Abstract
Background
Previous research in our cohort showed a delayed decline in functional status after first ischemic stroke. We compared the long-term trajectory of functional status before and after ischemic stroke.
Methods
The Northern Manhattan Study contains a prospective, population-based study of stroke-free individuals >40 years of age, followed for a median 11 years. The Barthel index (BI), a commonly used measure of activities of daily living, was assessed annually. Generalized estimating equations were used to assess functional decline over time before and beginning 6 months after stroke. Follow-up was censored at the time of recurrent stroke.
Results
Among 3298 participants, 210 had an ischemic stroke during follow-up and had post-stroke BI assessed. Mean age (+standard deviation) was 77+9 years, 38% were male, 52% were Hispanic, 37% had diabetes, and 31% had coronary artery disease. There was no difference in rate of functional decline over time before and after stroke (p=0.51), with a decline of 0.96 BI points per year before stroke (p<.0001) and 1.24 after stroke (p=0.001). However, when stratified by insurance status, among those with Medicaid or no insurance, in a fully adjusted model, there was a difference in slope before and after stroke (p=0.04), with a decline of 0.58 BI points per year before stroke (p=0.02) and 1.94 after stroke (p=0.001).
Conclusions
In this large, prospective, population-based study with long-term follow-up, there was a significantly steeper decline in functional status after ischemic stroke compared to before stroke among those with Medicaid or no insurance, after adjusting for confounders.
Keywords: Epidemiology, Disability, Rehabilitation
Introduction
The commonly held view is that stroke is a discrete event and that, following the 3-6 month recovery period after stroke, functional status would remain constant over time, or at best improve with rehabilitation, if no recurrent events occurred.1 However, stroke is caused by conditions, such as hypertension, diabetes, and hyperlipidemia, that may have an ongoing and cumulative effect on vessel dysfunction and may cause subclinical infarcts that can reduce functional status over the long term. Although the short-term effects of stroke on disability are well-described,2-9 the long-term course of functional status before and after stroke is less clear. Our prior research has shown that there was a steady linear decline in functional status over 5 years after stroke among those with Medicaid or no insurance.10 This decline occurred even when recurrent clinical stroke and MI were censored and in models adjusted for demographic variables, stroke severity, and vascular risk factors. An important unresolved question was whether the decline was due to the stroke (or mechanisms triggered by the stroke) or simply a result of aging and independent of any clinical stroke. To our knowledge, no prior study has compared the slope of functional status over time before and after ischemic stroke.
Our hypothesis was that the decline in functional status after recovery from stroke is steeper than before stroke, even in the absence of recurrent clinical strokes. Our objectives were to compare the annual decline in functional status, measured by the Barthel index (BI), a commonly used measure of activities of daily living, before and beginning 6 months after first ischemic stroke, adjusting for potential confounders. The modeling techniques proposed here to assess for a change in slope before and after vascular events have not been employed to examine this question. Also, we employed repeated measures of functional outcomes, which are rarely collected in healthy individuals but are collected in the Northern Manhattan Study (NOMAS).
Methods
NOMAS is a prospective, population-based cohort of 3,298 subjects, designed to describe the prevalence of vascular risk factors and incidence of vascular outcomes in a community-based sample of a racially and ethnically diverse population. The study was approved by the institutional review boards of Columbia University and the University of Miami, and informed consent was obtained from all participants.
Cohort selection
The cohort was recruited between 1993 and 2001 as described elsewhere.11 Subjects were enrolled if they: 1) were at least 40 years of age; 2) lived in a pre-defined geographic area of northern Manhattan for at least 3 months in a household with a telephone; and 3) did not have a history of stroke. Subjects were contacted by random digit dialing of both published and unpublished telephone numbers. The telephone response rate was 91% (9% refused to be screened), and 87% of eligible subjects indicated willingness to participate. The enrollment response rate was 75%, resulting in an overall response rate of 68%.
