Abstract
The brain responds to a fall in blood glucose by activating neuroendocrine mechanisms for its restoration. It is unclear whether the brain also responds to a fall in plasma free fatty acids (FFA) to activate mechanisms for its restoration. We examined whether lowering plasma FFA increases plasma corticosterone or catecholamine levels and, if so, whether the brain is involved in these responses. Plasma FFA levels were lowered in rats with three independent antilipolytic agents: nicotinic acid (NA), insulin, and the A1 adenosine receptor agonist SDZ WAG 994 with plasma glucose clamped at basal levels. Lowering plasma FFA with these agents all increased plasma corticosterone, but not catecholamine, within 1 h, accompanied by increases in plasma ACTH. These increases in ACTH or corticosterone were abolished when falls in plasma FFA were prevented by Intralipid during NA or insulin infusion. In addition, the NA-induced increases in plasma ACTH were completely prevented by administration of SSR149415, an arginine vasopressin receptor antagonist, demonstrating that the hypothalamus is involved in these responses. Taken together, the present data suggest that the brain may sense a fall in plasma FFA levels and activate the hypothalamic-pituitary-adrenal axis to increase plasma ACTH and corticosterone, which would help restore FFA levels. Thus, the brain may be involved in the sensing and control of circulating FFA levels.
The brain plays a crucial role in energy homeostasis. When the blood glucose level falls, the brain senses it and activates various neuroendocrine mechanisms to restore blood glucose levels (1). These responses, known as glucose counterregulatory responses, are critical for the survival of the brain and have been extensively studied. According to the “selfish brain” theory (2), the brain places the highest priority on covering its own energy requirements when regulating energy homeostasis, activating stress responses upon a fall in cerebral energy (i.e. ATP) levels and suppressing insulin secretion to allocate glucose to the brain rather than the periphery. Free fatty acids (FFA) are another major fuel in mammals. It is established that FFA, like glucose, are sensed by the brain, and excess supply of FFA to the brain can suppress food intake and reduce hepatic glucose output (3). However, it is unclear whether the brain responds to a fall in plasma FFA, as it does with glucose, to activate mechanisms for its restoration. There is ample evidence that acute depression (or elevation) of plasma FFA stimulates (or inhibits) growth hormone secretion (4, 5). Because growth hormone is known to increase lipolysis in adipocytes, these effects of FFA on growth hormone secretion provide a negative feedback mechanism in the regulation of plasma FFA. The underlying mechanisms appear to be complex (4), but the brain (i.e. hypothalamus) may be responsible for at least some FFA effects (6). In addition, there are scattered data showing that nicotinic acid (NA) administration, which decreases plasma FFA levels (7), was associated with increased plasma levels of epinephrine (8), glucagon (9), and cortisol (corticosterone in rodents) (10). Because these hormones are all lipolytic, these data raise the intriguing possibility that the brain senses a fall in plasma FFA level and mobilizes these lipolytic hormones to restore plasma FFA, i.e. the existence of FFA counterregulatory responses mediated by the brain. In the present study, we tested this idea in rats by examining whether lowering plasma FFA increases plasma corticosterone or catecholamine levels and, if so, whether the brain is involved in these responses.
Materials and Methods
Animals and catheterization
Male Wistar rats weighing 280–300 g were obtained from Simonsen (Gilroy, CA) and studied at least 5 d after arrival. Animals were housed under controlled temperature (22 ± 2 C) and lighting [12-h light (0600–1800 h), 12-h dark (1800–0600 h) cycle] with free access to water and standard rat chow. At least 4 d before the experiment, the animals were placed in individual cages with tail restraints as previously described (11, 12), which was required to protect tail blood-vessel catheters during the experiments. The animals were free to move about and were allowed unrestricted access to food and water. One tail-vein infusion catheter was placed the day before the experiment, and one tail-artery blood-sampling catheter was placed the morning of the experiment (∼0700 h). All procedures involving animals were approved by the Institutional Animal Care and Use Committee at the University of Southern California.
