Abstract
Insulin's trans-endothelial transport (TET) is critical for its metabolic action on muscle and involves trafficking of insulin bound to its receptor (or at high insulin concentrations, the IGF-I receptor) via caveolae. However, whether caveolae-mediated insulin TET involves actin cytoskeleton organization is unknown. Here we address whether insulin regulates actin filament organization in bovine aortic endothelial cells (bAEC) and whether this affects insulin uptake and TET. We found that insulin induced extensive cortical actin filament remodeling within 5 min. This remodeling was inhibited not only by disruption of actin microfilament organization but also by inhibition of phosphatidylinositol 3-kinase (PI3K) or by disruption of lipid rafts using respective specific inhibitors. Knockdown of either caveolin-1 or Akt using specific small interfering RNA also eliminated the insulin-induced cortical actin filament remodeling. Blocking either actin microfilament organization or PI3K pathway signaling inhibited both insulin uptake and TET. Disruption of actin microfilament organization also reduced the caveolin-1, insulin receptor, and IGF-I receptor located at the plasma membrane. Exposing bAEC for 6 h to either TNFα or IL-6 blocked insulin-induced cortical actin remodeling. Extended exposure (24 h) also inhibited actin expression at both mRNA and protein levels. We conclude that insulin-induced cortical actin filament remodeling in bAEC is required for insulin's TET in a PI3K/Akt and plasma membrane lipid rafts/caveolae-dependent fashion, and proinflammatory cytokines TNFα and IL-6 block this process.
To reach its cellular targets, insulin must first traverse the vascular endothelium to enter the tissue interstitium (1). For muscle, insulin transport from the plasma to the interstitial fluid compartment is rate limiting for insulin-induced glucose disposal (2, 3). Furthermore, insulin delivery to muscle interstitium is delayed in insulin-resistant subjects, suggesting that the vasculature contributes to muscle metabolic insulin resistance (4, 5). However, the pathway by which insulin transits the endothelium and the factors responsible for slowing transport with insulin resistance are poorly understood. We and others have reported that in cultured arterial endothelial cells, insulin's trans-endothelial transport (TET) is an insulin receptor-mediated process (6–8). In addition, inhibiting phosphatidylinositol 3-kinase (PI3K) signaling (9), interfering with caveolae formation, and exposure to either TNFα or IL-6 inhibit insulin transport by vascular endothelial cells (10).
Several laboratories have reported that insulin receptors (IR) colocalize with caveolae in the adipocyte plasma membrane (11, 12). Although this was not without some controversy, a recent electron microscope immunocytochemistry has convincingly shown that although IR are present throughout the plasma membrane, they are particularly concentrated at the neck of caveolae in 3T3-L1 adipocytes (13). IGF-I receptors (IGF-IR) appear to have similar lipid raft/caveolae localization in the plasma membrane (14). We and others have shown that disrupting lipid rafts with the cholesterol-depleting agent filipin inhibits insulin's uptake and TET (7, 8). More recently, we found that caveolin-1, a requisite structural protein of caveolae, is required for endothelial cell insulin uptake (10). Interestingly, caveolae-mediated simian virus 40 (SV40) uptake by CV-1 cells (15) was recently found to involve cortical actin polymerization that required tyrosine kinase activation (16). In adipocytes (17, 18) and muscle cells (19), insulin stimulates rapid cortical actin polymerization, and this is required for glucose transporter 4 (GLUT4) vesicle translocation to the plasma membrane. Whether insulin induces a similar cortical actin filament remodeling in vascular endothelial cells and whether such remodeling is necessary for caveolae-mediated insulin's transport is unknown.
In the present study, we examined the time and dose effect of insulin on cortical actin filament organization in cultured vascular endothelial cells and the effect of insulin-induced actin cytoskeleton remodeling on insulin transport. We also examined whether PI3K activation and maintenance of lipid raft integrity was necessary for insulin-induced cortical actin filament remodeling. Because insulin induces caveolin-1 trafficking to the plasma membrane (20) and caveolae appear to mediate insulin transport (10), we examined whether insulin-induced actin cytoskeleton reorganization is required for insulin-induced caveolin-1 as well as IR and IGF-IR trafficking to the plasma membrane. Finally, because we have previously reported that experimental endothelial cell insulin resistance, produced by exposure to proinflammatory cytokines IL-6 or TNFα significantly impedes insulin uptake by vascular endothelial cells (10), we tested whether IL-6 and TNFα affected insulin-induced cortical actin organization in vascular endothelial cells.
Materials and Methods
Cell culture
Bovine aortic endothelial cells (bAEC) (BioWhittaker, Inc., Walkersville, MD) (passage numbers 2–8) were grown in microvascular endothelial cell growth medium in eight-well slide chambers for immunocytochemical staining or in six-well plates for Western blot or real-time RT-PCR (see below).
Small interfering RNA (siRNA) design and transfection
The siRNA knockdown of either caveolin-1 or Akt was carried out as described previously (10, 20). Briefly, specific siRNA duplexes directed against either the target sequence 5′-CAGAAGGAACACACAGTTT-3′, which corresponds to bases 224–242 from the open reading frame of bovine caveolin-1 mRNA (10, 20), or the target sequence 5′-CCGAGGTGCTGGAGGACAA-3′, which corresponds to both the bases 1034–1052 from the open reading frame of bovine Akt1 mRNA and the bases 1136–1154 from the open reading frame of bovine Akt2 mRNA, were purchased from Dharmacon, Inc. (Lafayette, CO) along with a scrambled control siRNA (siCONTROL Non-Targeting siRNA no. 5). The bAEC were seeded and transfected with either the siRNA duplex against caveolin-1 or the siRNA duplex against Akt1/Akt2 or scrambled siRNA (as control) to a final concentration of 40 nm using Oligofectamine (Invitrogen, Carlsbad, CA) when cells reached approximately 30–50% confluence. Forty-eight hours after transfection, cells were either lysed for Western blotting or serum starved followed by treatment with 50 nm insulin for 5 min for immunocytochemical staining (see below).
