Abstract
MicroRNAs (miRNAs) regulate gene expression at the post-transcriptional level and are important for coordinating nervous system development and neuronal function in the mature brain. We have recently identified schizophrenia-associated alteration of cortical miRNA biogenesis and expression in post-mortem brain tissue with implications for the dysregulation of schizophrenia candidate genes. Although these changes were observed in the central nervous system, it is plausible that schizophrenia-associated miRNA expression signatures may also be detected in non-neural tissue. To explore this possibility, we investigated the miRNA expression profile of peripheral blood mononuclear cells (PBMCs) from 112 patients with schizophrenia and 76 non-psychiatric controls. miRNA expression analysis of total RNA conducted using commercial miRNA arrays revealed that 33 miRNAs were significantly downregulated after correction for multiple testing with a false discovery rate (FDR) of 0%, which increased to 83 when we considered miRNA with an FDR<5%. Seven miRNAs altered in microarray analysis of schizophrenia were also confirmed to be downregulated by quantitative real-time reverse transcription-polymerase chain reaction. A large subgroup consisting of 17 downregulated miRNAs is transcribed from a single imprinted locus at the maternally expressed DLK1-DIO3 region on chromosome 14q32. This pattern of differentially expressed miRNA in PBMCs may be indicative of significant underlying genetic or epigenetic alteration associated with schizophrenia.
Keywords: biomarker, gene silencing, microRNA, PBMCs, schizophrenia, 14q32
Introduction
Schizophrenia is a severely debilitating and complex neuropsychiatric disorder afflicting nearly one in 100 people throughout life.1 Although some gross neuroanatomical and histological features have been associated with schizophrenia, none are consistent enough to be considered diagnostic.2, 3, 4, 5, 6, 7 The scarcity of tangible pathology means that clinical diagnosis is based on descriptive criteria and requires persistent or episodic presence of certain symptoms over a defined period with associated disability and exclusion of other psychiatric conditions. As early intervention in schizophrenia is thought to be important in improving outcome, any delay in diagnosis or misdiagnosis owing to the inherent limitations of clinical descriptors may have a negative prognostic impact.8, 9 Consequently, accessible biological markers are needed to improve the timing and accuracy of diagnosis, and identify individuals at very high risk of schizophrenia. This information may also provide the opportunity to tailor treatment to the individual and enable more effective early intervention.10, 11, 12, 13 Despite having a strong genetic component, early expectations of a simple genetic diagnostic for schizophrenia have been thwarted as the heritability appears to involve a complex, heterogeneous mixture of common variants, each with small effect size.14
More recently, studies have attempted to move closer to the neuropathology of schizophrenia in an attempt to identify common pathways in the disorder. High-throughput gene expression analysis has revealed altered gene expression in post-mortem brain including the dorsolateral prefrontal cortex, superior temporal gyrus and amygdala, which are all functionally relevant to the pathology of schizophrenia.15, 16, 17, 18, 19, 20, 21, 22 Although no single gene has been found to be consistently altered across studies or brain regions, most report the enrichment of pathways relevant to the putative neurobiology of schizophrenia. In many instances, the extent and shape of gene alteration in post-mortem brain may imply the involvement of aberrant regulatory or epigenetic influences. In support of this hypothesis, we recently reported changes in cortical microRNA (miRNA) biogenesis and expression in schizophrenia.23, 24 These molecules strongly influence gene expression, particularly in the brain where they are thought to be involved in both neurodevelopment and in the regulation of synaptic structure and function.25, 26, 27, 28, 29
Although the pathophysiology of changes in cortical miRNA expression remains to be determined, it is plausible that alteration of miRNA expression extends beyond the brain and could provide useful peripheral biomarkers for schizophrenia that would be accessible for clinical use. miRNA molecules are highly stable30 and, owing to their regulatory function (predicted to regulate half of the human genome31), are arguably more informative and prognostic than gene expression. Certainly in the field of cancer biology, miRNA may be a more accurate predictor of tumor phenotype than its genotype.32, 33 miRNA profiling of tissues and body fluids has been instructive in a variety of cancer types including chronic lymphocytic leukemia and lung, prostate, bladder and breast cancer.34, 35, 36, 37 Perhaps more relevant to brain disorders like schizophrenia, miRNA profiling of whole blood in multiple sclerosis has revealed expression signatures associated with the disease and its remission status.38 Similarly, miRNA analysis in Alzheimer's disease has revealed significant patterns of altered expression in peripheral blood mononuclear cells (PBMCs)39 as well as in the cerebrospinal fluid and brain.40 PBMCs in particular represent an attractive alternative tissue for profiling active disease in living patients at statistically robust numbers. This accessible tissue can reflect global disease-associated changes in an underlying genetic disorder and may have special significance where immunological risk factors are believed to be involved. In schizophrenia, for example, prenatal maternal infection is thought to be an important risk factor41, 42, 43, 44 that may leave immunologically relevant expression signatures in PBMCs.
In this study, we examined miRNA expression in PBMCs derived from a cohort of schizophrenia patients and non-psychiatric controls, and identified a significant schizophrenia-associated reduction in 83 miRNAs. A large proportion of this expression signature was derived from a cluster of 17 miRNAs residing in a single imprinted domain on 14q32. This discovery has implications for our understanding of epigenetic regulation and inheritance in schizophrenia, and may also have applications as a novel biomarker for the disorder.
Materials and methods
Participant recruitment and clinical assessment protocol
Participants, the majority of whom identified as Caucasian, were recruited by the Australian Schizophrenia Research Bank and the Hunter DNA Bank. In this study, the cohorts consisted of 112 participants with a lifetime diagnosis of schizophrenia and 76 non-psychiatric controls that were deemed eligible for inclusion after assessment by qualified psychologists using the Diagnostic Interview for Psychosis.45 Participants were excluded if they were currently diagnosed with any organic brain disorders, substance dependence, brain injury that caused post-traumatic amnesia lasting >24 h, mental retardation (IQ<70), movement disorder or if they had electroconvulsive therapy within 6 months. These exclusion criteria were likewise used for non-psychiatric control subjects with the additional criterion that they had no personal or family history of either psychosis or bipolar disorder type 1.46 In addition, participants were required to speak English, and were aged between 18 and 65 years. Participants gave informed consent for inclusion in the study protocol, as approved by the institutional research ethics committees. All cases reported using antipsychotic medication at the time of interview and blood draw. Demographic and clinical variables of the cohort are summarized in Table 1 and described in detail in Supplementary Table 1.
Table 1. Summary statistics for clinical and demographic variables characterizing the schizophrenia and control cohort.
| Demographic/clinical variable | Summary statistics |
|---|---|
| Diagnosis: SZ/CTRL | 112/76 |
| Mean age | 39.4 |
| Gender: M/F | 101/82 |
| Mean age at the onset of illness | 24.2 |
| Family history of schizophrenia (cases): present/none | 41/55 |
| Family history of other psychosis (cases): present/none | 49/47 |
| Mean duration of illness | 16.4 |
| Mean RQI | 9.3 |
Abbreviations: CTRL, non-psychiatric control; F, female; M, male; RQI, RNA quality indicator; SZ, schizophrenia. Family history of other psychosis, reports any other psychiatric mental illness in any first- or second-degree relative.
Extraction of RNA and DNA from whole blood
Whole blood (20 ml) was collected from subjects and transported to the Australian Schizophrenia Research Bank for processing. PBMCs were extracted by centrifugation using Lymphoprep (Vital Diagnostics, Lincoln, RI, USA). Total RNA was extracted from PBMCs obtained using Trizol, as per the manufacturer's instructions (Invitrogen, Life Technologies, Carlsbad, CA, USA). RNA concentration was determined using a NanoDrop 2000 (Thermo Scientific, Delaware, ME, USA). Genomic DNA was extracted from PBMCs using a standard salt extraction method and quantified by PicoGreen assay (Invitrogen, San Diego, CA, USA). The integrity of randomly selected genomic DNA was checked by agarose gel electrophoresis.
RNA integrity
RNA integrity was assessed using an Experion microcapillary electrophoresis device according to the manufacturer's instructions (Bio-Rad, Hercules, CA, USA). The average RNA Quality Indicator for this cohort was 9.3. Samples with RNA Quality Indicator values ⩾6.3 were considered intact and therefore suitable for use in miRNA microarray experiments and quantitative real-time reverse transcription polymerase chain reaction (PCR).
miRNA expression profiling
Profiling of human miRNA expression (miRBase version 9.1) was achieved using the commercial miRNA microarray platform (Illumina, San Diego, CA, USA) as described previously.47 Microarray data was extracted and normalized with respect to the geometric mean of U44 and U49 small nucleolar RNA (snoRNA) expression. Analysis of variance was used for the evaluation of association between non-diagnosis variance (batch effects, gender and age) and miRNA expression. Age showed a significant correlation with miRNA expression and was therefore used as a covariate in the subsequent analysis. Differential expression analysis was performed using the significance analysis of microarray statistical analysis program (full academic version 2.23, website http://www-stat.stanford.edu/~tibs/SAM/).48 Normalized expression data for the differentially expressed miRNA is shown in Supplementary Table 2. Unsupervised hierarchical clustering was performed in Cluster (Stanford University, Palo Alto, CA, USA; Eisen et al49) and a heat map visualized through Java Treeview V.1.1.1.50 Data were log transformed and median centered by genes. Genes and arrays were clustered, correlation un-centered, by average linkage clustering.
Quantitative real-time reverse transcription PCR
Validation of differentially expressed miRNA was performed by quantitative real-time reverse transcription PCR on a subset of the cohort consisting of 91 participants (57 schizophrenia, 34 non-psychiatric controls), similarly to that described previously.24 Briefly, 500 ng of sample RNA was treated with DNAse-I (Invitrogen, Life Technologies) and multiplex reverse transcription performed with Superscript II reverse transcriptase (Invitrogen, Life Technologies), a 3 nM mix of miRNA sequence-specific primers and primers for U44 snRNA and U49 snoRNA (for primer sequences, see Supplementary Table 3). Seven miRNA were chosen for real-time validation on the basis of strong differential expression on the microarray and/or biological significance. Triplicate reactions were set up in a 96-well format using the epMotion 5070 automated pipetting system (Eppendorf, Hamburg, Germany) and carried out using the Applied Biosystems 7500 real-time PCR machine. Relative miRNA expression was determined with respect to the geometric mean of U44 and U49 snoRNAs by calculating the difference between the average cycle threshold (Ct) of the miRNA of interest and the geometric mean of the average Ct values of housekeeping snoRNA for each sample (ΔCt). Data were not normally distributed (as determined by GraphPad Prism version 5.0); therefore, the significance of differential expression was determined using a Mann–Whitney U-test (PASW Statistics 18).
