Abstract
Regulatory mechanisms of chondrocyte differentiation in the growth plate are incompletely understood. Here, we find that histone deacetylase 4 (HDAC4) is located in the nucleus of chondrocytes in the proliferation zone and relocates to the cytoplasm of chondrocytes in the prehypertrophic zone in vivo. This suggests that the relocation of HDAC4 from the nucleus to the cytoplasm may play a role during chondrocyte differentiation. Expression of active CaMKIV in chondrocytes promotes HDAC4 relocation into cytoplasm in primary chondrocytes. Conversely, HDAC4 relocation is blocked by a Ca2+/calmodulin-dependent kinase IV (CaMKIV) inhibitor. This indicates that CaMKIV signaling plays an important role in regulating HDAC4 relocation. In addition, CaMKIV is required for HDAC4 phosphorylation, which is required for HDAC4 association with the cytoplasmic protein 14-3-3. Active CaMKIV also stimulates runt-related transcription factor-2 (RunX2) and type X collagen (Col X) promoter activities and overcomes repression of these promoter activities by HDAC4. Furthermore, CaMKIV increases gene expression of the chondrocyte differentiation markers Ihh and Col X. Our results demonstrate that CaMKIV induces chondrocyte differentiation through regulation of HDAC4 subcellular relocation, from the nucleus to the cytoplasm, which results in increased activity of RunX2 and transition of chondrocytes from the proliferative to the prehypertrophic stage. Thus, CaMKIV plays an important regulatory role during chondrocyte differentiation.
Keywords: runt-related transcription factor-2
accumulating evidence indicates that active transcriptional repression by epigenetic regulation is an important component of many physiological events regulated at the level of gene expression. The repression of transcription is manifest at the level of chromatin structure where histone deacetylases (HDACs) are recruited to remove the acetyl groups from histones and create a repressive chromatin structure (3). In mammalian cells, two major classes of HDACs have been described so far. The class I HDACs (HDAC1, 2, 3, and 8) are widely expressed and consist mainly of a catalytic domain with low specificity (11). In contrast, the class II HDACs (HDAC4, 5, 6, and 7) display cell type restricted patterns of expression and contain an NH2-terminal extension that links them to specific transcription factors. This confers responsiveness to a variety of signal transduction pathways, thereby connecting the chromatin modification of targeted genes with the extracellular environment (5, 24).
HDAC4 has been specifically implicated in modulating cell growth and differentiation (2). A recent study has shown that HDAC4 null mice display premature ossification of developing bones due to ectopic and early onset chondrocyte hypertrophy (23). This is associated with a loss of inhibition of the activity of runt-related transcription factor-2 (RunX2), a transcription factor necessary for chondrocyte hypertrophy during endochondral bone formation. However, the detailed mechanism of chondrocyte hypertrophic regulation by HDAC4 is unclear. A surprising feature of HDAC4 is its ability to shuttle between the nucleus and cytoplasm (19). This feature could be unique to the class II enzymes since class I deacetylases do not show differential localization (4). Studies have shown that HDAC4 subcellular relocation plays an important role in muscle cell differentiation and neuronal cell death (4). Whether HDAC4 can shuttle between the nucleus and cytoplasm in chondrocytes and the role that this subcellular relocation might play in chondrocytes are unknown.
Ca2+/calmodulin-dependent kinase IV (CaMKIV) is a multifunctional serine/threonine protein kinase previously shown to regulate HDAC4 activity (16, 20). CaMKIV expression is restricted to a limited set of tissues and cell types (26). Previous studies have demonstrated that the Ca2+/calmodulin signaling pathways are important in the differentiation of the cells in which it is expressed, including cerebellar neurons, germ cells, muscle, and cardiomyocytes (1, 11, 16, 22, 28).
In this study we demonstrate that HDAC4 nuclear-cytoplasmic translocation controls chondrocyte differentiation during growth plate development through a Ca2+/calmodulin-dependent signaling pathway. By immunochemistry we show that HDAC4 is located in the nucleus of cells in the proliferation zone and relocates to the cytoplasm of the cells in the prehypertrophic zone of the growth plate. Inhibition of CaMKIV in prehypertrophic chondrocytes results in HDAC4 relocation from the cytoplasm to the nucleus. Conversely, activation of CaMKIV prevents nuclear entry of HDAC4 and enhances the binding of HDAC4 to the cytoplasmic binding protein 14-3-3 in a phosphorylation-dependent manner. Lack of nuclear HDAC4 results in increased activity of RunX2 and subsequent differentiation of proliferating chondrocytes to prehypertrophic chondrocytes. Together these data identify HDAC4 relocalization as a critical regulatory step in chondrocyte differentiation, which is mediated by CaMKIV signaling.
MATERIALS AND METHODS
DNA constructs and antibodies.
Green fluorescent protein (GFP)-HDAC4, Flag-HDAC4, and HDAC4D289E expression vectors were provided by Dr. A. R. Means (6), Dr. T. Kouzarides (16), and Dr. C. Brancolini (20). RunX2 and type X collagen (Col X) luciferase reporter constructs were a generous gift from Dr. G. S. Stein and Dr. H. Drissi (8, 9). Constitutively active CaMKIV (amino acids 1–313) and inactive CaMKIV (K75E) were provided by Dr. A. R. Means (6). Phosphoserine antibody was purchased from Zymed (90-0200, Carlsbad, CA) and RunX2 antibody was purchased from CeMines (catalog no. AB/RNT20, CeMines, Golden, CO). All other antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Primary cell culture and transfection.
Prehypertrophic chondrocytes were isolated from the cephalic part of 17-day-old embryonic chick sternum and cultured in F-12 media with 10% FBS (GIBCO BRL) as previously described (7). Briefly, the cephalic part of the sterna from 17-day-old embryonic chicks was subjected to enzymatic treatment with 0.1% trypsin (Sigma, St. Louis, MO), 0.3% collagenase (Worthington, Freehold, NJ), and 0.1% type 1 testicular hyaluronidase (Sigma) (dissociation medium). After an incubation of 30 min at 37°C, the dissociation medium was removed and replaced with fresh dissociation medium and incubated at 37°C for an additional hour. Chondrocytes were resuspended in plating medium plus 0.01% testicular hyaluronidase [plating medium: 10% fetal bovine serum in Ham's F-12 medium (GIBCO, Grand Island, NY)] at 37°C in an atmosphere containing 5% CO2. After culturing overnight, the medium of the chondrocyte culture was replaced with fresh medium without hyaluronidase. Medium was changed every other day. Chondrocytes were transfected using Lipofectamine 2000 (Invitrogen) as described in the manufacturer's protocol. Transfection efficiency was confirmed by real-time RT-PCR. The study has been approved by the Rhode Island Hospital Institutional Animal Care and Use Committee.
Western blot analysis and immunoprecipitation.