TET experiments
The experiments were performed as previously described (8). Briefly, bAEC were seeded onto Transwell inserts (6.5 mm diameter, 0.4 μm pore size, polyester membrane; Corning, Corning, NY) treated with human fibronectin (Sigma-Aldrich, St. Louis, MO). The trans-endothelial electrical resistance was monitored using an epithelial volt-ohmmeter (EVOM; WPI, Sarasota, FL) and EndOhm chamber (EndOhm; WPI) after a confluent monolayer was formed. After the trans-endothelial electrical resistance peaked, the plates with endothelial monolayers were washed twice at 37 C with endothelial basal medium (EBM), the fluid in the top chamber was replaced with the basal medium containing either fluorescein isothiocyanate (FITC)-insulin (1 μm) (Molecular Probes, Inc., Eugene, OR) or 125I-labeled [TyrA14] insulin (200 pm) (PerkinElmer, Boston, MA) with or without 2 μm unlabeled insulin or 25 nm latrunculin A (Calbiochem, La Jolla, CA) or 100 nm wortmannin (Sigma-Aldrich). In some experiments, FITC-inulin (1 μm) (Sigma-Aldrich) was used to verify the integrity of the endothelial monolayer. Two hundred microliters of fluid were removed from the bottom chamber, and this was replaced with 200 μl EBM to assure hydrostatic balance. The FITC-insulin concentration was quantitated using a fluorometer and that of 125I-labeled [TyrA14] insulin was measured in a γ-counter. The percentage of insulin added to the top chamber that was transported to the bottom chamber was calculated and presented in Results.
Western blotting
Western blotting was performed as described previously (10, 20). Briefly, after being blocked with Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE), membranes were incubated with mouse monoclonal antibody against β-actin (Sigma-Aldrich) or Akt1 (OriGene Technologies, Inc. Rockville, MD) or Akt2 (Santa Cruz Biotechnology, Santa Cruz, CA) and rabbit polyclonal antibody against glyceraldehyde-3-phosphate dehydrogenase (Sigma-Aldrich) overnight at 4 C. This was followed by incubation with a species-specific secondary antibody coupled to either IRDye 680LT or IRDye 800CW (LI-COR Biosciences). The membranes were scanned and quantified using an Odyssey LI-COR infrared imaging system (LI-COR Biosciences) following manufacturer's protocols.
Real-time RT-PCR
Total RNA was extracted from the cultured bAEC with the RNeasy kit (QIAGEN, Valencia, CA). A total of 2 μg total cellular RNA from each sample was reverse transcribed using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). The cDNA products were then amplified using the iQ SYBR green supermix and the iCycler apparatus (Bio-Rad) as described previously (10). For the amplification of the β-actin gene products, the following primers were designed: forward 5′-GTATGGGCATGCACTGTGTC-3′ and reverse 5′-GTGTCCGCCATCTACATCT-3′. And the β-actin mRNA levels were normalized to the housekeeping gene (GAPDH) mRNA (primers designed: forward 5′-GGGTCATCATCTCTGCACCT-3′ and reverse 5′-GGTCATAAGTCCCTCCACGA-3′) (Integrated DNA Technologies, Coralville, IA). Standard curves for each mRNA were generated by serial dilution of cDNA synthesized from the extracted total RNA and was included in each iCycler real-time PCR experiment. The specificity of the desired product was verified by the analysis of the melting curve.
Plasma membrane sheet preparation
Plasma membrane sheets were prepared as previously described (20, 21). Briefly, bAEC were washed once with ice-cold PBS followed by incubation on ice with 0.5 mg/ml poly-l-lysine in PBS for 30 sec. The cells were then washed three times with ice-cold hypotonic buffer [23 mm KCl, 10 mm HEPES (pH 7.5), 2 mm MgCl2, 1 mm EGTA]. The swollen cells were then sonicated for 3 sec (550 Fisher sonic dismembrator) in ice-cold sonication buffer [70 mm KCl, 30 mm HEPES (pH 7.5), 5 mm MgCl2, 3 mm EGTA, 1 mm dithiothreitol, 0.1 mm phenylmethylsulfonyl fluoride] followed by two washes with the ice-cold sonication buffer and used for immunocytochemical staining (see below).
Immunocytochemistry
Immunocytochemical staining was performed as described previously (10, 20). Briefly, after fixation, cells were washed three times in Tris-buffered saline (TBS), permeabilized in TBS containing Triton X-100 (0.05%) and 1% horse serum for 30 min at room temperature, and incubated with two different primary antibodies against two different target proteins (double labeling) overnight at 4 C. Primary antibodies used include mouse monoclonal anti-caveolin-1(BD Transduction Laboratories, Lexington, KY), rabbit polyclonal anti-caveolin-1 (Santa Cruz Biotechnology), mouse monoclonal anti-Akt1 (OriGene Technologies), mouse monoclonal anti-Akt2 (Santa Cruz Biotechnology), rabbit polyclonal anti-fluorescein (FITC) (Molecular Probes), and rabbit polyclonal anti-IRß and rabbit polyclonal anti-IGF-IRß (Santa Cruz Biotechnology). The cells were washed three times in PBS and then incubated with species-specific secondary antibodies conjugated with fluorochrome for 45 min at room temperature. The following secondary antibodies were used: donkey antirabbit IgG conjugated to Cy3 and donkey antimouse IgG conjugated to Cy2 (Jackson ImmunoResearch, West Grove, PA). Filamentous actin (F-actin) was stained using phalloidin-tetramethylrhodamine isothiocyanate (TRITC) (Sigma). The cells were washed three times in TBS and then coverslipped with the antifade mounting medium. For the quantitative analysis of F-actin stained by phalloidin-TRITC, bAEC were counted and seeded in 96-well plates with the same number of cells and then serum starved and pretreated with either inhibitors or vehicle for 30 min followed by 50 nm insulin treatment for 5 min, and then cells were fixed, permeabilized, washed, and stained with phalloidin-TRITC for 45 min before being measured using a fluorescent-detection microplate reader (Synergy 2; BioTek, Winooski, VT) (see figure legends for details).
Imaging
The double immunocytochemical labeling was examined simultaneously using a two-color Olympus BX50 WI confocal microscope equipped with krypton and argon laser as described previously (9, 10). Fluorescence intensity from both intact cell preparations and plasma membrane sheet preparations was quantified using Image J software (National Institutes of Health, Bethesda, MD) as previously described (20).
Statistical analysis
Data are presented as mean ± sem. Statistical comparisons among different groups were made using one-way ANOVA with post hoc testing performed by the method of Student-Newman-Keuls. Statistical significance was defined as P ≤ 0.05.
