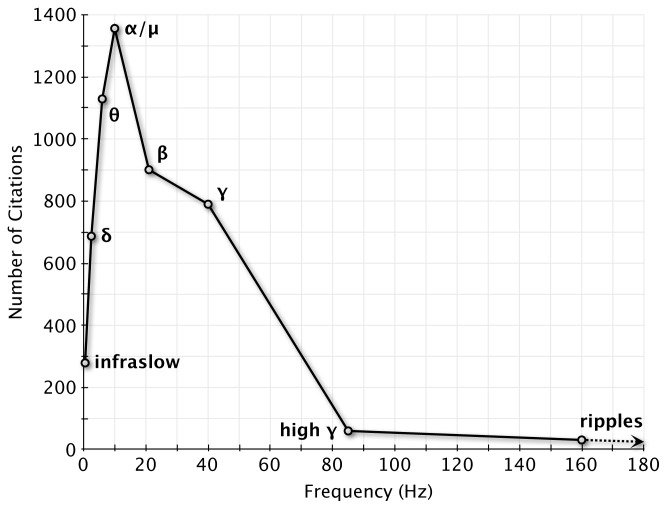
In their recent editorial, Nunez and Srinivasan (2010) assert that gamma band activity and intracranial recordings have been receiving an inordinate amount of attention in recent years. We agree that brain dynamics must be examined at all possible scales and across several frequency bands, and that it would be foolish to restrict our understanding of brain dynamics exclusively to intracranial recordings or higher frequency content. However, a number of points raised by the authors require further consideration.
How much emphasis is “too much”? A PubMed search of publications since 2007 reveals that the distribution of citations across frequency bands roughly follow their corresponding power distribution in the resting scalp EEG (see Figure 1), with 1,356 papers citing alpha or mu rhythms, while 790 cite gamma band activity and only 60 mention high gamma activity. Meanwhile, since 2007, 14,992 publications have cited EEG or MEG, while only 337 mention intracranial EEG1.
Fig. 1.
EEG/MEG citations in PubMed since 2007, visualized as a spectrum across frequencies. Number of citations were plotted as a function of frequency band centers, using the following definitions. Infraslow: 0.01–1 Hz, δ: 1–4 Hz, θ: 4–8 Hz, α/μ: 8–12 Hz, β: 12–30 Hz, γ: 30–50 Hz, high-γ: 50–120 Hz, and ripples: 120–200 Hz.
1. Historical Emphasis on Low Frequencies
Clearly, reports of alpha rhythms and scalp EEG/MEG still dominate the literature. As Nunez and Srinivasan (2010) point out, there is an extremely large body of literature describing the association of low-frequency (<20 Hz) activity with a variety of brain states, clinical conditions, and cognitive responses. Perhaps this should provide even stronger motivation to scrutinize higher frequencies, to explore the relatively uncharted territory at the frontier of neuroscience, to complement what is already known about evoked responses and lower frequency oscillations. In fact, one major reason that higher frequencies had been virtually ignored for decades is simply because this activity was either assumed to be entirely noise and therefore systematically filtered out, or deemed incompatible with the traditional phase-locked evoked response model, even in intracranial recordings. Additionally, advances in both computer technology as well as analytical techniques were necessary to bring the analysis of higher frequency brain activity to the mainstream.
The assertion of Nunez and Srinivasan (2010) that beta and gamma frequencies are “largely absent at the scalp” is somewhat puzzling. Hans Berger himself carefully documented and named the beta rhythm of scalp EEG in his earliest works (Berger 1929, 1930), which were soon replicated by Tönnies (1934), Jasper and Carmichael (1935), and Jasper and Andrews (1938). In the decades since, an enormous volume of literature has proliferated with numerous diverse experiments describing robust beta modulations recorded with scalp EEG (to cite only a few, Tallon-Baudry et al. 2001; Pfurtscheller et al. 2001, 2005; Muthukumaraswamy and Johnson 2004; Parkes et al. 2006) and MEG (Salmelin et al. 1995; Gross et al. 2001; Cheyne et al. 2003; Jurkiewicz et al. 2006; Dalal et al. 2008; Engel and Fries 2010).
As for gamma band activity, Nunez and Srinivasan (2010) remind us of the technical challenges facing its reliable detection in scalp recordings. Certainly, gamma band activity may suffer some additional attenuation at the scalp due to summation of neighboring sources with incoherent phases (Pfurtscheller and Cooper 1975), but the skull and scalp do not inherently form a lowpass filter as the electrical properties of the various head tissues do not vary appreciably across the 0–100,000 Hz range (Oostendorp et al. 2000). Furthermore, we must emphasize that both experimental designs and analysis strategies have evolved to overcome poor signal-to-noise ratios (SNRs) – even when noise power is a thousandfold greater than signal power in raw data – dramatically extending the usable bandwidth of electrophysiological recordings. Indeed, simple averaging across trials using raw data or band-limited power time courses decreases noise power in proportion to the number of trials acquired (Turetsky et al. 1988).
2. Dissociating Cortical Gamma and Muscle Contamination
Electromyographic (EMG) interference contains significant power in the gamma band and thereby poses a challenge for resolving cortical gamma rhythms. Granted, this challenge may prove too formidable to allow noninvasive recordings to reliably detect gamma events in single trials. It may likewise hinder detection of transient pathological oscillations that may arise from relatively small generators, as might arise in epilepsy (Bragin et al. 2002; Tao et al. 2007; Wu et al. 2008). However, event-related paradigms benefit from repeated responses that can then be statistically analyzed across trials.
Whitham et al. (2007), who are cited as evidence casting doubt on the cortical origin of event-related gamma rhythms, in fact report a false negative rather than a false positive in their attempt to resolve scalp gamma modulations in the presence of ordinary background EMG. That is to say, muscular activity did not seem to produce significant stimulus-locked artifacts, but rather generated more continuous background noise that obscured task-induced gamma modulations. However, by subsequently inducing scalp muscle paralysis and thereby significantly reducing background noise levels, they found quite robust gamma modulations, confirmed in a follow-up study (Pope et al. 2009). These studies and others (Goncharova et al. 2003; Whitham et al. 2008) furthermore demonstrate that scalp EMG does in fact manifest most strongly close to scalp muscles, resulting in enhanced noise power along the periphery of an EEG cap but less intense centrally.
