Abstract
Nuclear receptor transcriptional activity is enhanced by interaction with coactivators. The highly related nuclear receptor 5A (NR5A) subfamily members liver receptor homolog 1 and steroidogenic factor 1 bind to and activate several of the same genes, many of which are important for reproductive function. To better understand transcriptional activation by these nuclear receptors, we sought to identify interacting proteins that might function as coactivators. The LIM domain protein four and a half LIM domain 2 (FHL2) was identified as interacting with the NR5A receptors in a yeast two-hybrid screen of a human ovary cDNA library. FHL2, and the closely related FHL1, are both expressed in the rodent ovary and in granulosa cells. Small interfering RNA-mediated knockdown of FHL1 and FHL2 in primary mouse granulosa cells reduced expression of the NR5A target genes encoding inhibin-α and P450scc. In vitro assays confirmed the interaction between the FHL and NR5A proteins and revealed that a single LIM domain of FHL2 is sufficient for this interaction, whereas determinants in both the ligand binding domain and DNA binding domain of NR5A proteins are important. FHL2 enhances the ability of both liver receptor homolog 1 and steroidogenic factor 1 to activate the inhibin-α subunit gene promoter in granulosa cells and thus functions as a transcriptional coactivator. FHL2 also interacts with cAMP response element-binding protein and substantially augments activation of inhibin gene expression by the combination of NR5A receptors and forskolin, suggesting that FHL2 may facilitate integration of these two signals. Collectively these results identify FHL2 as a novel coactivator of NR5A nuclear receptors in ovarian granulosa cells and suggest its involvement in regulating target genes important for mammalian reproduction.
Nuclear receptors (NR) comprise a large class of DNA binding proteins (48 known human NR) that regulate gene transcription (1, 2). NR have a conserved structure that includes a DNA binding domain (DBD) composed of two zinc fingers and a carboxy-terminal ligand binding domain (LBD) composed of 12 alpha helices that form a hydrophobic pocket that in many cases binds a cognate ligand. NR are classified based on their binding to their DNA response elements: homodimers bind to inverted repeat response elements [i.e. estrogen receptor (ER), progesterone receptor, androgen receptor (AR)], heterodimers with retinoid X receptor as a partner bind to direct repeat response elements (i.e. retinoic acid receptor, peroxisomal proliferator-activated receptor), and NR monomers bind to half-site response elements (3). The transcriptional activation potential of many NR is dependent on the binding of small, lipophilic molecules to the LBD pocket. A small number of NR, most of which bind as monomers, are constitutively active and regulate gene transcription in the absence of exogenous ligand. These receptors are often referred to as orphan nuclear receptors (4).
Liver receptor homolog 1 (LRH-1) and steroidogenic factor 1 (SF-1) are members of the NR5A subfamily of NR (5, 6). The NR5A subfamily is also known as the FTZ-F1 family because the fushi tarazu segmentation gene in Drosophila melanogaster was the founding member of this gene family (7). Although LRH-1 and SF-1 are often characterized as orphan nuclear receptors, phospholipids have been found in the binding pockets of several NR5A LBD crystal structures, and these may serve as ligands that stabilize the folded structure of the receptor (8–10). SF-1 (NR5A1) is a transcriptional regulator of steroidogenic gene expression in endocrine tissues including the gonads, adrenal gland, and ventral medial hypothalamus. SF-1 knockout mice lack gonads and adrenal glands, highlighting the essential role that SF-1 plays in development (11). LRH-1 (NR5A2) is expressed in endoderm-derived tissues including the gonads, liver, pancreas, and intestine and is involved in processes including development, cell proliferation, and tumorogenesis (5, 12, 13). Initial work with LRH-1 was performed largely in the liver, in which it is a key regulator of the conversion of cholesterol to bile acid salts through the transcriptional control of the cytochrome P450 7A gene (CYP7A1) (5, 14, 15). Subsequently, LRH-1 expression was detected in ovarian granulosa cells in which SF-1 was already known to be expressed (16–18).
LRH-1 and SF-1 are highly related proteins with 96% amino acid similarity in the DBD and 75% amino acid similarity in the LBD. Consistent with the highly conserved DBD, LRH-1 and SF-1 regulate several of the same target genes including many that are involved in reproductive function. These include inhibin-α (19, 20) and cholesterol side-chain cleavage (CYP11A1, P450scc) (21, 22). Although they share homology and regulate many common target genes, LRH-1 and SF-1 have unique patterns of expression and regulation in the ovary (18, 23), and ovarian conditional knockout mice indicate that each is critically important for normal reproductive function and fertility (24, 25).
Coactivators interact with NR and enhance their activity by recruiting/interacting with components of the RNA polymerase II preinitiation complex, altering chromatin structure through histone modification or adding posttranslational modifications directly to the receptor (26). Coactivators such as steroid receptor coactivator 1 (SRC-1) and cAMP response element-binding protein (CREB) binding protein, which increase the activation potential of many nuclear receptors and are broadly expressed, coactivate LRH-1 and SF-1 on multiple target gene promoters including the inhibin-α gene (20, 27–30). Other, more specific NR5A coregulators have been reported, including multibridging factor 1 (31, 32), β-catenin (33, 34), peroxisome proliferator-activated receptor-γ coactivator-1α (35, 36), and proline-rich nuclear receptor coregulatory protein (37). In addition, the corepressors prospero-related homeobox 1 (38) and zinc finger protein 67 kDa (39) have been shown to modulate the activity of the NR5A nuclear receptors.
To better understand the transcriptional activation of the NR5A receptors within the reproductive axis, we performed a yeast two-hybrid screen of a human ovarian cDNA library to identify NR5A interacting proteins and thus possible coregulatory proteins. We identify the LIM domain proteins of the four and a half LIM (FHL) family, FHL1 and FHL2, as being expressed in the ovary and as interacting with LRH-1 and SF-1. The FHL domain family of multifunctional proteins is composed of five members (FHL1–4 and activator of cAMP response element modulator in the testes), with each gene displaying tissue specific expression. FHL2 is expressed in the heart, gonads, prostate, adrenal gland, and intestines (40, 41), whereas FHL1 is more broadly expressed in tissues including in the heart, gonads, muscle, kidney, lung, and brain (41). FHL3 expression is more restricted with high expression in skeletal muscle and lower expression in the heart (41, 42), whereas FHL4 and activator of cAMP response element modulator in the testes are exclusively expressed in germ cells of the testes (41, 43, 44). Each LIM domain is composed of two zinc fingers; however, the FHL proteins do not bind to DNA. Instead the FHL proteins are often characterized as protein interaction partners and are reported to act as tissue-specific coactivators [e.g. FHL2 is a known coactivator of the AR (45)]. Although FHL1 and FHL2 expression has been detected in the ovary (41, 46), a function in reproduction has not yet been established. We demonstrate in this report that knockdown of FHL1 and FHL2 decreases the expression of genes involved in mammalian reproduction and that FHL2 functions as a coactivator of the NR5A proteins in activating the inhibin-α subunit gene in ovarian granulosa cells. We also show that FHL2 interacts with CREB and may integrate multiple signaling pathways controlling inhibin gene expression.
Materials and Methods
Animals
CD1 mice (Harlan, Indianapolis, IN) were maintained in accordance with the policies of Northwestern University's Animal Care and Use Committee. Mice were housed at a constant temperature and humidity in a 12 h light, 12-h dark cycle with food and water available ad libitum. Mice were fed soy-free chow to limit exogenous phytoestrogen intake through food.
Plasmids
cDNA plasmids were kindly provided by the following people: mouse LRH-1, Dr. Alan Tall (Columbia University, New York, NY) (47); mouse SF-1, Dr. Larry Jameson (Northwestern University, Feinberg School of Medicine, Chicago, IL); human ERβ and human glucocorticoid receptor (GR), Dr. Peter Kopp (Northwestern University, Feinberg School of Medicine, Chicago, IL); mouse FHL1, Dr. Ju Chen (University of California, San Diego, La Jolla, CA); and rat CREB, Dr. Jon Kornhauser (Children's Hospital, Boston, MA). GAL4 activation assay fusion constructs were generated in the pBIND vector (CheckMate mammalian two-hybrid system; Promega, Madison, WI). Yeast two-hybrid bait constructs were generated in the pGBKT7-DBD vector (MatchMaker GAL4 Yeast Two-Hybrid Kit System 3; CLONTECH, Mountain View, CA). A human FHL2 construct was generated by digesting an FHL2 cDNA identified in the yeast two-hybrid assay followed by ligation into pcDNA3.1 (Invitrogen, Carlsbad, CA). Mouse FHL2 was generated by PCR using mouse ovary cDNA as a template. The PCR product was cloned into to the pCR2.1 TA cloning vector (Invitrogen) and then subcloned into pcDNA3.1. Glutathione-S-transferase (GST) expression vectors were generated by cloning inserts into the pGEX2T vector (GE Life Sciences, Pittsburgh, PA). Mutant constructs were generated with the QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA).
Yeast two-hybrid screens and assay
The yeast two-hybrid screen was performed using the MatchMaker GAL4 Yeast Two-Hybrid System 3 (CLONTECH) according to the manufacturer's protocol. A human ovary cDNA library (in pACT2 vector; CLONTECH) was screened with the LRH-1 K289R LBD/pGBKT7-DBD construct with a transformation efficiency of 3.2 × 104 CFU/μg DNA. Interaction colonies were isolated from SD-HIS/-TRP/-LEU containing 10 mm 3-amino-1,2,4-triazole. β-Galactosidase assays (o-nitrophenyl β-galactosidase substrate) were performed with 10 μg of mated yeast protein lysate in a 96-well plate format. Reactions were performed at 30 C for 60 min. Plates were read on a SpectraMax 384 Plus plate reader (Molecular Devices, Sunnyvale, CA) at 420 nm.