Results
Insulin induces rapid cortical actin filament remodeling in bAEC
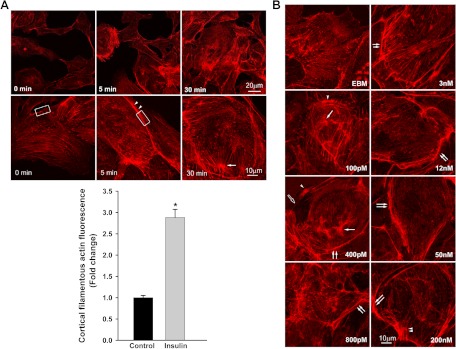
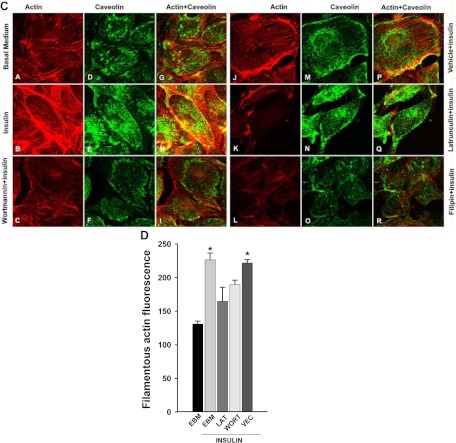
As shown in Fig. 1A, adding 50 nm insulin induced robust cortical actin filament remodeling. Stress fibers, which before giving insulin appeared uniformly distributed in the cytosol, reorganized into a thickened cortical band on or near the plasma membrane with spike-like mesh structures extending into the cytosol. These actin cytoskeletal changes occurred within 5 min of adding insulin and lasted for at least 30 min (Fig. 1A). This effect of insulin was dose dependent. Lower concentrations of insulin (∼100–400 pm) appeared to cause the stress fibers to form bundles in the cytosol with patch-like or thinner cortical bands (Fig. 1B). As the insulin concentration increased, the cortical actin filamentous band became more extensive and thicker with more spike-like mesh structures on or near the plasma membrane (Fig. 1B). The physiological significance of these remodeled structures of F-actin is not clear. Pretreatment of cells with latrunculin A, which specifically disrupts actin microfilament organization, substantially diminished insulin-induced cortical actin filament remodeling (Fig. 1C, micrograph K; also see Fig. 4). Figure 1C shows that insulin-induced cortical actin remodeling was accompanied by an increase of caveolin-1 at the cell periphery (20) and its colocalizing there with the actin mesh structure near or on the plasma membrane (Fig. 1C, micrographs H and P). Pretreatment of cells with latrunculin A not only interrupted insulin-induced cortical actin remodeling (Fig. 1C, micrograph K) but also reduced insulin-mediated increases in caveolin-1 at the plasma membrane (see Fig. 3, A and C; also see Fig. 1C, micrographs N and Q).
Fig. 1.
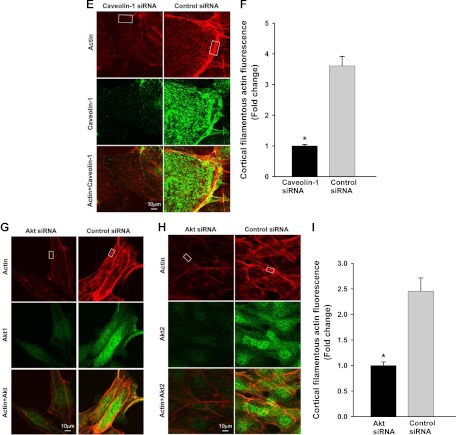
Insulin-induced cortical actin filament remodeling. bAEC were serum starved followed by treatment with insulin, and then cells were fixed and stained with phalloidin-TRITC. A, Representative confocal images of single optical sections reflecting the time course of 50 nm insulin-induced cortical actin filament remodeling; top panel, upper row, lower-magnification representative images; bottom row, higher-magnification representative images. Arrowheads denote the thickened cortical actin filament band, and an arrow denotes a spike-like mesh structure of cortical actin filaments. At 0 min, no insulin was added. Bottom panel, quantification of changes in the fluorescent intensity of F-actin in the cortical region on or near the plasma membrane of bAEC on the higher-magnification confocal images (indicated by the rectangular boxes in the images at 0 and 5 min, respectively) by using Image J software [n = 40 for each group; counting 10 cells randomly selected from either the control (at 0 min, i.e. no insulin added) or insulin-stimulated group (at 5 min after insulin added); the rectangular box was placed on the identifiable cortical regions]. *, P < 0.001 compared with the control group. B, Representative confocal images of single optical sections reflecting the dose response of cortical actin filament remodeling to insulin treatment for 5 min (the insulin dose is indicated in each micrograph). EBM indicates that cells were incubated in basal medium without insulin treatment. A closed arrow denotes the actin filamentous bundle, an open arrow denotes the thinner cortical actin filamentous band, an arrowhead denotes a patch-like structure of cortical actin filaments, and double arrowheads denote a spike-like mesh structure of cortical actin filaments. Double arrows denote thickened cortical actin bands. C and D, Effects of blocking PI3K insulin signaling pathway and interrupting actin filament organization or lipid rafts on insulin-induced cortical actin filament remodeling. C, bAEC were serum starved and then pretreated with either 100 nm wortmannin or 25 nm latrunculin A or 5 μg/ml filipin or vehicle (dimethylsulfoxide) or basal medium (EBM) for 30 min followed by 50 nm insulin treatment for 5 min and then fixed and processed for double staining for caveolin-1 (green, revealed by Cy2) and actin filaments (red, revealed by phalloidin-TRITC). Presented confocal images of single optical sections are representative of three independent experiments. D, bAEC seeded in 96-well plates with the same number of cells were serum starved and then pretreated with either 25 nm latrunculin A (LAT) or 100 nm wortmannin (WORT) or vehicle control (VEC) for 30 min followed by 50 nm insulin treatment for 5 min, and then cells were fixed and stained with phalloidin-TRITC for 45 min before being measured using a fluorescent-detection microplate reader. *, P < 0.05 compared with EBM, LAT, or WORT group, respectively; no significant difference was found between insulin-treated only (EBM+insulin) and VEC+insulin group or between remaining groups. E–I, The effects of knockdown of either caveolin-1 or Akt (both Akt1 and Akt2) on insulin-induced cortical actin filament remodeling; E, representative confocal images of single optical sections after siRNA knockdown of caveolin-1; F, quantification of changes in the fluorescent intensity of F-actin in the cortical region on or near the plasma membrane of bAEC after knockdown of caveolin-1; G and H, representative confocal images of single optical sections after siRNA knockdown of Akt (both Akt1 and Akt2) (G, stained for Akt1 with F-actin; H, stained for Akt2 with F-actin); I, quantification of changes in the fluorescent intensity of F-actin in the cortical region on or near the plasma membrane of bAEC after knockdown of the Akt. The quantitation was made on the confocal images (indicated by the rectangular boxes in the images, and the rectangular box was placed on the identifiable cortical regions) by using Image J software (n = 32 for each group; counting eight cells randomly selected from either the control siRNA-treated or specific siRNA-treated group, respectively). *, P < 0.05 compared with the control siRNA group. Experiments were repeated three times independently.