Experimental protocols
Experiments were conducted in the conscious state after an overnight fast. Starting at approximately 1300 h, animals received a constant infusion of NA (30 μmol/h), insulin (5 mU/kg·min), or SDZ WAG 994 (0.1 mg/kg·h) for 1 h to lower plasma FFA. NA and insulin inhibit lipolysis in adipocytes by inhibiting adenylate cyclase and activating phosphodiesterase, respectively, and decreasing cAMP. SDZ WAG 994, a selective A1 adenosine receptor agonist, inhibits lipolysis in adipocytes by activating adenosine receptors (13). Control animals received saline infusion. During the infusions, blood samples were collected at 15-min intervals for immediate measurement of the plasma glucose level. Exogenous glucose was infused (during NA or insulin infusion) at varying rates to maintain plasma glucose at basal levels. Additional blood samples were collected at various times for measurements of plasma FFA, catecholamines, ACTH, and corticosterone. The total volume of blood samples collected was less than 0.65 ml/100 g body weight (10% circulating blood volume) in all experiments and matched between control and treatment groups. Blood samples were stored at −80 C for later analysis.
In some experiments, insulin or NA infusion was combined with a constant infusion of Intralipid (20% wt/vol, 0.9 ml/h) and heparin (40 U/h with a bolus injection of 10 U at the beginning) to prevent a fall in plasma FFA level. In another experiment, animals were treated with antalarmin (Sigma-Aldrich, St. Louis, MO), a CRH receptor antagonist, or SSR149415 (SSR) (Axon Medchem, Groningen, The Netherlands), an arginine vasopressin (AVP) receptor (i.e. VP1b) antagonist, to inhibit hypothalamic control of ACTH secretion (see Results). These agents were dissolved in saline containing 5% dimethylsulfoxide and 5% Cremophor (Sigma-Aldrich) and infused for 1 h before the first basal blood sampling at −30 min (antalarmin, 20 mg/kg·h; SSR, 2 mg/h). SSR effects were short lived (14), and an additional dose of SSR (1 mg) was given at −10 min (i.e. 10 min before the start of NA infusion).
Assays
Plasma glucose was analyzed on a Beckman Glucose Analyzer II (Beckman, Fullerton, CA). Plasma FFA was measured using an acyl-coenzyme A oxidase-based colorimetric kit (Wako Chemicals, Inc., Richmond, VA) [coefficient of variation (CV), 1%; sensitivity, 1 μm]. Plasma ACTH, corticosterone, and catecholamines were analyzed using ELISA kits from ALPCO Diagnostics (Salem, NH) (CV, 7%; sensitivity, 0.22 pg/ml), AssayPro (St. Charles, MO) (CV, 7%; sensitivity, 0.3 ng/ml), and Rocky Mountain Diagnostics (Colorado Springs, CO) (CV, 10%; sensitivity, 0.03 ng/ml), respectively.
Statistical analysis
All data are expressed as means ± sem. The significance of differences in the mean values between groups was assessed by paired or unpaired Student's t tests, as indicated. A P value of less than 0.05 was considered to be statistically significant.
Results
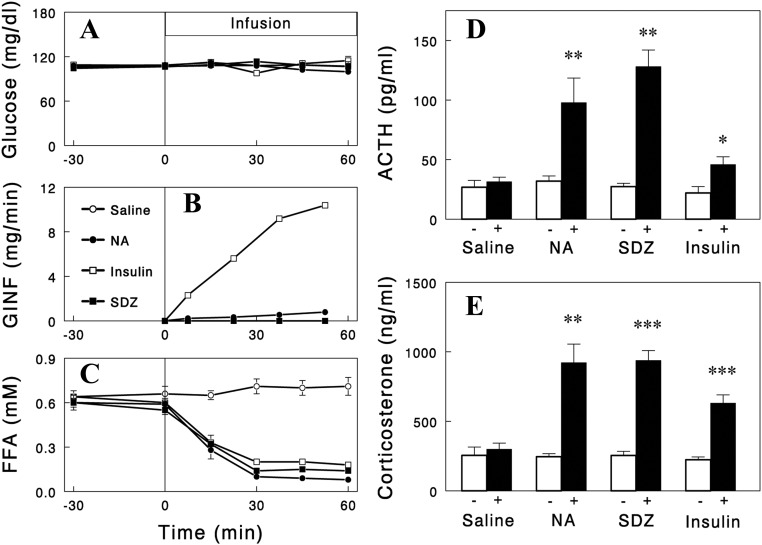
We first confirmed that NA infusion in rats decreased plasma FFA levels, which was associated with increased plasma levels of corticosterone (P < 0.05) (Fig. 1) but not catecholamines levels (epinephrine, 79 ± 19 and 96 ± 24 pg/ml; norepinephrine, 295 ± 44 and 384 ± 39 pg/ml for saline and NA infusion, respectively; P > 0.05). NA infusion also increased plasma ACTH levels (P < 0.05) (Fig. 1), suggesting that the NA-induced increase in corticosterone was mediated by ACTH. Plasma glucose was clamped at basal levels during NA infusion by glucose infusion (12); and therefore, the increases in plasma ACTH and corticosterone occurred without changes in plasma glucose.
