Abstract
AMP-activated protein kinase (AMPK) and the NAD+-dependent histone/protein deacetylase sirtuin 1 (SIRT1) are metabolic sensors that can increase each other's activity. They are also both activated by the antidiabetic drug metformin and downregulated in the liver under conditions of nutrient excess (e.g., hyperglycemia, high-fat diet, obesity). In these situations, the abundance of the tumor suppressor p53 is increased; however, the relevance of this to the changes in AMPK and SIRT1 is not known. In the present study we investigated this question in HepG2 cells under high glucose conditions. Metformin induced activation of AMPK and SIRT1 and decreased p53 protein abundance. It also decreased triglyceride accumulation and cytosolic oxidative stress (a trigger for p53 accumulation) and increased the deacetylation of p53 at a SIRT1-targeted site. The decrease in p53 abundance caused by metformin was abolished by inhibition of murine double minute 2 (MDM2), a ubiquitin ligase that mediates p53 degradation, as well as by overexpression of a dominant-negative AMPK or a shRNA-mediated knockdown of SIRT1. In addition, overexpression of p53 decreased SIRT1 gene expression and protein abundance, as well as AMPK activity in metformin-treated cells. It also diminished the triglyceride-lowering action of metformin, an effect that was rescued by incubation with the SIRT1 activator SRT2183. Collectively, these findings suggest the existence of a novel reciprocal interaction between AMPK/SIRT1 and p53 that may have implications for the pathogenesis and treatment of metabolic diseases.
Keywords: oxidative stress, nutrient excess, triglyceride accumulation, sirtuin 1
in response to a decrease in cellular energy state, activation of AMP-activated protein kinase (AMPK) stimulates processes such as fatty acid oxidation that generate ATP, and inhibits others that consume ATP such as protein and lipid synthesis (19, 56). Sirtuin 1 (SIRT1) is a NAD+-dependent histone protein deacetylase that is increased by caloric restriction and is thought to delay aging (13, 25) and counter cellular senescence (13, 25), a state that is characterized by permanent cell-cycle arrest and is associated with aging (52). The beneficial metabolic effects of both AMPK and SIRT1 activation include increased fatty acid oxidation, decreased hepatosteatosis, and improved insulin sensitivity and blood glucose levels (8, 12, 22, 35). Evidence has also been presented that AMPK and SIRT1 positively regulate each other [reviewed by Ruderman et al. (48)].
The tumor suppressor p53 is a stress-responsive transcription factor that has been studied extensively with regard to its role in DNA-damage response pathways and apoptosis (2). More recently it has also been examined in the context of metabolic dysfunction and cellular senescence. In rodents fed a high-fat, high-sucrose diet (39) or with obesity (57), the protein abundance of p53 in the liver is elevated, whereas AMPK and SIRT1 are downregulated (10, 15, 18, 59). Whether a linkage exists between diminished hepatic AMPK and SIRT1 and increased p53 protein under conditions of energy excess has yet to be examined.
In this study we investigated the relationship between AMPK-SIRT1 signaling and p53 abundance.1 Human hepatoma (HepG2) cells were exposed to glucose concentrations that have been shown to inhibit AMPK and SIRT1, induce lipid accumulation, and cause insulin resistance (49, 60). It is important to note that although HepG2 cells exhibit chromosomal (29) and some oncogenic gene abnormalities (23, 24, 61), they also express wild-type p53 (3, 21, 23) and show many of the metabolic characteristics of primary hepatocytes (46, 60, 62). Our data demonstrate that, in high glucose-exposed HepG2 cells, activation of AMPK and SIRT1 by metformin, a widely used antidiabetic drug and activator of AMPK (62), results in decreased p53 protein abundance. They also reveal that overexpression of p53 decreases SIRT1 abundance and diminishes the ability of metformin both to activate AMPK and to decrease cellular triglycerides. Overall, these findings suggest the existence of a reciprocal relationship between hepatic AMPK-SIRT1 signaling and p53 protein under conditions of nutrient excess and in response to metformin.
MATERIALS AND METHODS
Materials.