Chondrocytes were transfected with expression vectors, and 48 h after transfection, cells were washed in ice-cold PBS and lysed in RIPA buffer (50 mM Tris·HCl, pH 8.0, 150 mM NaCl, 5 mM EDTA, 1% NP-40) at 4°C for 30 min. Chondrocyte cytosol and nuclear fractions were separated with the Nuclear Extract Kit (catalog no. 40010, Active Motif, Carlsbad, CA) following the manufacturer's instructions. Lysates were cleared by centrifugation, diluted five times in RIPA buffer containing 0.1% NP-40, and incubated with 1 μg of either anti-14-3-3 or anti-HDAC4 goat polyclonal antibodies for 1 h. A 20-μl aliquot of protein A/G-Sepharose beads (Pharmacia) was added, and incubation continued for 3 h with rotation at 4°C. Precipitates were washed three times in ice-cold RIPA buffer and resuspended in loading buffer for SDS-PAGE. SDS-PAGE and Western blotting were performed according to standard procedures. Anti-HDAC4 antibody (sc-5245, Santa Cruz Biotechnology) or antiphosphoserine antibody (90-0200, Zymed) or anti-histone H3 (no. 4499, Cell Signaling Technology, Danvers, MA) was used for blotting at a concentration of 0.2 μg/ml.
Tibia growth plate was isolated by dissection of 17-day-old chicken embryos. Three zones (proliferating, maturation/prehypertrophy, and hypertrophy) of the growth plate were separated under dissection microscope as described previously (30). The protein extraction was obtained from the different zones of the growth plate for the Western blotting (17).
Real-time RT-PCR.
Real-time RT-PCR was performed as previously described (27). The primers were designed using Primers Express software (BioTools, Edmonton, AB, Canada). Primers used are as follows: Col X forward primers, sense AGT GCT GTC ATT GAT CTC ATG GA, antisense TCA GAG GAA TAG AGA CCA TTG GAT T; Sox 9 forward primers, sense TCG GTG AAG AAC GGG CAG TC, antisense TCG CTG ATG CTG GAG GAT GA; Ihh forward primers, sense GGG CGC TTT GTG GGG TGA T, antisense CGC CTC TCT TTC GGT CCC AGT; and 18S forward primers, sense CGG CTA CCA CAT CCA AGG AA, antisense GCT GGA ATT ACC GCG GCT. 18S RNA was amplified at the same time for each sample and used as an internal control. The cycle threshold values for 18S RNA, Col X, Ihh, and Sox 9 were measured and calculated with Opticon software. Relative transcript levels were calculated as × = 2 − ΔΔCt, in which ΔΔCt = ΔCt E − ΔCt C, and ΔCt E = Ctexp − Ct18S, and ΔCt C = CtC − Ct18S.
Reporter gene assays.
Chondrocytes were transiently transfected with 0.5 μg of RunX2 or Col X promoter luciferase reporter plasmid using 5 μl/well of Lipofectamine 2000 CD (Invitrogen) in 12-well plates. Cotransfections with promoters were performed with expression constructs for HDAC4, active CaMKIV, or inactive CaMKIV as indicated. Cells were washed 16 h after transfection and incubated for an additional 24 h, before harvesting in 1× reporter lysis buffer. RunX2 and Col X luciferase activities were read using the dual-luciferase reporter assay and normalized to Renilla activity (Dual-Luciferase Reporter Assay, Promega, Madison, WI).
Immunofluorescent staining.
The distribution of HDAC4 and RunX2 in the growth plate was detected using HDAC4 and RunX2 antibodies, respectively, with tissue from the C57BL/6 mouse 1 day after birth by immunofluorescent staining as previously described (27). Tissues were fixed overnight in 4% buffered paraformaldehyde at 4°C, dehydrated, and embedded in paraffin. Sections (5 μm thick) were deparaffinized, hydrated in xylene and an ethanol series, and washed twice in phosphate-buffered saline. The sections were analyzed by immunostaining using a goat polyclonal antibody against HDAC4 (1:100 dilution, catalog no. sc-5245, Santa Cruz Biotechnology) or a rabbit polyclonal antibody against RunX2 (1:200) overnight at 4°C. To detect the distribution of CaMKIV in the growth plate, sections were immunostained with a goat polyclonal antibody against CaMKIV overnight at 4°C (1:50, catalog no. sc-1546, Santa Cruz Biotechnology). The secondary antibody, rhodamine-conjugated donkey-anti-goat IgG (H+L) or FITC-conjugated donkey-anti-rabbit IgG (H+L) (Jackson ImmunoResearch Laboratories, West Grove, PA) was applied at 1:500 dilution in PBS containing 1% bovine serum albumin for 1 h. Normal goat IgG (sc-2028) or normal rabbit IgG (sc-2027) was used in place of primary antibody as negative control. Slides were mounted in FluorSave TM reagent (Calbiochem-Novabiochem, La Jolla, CA), and viewed under a fluorescent microscope (Nikon microscope E 800). To determine whether the relocation of HDAC4 is controlled by CaMKIV, prehypertrophic chondrocytes were seeded into eight-well chamber slides overnight and transfected with GFP-HDAC4 alone, GFP-HDAC4 with active CaMKIV, or GFP-HDAC4 with inactive CaMKIV for 48 h. The intracellular localization of HDAC4 was then determined under a fluorescent microscope (Nikon microscope E 800).
Statistical analysis.
The data represent means ± SD obtained from at least three independent experiments. Each experimental point was performed in triplicate. The means were compared using one-way analysis of variance (Bonferroni's multiple comparison test). P < 0.05 was considered significant.
RESULTS
Distribution of HDAC4, CaMKIV, and RunX2 in the growth plate.