Fig. 1.
Plasma glucose (A), glucose infusion rate (GINF) (B), plasma FFA (C), ACTH (D), and corticosterone (E) during an iv infusion of saline (control), NA (30 μmol/h), insulin (5 mU/kg·min), or SDZ WAG 994 (SDZ) (0.1 mg/kg·h). In the experiments with NA or insulin infusion, plasma glucose was maintained at basal levels by exogenous glucose infusion (12). The plasma corticosterone and ACTH levels shown represent the averages of two samples taken during the final 30 min (45- and 60-min samples for corticosterone; 30- and 60-min samples for ACTH). Data are means ± sem for five to eight experiments. *, P = 0.021; **, P < 0.01; ***, P < 0.001 vs. saline (paired two-tailed t test).
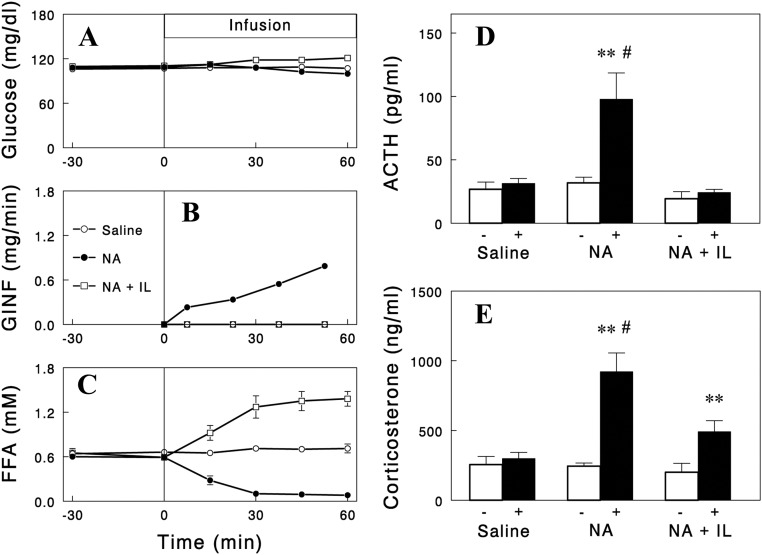
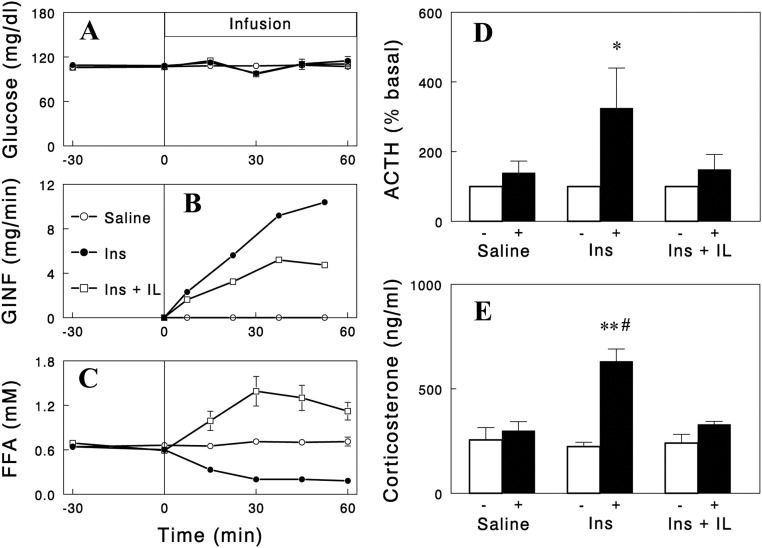
We next tested whether the effects of NA on ACTH and corticosterone were mediated by plasma FFA. For this, two independent approaches were used. First, we examined whether the NA effects are reproduced by lowering plasma FFA levels with other structurally and functionally unrelated antilipolytic agents. We employed insulin and the selective A1 adenosine receptor agonist SDZ WAG 994 (13) as tools to lower plasma FFA. Infusions of these agents decreased plasma FFA in time courses similar to that of NA with plasma glucose clamped during the insulin infusion. Importantly, similar to NA infusion, these two independent methods of lowering FFA increased plasma ACTH and corticosterone levels (Fig. 1), although the responses with insulin were smaller, possibly due to its additional effect to suppress the hypothalamic-pituitary-adrenal (HPA) axis (15). Second, we tested whether preventing a fall in plasma FFA levels during NA infusion with Intralipid infusion