A more in-depth examination of the topography of task-related gamma modulations can provide clues as to whether they originate from cortex or artifactual sources such as the scalp muscles or eyes (Reva and Aftanas 2004; Trujillo et al. 2005; Yuval-Greenberg et al. 2008; Jerbi et al. 2009a). Principal component analysis (Mäki and Ilmoniemi 2011), independent component analysis (Keren et al. 2010), and some source localization algorithms based on spatial filters analytically formalize such spatial distinctions. Spatial filters, in particular, reconstruct source activity with what is essentially a weighted average across EEG/MEG sensors in addition to averaging across trials. This allows us to not only examine the spatially distinct sources of cortical activity versus artifact, but to boost effective SNR as well (Sekihara et al. 2004; Ward et al. 1999; Väisänen and Malmivuo 2009). For even further sensitivity and generalizability, usually a large number of trials are acquired and statistics are computed across subjects. Indeed, these strategies seem to have the greatest success in resolving gamma activity at the scalp level (Dalal et al. 2008; Muthukumaraswamy 2010; Dockstader et al. 2010; Diwakar et al. 2011). Further confirmation on the possibilities and limitations of scalp recordings can be obtained from occasional opportunities to record them simultaneously with intracranial EEG (Dalal et al. 2008; Ball et al. 2009; Litvak et al. 2010; Rampp et al. 2010).
Given these considerations, our position is that there should be even more emphasis on gamma and beta, and researchers should take care not to filter this activity out upon acquisition, as any desired filtering for traditional analyses can now be easily done in post-processing software. Intracranial EEG, likewise, remains an underutilized technique to study human cognition, given the number of epilepsy surgery clinics around the world.
3. Intracranial EEG and Cortical Rhythms
Intracranial EEG (iEEG) in humans provides high-fidelity recordings of great clinical and research value. While intracranial recordings may occasionally be contaminated by eye muscle activity (Ball et al. 2009; Jerbi et al. 2009a; Kovach et al. 2011), these effects are predominantly restricted to recordings sites in the vicinity of the temporal pole and are efficiently reduced by using bipolar re-referencing strategies (Jerbi et al. 2009a). Certainly, these recordings are performed in patients with brain pathologies, so individual results are best interpreted in the context of converging evidence from other techniques; nevertheless, each patient’s pathology tends to be different, therefore findings that remain consistent across patients can be regarded with reasonable confidence (Jerbi et al. 2009b).
The spatial coverage of iEEG is inherently limited, and the contribution of brain regions distant from the implanted zone cannot be reliably assessed. Nevertheless, a specific region of the brain is usually targeted for implantation based on a clinical hypothesis from scalp EEG/MEG, other neuroimaging techniques, and neurological or neuropsychological symptoms; any cognitive experiment tends to be targeted based on this coverage as well. Moreover, a sufficiently large volume of cortex must be activated to produce a recordable signal at the scalp (Cooper et al. 1965; Nunez and Srinivasan 2006), further raising the likelihood of detection by at least one intracranial electrode that would provide a partial validation of analyses from scalp recordings. Furthermore, source localization techniques similar to those applied to scalp EEG/MEG are under development for increasing the effective spatial sampling of iEEG (Dümpelmann et al. 2009; Axmacher et al. 2010). These techniques may be able to extend spatial coverage somewhat beyond the limits of the targeted brain volume. Finally, depth electrodes can access deeper brain structures such as the hippocampus (Ekstrom et al. 2005; Axmacher et al. 2010), thalamus (Sarnthein et al. 2003; Hanajima et al. 2004), and sub-thalamic nucleus (Litvak et al. 2010; Hirschmann et al. 2010), all of which play critical roles in brain function but are currently difficult or impossible to resolve with scalp recordings.
The earliest reports of subdural recordings in humans have shown activity across a range of frequencies, including the alpha band (Scarff and Rahm 1941; Jasper and Penfield 1949; Cooper et al. 1965). Numerous experiments in recent years have also shown task-related alpha modulations in subdural surface grids (Arroyo et al. 1993; Toro et al. 1994; Towle et al. 1995; Crone et al. 1998; Ohara et al. 2000; Crone et al. 2001; Brunner et al. 2005; Dalal et al. 2008; Blakely et al. 2009; Edwards et al. 2009; Swann et al. 2009; Fukuda et al. 2010), including centimeter-scale coherence (Shen et al. 1999; Aoki et al. 2001; Brunner et al. 2005). Tremblay et al. (2004) reported decreases of alpha band power over frontal and motor cortex not only with finger movements but with observations of finger movements. Furthermore, alpha modulations have been observed in depth EEG recordings as well (Dalal et al. 2009; Vidal et al. 2010). A procedure called hemicraniectomy, in which a portion of skull is removed while leaving the scalp and dura, can be considered similar to dural recordings; Voytek et al. (2010) found that the procedure intensifies modulations across a broad range of frequencies – including the alpha band – relative to intact scalp EEG. However, bipolar montages, often used in intracranial recording, may inherently obscure diffuse alpha activity. Clearly, large-scale phenomena, by definition, cannot be observed with the small spatial coverage available in many human iEEG studies, but most of the experiments cited here typically recorded over wide regions of cortex and consequently revealed widespread alpha modulations; many of these studies simultaneously analyzed gamma band modulations also, finding effects that were more task-specific as well as more focal spatially and temporally. (Jerbi et al. 2009b).
Let us not forget one of the primary goals of brain mapping, and the decisive motivation to record human intracranial EEG – to provide important diagnostic information for patient treatment. Here, too, analysis of intracranial gamma band activity has proven critical. Functional gamma mapping appears to correlate favorably with results of electrocortical stimulation mapping of eloquent cortex (Towle et al. 2008; Wu et al. 2010; Roland et al. 2010), and can be performed far more quickly and with less stress for the patient. While lower-frequency modulations may be seen along with gamma band enhancements, their spatial extent is often larger, reducing their usefulness for planning of resective surgery. Certain kinds of pathology (tumors, epileptogenic zones) also manifest themselves with abnormally high gamma-band power (Jacobs et al. 2010b) and coherence (Le Van Quyen et al. 1997). Finally, intracranial gamma band modulations frequently show higher spatial and functional specificity than other metrics, properties that are essential for real-time and brain-computer interface applications (Leuthardt et al. 2004; Lachaux et al. 2007b; Miller et al. 2009).
4. Plausible Role for Faster Rhythms in Binding, Neuronal Communication, and Inhibition
Slow oscillations may not provide a plausible mechanism for inherently fast integration processes, considering that neuronal interactions occur at a millisecond timescale. Singer (1993) specifically hypothesized that, “oscillations in the α- and β-frequency range would be too slow to serve as carrier signal for binding at this level of processing,” especially if a few cycles of an oscillation are necessary, and proposed that the gamma range “appears as a good compromise between the opposing constraints to establish synchrony rapidly and with high temporal resolution on the one hand and over long distances on the other.” However, the role of gamma rhythms in long-distance binding remains controversial (Kopell et al. 2000), especially with respect to visual processing, as recent studies have provided evidence against gamma-mediated binding in V1 (Ray and Maunsell 2010; Lima et al. 2010).