Target prediction and functional pathway analysis
Putative target genes for schizophrenia-associated miRNA identified in this study were predicted using the miRanda51 and TargetScan (release 5.1)52 search algorithms (http://www.microrna.org/microrna/home.do; http://www.targetscan.org/). These putative target genes were further filtered by selecting genes containing five or more predicted binding sites either by the same or multiple miRNA as these genes are most likely to be regulated by the differentially expressed miRNA.53 Pathway analysis was then performed on a combined list of targets from both algorithms (union), followed by a more stringent pathways analysis on those targets that were common to both miRanda and TargetScan (intersection) using WebGestalt Gene Set Analysis Toolkit V.2 (http://bioinfo.vanderbilt.edu/webgestalt/).54 Visualization of pathways and networks from this information was facilitated by the construction of an ‘enrichment map' of significant pathways (P<0.05 Benjamini–Hochberg) containing seven or more genes per category (http://baderlab.org/Software/EnrichmentMap).55
Analysis of copy-number variation
Genomic copy-number status of the 14q32 region was investigated using commercial TaqMan copy-number assays (Applied Biosystems, Life Technologies, Carlsbad, CA, USA; Hs07397776_cn, Hs03879584_cn and Hs03889902_cn) corresponding to cytobands 14q32.2b, 14q32.2b and 14q32.31a, respectively (refer to Supplementary Figure 1 for details). Reactions were prepared as per the manufacturer's instructions using an EPMotion 5070 automated liquid handling device (Eppendorf) and carried out using an ABI7500 real-time PCR machine (Applied Biosystems). The CopyCaller software V.1.0 (Applied Biosystems) was used to calculate relative copy number at each of the three locations. Each sample was normalized to the copy-number reference assay (RNase P, part number 4 316 831), known to be present in two copies in a diploid genome.
Results
miRNA expression analysis
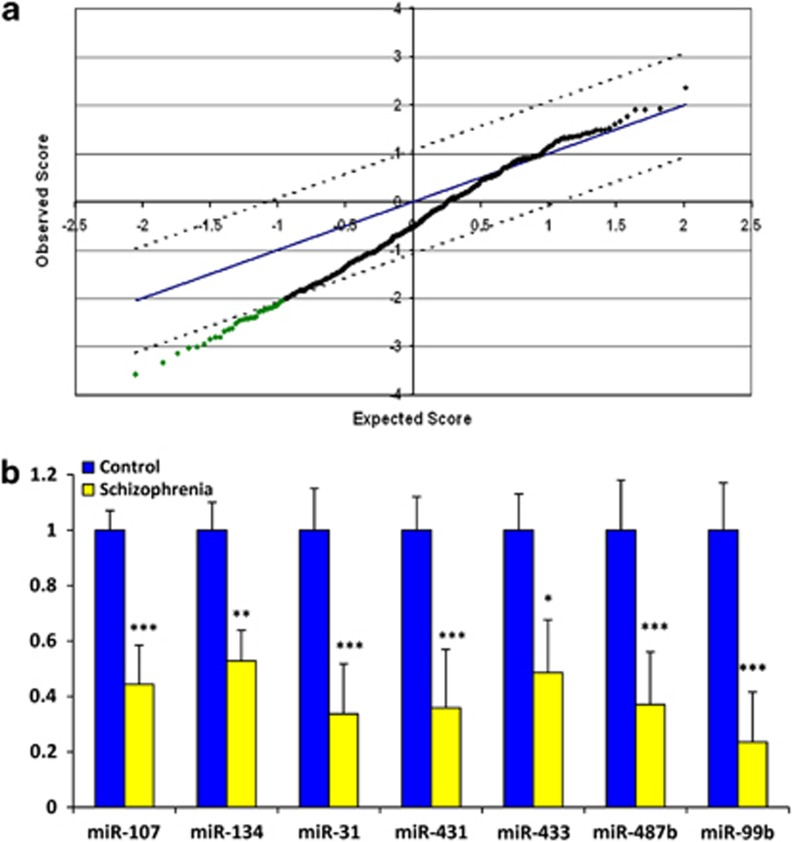
miRNA expression was analyzed in PBMCs of 112 patients with schizophrenia and 76 non-psychiatric controls using human miRNA array matrices (Illumina). After normalization and background subtraction, signals corresponding to 358 miRNAs or approximately 40% of the array content remained. Statistical analysis revealed 33 significantly downregulated miRNA at a false discovery rate (FDR) of 0% (Figure 1a) increasing to 83 at an FDR <5% (Table 2). This included a number of brain-enriched miRNAs, miR-134, miR-128 and miR-181b. By contrast, there were no miRNAs that were significantly increased according to these criteria. Seven of the differentially expressed miRNA were further analyzed by quantitative real-time reverse transcription PCR in a representative sample consisting of 57 schizophrenia cases and 34 controls. miRNA expression was normalized to the geometric mean of two constitutively expressed snoRNAs U44 and U49. These results were highly concordant with the microarray analysis with all seven of the miRNAs confirmed to be significantly downregulated in schizophrenia compared with controls (Figure 1b) (two-tailed Mann–Whitney U-test). These included miR-31 (2.97-fold, P<0.001), miR-431 (2.79-fold, P<0.001), miR-433 (2.06-fold, P=0.017), miR-107 (2.25-fold, P<0.001), miR-134 (1.89-fold, P=0.007), miR-99b (4.24-fold, P<0.001) and miR-487b (2.06-fold, P<0.001).
Figure 1.
Schizophrenia associated microRNA (miRNA) expression in peripheral blood mononuclear cells (PBMCs). (a) Significance analysis of microarray (SAM) plot of differentially expressed miRNA in PBMCs from participants with schizophrenia (n=112) compared with non-psychiatric controls (n=76). The central solid blue line indicates equal expression and the upper and lower dashed black lines indicate levels for significantly altered expression (false discovery rate (FDR) of 0%). (b) Quantitative real-time reverse transcription polymerase chain reaction (Q-PCR) validation of miRNA significantly downregulated by miRNA array analysis. Bars indicate mean fold change±s.e.m. of 91 samples (57 schizophrenia cases and 34 non-psychiatric controls). The control cohort is set at 1. *P<0.05; **P<0.01; ***P<0.001 by Mann–Whitney U-test.
Table 2. miRNA significantly downregulated in schizophrenia compared with non-psychiatric controls.
| miRNA ID | Fold change | q-Value | Chromosomal coordinates |
|---|---|---|---|
| hsa-miR-329 | 0.753 | 0 | hsa-miR-329-1 14: 101 493 122–101 493 201 [+] hsa-miR-329-2 14: 101 493 437–101 493 520 [+] |
| hsa-miR-31 | 0.754 | 0 | 9: 21 512 114–21 512 184 [−] |
| hsa-miR-409-3p | 0.757 | 0 | 14: 101 531 637–101 531 715 [+] |
| hsa-miR-224 | 0.762 | 0 | X: 151 127 050–151 127 130 [−] |
| hsa-miR-432 | 0.763 | 0 | 14: 101 350 820–101 350 913 [+] |
| hsa-miR-487b | 0.764 | 0 | 14: 101 512 792–101 512 875 [+] |
| hsa-miR-134 | 0.764 | 0 | 14: 101 521 024–101 521 096 [+] |
| hsa-miR-431 | 0.765 | 0 | 14: 101 347 344–101 347 457 [+] |
| hsa-miR-150* | 0.771 | 0 | 19: 50 004 042–50 004 125 [−] |
| hsa-miR-99b | 0.783 | 0 | 19: 52 195 865–52 195 934 [+] |
| hsa-miR-1275 | 0.798 | 0 | 6: 33 967 749–33 967 828 [−] |
| hsa-miR-335* | 0.799 | 0 | 7: 130 135 952–130 136 045 [+] |
| hsa-miR-200c | 0.813 | 0 | 12: 7 072 862–7 072 929 [+] |
| hsa-miR-486-3p | 0.815 | 0 | 8: 41 517 959–41 518 026 [−] |
| hsa-miR-29b-1* | 0.817 | 0 | 7: 130 562 218–130 562 298 [−] |
| hsa-miR-16-2* | 0.821 | 0 | 3: 160 122 533–160 122 613 [+] |
| hsa-miR-877 | 0.83 | 0 | 6: 30 552 109–30 552 194 [+] |
| hsa-miR-107 | 0.835 | 0 | 10: 91 352 504–91 352 584 [−] |
| hsa-miR-130b* | 0.839 | 0 | 22: 22 007 593–22 007 674 [+] |
| hsa-miR-544 | 0.843 | 0 | 14: 101 514 995–101 515 085 [+] |
| hsa-miR-342-5p | 0.844 | 0 | 14: 100 575 992–100 576 090 [+] |
| hsa-miR-148b | 0.844 | 0 | 12: 54 731 000–54 731 098 [+] |
| hsa-miR-625* | 0.856 | 0 | 14: 65 937 820–65 937 904 [+] |
| hsa-miR-28-3p | 0.858 | 0 | 3: 188 406 569–188 406 654 [+] |
| hsa-miR-576-5p | 0.86 | 0 | 4: 110 409 854–110 409 951 [+] |
| hsa-miR-151-3p | 0.864 | 0 | 8: 141 742 663–141 742 752 [−] |
| hsa-miR-28-5p | 0.869 | 0 | 3: 188 406 569–188 406 654 [+] |
| hsa-miR-664 | 0.871 | 0 | 1: 220 373 880–220 373 961 [−] |