Cell culture, transfection, and luciferase assay
GRMO2 cells (48) were maintained and transfected as previously described (49). Cells were transfected with 500 ng of the rat inhibin-α promoter (547 bp) fused to a luciferase reporter construct (inhibin-α luc) (50), 50 ng of LRH-1 or SF-1, and 50 ng of human FHL2 when necessary. DNA quantities were held constant with empty vector. GAL4 activation assays were performed with 500 ng of pG5-luc and 50 ng of LRH-1 or SF-1. Samples were assayed for luciferase activity using a Monolight 2010 luminometer (Analytical Luminescence Laboratory, San Diego, CA). Luciferase values were normalized to total protein content per well as assayed by a Bradford reagent (Bio-Rad Laboratories, Hercules, CA) per the manufacturer's protocol.
GST protein expression, purification, and pulldowns
GST fusion proteins were expressed in BL21 CodonPlus cells (Stratagene). Cultures were induced with 0.2 mm isopropyl β-d-1-thiogalactopyranoside and grown at 30 C for 1–2 h. Pelleted cells were resuspended in lysis buffer (50 mm Tris-Cl, pH 8.0; 150 mm NaCl; 1 mg/ml lysozyme; 50 μm ZnCl) and subjected to three rounds of sonication on ice: 1 × 20 sec, 2 × 10 sec. After sonication, the cells were lysed with Triton X-100 (1% final) and digested with deoxyribonuclease (50 μg/ml final) at 4 C with agitation for 30 min. Cell debris was harvested by centrifugation and the resulting supernatant was bound to 50% glutathione 4B sepharose (Pierce, Rockford, IL) overnight. Bound proteins were washed twice in a low-salt buffer (50 mm Tris-Cl, pH 8.0; 150 mm NaCl) and once in a high-salt buffer (50 mm Tris-Cl, pH 8.0; 500 mm NaCl). Proteins were stored at 4 C on the beads in low-salt buffer containing protease inhibitors. GST pulldowns were performed in a buffer containing 20 mm HEPES (pH 7.5), 50 mm KCl, 25 mm MgCl2, 1 mm dithiothreitol (DTT), 0.1 mm EDTA, and 0.15% Nonidet P-40. In vitro transcribed and translated proteins were generated with transcribed and translated T7 coupled reticulocyte lysate system (Promega) incorporating 35S-methionine (PerkinElmer, Norwalk, CT). A defined amount of each GST fusion protein bound to glutathione 4B sepharose was used in each pulldown, and the proteins were coincubated at 4 C for 2 h. Two microliters of each 35S-labeled protein were used in the pulldowns. The interacting protein complexes bound to the beads were washed three times with pulldown buffer and then boiled in a sodium dodecyl sulfate (SDS)-containing buffer. Eluted proteins were resolved on a 10% SDS-PAGE gel. Gels were fixed in isopropanol/water/acetic acid (25:65:10), impregnated in Amplify solution (GE Life Sciences), dried, and exposed to autoradiographic film at −80 C with intensifying screens.
Immunohistochemistry and immunocytochemistry
Ovaries were harvested from 18-d-old mice. Immunohistochemistry was performed as previously described (51) using an anti-FHL2 polyclonal antibody, diluted 1:2000 (kindly provided by Dr. Beat Schäfer, University Children's Hospital, Zurich, Switzerland) (40) or an anti-FHL1 polyclonal antibody diluted 1:1000 (no. 49241, Abcam, Cambridge, MA). For immunocytochemistry of endogenous or overexpressed proteins in GRMO2 cells, cells were plated overnight on glass coverslips. For detection of overexpressed proteins, cells were transfected with 500 ng of FLAG-tagged constructs for 24 h before immunocytochemistry detection. For endogenous and overexpressed proteins, cells were fixed in 1% paraformaldehyde and permeabilized in PBS/0.25% Triton X-100. Blocking was performed in PBS/0.25% Triton X-100 with 5% serum followed by incubation with primary antibody overnight at 4 C: anti-FHL2 1:600 (kindly provided by Dr. Roland Schüle, University of Freiburg, Freiburg, Germany) (52), anti-FLAG 1:250 (no. F3165; Sigma, St. Louis, MO). After an incubation with fluorophore-conjugated secondary antibodies (1:200; Sigma), the coverslips were incubated with fluorescent phallotoxins [phalloidin-TRITC (Sigma) or Alexa Fluor 488 phalloidin (Invitrogen)] to stain the actin filaments. Coverslips were mounted on slides with Vectastain [4′,6′-diamino-2-phenylindole (DAPI); Vector Laboratories, Burlingame, CA] for visualization of nuclei. Images were captured on a Leica DM5000B microscope (Leica Microsystems, Wetzlar, Germany).
Primary granulosa cell isolation and small interfering RNA (siRNA) knockdown
Primary granulosa cells were extracted from the ovaries of d 21–24 immature CD1 mice. Ovaries were trimmed of fat and collected into 4F media (1× DMEM/F12; 15 mm HEPES, pH 7.4; 5μg/m; apo-transferrin; 2μg/m; insulin; 40 ng/m; hydrocortisone; 2.43 g/liter sodium bicarbonate; 0.5× penicillin-streptomycin) containing 0.5 m sucrose and 10 mm EGTA, incubated at 37 C for 20 min to loosen cell junctions, and washed three times in cold 4F media. Cells were isolated by follicle puncture in 4F media using 26-gauge needles under a Reichert Stereo Star ZOOM dissection microscope (Reichert, Heidelberg, Germany). The extruded granulosa cells were collected via centrifugation in a swinging bucket rotor (500 × g, 10 min, Accuspin 3R; Fisher Scientific, Fair Lawn, NJ) and resuspended in 4F media containing 10% fetal bovine serum (FBS). The resuspended cells were filtered through a 40-μm nylon mesh strainer (Millipore, Bedford, MA) to remove oocytes and tissue pieces. The granulosa cells were plated in 4F media/10% FBS at an approximate density of 2 × 105 cells/well in a 12-well plate. Cells were allowed to adhere for 48 h and then transfected with 10 nm of each siRNA duplex pool (20 nm per well total with the amount held constant with control duplexes when necessary) (On-Target Plus Smartpool; Dharmacon, Lafayette, CO) using Lipofectamine RNAiMax reagent (Invitrogen) for 6 h. Cells were recovered in 4F media/10% FBS, and RNA or protein was extracted 72 h after transfection.
RNA extraction and PCR
RNA was extracted from primary mouse granulosa cells or from whole tissues using the QIAGEN RNAeasy kit (Valencia, CA). cDNA was generated using avian myeloblastosis virus-reverse transcriptase and random hexamer primers. Semiquantitative PCR was performed in the presence of 32P-deoxy-CTP radiolabeled nucleotides (Amersham Pharmacia Biotech, Piscataway, NJ). The resulting PCR products were electrophoresed on a 5% acrylamide gel. The gels were dried and exposed to autoradiographic film. Quantitative PCR (qPCR) was performed with SYBR green master mix (Applied Biosystems, Carlsbad, CA) with 100 ng of reverse-transcribed cDNA per well. Reactions were performed using a 7300 Realtime Machine (Applied Biosystems). The primers used in the semiquantitative PCR and qPCR assays are listed in Table 1.
Protein extraction and Western blotting
Whole-cell protein lysates were generated from primary granulosa cells as previously described (49). Protein concentrations were determined using a Bradford protein dye (Bio-Rad Laboratories). Then 13.5% SDS-PAGE gels were run with 100 μg of each protein lysate. Immunoblotting was performed as previously described with antibodies specific to each protein of interest [anti-FHL2, 1:8000 (kindly provided by Dr. R. Schüle, University of Freiburg, Freiburg, Germany)] (52), antiinhibin-α 1:1000 (kindly provided by Dr. W. Vale, Salk Institute, La Jolla, CA), and anti-actin 1:1500, no. A2066; Sigma). The nitrocellulose membranes were stripped with Re-Blot strong stripping solution (Millipore) after blotting with each primary antibody.
Chromatin immunoprecipitation (ChIP) assay
ChIP assays were performed with GRMO2 cells grown in 10-cm dishes in normal growth media. The cells were stimulated with 10 μm forskolin (FSK) or dimethylsulfoxide (1:1000) in growth media for 1 h at 37 C. The cells were washed with PBS and then cross-linked with 1% formaldehyde (in PBS) for 10 min at 37 C. The cross-linking was quenched with 125 mm glycine, on ice, for 5 min. The cells were washed and collected in PBS, pelleted by centrifugation, resuspended in lysis buffer (50 mm Tris-Cl, pH 7.9; 10 mm EDTA; 1% SDS; 1 mm DTT; protease inhibitors) and lysed for 10 min on ice. The cell debris was removed by centrifugation. The cross-linked chromatin was sheared via sonication to an average size of 500 bp or less (10 × 20 pulses on ice, 30% output). The sheared chromatin was diluted 1:10 in dilution buffer (20 mm Tris-HCl, pH 7.9; 150 mm NaCl; 2 mm EDTA; 0.5% Trion X-100; 1 mm DTT; 2 μg/ml sonicated salmon sperm; and protease inhibitors) and then precleared with 20 μl of a 50% protein A slurry (2 h at 4 C, nutating). The chromatin-protein complexes were incubated overnight at 4 C with the following antibodies: 2 μl FHL2 (no. 66399; Abcam), 2 μl LRH-1 (53), 2 μl SF-1 (no. PA1-800; Affinity BioReagents, Golden, CO), 10 μl CREB (no. sc-186; Santa Cruz Biotechnologies, Santa Cruz, CA) The antibody-bound complexes were pulled down (2 h, 4 C, nutating) with 40 μl of 50% protein A beads blocked with 100 μg of sonicated salmon sperm DNA and 100 μg BSA per 100 μl of 50% slurry beads. The beads were repeatedly washed to remove unbound chromatin-protein complexes (3 × 5min with 20 mm Tris-HCl, pH 8.0; 250 mm NaCl; 2 mm EDTA; 0.25% Nonidet P-40; 0.05% SDS; protease inhibitors; and 1× instant wash with Tris/EDTA). The chromatin-protein complexes were simultaneously eluted from the beads and reverse cross-linked at 65 C overnight followed by a proteinase K digestion for 30 min at 42 C. The DNA was extracted with phenol/chloroform/isoamyl alcohol followed by a sodium acetate and ethanol precipitation. The DNA was resuspended in 50 μl of water and used in subsequent qPCR reactions using ABI SYBR Green Mastermix (7300 Realtime Machine; Applied Biosystems) (for qPCR primer sequences, see Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org).
