Abstract
Objective
Fear reactivity during exposure is a commonly used indicator of learning and overall therapy outcome. The objective of this study was to assess the predictive value of fear reactivity during exposure using multimodal indicators and an advanced analytical design. We also investigated the degree to which treatment condition (cognitive training versus respiratory skill training) moderated fear reactivity and therapeutic outcome.
Method
Thirty-four patients with panic disorder and agoraphobia completed a total of 123 in-vivo exposure sessions, comprised of three weekly sessions and a fourth session 2 months following therapy completion. Sessions varied in length and phobic stimuli. Cardio-respiratory physiology (heart rate, PCO2, respiration rate) and experiential symptoms (panic symptoms and anxiety) were assessed repeatedly throughout exposure sessions, in addition to weekly assessments of panic cognitions, avoidance, and functioning.
Results
Panic symptomatology decreased substantially in both treatment conditions during therapy and follow-up. Significant cardio-respiratory and experiential reactivity was observed during all exposures, characterized by activation followed by reduction. Greater within-session activation of anxiety and panic symptoms was inversely related to improvement in panic symptoms severity, but neither physiological activation, nor within- or between-session reduction of either physiological or experiential variables was predictive of outcome. No moderating effects of treatment condition were found.
Conclusions
Fear activation and reduction during exposure are weak predictors of corrective learning and fear extinction. Clinical implications for exposure therapy and directions for future research are discussed.
Keywords: fear extinction, exposure, physiology, predictor, habituation, activation
Exposure therapy is a core element of behavior therapy for anxiety disorders. Confrontation with feared situations or sensations is thought to facilitate corrective learning by means of acquisition of fear-discordant information. The process of activation of the fear memory/structure, response to the stimulus, and the interpretation of the stimulus and response (Lang, 1971) has been hypothesized as a vital mechanism underlying extinction learning during exposure (e.g., Foa & Kozak, 1986).Specifically, activation is achieved by exposing the individual to cues related to the fear network, and is indexed by physiological (e.g., heart rate accelerations) and experiential (e.g., sensations of heart racing) reactivity that reaches beyond pre-exposure levels. Once activated, the integration of the corrective information/memory, which is incompatible with the components of the fear structure, takes places (Foa & McNally, 1996). Successful integration is indexed by the degree of fear decline during, as well as across, exposure sessions (termed within- and between-session reduction, respectively). Although there are a number of prevailing models on the mechanisms of fear extinction (e.g., Hofmann, 2008; Lissek et al., 2005; for reviews), the basic propositions of fear reactivity (activation and reduction) profoundly influence current clinical practice. Treatment manuals (e.g., Hope, Heimberg, & Turk, 2006, for social anxiety; Foa, Hembree, & Rothbaum, 2007, for PTSD; Antony, Craske, & Barlow, 2006, for specific phobia) often equate fear reactivity during exposure with treatment success (e.g., “These processes, decrease of distress within and between exposure sessions, are thought to indicate that the treatment is succeeding.” (Kozak & Foa, 1997, p. 97). Consequently, therapists should “emphasize the goal of remaining in the situation until her SUDS [subjective units of discomfort distress] decreases by at least 50%”. (Foa, Hembree, & Rothbaum, 2007, p. 72). Graphic displays of successful reactivity patterns are common, “with initial SUDS ratings [Y axis] to be rather high and then to decline over time [X axis].” (Hope et al., 2006, p.106).
Given the extensive clinical use of fear reactivity as a predictor of exposure success, one would expect there to be solid empirical evidence testifying to this relation. In a comprehensive review by Craske and colleagues (2008), the authors examined the ability of fear levels during exposure to predict therapeutic outcome. Overall support was weak across studies. For instance, the absence of within-session heart rate decreases during exposures in public-speaking anxious or acrophobic individuals did not result in poorer outcome (Tsao & Craske, 2000; Lang & Craske, 2000; Rachman, Craske, Tallman, & Solyom, 1986), while greater in-session reduction of heart rate in snake-phobic individuals (Rachman, Robinson, & Lopathka, 1987) was even associated with greater return of fear. However, Craske’s (2008) comparison of results across studies was hampered by the studies’ suboptimal methodologies, including lack of, or inadequate, physiological data, poor outcome measures, inadequate exposure situations or materials, or suboptimal timing of process and outcome measures.
Given the presumed importance of fear reactivity on the success of exposure treatments, and given the vital need for understanding the mechanisms explaining extinction learning in order to maximize treatment efficacy (Kazdin, 2007; McNally, 2007; Kendall et al., 2009), the primary aim of this study was to examine the predictive value of fear reactivity during repeated in-vivo exposures for patients suffering from panic disorder with agoraphobia. In an effort to improve upon methodological and analytic shortcomings of the prior research (see Craske et al., 2008), the following steps were taken. (1) We assessed indices of fear reactivity across two dimensions (both experiential and physiological), using multiple measures within these dimension. The physiological measures included carbon dioxide (PCO2) and respiration rate (RR), in addition to heart rate (HR). Conceptually, etiological models of panic focus on the role of respiration, specifically CO2, the importance of which has been substantiated by decades of experimental (Smits, Meuret, Zvolensky, Rosenfield, & Seidel, 2009; Dager et al., 1995; Maddock and Carter, 1991; Wilhelm et al., 2001; Blechert, Wilhelm, Meuret, Wilhelm, & Roth, 2010; Gorman et al., 2004; Papp et al., 1995; Meuret & Ritz, 2010 for a review) and clinical research (Meuret, Wilhelm, Ritz, & Roth, 2008, Meuret, Rosenfield, Hofmann, Suvak, & Roth, 2009; Meuret, Rosenfield, Seidel, Bhaskara, & Hofmann, 2010). In a recent ambulatory study, respiratory dysregulation emerged as a prime physiological indicator of impending panic attacks in the hour preceding naturally occurring panic attacks (Meuret, Rosenfield, et al., in press). Notwithstanding, with the exception of a few exposure studies in patients with specific phobias (Wilhelm & Roth, 1998; Alpers, Wilhelm, & Roth, 2005), there are no studies to date examining reactivity of respiration during exposure, either as a process measure or as a predictor of outcome. (2) In addition to measuring multiple indices of the fear network, the setting and duration of the exposures were tailored to best approximate the individual’s fear network. This was done with the intent to maximize the person-specific disconfirmatory process of their fear propositions. As proposed by Craske et al. (2008), exposures should be unrestrained in duration and incorporate a variety of situations (e.g., Marshall, 1985) to evoke a violation (or mismatch) of the expected adverse outcome and lack of occurrence thereof. (3) Reductions in panic disorder severity were assessed weekly during exposure and then two months following treatment termination. This approach allowed sufficient time to evaluate stable change (Shear, Clark, & Feske, 1998), rather than simply looking at temporary changes that may not reflect changes in the underlying fear structure (e.g., Baker et al., 2010). (4) Finally, initial severity and slopes of improvement over time were incorporated into our models, controlling for confounds between activation and initial severity, improving reliability of our outcome measure, and controlling for regression to the mean (Tabachnik & Fidell, 2007). This controls for the possibility that activation relates to improvement simply because it is related to initial severity, which itself is related to improvement in symptoms. Therefore, multilevel, longitudinal growth curve analyses were applied to determine whether experiential and physiological reactivity during exposure predicted slopes of improvement in outcome, as opposed to just predicting outcome at post- or follow-up. Since the slopes are based on multiple data points, they are more accurate and stable than single point outcomes at particular assessment points (e.g., Kraemer & Thiemann, 1989; Rauch et al., 2004)
A secondary aim of this study was to investigate whether theoretically distinct coping skills would differentially moderate fear response and reduction in panic disorder severity. This question is vital because exposure treatment is rarely provided in isolation from other treatment techniques (see for review, Meuret, Wolitzky-Taylor, Twohig, & Craske, in press). Moreover, past reports have raised concern about the effects of “arousal-dampening” coping strategies or safety behaviors such as breathing retraining (e.g., Craske, Rowe, Lewin, & Noriega-Dimitri, 1997; Rachman, Radomsky, & Shafran, 2008, for a review). However, empirical evidence for this thesis is surprisingly absent (see Meuret, Wilhelm, Ritz, & Roth, 2003, for a review), in part because proof of concept would require careful assessment and testing of successful skill acquisition (i.e., normalization of CO2) and subsequent application during exposure (i.e., less arousal during exposure; Meuret, Wolitzky-Taylor, et al., in press). The present study investigated the degree to which successful acquisition of respiratory skills was applied during exposure, dampens physiological response, and differentially influences the effect of fear reactivity on outcome. Examining the degree to which coping skills may differentially impact fear response and outcome will aid understanding the factors that contribute to treatment success.
Thus, the primary aims of the present investigation are 1) to examine the effect of fear reactivity on overall outcome from treatment for panic disorder, and 2) to determine if these effects differ depending on treatment modality (cognitive training vs. respiratory skills training).