HepG2 cells were purchased from American Type Culture Collection (Manassas, VA). DMEM and penicillin-streptomycin (PS) were from GIBCO (Grand Island, NY). FBS was from Thermo Scientific Hyclone (Logan, UT). d−(+)−Glucose solution, 45%, and metformin (1,1-bimethylbiguanide hydrochloride) were purchased from Sigma-Aldrich (St. Louis, MO). (±)−Nutlin-3 was from Cayman Chemical (Ann Arbor, MI). SRT2183 was provided by Sirtris Pharmaceuticals (Cambridge, MA). Lys382 acetyl-p53, acetyl-CoA carboxylase (ACC), AMPK, and Thr172 phospho-AMPKα (40H9) antibodies as well as secondary horseradish peroxidase-linked antibodies were purchased from Cell Signaling Technology (Danvers, MA). Ser79 phospho-ACC, acetyl-histone H3, and histone H3 (CT, pan, clone A3C) antibodies were from Upstate/Millipore (Temecula, CA). SIRT1 (H-300) and p53 (DO-1) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-β-actin was from Sigma-Aldrich.
Cell culture and treatments.
HepG2 cells were cultured in low-glucose (5.5 mM) DMEM supplemented with 10% FBS and 1% PS. Media were replaced every 24 h and cells were passaged upon reaching 80–90% confluency. Glucose-, pyruvate-, and FBS-free DMEM supplemented with 1% PS and glucose to a final concentration of 25 mM was used for all experimental incubations. Metformin was dissolved in H2O. Nutlin-3 and SRT2183 were dissolved in DMSO.
Adenoviruses and cell infection.
A replication-defective adenoviral vector expressing β-galactosidase (pCMV-β-gal) was used as a control (4). The recombinant adenoviral vector expressing a dominant negative mutant of AMPKα2 (DN-AMPK) was constructed from AMPKα2 bearing a mutation of lysine 45 to arginine (K45R) as described previously (40, 41, 63). Wild-type p53 was subcloned from a pCMV-p53 vector purchased from Clontech (Mountain View, CA), and the short hairpin RNA sequence used to knock down SIRT1 (shSIRT1) was generated as previously described (32). These two sequences were incorporated into an adenovirus vector as described by Cacicedo et al. (4). Cells were infected with approximately 50–200 plaque-forming units per cell for 24–48 h before the start of the experimental incubations.
Glucose assays.
Residual glucose concentration in the media was measured using an enzymatic glucose assay kit (GAHK-20) from Sigma. The assay was adapted for use in a 96-well plate by loading 2 μl sample or standard and 200 μl reagent per well, incubating for 15 min at room temperature, and reading at 340 nm in a spectrophotometer. Glucose concentrations were calculated from the standard curve linear regression.
SDS-PAGE and Western blot analysis.
Analyses were carried out as previously described (49, 60) with the following modifications: cells were washed once on ice with Dulbecco's PBS + 10 mM nicotinamide, lysed in buffer containing 20 mM Tris·HCl pH 8.0, 1% IGEPAL, 1 mM EGTA, 10 mM nicotinamide, 1 μM trichostatin A, 10 mM sodium butyrate, 1 mM PMSF, 1× phosphatase inhibitor cocktail 3 (Sigma), and 1× protease inhibitor cocktail containing 1 mM EDTA (Complete Mini, Roche, Basel, Switzerland). Nicotinamide was added to inhibit sirtuin deacetylase activity, and sodium butyrate and trichostatin A were added to inhibit other histone deacetylases (HDACs).
Detection of cytosolic oxidative stress.
Cells were incubated for 24 h in DMEM containing 25 mM glucose ± the indicated treatments. They were loaded with 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate dye (Invitrogen, Carlsbad, CA) during the last 30 min of this incubation, and analyses of DCF fluorescence were carried out as previously described (17).
Measurement of cellular triglyceride content.
Cellular triglyceride was determined in whole cell lysates (prepared as described above) using Infinity Triglycerides reagent (Thermo Fisher Scientific, Middletown, VA) as previously described (22, 60).
Real-time quantitative PCR.
Cells were washed once on ice with Dulbecco's PBS + 10 mM nicotinamide and immediately placed in TRIzol. RNA was extracted, 700 ng of total RNA were reverse transcribed using Moloney murine leukemia virus (MMLV; Invitrogen), and real-time RT-PCR was performed using SYBR Premix Extaq in a Cepheid Smart Cycler (Cepheid, Sunnyvale, CA). SIRT1 primers were as follows: forward, CACTGTGGTAGAGCTTGCAT, and reverse, ACACTCTCCCCAGTAGAAGT. Primers for the housekeeping gene RPS18 were as follows: forward, TCCAGCATATTTTGCGAGTACT, and reverse, CCACATGAGCATATCTTCGG. Data are expressed relative to the housekeeping gene and were calculated using the ΔΔCT method and are presented as fold change from control, within each time point.