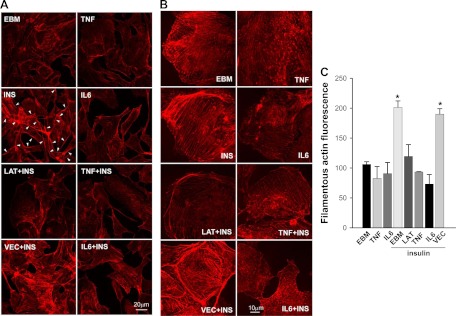
Fig. 4.
The effects of TNFα and IL-6 on insulin-induced cortical actin filament remodeling. The bAEC were serum starved for 24 h with or without TNFα or IL-6 (20 ng/ml) and then pretreated with or without 25 nm latrunclin A (LAT) or vehicle control (VEC) for 30 min followed or not by insulin (INS) treatment for 5 min. Cells were then fixed and stained with phalloidin-TRITC. A, Representative confocal images (single optical sections) at lower magnification. B, Representative confocal images (single optical sections) at higher magnification. Presented images are representative from two independent experiments. Arrowheads denote the insulin-induced cortical filamentous bands on the plasma membrane. For EBM, cells were incubated in basal medium only without insulin. C, The bAEC seeded in 96-well plates with same cell numbers in each well were serum-starved in the absence or presence of TNFα or IL-6 (20 ng/ml, each) for 6 h and then pretreated with either 25 nm latrunculin A (LAT) or vehicle control (VEC) for 30 min followed by 50 nm insulin treatment for 5 min. Cells were then fixed and stained with phalloidin-TRITC for 45 min before being measured using a fluorescent-detection microplate reader. *, P < 0.05 compared with EBM, TNF, IL-6, LAT+insulin, TNF+insulin, or IL-6+insulin group, respectively; no significant differences were seen between insulin-treated only (EBM+insulin) and VEC+insulin group or between the remaining groups.
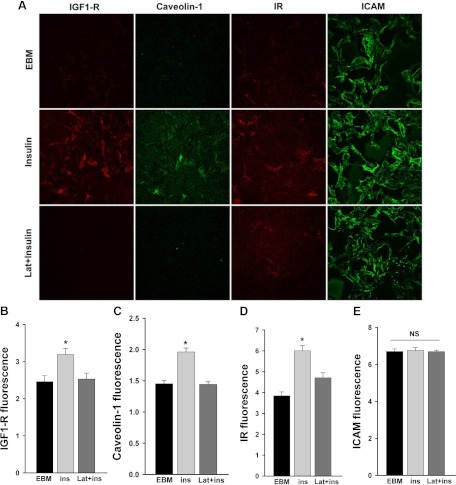
Fig. 3.
Latrunculin A inhibited insulin-induced caveolin-1 and IR/IGF-IR trafficking to the plasma membrane. After serum starvation for 24 h, cells were pretreated with 25 nm latrunculin A (Lat) for 30 min followed or not by insulin treatment for 30 min. Plasma membrane sheets were prepared followed by double staining for IGF-IR (red), caveolin-1 (green), IR (red), and ICAM (used as a control) (green). A, Representative confocal images of single optical sections from three independent experiments. For EBM, cells were incubated in basal medium only without insulin. B–E, Quantification of changes in the fluorescence intensity of IGF-IR, caveolin-1, IR, and ICAM, respectively. *, P < 0.05 compared with remaining groups. NS, Not statistically significant.
Because it has been reported that blocking the PI3K pathway with wortmannin inhibited insulin uptake (9) and disrupting lipid rafts using filipin inhibited both insulin uptake (8) and TET (7), we next examined the effects of wortmannin and filipin on insulin-induced cortical actin filament remodeling. Pretreatment of cells with wortmannin eliminated insulin-induced cortical actin filament remodeling (Fig. 1C, micrographs C, F, and I) and, like latrunculin A, diminished caveolin-1 localization at the plasma membrane (Fig. 1C, micrographs C, F, and I) (20). Likewise, disruption of the plasma membrane lipid rafts with filipin completely eliminated insulin-induced cortical actin remodeling and plasma membrane caveolin-1 localization (Fig. 1C, micrographs L, O, and R). To quantify these changes, we used a fluorescence-plate reader and measured the phalloidin-TRITC fluorescence 5 min after adding insulin. Figure 1D shows that within 5 min, insulin treatment increased the total amount of F-actin (also see Fig. 1A, bottom panel). Pretreatment of cells with either latrunculin A or wortmannin significantly reduced this insulin-induced actin polymerization. Filipin pretreatment caused cells to detach from the wells of 96-well plates, preventing this quantitation. The insulin-induced increase in F-actin detected by this increased fluorescence may reflect the thickened cortical actin filament band seen with confocal imaging (see Fig. 1, A and B). To confirm these findings with the chemical inhibitors, we further examined the effects of knockdown of either caveolin-1 (10, 20) (to reduce caveolae) or Akt (both Akt1 and Akt2) (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org) (to block the PI3K-Akt pathway) on insulin-induced cortical actin filament remodeling. Figure 1, E and F, shows that compared with a scrambled control siRNA, knockdown of caveolin-1 completely eliminated the insulin-induced cortical actin remodeling (Fig. 1E). Likewise, compared with the control group transfected with the scrambled siRNA, transfection of the specific siRNA against Akt (against both Akt1 and Akt2) not only strikingly reduced both Akt staining (for Akt1, see Fig. 1G; for Akt2, see Fig. H) but also almost completely abrogated the insulin-induced cortical actin remodeling (Fig. 1, G–I). These data were consistent with the observation in which treatment of bAEC with either wortmannin or filipin (see above) significantly reduced insulin-induced cortical actin filament remodeling. Taken together, these data strongly indicate that both lipid rafts/caveolae and the PI3K/Akt pathway play an important role in controlling insulin-induced cortical F-actin remodeling.