Methods
Study Design and Procedure
Participants
Participants were enlisted from the community through an outpatient center, the Center for Anxiety and Related Disorders (CARD) at Boston University, Massachusetts (n = 18, where the study was initiated) and through online and flyer advertisements at the Anxiety, Stress, and Chronic Disease Program at Southern Methodist University (SMU) in Dallas, Texas (n = 16, where the study was concluded). In this two-phase intervention, patients were randomized (within each site) to first receive five individual, weekly, one-hour sessions of respiratory skill training (CART) or cognitive skill training (CT) (Phase I – Skill Acquisition Training, Meuret et al., 20101), followed by three weekly sessions of in-vivo exposure (Phase II, Application Training). Randomization software was used to assign patients to condition. Thirty-four patients who had completed at least one exposure session were included in the data analysis of this study (CART = 17; CT = 17). Written consent was provided by all participants. All patients met the inclusion and exclusion criteria: a) minimum age of 18 years, b) current principal diagnosis (i.e., the disorder presently associated with the greatest life interference) of panic disorder with agoraphobia, c) being on a stable dose of psychotropic medication for at least three months before study initiation (if applicable), and agreement to continue this dose through the two month follow-up appointment, d) agreement not to initiate additional therapy until after the final follow-up appointment, and e) no indication of a history of bipolar disorder, psychotic disorder, suicidal intention, current substance abuse or dependence, or current organic mental disorder, serious unstable medical disease, respiratory illness, or seizures.
The patients were primarily white (91.2%), female (79.5%), well educated (M = 16.1 years of education, SD = 2.50, range: 12–20 years), and employed (76.5%). Fifteen patients were married. The sample had an average age of 32.9 years (SD = 9.03). Additional ethnic groups consisted of Hispanics (5.9%) and other (2.9%). Levels of agoraphobic avoidance were as followed: 2.9% reported mild, 26.5% moderate, 55.9% severe and 14.7% extreme levels, based on the agoraphobic avoidance item (item 4) of the Panic Disorder Severity Scale (PDSS; Shear et al., 1997). Fourteen participants met criteria for at least one other DSM-IV Axis-I diagnosis (anxiety disorder [26.5%], depression [5.9%], anxiety disorder and depression [8.8%]). The majority of the patients (58.8%) were on a stable dose of psychotropic medication (antidepressants [65.0%], benzodiazepines [70.0%], beta-blockers [5.0%]), with 30.0% of the participants taking more than one type of medication. The overall attrition rate for the exposure treatment was 11.8% (4 out of the 34 patients dropped out before the 2 month follow-up; CART: 17.6% [3 out of 17]; CT: 5.9% [1 out of 17]). Two additional patients from the CT condition did not return for the 2-month follow-up. MANOVAs, logistic regressions, t-tests, and chi-squares showed no treatment condition or site differences for the demographic or clinical variables prior to the first exposure session. Chi-squares showed no attrition differences due to treatment group (χ2 (1) = 1.13, p = .60, nor to site (BU = 2 out of 18, 11.1%; SMU = 2 out of 16, 12.5%; χ2 (1) = .02, p = .90). There were also no treatment group differences in number or duration of exposure sessions, t(32) = 1.19, p = .24 and t(32)=.89, p = .38, respectively. See Table 1 for details on demographic information and clinical characteristics.
Table 1.
Comparison between treatment conditions in demographic and clinical characteristics.
| CART (n = 17) |
CT (n = 17) |
|||||
|---|---|---|---|---|---|---|
| Variable | N(%) | M(SD) | N(%) | M(SD) | χ2(df) | t(df) |
| Patient Characteristics | ||||||
| Female | 13(76.6) | 14(82.4) | .18(1) | |||
| Age (years) | 31.24(7.82) | 34.59(10.06) | −1.09(32) | |||
| White | 15(88.2) | 16(94.1) | 1.03(2) | |||
| Family and social background | ||||||
| Education (years) | 16.24(2.61) | 15.88(2.45) | .41(32) | |||
| Married | 8(47.1) | 7(41.2) | 5.55(4) | |||
| Additional Axis I diagnosis | 6(35.3) | 8(57.1) | 1.48(1) | |||
| Use of psychotropic medication | 10(58.8) | 12(70.6) | .52(1) | |||
| Clinical baseline characteristics prior to exposure therapy | ||||||
| PDSS | 12.67(4.41) | 10.29(.60) | 10.78(28) | |||
| ASI | 28.16(12.16) | 26.72(15.36) | 0.27(29) | |||
| BSQ | 32.47(12.58) | 31.45(16.15) | 0.21(29) | |||
| MI-AAL | 69.12(35.37) | 53.73(19.44) | 1.50(29) | |||
| ACS | 74.82(24.87) | 78.99(13.44) | 0.59(29) | |||
| Physiological and experiential baseline characteristics prior to exposure session 1 | ||||||
| PCO2 | 38.33(2.73) | 35.13(5.58) | 2.08(29)* | |||
| HR | 72.00(11.00) | 73.06(12.82) | −.25(29) | |||
| RR | 11.07(4.29) | 15.87(3.63) | −3.32(29)** | |||
| DSM panic symptoms | 1.57(1.79) | 1.51(1.50) | .01(32) | |||
| Anxiety | 5.35(2.60) | 5.35(2.37) | .00(32) | |||
Note:
p < .05,
p < .01.
CART: Capnometry Assisted Breathing Retraining Therapy; CT: Cognitive Therapy. PDSS: Panic Disorder Severity Scale; ASI: Anxiety Sensitivity Index; BSQ: Body Sensations Questionnaire; MI-AAL: Mobility Inventory for Agoraphobia – When Alone; ACS: Anxiety Control Scale
Interventions
In-vivo exposure therapy (EXP)
Patients were randomly assigned to receive 4 weeks of either cognitive skills training (CT) or respiratory skill training (CART; see details below). After completing the skills training, they underwent three weekly in-vivo exposure sessions plus a fourth session at two-month follow-up. Exposure treatment was provided by trained clinicians (three were master level clinicians and one was a doctoral level clinician) and was conducted according to a structured manual (Craske, Barlow, & Meadows, 2000; in-vivo exposure component). In preparation for the in-vivo exposures, patients were given an in-depth explanation on the rationale of exposure therapy, explaining the necessity for recurring and extended exposure to their feared situations. Patients were encouraged to apply the respective coping skills (see details below) which they had acquired during the prior four weeks before entering the exposure phase of the treatment. No additional skills/behaviors were encouraged or provided. The exposure situations were based on the patient’s fear and avoidance hierarchy that was developed prior to the first exposure session. Patients were asked to rank and rate 10 situations that they either avoided or endured with great distress on a scale from 0 = do not avoid/no anxiety to 8 = always avoid/extreme anxiety. The fear-provoking situations in this study included driving, public transportation (e.g., subway, busses, boats, and trains), movie theaters, enclosed spaces (e.g., elevators, closets), tall buildings and bridges, and crowded places (e.g., restaurants and shopping malls). Patients were encouraged to choose the highest ranked situation that they felt they were able to enter and endure. The exposure situation varied at each of the exposures (i.e., different subway lines) to enhance inhibitory learning and insure a generalization effect (e.g., Bouton, 1993). Additionally, about half of the exposures (47.5%) took place without the presence of the therapist (if their presence was perceived as fear-reducing). In these cases, the therapist introduced the patient to the exposure situation, then left, and waited at an agreed location. Patients were asked to stay in the situation until they felt the situation no longer presented a substantial threat. A minimum duration of 30 minutes was encouraged. A total of 123 out of 136 possible sessions were performed (90.4%). Twenty-eight patients (82.4%) completed all four exposures, 2 (5.9%) completed 3 sessions, 1 (2.9%) completed 2 sessions, and 3 (8.8%) completed 1 session. The exposures lasted 62.2 minutes on average, with a range of 13 to 196 minutes, excluding baseline and recovery. Ninety-two percent of exposures lasted 30 minutes or more (range 16–196 minutes). The use of benzodiazepines prior to or during the exposures was strongly discouraged.
Capnometry-assisted breathing training
(CART; Meuret et al., 2008, Meuret, Rosenfield, et al., 2010) is based on the premise that basal or acute levels of hypocapnia (lower than normal levels of PCO2, < 36 mmHg) plays an important role in the etiology and maintenance of panic disorder. Consequently, the main focus of CART is to normalize PCO2 back into a normocapnic range. To achieve normocapnic levels, patients were trained to decrease tidal volume (i.e., breathe more shallowly) and respiration rate (i.e., breathe more slowly). The treatment consisted of four components: a) psychoeducation about the negative impact of hyperventilation and respiratory dysregulation on panic symptoms, b) raising awareness of possible maladaptive respiratory pattern, c) instruction in methods to control respiratory pattern, specifically PCO2, and d) training and reviewing during twice daily between-session exercises which focused on paced breathing and self-monitoring of PCO2 and RR.
Cognitive training
(CT) is based on the premise that maladaptive cognitions play an essential role in the etiology and maintenance of panic disorder (Clark, 1986). Consequently, patients were trained to identify and restructure catastrophic thought processes about panic related feared sensations using adaptive cognitions. CT consisted of four components: a) psychoeducation about the negative impact of maladaptive thoughts on symptoms, b) recognition of maladaptive thoughts and images related to the activation of panic attacks, c) training in substituting adaptive thoughts for maladaptive thoughts and images, and d) training in and reviewing of twice-daily between-session exercises which focused on substitutions for maladaptive cognitions.