In parallel, the theoretical interpretations and foundations of gamma-band activity in cerebral networks have diversified beyond the binding hypothesis. Recent reviews have stressed the important mechanistic role of gamma activity regarding selective neural communication, neural plasticity, and neural activation and inhibition (Fries 2009). GABAergic interneurons form one of the largest cell populations in cortex and are known to operate largely in the gamma band, appearing to provide a key role in sensory gating (Cardin et al. 2009); a recent MEG study found that resting GABA concentration in the visual cortex of individual subjects predicts the gamma oscillation frequency induced by visual stimuli (Muthukumaraswamy et al. 2009). All of these potential implications compel further investigation of the gamma band alongside other frequencies, to explore aspects of perception and cognition that may not be accessible to other techniques.
Nunez and Srinivasan (2010) contend that studies of conscious perception during binocular rivalry using steady-state visual evoked potentials (SSVEPs) constitute the consciousness studies that are most closely related to perceptual binding. However, the hypothesis of binding by synchrony was conceived as a computational solution that could explain how a limited number of neurons, by means of their temporal coordination, may represent the enormous variability of the environment. In contrast, exogenously driving the cortical response through steady-state visual stimulation is highly useful for studying conscious perception (Srinivasan et al. 1999; Cosmelli et al. 2004). This frequency-tagging of active neural networks constitutes a powerful tool to investigate consciousness but does not necessarily explain the underlying mechanism by which consciousness arises.
5. Link between Gamma & BOLD
Accumulating evidence over the last decade suggests that investigations of gamma-band neuronal activity might be key to bridging the gap between fMRI and electrophysiological research. Numerous studies have established a tight relationship between increases in the blood-oxygenation level-dependent (BOLD) signal and task-related increases in broadband gamma (~30–150 Hz) of the LFP in humans (Mukamel et al. 2005; Nir et al. 2007; Lachaux et al. 2007a) and in animals (Logothetis et al. 2001; Niessing et al. 2005). By contrast, alpha-band modulations often seem to be negatively correlated with simultaneously recorded BOLD responses (e.g., Laufs et al. 2003; Moosmann et al. 2003) but more spatially distributed.
A further indicator for the specificity of the coupling between gamma-band power and BOLD comes from recent reports indicating that positive and negative BOLD responses are associated respectively with increases and decreases of broadband gamma power in the primary visual cortex of monkeys (Shmuel et al. 2006). More recently, the coupling between negative BOLD responses and suppression of gamma power has also been suggested by direct electrophysiological recordings in the so-called default-mode network known to display BOLD deactivations during attention-demanding tasks. Several studies by our group and others show that execution of externally oriented attention-demanding tasks leads to suppressions of broad-band gamma power in specific default-mode network structures (Hayden et al. 2009; Lachaux et al. 2008; Ossandón et al. 2009; Jerbi et al. 2010). Therefore future studies of broadband gamma should improve our understanding of the neu-rophysiological basis of the BOLD signal and advance our understanding of the functional role of large-scale intrinsic networks such as the default-mode network.
6. Final Words
The term “gamma band” as currently used represents a very broad range of frequencies that likely encompasses a few different neural mechanisms, and we support Nunez and Srinivasan (2010) in cautioning against the temptation to rely on it as a “catch-all category.” The literature describing epilepsy-related high frequency oscillations has recognized some differences across this range, using the term ripples to describe activity between about 80–200 Hz but distinguishing them from “fast ripples” that appear to represent a distinct phenomenon between 250–500 Hz (Bragin et al. 1999). In the cognitive domain, more differentiation needs to be made between, for example, 40 Hz narrowband oscillations and broader 70–120 Hz power enhancements (Vidal et al. 2006; Hoogenboom et al. 2006; Wyart and Tallon-Baudry 2008; Crone et al. 2010), or even higher frequency phenomena of about 130–250 Hz (often also referred to as ripples) in the hippocampus and entorhinal cortex (Axmacher et al. 2008; Le Van Quyen et al. 2010) and 600 Hz somatosensory evoked potentials (Curio et al. 1994). A more nuanced view of these high frequencies should be considered, particularly in light of a recent study demonstrating the specificity of different subbands across the 60–500 Hz range to various cognitive tasks (Gaona et al. 2011). As suggested by Curio (2000) and Jacobs et al. (2010a), perhaps the EEG/MEG community should agree on more specific, consistent terminology to better differentiate the various high-frequency phenomena in the literature. In fact, if such distinctions were made, one could argue that alpha actually attracts a rather disproportionate amount of attention for encompassing only 4 Hz of the spectrum!
Figure 1 suggests that very low frequency phenomena (below 4 Hz) such as the slow cortical potential (Birbaumer et al. 1990; He et al. 2008) or infraslow fluctuations (e.g., Monto et al. 2008), as well as delta-band frequencies (e.g., Jerbi et al. 2007) may also deserve more studies and further evaluation. Ultimately, a more complete view of brain dynamics and cognition must come from examining activity across a broad range of frequencies. As Nunez and Srinivasan (2010) point out, cross-frequency interaction may provide a mechanism for inter-network communication during cognitive processing, and already studies on cross-frequency interaction from several laboratories have been rapidly elucidating the interplay between frequency bands (Canolty et al. 2006; Jensen and Colgin 2007; Monto et al. 2008; Osipova et al. 2008; de Lange et al. 2008; Jerbi and Bertrand 2009; Le Van Quyen et al. 2010; Dalal et al. 2010; Canolty and Knight 2010). It is therefore clear that the neural correlates of cognition are not confined to a specific frequency band and that the big picture can only be achieved by putting the pieces of the puzzle back together, i.e., not only including all frequencies of the spectrum but also various measures of brain responses across multiple spatial scales.
We shall also conclude with inspiration by Jacobs (2010), who notes that so much valuable information has been gained from simply opening up filters, using faster sampling rates, and examining the full frequency spectrum in subsequent analyses; indeed, her editorial closes with optimism that ever higher frequency activity reflecting ever earlier responses will arise from technical advances, not to the exclusion of lower frequency correlates, but rather in the context of them, and we couldn’t agree more.
Supplementary Material
Acknowledgments
This work was supported by grants to CMH from the Fondation Fyssen and to KJ from the Fondation pour la Recherche Médicale.
Footnotes
search included: intracranial electroencephalography, electrocorticography, stereoelectroencephalography, depth electroencephalography, and subdural recordings. See supplementary material for precise PubMed search queries.