Baseline assessment
All participants underwent a thorough baseline examination including comprehensive medical history, physical examination, review of medical records, and fasting blood samples. Standardized questions were adapted from the Centers for Disease Control and Prevention Behavioral Risk Factor Surveillance System. Race-ethnicity was based on self-identification modeled after the U.S. census. Smoking was defined as either nonsmoker or smoker (within the last year). Hypertension, coded as present or absent, was defined as a systolic blood pressure recording >140 mmHg or a diastolic blood pressure recording >90 mm Hg (based on the average of two blood pressure measurements) or the patient’s self-report of a history of hypertension or antihypertensive use. Diabetes mellitus was defined by self-report, fasting blood glucose level >126 mg/dL, or insulin/oral hypoglycemic use. Fasting total cholesterol was obtained using a Hitachi 705 automated spectrophotometer (Boehringer Mannheim, Mannheim, Germany). Hypercholesterolemia was defined as a patient’s self-report of hypercholesterolemia, use of lipid lowering therapy, or a fasting total cholesterol level >240 mg/dL. Moderate alcohol use was classified as 1 drink/month to 2 drinks/day. Physical activity was assessed using a questionnaire adapted from the National Health Interview Survey of the National Center for Health Statistics, and classified as any or none, as previously described.12
Prospective follow-up
Subjects were followed up annually by telephone. Only 2 subjects were completely lost to follow-up after their baseline examination, and the average annual contact rate was 99%. The telephone interview assessed any change in vital status, neurological symptoms and events, hospitalizations, and functional status via the BI. Previous research has demonstrated the reliability of phone assessments of functional status using the BI.13 For this analysis, the BI was analyzed as a continuous variable in order to increase power to describe the course of change over time.14
A positive screen for any potential neurological event was followed up by an in-person assessment to determine whether a stroke had occurred. We prospectively screened all admissions and discharges to detect hospitalizations and outcomes that may not have been captured by telephone interview. Nearly 70% of vascular events led to hospitalizations at Columbia University Medical Center. Hospital records were reviewed to classify all outcomes as previously reported.11 Stroke diagnostic evaluation included computerized tomography and/or magnetic resonance imaging of the brain, and a consensus of stroke neurologists assessed stroke subtype using modified Stroke Data Bank criteria and all available information, as described in a previous publication.15 Only individuals who had a first ischemic stroke during follow-up were included in this analysis.
Statistical analysis
Statistical analyses were conducted using SAS Version 9.1.3 (SAS Institute, Cary, NC). For descriptive purposes, means were calculated for continuous variables and proportions for categorical variables. The goal of the primary analysis was to determine whether functional status over time is different before and after ischemic stroke. Due to correlations among repeated measures of outcomes in the same individual, generalized estimating equations were used with an identity link function. Any BI assessments occurring within the 6 months after stroke were ignored, since the course of recovery during this period is well-documented,16, 17 and our interest was the long-term course of functional status after this initial period of recovery. Follow-up was censored at the time of recurrent stroke. The time variable was realigned, setting time of stroke as 0, with negative values reflecting years prior to stroke. The primary question was whether the time trend changed before versus after stroke. To conduct hypothesis testing, we set the model as follows:
where FU=time of follow-up assessment since stroke, poststroke=0 if the time of follow-up is before the stroke, and poststroke=1 if after the stroke. Note that β1 represents annual change in BI before stroke and β2 represents the additional annual change in BI after stroke. The above model gives a broken line as in Figure 1 at the time of stroke and the change in BI at the time of stroke equals β3. When β2 is zero, pre- and post-stroke slopes are the same and our hypothesis is β2=0.
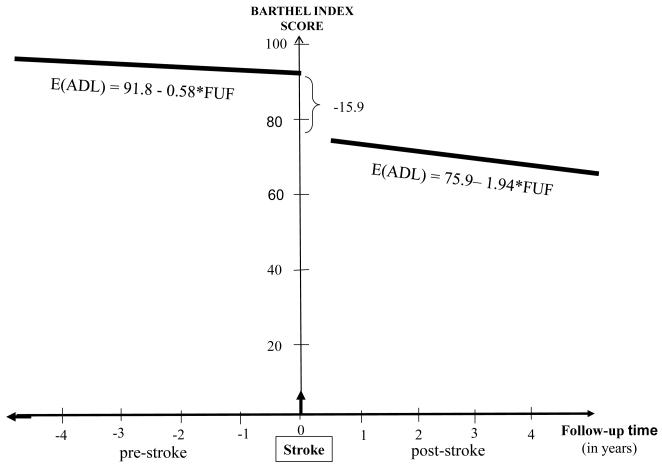
Figure. Conceptual depiction of the course of functional status before and after stroke among those with Medicaid or no insurance.
