Abstract
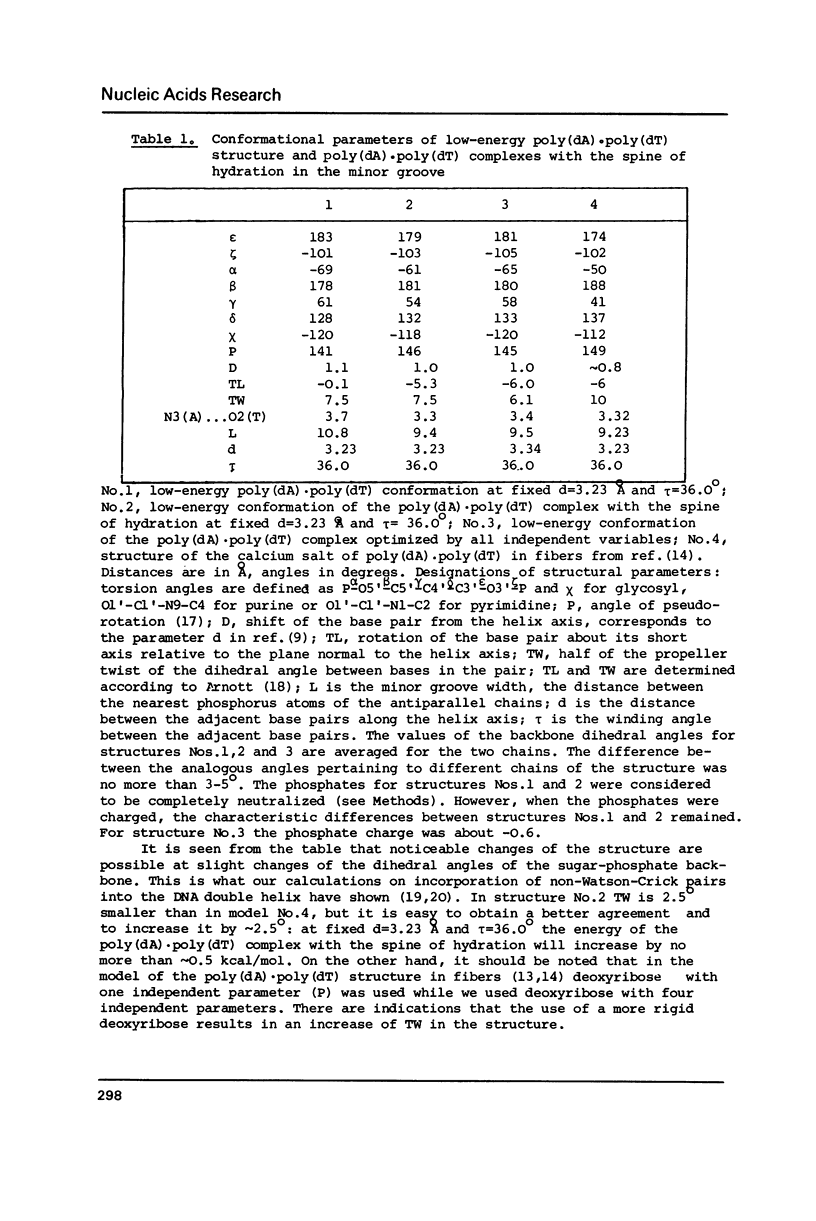
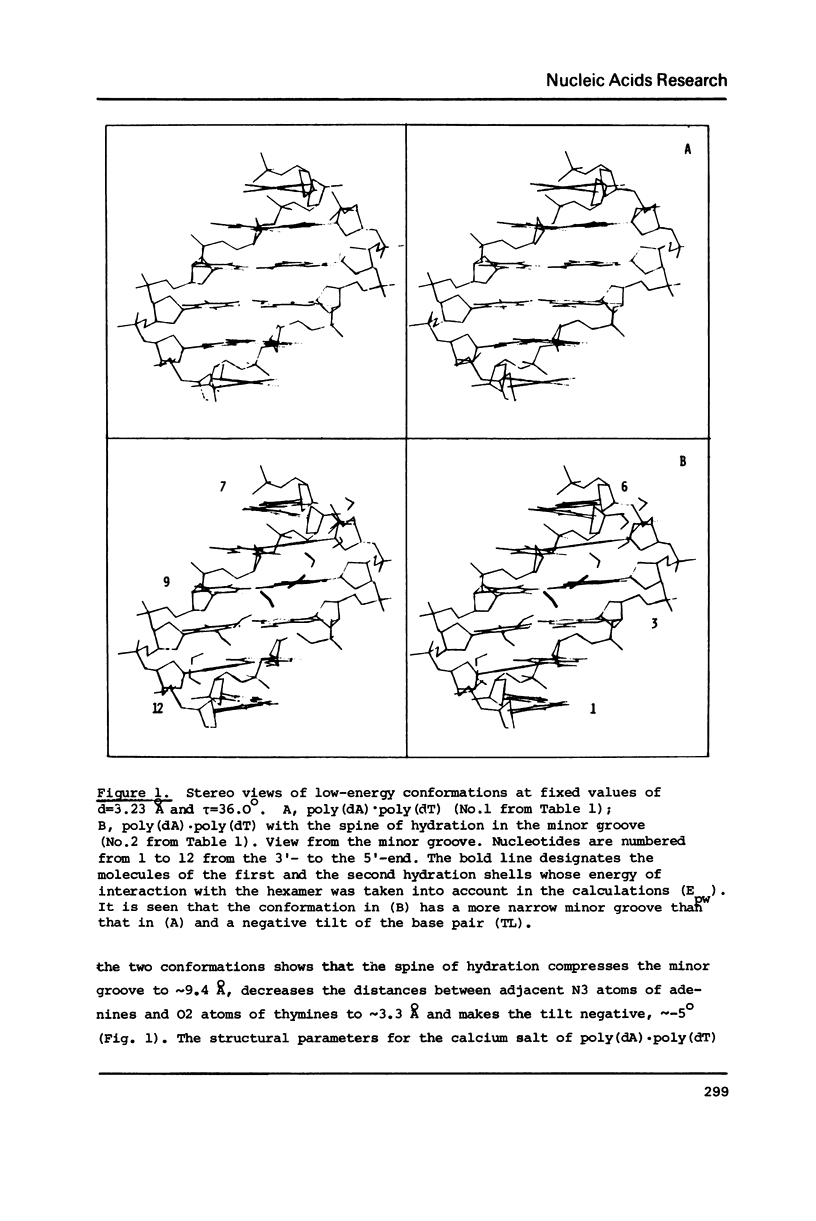
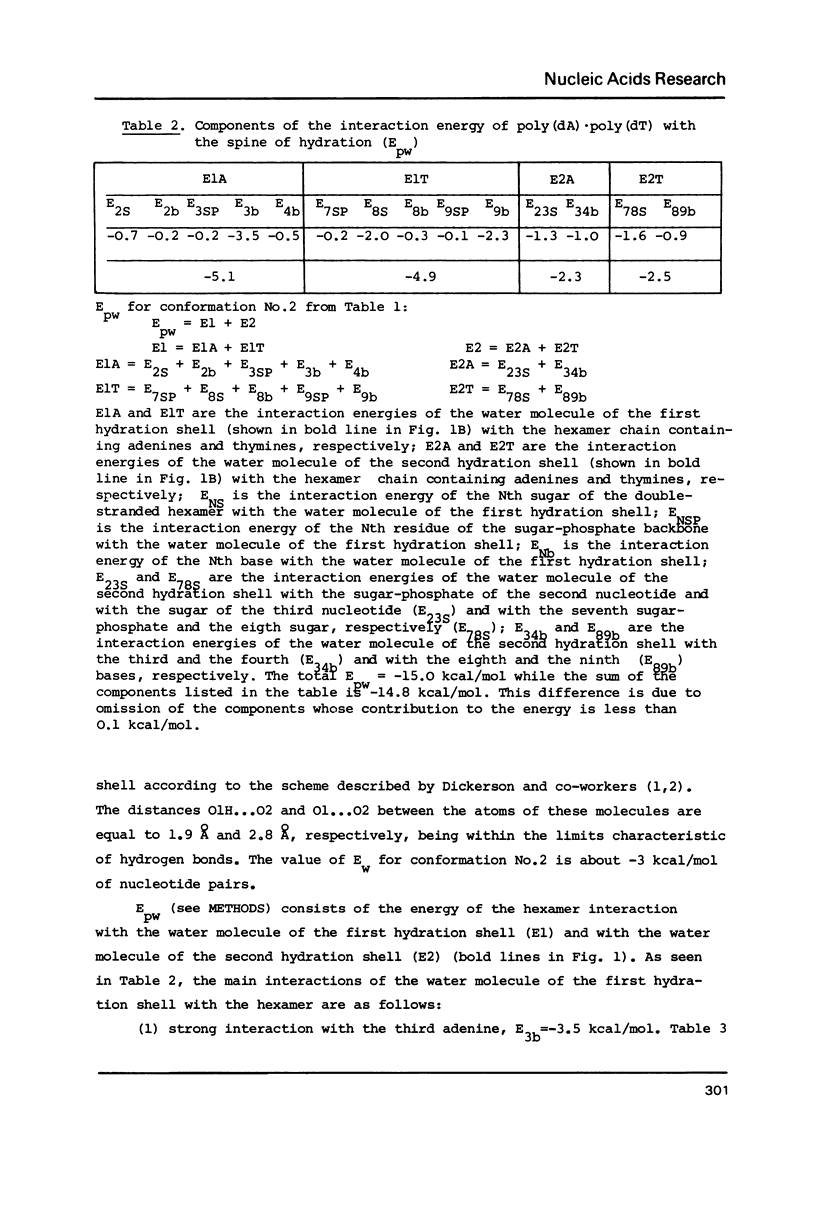
The results of the search for low-energy conformations of poly(dA).poly(dT) and of the poly(dA).poly(dT) "complex" with the spine of hydration similar to that found by Dickerson and co-workers (Kopka, M.L., Fratini, A.V., Drew, H.R. and Dickerson, R.E. (1983) J. Mol. Biol. 163, 129-146) in the minor groove of the CGCGAATTCGCG crystals are described. It is shown that the existence of such a spine in the minor groove of poly(dA).poly(dT) is energetically favourable. Moreover, the spine of hydration makes the polynucleotide conformation similar to the poly(dA).poly(dT) structure in fibers and to the conformation of the central part of CGCGAATTCGCG in crystals; it also acquires features characteristic of the structure of poly(dA).poly(dT) and DNA oligo(dA)-tracts in solution. It is shown that the existence of the TpA step in conformations characteristic of the poly(dA).poly(dT) complex with the spine of hydration is energetically unfavourable (in contrast to the ApT step) and therefore this step should result in destabilization of the spine of hydration in the DNA minor groove. Thus, it appears that the spine of hydration as described by Dickerson and co-workers is unlikely to exist in the poly d(A-T).poly d(A-T) structure. The data obtained permit us to interpret a large body of experimental facts concerning the unusual structure and properties of poly(dA).poly(dT) and oligo(dA)-tracts in DNA both in fibers and in solution. The results provide evidence of the existence of the minor groove spine of hydration both in fibers and in solution on A/T tracts of DNA which do not contain the TpA step. The spine plays an active role in the formation of the anomalous conformation of these tracts.