Disruption of actin microfilament organization inhibits insulin transport by vascular endothelial cells
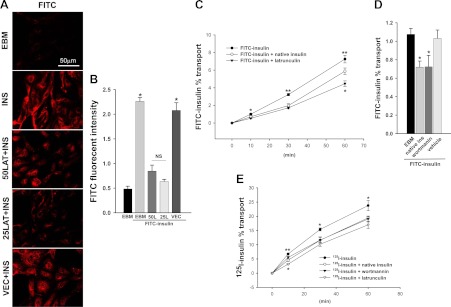
We next used latrunculin A to examine the effect of inhibiting actin microfilament organization on FITC-insulin uptake, the first step of insulin's TET. As shown in Fig. 2A, both 50 and 25 nm latrunculin A reduced FITC-insulin uptake compared with the vehicle control group (P < 0.05 for each) (Fig. 2B). Cell density was comparable between latrunculin A-treated and vehicle controls (latrunculin A at concentrations of 100 nm and higher caused obvious changes in cell morphology and a portion of cells detached from the slides). We further examined the effects of interruption of actin organization on insulin TET using a Transwell culture system as we described previously (8). We used two types of tracer-labeled insulin: FITC-insulin (Fig. 2, C and D) and 125I-labeled [TyrA14] insulin (Fig. 2E); the latter allowed us to examine insulin's TET in the physiological range of insulin concentration (200 pm). Figure 2C shows that the FITC-insulin (1 μΜ) added into the top chamber of the Transwell plates was transported across the bAEC monolayer and that the transport could be significantly inhibited by unlabeled insulin (2 μm) to a similar extent as we reported previously (8). Latrunculin A (25 nm) had an even more profound inhibitory effect on FITC-insulin transport, inhibiting insulin TET by approximately50% at 10 min and approximately 40% after 60 min, respectively. To address whether the residual transported FITC-insulin seen in the presence of latrunculin A could be due to insulin traversing the bAEC monolayer by nonspecific paracellular transport or leakiness of the monolayer, we measured the TET of 1 μm FITC-inulin. Inulin is a polysaccharide that is comparable to insulin in molecular mass (5.2 kDa) and has been extensively used as a control for assessment of the nonspecific transport (6–8). We observed that the transfer of FITC-inulin across the endothelial monolayer averaged approximately 4.2% over 60 min. This is similar to the rate of 1 μm FITC-insulin transfer seen when latrunculin A (25 nm) was added to Transwell plates (4.46 ± 0.31%; see Fig. 2C), suggesting that 25 nm latrunculin almost completely eliminated the insulin's TET. Similar inhibitory effects were seen when either insulin (2 μm) or latrunculin A (25 nm) was added to Transwell plates along with 200 pm [125I]insulin (Fig. 2E). Wortmannin also inhibited both FITC-insulin (1 μm) (Fig. 2D) and [125I]insulin (200 pm) TET (Fig. 2E). This is consistent with our previous observation that same dose of wortmannin inhibits FITC-insulin uptake by bAEC (9). In aggregate, these data (Figs. 1 and 2) suggest that actin microfilament reorganization occurs quickly in response to physiological insulin concentrations and is required for insulin uptake and TET. The findings further indicate that insulin-induced cortical actin remodeling is mediated at least in part by insulin signaling through PI3K. Interfering with this signaling coordinately not only impairs actin cytoskeletal remodeling but also inhibits insulin transport.
Fig. 2.
A and B, Dose response of FITC-insulin uptake to latrunculin A treatment. The bAEC were serum starved for 6 h and then pretreated with 0 (vehicle) and 25 and 50 nm latrunculin A (LAT or L), respectively, for 30 min followed by incubation with or without 50 nm FITC-insulin (INS) for 30 min. Cells then were fixed and stained using anti-FITC (red, revealed by Cy3) primary antibodies. A, Confocal images from single optical sections. B, The histograms indicate the quantitation of FITC-insulin fluorescence intensity observed in three experiments. *, P < 0.001 compared with EBM (incubated in basal medium without FITC-insulin), 50 nm LAT or 25 nm LAT group, respectively; no significant difference between FITC-insulin (INS)-treated only (EBM+FITC-insulin) and vehicle control (VEC) + FITC-insulin group or between remaining groups). C–E, Effects of either interruption of actin filament organization or blocking PI3K insulin signaling pathway on insulin's TET: time course for the transport of insulin across bAEC cultured on Transwell plates. Cells were exposed to either 1 μm FITC-insulin (C and D) or 200 pm [125I]insulin (E) alone or in the presence of either 2 μm unlabeled insulin or 25 nm latrunculin A (C and E) or 100 nm wortmannin (D and E) or vehicle control (D). C, Time course of percent transport of total added FITC-insulin. *, P < 0.05 compared with remaining groups, respectively; **, P < 0.001 compared with remaining groups, respectively; n = 8. D, Percent transport of total added FITC-insulin at 10 min. *, P < 0.05 compared with the control groups (EBM or vehicle), respectively; no significant difference between EBM and vehicle group; n = 4. E, Time course of percent transport of total added [125I]insulin. *, P < 0.01 compared with remaining groups, respectively; **, P < 0.001 compared with the latrunculin group and P < 0.05 compared with the remaining groups, respectively; n = 3.