Statistical analysis.
Results are reported as means ± SE. Statistical significance was determined by a two-tailed unpaired Student's t-test or ANOVA with Tukey's post hoc test. A level of P < 0.05 was considered statistically significant.
RESULTS
AMPK and SIRT1 are activated by metformin in high glucose-exposed HepG2 cells.
We first set out to confirm that metformin increases the activity of both AMPK and SIRT1 under conditions of nutrient excess. In initial studies, we measured the remaining glucose concentration in the media at 24 h. In cells incubated with a starting glucose concentration of 5.5 mM, glucose was completely depleted by 24 h. In contrast, at least 10 mM glucose remained at 24 h when the starting glucose concentration was 25 mM (data not shown). Under the high glucose conditions, the addition of 2 mM metformin increased the activities of AMPK, as assessed by phosphorylation of AMPK (Thr172) and its downstream target ACC (Ser79), and of SIRT1, as reflected by deacetylation of histone H3 (Fig. 1). Metformin increased AMPK activity (p-ACC and p-AMPK) under conditions of low glucose as well, but had no effect on SIRT1 activity, as evidenced by unchanged histone H3 acetylation (data not shown).
Fig. 1.
AMP-activated protein kinase (AMPK) and sirtuin 1 (SIRT1) activation by metformin. HepG2 cells were incubated in 25 mM glucose DMEM for 24 h with or without 2 mM metformin followed by whole cell lysis and Western blot analysis. A and B: representative blot from experiments repeated at least 3 times showing activation of AMPK evidenced by increased phosphorylated acetyl-CoA carboxylase (p-ACC) (Ser79) and p-AMPK (Thr172) and decreased NH2-terminal histone H3 acetylation (Ac-H3). C: densitometric analysis represented as fold change demonstrating increased p-ACC, increased p-AMPK, and reduced Ac-H3 in response to metformin, consistent with an increase in AMPK and SIRT1 activity. Results are means ± SE (n = 6); *P < 0.05.
Metformin triggers a decrease in p53 protein abundance that is dependent on AMPK and SIRT1.
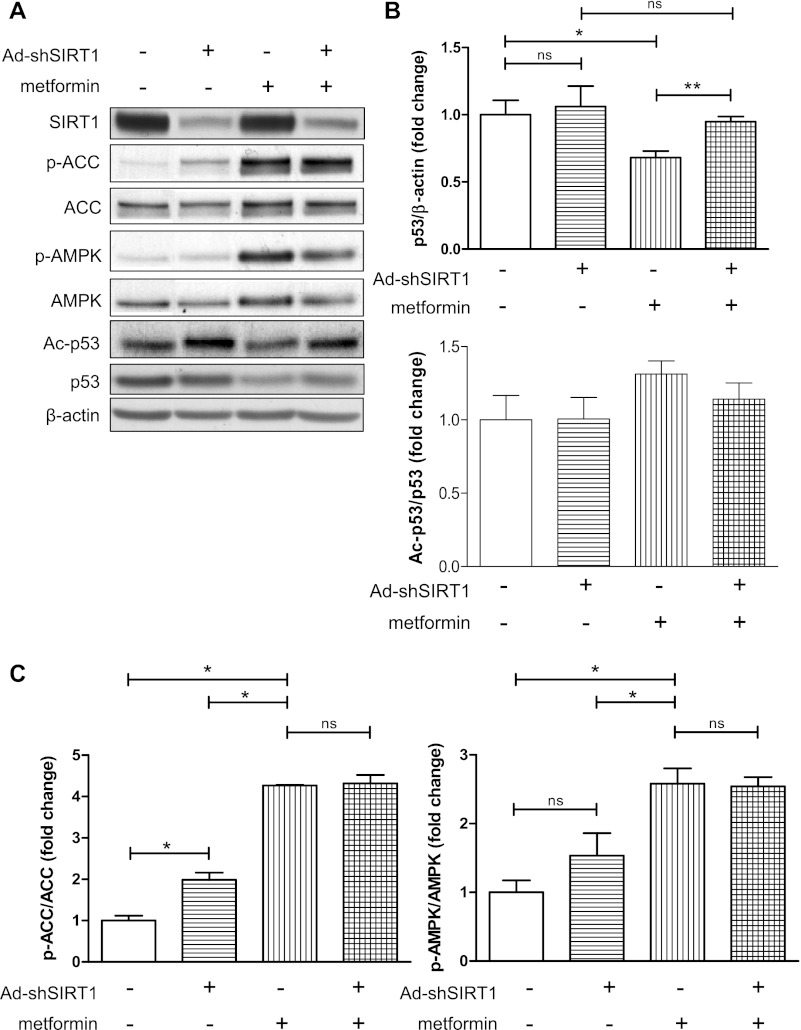
We next determined the effect of metformin on p53 abundance. Western blot analysis showed a dose-dependent decrease in p53 protein in response to metformin under high glucose conditions (Fig. 2A). Overexpression of a DN-AMPK increased p53 abundance under basal conditions and prevented the metformin-induced decrease in p53 (Fig. 2, B and C). DN-AMPK also attenuated the reduction in histone H3 deacetylation caused by metformin (Fig. 2, B and C), supporting a loss of SIRT1 activity in the absence of AMPK activation. Similarly, knockdown of SIRT1 by adenovirus-mediated expression of shRNA interference (shSIRT1) blunted the ability of metformin to decrease p53 protein abundance, although it did not significantly alter the acetylation status of p53 (Fig. 3, A and B). Importantly, knockdown of SIRT1 had no effect on metformin-induced AMPK activity (Fig. 3C), suggesting that metformin acts independently of SIRT1 to activate AMPK.