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altona C., Sundaralingam M. Conformational analysis of the sugar ring in nucleosides and nucleotides. A new description using the concept of pseudorotation. J Am Chem Soc. 1972 Nov 15;94(23):8205–8212. doi: 10.1021/ja00778a043. [DOI] [PubMed] [Google Scholar]
- Arnott S., Chandrasekaran R., Hall I. H., Puigjaner L. C. Heteronomous DNA. Nucleic Acids Res. 1983 Jun 25;11(12):4141–4155. doi: 10.1093/nar/11.12.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnott S. The geometry of nucleic acids. Prog Biophys Mol Biol. 1970;21:265–319. doi: 10.1016/0079-6107(70)90027-1. [DOI] [PubMed] [Google Scholar]
- Baase W. A., Johnson W. C., Jr Circular dichroism and DNA secondary structure. Nucleic Acids Res. 1979 Feb;6(2):797–814. doi: 10.1093/nar/6.2.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartenev V. N., Golovamov EuI, Kapitonova K. A., Mokulskii M. A., Volkova L. I., Skuratovskii IYa Structure of the B DNA cationic shell as revealed by an X-ray diffraction study of CsDNA. Sequence-specific cationic stabilization of B form DNA. J Mol Biol. 1983 Sep 5;169(1):217–234. doi: 10.1016/s0022-2836(83)80181-8. [DOI] [PubMed] [Google Scholar]
- Chuprina V. P., Poltev V. I. Alteration of the DNA double helix conformation upon incorporation of mispairs as revealed by energy computations and pathways of point mutations. Nucleic Acids Res. 1985 Jan 11;13(1):141–154. doi: 10.1093/nar/13.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuprina V. P., Poltev V. I. Possible conformations of double-helical polynucleotides containing incorrect base pairs. Nucleic Acids Res. 1983 Aug 11;11(15):5205–5222. doi: 10.1093/nar/11.15.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuprina V. P. Regularities in formation of the spine of hydration in the DNA minor groove and its influence on the DNA structure. FEBS Lett. 1985 Jul 1;186(1):98–102. doi: 10.1016/0014-5793(85)81347-8. [DOI] [PubMed] [Google Scholar]