abolishes the increases in plasma ACTH and corticosterone levels. Intralipid infusion significantly blunted the increases in plasma ACTH and corticosterone (Fig. 2). However, although the ACTH responses were completely blocked, there were some residual increases in corticosterone, suggesting a direct NA effect on corticosterone secretion independent of plasma FFA (or ACTH). In contrast, the increases in corticosterone during insulin infusion were completely abolished by Intralipid infusion (Fig. 3). Intralipid also completely prevented increases in ACTH during insulin infusion. Taken together, these data indicate that the falls in plasma FFA levels were largely responsible for the increases in plasma ACTH and corticosterone.
Fig. 2.
Effects of Intralipid (IL) (20%, 0.9 ml/h) and heparin (40 U/h) infusion on NA-induced changes in plasma glucose (A), glucose infusion rate (GINF) (B), plasma FFA (C), ACTH (D), and corticosterone (E). The data with saline or NA infusion are those in Fig. 1. Data are means ± sem for five to eight experiments. **, P < 0.01 vs. saline (paired two-tailed t test); #, P < 0.012 vs. NA + IL (unpaired two-tailed t test).
Fig. 3.
Effects of Intralipid (IL) (20%, 0.9 ml/h) and heparin (40 U/h) infusion on insulin (Ins)-induced changes in plasma glucose (A), glucose infusion rate (GINF) (B), plasma FFA (C), ACTH (D), and corticosterone (E). The data with saline or insulin infusion are those in Fig. 1. The ACTH data are presented as % basal, because basal ACTH levels were high in a couple of rats with IL infusion. Data are means ± sem for five to seven experiments. *, P = 0.021; **, P < 0.001 vs. saline (paired two-tailed t test); #, P < 0.01 vs. NA + IL (unpaired two-tailed t test).