In model building, we sequentially added groups of confounder variables defined by epidemiological relevance. The first model included no covariates, and successive models included demographic variables, vascular risk factors, and social variables. We present separate models by insurance status since our previous study showed a significant interaction between time of follow-up assessment and insurance status after first ischemic stroke and decline of functional status over time only among those with Medicaid or no insurance.10 Model diagnostics, including residual plots and goodness of fit measures, were used to evaluate the final model, including linearity of the time trends. We chose first-order autoregressive (AR-1) working correlation structure we chose the exchangeable (intraclass) structure and compared the QIC obtained with this model with one using the unstructured working correlation structure.
Results
Among all 3298 participants, 235 had a first ischemic stroke during a median of 11 years of follow-up, 210 of which were included in this analysis after excluding 51 participants who died within 6 months after stroke. These 210 participants, who provide the basis for these analyses, were followed for an average of 5.6 years before stroke and 4.9 years after stroke, and there were a total of 1694 pre-stroke BI assessments and 1390 post-stroke assessments among them. Baseline characteristics of the study population are described in Table 1.
Table 1. Baseline characteristics of study population.
| Number of participants, No. (%) | 210 (100) |
|---|---|
| Demographics: | |
| Age, mean (SD), y | 77.3 (9.0) |
| Male, No. (%) | 80 (38.1) |
| Non-Hispanic white, No. (%) | 44 (21.0) |
| Non-Hispanic black, No. (%) | 55 (26.2) |
| Hispanic, No. (%) | 109 (51.9) |
| Other race, No. (%) | 2 (1.0) |
| Received at least high school education, No. (%) | 86 (41.0) |
| Marital status, No. (%) married | 60 (28.6) |
| Risk factors, No. (%)* | |
| Moderate alcohol use, No. (%) | 58 (27.6) |
| Any physical activity, No. (%) | 121 (57.6) |
| Diabetes mellitus, No. (%) | 78 (37.1) |
| History of coronary artery disease, No. (%) | 64 (30.5) |
as defined in text
Table 2 describes an unadjusted model of the time trend of BI before and after stroke. There was no difference in functional decline over time before and after stroke (p= 0.6), with a decline of 0.84 BI points per year before stroke (p<0.0001) and 1.11 points after stroke (p=0.006). Stroke was associated with an immediate mean drop of 17.47 points on the BI scale (p<0.0001). When we adjusted for confounders, results were similar: there was no difference in functional decline over time before and after stroke (p=0.51), with a decline of 0.96 BI points per year before stroke (p<.0001) and 1.24 after stroke (p=0.001) in a fully adjusted model.
Table 2. Unadjusted and adjusted models examining the time trend of functional status before and after stroke.
| Unadjusted model | Adjusted for demographic variables‡ |
Fully adjusted model* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Change in Barthel index score |
Standard Error |
p-value | Change in Barthel index score |
Standa rd Error |
p-value | Change in Barthel index score |
Standard Error |
p-value |
| Annual change before stroke |
−0.84 | 0.15 | <0.0001 | −1.00 | 0.17 | <0.0001 | −0.96 | 0.18 | <0.0001 |
| Additional annual change after stroke |
−0.27 | 0.45 | 0.6 | −0.23 | 0.43 | 0.6 | −0.28 | 0.42 | 0.5 |
| Change in BI score at time of stroke |
−17.47 | 2.25 | <0.0001 | −17.34 | 2.30 | <0.0001 | −17.37 | 2.30 | <0.0001 |
Note: BI=Barthel index;
including age, sex, race-ethnicity, level of education, and insurance status, as defined in text;
including demographic variables as well as: alcohol use, physical activity, diabetes, marital status, and coronary artery disease
We created separate adjusted models examining the functional time trend before and after stroke, one for those with Medicare or private insurance, and one for those with Medicaid or no insurance (Table 3). Among those with Medicaid or no insurance, in a fully adjusted model, there was a difference in slope before and after stroke (p=0.04), with a decline of 0.58 BI points per year before stroke and 1.94 after stroke (Figure). However, among those with Medicare or private insurance, in a fully adjusted model, there was no difference in slope before and after stroke (p=0.3). By plotting the residuals against time and lowess curves, we found no evidence for non-linearity of the curves in these models.