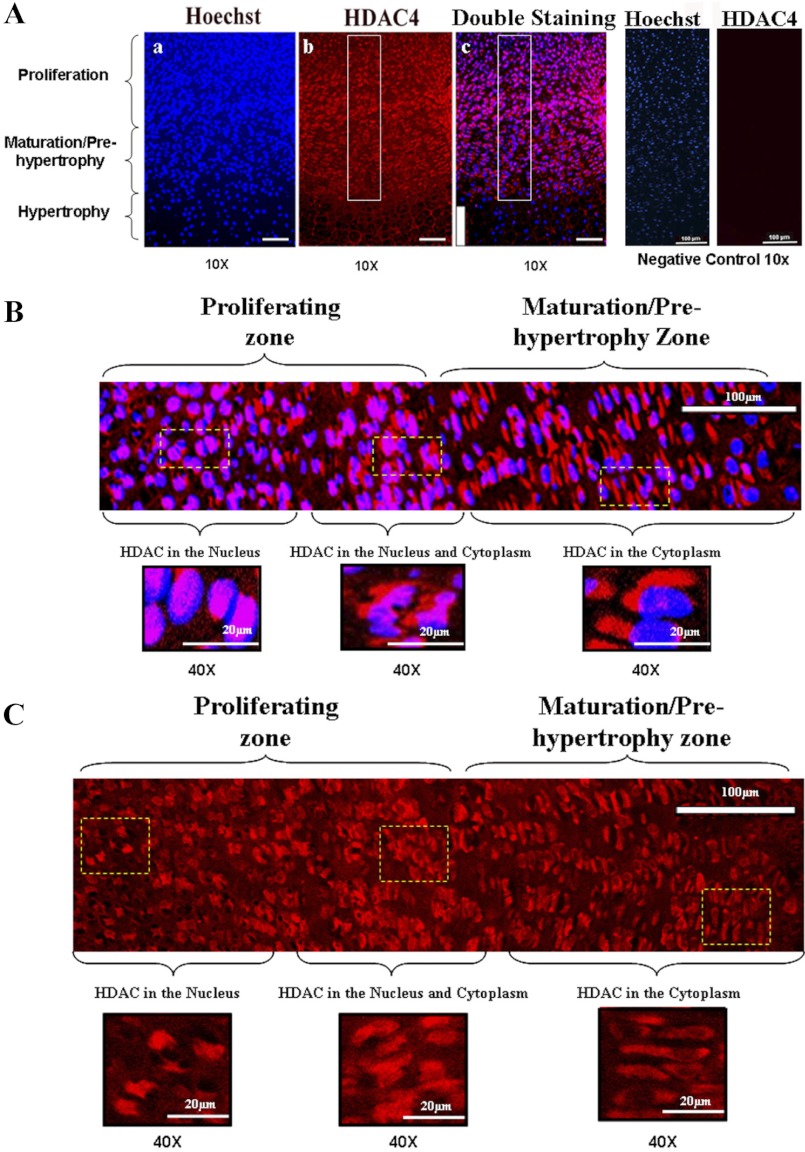
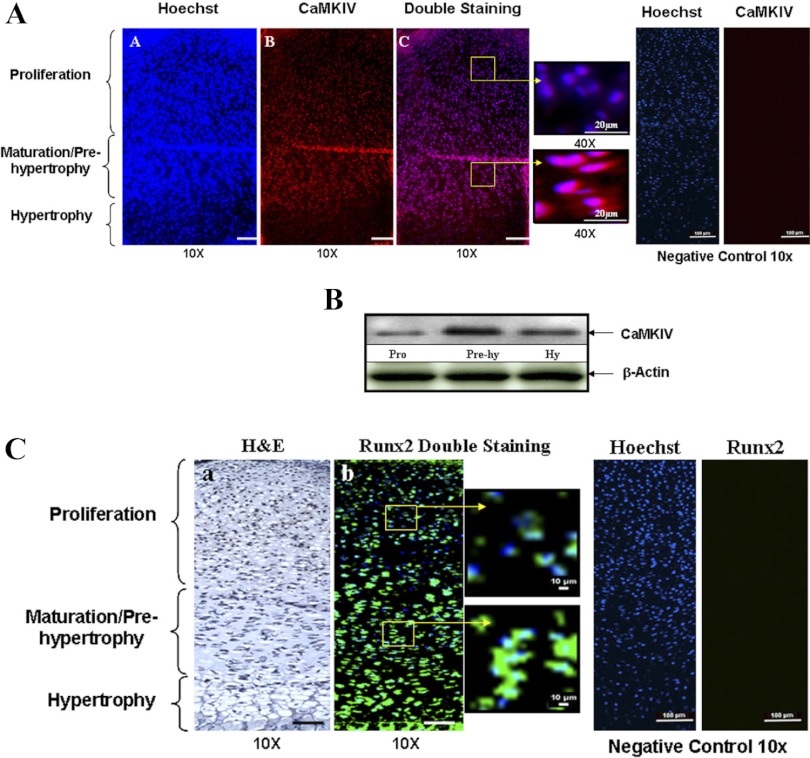
To determine the distribution of HDAC4, CaMKIV, and RunX2 in the growth plate, immunofluorescent staining was performed on mouse proximal tibia growth plate using antibodies against HDAC4, CaMKIV, and RunX2, respectively. Interestingly, we found that HDAC4 is located in the nucleus of cells in the proliferating zone and it is relocated in the cytoplasm of cells in the maturation/prehypertrophic zone (Fig. 1, A–C, red color). CaMKIV is strongly expressed in the maturation/prehypertrophic zone, while its expression is very low in the proliferation zone (Fig. 2A, red color). Western blotting results further demonstrate this distribution pattern of CaMKIV in the growth plate (Fig. 2B). Correlating with the distribution pattern of CaMKIV, RunX2 is present mainly in the prehypertrophic and hypertrophic zones, while it is weakly expressed in the proliferation zone (Fig. 2C, green color). Thus, the increased expression of CaMKIV and RunX2 during differentiation is correlated with the translocation of HDAC4 from the nucleus in the proliferating chondrocytes to the cytoplasm in the prehypertrophic chondrocytes.
Fig. 1.
The distribution of histone deacetylase 4 (HDAC4) in growth plate. Immunofluorescent histochemistry was performed with 5-μm-thick sections of the proximal tibia growth plate (from 1 day postpartum mice) using anti-HDAC4 antibody. A: in the proliferation zone, HDAC4 is located in the nucleus but it translocates gradually to the cytoplasm in the maturation/prehypertrophic zone. Normal goat IgG was used in place of HDAC4 primary antibody as negative control. No positive staining was detected for HDAC4 in the negative controls. Scale bar, 100 μm. B: the boxed region in Ac (top right) is represented at a higher magnification (×40). Scale bar, 20 μm. Blue, nucleus; red, HDAC4; purple, overlap of blue and red. C: the boxed region in Ab (top middle) is represented at a higher magnification (×40). Scale bar, 20 μm. Red, HDAC4.
Fig. 2.
The distribution of CaMKIV and runt-related transcription factor-2 (RunX2) in the growth plate. To determine the distribution of CaMKIV and RunX2 in the growth plate, immunofluorescent histochemistry was performed with 5-μm-thick sections of the proximal tibia growth plate (from 1 day postpartum mice) using anti-CaMKIV and RunX2 antibodies. A: strong staining for the CaMKIV is found in the maturation/prehypertrophic zone compared with the proliferation zone. The boxed region in Ac, left, is represented at a higher magnification in Ac, right. Scale bar, 100 μm. Blue, nucleus; red, HDAC4; purple, overlap of blue and red. Normal goat IgG was used in place of CaMKIV primary antibody as negative control. No positive staining was detected for CaMKIV in the negative controls. B: to further validate the distribution of CaMKIV, protein extracts were obtained from the different zones of the growth plate. A similar expression pattern of CaMKIV is detected by Western blotting with anti-CaMKIV antibody. Hy, hypertrophy. C: RunX2 is strongly expressed in the prehypertrophic and hypertrophic zones with a weaker staining found in the proliferation zone. The boxed region in Cb, left, is represented at a higher magnification in Cb, right. Normal rabbit IgG was used in place of RunX2 primary antibody as negative control. No positive staining was detected for RunX2 in the negative controls. Scale bar, 100 μm. Blue, nucleus; green, RunX2. H&E, hemotoxylin and eosin.
CaMKIV regulates HDAC4 cellular localization.
