Abstract
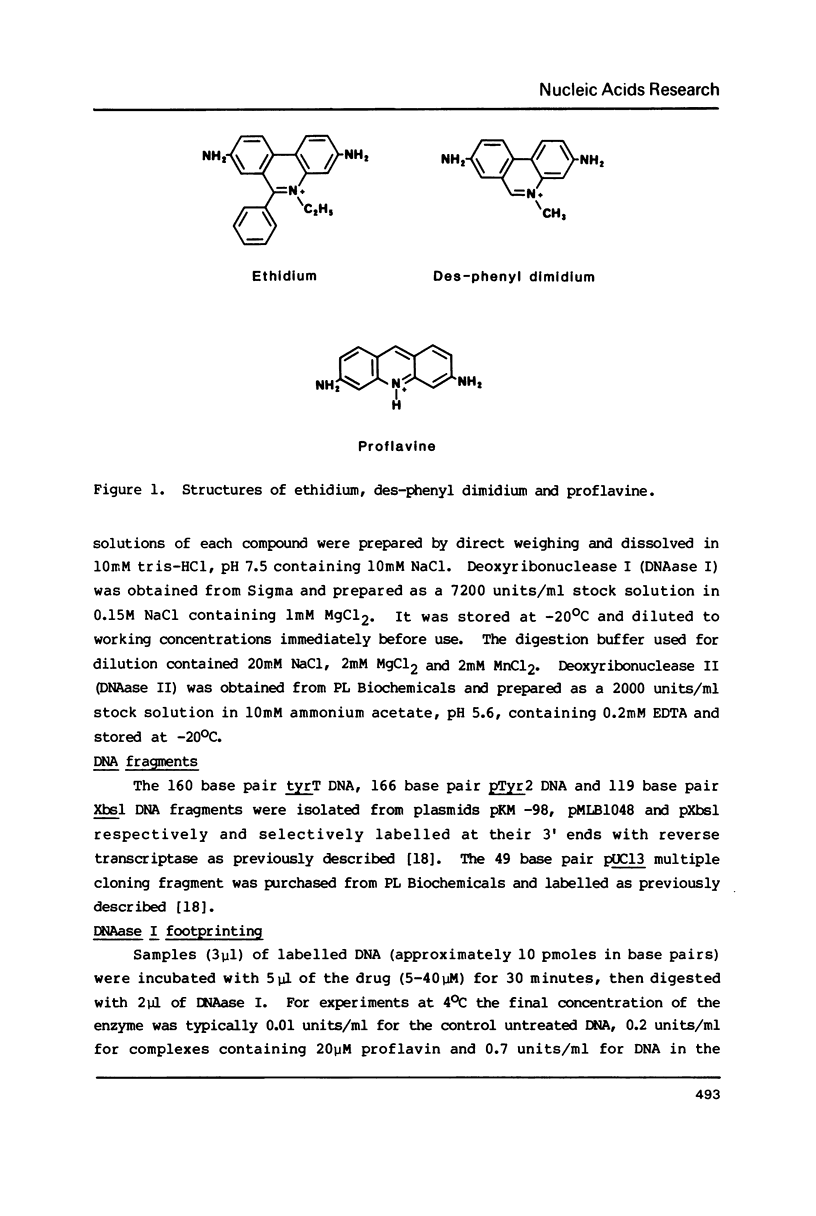
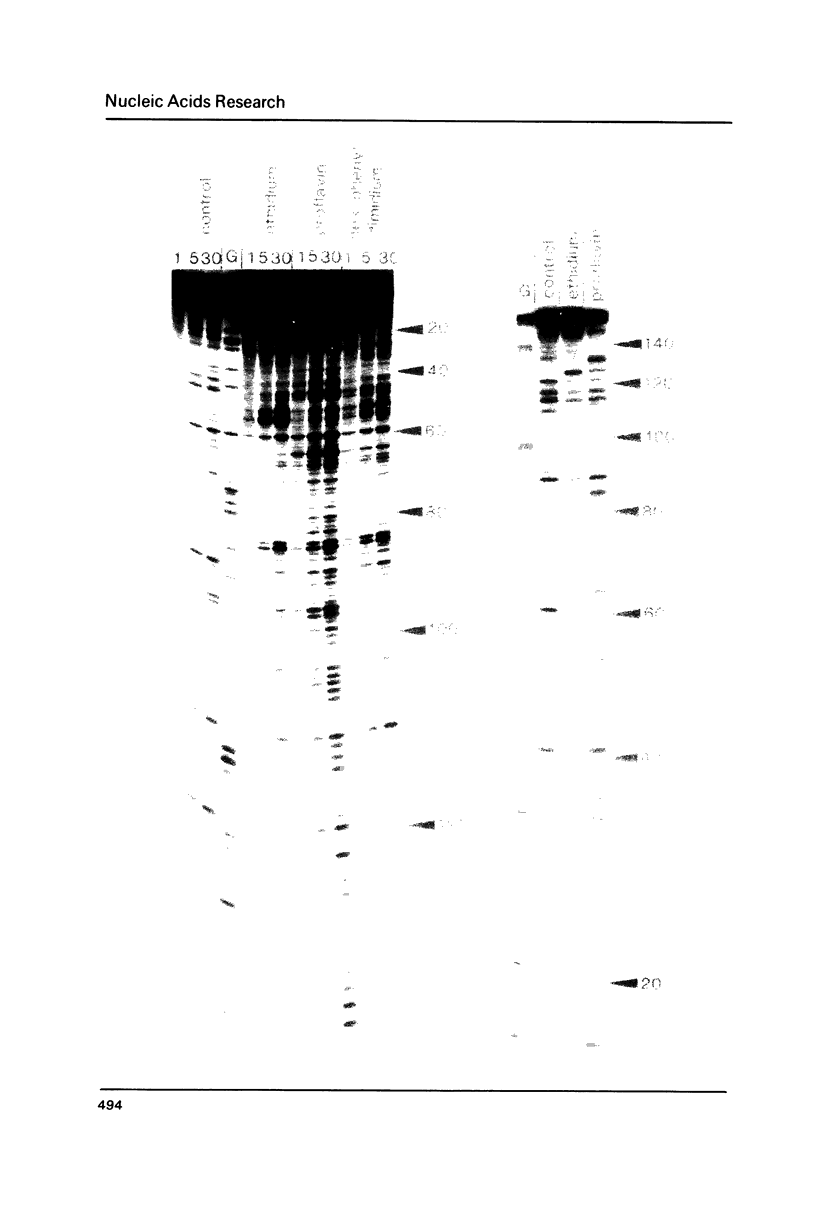
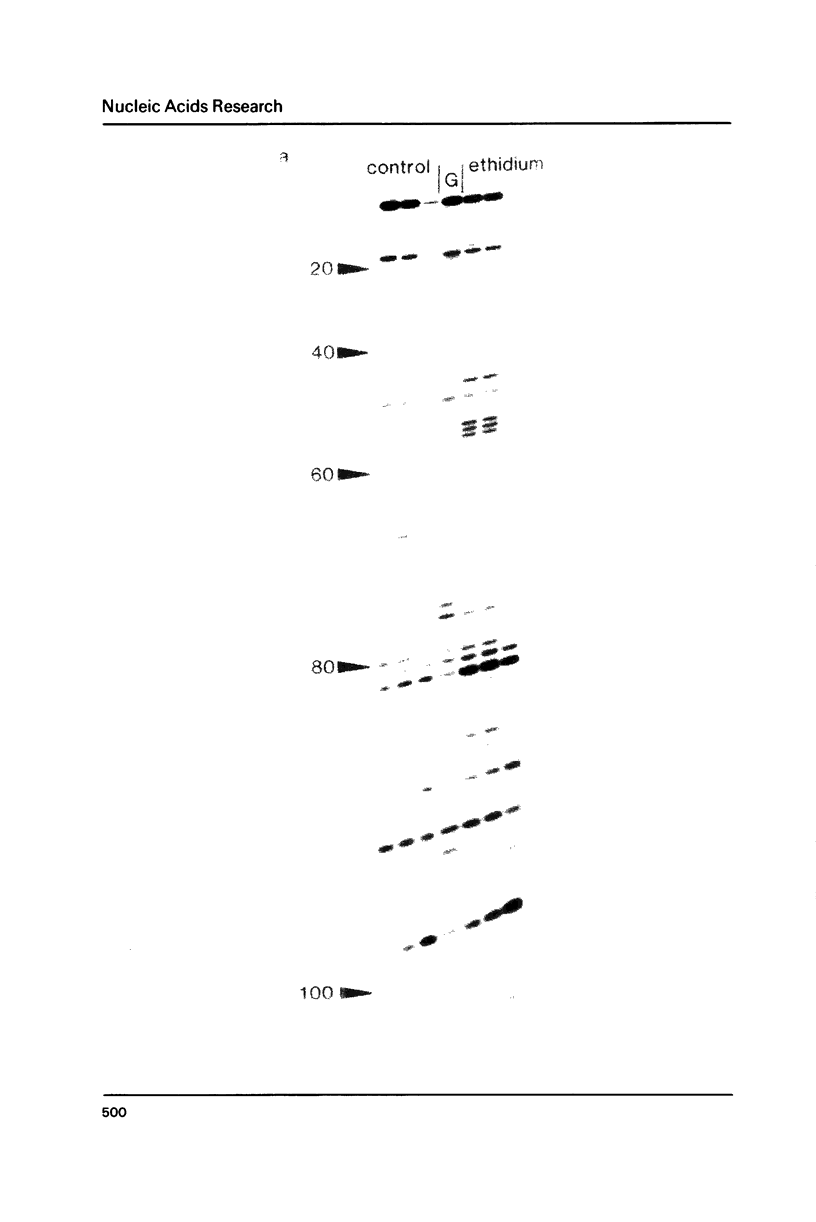
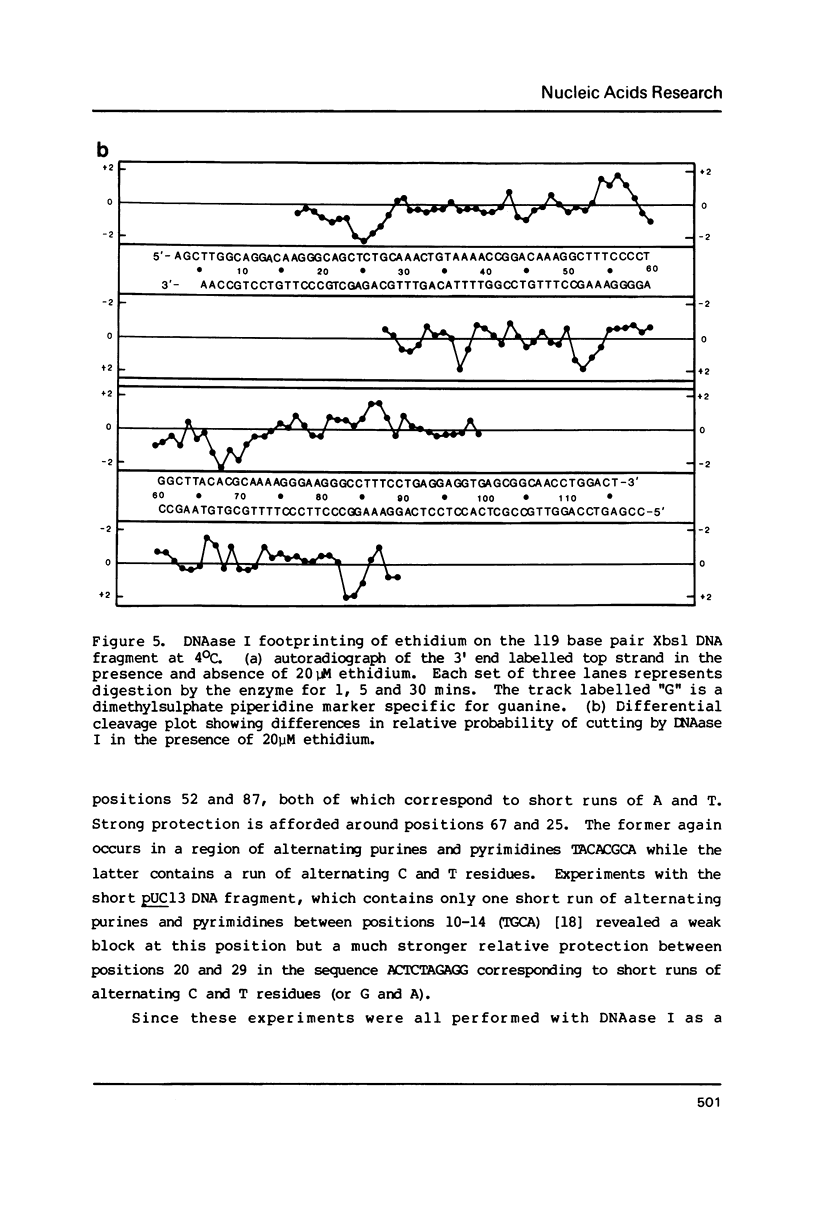
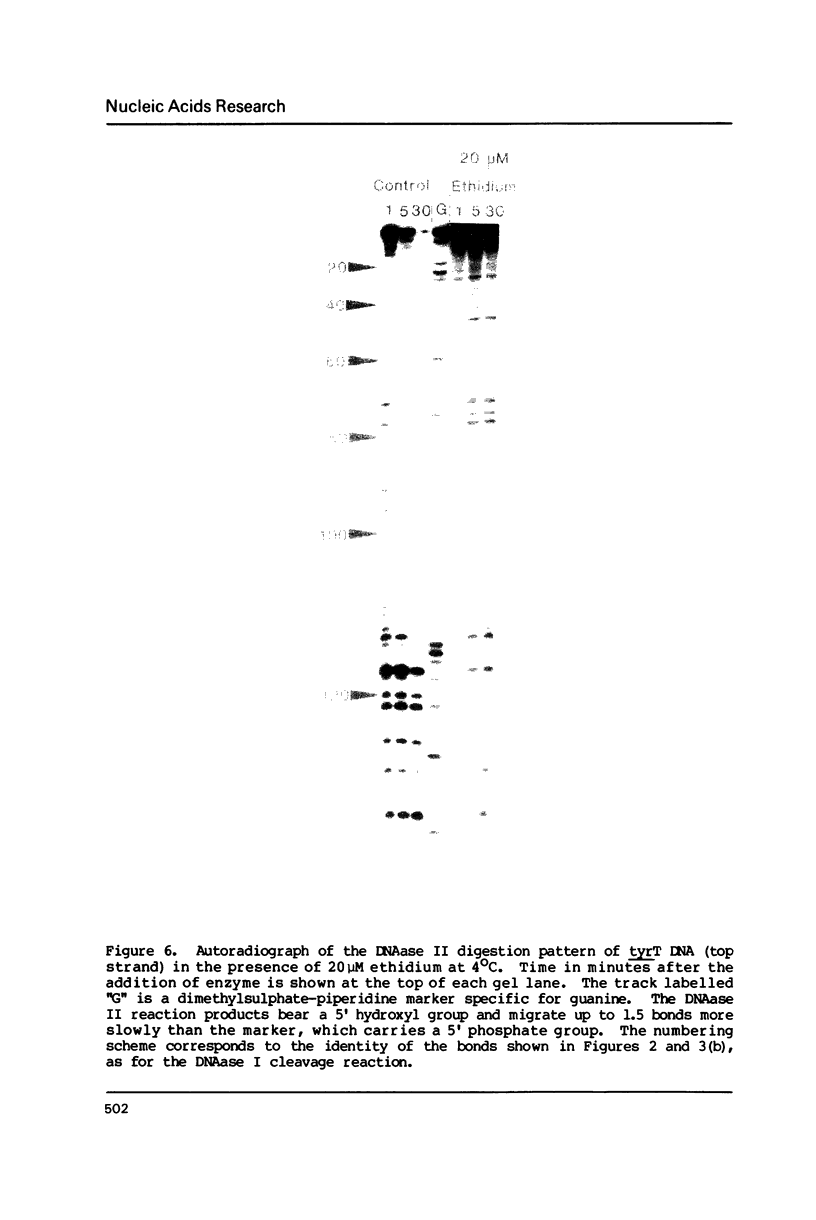
Footprinting experiments employing DNAase I have been performed at 4 degrees C. At this temperature several simple intercalating ligands, including both ethidium and proflavine, can be seen to induce marked changes in the pattern of cleavage. From an analysis of the changes in patterns of digestion by DNAase I we deduce that ethidium binds best to regions of mixed nucleotide sequence, especially those containing alternating purines and pyrimidines. Binding seems to be weakest to poly dA sequences which consequently appear as regions of relatively enhanced cleavage. Attempts to reproduce these changes using DNAase II as a footprinting tool were unsuccessful.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baguley B. C., Falkenhaug E. M. The interaction of ethidium with synthetic double-stranded polynucleotides at low ionic strength. Nucleic Acids Res. 1978 Jan;5(1):161–171. doi: 10.1093/nar/5.