- Diekmann S., Wang J. C. On the sequence determinants and flexibility of the kinetoplast DNA fragment with abnormal gel electrophoretic mobilities. J Mol Biol. 1985 Nov 5;186(1):1–11. doi: 10.1016/0022-2836(85)90251-7. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Travers A. A. DNA structural variations in the E. coli tyrT promoter. Cell. 1984 Jun;37(2):491–502. doi: 10.1016/0092-8674(84)90379-9. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Travers A. A. Structural junctions in DNA: the influence of flanking sequence on nuclease digestion specificities. Nucleic Acids Res. 1985 Jun 25;13(12):4445–4467. doi: 10.1093/nar/13.12.4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman P. J. Sequence dependence of the curvature of DNA: a test of the phasing hypothesis. Biochemistry. 1985 Dec 3;24(25):7033–7037. doi: 10.1021/bi00346a001. [DOI] [PubMed] [Google Scholar]
- Hinton D. M., Bode V. C. Purification of closed circular lambda deoxyribonucleic acid and its sedimentation properties as a function of Sodium chloride concentration and ethidium binding. J Biol Chem. 1975 Feb 10;250(3):1071–1079. [PubMed] [Google Scholar]
- Koo H. S., Wu H. M., Crothers D. M. DNA bending at adenine . thymine tracts. Nature. 1986 Apr 10;320(6062):501–506. doi: 10.1038/320501a0. [DOI] [PubMed] [Google Scholar]
- Kopka M. L., Fratini A. V., Drew H. R., Dickerson R. E. Ordered water structure around a B-DNA dodecamer. A quantitative study. J Mol Biol. 1983 Jan 5;163(1):129–146. doi: 10.1016/0022-2836(83)90033-5. [DOI] [PubMed] [Google Scholar]
- Leslie A. G., Arnott S., Chandrasekaran R., Ratliff R. L. Polymorphism of DNA double helices. J Mol Biol. 1980 Oct 15;143(1):49–72. doi: 10.1016/0022-2836(80)90124-2. [DOI] [PubMed] [Google Scholar]