| hsa-miR-128 | 0.874 | 0 | hsa-miR-128-1 2: 136 422 967–136 423 048 [+] hsa-miR-128-2 3: 35 785 968–35 786 051 [+] |
| hsa-miR-584 | 0.879 | 0 | 5: 148 441 876–148 441 972 [−] |
| hsa-miR-574-3p | 0.883 | 0 | 4: 38 869 653–38 869 748 [+] |
| hsa-miR-181a | 0.913 | 0 | hsa-miR-181a-1 1: 198 828 173–198 828 282 [−] hsa-miR-181a-2 9: 127 454 721–127 454 830 [+] |
| hsa-miR-30e* | 0.917 | 0 | 1: 41 220 027–41 220 118 [+] |
| hsa-miR-433 | 0.664 | 1.555 | 14: 101 348 223–101 348 315 [+] |
| hsa-miR-654-5p | 0.73 | 1.555 | 14: 101 506 556–101 506 636 [+] |
| hsa-miR-193b | 0.806 | 1.555 | 16: 14 397 824–14 397 906 [+] |
| hsa-miR-485-3p | 0.816 | 1.555 | 14: 101 521 756–101 521 828 [+] |
| hsa-miR-370 | 0.831 | 1.555 | 14: 101 377 476–101 377 550 [+] |
| hsa-miR-340* | 0.854 | 1.555 | 5: 179 442 303–179 442 397 [−] |
| hsa-miR-1271 | 0.854 | 1.555 | 5: 175 794 949–175 795 034 [+] |
| hsa-miR-151 | 0.855 | 1.555 | 8: 141 742 663–141 742 752 [−] |
| hsa-miR-15b* | 0.86 | 1.555 | 3: 160 122 376–160 122 473 [+] |
| hsa-miR-502-3p, hsa-miR-500* | 0.86 | 1.555 | X: 49 779 206–49 779 291 [+] |
| hsa-miR-27b | 0.905 | 1.555 | 9: 97 847 727–97 847 823 [+] |
| hsa-miR-199a-3p, hsa-miR-199b-3p | 0.922 | 1.555 | hsa-miR-199a-1 19: 10 928 102–10 928 172 [−] hsa-miR-199a-2 1: 172 113 675–172 113 784 [−] hsa-miR-199b 9: 131 007 000–131 007 109 [−] |
| hsa-miR-151-5p | 0.933 | 1.555 | 8: 141 742 663–141 742 752 [−] |
| hsa-miR-146a | 0.95 | 1.555 | 5: 159 912 359–159 912 457 [+] |
| hsa-miR-21 | 0.957 | 1.555 | 17: 57 918 627–57 918 698 [+] |
| hsa-miR-30d | 0.959 | 1.555 | 8: 135 817 119–135 817 188 [−] |
| hsa-miR-127-3p | 0.727 | 2.131 | 14: 101 349 316–101 349 412 [+] |
| hsa-miR-98 | 0.82 | 2.131 | X: 53 583 184–53 583 302 [−] |
| hsa-miR-328 | 0.864 | 2.131 | 16: 67 236 224–67 236 298 [−] |
| hsa-miR-181b | 0.875 | 2.131 | hsa-miR-181b-1 1: 198 828 002–198 828 111 [−] hsa-miR-181b-2 9: 127 455 989–127 456 077 [+] |
| hsa-miR-378 | 0.882 | 2.131 | 5: 149 112 388–149 112 453 [+] |
| hsa-miR-150 | 0.963 | 2.131 | 19: 50 004 042–50 004 125 [−] |
| hsa-miR-323-3p | 0.796 | 2.74 | 14: 101 492 069–101 492 154 [+] |
| hsa-miR-874 | 0.797 | 2.74 | 5: 136 983 261–136 983 338 [−] |
| hsa-miR-330-3p | 0.857 | 2.74 | 19: 46 142 252–46 142 345 [−] |
| hsa-miR-500 | 0.862 | 2.74 | X: 49 773 039–49 773 122 [+] |
| hsa-miR-181a-2* | 0.878 | 2.74 | 9: 127 454 721–127 454 830 [+] |
| hsa-miR-146b-5p | 0.912 | 2.74 | 10: 104 196 269–104 196 341 [+] |
| hsa-let-7b | 0.952 | 2.74 | 22: 46 509 566–46 509 648 [+] |
| hsa-miR-25 | 0.956 | 2.74 | 7: 99 691 183–99 691 266 [−] |
| hsa-miR-92a | 0.957 | 2.74 | hsa-miR-92a-1 13: 92 003 568–92 003 645 [+] hsa-miR-92a-2 X: 133 303 568–133 303 642 [−] |
| hsa-miR-410 | 0.812 | 3.288 | 14: 101 532 249–101 532 328 [+] |
| hsa-miR-221* | 0.815 | 3.288 | X: 45 605 585–45 605 694 [-] |
| hsa-miR-942 | 0.825 | 3.288 | 1: 117 637 265–117 637 350 [+] |
| hsa-miR-664* | 0.845 | 3.288 | 1: 220 373 880–220 373 961 [−] |
| hsa-miR-20b | 0.865 | 3.288 | X: 133 303 839–133 303 907 [−] |
| hsa-miR-628-3p | 0.867 | 3.288 | 15: 55 665 138–55 665 232 [−] |
| hsa-miR-152 | 0.892 | 3.288 | 17: 46 114 527–46 114 613 [−] |
| hsa-let-7d | 0.957 | 3.288 | 9: 96 941 116–96 941 202 [+] |
| hsa-miR-154 | 0.766 | 4.484 | 14: 101 526 092–101 526 175 [+] |
| hsa-miR-337-3p | 0.87 | 4.484 | 14: 101 340 830–101 340 922 [+] |
| hsa-miR-505 | 0.884 | 4.484 | X: 139 006 307–139 006 390 [−] |
| hsa-miR-625 | 0.897 | 4.484 | 14: 65 937 820–65 937 904 [+] |
| hsa-miR-22* | 0.898 | 4.484 | 17: 1 617 197–1 617 281 [−] |
| hsa-let-7g | 0.965 | 4.484 | 3: 52 302 294–52 302 377 [−] |
| hsa-miR-1301 | 0.869 | 4.853 | 2: 25 551 509–25 551 590 [−] |
| hsa-let-7d* | 0.905 | 4.853 | 9: 96 941 116–96 941 202 [+] |
| hsa-miR-766 | 0.93 | 4.853 | X: 11 8780 701–118 780 811 [−] |
| hsa-let-7a | 0.971 | 4.853 | hsa-let-7a-1 9: 96 938 239–96 938 318 [+] hsa-let7a-2 11: 12 2017 230–122 017 301 [−] hsa-let7a-3 22: 46 508 629–46 508 702 [+] |
Abbreviation: miRNA, microRNA.
Differentially expressed miRNA were ranked by significance and then by fold change. miRNA transcribed from the genomic region encompassing 14q32.2–q32.31 were identified with bold font. *Denotes star form of the mature miRNA.
Cluster analysis of schizophrenia-associated miRNA
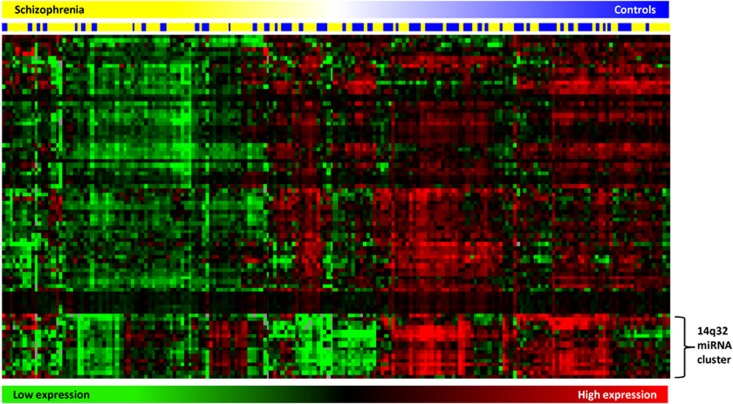
The miRNAs shown to be associated with schizophrenia in the microarray analysis were also subjected to hierarchical clustering (Figure 2). This segregated the participants into two main groups in which reduced expression was enriched within the schizophrenia cohort (Figure 2, left side) in contrast to the majority of controls, which were visually distinguished by their relatively higher expression (Figure 2, right side). Strikingly, a cluster of 17 of the most substantially downregulated miRNAs were also structurally associated by their genomic position on the long arm of chromosome 14 (14q32). These miRNA all reside within two closely neighboring segments each spanning about 40 kb and separated by ∼110 kb, at 14q32.2 and 14q32.31 (Figure 3). This cluster encodes at least 48 miRNA genes,56, 57 of which 30 mature miRNAs were expressed in the PBMCs. This indicated that approximately 53% of the miRNAs expressed at this locus were significantly downregulated in schizophrenia. Moreover, when we examined miRNAs expressed in this cluster that were not significantly altered after correction for multiple testing, a further 10 miRNAs or 33% (including miR-493*, miR-665, miR-379, miR-411, miR-376a, miR-376b, miR-376c, miR-495, miR-539, miR-889) also displayed an average reduction in schizophrenia consistent with the trend seen in the majority of miRNAs expressed from this region. This pattern of expression among the clustered miRNAs associated with schizophrenia was also highly consistent within individuals, suggesting that their transcription may be under the influence of shared regulatory mechanisms.
Figure 2.
Heatmap showing hierarchical clustering of significantly downregulated microRNA with diagnosis. Samples are color coded according to diagnosis (blue=control; yellow=schizophrenia). Green indicates low expression and red indicates high expression (Java Treeview).
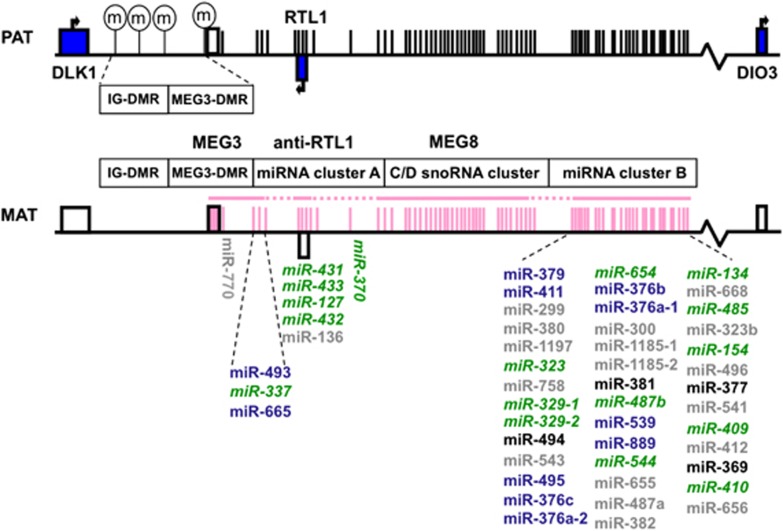
Figure 3.