Measures
Diagnostic Assessment
The Anxiety Disorders Interview Schedule for DSM-IV (ADIS-IV-L; DiNardo, Brown, & Barlow, 1994) was administered to verify panic disorder diagnoses and to assess for comorbidity at CARD, and the Structured Clinical Interview for DSM-IV Axis-I disorders (patient edition; SCID; First, Spitzer, Gibbon, & Williams, 1995) was administered at SMU. These wellestablished instruments have shown good interrater reliability (ADIS-IV-L: Brown, Di Nardo, Lehman, & Campbell, 2001; SCID: Williams et al., 1992). At CARD, the diagnoses were determined by agreement with senior and independent clinicians at weekly supervision meetings. At SMU, diagnostic reliability was established by having a second assessor rate 20% of randomly chosen interviews (ê= .93 for primary DSM-IV anxiety and mood disorders).
Treatment Outcome Measures
All measures, except the panic symptom severity, were assessed immediately prior to each exposure session, including the two-month follow. Panic disorder severity was assessed prior to the inception of the first exposure session (pre), after the last exposure session (post), and at the 2-month follow-up assessment.
Our primary outcome measure was the Panic Disorder Severity Scale (PDSS; Shear et al., 1997), a commonly used semi-structured interview to assess changes in panic disorder severity. The PDSS captures core disorder-specific characteristics, including the severity and intensity of panic attacks, anticipatory anxiety, sensational and situational avoidance, as well as social and occupational impairment. The response format of this 7-item, clinician rated scale ranges from 0 = “none” to 4 = “extreme or definite”. Two raters independently conducted the interviews, with the second rater being uninformed as to the patient’s group assignment. Interrater reliability in this study was excellent (ICC = .96-.97 from mid to follow-up). Henceforth, the PDSS will be referred to as panic disorder severity.
In addition to the PDSS, we assessed change in a series of secondary panic and anxiety outcome self-report questionnaires. The Anxiety Sensitivity Index (ASI; Reiss, Peterson, Gursky, & McNally, 1986) measures fear of symptoms linked to anxiety, and the potential undesirable effects of these symptoms. The five-point response format of this 16-item scale ranges from 0 = “very little” to 4 = “very much”. The measure has shown good internal consistency (á = .82; Telch, Shermis, & Lucas, 1989), test-retest reliability, and criterion validity (Reiss et al., 1986, respectively). The Body Sensations Questionnaire (BSQ; Chambless, Caputo, Bright, & Gallagher, 1984) measures bodily sensations that are associated with anxiety and distress. The response format of this 17-item questionnaire ranges from 1 = “not frightened or worried by this sensation” to 5 = “extremely frightened by this sensation”. The questionnaire has shown good internal consistency, moderate test-retest reliability, and good discriminative validity for individuals with agoraphobia in comparison to healthy control subjects (Chambless et al., 1984). The Mobility Inventory for Agoraphobia (MI-AAL; Chambless, Caputo, Jasin, Gracely, & Williams, 1985) assesses agoraphobic avoidance in different settings when alone. The response format of this 27-item inventory ranges from 1 = never avoid to 5 = always avoid. The MI-AAL has demonstrated good psychometric properties. The measure has shown excellent internal consistency, good to excellent 31-day test-retest reliability, good convergent validity (Marks and Mathews, 1979), and good discriminant validity (Chambless et al., 1985). The Anxiety Control Scale (ACS; Rapee, Craske, Brown, & Barlow, 1996) assesses perceived control that is associated with anxiety-provoking situations. The response format of this 30-item scale ranges from 0 = “strongly disagree” to 5 = “strongly agree”. The questionnaire has shown good internal consistency, test-retest reliability, convergent and discriminative validity (Rapee et al., 1996).
Measures of Within-Session Fear Reactivity
Physiological Indices
Cardio-respiratory data were collected during a five minute baseline just prior to exposure, continuously throughout the exposure, and during a five minute recovery period at the end of the exposure. The recovery assessment took place immediately after the patient left the fear-provoking (agoraphobic) situation. For example, if the exposure was on a commuter train, the recovery recording took place on the platform after exiting the train. All baseline and recovery recording periods complied with specific characteristics, such as a relatively quiet place with limited distraction and a sitting posture.
A portable, battery-run capnometry device (TIDALWAVE sp ®, Respironics, USA) was used to assess end-tidal PCO2 (a nonintrusive measure of CO2), respiration rate (RR), and heart rate (HR). The capnometry device captures exhaled gases through a nasal cannula for PCO2 analysis with the nondispersive infrared absorption technique. HR was measured by pulse wave photoplethysmography from the finger. Digital values of PCO2, RR, and HR were saved together with the time and date of the recording. The device complies with international standards for precision (Biedler et al., 2003). Physiological readings were masked to avoid providing feedback to the patient during exposure. Physiological data were edited for the potential influence with physical activity (see data reduction).
Experiential Indices
Intensity ratings for the 13 DSM-IV panic symptoms and anxiety were assessed by means of a self-report exposure log. Ratings on a scale of 0 = none to 10 = extreme were obtained at the 5 minute baseline recording, 30 minutes into the exposure (or earlier if exposure was briefer (e.g., elevator rides), 60 minutes into the exposure (if applicable), and at the end of exposure (recovery). At the latter assessment, patients were also asked to rate their maximum anxiety and DSM-IV symptoms experienced at any time during the exposure (i.e., peak ratings).
DSM-IV panic symptom ratings were averaged together to form a mean composite score, which henceforth will be referred to as DSM-IV panic symptoms. Anxiety was measured by a single item and rated on a scale from 0 (none) to 10 (extreme).
Data Reduction
Because physiological measures, in particular RR and HR, are highly responsive to physical activity, special attention was paid to limit data collection during periods of moderate or high physical activity. This was done by turning the device off (e.g., when changing subways, walking to or from exposure places). Additionally, data were inspected and edited for periods of moderate or high physical activity motion (measured by accelerometers, LifeShirt, Vivometrics, Inc.). Five out of the 123 exposure sessions were characterized by continuously high activity levels (e.g., when the exposure took place in a mall). For these, RR and HR were removed but PCO2 was retained, as the latter is less sensitive to phasic changes in movement. The data were then averaged in five minute intervals.
Analytic Strategy
Multilevel Modeling (MLM) was employed for all analyses. The repeated assessments of our variables over time comprised level 1 of these analyses, and these repeated assessments were nested within individuals (level 2). MLM accommodates both missing data and different numbers of assessments per session and per subject, and provides unbiased and accurate estimates of regression coefficients in samples as small as 30 subjects (Maas & Hox, 2005). It is the recommended method for analyzing psychiatric outcome data (Hamer & Simpson, 2009).
Treatment Efficacy Analyses
To examine treatment efficacy, we assessed the slope of change over time in our primary outcome variable, panic disorder severity (PDSS), and our secondary outcome measures (ASI, BSQ, MI-AAL, ACS), as well as in the physiological data (PCO2, RR, and HR) and the experiential variables (DSM-IV panic symptoms and anxiety). To allow for the change in outcome to “level off” over time, we modeled the change in outcome as curvilinear, using orthogonal polynomials for our linear and quadratic terms (Raudenbush & Lui, 2001). The quadratic term was dropped when it was not significant, and the model was rerun. A significant negative (or positive for PCO2) linear trend would indicate overall improvement over the course of treatment. Prior skills training (i.e., breathing therapy or cognitive therapy, referred to as “treatment condition”) and initial severity was included as level 2 moderators of the slopes of change over time.
Fear Reactivity as a Predictor of Improvement in Panic Symptom Severity over Time
To test the effects of fear activation and reduction on improvement in PDSS over time, we first calculated within-session activation and reduction of the physiological and experiential variables, and between session reduction for each patient. Within-session activation of physiological (PCO2, RR, and HR) and experiential variables (panic symptoms and anxiety) was calculated by subtracting the baseline value from the peak value during the exposure. Similarly, within-session reduction was calculated by subtracting the recovery score from the peak value during the exposure (Kozak, Foa, & Steketee, 1988). The PCO2 calculation was reversed since lower PCO2 is indicative of worse levels, not better, as in the other measures. The within-session measures of activation and reduction were averaged across the three exposure sessions (not including the follow-up exposure session) to obtain average activation and reduction. The follow-up exposure session was not included in the calculation of the average activation/reduction score since the PDSS assessment took place during the same session as exposure and hence could not have been impacted by the exposure during that session. Between-session reduction was calculated using MLM to determine the slope of change in peak values (minimum values for PCO2) during exposure across the 3 exposure sessions (not including follow-up). To determine if fear activation or reduction was related to improvement in PDSS over time, we used activation and reduction in each of the measures (PCO2, RR, HR, DSM-IV panic symptoms, and anxiety) as predictors of the slope of improvement in PDSS, each in a separate analysis. Further, since the relation between fear activation/reduction and outcome (PDSS) may be curvilinear (e.g., too much or too little activation may be less effective than medium levels of activation), we included a quadratic term for all analyses (activation/reduction squared) as an additional predictor of improvement in PDSS. When the quadratic trend was not significant, it was dropped from the analysis and the model was recomputed. Finally, to examine the effect of different coping skill training on the relation between fear activation/reduction and outcome, analyses included interactions between treatment condition and activation/reduction as predictors of slope of improvement in PDSS. Non-significant interactions were dropped before the final analyses were computed.