Disruption of actin microfilament organization inhibits the localization of caveolin-1, IR, and IGF-IR to the plasma membrane
Because we have previously shown that insulin TET involves its binding to the IR or, at high insulin concentrations, to the IGF-IR or IR/IGF-IR hybrids (8, 22) and that caveolin-1 is required for insulin transport (10), we next examined the effects of disruption of actin microfilament organization on insulin-induced localization of caveolin-1, IR, or IGF-IR to the plasma membrane of bAEC using a plasma membrane sheet preparation (10, 20). As shown in Fig. 3, insulin treatment of bAEC increased the amount of IGF-IR, caveolin-1, and IR on the plasma membrane (P < 0.05 for each) but did not affect plasma membrane intercellular adhesion molecule-1 (ICAM) content, which served as a control for the amount of membrane sheet (P > 0.05). However, pretreatment of cells with 25 nm latrunculin A almost completely blocked the insulin-induced increases of IGF-IR and caveolin-1 at the plasma membrane (Fig. 3, A–C) and significantly blunted plasma membrane IR increases by approximately 60% (Fig. 3, A and D; also see Fig. 1C, micrographs B, E, and H) without affecting the amount of ICAM (Fig. 3E). These data indicate that insulin-induced actin filament remodeling may facilitate either these proteins trafficking to or retention at the plasma membrane to mediate insulin transport (8, 10).
Proinflammatory cytokines TNFα and IL-6 inhibit insulin-induced cortical actin filament remodeling
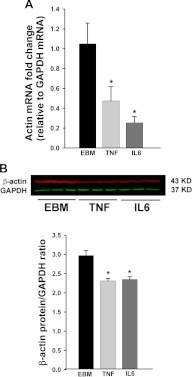
We have previously shown that treatment of bAEC with TNFα or IL-6 for either 6 h (9) or 24 h (10) not only significantly inhibits FITC-insulin uptake but also inhibits insulin-induced caveolin-1 localization at the plasma membrane (10). Figure 4 shows that, like latrunculin A, pretreatment of cells with either TNFα or IL-6 for 24 h completely blocked insulin-induced cortical actin remodeling. In addition, although pretreatment with TNFα or IL-6 for 6 h did not appear to significantly affect the amount of F-actin in the basal state, it completely blocked insulin-induced actin polymerization (Fig. 4C). Pretreatment of bAEC with TNFα or IL-6 for 24 h also significantly inhibited actin mRNA expression (Fig. 5A) and protein level (Fig. 5B). This did not appear to be due to diminished bAEC viability (10). This suggests that the reduction of actin expression caused by extended TNFα or IL-6 treatment may, at least in part, contribute to the inhibition of insulin-induced cortical actin remodeling by TNFα/IL-6.
Fig. 5.
Extended exposure of bAEC to TNFα or IL-6 inhibited β-actin expression at both mRNA and protein levels. Cultured bAEC were serum starved in the absence or presence of TNFα or IL-6 (20 ng/ml, each) for 24 h. Cells were then processed for real-time RT-PCR (A) or for Western blotting (B). *, P < 0.05 compared with EBM (incubated in basal medium only).
Discussion
The findings of the present study clearly demonstrate that insulin stimulates rapid cortical actin filament remodeling in vascular endothelial cells and that insulin-induced actin filament remodeling is required for insulin uptake and TET. This insulin-induced cortical actin filament remodeling is dependent on PI3K/Akt-mediated insulin signaling and on the integrity of plasma membrane lipid rafts or caveolae. Our findings also indicate that insulin-induced actin filament remodeling may facilitate insulin transit at least in part by promoting caveolin-1, IR, and IGF-IR trafficking to or sequestration at the plasma membrane. Finally, these data provide evidence that TNFα and IL-6 negatively affect insulin-induced cortical actin filament remodeling and even actin expression.
Insulin has been reported to induce rapid cortical actin filament remodeling (also referred to as membrane ruffling) in a variety of nonendothelial cell types including epidermoid-derived cells (23), muscle cells (19), and fat cells (17, 18). Interestingly, insulin-induced cortical actin filament remodeling has been found to correlate to an increased transport of nutrients such as amino acids (23) and glucose [the latter via increases in GLUT4 vesicle translocation to the plasma membrane] (17–19). Interruption of this insulin-induced cortical actin filament remodeling using specific inhibitors of actin polymerization can completely block insulin-induced amino acid transport (23) and significantly reduce cell surface GLUT4 localization and glucose transport (17–19). Certainly for GLUT4, cortical actin remodeling facilitates insulin-induced movement of transporting vesicles to the plasma membrane. Likewise, SV40 in CV-1 cells has also been reported to induce rapid cortical actin filament remodeling, and latrunculin A effectively blocks both actin filament remodeling and caveolae-mediated SV40 endocytosis (16). In aggregate, these data suggest that cortical actin filament remodeling may represent a common cellular pathway that mediates vesicular transport processes in a variety of cells.
In the present study, we observed that either inhibition of PI3K activity using wortmannin or knockdown of Akt using specific siRNA inhibited insulin-induced cortical actin filament remodeling (see Fig. 1, C, D, and G–I). Inhibition of PI3K also inhibited trans-endothelial insulin transport. We have previously observed that inhibition of either tyrosine kinases (by genistein) or PI3K (by wortmannin) significantly reduced FITC-insulin uptake by vascular endothelial cells, whereas inhibiting protein tyrosine phosphatase 1B stimulated insulin uptake (9). Thus, the PI3K/Akt dependency of insulin-induced cortical actin remodeling observed in the present study is consistent with our previous finding (9). The dependency of insulin-induced cortical actin filament remodeling on PI3K activity appears to be dependent on cell types. Wortmannin has been reported to completely block insulin-induced cortical actin filament remodeling in preadipocytes but was less effective in fully differentiated 3T3-L1 adipocytes (18). In CV-1 cells, SV40-induced cortical actin remodeling associated with increased caveolae-mediated SV40 uptake appears to not only be dependent on the activation of tyrosine kinases (16) but also to be regulated by a variety of serine/threonine kinases, although the precise regulatory mechanisms remain elusive (24).
In the present study, we observed that after insulin treatment, a portion of caveolin-1 associated with thickened cortical actin filamentous band at the cell periphery (Fig. 1C, micrograph H) and that pretreatment of bAEC with filipin, a sterol-binding agent, completely blocked insulin-induced cortical actin filament remodeling and disrupted the caveolin-1 localization. Knockdown of caveolin-1 using specific siRNA also completely blocked the insulin-induced cortical actin remodeling. These data suggest a relationship between the F-actin and lipid rafts/caveolae (Fig. 1C, micrographs L, O, and R, and Fig. 1E). Interestingly, cholesterol depletion from CV-1 cells not only effectively eliminated SV40-induced cortical actin filament remodeling but also inhibited caveolae-mediated SV40 uptake by the cells (16). Disrupting lipid rafts with the filipin also inhibits insulin's uptake and TET (7, 8). We have previously reported that knockdown of caveolin-1 not only significantly reduced both insulin receptor protein level and insulin-stimulated Akt phosphorylation but also inhibited insulin-induced caveolin-1 and IGF-IR translocation to the plasma membrane (10). Given that insulin-induced rapid cortical actin filament remodeling appears to be required for trans-endothelial insulin transport, it suggests that the caveolae-associated F-actin may play a role in regulation and/or assembly of specific cellular machinery essential for cellular insulin responsiveness (25).