- Marky L. A., Curry J., Breslauer K. J. Netropsin binding to polyd(AT) X polyd(AT) and to polydA X polydT: a comparative thermodynamic study. Prog Clin Biol Res. 1985;172B:155–173. [PubMed] [Google Scholar]
- Millane R. P., Walker J. K., Arnott S., Chandrasekaran R., Birdsall D. L., Ratliff R. L. Structure of a pleiomeric form of poly d(AT):poly d(AT). Nucleic Acids Res. 1984 Jul 11;12(13):5475–5493. doi: 10.1093/nar/12.13.5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck L. J., Wang J. C. Sequence dependence of the helical repeat of DNA in solution. Nature. 1981 Jul 23;292(5821):375–378. doi: 10.1038/292375a0. [DOI] [PubMed] [Google Scholar]
- Poltev V. I., Grokhlina T. I., Malenkov G. G. Hydration of nucleic acid bases studied using novel atom-atom potential functions. J Biomol Struct Dyn. 1984 Oct;2(2):413–429. doi: 10.1080/07391102.1984.10507576. [DOI] [PubMed] [Google Scholar]
- Rhodes D., Klug A. Sequence-dependent helical periodicity of DNA. Nature. 1981 Jul 23;292(5821):378–380. doi: 10.1038/292378a0. [DOI] [PubMed] [Google Scholar]
- Sarma M. H., Gupta G., Sarma R. H. Untenability of the heteronomous DNA model for poly(dA).poly(dT) in solution. This DNA adopts a right-handed B-DNA duplex in which the two strands are conformationally equivalent. A 500 MHz NMR study using one dimensional NOE. J Biomol Struct Dyn. 1985 Jun;2(6):1057–1084. doi: 10.1080/07391102.1985.10507624. [DOI] [PubMed] [Google Scholar]
- Strauss F., Gaillard C., Prunell A. Helical periodicity of DNA, Poly(dA) . poly(dT) and poly(dA-dT). poly(dA-dT) in solution. Eur J Biochem. 1981 Aug;118(2):215–222. doi: 10.1111/j.1432-1033.1981.tb06389.x. [DOI] [PubMed] [Google Scholar]