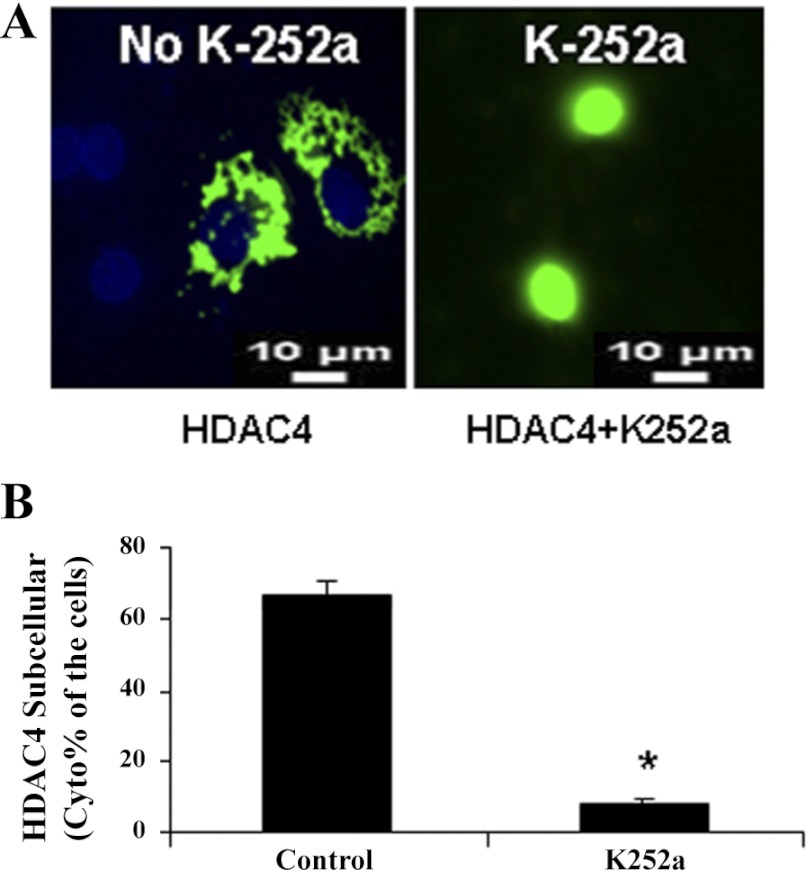
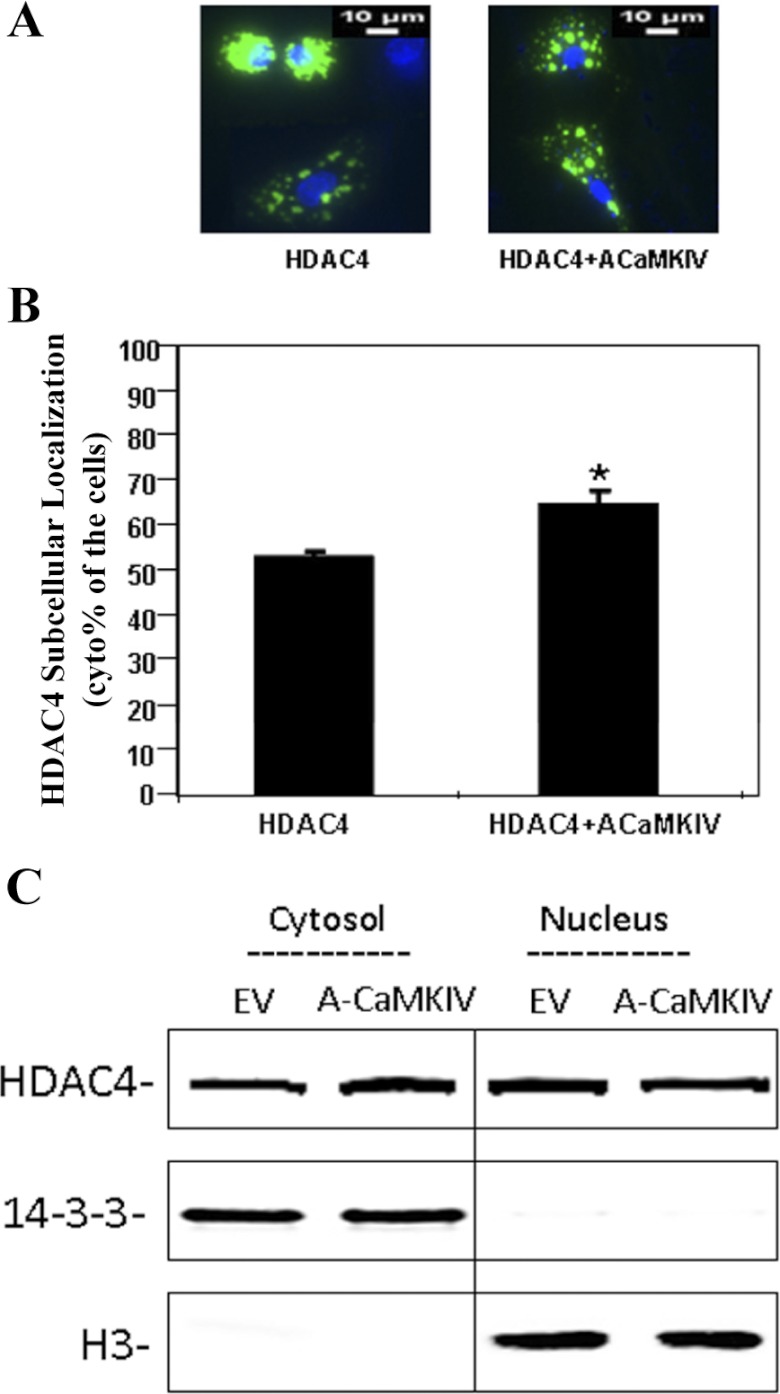
To determine whether HDAC4 translocation between the nucleus and the cytoplasm was regulated by CaMKIV during chondrocyte differentiation, we inhibited CaMKIV activities with a Ca2+/calmodulin signaling pathway inhibitor, K-252a, in primary prehypertrophic chondrocytes. Suppression of CaMKIV by K-252a caused a significant relocation of HDAC4 from the cytoplasm to the nucleus (Fig. 3, A and B). A similar result was also observed using staurosporine (20 μM), another CaMKIV inhibitor (data not shown). To perform a converse experiment, we transfected active CaMKIV into primary chondrocytes. Transfection with active CaMKIV causes an increase in the ratio of cytoplasmic to nuclear HDAC4 (Fig. 4, A and B). Transfection of inactive CaMKIV, however, causes no change in this ratio compared with HDAC4 transfection alone (data not shown). To verify the translocation of HDAC4 to the cytoplasm, immunoprecipitation was performed with an antibody against HDAC4. The level of HDAC4 is increased in the cytoplasmic fraction of chondrocytes in response to the transfection of active CaMKIV (Fig. 4C). In contrast, the transfection of inactive CaMKIV does not cause any change in the levels of cytoplasmic HDAC4 in chondrocytes. Furthermore, the level of HDAC4 is decreased in the nuclear fraction of chondrocytes in response to the transfection of active CaMKIV (Fig. 4C).
Fig. 3.