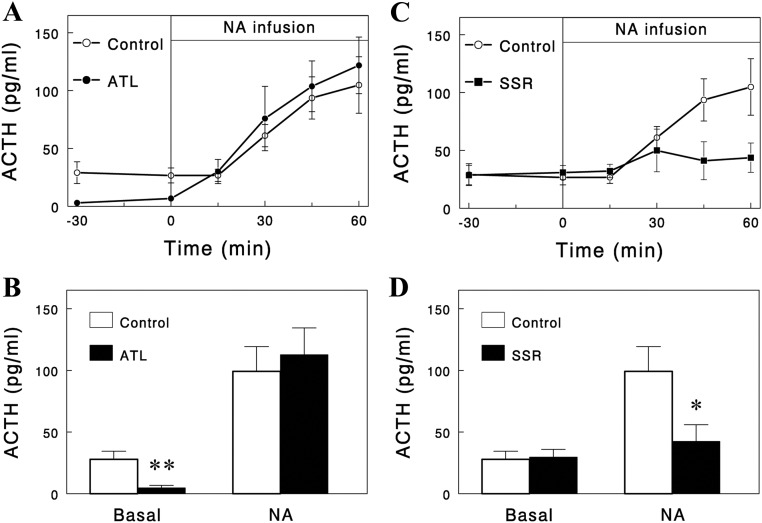
We further tested whether the effect of reduced plasma FFA to increase plasma ACTH involved the hypothalamus that controls ACTH secretion by the pituitary. The hypothalamic control of ACTH secretion occurs mainly via two neuropeptides: CRH and AVP. For inhibition of these controls, we employed the CRH receptor antagonist antalarmin (16) and the AVP receptor (i.e. VP1b) antagonist SSR (14). Antalarmin infusion profoundly decreased basal plasma ACTH levels but not the NA-induced increases (Fig. 4, A and B). In contrast, SSR had no effect on basal ACTH levels but almost completely blocked the NA-induced increases in ACTH (Fig. 4, C and D). These data clearly indicate that basal secretion of ACTH was under hypothalamic control via CRH, but NA (or low FFA)-induced stimulation of ACTH secretion was under control via AVP.
Fig. 4.
Effects of antalarmin (ATL) (A and B), a CRH receptor antagonist, and SSR (C and D), an AVP receptor antagonist, on basal and NA-increased plasma ACTH levels. The drugs were dissolved in saline containing 5% dimethylsulfoxide and 5% Cremophor and infused iv for 1 h (from −90 to −30 min; 20 mg/kg·h for ATL, 2 mg/h for SSR) before the first basal sampling at −30 min. SSR effects are short lived, and an additional dose of SSR (1 mg) was given at −10 min. In the bottom panels, ACTH levels with NA represent the averages of the values at 45 and 60 min. Data are means ± sem (n = 5, 5, and 9 for ATL, SSR, and control, respectively). *, P = 0.019; **, P = 0.004 vs. control (unpaired one-tailed t test).
Discussion
The present study demonstrates that lowering plasma FFA level using three structurally and functionally independent agents all increased plasma corticosterone levels, accompanied by increases in plasma ACTH levels, suggesting that a fall in plasma FFA level activates the HPA axis. This idea is further supported by the finding that the increases in plasma corticosterone and ACTH during insulin or NA infusion were abolished or significantly reduced by preventing a fall in plasma FFA level via Intralipid infusion. Also, the finding that NA-induced increases in plasma ACTH were completely abolished by inhibiting hypothalamic control of ACTH secretion via AVP strongly indicates that the hypothalamus is involved in these responses. Taken together, the present data suggest that the brain may sense a fall in plasma FFA levels and activate the HPA axis to increase plasma ACTH and corticosterone, which would help restore FFA levels. Thus, the brain may be involved in the sensing and control of circulating FFA levels.
Previous studies have shown that FFA, when injected into the brain, suppressed food intake and reduced hepatic glucose output (3, 17, 18). In addition, a recent study showed that starvation-induced increases in circulating FFA caused autophagy in hypothalamic neurons, which mobilized endogenous FFA to regulate neuronal activities (19). These findings support the notion that FFA can be directly sensed by cells and neurons in the brain. In the present study, although we demonstrate that the HPA axis was regulated by plasma FFA, we cannot exclude the possibility that the falls in plasma FFA levels were sensed outside the brain, and the information was transmitted to the brain via an afferent neural pathway or a humoral factor. Therefore, whether circulating FFA levels are directly sensed in the brain remains to be tested. Nonetheless, it is important to demonstrate in the present study that the brain was able to sense (directly or indirectly) and respond to falls in circulating FFA by activating the HPA axis. The activation of the HPA axis was independent of plasma glucose, because plasma glucose was clamped at basal levels by exogenous glucose infusion, if necessary, in all experiments. However, we cannot exclude the possibility that cerebral glucose or energy levels might be lowered by the employed agents or the falls in plasma FFA levels to activate the HPA axis.