The mechanism by which insulin-induced actin filament remodeling facilitates insulin transport is not clear. We have previously demonstrated that both vascular endothelial insulin uptake and TET are mediated by the IR, and at supraphysiological concentrations, the IGF-IR and IR/IGF-IR hybrids also contribute (8, 22). Very recently, we have also demonstrated that caveolin-1 is required for insulin uptake, suggesting that insulin transport is mediated by transporting caveolae vesicles (10). Others have recently demonstrated the dynamic nature of caveolae trafficking in living cells; i.e. about one third of total cellular caveolae engage in a rapid, local fission-fusion cycle with the plasma membrane (24), whereas the majority of stationary caveolae on the plasma membrane may serve as a physiological membrane reservoir to buffer acute mechanical stresses (26). In the present study, we observed that insulin increased the quantity of IR and IGF-IR and caveolin-1 on the plasma membrane (Fig. 3, A–E). Pretreatment of cells with latrunculin A significantly reduced this insulin effect (Fig. 3, A–E). This is consistent with our previous findings that insulin transport is mediated by IR/IGF-IR (8) and caveolin-1-containing vesicles (8, 10) and also suggests that in vascular endothelial cells, the cortical actin filament network may serve as a scaffold to enhance temporal and spatial location of the transporting vesicles and insulin signaling molecules as has been suggested for adipocytes and muscle cells (27).
It has recently been reported that with insulin resistance, insulin-induced Rac activation and actin filament remodeling in muscle cells are compromised (28). We have previously reported that TNFα or IL-6 significantly inhibits FITC-insulin uptake by bAEC (9, 10). In the present study, we found that pretreatment of cells with TNFα or IL-6 abolished insulin-induced cortical actin filament remodeling (Fig. 4). In addition, although extended treatment (24 h) with either TNFα or IL-6 decreased both β-actin mRNA and protein expression (Fig. 5), shorter exposures (6 h) did not (Fig. 4C), yet they still significantly impeded insulin-stimulated F-actin remodeling. Both TNFα and IL-6 significantly inhibit insulin-stimulated PI3K pathway activity in cultured aortic endothelial cells (29, 30), and this may be, at least in part, responsible for the interruption of insulin-induced cortical actin filament remodeling. These data are consistent with our previous finding that both TNFα and IL-6 interfere with insulin-stimulated caveolin-1, IR, and IGF-IR localization at the plasma membrane (10, 31).
In summary, we report what we believe to be the first observations that insulin induces rapid actin cytoskeletal reorganization in vascular endothelial cells. This occurs at physiological insulin concentrations. Furthermore, disruption of insulin-induced rapid cortical actin filament remodeling impedes insulin transport by vascular endothelial cells. Insulin-induced cortical actin remodeling requires insulin signaling via the PI3K/Akt pathway and is dependent on the integrity of lipid rafts/caveolae. Disruption of insulin-induced cortical actin remodeling also reduces caveolin-1, IR, and IGF-IR localization at the plasma membrane, which is needed for insulin transport. Vascular insulin resistance produced by TNFα or IL-6 also blocks insulin-induced cortical actin remodeling, and this may contribute to their inhibition of insulin transport (9, 10). Our results highlight that insulin appears to be an important regulator of endothelial cell actin cytoskeletal architecture and that disruption of actin cytoskeletal organization significantly impairs endothelial insulin transport. Improved understanding of insulin's effects on the vascular endothelial cell actin cytoskeleton reorganization may suggest new interventions for insulin resistance, type 2 diabetes, and their vascular sequelae.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants DK057878 and DK073059 and ADA 11-BS06 to E.J.B.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- bAEC
- Bovine aortic endothelial cells
- EBM
- endothelial basal medium
- F-actin
- filamentous actin
- FITC
- fluorescein isothiocyanate
- GLUT4
- glucose transporter 4
- ICAM
- intercellular adhesion molecule-1
- IGF-IR
- IGF-I receptor
- IR
- insulin receptors
- PI3K
- phosphatidylinositol 3-kinase
- siRNA
- small interfering RNA
- SV40
- simian virus 40
- TBS
- Tris-buffered saline
- TET
- trans-endothelial transport
- TRITC
- tetramethylrhodamine isothiocyanate.