References
- Aoki F, Fetz EE, Shupe L, Lettich E, Ojemann GA. Changes in power and coherence of brain activity in human sensorimotor cortex during performance of visuomotor tasks. BioSystems. 2001 Dec;63(1–3):89–99. doi: 10.1016/s0303-2647(01)00149-6. [DOI] [PubMed] [Google Scholar]
- Arroyo S, Lesser RP, Gordon B, Uematsu S, Jackson D, Webber R. Functional significance of the mu rhythm of human cortex: an electrophysiologic study with subdural electrodes. Electroencephalogr Clin Neurophysiol. 1993 Sep;87(3):76–87. doi: 10.1016/0013-4694(93)90114-b. [DOI] [PubMed] [Google Scholar]
- Axmacher N, Cohen MX, Fell J, Haupt S, Dümpelmann M, Elger CE, Schlaepfer TE, Lenartz D, Sturm V, Ranganath C. Intracranial EEG correlates of expectancy and memory formation in the human hippocampus and nucleus accumbens. Neuron. 2010 Feb;65(4):541–9. doi: 10.1016/j.neuron.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Axmacher N, Elger CE, Fell J. Ripples in the medial temporal lobe are relevant for human memory consolidation. Brain. 2008 Jul;131:1806–17. doi: 10.1093/brain/awn103. [DOI] [PubMed] [Google Scholar]
- Ball T, Kern M, Mutschler I, Aertsen A, Schulze-Bonhage A. Signal quality of simultaneously recorded invasive and non-invasive EEG. NeuroImage. 2009 Jul;46(3):708–16. doi: 10.1016/j.neuroimage.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Berger H. Über das elektrenkephalogramm des menschen. Arch Psychiat Nervenkr. 1929;87:527–570. [Google Scholar]
- Berger H. Über das elektrenkephalogramm des menschen. Zweite mitteilung. J Psychol Neurol. 1930;40:160–179. [Google Scholar]
- Birbaumer N, Elbert T, Canavan AG, Rockstroh B. Slow potentials of the cerebral cortex and behavior. Physiol Rev. 1990 Jan;70(1):1–41. doi: 10.1152/physrev.1990.70.1.1. [DOI] [PubMed] [Google Scholar]
- Blakely T, Miller KJ, Zanos SP, Rao RPN, Ojemann JG. Robust, long-term control of an electrocorticographic brain-computer interface with fixed parameters. Neurosurgical focus. 2009 Jul;27(1):E13. doi: 10.3171/2009.4.FOCUS0977. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Wilson CL, Fried I, Buzsáki G. High-frequency oscillations in human brain. Hippocampus. 1999 Jan;9(2):137–42. doi: 10.1002/(SICI)1098-1063(1999)9:2<137::AID-HIPO5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Bragin A, Mody I, Wilson CL, Engel J. Local generation of fast ripples in epileptic brain. J Neurosci. 2002 Mar;22(5):2012–21. doi: 10.1523/JNEUROSCI.22-05-02012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner C, Graimann B, Huggins JE, Levine SP, Pfurtscheller G. Phase relationships between different subdural electrode recordings in man. Neurosci Lett. 2005 Feb;375(2):69–74. doi: 10.1016/j.neulet.2004.11.052. [DOI] [PubMed] [Google Scholar]
- Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, Berger MS, Barbaro NM, Knight RT. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313:1626–1628. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canolty RT, Knight RT. The functional role of cross-frequency coupling. Trends Cogn Sci. 2010 Nov;14(11):506–15. doi: 10.1016/j.tics.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai L-H, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009 Jun;459(7247):663–7. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheyne D, Gaetz W, Garnero L, Lachaux J-P, Ducorps A, Schwartz D, Varela FJ. Neuromagnetic imaging of cortical oscillations accompanying tactile stimulation. Cogn Brain Res. 2003;17:599–611. doi: 10.1016/s0926-6410(03)00173-3. [DOI] [PubMed] [Google Scholar]
- Cooper R, Winter AL, Crow HJ, Walter WG. Comparison of subcortical, cortical and scalp activity using chronically indwelling electrodes in man. Electroencephalogr Clin Neurophysiol. 1965 Feb;18:217–28. doi: 10.1016/0013-4694(65)90088-x. [DOI] [PubMed] [Google Scholar]
- Cosmelli D, David O, Lachaux J-P, Martinerie J, Garnero L, Renault B, Varela F. Waves of consciousness: ongoing cortical patterns during binocular rivalry. NeuroImage. 2004 Sep;23(1):128–40. doi: 10.1016/j.neuroimage.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Crone NE, Boatman D, Gordon B, Hao L. Induced electrocorticographic gamma activity during auditory perception. Clin Neurophysiol. 2001;112:565–582. doi: 10.1016/s1388-2457(00)00545-9. [DOI] [PubMed] [Google Scholar]
- Crone NE, Korzeniewska A, Franaszczuk P. Cortical gamma responses: Searching high and low. Int J Psychophysiol. 2010 Nov; doi: 10.1016/j.ijpsycho.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone NE, Miglioretti DL, Gordon B, Sieracki JM, Wilson MT, Uematsu S, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. I. Alpha and beta event-related desynchronization. Brain. 1998;121:2271–2299. doi: 10.1093/brain/121.12.2271. [DOI] [PubMed] [Google Scholar]
- Curio G. Ain’t no rhythm fast enough: EEG bands beyond beta. J Clin Neurophysiol. 2000 Jul;17(4):339–40. doi: 10.1097/00004691-200007000-00001. [DOI] [PubMed] [Google Scholar]