Fig. 2.
Metformin decreases p53 abundance and is dependent on AMPK. A: HepG2 cells were incubated in 20–30 mM DMEM for 24 h with the indicated concentration of metformin. Representative blot and densitometric analysis showing dose-dependent effect of metformin on p53 abundance. Results are means ± SE (n = 3–4); *P < 0.05 for 0 vs. 1.5 mM metformin; **P < 0.01 for 0 vs. 2 mM metformin; P < 0.001 for linear trend. B: HepG2 cells were infected with adenovirus (Ad)-dominant negative (DN)-AMPK in 25 mM glucose DMEM for 48 h, followed by 24 h of incubation in fresh 25 mM glucose DMEM ± 1.5 mM metformin. Representative Western blot results are shown. C: densitometric analysis of p53 abundance and acetylation of histone-3 from blots shown in B. Results are means ± SE (n = 2–4). Significance: *P < 0.05, ***P < 0.001; ns, nonsignificant.
Fig. 3.
Metformin reduction in p53 abundance is dependent on SIRT1. A: HepG2 cells infected with short hairpin (sh)SIRT1-expressing adenovirus for 36 h before 24 h of incubation in fresh 25 mM glucose ± 2 mM metformin. Representative Western blots are shown. B: densitometric analysis of p53 acetylation and abundance from the experiment described in A. C: densitometric analysis of p-ACC and p-AMPK, indicators of AMPK activation, from the experiment described in A. Results are means ± SE (n = 3–4). Significance: *P < 0.05, **P < 0.01.
Metformin-induced decreases in p53 are associated with reduced oxidative stress, a decrease in its acetylation, and are attenuated by murine double minute 2 inhibition.
Various cellular stressors including oxidative stress can trigger p53 accumulation (2). To determine whether a decrease in oxidative stress occurs in response to metformin treatment under high glucose conditions, we assessed the production of cytosolic reactive oxygen species (ROS) using DCF fluorescence. Consistent with its observed effect on p53 abundance, metformin diminished cytosolic ROS production under high glucose (Fig. 4A). To determine whether the ability of metformin to decrease ROS production under high glucose conditions was dependent on AMPK, cells were infected with adenovirus (Ad)-DN-AMPK prior to the DCF experiment. The knockdown of AMPK resulted in increased ROS production, which was partially blunted by metformin treatment (Fig. 4B).
Fig. 4.