- Thomas G. J., Jr, Benevides J. M. An A-helix structure for poly(dA-dT) X poly(dA-dT). Biopolymers. 1985 Jun;24(6):1101–1105. doi: 10.1002/bip.360240613. [DOI] [PubMed] [Google Scholar]
- Wang J. C. Helical repeat of DNA in solution. Proc Natl Acad Sci U S A. 1979 Jan;76(1):200–203. doi: 10.1073/pnas.76.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartell R. M., Harrell J. T. Characteristics and variations of B-type DNA conformations in solution: a quantitative analysis of Raman band intensities of eight DNAs. Biochemistry. 1986 May 6;25(9):2664–2671. doi: 10.1021/bi00357a056. [DOI] [PubMed] [Google Scholar]
- Wilson W. D., Wang Y. H., Krishnamoorthy C. R., Smith J. C. Poly(dA).poly(dT) exists in an unusual conformation under physiological conditions: propidium binding to poly(dA).poly(dT) and poly[d(A-T)].poly[d(A-T)]. Biochemistry. 1985 Jul 16;24(15):3991–3999. doi: 10.1021/bi00336a029. [DOI] [PubMed] [Google Scholar]
- Wu H. M., Crothers D. M. The locus of sequence-directed and protein-induced DNA bending. Nature. 1984 Apr 5;308(5959):509–513. doi: 10.1038/308509a0. [DOI] [PubMed] [Google Scholar]
- Zhurkin V. B., Lysov Y. P., Florentiev V. L., Ivanov V. I. Torsional flexibility of B-DNA as revealed by conformational analysis. Nucleic Acids Res. 1982 Mar 11;10(5):1811–1830. doi: 10.1093/nar/10.5.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhurkin V. B., Poltev V. I., Florent'ev V. L. Atom-atomnye potentsial'nye funktsii dlia konformatsionnykh raschetov nukleinovykh kislot. Mol Biol (Mosk) 1980 Sep-Oct;14(5):1116–1130. [PubMed] [Google Scholar]
- Zimmer C., Wähnert U. Nonintercalating DNA-binding ligands: specificity of the interaction and their use as tools in biophysical, biochemical and biological investigations of the genetic material. Prog Biophys Mol Biol. 1986;47(1):31–112. doi: 10.1016/0079-6107(86)90005-2. [DOI] [PubMed] [Google Scholar]