- Curio G, Mackert BM, Burghoff M, Koetitz R, Abraham-Fuchs K, Härer W. Localization of evoked neuromagnetic 600 Hz activity in the cerebral somatosensory system. Electroencephalogr Clin Neurophysiol. 1994 Dec;91(6):483–7. doi: 10.1016/0013-4694(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Dalal SS, Baillet S, Adam C, Ducorps A, Schwartz D, Jerbi K, Bertrand O, Garnero L, Martinerie J, Lachaux J-P. Simultaneous MEG and intracranial EEG recordings during attentive reading. NeuroImage. 2009 May;45(4):1289–304. doi: 10.1016/j.neuroimage.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Dalal SS, Guggisberg AG, Edwards E, Sekihara K, Findlay AM, Canolty RT, Berger MS, Knight RT, Barbaro NM, Kirsch HE, Nagarajan SS. Five-dimensional neuroimaging: Localization of the time-frequency dynamics of cortical activity. NeuroImage. 2008;40:1686–1700. doi: 10.1016/j.neuroimage.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal SS, Hamamé CM, Eichenlaub J-B, Jerbi K. Intrinsic coupling between gamma oscillations, neuronal discharges, and slow cortical oscillations during human slow-wave sleep. J Neurosci. 2010 Oct;30(43):14285–7. doi: 10.1523/JNEUROSCI.4275-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange FP, Jensen O, Bauer M, Toni I. Interactions between posterior gamma and frontal alpha/beta oscillations during imagined actions. Frontiers in Human Neuroscience. 2008 Jan;2:7. doi: 10.3389/neuro.09.007.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwakar M, Huang M-X, Srinivasan R, Harrington DL, Robb A, Angeles A, Muzzatti L, Pakdaman R, Song T, Theilmann RJ, Lee RR. Dual-core beamformer for obtaining highly correlated neuronal networks in MEG. NeuroImage. 2011 Jan;54(1):253–63. doi: 10.1016/j.neuroimage.2010.07.023. [DOI] [PubMed] [Google Scholar]
- Dockstader C, Cheyne D, Tannock R. Cortical dynamics of selective attention to somatosensory events. NeuroImage. 2010 Jan;49(2):1777–85. doi: 10.1016/j.neuroimage.2009.09.035. [DOI] [PubMed] [Google Scholar]
- Dümpelmann M, Fell J, Wellmer J, Urbach H, Elger CE. 3D source localization derived from sub-dural strip and grid electrodes: a simulation study. Clin Neurophysiol. 2009 Jun;120(6):1061–9. doi: 10.1016/j.clinph.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Edwards E, Soltani M, Kim W, Dalal SS, Nagarajan SS, Berger MS, Knight RT. Comparison of time-frequency responses and the event-related potential to auditory speech stimuli in human cortex. J Neurophysiol. 2009 Jul;102(1):377–86. doi: 10.1152/jn.90954.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Caplan JB, Ho E, Shattuck K, Fried I, Kahana MJ. Human hippocampal theta activity during virtual navigation. Hippocampus. 2005;15(7):881–9. doi: 10.1002/hipo.20109. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P. Beta-band oscillations– signalling the status quo? Current Opinion in Neurobiology. 2010 Apr;20(2):156–65. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci. 2009;32:209–224. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Juhász C, Hoechstetter K, Sood S, Asano E. Somatosensory-related gamma-, beta-and alpha-augmentation precedes alpha- and beta-attenuation in humans. Clin Neurophysiol. 2010 Mar;121(3):366–75. doi: 10.1016/j.clinph.2009.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaona CM, Sharma M, Freudenburg ZV, Breshears JD, Bundy DT, Roland J, Barbour DL, Schalk G, Leuthardt EC. Nonuniform high-gamma (60–500 Hz) power changes dissociate cognitive task and anatomy in human cortex. J Neurosci. 2011 Feb;31(6):2091–2100. doi: 10.1523/JNEUROSCI.4722-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncharova II, McFarland DJ, Vaughan TM, Wolpaw JR. EMG contamination of EEG: spectral and topographical characteristics. Clin Neurophysiol. 2003 Sep;114(9):1580–93. doi: 10.1016/s1388-2457(03)00093-2. [DOI] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hämäläinen M, Timmermann L, Schnitzler A, Salmelin R. Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proc Natl Acad Sci USA. 2001 Jan;98(2):694–9. doi: 10.1073/pnas.98.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R, Chen R, Ashby P, Lozano AM, Hutchison WD, Davis KD, Dostrovsky JO. Very fast oscillations evoked by median nerve stimulation in the human thalamus and subthalamic nucleus. J Neurophysiol. 2004 Dec;92(6):3171–82. doi: 10.1152/jn.00363.2004. [DOI] [PubMed] [Google Scholar]
- Hayden BY, Smith DV, Platt ML. Electrophysiological correlates of default-mode processing in macaque posterior cingulate cortex. Proc Natl Acad Sci U S A. 2009 Apr;106(14):5948–53. doi: 10.1073/pnas.0812035106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Zempel JM, Smyth MD, Raichle ME. Electrophysiological correlates of the brain’s intrinsic large-scale functional architecture. Proc Natl Acad Sci USA. 2008 Oct;105(41):16039–44. doi: 10.1073/pnas.0807010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschmann J, Ozkurt TE, Butz M, Homburger M, Elben S, Hartmann CJ, Vesper J, Wojtecki L, Schnitzler A. Distinct oscillatory STN-cortical loops revealed by simultaneous MEG and local field potential recordings in patients with Parkinson’s disease. NeuroImage. 2010 Nov; doi: 10.1016/j.neuroimage.2010.11.063. [DOI] [PubMed] [Google Scholar]
- Hoogenboom N, Schoffelen J-M, Oostenveld R, Parkes LM, Fries P. Localizing human visual gamma-band activity in frequency, time and space. NeuroImage. 2006;29:764–773. doi: 10.1016/j.neuroimage.2005.08.043. [DOI] [PubMed] [Google Scholar]
- Jacobs J. Measuring cortical activity – we will only detect what we are looking for. Clin Neurophysiol. 2010 Mar;121(3):268–9. doi: 10.1016/j.clinph.2009.11.014. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Manning JR, Kahana MJ. “broadband” vs. “high gamma” electrocorticographic signals. J Neurosci. 2010a. http://www.jneurosci.org/cgi/data/30/19/6477/DC1/1.