References
- 1. Barrett EJ, Wang H, Upchurch CT, Liu Z. 2011. Insulin regulates its own delivery to skeletal muscle by feed-forward actions on the vasculature. Am J Physiol Endocrinol Metab 301:E252–E263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang YJ, Hope ID, Ader M, Bergman RN. 1989. Insulin transport across capillaries is rate limiting for insulin action in dogs. J Clin Invest 84:1620–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miles PD, Levisetti M, Reichart D, Khoursheed M, Moossa AR, Olefsky JM. 1995. Kinetics of insulin action in vivo. Identification of rate-limiting steps. Diabetes 44:947–953 [DOI] [PubMed] [Google Scholar]
- 4. Miles PD, Li S, Hart M, Romeo O, Cheng J, Cohen A, Raafat K, Moossa AR, Olefsky JM. 1998. Mechanisms of insulin resistance in experimental hyperinsulinemic dogs. J Clin Invest 101:202–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sjöstrand M, Gudbjörnsdottir S, Holmäng A, Lönn L, Strindberg L, Lönnroth P. 2002. Delayed transcapillary transport of insulin to muscle interstitial fluid in obese subjects. Diabetes 51:2742–2748 [DOI] [PubMed] [Google Scholar]
- 6. King GL, Johnson SM. 1985. Receptor-mediated transport of insulin across endothelial cells. Science 227:1583–1586 [DOI] [PubMed] [Google Scholar]
- 7. Schnitzer JE, Oh P, Pinney E, Allard J. 1994. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J Cell Biol 127:1217–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang H, Liu Z, Li G, Barrett EJ. 2006. The vascular endothelial cell mediates insulin transport into skeletal muscle. Am J Physiol Endocrinol Metab 291:E323–E332 [DOI] [PubMed] [Google Scholar]
- 9. Wang H, Wang AX, Liu Z, Barrett EJ. 2008. Insulin signaling stimulates insulin transport by bovine aortic endothelial cells. Diabetes 57:540–547 [DOI] [PubMed] [Google Scholar]
- 10. Wang H, Wang AX, Barrett EJ. 2011. Caveolin-1 is required for vascular endothelial insulin uptake. Am J Physiol Endocrinol Metab 300:E134–E144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gustavsson J, Parpal S, Karlsson M, Ramsing C, Thorn H, Borg M, Lindroth M, Peterson KH, Magnusson KE, Strâlfors P. 1999. Localization of the insulin receptor in caveolae of adipocyte plasma membrane. FASEB J 13:1961–1971 [PubMed] [Google Scholar]
- 12. Kabayama K, Sato T, Saito K, Loberto N, Prinetti A, Sonnino S, Kinjo M, Igarashi Y, Inokuchi J. 2007. Dissociation of the insulin receptor and caveolin-1 complex by ganglioside GM3 in the state of insulin resistance. Proc Natl Acad Sci USA 104:13678–13683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Foti M, Porcheron G, Fournier M, Maeder C, Carpentier JL. 2007. The neck of caveolae is a distinct plasma membrane subdomain that concentrates insulin receptors in 3T3–L1 adipocytes. Proc Natl Acad Sci USA 104:1242–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huo H, Guo X, Hong S, Jiang M, Liu X, Liao K. 2003. Lipid rafts/caveolae are essential for insulin-like growth factor-1 receptor signaling during 3T3-L1 preadipocyte differentiation induction. J Biol Chem 278:11561–11569 [DOI] [PubMed] [Google Scholar]
- 15. Pelkmans L, Kartenbeck J, Helenius A. 2001. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat Cell Biol 3:473–483 [DOI] [PubMed] [Google Scholar]
- 16. Pelkmans L, Püntener D, Helenius A. 2002. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science 296:535–539 [DOI] [PubMed] [Google Scholar]
- 17. Wang Q, Bilan PJ, Tsakiridis T, Hinek A, Klip A. 1998. Actin filaments participate in the relocalization of phosphatidylinositol3-kinase to glucose transporter-containing compartments and in the stimulation of glucose uptake in 3T3-L1 adipocytes. Biochem J 331(Pt 3):917–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kanzaki M, Pessin JE. 2001. Insulin-stimulated GLUT4 translocation in adipocytes is dependent upon cortical actin remodeling. J Biol Chem 276:42436–42444 [DOI] [PubMed] [Google Scholar]
- 19. Tong P, Khayat ZA, Huang C, Patel N, Ueyama A, Klip A. 2001. Insulin-induced cortical actin remodeling promotes GLUT4 insertion at muscle cell membrane ruffles. J Clin Invest 108:371–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang H, Wang AX, Liu Z, Chai W, Barrett EJ. 2009. The trafficking/interaction of eNOS and caveolin-1 induced by insulin modulates endothelial nitric oxide production. Mol Endocrinol 23:1613–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Robinson LJ, Pang S, Harris DS, Heuser J, James DE. 1992. Translocation of the glucose transporter (GLUT4) to the cell surface in permeabilized 3T3-L1 adipocytes: effects of ATP insulin, and GTP gamma S and localization of GLUT4 to clathrin lattices. J Cell Biol 117:1181–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li G, Barrett EJ, Wang H, Chai W, Liu Z. 2005. Insulin at physiological concentrations selectively activates insulin but not insulin-like growth factor I (IGF-I) or insulin/IGF-I hybrid receptors in endothelial cells. Endocrinology 146:4690–4696 [DOI] [PubMed] [Google Scholar]
- 23. Goshima K, Masuda A, Owaribe K. 1984. Insulin-induced formation of ruffling membranes of KB cells and its correlation with enhancement of amino acid transport. J Cell Biol 98:801–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pelkmans L, Zerial M. 2005. Kinase-regulated quantal assemblies and kiss-and-run recycling of caveolae. Nature 436:128–133 [DOI] [PubMed] [Google Scholar]
- 25. Kanzaki M, Pessin JE. 2002. Caveolin-associated filamentous actin (Cav-actin) defines a novel F-actin structure in adipocytes. J Biol Chem 277:25867–25869 [DOI] [PubMed] [Google Scholar]
- 26. Sinha B, Köster D, Ruez R, Gonnord P, Bastiani M, Abankwa D, Stan RV, Butler-Browne G, Vedie B, Johannes L, Morone N, Parton RG, Raposo G, Sens P, Lamaze C, Nassoy P. 2011. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell 144:402–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zaid H, Antonescu CN, Randhawa VK, Klip A. 2008. Insulin action on glucose transporters through molecular switches, tracks and tethers. Biochem J 413:201–215 [DOI] [PubMed] [Google Scholar]
- 28. JeBailey L, Wanono O, Niu W, Roessler J, Rudich A, Klip A. 2007. Ceramide- and oxidant-induced insulin resistance involve loss of insulin-dependent Rac-activation and actin remodeling in muscle cells. Diabetes 56:394–403 [DOI] [PubMed] [Google Scholar]
- 29. Li G, Barrett EJ, Barrett MO, Cao W, Liu Z. 2007. Tumor necrosis factor-α induces insulin resistance in endothelial cells via a p38 mitogen-activated protein kinase-dependent pathway. Endocrinology 148:3356–3363 [DOI] [PubMed] [Google Scholar]
- 30. Yuen DY, Dwyer RM, Matthews VB, Zhang L, Drew BG, Neill B, Kingwell BA, Clark MG, Rattigan S, Febbraio MA. 2009. Interleukin-6 attenuates insulin-mediated increases in endothelial cell signaling but augments skeletal muscle insulin action via differential effects on tumor necrosis factor-alpha expression. Diabetes 58:1086–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang H, Wang AX, Barrett EJ. 2009. Insulin promotes and cytokines inhibit both trafficking of IR and IGF-IR to the plasma membrane and endothelial cell insulin transport. Diabetes 58:A333 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.