Because we performed numerous analyses to test each of the hypotheses, we adopted an alpha level of p≤.01 to partially correct for the multiple significance tests conducted. We chose not to examine the effect of fear activation/reduction on the secondary outcome measures since this would have greatly increased the number of significance tests performed (15 for each secondary outcome measure), thus risking further inflation of Type 1 error. Power analyses using the multilevel power analysis program PinT (Snijders & Bosker, 1993) confirmed that we had sufficient power (greater than .80) to detect a medium effect size for a predictor of the slope of change in PDSS over time. However, our power to detect a medium effect size for treatment condition moderating the effect of activation/habituation on outcome was below .80 (approximately .68). Thus, the analyses involving how the interaction of treatment condition and activation/habituation impact improvement in outcome should be considered exploratory.
Results
Results for the first four weeks of treatment (CART vs. CT training before the exposure sessions) are reported elsewhere (Meuret et al., 2010). The current study reports results over the following 3 weeks of exposure sessions, plus the 2 month follow-up.
Overall Treatment Efficacy
Change in Primary and Secondary Outcome Measures
Significant improvements were found for the primary and all secondary outcome measures (PDSS, ASI, BSQ, MI-AAL, ACS) across exposure sessions in both treatment conditions, as demonstrated by significant linear slopes over time (ps ≤ .01; Table 2). None of the measures displayed a significant curvilinear effect over time, indicating that the reductions of panic symptoms and anxiety continued through the 2-month follow-up.
Table 2.
Between-session analyses: Slopes of change over time
| Slopes |
Estimated Effect Size |
|||
|---|---|---|---|---|
| CART | CT | CART | CT | |
| Outcome Measures | ||||
| PDSS | −3.36** | −1.47** | 2.66 | 1.21 |
| ASIa | −1.76** | −1.68** | 1.10 | 1.05 |
| BSQ | −2.38** | −2.43** | 1.11 | 1.13 |
| MI-AALa | −3.40** | −3.13** | 3.57 | 3.29 |
| ACS | 4.50** | 3.39** | 1.65 | 1.24 |
| Physiological Measures | ||||
| PCO2a | .31 | −.47 | n/a | n/a |
| HRa | .04 | −1.13 | n/a | n/a |
| RRa | .45 | .47 | n/a | n/a |
| Experiential Measures | ||||
| DSM-IV panic symptomsa | −.21** | −.22** | .94 | 1.25 |
| Anxietya | −.88** | −.97** | 1.87 | 2.09 |
Note:
p ≤ .01. Effect sizes are estimated as β1/τ11 for the linear slope, and reported only for the significant slopes.
Moderated by pretreatment levels (i.e., initial severity) of the outcome variable (p < .01).
ASI: Anxiety Sensitivity Index; BSQ: Body Sensations Questionnaire; ACS: Anxiety Control Scale; MI-AAL: Mobility Inventory for Agoraphobia – When Alone; PDSS: Panic Disorder Severity Scale.
Change in Baseline Experiential and Physiological Indices
Baseline levels of the physiological variables (PCO2, RR, HR), measured at the beginning of each exposure session, did not change significantly from pre-exposure to follow-up. However, CART patients had PCO2 levels that were substantially higher, and in normocapnic range, compared to CT patients (p<.01), due to the prior respiratory training which was designed to correct chronically low PCO2 levels. On the other hand, significant improvement across exposure sessions was observed for both DSM-IV panic symptoms and anxiety (ps<.01; Table 2).
Treatment Condition and Pre-Treatment Severity of Symptoms as Moderators
Treatment condition did not moderate the slopes of change for the primary or secondary outcome measures, the physiological measures, nor experiential measures (ps>.01). However, pre-treatment severity levels were significantly related to rate of improvement for ASI, the MI-AAL, as well as all the physiological and experiential measures (all ps<.01; see Table 2). For each measure, greater initial severity was related to faster rates of improvement in the measure over the course of the exposure therapy.
Fear Reactivity during Exposure Sessions
Table 3 displays the average amount of within-session fear activation and reduction for each of the physiological and experiential variables during exposure. The amount of activation was significantly greater than 0 for all of the measures (ps<.001). Drops in PCO2 (from baseline to minimum) averaged almost 5 mmHg (range 1.19 to 14.86), indicating a substantial hyperventilatory response. Average HR increases were close to 12 beats per minute (range 0.88 to 28.34), and average RR increased almost 6 breaths/min. For the experiential variables, anxiety ratings increased from an average of 4.35 at baseline to an average of 5.32 at their maximum (22% increase). Intensity of the DSM-IV panic symptoms increased from an average of 1.23 at baseline to an average of 1.94 at maximum (58% increase). The seemingly small absolute change in the DSM-IV panic symptoms can be attributed to it being an average activation across the 13 DSM-IV panic symptoms, many of which where not reported to be present (and hence had no activation). There were no treatment condition differences in average activation for any of the physiological or experiential measures.
Table 3.
Mean (and SD) for within-session and between-session activation and reduction.
| Within-Session Activation |
Within-Session Reduction |
Between-Session Reductiona |
|
|---|---|---|---|
| Physiological Measures | |||
| PCO2 | 4.78(2.89)** | 2.43(2.84)** | 0.39(0.52) |
| HR | 11.79(6.06)** | 5.75(6.09)** | −0.51(1.00) |
| RR | 5.74(3.50)** | 4.91(3.66)** | −1.04(0.22)** |
| Experiential Measures | |||
| DSM-IV panic symptoms | 0.71(0.54)** | 0.28(0.40)** | 0.61(0.45) |
| Anxiety | 0.97(1.12)** | −0.93(0.93)** | 0.30(0.11)** |
Note:
p ≤ .01.
Between-session reduction is slope of change over the 3 weekly exposure sessions.
Fear Reactivity: Predictive Value
Within-session activation as a predictor of reductions in panic disorder severity
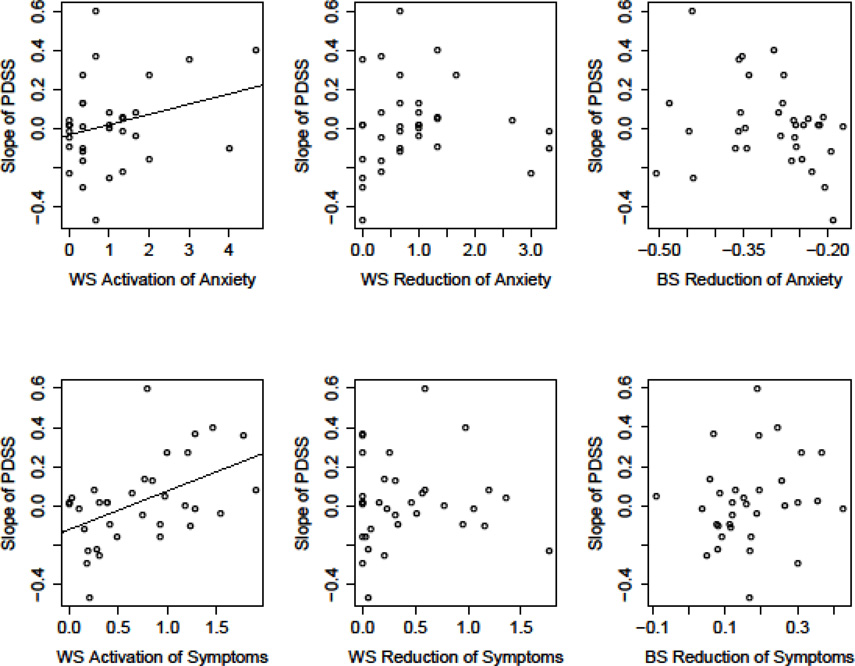
Amount of activation of the physiological variables (PCO2, HR, RR) was not a significant predictor of outcome (i.e., slope of change in PDSS). However, activation of the experiential variables was related to outcome. That is, higher levels of activation were related to slower rates of reductions in panic disorder severity for both anxiety, b = .13, t (66) = 2.52, p = .01, and DSM-IV panic symptoms, b = .18, t (60) = 3.47, p = .001 (Figure 1, left column). Performing these same analyses, but using the patient’s retrospective rating of their maximum anxiety and DSM-IV symptoms to calculate activation, yielded identical results.