The effect of metformin on p53 abundance is associated with decreased oxidative stress, murine double minute 2 (MDM2)-mediated degradation, and lysine 382 deacetylation. A: HepG2 cells were incubated in 25 mM glucose ± 1 mM metformin for 24 h, after which reactive oxygen species (ROS) production was measured using DCF fluorescence; n = 3 per data point. B: cells were infected with Ad-DN-AMPK or Ad-β-galactosidase in 25 mM glucose DMEM for 48 h, followed by 24 h of incubation in fresh 25 mM glucose DMEM ± 1.5 mM metformin, after which ROS production was measured using DCF fluorescence; n = 6–9 per data point. Significance: *P < 0.05 vs. all other treatments, †P < 0.05 vs. Ad-β-galactosidase treatment. C: cells were incubated for 24 h in 25 mM glucose with or without coincubation with the MDM2 inhibitor nutlin-3 (5 μM) or metformin (2 mM) followed by Western blot analysis. A representative blot is shown demonstrating that in the presence of nutlin-3, metformin no longer significantly decreases p53 abundance. Graph shows fold change of p53 relative to control. Results are means ± SE (n = 2 for controls, n = 4 for those with nutlin-3). D: cells were incubated for 24 h in 25 mM glucose with or without coincubation with nutlin-3 (5 μM), after which ROS production was measured using DCF fluorescence; results are means ± SE (n = 6). E: cells were infected with a p53-expressing adenovirus in 25 mM glucose for 24 h, followed by incubation in fresh 25 mM glucose for 24 h with or without metformin (2 mM). A representative blot is shown and quantification demonstrating a decrease in p53 acetylation at a SIRT1-targeted site (Lys382); results are means ± SE (n = 6). Significance: *P < 0.05, ***P < 0.001.
In conjunction with the degree of cellular stress, the abundance of p53 is regulated by the rate of its degradation by the ubiquitin ligase murine double minute 2 (MDM2) (20). To determine whether MDM2-mediated p53 degradation contributes to the observed effect of metformin, we incubated the cells with nutlin-3, a pharmacological inhibitor of MDM2 (50). As shown in Fig. 4C, the decrease in p53 abundance that occurs in response to metformin treatment was abolished in the presence of nutlin-3. Overexpression of p53 with nutlin-3 treatment had no significant effect on ROS production (Fig. 4D).
It has been reported that acetylation makes p53 more resistant to ubiquitination by MDM2 and increases its half-life in vivo (33). SIRT1 has been shown to deacetylate p53 at lysine 382 (36, 51), a known ubiquitination site (42, 47). By overexpressing wild-type p53 in the HepG2 cells, we were able to detect a decrease in lysine 382 acetylation of p53 in response to metformin treatment, consistent with an increase in SIRT1-mediated deacetylation (Fig. 4E). Overall, these results suggest that activation of AMPK and SIRT1 by metformin decreases p53 abundance by reducing oxidative stress, decreasing lysine 382 acetylation, and increasing MDM2-mediated degradation.
Overexpression of p53 decreases SIRT1 abundance and attenuates the effects of metformin on AMPK activation and cellular triglycerides.
Overexpression of p53 decreased SIRT1 protein abundance and attenuated metformin-induced AMPK and ACC phosphorylation (Fig. 5, A and B). In addition, treatment with nutlin-3, which increased the abundance of p53 (Fig. 4C), reduced the transcription of SIRT1 as evidenced by reduced SIRT gene expression at both 12 and 16 h (Fig. 5C). Since both AMPK and SIRT1 have lipid-lowering effects in vitro and in vivo (8, 12, 22, 35, 60, 62), we hypothesized that p53 overexpression would attenuate the lipid-lowering effect of metformin. As shown in Fig. 6, the ability of metformin to inhibit triglyceride accumulation was blunted in HepG2 cells in which p53 was overexpressed.
Fig. 5.
Overexpression of p53 decreases SIRT1 abundance and diminishes AMPK activation by metformin. Cells infected with adenovirus Ad-β-gal (control) or Ad-p53 for 24 h in 25 mM glucose, followed by 24 h incubation in 25 mM glucose with or without 2 mM metformin. A: representative Western blot demonstrating the inhibitory effects of Ad-p53 on SIRT1 abundance and phosphorylation of AMPK (Thr172) and ACC (Ser79) in response to metformin. B: densitometric analysis of SIRT1 abundance, p-AMPK/AMPK, and p-ACC/ACC. C: RT-PCR analysis of SIRT1 gene expression in response to p53 overexpression with nutlin-3. Results are means ± SE (n = 3–10); *P < 0.05 and ***P < 0.001.
Fig. 6.
