonstrate similar inhibition on T cell proliferation as seen with WT BMDCs. BMDCs were exposed to N. gonorrhoeae at different MOIs with or without OVA for 24 hours and then co-cultured with CFSE-loaded OT-II T cells for seven days. T cell proliferation to OVA was assessed by flow cytometric analysis. Percent proliferation of T cells normalized to OVA-DC-induced T cell proliferation. Data are mean ± standard deviation (N = 3).
(TIF)
CD4+ T cell apoptosis is unchanged by BMDC exposure to N. gonorrhoeae in the absence of antigen. Caspase 3&7 activity (FLICA) form CD4+ Vβ5+ OT-II T cells following co-culture with medium or N. gonorrhoeae (MOI = 1) pulsed BMDCs. Percentage of apoptotic CD4+ Vβ5+ OT-II T cells following co-cultured with BMDCs for 24 hours. Data are mean ± standard deviation (N = 4 replicates).
(TIFF)
Construction of OpaB(OVA(257–264;323–339)) -expressing N. gonorrhoeae FA1090 strain. N. gonorrhoeae OpaB containing intermediate plasmid constructs used to generate an OpaB (OVA(257–264;323–339))-expressing N. gonorrhoeae strain are shown (designated pJAD). Oligonucleotides used to amplify segments of OpaB or insert sequences encoding amino acids 323 to 339 of G. gallus ovalbumin are designated OVA-1 to OVA-6.
(TIF)
Expression of inflammatory genes was upregulated in BMDCs 24 hours post N. gonorrhoeae exposure. Representative scatter plots of gene expression (qRT-PCR arrays) from A) medium only versus OVA-pulsed BMDCs and B) OVA-pulsed BMDCs versus N. gonorrhoeae (MOI = 1) with OVA, N = 3.
(TIFF)