CaMKIV inhibition by K-252a results in relocation of HDAC4 from the cytoplasm to the nucleus. A: green fluorescent protein (GFP)-HDAC4 was transfected into chicken prehypertrophic chondrocytes. Forty-eight hours later, chondrocytes were treated with K-252a (1 μM), an inhibitor of the Ca2+/calmodulin signaling pathway, for 1 h. B: the percentage of cells that only have cytoplasmic localization of HDAC4 was detected using fluorescence microscopy. Approximately 300 cells from 3 independent experiments were scored. Nuclei were visualized by DAPI staining. The quantitative analysis is shown below the representative images. Data are expressed as means ± SD. *Statistically significant difference (P < 0.01).
Fig. 4.
HDAC4 nuclear-cytoplasmic shuttling is dependent on the Ca2+/calmodulin signaling pathway. Chondrocytes were transfected with HDAC4 or cotransfected with HDAC4 and active CaMKIV. Forty-eight hours later, the cells were stained with Hoechst dye for 10 min. Nuclei were defined with Hoechst staining. The location of HDAC4 was detected by fluorescence microscopy. A: active (A)CaMKIV increases subcellular location of HDAC4 from the nucleus to the cytoplasm. B: percentage of cytoplasmic staining is shown. Approximately 300 cells from 3 independent experiments were scored. Data are expressed as means ± SD (*P < 0.01). C: after 48 h of transfection, cytoplasmic and nuclear lysates from the cells were immunoprecipitated with anti-HDAC4 antibody followed by Western blot analysis with HDAC4 antibody. 14-3-3 and histone 3 are shown as a loading control for the cytoplasmic and nuclear fraction, respectively. EV, empty vector.
HDAC4 is phosphorylated by CaMKIV and associates with 14-3-3 protein in a phosphorylation-dependent manner.
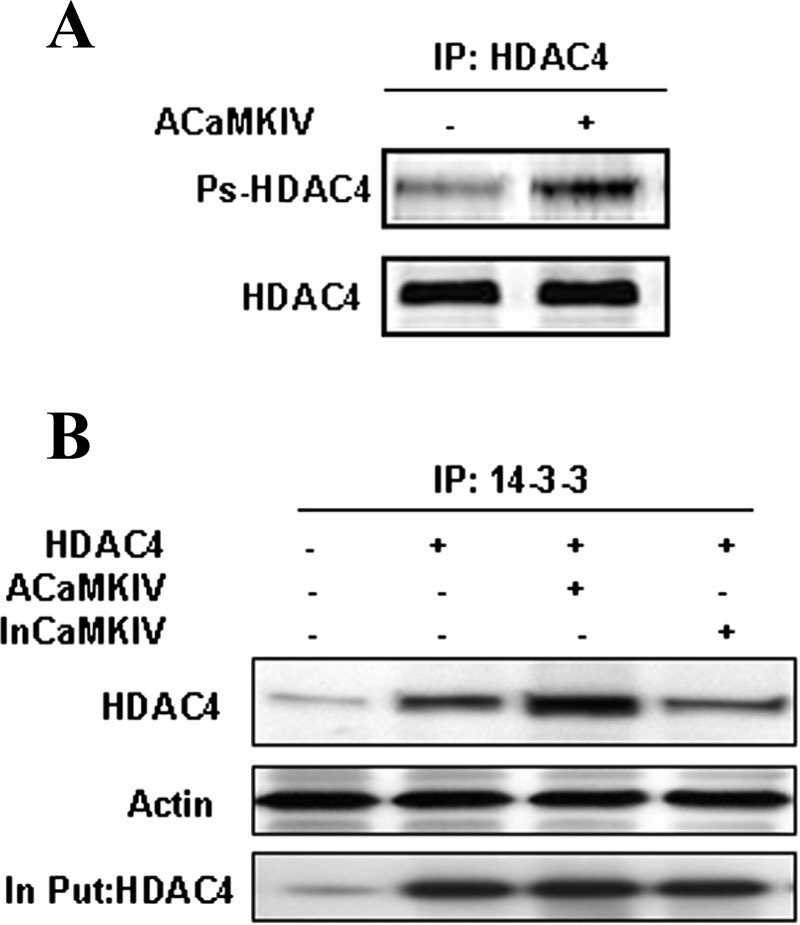
To determine whether CaMKIV expression triggers HDAC4 phosphorylation in chondrocytes, we performed Western blot analysis with antiphosphoserine antibody after immunoprecipitating HDAC4 from the cells transfected with active CaMKIV. Transfection of active CaMKIV increases the level of phosphorylated HDAC4 in chondrocytes (Fig. 5A).
Fig. 5.
HDAC4 interacts with 14-3-3 proteins in a phosphorylation-dependent manner. A: CaMKIV phosphorylated HDAC4 in chondrocytes. Chicken primary chondrocytes were cotransfected with HDAC4 and active CaMKIV. Forty-eight hours after transfection, whole cell lysates were collected and immunoprecipitated (IP) with HDAC4 antibody and followed by Western blot analysis with antiphosphoserine antibody and HDAC4 antibody. B: 14-3-3 interacts with HDAC4. Chondrocytes were transfected with HDAC4 and active CaMKIV or inactive (in)CaMKIV for 48 h. Anti-14-3-3 immunoprecipitates were analyzed with antibody to HDAC4. Equal input amounts used for immunoprecipitation are shown by blotting with actin and HDAC4 antibody.
To determine whether phosphorylated HDAC4 binds to a cytoplasmic protein 14-3-3, thereby increasing its level in the cytoplasm, we extracted cytoplasmic fractions from chondrocytes transfected with active or inactive CaMKIV. 14-3-3 immunoprecipitates were analyzed by Western blotting using the HDAC4 antibody (Fig. 5B). The cytoplasmic level of HDAC4 associated with 14-3-3 protein is increased in chondrocytes transfected with active CaMKIV, but not in those transfected with inactive CaMKIV.
CaMKIV overcomes HDAC4 repression of RunX2 and Col X promoters and induces chondrocyte differentiation.