Increasing p53 abundance attenuates the effect of metformin on cellular triglycerides. HepG2 cells infected with adenovirus Ad-β-gal (control) or Ad-p53 for 24 h in 25 mM glucose media, followed by 24 h incubation in 25 mM glucose with or without 2 mM metformin. Triglyceride levels from a representative experiment are shown. Results are means ± SE (n = 4–6); *P < 0.05 and ***P < 0.001.
Metformin effects are partially restored by the SIRT1 activator SRT2183 in cells overexpressing p53.
We next tested whether SIRT1 activation in cells overexpressing p53 would restore the effects of metformin on AMPK activity and triglyceride accumulation. Coincubation with the SIRT1 activator SRT2183 restored some, but not all, of the effects of metformin in these cells, as evidenced by their decreased triglyceride content and increased AMPK phosphorylation (Fig. 7). Although no effect on ACC phosphorylation was observed (Fig. 7A), these results support the notion that p53 overexpression attenuates the effects of metformin in part by decreasing SIRT1 activity.
Fig. 7.
The effects of metformin are partially restored by the SIRT1 activator SRT2183. A: representative Western blot demonstrating partial restoration of p-AMPK with SRT2183, with no apparent effect on Ac-H3 or Ac-p53. B: triglyceride content in cellular lysates is reported in the graph. C: densitometric analysis of p-AMPK/AMPK. Results are means ± SE (n = 4); *P < 0.05, ***P < 0.001.
DISCUSSION
In this study, we investigated the interactions between AMPK/SIRT1 signaling and p53 protein abundance in HepG2 cells under conditions of nutrient excess. The results demonstrate that activation of AMPK and SIRT1 in response to metformin treatment decreases p53 abundance. They also reveal that metformin diminishes oxidative stress and enhances p53 deacetylation, likely contributing to the MDM2-mediated increase in p53 degradation. Conversely, we showed that overexpression of p53 diminishes SIRT1 abundance and prevents metformin-induced activation of AMPK and reductions in triglyceride content.
The findings that AMPK activation by metformin diminishes p53 abundance and oxidative stress are in agreement with those of Eid et al. (11), who reported that AMPK activation by AICAR (5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside) and by overexpression of wild-type AMPKα2 decreased p53 abundance in glomerular epithelial cells under high glucose conditions. They also showed that this effect was mediated by an AMPK-induced decrease in Nox4 expression that inhibited ROS production by NADPH oxidase. In addition, several other groups have reported an inhibitory effect of AMPK on NADPH oxidase activity (11, 45, 54). To our knowledge, this report and that of Eid et al. (11) are the only studies to date in which the effect of AMPK activation on p53 abundance was examined under high glucose conditions. In contrast, in response to extreme glucose deprivation, it has been reported that AMPK activation promotes cellular survival by phosphorylation and activation of p53 (27, 28).
We found that inhibition of the ubiquitin ligase MDM2 with nutlin-3 abolished the p53-lowering effect of metformin. Although many factors may contribute to the stability of the p53-MDM2 interaction, including posttranslational modifications of p53, some studies suggest that modifications of MDM2 may play a role (37, 55). In particular, it was recently reported that the mammalian target of rapamycin (mTOR)-ribosomal protein S6 kinase 70-kDa polypeptide 1 (S6K1) pathway (mTORC1) contributes to serine 163 phosphorylation of MDM2, resulting in inhibition of MDM2-mediated p53 ubiquitination and degradation (31). Although the effect of AMPK on mTOR/S6K1 was not examined in this study, it is known that AMPK phosphorylates and activates tuberous sclerosis 2 (TSC2), which in turn leads to inhibition of mTOR-S6K1 signaling (26). Thus it is plausible that AMPK activation could increase MDM2-mediated degradation of p53 by decreasing S6K1 activity, although this remains to be proven.
We also investigated whether metformin increased the deacetylation of p53, perhaps making it more susceptible to MDM2 degradation. In control cells, metformin caused a robust reduction in p53 abundance but had no measureable effect on p53 acetylation (Fig. 3). It is plausible that metformin caused the deacetylation of p53 in these cells, contributing to p53 degradation. Importantly, in cells overexpressing wild-type p53 and resistant to degradation of p53, metformin decreased lysine 382-acetylated p53 (Fig. 4E). This experimental model provides evidence for a role of metformin in decreasing p53. Furthermore, the striking reduction in p53 protein abundance by metformin was abolished by knockdown of SIRT1. These findings support the notion that SIRT1 activity is enhanced and contributes to metformin-induced decreases in p53 stability. However, we cannot entirely rule out effects of other HDACs, histone acetyltransferases (HATs), or other sirtuins.