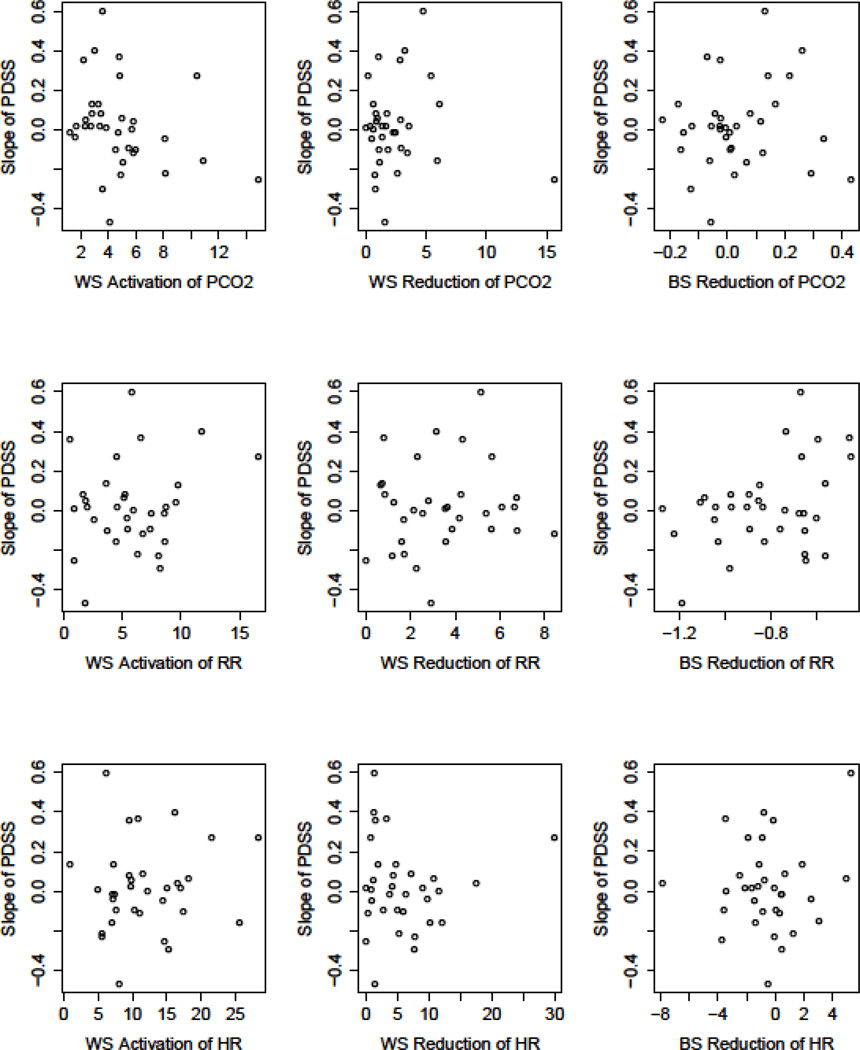
Figure 1.
Scatterplots depicting the predictive value of physiological (PCO2, RR, HR) and experiential (anxiety, DSM-IV panic symptoms) reactivity on panic symptom severity (PDSS) reductions. Slope of PDSS (on y-axis) represents the slope of change from pre –through follow-up. Values for fear reactivity (x-axis) represent average change for within session (WS) activation (peak minus baseline), WS reduction (peak minus recovery), and between-session (BS) reduction (slope of peak levels across session). Calculations for PCO2 were reversed since higher numbers for PCO2 reflect lesser (not greater) pathology. Only the relations between anxiety and DSM-IV symptoms with reductions in PDSS were significant (p≤.01; indicated as regression line).
Since the baseline levels of the physiological and experiential measures may have reflected some initial activation due to anticipatory anxiety (Craske et al., 2008), we also investigated alternative experiential and physiological baseline values, values that were unlikely to be affected by anticipatory anxiety. These values were taken from the 5-minute baseline recording of the last homework exercise of the CART/CT skill training (see Meuret, Rosenfield, et al., 2010). Activation at each exposure session was then calculated as the peak levels of each measure during the exposure session minus the “alternative baseline value”. This measure of activation was not significantly related to rates of improvement in PDSS.
Within-session fear reduction as a predictor of decline in panic disorder severity
The average amount of fear reduction was significantly greater than 0 for all the physiological and experiential variables (ps<.001; Table 3). However, the amount of reduction was significantly less than the amount of activation for PCO2 and HR (ps<.001), but not significantly less for RR, anxiety, or DSM-IV panic symptoms.
Fear reduction was not related to the slope of change in PDSS (ps ≥ .08) for any of the physiological or experiential variables (Figure 1, middle column). To further explore the relation of fear reduction on decline in PDSS, we calculated an alternative measure: reduction from the beginning of exposure to the end of exposure (recovery minus baseline), indicating the degree to which a person returned to (or bettered) baseline levels of symptoms. This measure of fear reduction was also unrelated to rates of decrease in PDSS (ps ≥.32).
Between-session reduction as a predictor of reductions in panic disorder severity
Between-session fear reduction (e.g., the slope of change in the peak levels of the physiological and experiential variables over the 3 exposure sessions) was not related to change in PDSS over time (Figure 1, right column). Additionally, the slope of change of the baseline level (assessed at the beginning of each exposure session), and the recovery levels (assessed at the end of each exposure session) of the experiential and physiological measures were not found to be related to treatment outcome.
Additional Analyses
In the previous analyses, we used average activation and average reduction over the three exposure sessions to predict decreases in PDSS. However, it could be that decreases (or increases) in activation/reduction may be more important than average level in determining slope of decrease in PDSS. Thus, we employed MLM to calculate the slopes of change in activation and reduction of the five physiological/experiential measures across the 3 exposure sessions. We then used the slope of change in activation and reduction for each of the five measures as predictors of change in PDSS. A separate analysis was conducted for each variable, and for activation and reduction of that variable. None were significant predictors of rates of reductions in panic disorder severity. Thus, neither changes in fear activation nor reduction appear to be related to rates of improvement in outcome.
It is also possible that activation or reduction at certain sessions (e.g., the first exposure session) might be more important in predicting outcome than the average activation or reduction across the 3 sessions. For example, Pitman and colleagues (1996) found heart rate activation during the first, but not consecutive sessions, to be related to reductions in PTSD symptoms (see also Rauch et al., 2004). Thus, we tested whether activation (or reduction) at any of the sessions predicted rate of change in PDSS over and above average activation/reduction. None did. Thus, activation/reduction at individual exposure sessions did not predict outcome differently than the average level of activation/reduction.
We also tested the possibility that the interaction of activation and reduction might be important in predicting rate of decrease in PDSS. For example, high levels of activation may be related to faster decreases in PDSS when coupled with high levels of reduction, but not when it occurs with low reduction. However, the interaction of activation and reduction was also not a significant predictor of slope of decrease in PDSS for any of the physiological or experiential variables.
In addition, although the use of any arousal-reducing medication (i.e., benzodiazepines and beta-blockers) prior to exposure was strongly discouraged, some patients may have taken them, which could have impacted their fear reactivity during exposure. We thus investigated whether benzodiazepines/beta-blockers moderated any of the relations between fear reactivity and outcome, and found no significant effects (ps>.10). Finally, we examined whether average duration of exposure sessions (in min), or peak levels during exposure, were predictive of outcome. Again, none of these were found to be significantly related to rates in change in outcome.
Discussion
The primary aim of this study was to investigate whether experiential and/or physiological reactivity during exposure therapy predicted changes in outcomes for panic patients. Significant cardio-respiratory and experiential reactivity was observed across exposures, characterized by activation followed by reduction. Thus, the criteria for successful fear reactivity were clearly met. Between-session reduction of maximum levels of the physiological and experiential variables during exposure showed overall declines in some measures (RR and anxiety), but not in others (HR, PCO2, and DSM symptoms). Thus, between-session reduction was less robust than within session activation and habituation, probably because exposure situations and length were purposely varied to maximize disconfirmation of the individual’s fear propositions. Comparable findings for a lack of between-session reduction for HR, but significant reductions for fear ratings, were found in study by Kozak et al. (1988) on psychophysiological indicators of emotional processing in patients with obsessive-compulsive disorder.
Despite successful fear activation and reduction, only 2 out of 15 indicators of fear reactivity were predictive of outcome. Neither within-session nor between-session reduction for either physiological (PCO2, HR, RR) or experiential (anxiety, DSM IV panic symptoms) measures were related to changes in panic disorder severity. Further, none of the physiological indices of within-session activation were related to outcome. These findings replicate in part the findings of previous studies (e.g., Tsao & Craske, 2000; Lang & Craske, 2000; Rachman et al., 1986). The only significant predictors of outcome were activation of anxiety and DSM-IV panic symptoms, with greater levels of activation resulting in poorer outcome (i.e., lesser reductions in panic symptom severity). Thus, these findings are in direct contrast with common beliefs that greater levels of fear activation will result in greater fear reduction and learning, but also question whether enhanced adrenergic activity, which has been posited as a potential extinction facilitator in rats (Cain et al. (2004), extends to human learning. However, it is important to note that activation per se is not predictive of poorer outcome. Baseline anxiety and panic symptoms just prior to each exposure session were already elevated (i.e., M = 5.35 and 1.54 for anxiety and panic symptom) over the non-exposure “alternative baseline values” (M = 3.35 and 0.75 for anxiety and panic symptoms), indicative of anticipatory anxiety. Thus, activation during exposure represents additional activation over and above fear reactivity due to anticipatory anxiety. While this study is the first to demonstrate adverse effects of greater experiential activation during exposure therapy, similar adverse effects of high baseline levels of severity on outcome have been reported previously. For instance, higher baseline misappraisal was related to poorer outcome in cognitive/behavioral interventions (Keijsers et al., 1994; Hicks et al., 2005; Schneider and Schulte, 2008). Likewise, higher initial levels of catastrophic cognitions were predictive of poorer outcome (i.e., reduction in panic disorder severity) in patients receiving CT, but not CART (Meuret, Hofmann, & Rosenfield, 2010).