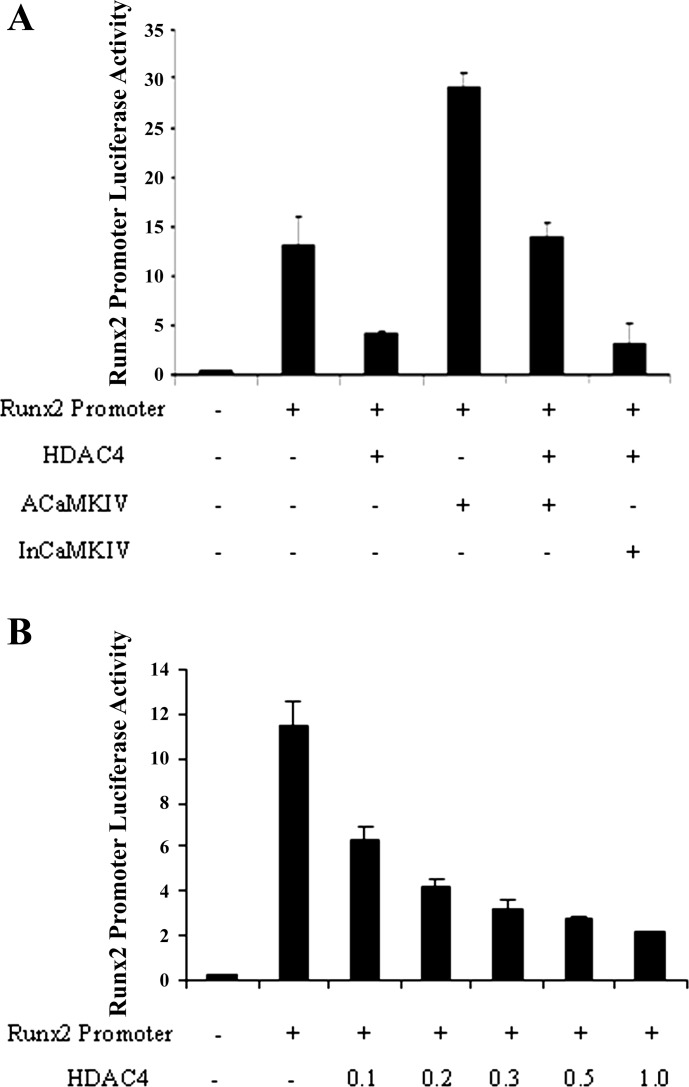
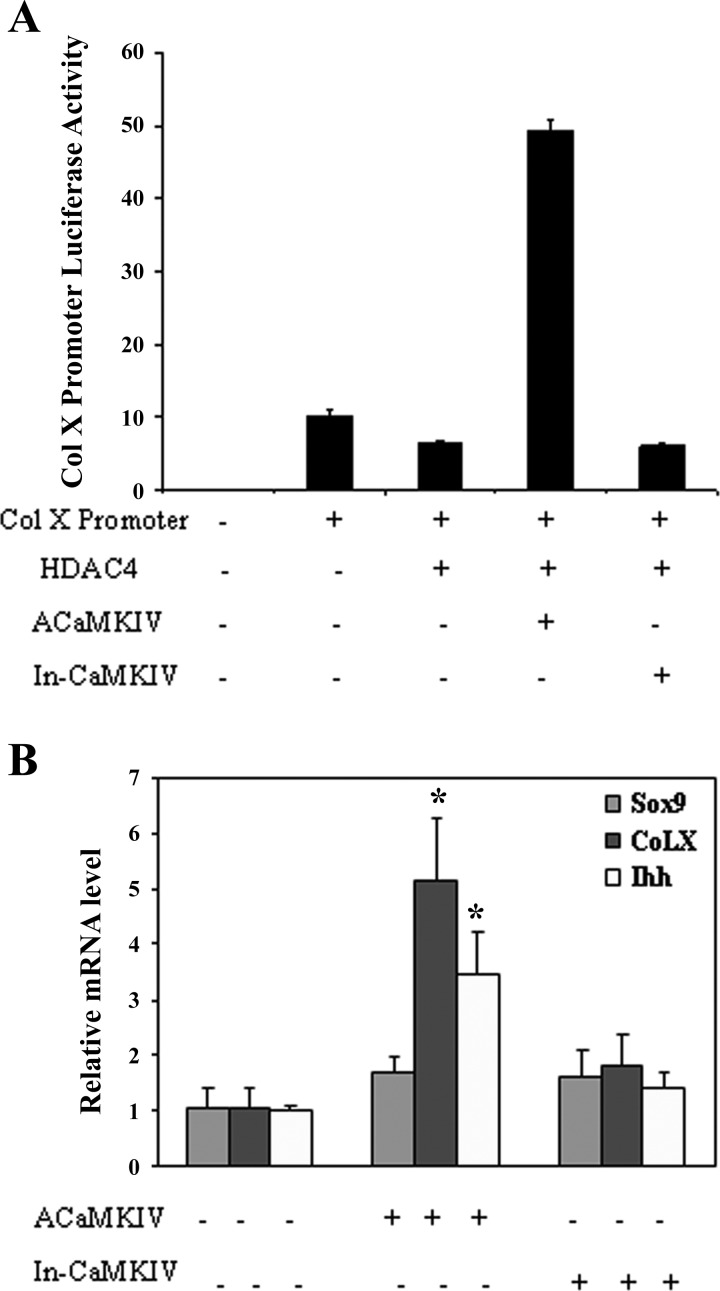
To determine whether CaMKIV is involved in HDAC4 regulation of RunX2 promoter activity, we cotransfected RunX2 promoter with active CaMKIV or inactive CaMKIV and performed dual luciferase assays. As shown in Fig. 6B, HDAC4 inhibits RunX2 promoter activity in a dose-dependent manner (Fig. 6B). This inhibition is reversed by transfection of active CaMKIV, but not by inactive CaMKIV (Fig. 6A). Transfection of active CaMKIV also greatly increases the promoter activity of Col X, while inactive CaMKIV fails to do so (Fig. 7A).
Fig. 6.
CaMKIV stimulates RunX2 promoter activity and overcomes HDAC4 repression of RunX2 promoter. Chondrocytes were transiently transfected with RunX2 promoter and expression vectors encoding active CaMKIV or inactive CaMKIV as indicated in the presence or absence of HDAC4. A: HDAC4 inhibits RunX2 promoter activity while CaMKIV stimulates RunX2 promoter activity and overcomes HDAC4 repression of the RunX2 promoter. B: HDAC4 inhibits RunX2 promoter activity in a dose-dependent manner. Data are presented as means ± SD. All transfections were performed in triplicate.
Fig. 7.
CaMKIV stimulates collagen type X (Col X) promoter activity and induces expression of chondrocyte differentiation markers. A: chondrocytes were transiently transfected with Col X promoter and expression vectors encoding active CaMKIV or inactive CaMKIV as indicated in the presence or absence of HDAC4. CaMKIV overcomes HDAC4 repression of Col X promoter activity. B: HDAC4 was transfected into chondrocytes with active CaMKIV or inactive CaMKIV. Forty-eight hours later, RNA was isolated and qRT-PCR was used to quantitate expression of Col X, Ihh, and Sox 9. Values represent means ± SD from 3 independent experiments. *P < 0.05.
To determine whether CaMKIV is involved in stimulating chondrocyte differentiation, we quantified the mRNA levels of chondrocyte differentiation markers including Sox 9, Ihh, and Col X using real-time RT-PCR. While active CaMKIV has no significant effect on the expression of Sox 9, a proliferating chondrocyte marker, it significantly increases the expression of Ihh, a prehypertrophic chondrocyte marker, and Col X, a hypertrophic chondrocyte marker (Fig. 7B).
DISCUSSION
The chondrocyte differentiation process is exquisitely regulated during growth plate development. Previous studies have shown that HDAC4 plays a vital role in skeletal growth and development (23). Mice lacking HDAC4 die perinatally owing to abnormal chondrocyte hypertrophy which results in ectopic and premature ossification of endochondral bones (23). Our findings present a mechanism for HDAC4 regulation of chondrocyte differentiation through the Ca2+/calmodulin signaling pathway.