Table 3. Adjusted models examining the time trend of functional status before and after stroke, stratified by insurance status.†.
| Medicare or private insurance | Medicaid or no insurance | |||||
|---|---|---|---|---|---|---|
| Variable | Change in Barthel index |
Standard error |
p-value | Change in Barthel index |
Standard error |
p-value |
| Annual change before stroke | −1.16 | 0.25 | <0.0001 | −0.58 | 0.25 | 0.02 |
| Additional annual change after stroke |
0.51 | 0.52 | 0.3 | −1.36 | 0.68 | 0.04 |
| Change in BI score at time of stroke |
−18.64 | 3.29 | <0.0001 | −15.90 | 3.10 | <0.0001 |
Adjusted for age, sex, race-ethnicity, level of education, alcohol use, physical activity, diabetes, marital status, and coronary artery disease, as defined in text.
Discussion
In this large, prospective, population-based study with long-term follow-up, there was a significantly steeper decline in functional status after recovery from ischemic stroke compared to before stroke among those with Medicaid or no insurance, after adjusting for confounders. Specifically, the decline was more than three times as steep after stroke (1.94 BI points per year) compared to before stroke (0.58 BI points per year). No other study has modeled the trajectory of functional status before and after stroke using analysis of repeated measures of function, while adjusting for confounders. Since recurrent strokes were censored in this analysis, it is likely that some aspect of the first stroke, or the effect of clinically silent infarcts, causes the decline, and not only aging. Furthermore, the trajectory of functional decline was linear and not stepwise, suggesting that the decline may be due to ongoing effects of chronic exposures rather than episodic drops in function from discrete events. We found the functional decline among those with Medicaid or no insurance, possibly reflecting poorer access to health care,18, 19 poorer management of risk factors,19-21 or other effects of lower socioeconomic status.22, 23 The effect of insurance status was similar to that seen in previous research on functional status and quality of life in NOMAS,10, 24 and further research may clarify mechanisms of this effect.
There are several biological mechanisms through which ischemic stroke may cause delayed functional decline. The ischemic penumbra undergoes delayed neuronal death through apoptosis and necrosis that can take place over days to weeks after the stroke,25 which may cause delayed functional decline by delayed extension of infarcted tissue. Longer-term decline may be caused by other factors. For example, a single ischemic stroke may cause changes in inflammatory profiles26 that may have an ongoing deleterious effect on brain structure and function. In animal models, stroke sets in motion an endogenous inflammatory cascade that contributes to ongoing brain damage beyond the effect of ischemia alone.27 Changes in inflammatory profiles resulting from ischemic stroke may have a delayed and longstanding effect that may persist years after stroke.28 Such changes may cause subclinical, insidious decline in brain function that compromises functional status. Alternatively, static functional impairment after stroke may lead to progressive cardiovascular impairment and reduced fitness, which adversely effects performance in ADLs.29 Further research may clarify the mechanisms of the decline seen in this study.
Clinically silent infarcts may also account for the decline in function seen after stroke in this study. Silent infarcts are at least 5 times as prevalent as clinical strokes,30 share the same risk factors as clinical strokes,31, 32 accumulate over time in the absence of clinical strokes,33 and are associated with stroke and cognitive impairment.34, 35 Asymptomatic brain MRI abnormalities, including white matter hyperintensities and infarcts, have been associated with functional impairment cross-sectionally,36 at 3 months,37 and over 4 years of follow-up.38
Advantages of this study include its population-based design and random sampling techniques, which allow for good generalizability to the urban, multi-ethnic source population. Furthermore, NOMAS follows a large sample of individuals with little loss to follow-up, and its repeated measures of a well-validated functional scale are a unique strength that allows detailed modeling of the trajectory of functional status over time. Also, the identification and classification of vascular events such as stroke is thorough and validated, which allows one to answer focused questions about the effects of first ischemic stroke.