Chronic elevations of circulating FFA levels cause metabolic derangements, such as insulin resistance, and contribute to the metabolic syndrome (20). Our findings have an important implication for the regulation of circulating FFA levels. Although elevated FFA levels can be attributed to a primary change in adipocytes (20), our data suggest another potential site of regulation: the brain. It may be possible that any impairment in brain sensing of circulating FFA (21) unnecessarily increases HPA-axis activity and thus lipolysis to elevate circulating FFA levels. Our findings also have an important implication for NA or other lipid drugs acting by lowering plasma FFA levels (7, 13). Our data suggest that significant HPA-axis activation can occur during treatments with these drugs (when plasma FFA levels fall too low), causing FFA rebound and/or compromising beneficial effects of lowering plasma FFA (22). Treatment schemes avoiding or inhibiting such unwanted effects would help increase drug efficacy. Finally, the activation of the HPA axis at low FFA levels may have been a serious confounding factor in many previous studies, in which NA or other FFA-lowering agents were used to address the role of plasma FFA. This may also be the case in studies in which plasma FFA levels were not controlled. For example, previous studies showed that a hyperinsulinemic euglycemic clamp increased plasma cortisol (23) or corticosterone (24). Because plasma glucose was clamped, these responses were attributed to the direct effect of insulin per se. However, FFA levels were not controlled in these studies, and the responses might well have been due to a fall in plasma FFA expected during insulin infusion, as demonstrated in the present study.
A previous human study demonstrated that Intralipid infusion, which raised plasma FFA above basal levels, decreased plasma ACTH and cortisol levels (25), consistent with the idea of the regulation of the HPA axis by plasma FFA levels. In contrast, a rat study (26) reported that elevation of plasma FFA with Intralipid increased plasma ACTH and corticosterone levels. The reason for this discrepancy is unclear. Interpretation of data with Intralipid infusion or elevated plasma FFA may be complicated by potential nonspecific effects arising from excessive increases in plasma FFA and/or triglyceride pools. In our experiments, when used under conditions of decreased plasma FFA, Intralipid was clearly inhibitory on HPA-axis activity.
The Intralipid dose employed in the present study was larger than that required to maintain basal FFA levels during NA or insulin infusion, resulting in plasma FFA significantly higher than basal levels (Figs. 3 and 4). When Intralipid was infused at a lower rate to maintain plasma FFA at basal levels during NA infusion, the ACTH and corticosterone responses were not fully abolished (data not shown). To understand these data, it should be noted that Intralipid is mainly composed of plant-derived essential FFA (C18:2 and C18:3) (27), whereas rat plasma contains mainly nonessential FFA, such as palmitate (C16:0) and oleate (C18:1) (28). Therefore, when total FFA levels were maintained at basal, plasma levels of nonessential FFA might be still significantly below their basal levels (whereas those of essential FFA might be above their basal levels), as demonstrated by a previous study in rats (28). This may explain why ACTH and corticosterone responses were not abolished at basal FFA levels. In contrast, when total plasma FFA levels were elevated substantially above basal (Fig. 3), plasma levels of nonessential FFA might be maintained at or above basal, abolishing ACTH and corticosterone responses. Thus, our data are consistent with the notion that the activation of the HPA axis might be due to reduced plasma levels of nonessential (e.g. oleate) but not total FFA. Regarding this, Obici et al. (17) showed that central administration of oleate, but not octanoate (C8:0), decreased food intake and hepatic glucose production, suggesting that oleate, but not octanoate, was sensed by the brain. A subsequent study by Ross et al. (18) showed that similar responses occurred with palmitate (although it was substantially less potent) but not with linoleate (C18:2). Therefore, there is the intriguing possibility that specific (e.g. oleate and/or palmitate), but not all, circulating FFA are involved in the regulation of HPA-axis activity. Further study is warranted to test this important issue.
Acknowledgments
We thank Dr. Joyce Richey and Dr. Richard Watanabe at University of Southern California, Keck School of Medicine for their critical reading of the manuscript.
Present address for K.-S.O.: Research Center for Women's Diseases, Sookmyung Women's University, Cheongpa-ro 47-gil 100, Yongsan-gu, Seoul 140-742, Korea.