Statistical analysis
Statistics were performed using GraphPad Prism 4.02 for Windows (GraphPad Software, La Jolla, CA). The data were analyzed using a two-tailed, paired Student's t test. P < 0.05 was considered statistically significant.
Results
NR5A proteins potentially interact with FHL2
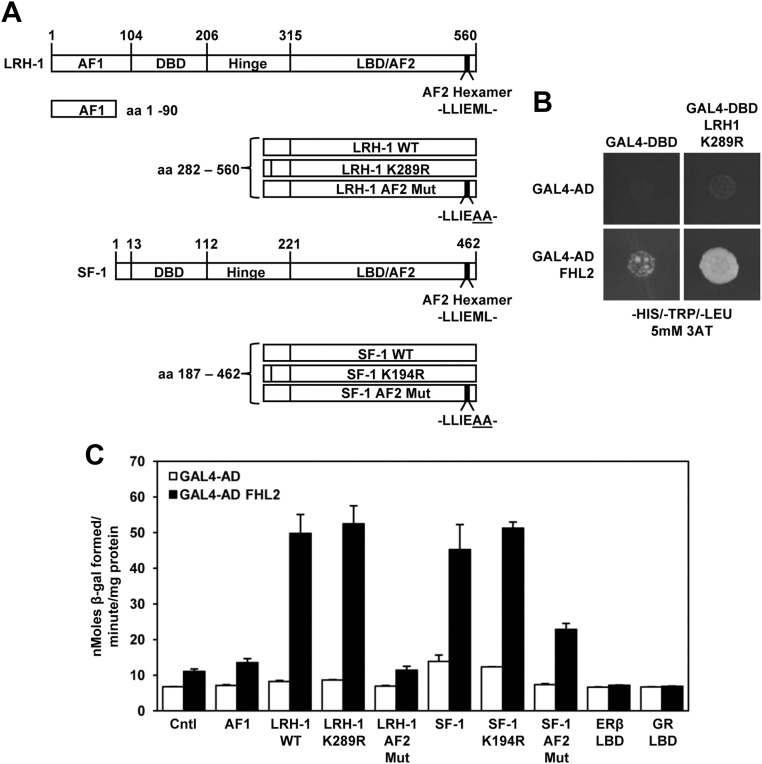
Proteins that might modulate NR5A function were identified in a yeast two-hybrid interaction assay. A human ovary library was screened with an LRH-1 carboxy terminus construct containing a mutation of a known small ubiquitin-related modifier acceptor site (SUMOylation site) (LRH-1 K289R LBD, Fig. 1A) (54). This construct was used in the yeast two-hybrid screen because it had robust activity in a GAL4 activation assay in granulosa cells compared with a wild-type (WT) construct (Supplemental Fig. 1). Six of 120 isolated and sequenced cDNA clones were identified as the multifunctional protein FHL2 (Fig. 1B, yeast growth assay) (40). The entire FHL2 open reading frame was present in the clones isolated.
Fig. 1.
FHL2 interacts with the NR5A proteins in yeast. A, Schematic representation of the LRH-1 and SF-1 constructs used in the yeast two-hybrid assays. B, Yeast two-hybrid growth assay in which the GAL4-DBD vector expresses tryptophan (TRP+), GAL4-AD vector expresses leucine (LEU+), with a positive interaction activating an endogenous histidine (HIS+) reporter in the yeast. A positive interaction is indicated by yeast growth on a synthetic medium lacking HIS, TRP, and LEU. 3-Amino-1,2,4-triazole was included in the medium to block leaky HIS expression. C, β-Gal assays performed with yeast cell lysates. The data represent the mean ± sem of three independent experiments performed in duplicate.
Because the yeast two-hybrid screen was performed with the LRH-1K289R LBD construct, we asked whether FHL2 could also interact with WT LRH-1 LBD. Yeast β-galactosidase (β-gal) assays demonstrated that the strength of interaction between FHL2 and LRH-1 WT LBD or LRH-1 K289R LBD was similar (Fig. 1C, 49.8 ± 5.3 vs. 52.5 ± 5.0 β-gal specific activity units, respectively). Therefore, the presence of the LRH-1 small ubiquitin-related modifier (SUMO) acceptor site does not affect the interaction with FHL2 in yeast cells. Because the LRH-1 construct used had 75% amino acid similarity (55% amino acid identity) with the equivalent region of SF-1, we asked whether FHL2 could also interact with SF-1. The β-gal-specific activity generated between the interaction of FHL2 and WT SF-1 LBD or SF-1 K194R LBD (mutation of a known SF-1 SUMO acceptor site) (54, 55) was 45.2 ± 7.0 and 51.3 ± 1.7, respectfully, demonstrating that FHL2 also interacts with SF-1 in yeast cells (Fig. 1C).
Many coregulatory proteins are known to interact with the activation function 1 and/or activation function 2 (AF2) region of nuclear receptors. For example, mutation of the second pair of hydrophobic residues in the AF2 hexamer (Fig. 1A) has previously been shown to abolish interaction between SF-1 and SRC-1 (27). Mutation of the LRH-1 or SF-1 AF2 region (LLIEML to LLIEAA) substantially decreased the interaction with FHL2 (Fig. 1C). FHL2 did not interact with the ERβ or GR LBD in the yeast β-gal assays (Fig. 1C), demonstrating specificity of the NR5A interaction. However, FHL2 has been previously reported to interact with the AR (45).
FHL proteins are expressed in granulosa cells
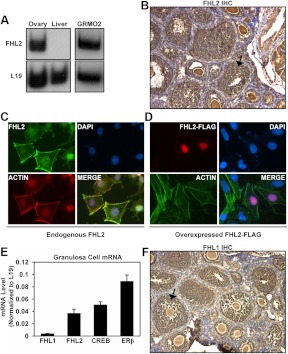
As noted above, we isolated full-length FHL2 from the human ovary cDNA library. Previous reports have demonstrated that FHL2 is expressed in the ovary, but these studies did not examine the localization of FHL2 in the ovary. To confirm the expression of FHL2 in the rodent ovary, we performed semiquantitative RT-PCR using FHL2-specific primers and mouse ovary or liver cDNA. FHL2 was expressed in the ovary but not in the liver, as previously reported in mouse tissues (Fig. 2A) (41). In addition, FHL2 was expressed in GRMO2 cells, which are an immortalized mouse ovarian granulosa cell line (Fig. 2A, right panel). Using immunohistochemistry, we demonstrated that FHL2 protein is expressed in the ovary predominantly in the granulosa cells and oocytes of follicles of all sizes, with lesser expression in thecal cells (Fig. 2B, with the negative control located in Supplemental Fig. 2A).
Fig. 2.
FHL family proteins are expressed in granulosa cells. A, Semiquantitative RT-PCR to detect FHL2 or RPL19 mRNA expression in mouse tissues and GRMO2 cells. B, Immunohistochemistry with an antibody against FHL2 (40) on a paraffin-embedded d 18 mouse ovary section. Closed arrow indicates granulosa cells. C, Immunocytochemistry on GRMO2 cells using an antibody against FHL2 (green) (52). The nuclei were detected with DAPI (blue) and actin filaments were detected with phalloidin (red). D, Immunocytochemistry on GRMO2 cells using an antibody against FLAG to detect overexpressed FHL2-FLAG (red). The nuclei were detected with DAPI (blue) and actin filaments were detected with phalloidin (green). E, Quantitative RT-PCR analyzing the relative mRNA expression of genes in primary mouse granulosa cells. The data represent the mean ± sem of three independent experiments. F, Immunohistochemistry with an antibody against FHL1 on a paraffin-embedded d 18 mouse ovary section. Closed arrow indicates granulosa cells. Negative controls are found in Supplemental Fig. 2.
FHL2 is known to be present and have functions both in the cell nucleus and cytoplasm (52). Immunofluorescence localized endogenous FHL2 protein to both the nucleus and cytoplasm of GRMO2 cells (Fig. 2C). The extranuclear FHL2 expression overlaps with actin filaments stained with phalloidin, with condensed punctuate staining at the end of stress fibers. FHL2 is known to interact with α- and β-integrin chains (56) as well as focal adhesion kinase (57); thus, these punctuate structures are likely focal adhesion complexes. Overexpressed FHL2-FLAG is expressed predominantly in the nucleus of GRMO2 cells (Fig. 2D).
FHL2 belongs to a family of proteins (FHL1–5) that display tissue specific but, in some cases, overlapping expression. The FHL2 family member FHL1 is also reported to be expressed in the ovary (41). We demonstrated that FHL1 is expressed in primary mouse granulosa cells, albeit at a much lower level than FHL2 (Fig. 2E). Similar to FHL2, FHL1 protein is expressed predominantly in the granulosa cells and oocytes of follicles of all sizes, with lesser expression in thecal cells (Fig. 2F, with the negative control located in Supplemental Fig. 2B).