Our studies show that CaMKIV is strongly expressed in prehypertrophic chondrocytes where HDAC4 is located to the cytoplasm. On the other hand, CaMKIV is weakly expressed in proliferating chondrocytes where HDAC4 is located in the nucleus. This indicates that CaMKIV may be involved in dynamic regulation of HDAC4 translocation between the cytoplasm and the nucleus in chondrocytes. Consistent with this hypothesis, we demonstrated that suppression of the Ca2+/calmodulin signaling pathway by the inhibitor K-252a allows HDAC4 to relocate to the nucleus, while expression of active CaMKIV promotes HDAC4 relocation from the nucleus to the cytoplasm. Previously, it has been shown that HDAC4 expressed in skeletal muscle (25) has the ability to shuttle between the nucleus and cytoplasm (19) and that such shuttling is regulated by CaMKIV in muscle cells (16). Our findings suggest that CaMKIV also regulates HDAC4 subcellular translocalization during chondrocyte differentiation.
A unique feature of class II HDACs that distinguishes them from class I HDACs is their ability to shuttle between the nucleus and cytoplasm (10, 13, 31), allowing their activity to be regulated by phosphorylation of conserved serine residues and nuclear export (16). HDAC4 can be phosphorylated by many types of kinases such as PKDs (18) and CaMKs (4, 15). We found that not only does CaMKIV stimulate HDAC4 relocation to the cytoplasm, it also leads to an increase in phosphorylated HDAC4, stimulation of RunX2 promoter activity, and stimulation of chondrocyte differentiation. Active CaMKIV increases expression of Ihh and Col X, markers for prehypertrophic and hypertrophic chondrocytes, respectively. In contrast, CAMKIV has no effect on Sox 9, a marker of proliferating chondrocytes. Taken together, these data strongly support the conclusion that the CaMKIV signaling pathway plays an important role in regulating chondrocyte differentiation through controlling HDAC4 phosphorylation and relocation.
In this study we demonstrated that activation of the Ca2+/calmodulin signaling pathway via CaMKIV phosphorylates HDAC4 and promotes the binding of 14-3-3 and HDAC4 in the cytoplasm of chondrocytes. Several lines of evidence have demonstrated that the family of 14-3-3 proteins has the ability to recognize phosphorylated proteins and can bind with them in the cytoplasm (21, 29), thereby sequestering them in the cytoplasm (12, 16). Studies using a yeast two-hybrid system with mammalian cell extracts demonstrated that HDAC4 is involved in 14-3-3 binding and is phosphorylated by CaMKIV (12, 16). In muscle cells, activation of the Ca2+/calmodulin signaling pathway via CaMKIV prevents nuclear entry of HDAC4 and enhances the binding of 14-3-3 to HDAC4 in cytoplasm, which releases the inhibition of myocyte differentiation (16). We have shown that these events occur in chondrocytes, which is of critical importance in understanding the regulation of chondrocyte differentiation during the growth plate development. Our data strongly support a mechanism by which HDAC4 phosphorylation by CaMKIV results in cytoplasmic accumulation of HDAC4 via 14-3-3 binding, which releases the repression of RunX2 activity and leads to chondrocyte differentiation.
RunX2 is a transcription factor that belongs to the RunX family and is necessary for osteoblast differentiation in vivo (14). RunX2 has also been shown to play a critical role in promoting cellular differentiation in chondrocytes (23). One important role for HDAC4 in chondrocytes is to repress hypertrophic differentiation by interacting with and inhibiting the activity of RunX2 protein (23). We found that RunX2 is strongly expressed in prehypertrophic and hypertrophic chondrocytes where HDAC4 is localized to the cytoplasm. Conversely, RunX2 is only weakly expressed in proliferating chondrocytes where HDAC4 is localized to the nucleus. Indeed, the RunX2 promoter activity is suppressed by HDAC4 in a dosage-dependent manner. Such suppression can be relieved by expression of active CaMKIV, but not by inactive CaMKIV. These data suggest that one of the mechanisms for CaMKIV to regulate chondrocyte differentiation is through modulating RunX2 promoter activities via HDAC4 subcellular localization.
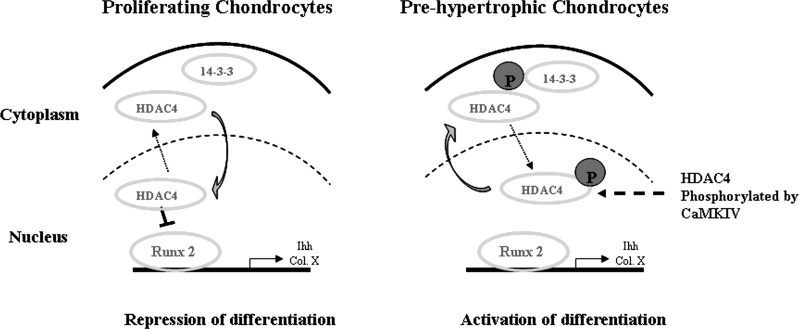
In conclusion, our data support the following model for CaMKIV to regulate chondrocyte differentiation (Fig. 8). HDAC4 is located in the nucleus in proliferating chondrocytes and relocates to the cytoplasm in the maturation/prehypertrophic chondrocytes during growth plate development. CaMKIV induces chondrocyte differentiation through phosphorylation of HDAC4, which leads to its translocation to the cytoplasm. The serines of HDAC4, once phosphorylated, become attachment sites for 14-3-3 chaperone proteins, which retain HDAC4 in the cytoplasm thereby relieving HDAC4 transcriptional repression of RunX2. Overall, these findings indicate that HDAC4 nuclear-cytoplasmic shuttling controls chondrocyte differentiation in the growth plate, which is dependent on the Ca2+/calmodulin signaling pathway.
Fig. 8.