- Jacobs J, Zijlmans M, Zelmann R, Chatillon C-E, Hall J, Olivier A, Dubeau F, Gotman J. High-frequency electroencephalographic oscillations correlate with outcome of epilepsy surgery. Ann Neurol. 2010b Feb;67(2):209–20. doi: 10.1002/ana.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper H, Penfield W. Electrocorticograms in man: effect of voluntary movement upon the electrical activity of the precentral gyrus. Archive für Psychiatrie und Zeitsehrift Neurologie. 1949 Apr;183(1):163–174. [Google Scholar]
- Jasper HH, Andrews HL. Electroencephalography: III. Normal differentiation of occipital and precentral regions in man. Archives of Neurology & Psychiatry. 1938 Jan;39(1):96. [Google Scholar]
- Jasper HH, Carmichael L. Electrical potentials from the intact human brain. Science. 1935 Jan;81(2089):51–3. doi: 10.1126/science.81.2089.51. [DOI] [PubMed] [Google Scholar]
- Jensen O, Colgin LL. Cross-frequency coupling between neuronal oscillations. Trends Cogn Sci (Regul Ed) 2007 Jul;11(7):267–9. doi: 10.1016/j.tics.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Jerbi K, Bertrand O. Cross-frequency coupling in parieto-frontal oscillatory networks during motor imagery revealed by magnetoencephalography. Front Neurosci. 2009 May;3(1):3–4. doi: 10.3389/neuro.01.011.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerbi K, Freyermuth S, Dalal S, Kahane P, Bertrand O, Berthoz A, Lachaux J-P. Saccade related gamma-band activity in intracerebral EEG: dissociating neural from ocular muscle activity: dissociating neural from ocular muscle activity. Brain Topogr. 2009a Jun;22(1):18–23. doi: 10.1007/s10548-009-0078-5. [DOI] [PubMed] [Google Scholar]
- Jerbi K, Lachaux J-P, N’Diaye K, Pantazis D, Leahy RM, Garnero L, Baillet S. Coherent neural representation of hand speed in humans revealed by meg imaging. Proc Natl Acad Sci USA. 2007 May;104(18):7676–81. doi: 10.1073/pnas.0609632104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerbi K, Ossandón T, Hamamé CM, Senova S, Dalal SS, Jung J, Minotti L, Bertrand O, Berthoz A, Kahane P, Lachaux J-P. Task-related gamma-band dynamics from an intracerebral perspective: review and implications for surface EEG and MEG. Human brain mapping. 2009b Jun;30(6):1758–71. doi: 10.1002/hbm.20750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerbi K, Vidal JR, Ossandón T, Dalal SS, Jung J, Hoffmann D, Minotti L, Bertrand O, Kahane P, Lachaux J-P. Exploring the electrophysiological correlates of the default-mode network with intracerebral EEG. Frontiers in Systems Neuroscience. 2010 Jan;4:27. doi: 10.3389/fnsys.2010.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkiewicz MT, Gaetz WC, Bostan AC, Cheyne D. Post-movement beta rebound is generated in motor cortex: Evidence from neuromagnetic recordings. NeuroImage. 2006;32:1281–1289. doi: 10.1016/j.neuroimage.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Keren AS, Yuval-Greenberg S, Deouell LY. Saccadic spike potentials in gamma-band EEG: characterization, detection and suppression. NeuroImage. 2010 Feb;49(3):2248–63. doi: 10.1016/j.neuroimage.2009.10.057. [DOI] [PubMed] [Google Scholar]
- Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci USA. 2000 Feb;97(4):1867–72. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach CK, Tsuchiya N, Kawasaki H, Oya H, Howard MA, Adolphs R. Manifestation of ocular-muscle emg contamination in human intracranial recordings. NeuroImage. 2011 Jan;54(1):213–33. doi: 10.1016/j.neuroimage.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaux J-P, Fonlupt P, Kahane P, Minotti L, Hoffmann D, Bertrand O, Baciu M. Relationship between task-related gamma oscillations and BOLD signal: New insights from combined fMRI and intracranial EEG. Hum Brain Mapp. 2007a;28:1368–1375. doi: 10.1002/hbm.20352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaux J-P, Jerbi K, Bertrand O, Minotti L, Hoffmann D, Schoendorff B, Kahane P. A blueprint for real-time functional mapping via human intracranial recordings. PLoS One. 2007b;2:e1094. doi: 10.1371/journal.pone.0001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaux JP, Jung J, Mainy N, Dreher JC, Bertrand O, Baciu M, Minotti L, Hoffmann D, Kahane P. Silence is golden: Transient neural deactivation in the prefrontal cortex during attentive reading. Cereb Cortex. 2008;18:443–450. doi: 10.1093/cercor/bhm085. [DOI] [PubMed] [Google Scholar]
- Laufs H, Kleinschmidt A, Beyerle A, Eger E, Salek-Haddadi A, Preibisch C, Krakow K. EEG-correlated fMRI of human alpha activity. NeuroImage. 2003 Aug;19(4):1463–76. doi: 10.1016/s1053-8119(03)00286-6. [DOI] [PubMed] [Google Scholar]
- Le Van Quyen M, Adam C, Lachaux JP, Martinerie J, Baulac M, Renault B, Varela FJ. Temporal patterns in human epileptic activity are modulated by perceptual discriminations. NeuroReport. 1997 May;8(7):1703–10. doi: 10.1097/00001756-199705060-00028. [DOI] [PubMed] [Google Scholar]
- Le Van Quyen M, Staba R, Bragin A, Dickson C, Valderrama M, Fried I, Engel J. Large-scale microelectrode recordings of high-frequency gamma oscillations in human cortex during sleep. J Neurosci. 2010;30:7770–7782. doi: 10.1523/JNEUROSCI.5049-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuthardt EC, Schalk G, Wolpaw JR, Ojemann JG, Moran DW. A brain-computer interface using electrocorticographic signals in humans. J Neural Eng. 2004;1:63–71. doi: 10.1088/1741-2560/1/2/001. [DOI] [PubMed] [Google Scholar]
- Lima B, Singer W, Chen N-H, Neuenschwander S. Synchronization dynamics in response to plaid stimuli in monkey V1. Cereb Cortex. 2010 Jul;20(7):1556–73. doi: 10.1093/cercor/bhp218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak V, Eusebio A, Jha A, Oostenveld R, Barnes GR, Penny WD, Zrinzo L, Hariz MI, Limousin P, Friston KJ, Brown P. Optimized beam-forming for simultaneous MEG and intracranial local field potential recordings in deep brain stimulation patients. NeuroImage. 2010 May;50(4):1578–88. doi: 10.1016/j.neuroimage.2009.12.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Mäki H, Ilmoniemi RJ. Projecting out muscle artifacts from TMS-evoked EEG. NeuroImage. 2011 Feb;54(4):2706–10. doi: 10.1016/j.neuroimage.2010.11.041. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Zanos S, Fetz EE, den Nijs M, Ojemann JG. Decoupling the cortical power spectrum reveals real-time representation of individual finger movements in humans. J Neurosci. 2009 Mar;29(10):3132–7. doi: 10.1523/JNEUROSCI.5506-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monto S, Palva S, Voipio J, Palva JM. Very slow EEG fluctuations predict the dynamics of stimulus detection and oscillation amplitudes in humans. J Neurosci. 2008 Aug;28(33):8268–72. doi: 10.1523/JNEUROSCI.1910-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosmann M, Ritter P, Krastel I, Brink A, Thees S, Blankenburg F, Taskin B, Obrig H, Villringer A. Correlates of alpha rhythm in functional magnetic resonance imaging and near infrared spectroscopy. NeuroImage. 2003 Sep;20(1):145–58. doi: 10.1016/s1053-8119(03)00344-6. [DOI] [PubMed] [Google Scholar]