Knockdown of FHL proteins attenuates ovarian gene expression
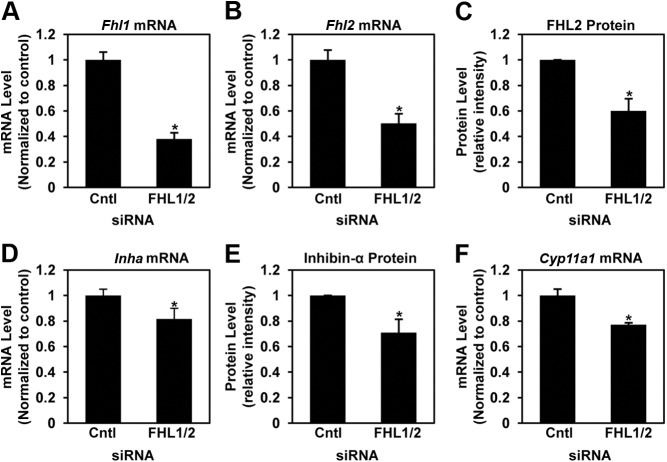
To determine whether FHL1 and FHL2 have a functional role in gene regulation in the ovary through their interaction with NR5A receptors, we performed siRNA-mediated knockdown experiments in primary mouse granulosa cells and examined the effect on the expression of several NR5A target genes. Both inhibin-α (Inha) and p450scc (Cyp11a1) are known to have NR5A binding sites and be regulated by NR5A proteins (19, 22). Upon cotransfection of the FHL1 and FHL2 siRNA smart pools, endogenous FHL1 mRNA expression was reduced by 62% (Fig. 3A), whereas FHL2 mRNA levels were reduced by 49% (Fig. 3B) compared with control siRNA. Each siRNA smart pool was independently verified for the ability to specifically reduce the mRNA level of the targeted gene: Fhl1 = 63% and Fhl2 = 47% (data not shown). The reduction in Fhl2 mRNA levels resulted in a 40% decrease in protein level (Fig. 3C). The combined knockdown of Fhl1 and Fhl2 resulted in a small but significant 18% reduction in Inha mRNA expression (Fig. 3D). The inhibin-α protein level (pro-inhibin-α) was significantly decreased by 29% when FHL1 and FHL2 were simultaneously knocked down (Fig. 3E). The NR5A target gene Cyp11a1 mRNA expression was also reduced by 23% after cotransfection of FHL1 and FHL2 siRNA smart pools compared with control siRNA (Fig. 3F). Thus, FHL1 and FHL2 appear to be important for the normal expression of these target genes in ovarian granulosa cells.
Fig. 3.
Knockdown of FHL1/2 decreases the expression of NR5A target genes in granulosa cells. Fhl1 (A) and Fhl2 (B) mRNA expression in primary mouse granulosa cells were quantified by quantitative RT-PCR after siRNA-mediated knockdown of FHL1 and FHL2. The mRNA level of each sample was normalized to the internal control RPL19 and plotted as fold over control siRNA. The data represent the mean ± sem of three independent experiments. C, Densinometric quantification of FHL2 protein levels normalized to actin protein levels, as analyzed by immunoblotting (not shown), after siRNA-mediated knockdown of FHL1 and FHL2. The data are plotted as fold over control siRNA and represents the mean ± sem of at least three independent experiments. Inha mRNA (D), inhibin-α protein (E), and Cyp11a1 (F) mRNA was analyzed and plotted as described in A–C. Bars marked by asterisk are significantly different (P < 0.05) from the corresponding control in the same graph.
FHL family proteins interact with NR5A proteins through specific domains
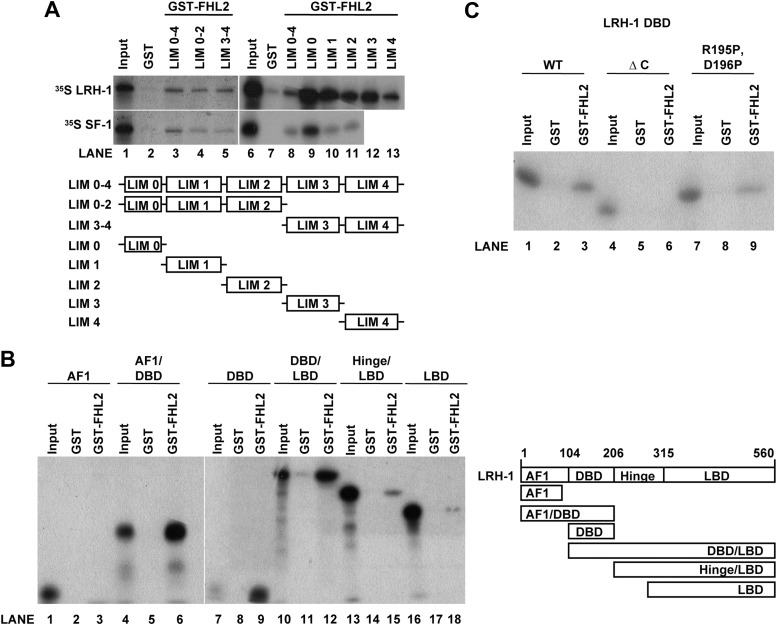
To ascertain whether the FHL proteins interact with LRH-1 or SF-1 in vitro, GST pulldown assays were performed. Recombinant FHL1 and FHL2 were purified as GST fusion proteins from Escherichia coli cells. GST-FHL1 and GST-FHL2 interacted with in vitro-transcribed and -translated 35S-methionine-labeled LRH-1 and SF-1 (Fig. 4). GST-FHL2 did not interact with 35S-methionine GR (Fig. 4, bottom, left panel), indicating that the interaction with the NR5A proteins was specific, as was observed in the yeast interaction studies (the GST-FHL1/GR interaction was not examined). Because granulosa cells express far more FHL2 than FHL1 (Fig. 2E) and because only FHL2 was identified in the initial yeast two-hybrid screen, we focused our remaining studies on FHL2.
Fig. 4.
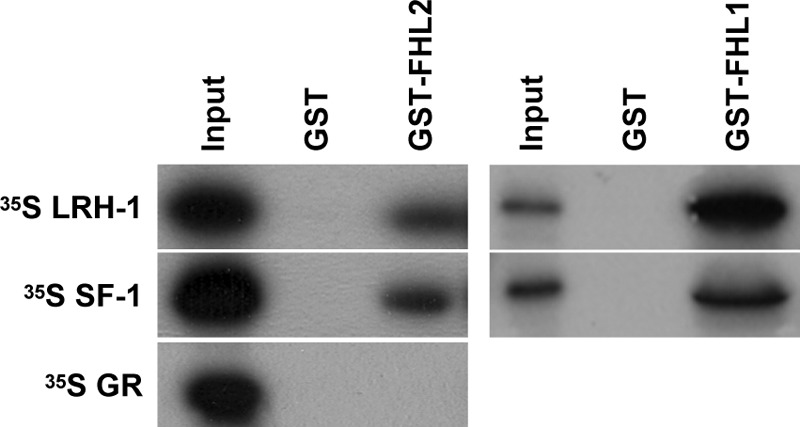
FHL and NR5A proteins interact in vitro. GST pulldown assays were performed with full-length GST-FHL2 (left panels) or GST-FHL1 (right panels). Ten percent of the input sample was run as a positive control, whereas GST alone was used as a negative control.
FHL2 is composed of four and a half highly homologous LIM domains, with each consisting of two zinc fingers (Fig. 5A). The FHL2 amino terminal LIM domains (LIM 0–2) and carboxy terminal LIM domains (LIM 3–4) were purified as GST fusion proteins, and we found that LRH-1 and SF-1 interacted with both the amino and carboxy terminal LIM domains of FHL2 (Fig. 5A, lanes 1–5). The LIM domains were next purified as individual GST fusion proteins (GST-LIM 0, GST-LIM 1, GST-LIM 2, GST-LIM 3, and GST-LIM 4) and used in pulldown assays. LRH-1 and SF-1 interacted with the single zinc finger, half LIM domain (Fig. 5A, lane 9) as well as the individual full LIM domains (Fig. 5A, lanes 6–13; SF-1 was not tested with GST-LIM 3 or GST-LIM 4). Thus, each of the individual LIM domains is capable of independent interaction with the NR5A receptors.
Fig. 5.
Mapping of the interaction domains between FHL2 and NR5A proteins. GST pulldown assays were performed with GST fused to different LIM regions of FHL2 and incubated with full-length SF-1 or LRH-1 (A) or GST fused to full-length FHL2 and incubated with the indicated domains of LRH-1 (B and C). Ten percent of the input sample was run as a positive control, whereas GST alone was used as a negative control. Images taken from different gels are indicated by a break between the panels.
To explore which region of the NR5A proteins interacts with FHL2, deletion constructs were generated that spanned LRH-1 (Fig. 5B). A very weak interaction was present between LRH-1 AF1 and FHL2 in yeast assays (Fig. 1C), but this interaction was not detected in the GST pulldowns (Fig. 5B, lanes 1–3). However, GST-FHL2 did interact with the LRH-1 DBD when it was expressed as an independent domain (Fig. 5B, lanes 7–9) or as part of the amino terminal (Fig. 5B, lanes 4–6) or carboxy terminal (Fig. 5B, lanes 10–12) domains of the protein. In agreement with the yeast β-gal data (Fig. 1C), the LRH-1 LBD also interacted with GST-FHL2 in the absence of the LRH-1 DBD (Fig. 5B, lanes 13–18). Thus, both the DBD and LBD of LRH-1 appear to facilitate interaction with FHL2. Although the LBD association is typical of many coactivators, the DBD interaction is rather unique and was explored further.
The NR5A DBD is composed of a core DBD containing two zinc fingers followed by a carboxy terminal α-helix (FTZ-F1 helix) that is necessary for protein-DNA complex stability (49, 58). We previously hypothesized that the FTZ-F1 helix may be an interaction surface for protein association (49). Consistent with this hypothesis, GST-FHL2 did not interact with the LRH-1 DBD when the FTZ-F1 helix was deleted (LRH-1 DBD ΔC) (Fig. 5C, lanes 4–6). When the FTZ-F1 helix was disrupted by a mutation introducing two proline residues (LRH-1 R195P, D196P), the interaction with GST-FHL2 was decreased compared with WT LRH-1 DBD (Fig. 5C, lanes 7–9). This same mutation is known to reduce the transcriptional potential of SF-1 (49).