This work was supported by the National Institutes of Health Grant DK090749 (to J.H.Y.) by and the Basic Science Research Program through the National Research Foundation of Korea Ministry of Education, Science, and Technology Grant 2009-0073746 (to I.K.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AVP
- Arginine vasopressin
- CV
- coefficient of variation
- FFA
- free fatty acid
- HPA
- hypothalamic-pituitary-adrenal
- NA
- nicotinic acid
- SSR
- SSR149415.
References
- 1. Cryer PE, Gerich JE. 1985. Glucose counterregulation, hypoglycemia, and intensive insulin therapy in diabetes mellitus. N Engl J Med 313:232–241 [DOI] [PubMed] [Google Scholar]
- 2. Peters A, Kubera B, Hubold C, Langemann D. 2011. The selfish brain: stress and eating behavior. Front Neurosci 5:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lam TK, Schwartz GJ, Rossetti L. 2005. Hypothalamic sensing of fatty acids. Nat Neurosci 8:579–584 [DOI] [PubMed] [Google Scholar]
- 4. Kreitschmann-Andermahr I, Suarez P, Jennings R, Evers N, Brabant G. 2010. GH/IGF-I regulation in obesity—mechanisms and practical consequences in children and adults. Horm Res Paediatr 73:153–160 [DOI] [PubMed] [Google Scholar]
- 5. Reynaert R, De Paepe M, Marcus S, Peeters G. 1975. Influence of serum free fatty acid levels on growth hormone secretion in lactating cows. J Endocrinol 66:213–224 [DOI] [PubMed] [Google Scholar]
- 6. Briard N, Rico-Gomez M, Guillaume V, Sauze N, Vuaroqueaux V, Dadoun F, Le Bouc Y, Oliver C, Dutour A. 1998. Hypothalamic mediated action of free fatty acid on growth hormone secretion in sheep. Endocrinology 139:4811–4819 [DOI] [PubMed] [Google Scholar]
- 7. Carlson LA, Oro L. 1962. The effect of nicotinic acid on the plasma free fatty acid; demonstration of a metabolic type of sympathicolysis. Acta Med Scand 172:641–645 [DOI] [PubMed] [Google Scholar]
- 8. Watt MJ, Holmes AG, Steinberg GR, Mesa JL, Kemp BE, Febbraio MA. 2004. Reduced plasma FFA availability increases net triacylglycerol degradation, but not GPAT or HSL activity, in human skeletal muscle. Am J Physiol Endocrinol Metab 287:E120–E127 [DOI] [PubMed] [Google Scholar]
- 9. Quabbe HJ, Luyckx AS, L'age M, Schwarz C. 1983. Growth hormone, cortisol, and glucagon concentrations during plasma free fatty acid depression: different effects of nicotinic acid and an adenosine derivative (BM 11.189). J Clin Endocrinol Metab 57:410–414 [DOI] [PubMed] [Google Scholar]
- 10. Pereira JN. 1967. The plasma free fatty acid rebound induced by nicotinic acid. J Lipid Res 8:239–244 [PubMed] [Google Scholar]
- 11. Youn JH, Buchanan TA. 1993. Fasting does not impair insulin-stimulated glucose uptake but alters intracellular glucose metabolism in conscious rats. Diabetes 42:757–763 [DOI] [PubMed] [Google Scholar]
- 12. Choi S, Yoon H, Oh KS, Oh YT, Kim YI, Kang I, Youn JH. 2011. Widespread effects of nicotinic acid on gene expression in insulin-sensitive tissues: implications for unwanted effects of nicotinic acid treatment. Metabolism 60:134–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cox BF, Clark KL, Perrone MH, Welzel GE, Greenland BD, Colussi DJ, Merkel LA. 1997. Cardiovascular and metabolic effects of adenosine A1-receptor agonists in streptozotocin-treated rats. J Cardiovasc Pharmacol 29:417–426 [DOI] [PubMed] [Google Scholar]