- Mukamel R, Gelbard H, Arieli A, Hasson U, Fried I, Malach R. Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science. 2005;309:951–954. doi: 10.1126/science.1110913. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD. Functional properties of human primary motor cortex gamma oscillations. J Neurophysiol. 2010 Nov;104(5):2873–85. doi: 10.1152/jn.00607.2010. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Edden RAE, Jones DK, Swettenham JB, Singh KD. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Natl Acad Sci USA. 2009 May;106(20):8356–61. doi: 10.1073/pnas.0900728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Johnson BW. Primary motor cortex activation during action observation revealed by wavelet analysis of the EEG. Clin Neurophysiol. 2004 Aug;115(8):1760–6. doi: 10.1016/j.clinph.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RAW. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. 2005;309:948–951. doi: 10.1126/science.1110948. [DOI] [PubMed] [Google Scholar]
- Nir Y, Fisch L, Mukamel R, Gelbard-Sagiv H, Arieli A, Fried I, Malach R. Coupling between neuronal firing rate, gamma LFP, and BOLD fMRI is related to interneuronal correlations. Curr Biol. 2007 Aug;17(15):1275–85. doi: 10.1016/j.cub.2007.06.066. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R. Electric Fields of the Brain: The Neurophysics of EEG. 2. New York: Oxford University Press; 2006. [Google Scholar]
- Nunez PL, Srinivasan R. Scale and frequency chauvinism in brain dynamics: too much emphasis on gamma band oscillations. Brain Struct Funct. 2010 Oct;215:67–71. doi: 10.1007/s00429-010-0277-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara S, Ikeda A, Kunieda T, Yazawa S, Baba K, Nagamine T, Taki W, Hashimoto N, Mihara T, Shibasaki H. Movement-related change of electrocorticographic activity in human supplementary motor area proper. Brain. 2000;123:1203–1215. doi: 10.1093/brain/123.6.1203. [DOI] [PubMed] [Google Scholar]
- Oostendorp TF, Delbeke J, Stegeman DF. The conductivity of the human skull: Results of in vivo and in vitro measurements. IEEE Trans Biomed Eng. 2000 Nov;47(11):1487–92. doi: 10.1109/TBME.2000.880100. [DOI] [PubMed] [Google Scholar]
- Osipova D, Hermes D, Jensen O. Gamma power is phase-locked to posterior alpha activity. PLoS ONE. 2008 Jan;3(12):e3990. doi: 10.1371/journal.pone.0003990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossandón T, Jerbi K, Bayle D, Bertrand O, Kahane P, Lachaux J-P. Soc Neurosci Abstr (Program No 804.10) SfN 2009. Chicago, IL: 2009. Task-related gamma band suppressions: a plausible electrophysiological correlate of the default-mode network? [Google Scholar]
- Parkes LM, Bastiaansen MCM, Norris DG. Combining EEG and fMRI to investigate the post-movement beta rebound. NeuroImage. 2006 Feb;29(3):685–96. doi: 10.1016/j.neuroimage.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Cooper R. Frequency dependence of the transmission of the EEG from cortex to scalp. Electroencephalogr Clin Neurophysiol. 1975;38:93–96. doi: 10.1016/0013-4694(75)90215-1. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Krausz G, Neuper C. Mechanical stimulation of the fingertip can induce bursts of beta oscillations in sensorimotor areas. J Clin Neurophysiol. 2001 Nov;18(6):559–64. doi: 10.1097/00004691-200111000-00006. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C, Brunner C, da Silva FL. Beta rebound after different types of motor imagery in man. Neurosci Lett. 2005 Apr;378(3):156–9. doi: 10.1016/j.neulet.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Pope KJ, Fitzgibbon SP, Lewis TW, Whitham EM, Willoughby JO. Relation of gamma oscillations in scalp recordings to muscular activity. Brain topography. 2009 Jun;22(1):13–7. doi: 10.1007/s10548-009-0081-x. [DOI] [PubMed] [Google Scholar]
- Rampp S, Kaltenhäuser M, Weigel D, Buchfelder M, Blümcke II, Dörfler A, Stefan H. MEG correlates of epileptic high gamma oscillations in invasive EEG. Epilepsia. 2010 Aug;51(8):1638–42. doi: 10.1111/j.1528-1167.2010.02579.x. [DOI] [PubMed] [Google Scholar]
- Ray S, Maunsell JHR. Differences in gamma frequencies across visual cortex restrict their possible use in computation. Neuron. 2010 Sep;67(5):885–96. doi: 10.1016/j.neuron.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reva NV, Aftanas LI. The coincidence between late non-phase-locked gamma synchronization response and saccadic eye movements. Int J Psychophysiol. 2004 Feb;51(3):215–22. doi: 10.1016/j.ijpsycho.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Roland J, Brunner P, Johnston J, Schalk G, Leuthardt EC. Passive real-time identification of speech and motor cortex during an awake craniotomy. Epilepsy Behav. 2010 May;18(1–2):123–8. doi: 10.1016/j.yebeh.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Hämäläinen M, Kajola M, Hari R. Functional segregation of movement-related rhythmic activity in the human brain. NeuroImage. 1995 Dec;2(4):237–43. doi: 10.1006/nimg.1995.1031. [DOI] [PubMed] [Google Scholar]
- Sarnthein J, Morel A, Stein AV, Jeanmonod D. Thalamic theta field potentials and EEG: high thalamocortical coherence in patients with neurogenic pain, epilepsy and movement disorders. Thalamus Rel Sys. 2003;2(03):231–238. [Google Scholar]
- Scarff J, Rahm W. The human electrocorticogram: A report of spontaneous electrical potentials obtained from the exposed human brain. J Neurophysiol. 1941;4(5):418. [Google Scholar]
- Sekihara K, Nagarajan SS, Poeppel D, Marantz A. Asymptotic SNR of scalar and vector minimum-variance beamformers for neuromagnetic source reconstruction. IEEE Trans Biomed Eng. 2004;51:1726–1734. doi: 10.1109/TBME.2004.827926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B, Nadkarni M, Zappulla RA. Spectral modulation of cortical connections measured by EEG coherence in humans. Clin Neurophysiol. 1999 Jan;110(1):115–25. doi: 10.1016/s0013-4694(98)00104-7. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci. 2006 Apr;9(4):569–77. doi: 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]