Limitations of this study include the lack of data on outpatient longer-term rehabilitation, physical, or occupational therapy received after stroke, which may have an impact on outcomes. Another limitation is that covariates, including medical risk factors, demographic information, and insurance status, are assessed at entry into the study and not at the time of stroke. Hence, it is the association of these factors at study entry with long-term outcomes that is being assessed. The advantage of this fact is that the timing of data collection is appropriate to the study question, which focuses on baseline predictors of long-term outcome. Furthermore, the status of most of the covariates is unlikely to change over time, especially demographic characteristics and some medical and social risk factors. The advantage of the modeling strategy is that it allows one to assess whether the slope of decline is different before and after an event. However, the model assumes that the effect of baseline factors is the same before and after stroke, which may not be the case. Finally, the decline in functional status applies to older stroke patients as seen in this cohort, but these findings cannot be directly applied to younger patients, and more research is needed on long-term functional outcomes in younger stroke survivors.
In summary, we have found epidemiological evidence of a long-term and linear decline in functional status after stroke, at least among those with limited resources, which is more than 3 times as steep as the decline before stroke. This decline was seen even in the absence of recurrent clinical strokes. Considering these findings, it is possible that stroke may be not only a discrete event but also an ongoing, chronic condition with effects on function. In terms of functional status, strokes may be considered as events that occur on a continuum of functional decline. Further research is needed to clarify the cause of this decline, and to identify the elements of ischemic stroke that would result in such a long-term decline, even in the absence of recurrent events.
Acknowledgements
Mitchell Elkind had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Sources of funding
This work was supported by grants from the National Institute of Neurological Disorders and Stroke (R01 NS48134, MSVE; R37 29993, RLS/MSVE).
Footnotes
Disclosures
Mandip S. Dhamoon reports no disclosures.
Yeseon Park Moon reports no disclosures.
Myunghee C. Paik reports no disclosures.
Ralph L. Sacco has received research grants from NINDS.
Mitchell S. V. Elkind serves as Resident and Fellow Section Editor for Neurology; serves as a consultant to GlaxoSmithKline, Organon, and Jarvik Heart, and Tethys Bioscience, Inc.; receives research support from diaDexus, Inc., Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership, and from the NIH/NINDS [R01 NS050724 (PI), NS048134 (PI), P50 NS049060 (Project PI), R27 NS029993 (Co-PI), R01 NS55809 (Co-I) and R01 NS062820 (Co-I)].
None of the authors has a financial relationship relevant to the topic of the manuscript.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaplan PE. Rehabilitation of stroke. Butterworth-Heinemann; Burlington, MA: 2003. [Google Scholar]
- 2.Andrews K, Brocklehurst JC, Richards B, Laycock PJ. The rate of recovery from stroke - and its measurement. Int Rehabil Med. 1981;3:155–161. doi: 10.3109/03790798109166795. [DOI] [PubMed] [Google Scholar]
- 3.Dignan MB, Howard G, Toole JF, Becker C, McLeroy KR. Evaluation of the north carolina stroke care program. Stroke. 1986;17:382–386. doi: 10.1161/01.str.17.3.382. [DOI] [PubMed] [Google Scholar]
- 4.Dombovy ML, Sandok BA, Basford JR. Rehabilitation for stroke: A review. Stroke. 1986;17:363–369. doi: 10.1161/01.str.17.3.363. [DOI] [PubMed] [Google Scholar]