- 14. Chen J, Young S, Subburaju S, Sheppard J, Kiss A, Atkinson H, Wood S, Lightman S, Serradeil-Le Gal C, Aguilera G. 2008. Vasopressin does not mediate hypersensitivity of the hypothalamic pituitary adrenal axis during chronic stress. Ann NY Acad Sci 1148:349–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bohringer A, Schwabe L, Richter S, Schachinger H. 2008. Intranasal insulin attenuates the hypothalamic-pituitary-adrenal axis response to psychosocial stress. Psychoneuroendocrinology 33:1394–1400 [DOI] [PubMed] [Google Scholar]
- 16. Webster EL, Lewis DB, Torpy DJ, Zachman EK, Rice KC, Chrousos GP. 1996. In vivo and in vitro characterization of antalarmin, a nonpeptide corticotropin-releasing hormone (CRH) receptor antagonist: suppression of pituitary ACTH release and peripheral inflammation. Endocrinology 137:5747–5750 [DOI] [PubMed] [Google Scholar]
- 17. Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. 2002. Central administration of oleic acid inhibits glucose production and food intake. Diabetes 51:271–275 [DOI] [PubMed] [Google Scholar]
- 18. Ross RA, Rossetti L, Lam TK, Schwartz GJ. 2010. Differential effects of hypothalamic long-chain fatty acid infusions on suppression of hepatic glucose production. Am J Physiol Endocrinol Metab 299:633–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaushik S, Rodriguez-Navarro JA, Arias E, Kiffin R, Sahu S, Schwartz GJ, Cuervo AM, Singh R. 2011. Autophagy in hypothalamic AgRP neurons regulates food intake and energy balance. Cell Metab 14:173–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. DeFronzo RA. 2004. Dysfunctional fat cells, lipotoxicity and type 2 diabetes. Int J Clin Pract Suppl 143:9–21 [DOI] [PubMed] [Google Scholar]
- 21. Morgan K, Obici S, Rossetti L. 2004. Hypothalamic responses to long-chain fatty acids are nutritionally regulated. J Biol Chem 279:31139–31148 [DOI] [PubMed] [Google Scholar]
- 22. Poynten AM, Gan SK, Kriketos AD, O'Sullivan A, Kelly JJ, Ellis BA, Chisholm DJ, Campbell LV. 2003. Nicotinic acid-induced insulin resistance is related to increased circulating fatty acids and fat oxidation but not muscle lipid content. Metabolism 52:699–704 [DOI] [PubMed] [Google Scholar]
- 23. Fruehwald-Schultes B, Kern W, Bong W, Wellhoener P, Kerner W, Born J, Fehm HL, Peters A. 1999. Supraphysiological hyperinsulinemia acutely increases hypothalamic-pituitary-adrenal secretory activity in humans. J Clin Endocrinol Metab 84:3041–3046 [DOI] [PubMed] [Google Scholar]
- 24. Chan O, Inouye K, Akirav E, Park E, Riddell MC, Vranic M, Matthews SG. 2005. Insulin alone increases hypothalamo-pituitary-adrenal activity, and diabetes lowers peak stress responses. Endocrinology 146:1382–1390 [DOI] [PubMed] [Google Scholar]
- 25. Lanfranco F, Giordano R, Pellegrino M, Gianotti L, Ramunni J, Picu A, Baldi M, Ghigo E, Arvat E. 2004. Free fatty acids exert an inhibitory effect on adrenocorticotropin and cortisol secretion in humans. J Clin Endocrinol Metab 89:1385–1390 [DOI] [PubMed] [Google Scholar]
- 26. Widmaier EP, Rosen K, Abbott B. 1992. Free fatty acids activate the hypothalamic-pituitary-adrenocortical axis in rats. Endocrinology 131:2313–2318 [DOI] [PubMed] [Google Scholar]
- 27. van Kempen AA, van der Crabben SN, Ackermans MT, Endert E, Kok JH, Sauerwein HP. 2006. Stimulation of gluconeogenesis by intravenous lipids in preterm infants: response depends on fatty acid profile. Am J Physiol Endocrinol Metab 290:723–730 [DOI] [PubMed] [Google Scholar]
- 28. Stein DT, Stevenson BE, Chester MW, Basit M, Daniels MB, Turley SD, McGarry JD. 1997. The insulinotropic potency of fatty acids is influenced profoundly by their chain length and degree of saturation. J Clin Invest 100:398–403 [DOI] [PMC free article] [PubMed] [Google Scholar]