- Singer W. Synchronization of cortical activity and its putative role in information processing and learning. Annu Rev Physiol. 1993 Jan;55:349–74. doi: 10.1146/annurev.ph.55.030193.002025. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Russell DP, Edelman GM, Tononi G. Increased synchronization of neuromagnetic responses during conscious perception. J Neurosci. 1999 Jul;19(13):5435–48. doi: 10.1523/JNEUROSCI.19-13-05435.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann N, Tandon N, Canolty R, Ellmore TM, McEvoy LK, Dreyer S, Disano M, Aron AR. Intracranial EEG reveals a time- and frequency-specific role for the right inferior frontal gyrus and primary motor cortex in stopping initiated responses. J Neurosci. 2009 Oct;29(40):12675–85. doi: 10.1523/JNEUROSCI.3359-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Fischer C. Oscillatory synchrony between human extrastriate areas during visual short-term memory maintenance. J Neurosci. 2001;21:RC177, 1–5. doi: 10.1523/JNEUROSCI.21-20-j0008.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao JX, Baldwin M, Hawes-Ebersole S, Ebersole JS. Cortical substrates of scalp EEG epileptiform discharges. J Clin Neurophysiol. 2007 Apr;24(2):96–100. doi: 10.1097/WNP.0b013e31803ecdaf. [DOI] [PubMed] [Google Scholar]
- Tönnies J. Die unipolare ableitung elektrischer spannungen vom menschlichen gehirn. Naturwissenschaften. 1934;22:411–414. [Google Scholar]
- Toro C, Deuschl G, Thatcher R, Sato S, Kufta C, Hallett M. Event-related desynchronization and movement-related cortical potentials on the ECoG and EEG. Electroencephalogr Clin Neurophysiol. 1994 Oct;93(5):380–9. doi: 10.1016/0168-5597(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Towle VL, Cohen S, Alperin N, Hoffmann K, Cogen P, Milton J, Grzesczcuk R, Pelizzari C, Syed I, Spire JP. Displaying electrocorticographic findings on gyral anatomy. Electroencephalogr Clin Neurophysiol. 1995 Apr;94(4):221–8. doi: 10.1016/0013-4694(95)98474-m. [DOI] [PubMed] [Google Scholar]
- Towle VL, Yoon H-A, Castelle M, Edgar JC, Biassou NM, Frim DM, Spire J-P, Kohrman MH. ECoG gamma activity during a language task: differentiating expressive and receptive speech areas. Brain. 2008 Aug;131:2013–27. doi: 10.1093/brain/awn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay C, Robert M, Pascual-Leone A, Lepore F, Nguyen DK, Carmant L, Bouthillier A, Théoret H. Action observation and execution: intracranial recordings in a human subject. Neurology. 2004 Sep;63(5):937–8. doi: 10.1212/01.wnl.0000137111.16767.c6. [DOI] [PubMed] [Google Scholar]
- Trujillo LT, Peterson MA, Kaszniak AW, Allen JJB. EEG phase synchrony differences across visual perception conditions may depend on recording and analysis methods. Clin Neurophysiol. 2005 Jan;116(1):172–89. doi: 10.1016/j.clinph.2004.07.025. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Raz J, Fein G. Noise and signal power and their effects on evoked potential estimation. Electroencephalogr Clin Neurophysiol. 1988 Jan;71(4):310–8. doi: 10.1016/0168-5597(88)90032-9. [DOI] [PubMed] [Google Scholar]
- Väisänen O, Malmivuo J. Improving the SNR of EEG generated by deep sources with weighted multielectrode leads. J Physiol Paris. 2009 Oct;103(6):306–14. doi: 10.1016/j.jphysparis.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Vidal JR, Chaumon M, O’Regan JK, Tallon-Baudry C. Visual grouping and the focusing of attention induce gamma-band oscillations at different frequencies in human magnetoencephalogram signals. J Cogn Neurosci. 2006;18:1850–1862. doi: 10.1162/jocn.2006.18.11.1850. [DOI] [PubMed] [Google Scholar]
- Vidal JR, Ossandón T, Jerbi K, Dalal SS, Minotti L, Ryvlin P, Kahane P, Lachaux J-P. Category-specific visual responses: an intracranial study comparing gamma, beta, alpha, and ERP response selectivity. Front Hum Neurosci. 2010 Nov;4:195. doi: 10.3389/fnhum.2010.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek B, Secundo L, Bidet-Caulet A, Scabini D, Stiver SI, Gean AD, Manley GT, Knight RT. Hemicraniectomy: a new model for human electro-physiology with high spatio-temporal resolution. J Cogn Neurosci. 2010 Nov;22(11):2491–502. doi: 10.1162/jocn.2009.21384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward DM, Jones RD, Bones PJ, Carroll GJ. Enhancement of deep epileptiform activity in the EEG via 3-D adaptive spatial filtering. IEEE Trans Biomed Eng. 1999 Jun;46(6):707–16. doi: 10.1109/10.764947. [DOI] [PubMed] [Google Scholar]
- Whitham EM, Lewis T, Pope KJ, Fitzgibbon SP, Clark CR, Loveless S, Delosangeles D, Wallace AK, Broberg M, Willoughby JO. Thinking activates EMG in scalp electrical recordings. Clin Neurophysiol. 2008;119:1166–1175. doi: 10.1016/j.clinph.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Whitham EM, Pope KJ, Fitzgibbon SP, Lewis T, Clark CR, Loveless S, Broberg M, Wallace A, De-LosAngeles D, Lillie P, Hardy A, Fronsko R, Pulbrook A, Willoughby JO. Scalp electrical recording during paralysis: quantitative evidence that EEG frequencies above 20 Hz are contaminated by EMG. Clin Neurophysiol. 2007 Aug;118(8):1877–88. doi: 10.1016/j.clinph.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Wu JY, Koh S, Sankar R, Mathern GW. Paroxysmal fast activity: an interictal scalp EEG marker of epileptogenesis in children. Epilepsy Res. 2008 Nov;82(1):99–106. doi: 10.1016/j.eplepsyres.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Wisneski K, Schalk G, Sharma M, Roland J, Breshears J, Gaona C, Leuthardt EC. Electrocorticographic frequency alteration mapping for extraoperative localization of speech cortex. Neurosurgery. 2010 Jan;66(2):E407–9. doi: 10.1227/01.NEU.0000345352.13696.6F. [DOI] [PubMed] [Google Scholar]
- Wyart V, Tallon-Baudry C. Neural dissociation between visual awareness and spatial attention. J Neurosci. 2008 Mar;28(10):2667–79. doi: 10.1523/JNEUROSCI.4748-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuval-Greenberg S, Tomer O, Keren AS, Nelken I, Deouell LY. Transient induced gamma-band response in EEG as a manifestation of miniature saccades. Neuron. 2008;58:429–441. doi: 10.1016/j.neuron.2008.03.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.