FHL2 coactivates the NR5A family of NR in granulosa cells
Because we determined that NR5A target gene expression was reduced after FHL2 knockdown in granulosa cells and we identified in vitro interactions between FHL2 and the NR5A proteins, we asked whether FHL2 had a functional effect on the transactivation potential of LRH-1 or SF-1. GRMO2 cells were transfected with a 547-bp proximal promoter construct of the rat inhibin-α subunit gene fused upstream of a luciferase reporter cassette (inhibin-α luc) (50). GRMO2 cells were cotransfected with LRH-1 alone or in combination with FHL2. FHL2 alone did not activate inhibin-α luc (Fig. 6A). LRH-1 transactivated inhibin-α luc 4.8-fold over basal levels and the activation was increased to 7.4-fold over basal when FHL2 was coexpressed. Similar coactivation results were obtained with SF-1 (Fig. 6B). SF-1 activated inhibin-α luc 2.3-fold over basal levels, and the addition of FHL2 increased the activation to 3.7-fold over basal. Therefore, FHL2 coactivates the NR5A nuclear receptors on the proximal promoter of the inhibin-α subunit gene in granulosa cells.
Fig. 6.
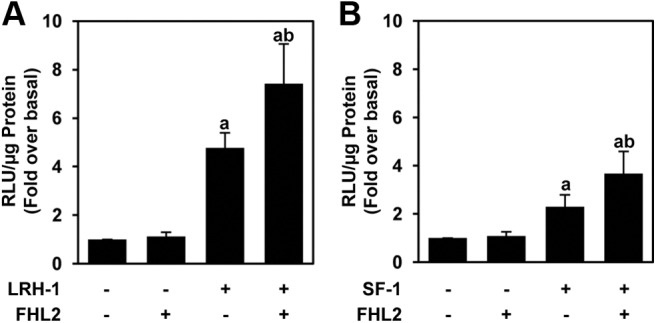
FHL2 coactivates the NR5A proteins in granulosa cells. GRMO2 cells were cotransfected with inhibin-α luc, FHL2, NR5A receptor, or the combination. A, Results for LRH-1. B, Results for SF-1. The data represent the mean ± sem of at least 11 experiments performed in triplicate. Bars with different letters are significantly different from each other by a value of P ≤ 0.05.
FHL2 interacts with CREB and enhances synergism between NR5A and cAMP regulation of the inhibin-α promoter
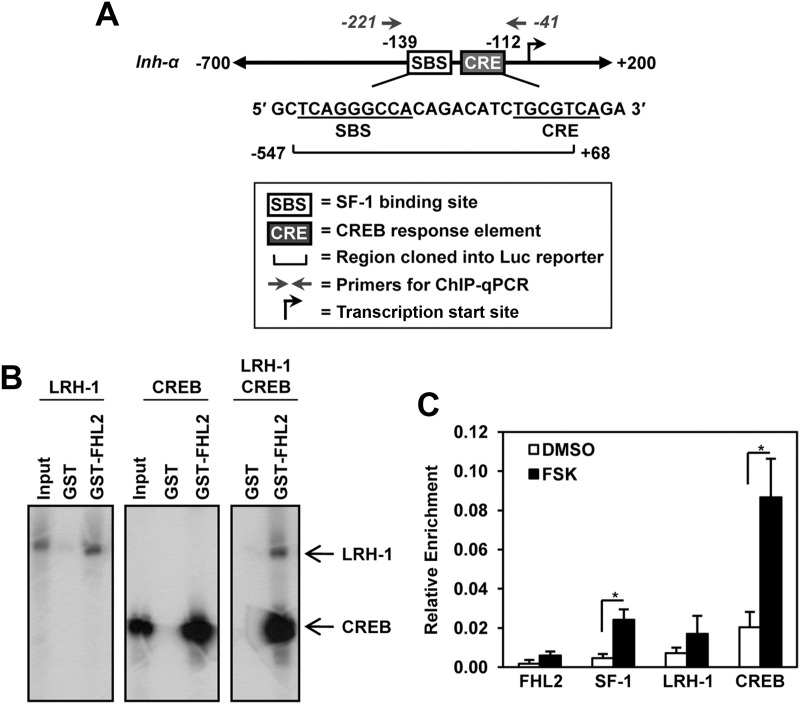
FHL2 has been reported to interact with and coactivate CREB (41). We confirmed the interaction of FHL2 and CREB in yeast cell lysates using β-gal assays and in vitro using GST pulldown assays (Supplemental Fig. 3). The NR5A proteins bind to the inhibin-α promoter at a conserved site (the SF-1 binding site that is 8 bp 5′ to a conserved cAMP response element that binds CREB (19, 20, 50) (Fig. 7A). Previous work indicated that NR5A proteins and cAMP signaling exhibit synergism in activating the inhibin-α promoter and that the NR5A proteins do not activate the promoter if the CRE site is mutated (20), leading to the conclusion that there is an integration between the NR5A and CREB responses. Such integration could occur in part through a bridging protein such as FHL2. LRH-1 and CREB individually interact with GST-FHL2, and they both interact with GST-FHL2 when present in the same pulldown (Fig. 7B). The simultaneous, direct interaction of these factors with GST-FHL2 indicates that they do not compete for binding to the same interaction surface of FHL2. This is likely because the multiple LIM domains each have the ability to bind to both proteins. To test whether these factors are simultaneously recruited to the inhibin-α promoter in granulosa cells, we performed ChIP experiments in GRMO2 cells (Fig. 7C). We found that FHL2 is recruited to the promoter along with both SF-1/LRH-1 and CREB and that this recruitment increases after the activation of the protein kinase A (PKA) pathway with the adenylate cyclase activator FSK.
Fig. 7.
FHL2 interacts with both NR5A receptors and CREB and is recruited to the inhibin promoter with these factors after FSK stimulation. A, Gene structure schematic of the mouse inhibin-α promoter. The NR5A and CREB response elements and the promoter region included in the luciferase reporter construct are indicated. The ChIP qPCR primer position is also indicated. B, LRH-1 and CREB do not compete for interaction with FHL2. GST pulldown was performed with full-length GST-FHL2. Ten percent of the input sample was run as a control. C, ChIP assay showing the relative enrichment of FHL2, NR5A receptors, and CREB recruited to the inhibin-α promoter after FSK stimulation in GRMO2 cells. The data are plotted with the IgG immunoprecipitations subtracted as background. The data represent the mean ± sem of at least three experiments. The asterisk marks the factors with significantly different relative enrichment (P < 0.05) between dimethylsulfoxide (DMSO) and FSK treatment.
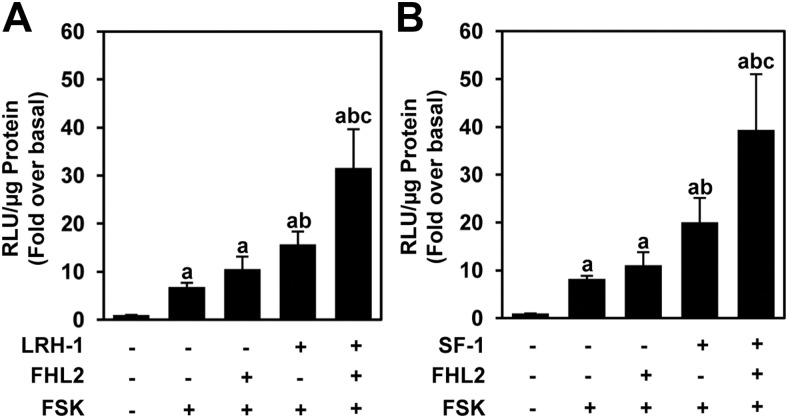
We next tested the functional effect of this corecruitment by asking whether FHL2 could coactivate LRH-1 after the activation of the PKA pathway. Stimulation of the PKA pathway with FSK, which is known to increase CREB phosphorylation, increased the activation of inhibin-α luc. We did not observe a significant increase in this response in the presence of cotransfected FHL2 (Fig. 8A). However, the combination of LRH-1 and FSK increased the activation of inhibin-α luc to 15.7-fold over basal activity, which was increased to 31.5-fold over basal activity upon the addition of FHL2 (Fig. 8A). Similar results were generated with FSK stimulation in the presence of both SF-1 and FHL2 (Fig. 8B). Thus, FHL2 augments the combined response to NR5A receptors and the cAMP-PKA-CREB pathway and may serve to integrate the activity of these two important regulators of reproductive hormone gene expression.
Fig. 8.
FHL2 augments inhibin-α promoter activity in the presence of NR5A receptors and FSK. GRMO2 cells were cotransfected with inhibin-α luc and LRH-1 and FHL2 (A) or SF-1 and FHL2 (B). Twenty-four hours after transfection, the cells were stimulated with 10 μm FSK for 6 h. The data represent the mean ± sem of at least eight experiments performed in triplicate. Bars with different letters are significantly different from each other by a value of P ≤ 0.05.
Discussion
We have identified FHL2 as an interactor of the NR5A nuclear receptors LRH-1 and SF-1, shown that FHL2 coactivates the NR5A receptors on the inhibin-α subunit promoter, and demonstrated that FHL2 interacts with CREB and enhances the combined effects of NR5A receptors and cAMP/CREB signaling on the inhibin-α subunit gene. FHL2 is a multifunctional protein that can shuttle between the cytoplasm and nucleus. FHL2 is a major component of cytoskeletal architecture, interacting with actin filaments (59) and the cytoplasmic tails of multiple integrin chains (56, 60). Stimulation of cells with serum or activation of Rho GTPases causes translocation of FHL2 from the cytoplasm to the nucleus (52, 61). Once in the nucleus, FHL2 stimulates the transcription of genes involved in cell proliferation and tumorogenesis (40, 52, 62), bone formation (63, 64), and cardiac function. Transcription factors reported to be coactivated by FHL2 include the AR (45), CREB (41), the aryl hydrocarbon receptor (65), activator protein 1 (61), and Wilm's tumor 1 (WT-1) (46).