A secondary aim was to examine whether theoretically-distinct coping skills would moderate fear response and reductions in panic disorder severity. Notably, none of the observed patterns were moderated by treatment condition. That is, patients showed significant improvements in all panic measures over time, independent of treatment condition. Furthermore, neither strategy was more effective in influencing physiological reactivity, in that neither physiological nor experiential reactivity predicted outcome differently among treatment conditions. As noted earlier, it has often been theorized, but never empirically tested, that influencing ones breathing would suppress sympathetic activation. Such suppression has been speculated to inhibit successful fear processing, resulting in poorer outcome (e.g., Schmidt et al., 2000; Craske et al., 1997). Aversive effects of such kind were not evident in our data. However, patients did differ significantly with respect to absolute levels of PCO2. That is, patients with prior training in respiratory regulation had normocapnic levels at baseline and hyperventilated less (i.e., PCO2 levels were closer to normal) during exposure. This finding indicates that patients with prior training in CART successfully applied their previously learned skills, albeit not succeeding in abating the remarkable reactivity of CO2 during fearful states. While this study marks the first to assess changes in PCO2 during in vivo-exposure in PD patients, one other study assessed PCO2 in the context of driving phobia patients. A similar pattern of reactivity during exposure emerged (Alpers et al., 2005). Hyperventilation is often driven by deeper, but not faster breathing, as has been shown in other studies with anxious patients (e.g., Ritz, Wilhelm, Meuret, Gerlach, & Roth, 2009; Ayala, Meuret, & Ritz, 2010), thus making it less plausible that traditional breathing training, which teaches slow and deep breathing, can buffer arousal (e.g., Conrad et al., 2007; Meuret et al., 2003, for a review). Interestingly, neither peak levels of PCO2 during exposure, nor reactivity, were predictive of outcome in either condition. However, clinical and basic scientific evidence points to an active role for carbon dioxide in organ injury, in which raised concentrations of carbon dioxide are protective, and low concentrations to be injurious (e.g., Laffey & Kavanagh, Lancet, 1999; Laffey & Kavanagh, 2002). Thus, the extent to which therapies such as CART might lead to better general health outcomes, and perhaps to superior long-term prevention of panic relapse, requires further research.
“The golden rule is to try never to leave a situation until the fear is going down,” (original emphasis, Mathews, Gelder, & Johnston, 1981, p. 182). The findings of this study lend little support to the notion that the therapist must wait for fear (or arousal) to decline substantially during exposure session, since greater reductions in arousal were not related to better outcomes. The degree to which no reduction might be related to poor outcome could not be assessed in this study as fear reduction (both experiential and physiological) was present for all patients. Thus, we cannot determine whether our findings are at odds with commonly used statements in treatment manuals for exposure therapy (e.g., “As with an in vivo exercise, if an imaginal exposure exercise does not both activate fear and allow it to decrease, it is unlikely to be therapeutic.” (Kozak & Foa, 1997, p. 75). However, at the minimum we can qualify such statements by adding that although activation and reduction of fear may be therapeutic, the amount of such changes does not seem to matter. Indeed, our findings suggest that neither reductions in anxiety or symptoms during exposure nor such reductions across sessions are related to the degree of overall treatment gain for a given patient. Undoubtedly, the general practice of monitoring patients’ distress levels and waiting for them to decline is of great face validity and satisfaction to patients and therapists alike. This common practice may enhance a sense of control, self-efficacy, or accomplishment. However, at the same time, it may result in distraction, an illusion of competency, and even conscious or unconscious experiential avoidance. By contrast, a lack of reduction may evoke an unnecessary sense of failure in both clients and therapist, as it is often considered equivalent to escape (mentally or physically), thus strengthening agoraphobic behavior, sensitizing fear, and preventing decrements in anxiety which would result from prolonged exposure (e.g., Wilson & O’Leary, 1980). However, duration of exposure was also not a determinant of outcome in the present study, suggesting that prolonged exposure is not necessarily more effective than shorter exposures.
Together, the findings of this study suggest reconsideration of the (over) reliance on fear expression as an index of corrective learning. Of greater importance to corrective learning, and thus extinction, may indeed be the successful violation of the expectancy that an aversive event will occur, followed by the actual experience (and realization) that it did not (Lovibond & Shanks, 2002). Therefore, it may be argued that extinction learning and exposure therapy is a form of cognitive process that specifically changes harm expectancy (Hofmann, 2008; Lovibond, 2004). This is consistent with the view that extinction results in new learning about CS-US expectancy (namely that the CS no longer signals a US), which competes with the previously learned knowledge (namely that the US is followed by the CS; for a review, see Myers and Davis, 2002).
As noted by Craske and colleagues (2008), in an effort to move “away from initial activation followed by fear reduction as the guiding principle of exposure therapy”, an inhibitory learning based approach may be more applicable. Strategies for enhancing inhibitory learning for providing potent expectancy mismatches, include varied exposure contexts, stimuli, and duration (Craske et al., 2008 for a review). Recent developments emphasize the importance of fear and distress tolerance as a means of moving past patients’ fixation on eliminating fear/symptoms (Forsyth, Eifert, & Barrios, 2006; Hayes, Strosahl, & Wilson, 1999). Willingness to experience and tolerate anxiety symptoms, rather than trying to reduce or eliminate them, may provide the necessary space for learning new aspects and values of the feared situation. Preliminary support for tolerating elevated as well as varying levels of fear to facilitating corrective learning comes from a recent study illustrating that sustained self-reported fear levels (i.e., lack of habituation) (Culver, Stoyanova, & Craske, 2012) as well as fear variability during exposure were related to greater fear reduction in individuals fearful of public speaking (Culver, Stoyanova, & Craske, 2012) or contamination (Kircanski, Mortazavi, Castriotta, Baker, Mystkowski, Yi, & Craske, 2012). While performance (i.e., observed levels of change) may generally be viewed as an unreliable index of learning (Bjork & Bjork, 2006), limiting excessive fear activation seems advisable to prevent lesser improvement, as shown by the results of the present study.
Several limitations need to be taken into consideration when interpreting the results. Our sample was relatively homogeneous, consisting primarily of white females who were employed and relatively well educated. It remains to be seen whether these same findings would hold in a more heterogeneous population. Patients in this study were strongly encouraged to refrain from any medication with arousal-lowering potency, we failed to obtain written records on their use. Albeit, no differential effect on outcome for users versus non-user was found for any variable, medication use should be closely monitored in future studies. Further, only a limited number of assessments of the self-report measures were obtained during the exposures, compared to the continued assessment of physiology, thereby limiting our ability to assess the exact maximum of activation for these variables. We chose to refrain from more frequent experiential assessments as they can distract from fully experiencing the exposure and because verbalization is known to affect physiological responding (Wilhelm & Roth, 2001). Patients in this study were additionally asked to retrospectively report their peak level of anxiety and symptoms, which resulted in identical findings for the effects of activation. Future studies should consider the use of electronic diaries with timed prompts (Newman, Kenardy, Herman, & Taylor, 1997) to allow for more repeated assessments of experiential variables without the need for verbalization or the presence of the therapist during exposure. Similarly, while we have a reliable index of successful application of respiratory coping skills (i.e., degree to which CO2 changes during exposure), we know little about the extent to which cognitive restructuring was applied. Monitoring of thought patterns during exposure, using electronic diaries, could inform us about the predictive value of such techniques. Also, our protocol with prior skill training is a specific one that may not generalize to other cognitive and/or behavior interventions, exposure only treatments, treatments where skill training is combined with exposure, or treatments that use constant exposure (same feared stimuli/situations across exposure sessions). Lastly, future studies should examine whether symptom clusters or subtypes (e.g., respiratory, cognitive) differentially a) influence the relation of fear reactivity and outcome and b) respond to a certain treatment type.
In sum, our findings suggest that the expression of fear reactivity during exposure constitutes a weak predictor of corrective learning and fear extinction. More research is needed to examine the underlying mechanism of corrective learning during exposure across therapy types. More tools to assess the generation of competing, non-threat associations during exposure are needed to replace the reliance on performance measures.
Acknowledgments
Writing of this article was partly supported by an R01HL089761-01A1 grant from the National Institutes of Health (Meuret, Rosenfield) and the Beth and Russell Siegelman Foundation (Meuret). Dr. Hofmann is supported by NIMH Grant MH079236. He is also a paid consultant of Organon (Schering-Plough).
We gratefully acknowledge Dr. Thomas Ritz for his helpful feedback on an earlier draft of this article. We would further like to acknowledge the therapists, especially Lavanya Bhaskara, clinical interviewers, and independent assessors that were involved in this study.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/ccp
Detailed information on the original sample, including a flow chart, can be viewed at Meuret et al. 2010.
Contributor Information
Alicia E. Meuret, Southern Methodist University
Anke Seidel, Southern Methodist University.
Benjamin Rosenfield, University of Minnesota.
Stefan G. Hofmann, Boston University
David Rosenfield, Southern Methodist University.