- 5.de Groot-Driessen D, van de Sande P, van Heugten C. Speed of finger tapping as a predictor of functional outcome after unilateral stroke. Arch Phys Med Rehabil. 2006;87:40–44. doi: 10.1016/j.apmr.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 6.Pan SL, Lien IN, Yen MF, Lee TK, Chen TH. Dynamic aspect of functional recovery after stroke using a multistate model. Arch Phys Med Rehabil. 2008;89:1054–1060. doi: 10.1016/j.apmr.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 7.Hankey GJ, Spiesser J, Hakimi Z, Bego G, Carita P, Gabriel S. Rate, degree, and predictors of recovery from disability following ischemic stroke. Neurology. 2007;68:1583–1587. doi: 10.1212/01.wnl.0000260967.77422.97. [DOI] [PubMed] [Google Scholar]
- 8.Johnston M, Pollard B, Morrison V, MacWalter R. Functional limitations and survival following stroke: Psychological and clinical predictors of 3-year outcome. Int J Behav Med. 2004;11:187–196. doi: 10.1207/s15327558ijbm1104_1. [DOI] [PubMed] [Google Scholar]
- 9.Jorgensen HS, Reith J, Nakayama H, Kammersgaard LP, Raaschou HO, Olsen TS. What determines good recovery in patients with the most severe strokes? The copenhagen stroke study. Stroke. 1999;30:2008–2012. doi: 10.1161/01.str.30.10.2008. [DOI] [PubMed] [Google Scholar]
- 10.Dhamoon MS, Moon YP, Paik MC, Boden-Albala B, Rundek T, Sacco RL, et al. Long-term functional recovery after first ischemic stroke: The northern manhattan study. Stroke. 2009;40:2805–2811. doi: 10.1161/STROKEAHA.109.549576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sacco RL, Anand K, Lee HS, Boden-Albala B, Stabler S, Allen R, et al. Homocysteine and the risk of ischemic stroke in a triethnic cohort: The northern manhattan study. Stroke. 2004;35:2263–2269. doi: 10.1161/01.STR.0000142374.33919.92. [DOI] [PubMed] [Google Scholar]
- 12.Sacco RL, Gan R, Boden-Albala B, Lin IF, Kargman DE, Hauser WA, et al. Leisure-time physical activity and ischemic stroke risk: The northern manhattan stroke study. Stroke. 1998;29:380–387. doi: 10.1161/01.str.29.2.380. [DOI] [PubMed] [Google Scholar]
- 13.Shinar D, Gross CR, Bronstein KS, Licata-Gehr EE, Eden DT, Cabrera AR, et al. Reliability of the activities of daily living scale and its use in telephone interview. Arch Phys Med Rehabil. 1987;68:723–728. [PubMed] [Google Scholar]
- 14.Kaambwa B, Billingham L, Bryan S. Mapping utility scores from the barthel index. Eur J Health Econ. 2011 Nov 2; doi: 10.1007/s10198-011-0364-5. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 15.Gan R, Sacco RL, Kargman DE, Roberts JK, Boden-Albala B, Gu Q. Testing the validity of the lacunar hypothesis: The northern manhattan stroke study experience. Neurology. 1997;48:1204–1211. doi: 10.1212/wnl.48.5.1204. [DOI] [PubMed] [Google Scholar]
- 16.Verheyden G, Nieuwboer A, De Wit L, Thijs V, Dobbelaere J, Devos H, et al. Time course of trunk, arm, leg, and functional recovery after ischemic stroke. Neurorehabil Neural Repair. 2008;22:173–179. doi: 10.1177/1545968307305456. [DOI] [PubMed] [Google Scholar]
- 17.Jorgensen HS, Nakayama H, Raaschou HO, Vive-Larsen J, Stoier M, Olsen TS. Outcome and time course of recovery in stroke. Part ii: Time course of recovery. The copenhagen stroke study. Arch Phys Med Rehabil. 1995;76:406–412. doi: 10.1016/s0003-9993(95)80568-0. [DOI] [PubMed] [Google Scholar]
- 18.Giacovelli JK, Egorova N, Nowygrod R, Gelijns A, Kent KC, Morrissey NJ. Insurance status predicts access to care and outcomes of vascular disease. J Vasc Surg. 2008;48(4):905–11. doi: 10.1016/j.jvs.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calman NS, Golub M, Ruddock C, Le L, Hauser D. Separate and unequal care in new york city. J Health Care Law Policy. 2006;9:105–120. [PubMed] [Google Scholar]
- 20.Mold F, McKevitt C, Wolfe C. A review and commentary of the social factors which influence stroke care: Issues of inequality in qualitative literature. Health Soc Care Community. 2003;11:405–414. doi: 10.1046/j.1365-2524.2003.00443.x. [DOI] [PubMed] [Google Scholar]
- 21.Kiely DK, Morris JN, Morris SA, Cupples LA, Ooi WL, Sherwood S. The effect of specific medical conditions on functional decline. J Am Geriatr Soc. 1997;45:1459–1463. doi: 10.1111/j.1532-5415.1997.tb03196.x. [DOI] [PubMed] [Google Scholar]