Genomic organization of 14q32 microRNA (miRNA) clusters neighboring the DLK1-DIO3 imprinted domain. DLK1, RTL1 and DIO3 (blue rectangles) are paternally expressed genes. The maternally expressed non-coding RNA genes (pink) include maternally expressed gene 3 (MEG3), anti-RTL1 (encodes the smaller miRNA cluster A), maternally expressed gene 8 (MEG8) (encodes small nucleolar RNA (snoRNA) cluster) and the larger miRNA cluster B. The intergenic germline derived differentially methylated region (IG-DMR) is methylated on the paternal chromosome denoted by the encircled letter m. The 14q32 miRNA clusters are arranged in two segments: each separated by a C/D snoRNA cluster. miRNA in dark green italic font were significantly downregulated in schizophrenia and miRNA in blue exhibited a trend for downregulation in schizophrenia. miRNA in black were expressed but not differentially expressed, and in gray were not expressed. This figure was adapted from Royo and Cavaille64 and Hagan et al.103
Copy-number analysis
To investigate whether the downregulation of miRNA on chromosome 14q32.31–32.2 is related to structural variation, we analyzed a subset of samples including 57 controls and 81 cases using commercially available TaqMan copy-number assays (Applied Biosystems). Amplicons specific for sequence within maternally expressed gene 3, cluster A and cluster B of the 14q32 region, were analyzed relative to the endogenous control RNase-P. No significant deletion or amplification events were detected in this region in either cases or controls (Supplementary Figure 1).
Target gene and pathway analysis
To gain some appreciation of the biological implications of the schizophrenia-associated changes in miRNA expression, we examined predicted miRNA targets and their associated pathways to see whether any patterns emerged. The more miRNA binding sites a gene has within its 3′-untranslated region, the more likely it is to be post-transcriptionally regulated.53 The target prediction algorithms miRanda51 and TargetScan52 are capable of identifying those miRNA that bind multiple times within the one 3′-untranslated region. As such, this information was used to predict target genes that were most likely to be heavily regulated by the differentially expressed miRNA. Owing to a high degree of false positives, the list of target genes was filtered to only include those genes that were targeted by five or more miRNA, or contained five or more binding sites for a particular miRNA within its 3′-untranslated region. This target analysis process was performed on the 17 miRNA displaying altered expression in the 14q32 region. The miRanda and TargetScan lists were then combined to form a union of targets predicted by both algorithms in addition to a more stringent analysis involving the intersection of targets identified by both miRanda and TargetScan. Selected genes were then collectively subjected to pathway analysis using the Gene Set Analysis Toolkit V.2 from WebGestalt (http://bioinfo.vanderbilt.edu/webgestalt).54 The union of miRanda and TargetScan revealed a number of significantly enriched pathways with relevance to neural connectivity and synaptic plasticity, including axon guidance, long-term potentiation, focal adhesion, neurotrophin, ErbB, calcium and mitogen-activated protein kinase signaling (Table 3 and Supplementary Figure 2). Furthermore, the more stringent intersection approach, using genes predicted to be targets by both algorithms, also suggested the involvement of neurologically relevant pathways as well as pathways relevant to immune system function (Table 4 and Supplementary Figure 3).
Table 3. Pathways enriched with target genes predicted by a union of miRanda and TargetScan target lists for the 14q32 cluster.
| Category | Term | n | % | P-value | Benjamini |
|---|---|---|---|---|---|
| KEGG_PATHWAY | Pathways in cancer | 108 | 0.3 | <0.000 | <0.000 |
| KEGG_PATHWAY | Axon guidance | 54 | 0.1 | <0.000 | <0.000 |
| KEGG_PATHWAY | Adherens junction | 36 | 0.1 | <0.000 | <0.000 |
| KEGG_PATHWAY | Regulation of actin cytoskeleton | 72 | 0.2 | <0.000 | <0.000 |
| KEGG_PATHWAY | Focal adhesion | 68 | 0.2 | <0.000 | <0.000 |
| KEGG_PATHWAY | Chronic myeloid leukemia | 32 | 0.1 | <0.000 | <0.000 |
| KEGG_PATHWAY | Insulin signaling pathway | 48 | 0.1 | <0.000 | <0.000 |
| KEGG_PATHWAY | Renal cell carcinoma | 30 | 0.1 | <0.000 | <0.000 |
| KEGG_PATHWAY | ErbB signaling pathway | 34 | 0.1 | <0.000 | 0.001 |
| KEGG_PATHWAY | MAPK signaling pathway | 79 | 0.2 | <0.000 | 0.001 |
| KEGG_PATHWAY | Pancreatic cancer | 29 | 0.1 | <0.000 | 0.0016 |
| KEGG_PATHWAY | Ubiquitin-mediated proteolysis | 46 | 0.1 | <0.000 | 0.0018 |
| KEGG_PATHWAY | Wnt signaling pathway | 49 | 0.1 | <0.000 | 0.0024 |
| KEGG_PATHWAY | Prostate cancer | 33 | 0.1 | <0.000 | 0.0023 |
| KEGG_PATHWAY | Long-term potentiation | 27 | 0.1 | <0.000 | 0.0028 |
| KEGG_PATHWAY | Glioma | 25 | 0.1 | <0.000 | 0.0049 |
| KEGG_PATHWAY | Neurotrophin signaling pathway | 41 | 0.1 | <0.000 | 0.0046 |
| KEGG_PATHWAY | Colorectal cancer | 30 | 0.1 | <0.000 | 0.0078 |
| KEGG_PATHWAY | mTOR signaling pathway | 21 | 0.1 | 0.0011 | 0.0110 |
| KEGG_PATHWAY | Endocytosis | 54 | 0.1 | 0.0012 | 0.0110 |
| KEGG_PATHWAY | Melanogenesis | 33 | 0.1 | 0.0015 | 0.0130 |
| KEGG_PATHWAY | Calcium signaling pathway | 50 | 0.1 | 0.0039 | 0.0320 |
| KEGG_PATHWAY | Non-small-cell lung cancer | 20 | 0.1 | 0.0047 | 0.0370 |
| KEGG_PATHWAY | Progesterone-mediated oocyte maturation | 28 | 0.1 | 0.0053 | 0.0400 |
| KEGG_PATHWAY | TGF-β signaling pathway | 28 | 0.1 | 0.0063 | 0.0460 |
| KEGG_PATHWAY | Melanoma | 24 | 0.1 | 0.0064 | 0.0450 |
| KEGG_PATHWAY | T-cell receptor signaling pathway | 33 | 0.1 | 0.0067 | 0.0450 |
| KEGG_PATHWAY | Jak-STAT signaling pathway | 44 | 0.1 | 0.0070 | 0.0450 |
| KEGG_PATHWAY | Dilated cardiomyopathy | 29 | 0.1 | 0.0073 | 0.0460 |
| KEGG_PATHWAY | HCM | 27 | 0.1 | 0.0089 | 0.0540 |
| KEGG_PATHWAY | Oocyte meiosis | 33 | 0.1 | 0.0090 | 0.0530 |
Abbreviations: HCM, hypertrophic cardiomyopathy; KEGG, Kyoto Encyclopedia of Genes and Genomes; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; TGF, tumor growth factor.
Enriched KEGG pathways identified by WebGestalt gene set analysis using predicted target genes. The list was compiled from an union of the miRanda and TargetScan predicted targets of 17 members of the miR-14q32 cluster differentially expressed in schizophrenia. n, number of input genes in pathway (filtered to only show pathways represented by ⩾7 target genes). %, percentage of total target genes analyzed (∼3600 gene IDs) that are represented in this pathway.
Table 4. Pathways enriched with target genes predicted by an intersection of miRanda and TargetScan target lists for the 14q32 cluster.