References
- Alpers GW, Wilhelm FH, Roth WT. Psychophysiological assessment during exposure in driving phobic patients. Journal of Abnormal Psychology. 2005;114:126–139. doi: 10.1037/0021-843X.114.1.126. [DOI] [PubMed] [Google Scholar]
- Antony MM, Craske MG, Barlow DH. Mastering your fears and phobias: Client workbook. 2nd Ed. New York, NY: Oxford University Press; 2006. [Google Scholar]
- Ayala ES, Meuret AE, Ritz T. Confrontation with blood and disgust stimuli precipitates respiratory dysregulation in blood-injection-injury phobia. Biological Psychology. 2010;84:88–97. doi: 10.1016/j.biopsycho.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Baker A, Mystkowski J, Culver N, Yi R, Mortazavi A, Craske MG. Does habituation matter? Emotional processing theory and exposure therapy for acrophobia. Behavior Research and Therapy. 2010;48:1139–1143. doi: 10.1016/j.brat.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedler AE, Wilhelm W, Kreuer S, Soltesz S, Bach F, Mertzlufft FO, et al. Accuracy of portable quantitative capnometers and capnographs under prehospital conditions. The American Journal of Emergency Medicine. 2003;21:520–524. doi: 10.1016/j.ajem.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Bjork RA, Bjork EL. Optimizing treatment and instruction: Implications of a new theory of disuse. In: Nilson L-G, Ohta N, editors. Memory and society: Psychological prespectives. New York, NY: Psychological Press; 2006. pp. 116–140. [Google Scholar]
- Blechert J, Wilhelm FH, Meuret AE, Wilhelm EM, Roth WT. Respiratory, autonomic, and experiential responses to repeated inhalations of 20% CO2 enriched air in panic disorder, social phobia, and healthy controls. Biological Psychology. 2010;84:104–111. doi: 10.1016/j.biopsycho.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TA, Di Nardo PA, Lehman CL, Campbell LA. Reliability of DSM-IV anxiety and mood disorders: Implications for the classification of emotional disorders. Journal of Abnormal Psychology. 2001;110:49–58. doi: 10.1037//0021-843x.110.1.49. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychological Bulletin. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Cain CK, Blouin AM, Barad M. Adrenergic transmission facilitates extinction of conditional fear in mice. Learning and Memory. 2004;11:179–187. doi: 10.1101/lm.71504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambless DL, Caputo GC, Bright P, Gallagher R. Assessment of fear of fear in agoraphobics: The body sensations questionnaire and the agoraphobic cognitions questionnaire. Journal of Consulting and Clinical Psychology. 1984;52:1090–1097. doi: 10.1037//0022-006x.52.6.1090. [DOI] [PubMed] [Google Scholar]
- Chambless DL, Caputo GC, Jasin SE, Gracely EJ, Williams C. The mobility inventory for agoraphobia. Behaviour Research and Therapy. 1985;23:35–44. doi: 10.1016/0005-7967(85)90140-8. [DOI] [PubMed] [Google Scholar]
- Clark DM. A cognitive approach to panic. Behavior Research and Therapy. 1986;24:461–470. doi: 10.1016/0005-7967(86)90011-2. [DOI] [PubMed] [Google Scholar]
- Conrad A, Müller A, Doberenz S, Kim S, Meuret AE, Wollburg E, Roth WT. Psychophysiological effects of breathing instructions for stress management. Applied Psychophysiology Biofeedback. 2007;32:89–98. doi: 10.1007/s10484-007-9034-x. [DOI] [PubMed] [Google Scholar]
- Craske MG, Barlow DH, Meadows E. Mastery of your anxiety and panic: Therapist guide for anxiety, panic, and agoraphobia (MAP-3) San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, Baker A. Optimizing inhibitory learning during exposure therapy. Behaviour Research & Therapy. 2008;46:5–27. doi: 10.1016/j.brat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Craske MG, Rowe M, Lewin M, Noriega-Dimitri R. Interoceptive exposure versus breathing retraining within cognitive-behavioral therapy for panic disorder with agoraphobia. British Journal of Clinical Psychology. 1997;36:85–99. doi: 10.1111/j.2044-8260.1997.tb01233.x. [DOI] [PubMed] [Google Scholar]
- Culver NC, Stoyanova M, Craske MG. Emotional variability and sustained arousal during exposure. Journal of Behavior Therapy and Experimental Psychiatry. 2012;43:787–793. doi: 10.1016/j.jbtep.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Dager SR, Strauss WL, Marro KI, Richards TL, Metzger GD, Artru AA. Proton magnetic resonance spectroscopy investigation of hyperventilation in subjects with panic disorder and comparison subjects. American Journal of Psychiatry. 1995;152:666–672. doi: 10.1176/ajp.152.5.666. [DOI] [PubMed] [Google Scholar]
- DiNardo SC, Brown TA, Barlow DH. Anxiety Disorders Interview Schedule for DSM-IV: lifetime version (ADIS-IV-L) San Antonio, TX: Psychological Corporation; 1994. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM–IV (SCID–I/P Version 2.0) New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- Foa EB, Kozak MJ. Emotional processing of fear: Exposure to corrective information. Psychological Bulletin. 1986;99:20–35. [PubMed] [Google Scholar]
- Foa EB, McNally RJ. Mechanisms of change in exposure therapy. In: Rapee M, editor. Current controversies in the anxiety disorders. New York, NY: Guilford Press; 1996. pp. 329–343. [Google Scholar]
- Foa EB, Hembree EA, Rothbaum BO. Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences Therapist Guide. New York, NY: Oxford University Press; 2007. [Google Scholar]
- Forsyth JP, Eifert GH, Barrios V. Fear conditioning in an emotion regulation context: A fresh perspective on the origins of anxiety disorders. In: Craske MG, Hermans D, Vansteenwegen D, editors. Fear and learning: From basic processes to clinical applications. Washington, DC: American Psychological Association; 2006. pp. 133–153. [Google Scholar]
- Gorman JM, Martinez J, Coplan JD, Kent J, Kleber M. The effect of successful treatment on the emotional and physiological response to carbon dioxide inhalation in patients with panic disorder. Biological Psychiatry. 2004;56:862–867. doi: 10.1016/j.biopsych.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Hamer RM, Simpson PM. Last observation carried forward versus mixed models in the analysis of psychiatric clinical trials. American Journal of Psychiatry. 2009;166:639–641. doi: 10.1176/appi.ajp.2009.09040458. [DOI] [PubMed] [Google Scholar]
- Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: An experiential approach to behavior change. New York, NY: Guilford Press; 1999. [Google Scholar]
- Hicks TV, Leitenberg H, Barlow DH, Gorman JM, Shear MK, Woods SW. Physical, mental, and social catastrophic cognitions as prognostic factors in cognitive-behavioral and pharmacological treatments for panic disorder. Journal of Consulting and Clinical Psychology. 2005;73:506–514. doi: 10.1037/0022-006X.73.3.506. [DOI] [PubMed] [Google Scholar]
- Hofmann SG. Cognitive processes during fear acquisition and extinction in animals and humans: Implications for exposure therapy of anxiety disorder. Clinical Psychology Review. 2008;28:200–211. doi: 10.1016/j.cpr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope DA, Heimberg RG, Turk CL. Therapist guide for Managing social anxiety: A cognitive-behavioral therapy approach. New York, NY: Oxford University Press; 2006. [Google Scholar]
- Kazdin AE. Mediators and mechanisms of change in psychotherapy research. Annual Review of Clinical Psychology. 2007;3:1–27. doi: 10.1146/annurev.clinpsy.3.022806.091432. [DOI] [PubMed] [Google Scholar]
- Keijsers GP, Hoogduin CA, Schaap CP. Predictors of treatment outcome in the behavioural treatment of obsessive-compulsive disorder. The British Journal of Psychiatry. 1994;165:781–786. doi: 10.1192/bjp.165.6.781. [DOI] [PubMed] [Google Scholar]
- Kendall PC, Comer JS, Marker CD, Creed TA, Puliafico AC, Hughes AA, Martin ED, Suveg C, Hudson J. In-session exposure tasks and therapeutic alliance across the treatment of childhood anxiety disorders. Journal of Consulting and Clinical Psychology. 2009;77:517–525. doi: 10.1037/a0013686. [DOI] [PubMed] [Google Scholar]
- Kircanski K, Mortazavi A, Castriotta N, Baker AS, Mystkowski JL, Yi R, Craske MG. Challenges to the traditional exposure paradigm: Variability in exposure therapy for contamination fears. Journal of Behavior Therapy and Experimental Psychiatry. 2012;43:745–751. doi: 10.1016/j.jbtep.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Kozak MJ, Foa EB, Steketee G. Process and outcome of exposure treatment with obsessive-compulsives: Psychophysiological indicators of emotional processing. Behavior Therapy. 1988;19:157–169. [Google Scholar]
- Kozak MJ, Foa EB. Mastery of obsessive compulsive disorder: A cognitive behavioural approach - Therapist guide. New York: Oxford University Press; 1997. [Google Scholar]
- Kraemer HC, Thiemann S. A strategy to use soft data effectively in randomized controlled clinical trials. Journal of Consulting and Clinical Psychology. 1989;57:148–154. doi: 10.1037//0022-006x.57.1.148. [DOI] [PubMed] [Google Scholar]
- Laffey JG, Kavanagh BP. Hypocapnia. New England Journal of Medicine. 2002;347:43–53. doi: 10.1056/NEJMra012457. [DOI] [PubMed] [Google Scholar]
- Lang AJ, Craske MG. Manipulations of exposure-based therapy to reduce return of fear: A replication. Behaviour Research & Therapy. 2000;38:1–12. doi: 10.1016/s0005-7967(99)00031-5. [DOI] [PubMed] [Google Scholar]