Two groups have generated FHL2 knockout mice, and both strains are viable (66, 67). Although FHL2 is expressed in the myocardium of the developing murine heart, these mice have largely normal cardiac development (66, 67). Slight functional abnormalities were reported in the FHL2 knockout mice including exaggerated cardiac hypertrophy in response to a sustained β-adrenergic stimulus (67), osteopenia due to reduced activity of osteoblasts (63), and impaired wound healing due to a slow cell migration resulting from decreased expression of α-smooth muscle actin and decreased effector functions of focal adhesion kinase including reduced expression of p130cas and decreased activity of Rac GTPase (68). The FHL2 knockout mice are fertile, but reproductive function in these mice has not been carefully examined. It is postulated that the lack of a severe phenotype in these mice may be due to functional redundancy with other FHL proteins, including FHL1, which are expressed in the same tissues.
Partial knockdown of FHL1 and FHL2 in primary granulosa cells resulted in a significant decrease in NR5A target gene expression. We were unable to achieve a full knockdown of FHL1 and FHL2, perhaps because of the specific duplexes used or perhaps because of the inefficiency in transfecting these primary cells, and this likely contributes to the modest impact on target gene expression. In addition, we know that the promoters of these genes are under complex and multifactorial regulation (20), so it is perhaps not surprising that knockdown of this single factor did not have a larger effect. Nonetheless, these studies suggest that FHL family proteins are necessary for full ovarian expression of genes that play key roles in reproductive function.
FHL2 interacts with the NR5A nuclear receptors both in vivo and in vitro. We determined that LRH-1 and SF-1 interact with full-length FHL2 and that a single LIM domain is sufficient for the interaction. The FHL2 LIM domains required for protein interaction vary, depending on the partner protein. The AR was shown to interact with FHL2 LIM 0–2 and LIM 3–4, but individual LIM domain interactions were not analyzed (45). The interaction between FHL2 and β-catenin requires all of the FHL2 LIM domains (69, 70), but the interaction between FHL2 and WT-1 requires only LIM 0–2 (46). The interaction between FHL2 and ERK-2 is strongest with LIM 1–4, but an interaction was also detected with only LIM 2 (71). There are 58 known human LIM domain proteins, which are classified into three groups: LIM only proteins, LIM homeodomain proteins, and LIM kinase proteins (72). The FHL family members are LIM-only proteins, and their effects on gene regulation are likely indirect and are thought to be mediated through protein-protein interactions, such as those demonstrated here for LRH-1 and SF-1.
The NR5A nuclear receptors, like other nuclear receptors, have an AF2 hexamer located near the carboxy terminal end of the LBD (27, 73), and this forms a docking site for coactivators. Yeast β-gal assays demonstrate that this AF2 hexamer is required for the full interaction of both LRH-1 and SF-1 with FHL2. However, we determined that FHL2 also interacts with the FTZ-F1 helix in the NR5A DBD. The FTZ-F1 helix forms a hydrophobic patch on the surface of the DBD that was previously hypothesized to act as a docking site for interacting factors (49). Other coregulatory proteins are reported to interact with the NR5A DBD including WT-1 (74) and multibridging factor 1 (31, 32). ER and GR both have a highly conserved AF2 hexamer but lack the FTZ-1 helix, and neither interacts with FHL2. Thus, the FTZ-F1 helix may provide an additional surface contributing important specificity to the interaction between NR5A receptors and FHL2.
Well-characterized coactivators of LRH-1 and SF-1 include SRC-1 and CREB binding protein (20, 27–30). The transcriptional effects of these coactivators are mediated, in part, by their histone acetyltransferase activity (75). FHL2 is different from these types of coactivators because it possesses transcriptional coactivation capability but has no known inherent enzymatic activity. A well-documented characteristic of LIM domains is that they facilitate protein-protein interactions (72, 76), and FHL2 is often referred to as an adaptor or scaffolding protein (77, 78). It is through this scaffolding mechanism that FHL2 likely integrates transcriptional responses on the inhibin-α promoter through interactions with proteins such as the NR5A receptors and CREB. Interestingly, FHL2 has recently been shown to interact with and corepress the NR4A orphan receptor Nur77 (79), which is implicated in the negative regulation of inhibin gene expression after ovulation (80). Therefore, FHL2 may be a central interaction partner for the complex of proteins that regulate inhibin-α subunit gene expression in granulosa cells.
Collectively the results presented in this paper identify FHL2 as a novel coactivator of the NR5A family of nuclear receptors in ovarian granulosa cells and suggest roles for FHL2 and related LIM domain proteins in regulating mammalian reproduction.
Supplementary Material
Acknowledgments
We thank Dr. Ishwar Radhakrishnan (Northwestern University, Evanston, IL) for technical guidance and helpful discussions throughout this project and Dr. Rolande Schüle (University of Freiburg, Freiburg, Germany) and Dr. Beat Schäfer (University Children's Hospital, Zurich, Switzerland) for anti-FHL2 antibodies.
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Insitutes of Health through Cooperative Agreement U54 HD41857 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (to K.E.M.) and National Institutes of Health Reproductive Biology Training Grant T32 HD007068 predoctoral fellowship (to C.K.M.).
Disclosure Summary: K.E.M. has received grant support for the work presented in this manuscript (National Institute of Child Health and Human Development/National Insitutes of Health Cooperative Agreement U54 HD41857 from 2008 to 2013). C.K.M. has nothing to disclose.
Footnotes
- AF2
- Activation function 2
- AR
- androgen receptor
- ChIP
- chromatin immunoprecipitation
- CREB
- cAMP response element-binding protein
- DAPI
- 4′,6′-diamino-2-phenylindole
- DBD
- DNA binding domain
- DTT
- dithiothreitol
- ER
- estrogen receptor
- FBS
- fetal bovine serum
- FHL
- four and a half LIM
- FSK
- forskolin
- β-gal
- β-galactosidase
- GR
- glucocorticoid receptor
- GST
- glutathione-S-transferase
- LBD
- ligand binding domain
- LRH-1
- liver receptor homolog 1
- NR
- nuclear receptor
- PKA
- protein kinase A
- qPCR
- quantitative PCR
- SDS
- sodium dodecyl sulfate
- SF-1
- steroidogenic factor 1
- siRNA
- small interfering RNA
- SRC-1
- steroid receptor coactivator 1
- SUMO
- small ubiquitin-related modifier
- WT
- wild type
- WT-1
- Wilm's tumor 1.
References
- 1. 1999. A unified nomenclature system for the nuclear receptor superfamily. Cell 97:161–163 [DOI] [PubMed] [Google Scholar]
- 2. McEwan IJ. 2009. Nuclear receptors: one big family. Methods Mol Biol 505:3–18 [DOI] [PubMed] [Google Scholar]
- 3. Tsai MJ, O'Malley BW. 1994. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem 63:451–486 [DOI] [PubMed] [Google Scholar]
- 4. Benoit G, Cooney A, Giguere V, Ingraham H, Lazar M, Muscat G, Perlmann T, Renaud JP, Schwabe J, Sladek F, Tsai MJ, Laudet V. 2006. International Union of Pharmacology. LXVI. Orphan nuclear receptors. Pharmacol Rev 58:798–836 [DOI] [PubMed] [Google Scholar]
- 5. Nitta M, Ku S, Brown C, Okamoto AY, Shan B. 1999. CPF: an orphan nuclear receptor that regulates liver-specific expression of the human cholesterol 7α-hydroxylase gene. Proc Natl Acad Sci USA 96:6660–6665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Honda S, Morohashi K, Nomura M, Takeya H, Kitajima M, Omura T. 1993. Ad4BP regulating steroidogenic P-450 gene is a member of steroid hormone receptor superfamily. J Biol Chem 268:7494–7502 [PubMed] [Google Scholar]
- 7. Ueda H, Sonoda S, Brown JL, Scott MP, Wu C. 1990. A sequence-specific DNA-binding protein that activates fushi tarazu segmentation gene expression. Genes Dev 4:624–635 [DOI] [PubMed] [Google Scholar]
- 8. Krylova IN, Sablin EP, Moore J, Xu RX, Waitt GM, MacKay JA, Juzumiene D, Bynum JM, Madauss K, Montana V, Lebedeva L, Suzawa M, Williams JD, Williams SP, Guy RK, Thornton JW, Fletterick RJ, Willson TM, Ingraham HA. 2005. Structural analyses reveal phosphatidyl inositols as ligands for the NR5 orphan receptors SF-1 and LRH-1. Cell 120:343–355 [DOI] [PubMed] [Google Scholar]
- 9. Ortlund EA, Lee Y, Solomon IH, Hager JM, Safi R, Choi Y, Guan Z, Tripathy A, Raetz CR, McDonnell DP, Moore DD, Redinbo MR. 2005. Modulation of human nuclear receptor LRH-1 activity by phospholipids and SHP. Nat Struct Mol Biol 12:357–363 [DOI] [PubMed] [Google Scholar]