| Category | Term | n | % | P-value | Benjamini |
|---|---|---|---|---|---|
| KEGG_PATHWAY | Pathways in cancer | 31 | 3.8 | <0.0001 | <0.0001 |
| KEGG_PATHWAY | Prostate cancer | 13 | 1.6 | <0.0001 | <0.0001 |
| KEGG_PATHWAY | MAPK signaling pathway | 21 | 2.6 | <0.0001 | <0.0001 |
| KEGG_PATHWAY | Focal adhesion | 18 | 2.2 | <0.0001 | <0.0001 |
| KEGG_PATHWAY | Melanoma | 11 | 1.4 | <0.0001 | <0.0001 |
| KEGG_PATHWAY | Adherens junction | 11 | 1.4 | <0.0001 | <0.0001 |
| KEGG_PATHWAY | Endocytosis | 16 | 2.0 | <0.0001 | <0.0001 |
| KEGG_PATHWAY | mTOR signaling pathway | 9 | 1.1 | <0.0001 | <0.0001 |
| KEGG_PATHWAY | Non-small-cell lung cancer | 9 | 1.1 | <0.0001 | <0.0001 |
| KEGG_PATHWAY | Long-term potentiation | 10 | 1.2 | <0.0001 | <0.0001 |
| KEGG_PATHWAY | Chronic myeloid leukemia | 10 | 1.2 | <0.0001 | <0.0001 |
| KEGG_PATHWAY | Insulin signaling pathway | 13 | 1.6 | <0.0001 | <0.0001 |
| KEGG_PATHWAY | Regulation of actin cytoskeleton | 16 | 2.0 | <0.0001 | <0.0001 |
| KEGG_PATHWAY | Glioma | 9 | 1.1 | <0.0001 | <0.0001 |
| KEGG_PATHWAY | Progesterone-mediated oocyte maturation | 10 | 1.2 | <0.0001 | <0.0001 |
| KEGG_PATHWAY | ErbB signaling pathway | 10 | 1.2 | <0.0001 | <0.0001 |
| KEGG_PATHWAY | Renal cell carcinoma | 9 | 1.1 | <0.0001 | <0.0001 |
| KEGG_PATHWAY | Jak-STAT signaling pathway | 13 | 1.6 | <0.0001 | <0.0001 |
| KEGG_PATHWAY | Pancreatic cancer | 9 | 1.1 | <0.0001 | <0.0001 |
| KEGG_PATHWAY | Neurotrophin signaling pathway | 11 | 1.4 | <0.0001 | <0.0001 |
| KEGG_PATHWAY | Colorectal cancer | 9 | 1.1 | <0.0001 | <0.0001 |
| KEGG_PATHWAY | Small-cell lung cancer | 9 | 1.1 | <0.0001 | <0.0001 |
| KEGG_PATHWAY | Calcium signaling pathway | 13 | 1.6 | <0.0001 | <0.0001 |
| KEGG_PATHWAY | TGF-β signaling pathway | 9 | 1.1 | <0.0001 | <0.0001 |
| KEGG_PATHWAY | Dilated cardiomyopathy | 9 | 1.1 | <0.0001 | <0.0001 |
| KEGG_PATHWAY | Phosphatidylinositol signaling system | 8 | 1.0 | <0.0001 | 0.0001 |
| KEGG_PATHWAY | Fcγ R-mediated phagocytosis | 9 | 1.1 | <0.0001 | 0.0001 |
| KEGG_PATHWAY | Acute myeloid leukemia | 7 | 0.9 | <0.0001 | 0.0002 |
| KEGG_PATHWAY | HCM | 8 | 1.0 | 0.0001 | 0.0002 |
| KEGG_PATHWAY | Melanogenesis | 9 | 1.1 | <0.0001 | 0.0002 |
| KEGG_PATHWAY | Axon guidance | 10 | 1.2 | 0.0001 | 0.0002 |
| KEGG_PATHWAY | Apoptosis | 8 | 1.0 | 0.0002 | 0.0003 |
| KEGG_PATHWAY | Neuroactive ligand–receptor interaction | 14 | 1.7 | 0.0002 | 0.0003 |
| KEGG_PATHWAY | Vascular smooth muscle contraction | 9 | 1.1 | 0.0002 | 0.0003 |
| KEGG_PATHWAY | Long-term depression | 7 | 0.9 | 0.0003 | 0.0004 |
| KEGG_PATHWAY | Wnt signaling pathway | 10 | 1.2 | 0.0004 | 0.0006 |
| KEGG_PATHWAY | ARVC | 7 | 0.9 | 0.0004 | 0.0006 |
| KEGG_PATHWAY | Fcɛ RI signaling pathway | 7 | 0.9 | 0.0005 | 0.0007 |
| KEGG_PATHWAY | GnRH signaling pathway | 8 | 1.0 | 0.0005 | 0.0007 |
| KEGG_PATHWAY | Tight junction | 9 | 1.1 | 0.0007 | 0.0009 |
| KEGG_PATHWAY | Chemokine signaling pathway | 11 | 1.4 | 0.0007 | 0.0009 |
| KEGG_PATHWAY | T-cell receptor signaling pathway | 8 | 1.0 | 0.0007 | 0.0009 |
| KEGG_PATHWAY | Ubiquitin-mediated proteolysis | 9 | 1.1 | 0.0009 | 0.0011 |
| KEGG_PATHWAY | Oocyte meiosis | 8 | 1.0 | 0.001 | 0.0012 |
| KEGG_PATHWAY | Gap junction | 7 | 0.9 | 0.0012 | 0.0014 |
| KEGG_PATHWAY | Leukocyte transendothelial migration | 8 | 1.0 | 0.0013 | 0.0015 |
| KEGG_PATHWAY | Natural killer cell-mediated cytotoxicity | 8 | 1.0 | 0.0033 | 0.0037 |
| KEGG_PATHWAY | Lysosome | 7 | 0.9 | 0.0052 | 0.0056 |
| KEGG_PATHWAY | Huntington's disease | 9 | 1.1 | 0.0063 | 0.0067 |
| KEGG_PATHWAY | CAMs | 7 | 0.9 | 0.0106 | 0.011 |
| KEGG_PATHWAY | Cytokine–cytokine receptor interaction | 10 | 1.2 | 0.0227 | 0.0231 |
| KEGG_PATHWAY | Metabolic pathways | 29 | 3.6 | 0.0268 | 0.0268 |
Abbreviations: ARVC, arrhythmogenic right ventricular cardiomyopathy; CAM, cell adhesion molecules; GnRH, gonadotropin-releasing hormone; HCM, hypertrophic cardiomyopathy; KEGG, Kyoto Encyclopedia of Genes and Genomes; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin.
Enriched KEGG pathways identified by WebGestalt gene set analysis using predicted target genes. The list was compiled from an intersection of the miRanda and TargetScan predicted targets of 17 members of the miR-14q32 cluster differentially expressed in schizophrenia. n, number of input genes in pathway (filtered to only show pathways represented by ⩾7 target genes). %, percentage of total target genes analyzed (∼810 gene IDs) that are represented in this pathway.
Discussion
A subset of brain-enriched miRNA genes located within a large imprinted cluster on chromosome 14q32 were differentially expressed in schizophrenia
Analysis of post-mortem miRNA expression has previously reported schizophrenia-associated changes in both the superior temporal gyrus and prefrontal cortex.24, 47, 58 Although miRNA expression typically displays a stage- and tissue-specific expression pattern, we identified several brain-enriched miRNAs including miR-134, miR-128 and miR-181b that were also differentially expressed in PBMCs from individuals with schizophrenia. It is plausible that these represent a neurologically relevant signature reflecting the prevailing neuropsychiatric phenotype. The reduction of miR-134 was particularly significant not only because of its role in brain development and function,28, 59, 60 but also as a consequence of its position in a cluster of differentially expressed miRNAs on chromosome 14q32.
A large proportion of the miRNAs found to be differentially expressed in schizophrenia are encoded within this large cluster on chromosome 14q32, which collectively comprises over 5% of known human miRNA genes61, 62, 63 and about 40–50% of all known eutherian-specific miRNA.64 Significantly, this locus on 14q32, known as the DLK1-DIO3 region, is imprinted such that the associated miRNA cluster is only expressed from the maternal chromosome.64 In mice, the syntenic region on chromosome 12 also resides within an imprinted domain expressed exclusively from the maternal chromosome.57 The miRNAs are encoded by two clusters located in close proximity to each other, the smaller of the two at 14q32.2 contains 10 miRNA, which reside within the retrotransposon-like gene RTL1 (in antisense), whereas the larger cluster also known as the miR379-410 cluster contains approximately 40 miRNAs. Although the nature of their organization is not entirely clear in human beings, some of the miRNA in this region could be processed from a single large polycistron such as Mirg (miRNA containing gene, also known as Meg9), which appears to be the case in the mouse. However, it seems more likely that the majority of miRNA in this cluster are encoded by tandem arrays of related intronic sequences.57, 65
Regulation of the 14q32 miRNA cluster
As more than 85% of the miRNAs expressed in this region showed a trend for downregulation in schizophrenia, it is reasonable to suggest that these molecules may be co-regulated by a shared mechanism associated with the disorder. This could relate to a number of common factors that affect transcriptional activity or miRNA processing in this region. Why the remaining miRNAs expressed from this cluster were neutral with respect to disease status remains to be determined, but it could be that the genes encoding these molecules are responding more to differences in their local transcriptional environment, which over-ride the prevailing cluster-wide influence. They may also display isolated differences in their splicing or primary transcript processing, or pre-miRNA cleavage and maturation. It is also possible that the schizophrenia-associated regulatory influence seen for most of the expressed miRNA extends beyond the miRNA clusters to affect protein coding and other non-coding mRNAs in the region; however, there are no reports to this effect in the schizophrenia literature. A genome-wide study of MS from our laboratory suggests that the protein-coding genes in this domain (DLK1, RTL1, DIO3 and MEG3) are not expressed in lymphocytes.66
Studies in the mouse suggest that the expression of miRNA clustered on 14q32 in human beings may be particularly sensitive to changes in the miRNA biogenesis pathway. For example, DiGeorge syndrome critical region gene 8 (DGCR8) heterozygous knockout mice were shown to have significantly reduced levels of mature miRNA expression from the syntenic region on chromosome 12 in both the prefrontal cortex and hippocampus.67 The product of this gene, one of many depleted in the 22q11 microdeletion syndromes, is an important component of the microprocessor complex involved in pri-miRNA cleavage in the nucleus. Interestingly, these miRNA were also over represented (∼25%) among miRNA showing downregulation in dopaminergic neurons from the striatum of Ago2-deficient mice.68 A large proportion of miRNA from this cluster also contain primary and secondary structural features associated with dicer-independent/Ago2-slicer activity-dependent processing.69 This includes the preponderance for an A/U base at the 5′ terminal in the mature sequence (80%) and the preference for miRNA residing in the 3p side of precursor hairpins (∼70%).68, 70 This suggested that Ago2-dependent pre-miRNA processing69, 71 is particularly important for the biogenesis of miRNA in this cluster and may be associated with changes we observed in schizophrenia. Another possibility is that a 14q32 cluster-associated transcription factor or other regulatory molecule is responsible for the change observed in schizophrenia. One example of this is the Ca2+-activated transcription factor known as myocyte enhancer factor-2 (MEF2), which has been shown to positively regulate expression of miRNA in this cluster.56 MEF2 isoforms have also been shown to regulate neuronal development through their role in supporting newly differentiated neurons and synapse formation.72, 73, 74, 75, 76 We have also shown that expression of the MEF2D isoform is upregulated in differentiating neurons in response to a reduction in miR-17 family miRNA expression.77 Lower expression of MEF2 in PBMC could potentially contribute to the reduction in transcription of this cluster in schizophrenia.