- Lang PJ. The application of psychophysiological methods to the study of psychotherapy and behavior modification. In: Bergin A, Garfield S, editors. Handbook of psychotherapy and behavior change. New York, NY: Wiley-Blackwell; 1971. [Google Scholar]
- Lissek S, Powers AS, McClure EB, Phelps EA, Woldehawariat G, Grillon C, Pine DS. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behaviour Research and Therapy. 2005;43:1391–1424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Lovibond PF, Shanks DR. The role of awareness in Pavlovian conditioning: empirical evidence and theoretical implications. Journal of Experimental Psychology: Animal Behavior Processes. 2002;28:3–26. [PubMed] [Google Scholar]
- Lovibond PF. Cognitive processes in extinction. Learning and Memory. 2004;11:495–500. doi: 10.1101/lm.79604. [DOI] [PubMed] [Google Scholar]
- Maas CJM, Hox JJ. Sufficient Sample Sizes for Multilevel Modeling. Methodology: European Journal of Research Methods for the Behavioral and Social Sciences. 2005;1:85–91. [Google Scholar]
- Maddock RJ, Carter CS. Hyperventilation-induced panic attacks in panic disorder with agoraphobia. Biological Psychiatry. 1991;29:843–854. doi: 10.1016/0006-3223(91)90051-m. [DOI] [PubMed] [Google Scholar]
- Marks IM, Mathews AM. Brief standard self-rating for phobia patients. Behaviour Research and Therapy. 1979;17:263–267. doi: 10.1016/0005-7967(79)90041-x. [DOI] [PubMed] [Google Scholar]
- Marshall WL. The effects of variable exposure in flooding therapy. Behavior Therapy. 1985;16:117–135. [Google Scholar]
- Mathews AM, Gelder MG, Johnston DW. Agoraphobia, nature and treatment. New York, NY: Guilford Press; 1981. [Google Scholar]
- McNally RJ. Mechanisms of exposure therapy: how neuroscience can improve psychological treatments for anxiety disorders. Clinical Psychological Review. 2007;27:750–759. doi: 10.1016/j.cpr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Meuret AE, Rosenfield D, Hofmann SG, Suvak MK, Roth WT. Changes in Respiration Mediate Changes in Fear of Bodily Sensations in Panic Disorder. Journal of Psychiatric Research. 2009;43:634–641. doi: 10.1016/j.jpsychires.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret AE, Rosenfield D, Wilhelm FH, Zhou E, Conrad A, Ritz T, Roth WT. Do unexpected panic attacks occur spontaneously? Biological Psychiatry. doi: 10.1016/j.biopsych.2011.05.027. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret AE, Wilhelm FH, Ritz T, Roth WT. Feedback of end-tidal PCO2 as a therapeutic approach for panic disorder. Journal of Psychiatric Research. 2008;42:560–568. doi: 10.1016/j.jpsychires.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret AE, Hofmann SG, Rosenfield D. Catastrophic Appraisal and Perceived Control as Moderators of Treatment Response in Panic Disorder. International Journal of Cognitive Therapy. 2010;3:262–277. doi: 10.1521/ijct.2010.3.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret AE, Rosenfield D, Seidel A, Bhaskara L, Hofmann SG. Respiratory and cognitive mediators of treatment change in panic disorder: evidence for intervention specificity. Journal of Consulting and Clinical Psychology. 2010;78:691–704. doi: 10.1037/a0019552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret AE, Wilhelm FH, Ritz T, Roth WT. Breathing training for treating panic disorder. Useful intervention or impediment? Behavior Modification. 2003;27:731–754. doi: 10.1177/0145445503256324. [DOI] [PubMed] [Google Scholar]
- Meuret AE, Ritz T. Hyperventilation in panic disorder and asthma: Empirical evidence and clinical strategies. International Journal of Psychophysiology. 2010;78:68–79. doi: 10.1016/j.ijpsycho.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuret AE, Wolitzky-Taylor KB, Twohig MP, Craske MG. Coping skills and exposure therapy in panic disorder and agoraphobia: Latest advances and future directions. Behavior Therapy. doi: 10.1016/j.beth.2011.08.002. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–684. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Newman MG, Kenardy J, Herman S, Taylor CB. Comparison of palmtop-computer-assisted brief cognitive-behavioral treatment to cognitive-behavioral treatment for panic disorder. Journal of Consulting and Clinical Psychology. 1997;65:178–183. doi: 10.1037//0022-006x.65.1.178. [DOI] [PubMed] [Google Scholar]
- Papp LA, Welkowitz LA, Martinez JM, Klein DF, Browne S, Gorman JM. Instructional set does not alter outcome of respiratory challenges in panic disorder. Biological Psychiatry. 1995;38:826–830. doi: 10.1016/0006-3223(95)00047-X. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Orr SP, Altman B, Longpre RE. Emotional processing and outcome of imaginal flooding therapy in Vietnam Veterans with chronic post-traumatic stress disorder. Comprehensive Psychiatry. 1996;37:409–418. doi: 10.1016/s0010-440x(96)90024-3. [DOI] [PubMed] [Google Scholar]
- Rachman S, Craske M, Tallman K, Solyom C. Does escape behavior strengthen agoraphobic avoidance? A replication. Behavior Therapy. 1986;17:366–384. [Google Scholar]
- Rachman S, Robinson S, Lopatka C. Is incomplete fear-reduction followed by a return of fear? Behaviour Research & Therapy. 1987;25:67–69. doi: 10.1016/0005-7967(87)90116-1. [DOI] [PubMed] [Google Scholar]
- Rachman S, Radomsky AS, Shafran R. Safety behaviour: a reconsideration. Behaviour Research and Therapy. 2008;46:163–173. doi: 10.1016/j.brat.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Rapee RM, Craske MG, Brown TA, Barlow DH. Measurement of perceived control over anxiety-related events. Behavior Therapy. 1996;27:279–293. [Google Scholar]
- Rauch SAM, Foa EB, Furr JM, Filip JC. Imagery vividness and perceived anxious arousal in prolonged exposure treatment for PTSD. Journal of Traumatic Stress. 2004;17:461–465. doi: 10.1007/s10960-004-5794-8. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Liu X. Effects of study duration, frequency of observation, and sample size on power in studies of group differences in polynomial change. Psychological Methods. 2001;6:387–401. [PubMed] [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behaviour Research and Therapy. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Ritz T, Wilhelm FH, Meuret AE, Gerlach AL, Roth WT. Do blood phobia patients hyperventilate during exposure by breathing faster, deeper, or both? Depression and Anxiety. 2009;26:E60–E67. doi: 10.1002/da.20466. [DOI] [PubMed] [Google Scholar]
- Schneider R, Schulte D. Catastrophic associations predict level of change in anxiety sensitivity in response to cognitive-behavioural treatment for panic. Behaviour Research and Therapy. 2008;46:557–572. doi: 10.1016/j.brat.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Shear MK, Brown TA, Barlow DH, Money R, Sholomskas DE, Woods SW, et al. Multicenter collaborative panic disorder severity scale. American Journal of Psychiatry. 1997;154:1571–1575. doi: 10.1176/ajp.154.11.1571. [DOI] [PubMed] [Google Scholar]
- Shear MK, Clark D, Feske U. The road of recovery in panic disorder: Response, Remission, and Relapse. Journal of Clinical Psychiatry. 1998;59:4–8. [PubMed] [Google Scholar]
- Smits JA, Meuret AE, Zvolensky MJ, Rosenfield D, Seidel A. The effects of acute exercise on CO(2) challenge reactivity. Journal of Psychiatric Research. 2009;43:446–454. doi: 10.1016/j.jpsychires.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Woolaway-Bickel K, Trakowski J, Santiago H, Storey J, Koselka M, Cook J. Dismantling cognitive-behavioral treatment for panic disorder: Questioning the utility of breathing retraining. Journal of Consulting and Clinical Psychology. 2000;68:417–424. doi: 10.1037//0022-006x.68.3.417. [DOI] [PubMed] [Google Scholar]
- Snijders TA, Bosker RJ. Standard errors and sample sizes for two-level research. Journal of Educational Statistics. 1993;18:237–259. [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. 5th ed. Boston, MA: Allyn and Bacon; 2007. [Google Scholar]
- Telch MJ, Shermis MD, Lucas JA. Anxiety sensitivity: Unitary personality trait or domain-specific appraisals? Journal of Anxiety Disorders. 1989;3:25–32. [Google Scholar]
- Tsao JCI, Craske MG. Timing of treatment and return of fear: Effects of massed, uniform-, and expanding-spaced exposure schedules. Behavior Therapy. 2000;31:479–497. [Google Scholar]
- Wilhelm FH, Roth WT. Taking the laboratory to the skies: ambulatory assessment of self-report, autonomic, and respiratory responses in flying phobia. Psychophysiology. 1998;35:596–606. doi: 10.1017/s0048577298970196. [DOI] [PubMed] [Google Scholar]
- Wilhelm FH, Gerlach AL, Roth WT. Slow recovery from voluntary hyperventilation in panic disorder. Psychosomatic Medicine. 2001;63:638–649. doi: 10.1097/00006842-200107000-00017. [DOI] [PubMed] [Google Scholar]
- Wilhelm FH, Roth WT. The somatic symptom paradox in DSM-IV anxiety disorders: suggestions for a clinical focus in psychophysiology. Biological Psychology. 2001;57:105–140. doi: 10.1016/s0301-0511(01)00091-6. [DOI] [PubMed] [Google Scholar]
- Williams JBW, Gibbon M, First MB, Spitzer RL, Davis M, Borus J, et al. The Structured Clinical Interview for DSM-III-R (SCID) II. Multi-site test-retest reliability. Archives of General of Psychiatry. 1992;49:630–636. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]
- Wilson GT, O’Leary D. Principles of behavior therapy. Englewood Cliffs, NJ: Prentice-Hall; 1980. [Google Scholar]