A model of Ca2+/calmodulin-dependent HDAC4 nuclear-cytoplasmic relocation controlling chondrocyte differentiation. In proliferating chondrocytes, HDAC4 inhibits the expression of RunX2 in the nucleus, which represses differentiation. In the prehypertrophic chondrocytes, HDAC4 is phosphorylated by CaMKIV and relocates and binds to 14-3-3 protein in the cytoplasm. Cytosolic accumulation of HDAC4 increases RunX2 expression resulting in chondrocyte differentiation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Y.G., Q.C., M.P., R.T., X.W., and L.W. conception and design of the research; Y.G., X.Y., P.H., and L.W. performed the experiments; Y.G., Q.C., M.P., X.W., T.Z., and L.W. analyzed the data; Y.G., Q.C., T.Z., and L.W. interpreted the results of the experiments; Y.G. and L.W. prepared the figures; Y.G., X.Y., P.H., R.T., X.W., and L.W. drafted the manuscript; Y.G., Q.C., M.P., R.T., X.W., T.Z., and L.W. edited and revised the manuscript; Y.G., Q.C., X.Y., P.H., M.P., R.T., X.W., T.Z., and L.W. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The project was supported by Grant R01AR059142 and 5R03AR052479 from National Institutes of Health (NIH)/National Institute of Arthritis and Musculoskeletal and Skin Diseases, AG17021 and AG14399 from NIH/National Institute on Aging, P20RR024484 from NIH/National Center for Research Resources, Aircast Foundation, and Arthritis National Research Foundation, National Science Foundation 81071495 and 81171676, and SXNSF 2011011042. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
REFERENCES
- 1.Anderson KA, Ribar TJ, Illario M, Means AR. Defective survival and activation of thymocytes in transgenic mice expressing a catalytically inactive form of Ca2+/calmodulin-dependent protein kinase IV. Mol Endocrinol 11: 725–737, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Backs J, Song K, Bezprozvannaya S, Chang S, Olson EN. CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J Clin Invest 116: 1853–1864, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger SL. The complex language of chromatin regulation during transcription. Nature 447: 407–412, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Bolger TA, Yao TP. Intracellular trafficking of histone deacetylase 4 regulates neuronal cell death. J Neurosci 25: 9544–9553, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang S, McKinsey TA, Zhang CL, Richardson JA, Hill JA, Olson EN. Histone deacetylases 5 and 9 govern responsiveness of the heart to a subset of stress signals and play redundant roles in heart development. Mol Cell Biol 24: 8467–8476, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatila T, Anderson KA, Ho N, Means AR. A unique phosphorylation-dependent mechanism for the activation of Ca2+/calmodulin-dependent protein kinase type IV/GR. J Biol Chem 271: 21542–21548, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Chen Q, Johnson DM, Haudenschild DR, Goetinck PF. Progression and recapitulation of the chondrocyte differentiation program: cartilage matrix protein is a marker for cartilage maturation. Dev Biol 172: 293–306, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Dong YF, Soung DY, Schwarz EM, O'Keefe RJ, Drissi H. Wnt induction of chondrocyte hypertrophy through the RunX2 transcription factor. J Cell Physiol 208: 77–86, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Drissi H, Luc Q, Shakoori R, Chuva De Sousa Lopes S, Choi JY, Terry A, Hu M, Jones S, Neil JC, Lian JB, Stein JL, Van Wijnen AJ, Stein GS. Transcriptional autoregulation of the bone related CBFA1/RUNX2 gene. J Cell Physiol 184: 341–350, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Emiliani S, Fischle W, Van Lint C, Al-Abed Y, Verdin E. Characterization of a human RPD3 ortholog, HDAC3. Proc Natl Acad Sci USA 95: 2795–2800, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grozinger CM, Hassig CA, Schreiber SL. Three proteins define a class of human histone deacetylases related to yeast Hda1p. Proc Natl Acad Sci USA 96: 4868–4873, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grozinger CM, Schreiber SL. Regulation of histone deacetylase 4 and 5 and transcriptional activity by 14-3-3-dependent cellular localization. Proc Natl Acad Sci USA 97: 7835–7840, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu E, Chen Z, Fredrickson T, Zhu Y, Kirkpatrick R, Zhang GF, Johanson K, Sung CM, Liu R, Winkler J. Cloning and characterization of a novel human class I histone deacetylase that functions as a transcription repressor. J Biol Chem 275: 15254–15264, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Lee HW, Suh JH, Kim HN, Kim AY, Park SY, Shin CS, Choi JY, Kim JB. Berberine promotes osteoblast differentiation by RunX2 activation with p38 MAPK. J Bone Miner Res 23: 1227–1237, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Liu A, Wang B, Niswander LA. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development 132: 3103–3111, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Miska EA, Langley E, Wolf D, Karlsson C, Pines J, Kouzarides T. Differential localization of HDAC4 orchestrates muscle differentiation. Nucleic Acids Res 29: 3439–3447, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Namdari S, Wei L, Moore D, Chen Q. Reduced limb length and worsened osteoarthritis in adult mice after genetic inhibition of p38 MAP kinase activity in cartilage. Arthritis Rheum 58: 3520–3529, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishino TG, Miyazaki M, Hoshino H, Miwa Y, Horinouchi S, Yoshida M. 14-3-3 regulates the nuclear import of class IIa histone deacetylases. Biochem Biophys Res Commun 377: 852–856, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Paroni G, Fontanini A, Cernotta N, Foti C, Gupta MP, Yang XJ, Fasino D, Brancolini C. Dephosphorylation and caspase processing generate distinct nuclear pools of histone deacetylase 4. Mol Cell Biol 27: 6718–6732, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paroni G, Mizzau M, Henderson C, Del Sal G, Schneider C, Brancolini C. Caspase-dependent regulation of histone deacetylase 4 nuclear-cytoplasmic shuttling promotes apoptosis. Mol Biol Cell 15: 2804–2818, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng CY, Graves PR, Thoma RS, Wu Z, Shaw AS, Piwnica-Worms H. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science 277: 1501–1505, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Ribar TJ, Rodriguiz RM, Khiroug L, Wetsel WC, Augustine GJ, Means AR. Cerebellar defects in Ca2+/calmodulin kinase IV-deficient mice. J Neurosci 20: RC107, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vega RB, Matsuda K, Oh J, Barbosa AC, Yang X, Meadows E, McAnally J, Pomajzl C, Shelton JM, Richardson JA, Karsenty G, Olson EN. Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell 119: 555–566, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Verdin E, Dequiedt F, Kasler HG. Class II histone deacetylases: versatile regulators. Trends Genet 19: 286–293, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Wang AH, Kruhlak MJ, Wu J, Bertos NR, Vezmar M, Posner BI, Bazett-Jones DP, Yang XJ. Regulation of histone deacetylase 4 by binding of 14-3-3 proteins. Mol Cell Biol 20: 6904–6912, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang D, Chang PS, Wang Z, Sutherland L, Richardson JA, Small E, Krieg PA, Olson EN. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell 105: 851–862, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Wei LKK, Lee M, Wei X, Pei M, Sun X, Terek R, Chen Q. Stimulation of chondrocyte hypertrophy by chemokine stromal cell-derived factor 1 in the chondro-osseous junction during endochondral bone formation. Dev Biol 341: 236–245, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu JY, Gonzalez-Robayna IJ, Richards JS, Means AR. Female fertility is reduced in mice lacking Ca2+/calmodulin-dependent protein kinase IV. Endocrinology 141: 4777–4783, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Zeng Y, Forbes KC, Wu Z, Moreno S, Piwnica-Worms H, Enoch T. Replication checkpoint requires phosphorylation of the phosphatase Cdc25 by Cds1 or Chk1. Nature 395: 507–510, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Chen Q. Changes of matrilin forms during endochondral ossification. Molecular basis of oligomeric assembly. J Biol Chem 275: 32628–32634, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Zhao X, Ito A, Kane CD, Liao TS, Bolger TA, Lemrow SM, Means AR, Yao TP. The modular nature of histone deacetylase HDAC4 confers phosphorylation-dependent intracellular trafficking. J Biol Chem 276: 35042–35048, 2001 [DOI] [PubMed] [Google Scholar]