Function of 14q32 miRNA cluster in brain function and development
The miR-379/410 cluster has been shown to follow a similar stage and tissue-specific expression pattern as a colocalized cluster of C/D snoRNAs derived from a single intronic transcript known as maternally expressed gene 8 (RNA imprinted and accumulated in the nucleus on mouse chromosome 12).62 This is detectable throughout the developing mouse embryo and the placenta, is enriched in the brain in adult mice29, 57, 62, 78 and may be significant for the development of higher brain functions in eutherian mammals.79 The neural function of this cluster is supported by recent research suggesting that coordinated expression of these miRNA, including miR-134 and miR-329, is important for brain function and development. Neuronal activity in rats trigger MEF2, a neuronal-activity Ca2+-dependent transcription factor (described above), to bind to sequence upstream of the larger of the two clusters and induce expression of all pre-miRNAs within it.56 At least three members of the co-regulated cluster, including miR-329 and miR-134, that we see altered in schizophrenia, appear to regulate hippocampal neuron dendrite morphology.56 MiR-134 has also been shown to repress dendritic development by targeting LimK128 and to activate it through targeting Pumilio2.56 Interestingly, miR-134 expression is upregulated in the rat hippocampus and amygdala following acute stress, but is decreased during chronic stress.60 A predicted target of miR-134, splicing factor SC35, has been shown to be upregulated after stress and is believed to promote alternative splicing of acetylcholinesterase mRNA. Therefore, it is possible that miR-134 and other related miRNAs from the 14q32 cluster function as stress-dependent regulators of neurotransmission and synaptic plasticity. This is supported by the pathway analysis of target genes predicted for differentially expressed miRNA in this cluster, which included axon guidance, regulation of the actin cytoskeleton, long-term potentiation, long-term depression, neuroactive ligand–receptor interaction, focal adhesion, neurotrophin, mammalian target of rapamycin, calcium, mitogen-activated protein kinase and ErbB signaling pathways. It was also broadly consistent with previous pathway analysis of the human 14q32 cluster and the syntenic miRNA cluster in the mouse, with enrichment of target genes involved in neurogenesis (including the biological processes in axon guidance), embryonic development and more general developmental processes.79 Another interesting theme emerging from the pathway analysis was the large number of immunologically relevant processes including endocytosis, Fcγ R-mediated phagocytosis, Fcɛ RI signaling pathway, chemokine signaling pathway, T-cell receptor signaling pathway, leukocyte transendothelial migration and natural killer cell-mediated cytotoxicity. Given the evidence suggesting a link between early environmental exposure to infection/inflammation and schizophrenia, the alteration of miRNA expression in this tissue and their influence in these pathways could have functional significance for the immune system in individuals with the disorder. The expression of miRNA in this cluster was also shown to be highly associated with cellular pluripotency.80 If this cluster is involved in the regulation and maintenance of stem cell behavior, the reduction observed in schizophrenia could have broad developmental implications for both the immune and nervous systems.
Differentially expressed miRNA are contained within an imprinted locus
Maternal specific expression of this region is regulated by imprinting centers defined by an intergenic germline derived differentially methylated region (DMR) and the post-fertilization derived MEG3-DMR (shown in Figure 3).81 Differential expression of miRNA clustered in this imprinted region raises the possibility that there is a schizophrenia-associated parent of origin effect influencing the miRNA expression from this cluster similar to that observed at a number of other genes including the 5-hydroxytryptamine type 2A receptor82 and GABAA receptor β2.83 Imprinting at the 14q32 region has also been implicated in bipolar disorder84, 85 and anxiety.86 Although no gender bias was observed in the expression of the miRNA cluster, specific details about participants' parental disease status were not sufficient to derive parent of origin effect.
Many characteristics of the imprinted locus on 14q32 parallel the genetic landscape at 15q11–13, known for its involvement in Prader–Willi Syndrome). This neurodevelopmental disorder arises from monoallelic expression at 15q11–13 as a result of deletion of the paternal copy of the imprinted region (delPWS), maternal uniparental disomy (mat-upd-15), as well as abnormal imprinting (reviewed by Buiting87). The 14q32 and 15q11–13 regions are similar in that they are both developmentally regulated via an imprinting center, and contain both maternally and paternally expressed genes. The imprinted regions of both loci also encode C/D snoRNAs that function as guides for epigenetic modification of other RNAs. The snoRNAs in these loci are atypical in that they lack complementarity to their neighboring RNA. These small non-coding RNA molecules, exclusive to eutherians, are also brain enriched and transcribed in the same direction, possibly by a single transcription unit.62, 64, 88, 89, 90 In further support of a role for these imprinted loci in neuronal development, Leung et al.90 observed developmentally regulated chromatin decondensation of snoRNA clusters at 15q11–13 and 14q32 in postnatal mouse and human neurons.
Individuals with Prader–Willi Syndrome also share some phenotypic features with schizophrenia such as cognitive deficits, behavioral problems and, in some cases, autistic symptoms and psychosis.91, 92, 93, 94, 95, 96, 97 The 14q32 locus has also been implicated in other disorders with impaired development and behavioral phenotypes.98 Rare copy-number variations at 14q32 and 15q11–13 have also been reported in schizophrenia.99, 100, 101, 102 Although our analysis of copy number in this region did not identify structural variants, this genomic region clearly has the potential to convey risk for neurodevelopmental disorders including schizophrenia.
Conclusions
In this study, we examined schizophrenia-associated miRNA expression and identified a significant correlation between miRNAs clustered in a small imprinted domain on 14q32. Although the cause of this anomaly is not known, it may be due to an alteration in transcriptional regulation or processing. The miRNAs encoded in this cluster evolved in eutherian mammals and appear to have a significant role in brain development and function. And while the significance of their downregulation in PBMCs in schizophrenia is less clear, there are implications for lymphocyte function that could contribute to the risk for the disorder. Alternatively, this phenomenon may be a vestige of broader alteration to post-transcriptional regulation, with more relevance to neurodevelopment. Regardless of the functional significance, the miRNA expression signatures observed in PBMCs may have the potential to serve as biomarkers of schizophrenia and associated phenotypes.
Acknowledgments
This study was supported by the Schizophrenia Research Institute and the Neurobehavioral Genetics Unit, utilizing funding from NSW Health and an MC Ainsworth Research Fellowship in Epigenetics (MC); a NARSAD Young Investigator Award; a Hunter Medical Research Institute project grant; an NHMRC Project Grant 631057. Samples and clinical and demographic data for this study were provided by the Australian Schizophrenia Research Bank. PBMCs were processed by Melissa Tooney and Janelle Collins-Langworthy. The ASRB is supported by the NHMRC, the Pratt Foundation, Ramsay Health Care and the Schizophrenia Research Institute.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
Supplementary Material
References
- Saha S, Chant D, Welham J, McGrath J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005;2:e141. doi: 10.1371/journal.pmed.0020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nopoulos P, Torres I, Flaum M, Andreasen NC, Ehrhardt JC, Yuh WT. Brain morphology in first-episode schizophrenia. Am J Psychiatry. 1995;152:1721–1723. doi: 10.1176/ajp.152.12.1721. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Shenton ME, O'Donnell BF, Faux SF, Kikinis R, Nestor PG, et al. Auditory P300 abnormalities and left posterior superior temporal gyrus volume reduction in schizophrenia. Arch Gen Psychiatry. 1993;50:190–197. doi: 10.1001/archpsyc.1993.01820150036003. [DOI] [PubMed] [Google Scholar]
- Suddath RL, Christison GW, Torrey EF, Casanova MF, Weinberger DR. Anatomical abnormalities in the brains of monozygotic twins discordant for schizophrenia. N Engl J Med. 1990;322:789–794. doi: 10.1056/NEJM199003223221201. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Hall J, McIntosh AM, Cunningham-Owens DG, Johnstone EC. Neuroimaging and molecular genetics of schizophrenia: pathophysiological advances and therapeutic potential. Br J Pharmacol. 2008;153 (Suppl 1:S120–S124. doi: 10.1038/sj.bjp.0707655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes E, Tsang TM, Huang JT, Leweke FM, Koethe D, Gerth CW, et al. Metabolic profiling of CSF: evidence that early intervention may impact on disease progression and outcome in schizophrenia. PLoS Med. 2006;3:e327. doi: 10.1371/journal.pmed.0030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killackey E, Yung AR. Effectiveness of early intervention in psychosis. Curr Opin Psychiatry. 2007;20:121–125. doi: 10.1097/YCO.0b013e328017f67d. [DOI] [PubMed] [Google Scholar]
- Kawanishi Y, Tachikawa H, Suzuki T. Pharmacogenomics and schizophrenia. Eur J Pharmacol. 2000;410:227–241. doi: 10.1016/s0014-2999(00)00817-7. [DOI] [PubMed] [Google Scholar]
- Lakhan SE, Kramer A. Schizophrenia genomics and proteomics: are we any closer to biomarker discovery. Behav Brain Funct. 2009;5:2. doi: 10.1186/1744-9081-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz E, Bahn S. Biomarker discovery in psychiatric disorders. Electrophoresis. 2008;29:2884–2890. doi: 10.1002/elps.200700710. [DOI] [PubMed] [Google Scholar]
- Schwarz E, Bahn S. The utility of biomarker discovery approaches for the detection of disease mechanisms in psychiatric disorders. Br J Pharmacol. 2008;153 (Suppl 1:S133–S136. doi: 10.1038/sj.bjp.0707658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Owen MJ. Genes for schizophrenia? Recent findings and their pathophysiological implications. Lancet. 2003;361:417–419. doi: 10.1016/S0140-6736(03)12379-3. [DOI] [PubMed] [Google Scholar]