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresloff J. L., Crothers D. M. Equilibrium studies of ethidium--polynucleotide interactions. Biochemistry. 1981 Jun 9;20(12):3547–3553. doi: 10.1021/bi00515a038. [DOI] [PubMed] [Google Scholar]
- Drew H. R. Structural specificities of five commonly used DNA nucleases. J Mol Biol. 1984 Jul 15;176(4):535–557. doi: 10.1016/0022-2836(84)90176-1. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Travers A. A. DNA structural variations in the E. coli tyrT promoter. Cell. 1984 Jun;37(2):491–502. doi: 10.1016/0092-8674(84)90379-9. [DOI] [PubMed] [Google Scholar]
- Fox K. R., Howarth N. R. Investigations into the sequence-selective binding of mithramycin and related ligands to DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8695–8714. doi: 10.1093/nar/13.24.8695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K. R., Waring M. J., Brown J. R., Neidle S. DNA sequence preferences for the anti-cancer drug mitoxanthrone and related anthraquinones revealed by DNase I footprinting. FEBS Lett. 1986 Jul 7;202(2):289–294. doi: 10.1016/0014-5793(86)80703-7. [DOI] [PubMed] [Google Scholar]
- Fox K. R., Waring M. J. DNA structural variations produced by actinomycin and distamycin as revealed by DNAase I footprinting. Nucleic Acids Res. 1984 Dec 21;12(24):9271–9285. doi: 10.1093/nar/12.24.9271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K. R., Waring M. J. Nucleotide sequence binding preferences of nogalamycin investigated by DNase I footprinting. Biochemistry. 1986 Jul 29;25(15):4349–4356. doi: 10.1021/bi00363a026. [DOI] [PubMed] [Google Scholar]
- Goodisman J., Dabrowiak J. C. Theoretical analysis of the footprinting experiment. J Biomol Struct Dyn. 1985 Feb;2(5):967–979. doi: 10.1080/07391102.1985.10507613. [DOI] [PubMed] [Google Scholar]
- Krugh T. R., Reinhardt C. G. Evidence for sequence preferences in the intercalative binding of ethidium bromide to dinucleoside monophosphates. J Mol Biol. 1975 Sep 15;97(2):133–162. doi: 10.1016/s0022-2836(75)80031-3. [DOI] [PubMed] [Google Scholar]