- 10. Wang W, Zhang C, Marimuthu A, Krupka HI, Tabrizizad M, Shelloe R, Mehra U, Eng K, Nguyen H, Settachatgul C, Powell B, Milburn MV, West BL. 2005. The crystal structures of human steroidogenic factor-1 and liver receptor homologue-1. Proc Natl Acad Sci USA 102:7505–7510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Luo X, Ikeda Y, Parker KL. 1994. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77:481–490 [DOI] [PubMed] [Google Scholar]
- 12. Schoonjans K, Dubuquoy L, Mebis J, Fayard E, Wendling O, Haby C, Geboes K, Auwerx J. 2005. Liver receptor homolog 1 contributes to intestinal tumor formation through effects on cell cycle and inflammation. Proc Natl Acad Sci USA 102:2058–2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gu P, Goodwin B, Chung AC, Xu X, Wheeler DA, Price RR, Galardi C, Peng L, Latour AM, Koller BH, Gossen J, Kliewer SA, Cooney AJ. 2005. Orphan nuclear receptor LRH-1 is required to maintain Oct4 expression at the epiblast stage of embryonic development. Mol Cell Biol 25:3492–3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, Maloney PR, Willson TM, Kliewer SA. 2000. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell 6:517–526 [DOI] [PubMed] [Google Scholar]
- 15. Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, Mangelsdorf DJ. 2000. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell 6:507–515 [DOI] [PubMed] [Google Scholar]
- 16. Boerboom D, Pilon N, Behdjani R, Silversides DW, Sirois J. 2000. Expression and regulation of transcripts encoding two members of the NR5A nuclear receptor subfamily of orphan nuclear receptors, steroidogenic factor-1 and NR5A2, in equine ovarian cells during the ovulatory process. Endocrinology 141:4647–4656 [DOI] [PubMed] [Google Scholar]
- 17. Liu DL, Liu WZ, Li QL, Wang HM, Qian D, Treuter E, Zhu C. 2003. Expression and functional analysis of liver receptor homologue 1 as a potential steroidogenic factor in rat ovary. Biol Reprod 69:508–517 [DOI] [PubMed] [Google Scholar]
- 18. Hinshelwood MM, Repa JJ, Shelton JM, Richardson JA, Mangelsdorf DJ, Mendelson CR. 2003. Expression of LRH-1 and SF-1 in the mouse ovary: localization in different cell types correlates with differing function. Mol Cell Endocrinol 207:39–45 [DOI] [PubMed] [Google Scholar]
- 19. Ito M, Park Y, Weck J, Mayo KE, Jameson JL. 2000. Synergistic activation of the inhibin α-promoter by steroidogenic factor-1 and cyclic adenosine 3′,5′-monophosphate. Mol Endocrinol 14:66–81 [DOI] [PubMed] [Google Scholar]
- 20. Weck J, Mayo KE. 2006. Switching of NR5A proteins associated with the inhibin α-subunit gene promoter after activation of the gene in granulosa cells. Mol Endocrinol 20:1090–1103 [DOI] [PubMed] [Google Scholar]
- 21. Kim JW, Havelock JC, Carr BR, Attia GR. 2005. The orphan nuclear receptor, liver receptor homolog-1, regulates cholesterol side-chain cleavage cytochrome p450 enzyme in human granulosa cells. J Clin Endocrinol Metab 90:1678–1685 [DOI] [PubMed] [Google Scholar]
- 22. Watanabe N, Inoue H, Fujii-Kuriyama Y. 1994. Regulatory mechanisms of cAMP-dependent and cell-specific expression of human steroidogenic cytochrome P450scc (CYP11A1) gene. Eur J Biochem 222:825–834 [DOI] [PubMed] [Google Scholar]
- 23. Falender AE, Lanz R, Malenfant D, Belanger L, Richards JS. 2003. Differential expression of steroidogenic factor-1 and FTF/LRH-1 in the rodent ovary. Endocrinology 144:3598–3610 [DOI] [PubMed] [Google Scholar]
- 24. Duggavathi R, Volle DH, Mataki C, Antal MC, Messaddeq N, Auwerx J, Murphy BD, Schoonjans K. 2008. Liver receptor homolog 1 is essential for ovulation. Genes Dev 22:1871–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pelusi C, Ikeda Y, Zubair M, Parker KL. 2008. Impaired follicle development and infertility in female mice lacking steroidogenic factor 1 in ovarian granulosa cells. Biol Reprod 79:1074–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kininis M, Kraus WL. 2008. A global view of transcriptional regulation by nuclear receptors: gene expression, factor localization, and DNA sequence analysis. Nucl Recept Signal 6:e005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Crawford PA, Polish JA, Ganpule G, Sadovsky Y. 1997. The activation function-2 hexamer of steroidogenic factor-1 is required, but not sufficient for potentiation by SRC-1. Mol Endocrinol 11:1626–1635 [DOI] [PubMed] [Google Scholar]
- 28. Ito M, Yu RN, Jameson JL. 1998. Steroidogenic factor-1 contains a carboxy-terminal transcriptional activation domain that interacts with steroid receptor coactivator-1. Mol Endocrinol 12:290–301 [DOI] [PubMed] [Google Scholar]
- 29. Monté D, DeWitte F, Hum DW. 1998. Regulation of the human P450scc gene by steroidogenic factor 1 is mediated by CBP/p300. J Biol Chem 273:4585–4591 [DOI] [PubMed] [Google Scholar]
- 30. Xu PL, Liu YQ, Shan SF, Kong YY, Zhou Q, Li M, Ding JP, Xie YH, Wang Y. 2004. Molecular mechanism for the potentiation of the transcriptional activity of human liver receptor homolog 1 by steroid receptor coactivator-1. Mol Endocrinol 18:1887–1905 [DOI] [PubMed] [Google Scholar]
- 31. Kabe Y, Goto M, Shima D, Imai T, Wada T, Morohashi K, Shirakawa M, Hirose S, Handa H. 1999. The role of human MBF1 as a transcriptional coactivator. J Biol Chem 274:34196–34202 [DOI] [PubMed] [Google Scholar]
- 32. Brendel C, Gelman L, Auwerx J. 2002. Multiprotein bridging factor-1 (MBF-1) is a cofactor for nuclear receptors that regulate lipid metabolism. Mol Endocrinol 16:1367–1377 [DOI] [PubMed] [Google Scholar]
- 33. Botrugno OA, Fayard E, Annicotte JS, Haby C, Brennan T, Wendling O, Tanaka T, Kodama T, Thomas W, Auwerx J, Schoonjans K. 2004. Synergy between LRH-1 and β-catenin induces G1 cyclin-mediated cell proliferation. Mol Cell 15:499–509 [DOI] [PubMed] [Google Scholar]
- 34. Yumoto F, Nguyen P, Sablin EP, Baxter JD, Webb P, Fletterick RJ. 2012. Structural basis of coactivation of liver receptor homolog-1 by β-catenin. Proc Natl Acad Sci USA 109:143–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shin DJ, Osborne TF. 2008. Peroxisome proliferator-activated receptor-γ coactivator-1α activation of CYP7A1 during food restriction and diabetes is still inhibited by small heterodimer partner. J Biol Chem 283:15089–15096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yazawa T, Inaoka Y, Okada R, Mizutani T, Yamazaki Y, Usami Y, Kuribayashi M, Orisaka M, Umezawa A, Miyamoto K. 2010. PPAR-γ coactivator-1α regulates progesterone production in ovarian granulosa cells with SF-1 and LRH-1. Mol Endocrinol 24:485–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhou D, Quach KM, Yang C, Lee SY, Pohajdak B, Chen S. 2000. PNRC: a proline-rich nuclear receptor coregulatory protein that modulates transcriptional activation of multiple nuclear receptors including orphan receptors SF1 (steroidogenic factor 1) and ERRα1 (estrogen related receptor α-1). Mol Endocrinol 14:986–998 [DOI] [PubMed] [Google Scholar]
- 38. Qin J, Gao DM, Jiang QF, Zhou Q, Kong YY, Wang Y, Xie YH. 2004. Prospero-related homeobox (Prox1) is a corepressor of human liver receptor homolog-1 and suppresses the transcription of the cholesterol 7-α-hydroxylase gene. Mol Endocrinol 18:2424–2439 [DOI] [PubMed] [Google Scholar]
- 39. Borud B, Mellgren G, Lund J, Bakke M. 2003. Cloning and characterization of a novel zinc finger protein that modulates the transcriptional activity of nuclear receptors. Mol Endocrinol 17:2303–2319 [DOI] [PubMed] [Google Scholar]
- 40. Genini M, Schwalbe P, Scholl FA, Remppis A, Mattei MG, Schäfer BW. 1997. Subtractive cloning and characterization of DRAL, a novel LIM-domain protein down-regulated in rhabdomyosarcoma. DNA Cell Biol 16:433–442 [DOI] [PubMed] [Google Scholar]
- 41. Fimia GM, De Cesare D, Sassone-Corsi P. 2000. A family of LIM-only transcriptional coactivators: tissue-specific expression and selective activation of CREB and CREM. Mol Cell Biol 20:8613–8622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Turner J, Nicholas H, Bishop D, Matthews JM, Crossley M. 2003. The LIM protein FHL3 binds basic Krüppel-like factor/Krüppel-like factor 3 and its co-repressor C-terminal-binding protein 2. J Biol Chem 278:12786–12795 [DOI] [PubMed] [Google Scholar]
- 43. Morgan MJ, Madgwick AJ. 1999. The fourth member of the FHL family of LIM proteins is expressed exclusively in the testis. Biochem Biophys Res Commun 255:251–255 [DOI] [PubMed] [Google Scholar]
- 44. Fimia GM, De Cesare D, Sassone-Corsi P. 1999. CBP-independent activation of CREM and CREB by the LIM-only protein ACT. Nature 398:165–169 [DOI] [PubMed] [Google Scholar]