- Altar CA, Jurata LW, Charles V, Lemire A, Liu P, Bukhman Y, et al. Deficient hippocampal neuron expression of proteasome, ubiquitin, and mitochondrial genes in multiple schizophrenia cohorts. Biol Psychiatry. 2005;58:85–96. doi: 10.1016/j.biopsych.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Aston C, Jiang L, Sokolov BP. Microarray analysis of postmortem temporal cortex from patients with schizophrenia. J Neurosci Res. 2004;77:858–866. doi: 10.1002/jnr.20208. [DOI] [PubMed] [Google Scholar]
- Bowden NA, Scott RJ, Tooney PA. Altered gene expression in the superior temporal gyrus in schizophrenia. BMC Genom. 2008;9:199. doi: 10.1186/1471-2164-9-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, et al. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci USA. 2001;98:4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P. Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron. 2000;28:53–67. doi: 10.1016/s0896-6273(00)00085-4. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Barrett T, Cheadle C, Sokolov BP, Wood WH, III, Donovan DM, et al. Application of cDNA microarrays to examine gene expression differences in schizophrenia. Brain Res Bull. 2001;55:641–650. doi: 10.1016/s0361-9230(01)00522-6. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Crook JM, Hyde TM, Kleinman JE, Weinberger DR, Becker KG, et al. Microarray analysis of gene expression in the prefrontal cortex in schizophrenia: a preliminary study. Schizophr Res. 2002;58:11–20. doi: 10.1016/s0920-9964(01)00377-2. [DOI] [PubMed] [Google Scholar]
- Weidenhofer J, Bowden NA, Scott RJ, Tooney PA. Altered gene expression in the amygdala in schizophrenia: up-regulation of genes located in the cytomatrix active zone. Mol Cell Neurosci. 2006;31:243–250. doi: 10.1016/j.mcn.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Beveridge NJ, Gardiner E, Carroll AP, Tooney PA, Cairns MJ. Schizophrenia is associated with an increase in cortical microRNA biogenesis. Mol Psychiatry. 2009;15:1176–1189. doi: 10.1038/mp.2009.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge NJ, Tooney PA, Carroll AP, Gardiner E, Bowden N, Scott RJ, et al. Dysregulation of miRNA 181b in the temporal cortex in schizophrenia. Hum Mol Genet. 2008;17:1156–1168. doi: 10.1093/hmg/ddn005. [DOI] [PubMed] [Google Scholar]
- Farh KK, Grimson A, Jan C, Lewis BP, Johnston WK, Lim LP, et al. The widespread impact of mammalian microRNAs on mRNA repression and evolution. Science (New York, NY) 2005;310:1817–1821. doi: 10.1126/science.1121158. [DOI] [PubMed] [Google Scholar]
- Fiore R, Siegel G, Schratt G. MicroRNA function in neuronal development, plasticity and disease. Biochim Biophys Acta. 2008;1779:471–478. doi: 10.1016/j.bbagrm.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Krichevsky AM, Sonntag KC, Isacson O, Kosik KS. Specific microRNAs modulate embryonic stem cell-derived neurogenesis. Stem cells (Dayton, OH) 2006;24:857–864. doi: 10.1634/stemcells.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, et al. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439:283–289. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- Sempere LF, Freemantle S, Pitha-Rowe I, Moss E, Dmitrovsky E, Ambros V. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 2004;5:R13. doi: 10.1186/gb-2004-5-3-r13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mraz M, Malinova K, Mayer J, Pospisilova S. MicroRNA isolation and stability in stored RNA samples. Biochem Biophys Res Commun. 2009;390:1–4. doi: 10.1016/j.bbrc.2009.09.061. [DOI] [PubMed] [Google Scholar]
- Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodes MJ, Caraballo M, Suciu D, Munro S, Kumar A, Anderson B. Detection of cancer with serum miRNAs on an oligonucleotide microarray. PLoS One. 2009;4:e6229. doi: 10.1371/journal.pone.0006229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- Calin GA, Liu CG, Sevignani C, Ferracin M, Felli N, Dumitru CD, et al. MicroRNA profiling reveals distinct signatures in B cell chronic lymphocytic leukemias. Proc Natl Acad Sci USA. 2004;101:11755–11760. doi: 10.1073/pnas.0404432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenkiron C, Goldstein LD, Thorne NP, Spiteri I, Chin SF, Dunning MJ, et al. MicroRNA expression profiling of human breast cancer identifies new markers of tumor subtype. Genome Biol. 2007;8:R214. doi: 10.1186/gb-2007-8-10-r214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Guo N. MicroRNA expression and its implications for the diagnosis and therapeutic strategies of breast cancer. Cancer Treat Rev. 2009;35:328–334. doi: 10.1016/j.ctrv.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Iorio MV, Casalini P, Tagliabue E, Menard S, Croce CM. MicroRNA profiling as a tool to understand prognosis, therapy response and resistance in breast cancer. Eur J Cancer. 2008;44:2753–2759. doi: 10.1016/j.ejca.2008.09.037. [DOI] [PubMed] [Google Scholar]
- Cox MB, Cairns MJ, Gandhi KS, Carroll AP, Moscovis S, Stewart GJ, et al. MicroRNAs miR-17 and miR-20a inhibit T cell activation genes and are under-expressed in MS whole blood. PLoS One. 2010;5:e12132. doi: 10.1371/journal.pone.0012132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipper HM, Maes OC, Chertkow HM, Wang E. MicroRNA expression in Alzheimer blood mononuclear cells. Gene Regul Syst Biol. 2007;1:263–274. doi: 10.4137/grsb.s361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogswell JP, Ward J, Taylor IA, Waters M, Shi Y, Cannon B, et al. Identification of miRNA changes in Alzheimer's disease brain and CSF yields putative biomarkers and insights into disease pathways. J Alzheimer's Dis. 2008;14:27–41. doi: 10.3233/jad-2008-14103. [DOI] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Huang H, Oishi K, Mori S, et al. Maternal infection leads to abnormal gene regulation and brain atrophy in mouse offspring: implications for genesis of neurodevelopmental disorders. Schizophr Res. 2008;99:56–70. doi: 10.1016/j.schres.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strous RD, Shoenfeld Y. Schizophrenia, autoimmunity and immune system dysregulation: a comprehensive model updated and revisited. J Autoimmun. 2006;27:71–80. doi: 10.1016/j.jaut.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Yolken RH, Torrey EF. Viruses, schizophrenia, and bipolar disorder. Clin Microbiol Rev. 1995;8:131–145. doi: 10.1128/cmr.8.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle DJ, Jablensky A, McGrath JJ, Carr V, Morgan V, Waterreus A, et al. The diagnostic interview for psychoses (DIP): development, reliability and applications. Psychol Med. 2006;36:69–80. doi: 10.1017/S0033291705005969. [DOI] [PubMed] [Google Scholar]
- Loughland C, Draganic D, McCabe K, Richards J, Nasir A, Allen J, et al. The Australian Schizophrenia Research Bank: a database of comprehensive clinical, endophenotypic and genetic data for aetiological studies of schizophreniaAust N Z J Psychiatry2010441029–1035. [DOI] [PubMed] [Google Scholar]
- Santarelli DM, Beveridge NJ, Tooney PA, Cairns MJ. Upregulation of dicer and microRNA expression in the dorsolateral prefrontal cortex Brodmann area 46 in schizophrenia. Biol Psychiatry. 2011;69:180–187. doi: 10.1016/j.biopsych.2010.09.030. [DOI] [PubMed] [Google Scholar]
- Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha AJ. Java Treeview—extensible visualization of microarray data. Bioinformatics (Oxford, England) 2004;20:3246–3248. doi: 10.1093/bioinformatics/bth349. [DOI] [PubMed] [Google Scholar]
- Enright JB, Aravin AJ, Tuschl T, Sander C, Marks DS. Human microRNA targets. PLoS Biol. 2004;2:e363. doi: 10.1371/journal.pbio.0020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Hon LS, Zhang Z. The roles of binding site arrangement and combinatorial targeting in microRNA repression of gene expression. Genome Biol. 2007;8:R166. doi: 10.1186/gb-2007-8-8-r166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Kirov S, Snoddy J. WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res. 2005;33:W741–W748. doi: 10.1093/nar/gki475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merico D, Isserlin R, Stueker O, Emili A, Bader GD. Enrichment map: a network-based method for gene-set enrichment visualization and interpretation. PLoS One. 2010;5:e13984. doi: 10.1371/journal.pone.0013984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore R, Khudayberdiev S, Christensen M, Siegel G, Flavell SW, Kim TK, et al. Mef2-mediated transcription of the miR379-410 cluster regulates activity-dependent dendritogenesis by fine-tuning Pumilio2 protein levels. EMBO J. 2009;28:697–710. doi: 10.1038/emboj.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz H, Royo H, Bortolin ML, Lin SP, Ferguson-Smith AC, Cavaille J. A large imprinted microRNA gene cluster at the mouse Dlk1-Gtl2 domain. Genome Res. 2004;14:1741–1748. doi: 10.1101/gr.2743304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins DO, Jeffries CD, Jarskog LF, Thomson JM, Woods K, Newman MA, et al. microRNA expression in the prefrontal cortex of individuals with schizophrenia and schizoaffective disorder. Genome Biol. 2007;8:R27. doi: 10.1186/gb-2007-8-2-r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khudayberdiev S, Fiore R, Schratt G. MicroRNA as modulators of neuronal responses. Commun Integr Biol. 2009;2:411–413. doi: 10.4161/cib.2.5.8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerson A, Cacheaux L, Goosens KA, Sapolsky RM, Soreq H, Kaufer D. Changes in brain microRNAs contribute to cholinergic stress reactions. J Mol Neurosci. 2010;40:47–55. doi: 10.1007/s12031-009-9252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaille J. MicroRNAs: biosynthesis: mechanisms of action and biological functions. Ann Pathol. 2007;27:1S31–1S32. [PubMed] [Google Scholar]
- Cavaille J, Seitz H, Paulsen M, Ferguson-Smith AC, Bachellerie JP. Identification of tandemly-repeated C/D snoRNA genes at the imprinted human 14q32 domain reminiscent of those at the Prader–Willi/Angelman syndrome region. Hum Mol Genet. 2002;11:1527–1538. doi: 10.1093/hmg/11.13.1527. [DOI] [PubMed] [Google Scholar]
- Kircher M, Bock C, Paulsen M. Structural conservation versus functional divergence of maternally expressed microRNAs in the Dlk1/Gtl2 imprinting region. BMC Genom. 2008;9:346. doi: 10.1186/1471-2164-9-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royo H, Cavaille J. Non-coding RNAs in imprinted gene clusters. Biology of the Cell/Under the Auspices of the Eur Cell Biology Organization. 2008;100:149–166. doi: 10.1042/BC20070126. [DOI] [PubMed] [Google Scholar]