- Lane M. J., Dabrowiak J. C., Vournakis J. N. Sequence specificity of actinomycin D and Netropsin binding to pBR322 DNA analyzed by protection from DNase I. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3260–3264. doi: 10.1073/pnas.80.11.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LePecq J. B., Paoletti C. A fluorescent complex between ethidium bromide and nucleic acids. Physical-chemical characterization. J Mol Biol. 1967 Jul 14;27(1):87–106. doi: 10.1016/0022-2836(67)90353-1. [DOI] [PubMed] [Google Scholar]
- Leupin W., Feigon J., Denny W. A., Kearns D. R. Substituent effects on the binding of ethidium and its derivatives to natural DNA. Biophys Chem. 1985 Oct;22(4):299–305. doi: 10.1016/0301-4622(85)80053-3. [DOI] [PubMed] [Google Scholar]
- Low C. M., Drew H. R., Waring M. J. Sequence-specific binding of echinomycin to DNA: evidence for conformational changes affecting flanking sequences. Nucleic Acids Res. 1984 Jun 25;12(12):4865–4879. doi: 10.1093/nar/12.12.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W., Crothers D. M. Studies of the binding of actinomycin and related compounds to DNA. J Mol Biol. 1968 Jul 28;35(2):251–290. doi: 10.1016/s0022-2836(68)80024-5. [DOI] [PubMed] [Google Scholar]
- Reinhardt C. G., Krugh T. R. A comparative study of ethidium bromide complexes with dinucleotides and DNA: direct evidence for intercalation and nucleic acid sequence preferences. Biochemistry. 1978 Nov 14;17(23):4845–4854. doi: 10.1021/bi00616a001. [DOI] [PubMed] [Google Scholar]
- Van Dyke M. M., Dervan P. B. Echinomycin binding sites on DNA. Science. 1984 Sep 14;225(4667):1122–1127. doi: 10.1126/science.6089341. [DOI] [PubMed] [Google Scholar]
- Van Dyke M. W., Dervan P. B. Methidiumpropyl-EDTA.Fe(II) and DNase I footprinting report different small molecule binding site sizes on DNA. Nucleic Acids Res. 1983 Aug 25;11(16):5555–5567. doi: 10.1093/nar/11.16.5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke M. W., Hertzberg R. P., Dervan P. B. Map of distamycin, netropsin, and actinomycin binding sites on heterogeneous DNA: DNA cleavage-inhibition patterns with