- 45. Müller JM, Isele U, Metzger E, Rempel A, Moser M, Pscherer A, Breyer T, Holubarsch C, Buettner R, Schüle R. 2000. FHL2, a novel tissue-specific coactivator of the androgen receptor. EMBO J 19:359–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Du X, Hüblitz P, Gunther T, Wilhelm D, Englert C, Schüle R. 2002. The LIM-only coactivator FHL2 modulates WT1 transcriptional activity during gonadal differentiation. Biochim Biophys Acta 1577:93–101 [DOI] [PubMed] [Google Scholar]
- 47. Luo Y, Liang CP, Tall AR. 2001. The orphan nuclear receptor LRH-1 potentiates the sterol-mediated induction of the human CETP gene by liver X receptor. J Biol Chem 276:24767–24773 [DOI] [PubMed] [Google Scholar]
- 48. Vanderstichele H, Delaey B, de Winter J, de Jong F, Rombauts L, Verhoeven G, Dello C, van de Voorde A, Briers T. 1994. Secretion of steroids, growth factors, and cytokines by immortalized mouse granulosa cell lines. Biol Reprod 50:1190–1202 [DOI] [PubMed] [Google Scholar]
- 49. Little TH, Zhang Y, Matulis CK, Weck J, Zhang Z, Ramachandran A, Mayo KE, Radhakrishnan I. 2006. Sequence-specific deoxyribonucleic acid (DNA) recognition by steroidogenic factor 1: a helix at the carboxy terminus of the DNA binding domain is necessary for complex stability. Mol Endocrinol 20:831–843 [DOI] [PubMed] [Google Scholar]
- 50. Pei L, Dodson R, Schoderbek WE, Maurer RA, Mayo KE. 1991. Regulation of the α inhibin gene by cyclic adenosine 3′,5′-monophosphate after transfection into rat granulosa cells. Mol Endocrinol 5:521–534 [DOI] [PubMed] [Google Scholar]
- 51. Trombly DJ, Woodruff TK, Mayo KE. 2009. Suppression of Notch signaling in the neonatal mouse ovary decreases primordial follicle formation. Endocrinology 150:1014–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Müller JM, Metzger E, Greschik H, Bosserhoff AK, Mercep L, Buettner R, Schüle R. 2002. The transcriptional coactivator FHL2 transmits Rho signals from the cell membrane into the nucleus. EMBO J 21:736–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Galarneau L, Paré JF, Allard D, Hamel D, Levesque L, Tugwood JD, Green S, Bélanger L. 1996. The α1-fetoprotein locus is activated by a nuclear receptor of the Drosophila FTZ-F1 family. Mol Cell Biol 16:3853–3865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lee MB, Lebedeva LA, Suzawa M, Wadekar SA, Desclozeaux M, Ingraham HA. 2005. The DEAD-box protein DP103 (Ddx20 or Gemin-3) represses orphan nuclear receptor activity via SUMO modification. Mol Cell Biol 25:1879–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chen WY, Lee WC, Hsu NC, Huang F, Chung BC. 2004. SUMO modification of repression domains modulates function of nuclear receptor 5A1 (steroidogenic factor-1). J Biol Chem 279:38730–38735 [DOI] [PubMed] [Google Scholar]
- 56. Wixler V, Geerts D, Laplantine E, Westhoff D, Smyth N, Aumailley M, Sonnenberg A, Paulsson M. 2000. The LIM-only protein DRAL/FHL2 binds to the cytoplasmic domain of several α and β integrin chains and is recruited to adhesion complexes. J Biol Chem 275:33669–33678 [DOI] [PubMed] [Google Scholar]
- 57. Gabriel B, Mildenberger S, Weisser CW, Metzger E, Gitsch G, Schüle R, Müller JM. 2004. Focal adhesion kinase interacts with the transcriptional coactivator FHL2 and both are overexpressed in epithelial ovarian cancer. Anticancer Res 24:921–927 [PubMed] [Google Scholar]
- 58. Solomon IH, Hager JM, Safi R, McDonnell DP, Redinbo MR, Ortlund EA. 2005. Crystal structure of the human LRH-1 DBD-DNA complex reveals Ftz-F1 domain positioning is required for receptor activity. J Mol Biol 354:1091–1102 [DOI] [PubMed] [Google Scholar]
- 59. Coghill ID, Brown S, Cottle DL, McGrath MJ, Robinson PA, Nandurkar HH, Dyson JM, Mitchell CA. 2003. FHL3 is an actin-binding protein that regulates α-actinin-mediated actin bundling: FHL3 localizes to actin stress fibers and enhances cell spreading and stress fiber disassembly. J Biol Chem 278:24139–24152 [DOI] [PubMed] [Google Scholar]
- 60. Samson T, Smyth N, Janetzky S, Wendler O, Müller JM, Schüle R, von der Mark H, von der Mark K, Wixler V. 2004. The LIM-only proteins FHL2 and FHL3 interact with α- and β-subunits of the muscle α7β1 integrin receptor. J Biol Chem 279:28641–28652 [DOI] [PubMed] [Google Scholar]
- 61. Morlon A, Sassone-Corsi P. 2003. The LIM-only protein FHL2 is a serum-inducible transcriptional coactivator of AP-1. Proc Natl Acad Sci USA 100:3977–3982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Labalette C, Nouët Y, Sobczak-Thepot J, Armengol C, Levillayer F, Gendron MC, Renard CA, Regnault B, Chen J, Buendia MA, Wei Y. 2008. The LIM-only protein FHL2 regulates cyclin D1 expression and cell proliferation. J Biol Chem 283:15201–15208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Günther T, Poli C, Müller JM, Catala-Lehnen P, Schinke T, Yin N, Vomstein S, Amling M, Schüle R. 2005. Fhl2 deficiency results in osteopenia due to decreased activity of osteoblasts. EMBO J 24:3049–3056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Govoni KE, Baylink DJ, Chen J, Mohan S. 2006. Disruption of four-and-a-half LIM 2 decreases bone mineral content and bone mineral density in femur and tibia bones of female mice. Calcif Tissue Int 79:112–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kollara A, Brown TJ. 2009. Modulation of aryl hydrocarbon receptor activity by four and a half LIM domain 2. Int J Biochem Cell Biol 41:1182–1188 [DOI] [PubMed] [Google Scholar]
- 66. Chu PH, Bardwell WM, Gu Y, Ross J, Jr, Chen J. 2000. FHL2 (SLIM3) is not essential for cardiac development and function. Mol Cell Biol 20:7460–7462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kong Y, Shelton JM, Rothermel B, Li X, Richardson JA, Bassel-Duby R, Williams RS. 2001. Cardiac-specific LIM protein FHL2 modifies the hypertrophic response to β-adrenergic stimulation. Circulation 103:2731–2738 [DOI] [PubMed] [Google Scholar]
- 68. Wixler V, Hirner S, Müller JM, Gullotti L, Will C, Kirfel J, Günther T, Schneider H, Bosserhoff A, Schorle H, Park J, Schüle R, Buettner R. 2007. Deficiency in the LIM-only protein Fhl2 impairs skin wound healing. J Cell Biol 177:163–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wei Y, Renard CA, Labalette C, Wu Y, Lévy L, Neuveut C, Prieur X, Flajolet M, Prigent S, Buendia MA. 2003. Identification of the LIM protein FHL2 as a coactivator of β-catenin. J Biol Chem 278:5188–5194 [DOI] [PubMed] [Google Scholar]
- 70. Martin B, Schneider R, Janetzky S, Waibler Z, Pandur P, Kühl M, Behrens J, von der Mark K, Starzinski-Powitz A, Wixler V. 2002. The LIM-only protein FHL2 interacts with β-catenin and promotes differentiation of mouse myoblasts. J Cell Biol 159:113–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Purcell NH, Darwis D, Bueno OF, Müller JM, Schüle R, Molkentin JD. 2004. Extracellular signal-regulated kinase 2 interacts with and is negatively regulated by the LIM-only protein FHL2 in cardiomyocytes. Mol Cell Biol 24:1081–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kadrmas JL, Beckerle MC. 2004. The LIM domain: from the cytoskeleton to the nucleus. Nat Rev Mol Cell Biol 5:920–931 [DOI] [PubMed] [Google Scholar]
- 73. Sablin EP, Krylova IN, Fletterick RJ, Ingraham HA. 2003. Structural basis for ligand-independent activation of the orphan nuclear receptor LRH-1. Mol Cell 11:1575–1585 [DOI] [PubMed] [Google Scholar]
- 74. Nachtigal MW, Hirokawa Y, Enyeart-VanHouten DL, Flanagan JN, Hammer GD, Ingraham HA. 1998. Wilms' tumor 1 and Dax-1 modulate the orphan nuclear receptor SF-1 in sex-specific gene expression. Cell 93:445–454 [DOI] [PubMed] [Google Scholar]
- 75. Jenster G, Spencer TE, Burcin MM, Tsai SY, Tsai MJ, O'Malley BW. 1997. Steroid receptor induction of gene transcription: A two-step model. Proc Natl Acad Sci USA 94:7879–7884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dawid IB, Breen JJ, Toyama R. 1998. LIM domains: multiple roles as adapters and functional modifiers in protein interactions. Trends Genet 14:156–162 [DOI] [PubMed] [Google Scholar]
- 77. Johannessen M, Møller S, Hansen T, Moens U, Van Ghelue M. 2006. The multifunctional roles of the four-and-a-half-LIM only protein FHL2. Cell Mol Life Sci 63:268–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. El Mourabit H, Müller S, Tunggal L, Paulsson M, Aumailley M. 2004. Analysis of the adaptor function of the LIM domain-containing protein FHL2 using an affinity chromatography approach. J Cell Biochem 92:612–625 [DOI] [PubMed] [Google Scholar]
- 79. Kurakula K, van der Wal E, Geerts D, van Tiel CM, de Vries CJM. 2011. FHL2 protein is a novel co-repressor of nuclear receptor Nur77. J Biol Chem 286:44336–44343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Meldi KM, Pearse WB, Burkart AD, Mayo KE. 2010. Regulation of the inhibin alpha subunit gene by the NR4A orphan nuclear receptors in ovarian granulosa cells. Endocr Rev 31:P2–P17 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.