- Seitz H, Youngson N, Lin SP, Dalbert S, Paulsen M, Bachellerie JP, et al. Imprinted microRNA genes transcribed antisense to a reciprocally imprinted retrotransposon-like gene. Nat Genet. 2003;34:261–262. doi: 10.1038/ng1171. [DOI] [PubMed] [Google Scholar]
- Riveros C, Mellor D, Gandhi KS, McKay FC, Cox MB, Berretta R, et al. A transcription factor map as revealed by a genome-wide gene expression analysis of whole-blood mRNA transcriptome in multiple sclerosis. PLoS One. 2010;5:e14176. doi: 10.1371/journal.pone.0014176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark KL, Xu B, Bagchi A, Lai WS, Liu H, Hsu R, et al. Altered brain microRNA biogenesis contributes to phenotypic deficits in a 22q11-deletion mouse model. Nat Genet. 2008;40:751–760. doi: 10.1038/ng.138. [DOI] [PubMed] [Google Scholar]
- Schaefer A, Im HI, Veno MT, Fowler CD, Min A, Intrator A, et al. Argonaute 2 in dopamine 2 receptor-expressing neurons regulates cocaine addiction. J Exp Med. 2010;207:1843–1851. doi: 10.1084/jem.20100451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederichs S, Haber DA. Dual role for argonautes in microRNA processing and posttranscriptional regulation of microRNA expression. Cell. 2007;131:1097–1108. doi: 10.1016/j.cell.2007.10.032. [DOI] [PubMed] [Google Scholar]
- Frank F, Sonenberg N, Nagar B. Structural basis for 5′-nucleotide base-specific recognition of guide RNA by human AGO2. Nature. 2010;465:818–822. doi: 10.1038/nature09039. [DOI] [PubMed] [Google Scholar]
- O'Carroll D, Mecklenbrauker I, Das PP, Santana A, Koenig U, Enright AJ, et al. A Slicer-independent role for Argonaute 2 in hematopoiesis and the microRNA pathway. Genes Dev. 2007;21:1999–2004. doi: 10.1101/gad.1565607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam BY, Chawla S. MEF2D expression increases during neuronal differentiation of neural progenitor cells and correlates with neurite length. Neurosci Lett. 2007;427:153–158. doi: 10.1016/j.neulet.2007.09.030. [DOI] [PubMed] [Google Scholar]
- Linseman DA, Bartley CM, Le SS, Laessig TA, Bouchard RJ, Meintzer MK, et al. Inactivation of the myocyte enhancer factor-2 repressor histone deacetylase-5 by endogenous Ca(2+) //calmodulin-dependent kinase II promotes depolarization-mediated cerebellar granule neuron survival. J Biol Chem. 2003;278:41472–41481. doi: 10.1074/jbc.M307245200. [DOI] [PubMed] [Google Scholar]
- Mao Z, Bonni A, Xia F, Nadal-Vicens M, Greenberg ME. Neuronal activity-dependent cell survival mediated by transcription factor MEF2. Science (New York, NY) 1999;286:785–790. doi: 10.1126/science.286.5440.785. [DOI] [PubMed] [Google Scholar]
- Mao Z, Wiedmann M. Calcineurin enhances MEF2 DNA binding activity in calcium-dependent survival of cerebellar granule neurons. J Biol Chem. 1999;274:31102–31107. doi: 10.1074/jbc.274.43.31102. [DOI] [PubMed] [Google Scholar]
- Okamoto S, Krainc D, Sherman K, Lipton SA. Antiapoptotic role of the p38 mitogen-activated protein kinase-myocyte enhancer factor 2 transcription factor pathway during neuronal differentiation. Proc Natl Acad Sci USA. 2000;97:7561–7566. doi: 10.1073/pnas.130502697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge NJ, Tooney PA, Carroll AP, Tran N, Cairns MJ. Down-regulation of miR-17 family expression in response to retinoic acid induced neuronal differentiation. Cell Signal. 2009;21:1837–1845. doi: 10.1016/j.cellsig.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Wheeler G, Ntounia-Fousara S, Granda B, Rathjen T, Dalmay T. Identification of new central nervous system specific mouse microRNAs. FEBS Lett. 2006;580:2195–2200. doi: 10.1016/j.febslet.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Glazov EA, McWilliam S, Barris WC, Dalrymple BP. Origin, evolution, and biological role of miRNA cluster in DLK-DIO3 genomic region in placental mammals. Mol Biol Evol. 2008;25:939–948. doi: 10.1093/molbev/msn045. [DOI] [PubMed] [Google Scholar]
- Liu L, Luo GZ, Yang W, Zhao X, Zheng Q, Lv Z, et al. Activation of the imprinted Dlk1-Dio3 region correlates with pluripotency levels of mouse stem cells. J Biol Chem. 2010;285:19483–19490. doi: 10.1074/jbc.M110.131995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagami M, O'Sullivan MJ, Green AJ, Watabe Y, Arisaka O, Masawa N, et al. The IG-DMR and the MEG3-DMR at human chromosome 14q32.2: hierarchical interaction and distinct functional properties as imprinting control centers. PLoS Genet. 2010;6:e1000992. doi: 10.1371/journal.pgen.1000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunzel R, Blumcke I, Cichon S, Normann S, Schramm J, Propping P, et al. Polymorphic imprinting of the serotonin-2A (5-HT2A) receptor gene in human adult brain. Brain Res. 1998;59:90–92. doi: 10.1016/s0169-328x(98)00146-6. [DOI] [PubMed] [Google Scholar]
- Pun FW, Zhao C, Lo WS, Ng SK, Tsang SY, Nimgaonkar V, et al. Imprinting in the schizophrenia candidate gene GABRB2 encoding GABA(A) receptor beta(2) subunit. Mol Psychiatry. 2010;16:557–568. doi: 10.1038/mp.2010.47. [DOI] [PubMed] [Google Scholar]
- Cichon S, Schumacher J, Muller DJ, Hurter M, Windemuth C, Strauch K, et al. A genome screen for genes predisposing to bipolar affective disorder detects a new susceptibility locus on 8q. Hum Mol Genet. 2001;10:2933–2944. doi: 10.1093/hmg/10.25.2933. [DOI] [PubMed] [Google Scholar]
- Segurado R, Detera-Wadleigh SD, Levinson DF, Lewis CM, Gill M, Nurnberger JI, Jr, et al. Genome scan meta-analysis of schizophrenia and bipolar disorder, part III: bipolar disorder. Am J Hum Genet. 2003;73:49–62. doi: 10.1086/376547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middeldorp CM, Hottenga JJ, Slagboom PE, Sullivan PF, de Geus EJ, Posthuma D, et al. Linkage on chromosome 14 in a genome-wide linkage study of a broad anxiety phenotype. Mol Psychiatry. 2008;13:84–89. doi: 10.1038/sj.mp.4002061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buiting K. Prader–Willi syndrome and Angelman syndrome. Am J Med Genet C. 2010;154C:365–376. doi: 10.1002/ajmg.c.30273. [DOI] [PubMed] [Google Scholar]
- de los Santos T, Schweizer J, Rees CA, Francke U. Small evolutionarily conserved RNA, resembling C/D box small nucleolar RNA, is transcribed from PWCR1, a novel imprinted gene in the Prader–Willi deletion region, which is highly expressed in brain. Am J Hum Genet. 2000;67:1067–1082. doi: 10.1086/303106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone KA, DuBose AJ, Futtner CR, Elmore MD, Brannan CI, Resnick JL. A human imprinting centre demonstrates conserved acquisition but diverged maintenance of imprinting in a mouse model for Angelman syndrome imprinting defects. Hum Mol Genet. 2006;15:393–404. doi: 10.1093/hmg/ddi456. [DOI] [PubMed] [Google Scholar]
- Leung KN, Vallero RO, DuBose AJ, Resnick JL, LaSalle JM. Imprinting regulates mammalian snoRNA-encoding chromatin decondensation and neuronal nucleolar size. Hum Mol Genet. 2009;18:4227–4238. doi: 10.1093/hmg/ddp373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy SB, Dykens E, Williams CA. Prader–Willi and Angelman syndromes: sister imprinted disorders. Am J Med Genet. 2000;97:136–146. doi: 10.1002/1096-8628(200022)97:2<136::aid-ajmg5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Veltman MW, Craig EE, Bolton PF. Autism spectrum disorders in Prader–Willi and Angelman syndromes: a systematic review. Psychiatric Genet. 2005;15:243–254. doi: 10.1097/00041444-200512000-00006. [DOI] [PubMed] [Google Scholar]
- Whittington J, Holland A, Webb T, Butler J, Clarke D, Boer H. Cognitive abilities and genotype in a population-based sample of people with Prader–Willi syndrome. J Intellect Disabil Res. 2004;48:172–187. doi: 10.1111/j.1365-2788.2004.00556.x. [DOI] [PubMed] [Google Scholar]
- Holland AJ, Whittington JE, Butler J, Webb T, Boer H, Clarke D. Behavioural phenotypes associated with specific genetic disorders: evidence from a population-based study of people with Prader–Willi syndrome. Psychol Med. 2003;33:141–153. doi: 10.1017/s0033291702006736. [DOI] [PubMed] [Google Scholar]
- Jauregi J, Arias C, Vegas O, Alen F, Martinez S, Copet P, et al. A neuropsychological assessment of frontal cognitive functions in Prader–Willi syndrome. J Intellect Disabil Res. 2007;51:350–365. doi: 10.1111/j.1365-2788.2006.00883.x. [DOI] [PubMed] [Google Scholar]
- Relkovic D, Doe CM, Humby T, Johnstone KA, Resnick JL, Holland AJ, et al. Behavioural and cognitive abnormalities in an imprinting centre deletion mouse model for Prader–Willi syndrome. Eur J Neurosci. 2010;31:156–164. doi: 10.1111/j.1460-9568.2009.07048.x. [DOI] [PubMed] [Google Scholar]
- Soni S, Whittington J, Holland AJ, Webb T, Maina EN, Boer H, et al. The phenomenology and diagnosis of psychiatric illness in people with Prader–Willi syndrome. Psychol Med. 2008;38:1505–1514. doi: 10.1017/S0033291707002504. [DOI] [PubMed] [Google Scholar]
- Zollino M, Seminara L, Orteschi D, Gobbi G, Giovannini S, Della Giustina E, et al. The ring 14 syndrome: clinical and molecular definition. Am J Med Genet A. 2009;149A:1116–1124. doi: 10.1002/ajmg.a.32831. [DOI] [PubMed] [Google Scholar]
- Kirov G, Grozeva D, Norton N, Ivanov D, Mantripragada KK, Holmans P, et al. Support for the involvement of large copy number variants in the pathogenesis of schizophrenia. Hum Mol Genet. 2009;18:1497–1503. doi: 10.1093/hmg/ddp043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Rujescu D, Cichon S, Pietilainen OP, Ingason A, Steinberg S, et al. Large recurrent microdeletions associated with schizophrenia. Nature. 2008;455:232–236. doi: 10.1038/nature07229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon HJ, Yim SV, Lee WK, Jeon YW, Kim YH, Ko YJ, et al. Identification of DNA copy-number aberrations by array-comparative genomic hybridization in patients with schizophrenia. Biochem Biophys Res Commun. 2006;344:531–539. doi: 10.1016/j.bbrc.2006.03.156. [DOI] [PubMed] [Google Scholar]
- Xu B, Roos JL, Levy S, van Rensburg EJ, Gogos JA, Karayiorgou M. Strong association of de novo copy number mutations with sporadic schizophrenia. Nat Genet. 2008;40:880–885. doi: 10.1038/ng.162. [DOI] [PubMed] [Google Scholar]
- Hagan JP, O'Neill BL, Stewart CL, Kozlov SV, Croce CM. At least ten genes define the imprinted Dlk1-Dio3 cluster on mouse chromosome 12qF1. PLoS One. 2009;4:e4352. doi: 10.1371/journal.pone.0004352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.