methidiumpropyl-EDTA.Fe(II). Proc Natl Acad Sci U S A. 1982 Sep;79(18):5470–5474. doi: 10.1073/pnas.79.18.5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakelin L. P., Waring M. J. The unwinding of circular deoxyribonucleic acid by phenanthridinium drugs: structure-activity relations for the intercalation reaction. Mol Pharmacol. 1974 May;10(3):544–561. [PubMed] [Google Scholar]
- Waring M. J. Complex formation between ethidium bromide and nucleic acids. J Mol Biol. 1965 Aug;13(1):269–282. doi: 10.1016/s0022-2836(65)80096-1. [DOI] [PubMed] [Google Scholar]
- Wilson W. R., Baguley B. C., Wakelin L. P., Waring M. J. Interaction of the antitumor drug 4'-(9-acridinylamino)methanesulfon-m-anisidide and related acridines with nucleic acids. Mol Pharmacol. 1981 Sep;20(2):404–414. [PubMed] [Google Scholar]
- Winkle S. A., Rosenberg L. S., Krugh T. R. On the cooperative and noncooperative binding of ethidium to DNA. Nucleic Acids Res. 1982 Dec 20;10(24):8211–8223. doi: 10.1093/nar/10.24.8211. [DOI] [PMC free article] [PubMed] [Google Scholar]