- 22.Grau AJ, Ling P, Palm F, Urbanek C, Becher H, Buggle F. Childhood and adult social conditions and risk of stroke. Cerebrovasc Dis. 2012;33:385–391. doi: 10.1159/000336331. [DOI] [PubMed] [Google Scholar]
- 23.Addo J, Ayerbe L, Mohan KM, Crichton S, Sheldenkar A, Chen R, et al. Socioeconomic status and stroke: An updated review. Stroke. 2012;43:1186–1191. doi: 10.1161/STROKEAHA.111.639732. [DOI] [PubMed] [Google Scholar]
- 24.Dhamoon MS, Moon YP, Paik MC, Boden-Albala B, Rundek T, Sacco RL, et al. Quality of life declines after first ischemic stroke. The northern manhattan study. Neurology. 2010;75:328–334. doi: 10.1212/WNL.0b013e3181ea9f03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaushal V, Schlichter LC. Mechanisms of microglia-mediated neurotoxicity in a new model of the stroke penumbra. J Neurosci. 2008;28:2221–2230. doi: 10.1523/JNEUROSCI.5643-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaremba J, Losy J. Early tnf-alpha levels correlate with ischaemic stroke severity. Acta Neurol Scand. 2001;104:288–295. doi: 10.1034/j.1600-0404.2001.00053.x. [DOI] [PubMed] [Google Scholar]
- 27.Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, et al. Regulatory t cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- 28.Theodorou GL, Marousi S, Ellul J, Mougiou A, Theodori E, Mouzaki A, et al. T helper 1 (th1)/th2 cytokine expression shift of peripheral blood cd4+ and cd8+ t cells in patients at the post-acute phase of stroke. Clin Exp Immunol. 2008;152:456–463. doi: 10.1111/j.1365-2249.2008.03650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ivey FM, Macko RF, Ryan AS, Hafer-Macko CE. Cardiovascular health and fitness after stroke. Top Stroke Rehabil. 2005;12:1–16. doi: 10.1310/GEEU-YRUY-VJ72-LEAR. [DOI] [PubMed] [Google Scholar]
- 30.Vermeer SE, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MM. Prevalence and risk factors of silent brain infarcts in the population-based rotterdam scan study. Stroke. 2002;33:21–25. doi: 10.1161/hs0102.101629. [DOI] [PubMed] [Google Scholar]
- 31.Vermeer SE, Longstreth WT, Jr., Koudstaal PJ. Silent brain infarcts: A systematic review. Lancet Neurol. 2007;6:611–619. doi: 10.1016/S1474-4422(07)70170-9. [DOI] [PubMed] [Google Scholar]
- 32.Hajjar I, Quach L, Yang F, Chaves PH, Newman AB, Mukamal K, et al. Hypertension, white matter hyperintensities, and concurrent impairments in mobility, cognition, and mood: The cardiovascular health study. Circulation. 2011;123:858–865. doi: 10.1161/CIRCULATIONAHA.110.978114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Longstreth WT, Jr., Arnold AM, Kuller LH, Bernick C, Lefkowitz DS, Beauchamp NJ, et al. Progression of magnetic resonance imaging-defined brain vascular disease predicts vascular events in elderly: The cardiovascular health study. Stroke. 2011;42:2970–2972. doi: 10.1161/STROKEAHA.111.622977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hachinski V. World stroke day 2008: “Little strokes, big trouble. Stroke. 2008;39:2407–2420. doi: 10.1161/STROKEAHA.108.531681. [DOI] [PubMed] [Google Scholar]
- 35.Longstreth WT, Jr., Dulberg C, Manolio TA, Lewis MR, Beauchamp NJ, Jr., O’Leary D, et al. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: The cardiovascular health study. Stroke. 2002;33:2376–2382. doi: 10.1161/01.str.0000032241.58727.49. [DOI] [PubMed] [Google Scholar]
- 36.Baune BT, Schmidt WP, Roesler A, Berger K. Functional consequences of subcortical white matter lesions and mri-defined brain infarct in an elderly general population. J Geriatr Psychiatry Neurol. 2009;22:266–273. doi: 10.1177/0891988709342722. [DOI] [PubMed] [Google Scholar]
- 37.Pohjasvaara TI, Jokinen H, Ylikoski R, Kalska H, Mantyla R, Kaste M, et al. White matter lesions are related to impaired instrumental activities of daily living poststroke. J Stroke Cerebrovasc Dis. 2007;16:251–258. doi: 10.1016/j.jstrokecerebrovasdis.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Rosano C, Kuller LH, Chung H, Arnold AM, Longstreth WT, Jr., Newman AB. Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. J Am Geriatr Soc. 2005;53:649–654. doi: 10.1111/j.1532-5415.2005.53214.x. [DOI] [PubMed] [Google Scholar]