The increase in ROS production caused by AMPK knockdown with Ad-DN-AMPK is in agreement with previous reports that AMPK activation can reduce ROS generation (16). In DN-AMPK cells, metformin was effective in reducing ROS, but not to the level of control cells; thus metformin may act independently of, as well as through AMPK to attenuate ROS production. Pharmacologic overexpression of p53 by nutlin-3 had no effect on ROS (Fig. 4D), indicating that, although oxidative stress increases p53 (2), p53 does not affect ROS generation. Nutlin-3 treatment had no effect on ROS production (Fig. 4D).
Numerous studies have shown that AMPK activation can lead to increased SIRT1 activity (5–7, 9, 14), with two of them demonstrating that metformin increases the NAD+/NADH ratio and SIRT1 abundance and activity (5, 7). Under control conditions, we did not observe an increase in SIRT1 protein; however, metformin did decrease histone H3 acetylation, consistent with an increase in SIRT1 activity. On the other hand, in cells incubated with Ad-DN-AMPK it no longer had this effect (Fig. 2, B and C). In contrast, metformin-induced increases in p-AMPK and p-ACC (indicators of AMPK activity) were unchanged when SIRT1 was knocked down (Fig. 3C). These findings suggest both that metformin can activate AMPK independently of SIRT1 and that it acts primarily through AMPK when it increases SIRT1 activity. These data corroborate the findings of Caton et al. (7), which demonstrated that compound C blunted the metformin-induced increase in SIRT1 activity. Although metformin appears to be acting through AMPK, it is also noteworthy that several reports have demonstrated that SIRT1 activation or overexpression can also activate AMPK (22, 32, 53).
The findings discussed thus far support the notion of an inhibitory effect of AMPK/SIRT1 activation on the abundance of p53 protein in HepG2 cells under high glucose conditions. We also investigated the effect of p53 overexpression and found that it decreased SIRT1 abundance and AMPK activity in metformin-treated cells. Since SIRT1 has been shown to regulate LKB1 (32), an upstream kinase for AMPK, it is likely that the observed decrease in SIRT1 protein caused by p53 could have contributed to the diminished AMPK activation. In further studies, we found an attenuation of the triglyceride-lowering effect of metformin in cells overexpressing p53 that was reversed by coincubation with the SIRT1 activator SRT2183. Acetylation status of p53 and histone H3 did not reflect SIRT1 activation; however, there is debate as to the effectiveness of this compound to directly activate SIRT1 (38, 44). There was, however, a modest but significant increase in AMPK phosphorylation with SRT2183 (Fig. 7B), possibly contributing to the reduction in triglyceride. These results suggest that p53 interferes with the triglyceride-lowering effect of metformin by decreasing AMPK activation and SIRT1 protein. Although not directly tested, the latter effect could have been mediated by p53-induced transcription of miR-34a, a microRNA that inhibits SIRT1 expression by binding to the 3′-untranslated region (UTR) of the SIRT1 transcript (58), or by direct p53-induced repression of the SIRT1 promoter that occurs under conditions of nutrient abundance (43).
Taken together, these results suggest the existence of a novel reciprocal relationship between p53 and the AMPK-SIRT1 signaling cycle. Increasing AMPK and SIRT1 activity under conditions of nutrient excess diminishes p53 abundance and cellular triglycerides, whereas increasing p53 dampens AMPK-SIRT1 signaling and blunts the triglyceride-lowering effect of metformin. In response to metformin, AMPK is the primary target, leading to increased activation of SIRT1. However, both molecules are necessary for the metformin-induced reduction in p53 protein abundance. The experiments presented here were limited to HepG2 cells; however, our novel data are consistent with a growing body of literature regarding AMPK, SIRT1, p53, and metabolic abnormalities. In accordance with this model, the phosphorylation of AMPK and the abundance of SIRT1 are diminished in the liver following high-fat feeding (1, 10, 18), whereas p53 protein abundance is increased (39). Hepatic p53 abundance is also elevated in two distinct models of hepatosteatosis, ob/ob mice and transgenic mice overexpressing sterol regulatory element-binding protein-1 (SREBP-1) (57). Furthermore, the transcription of miR-34a, which is regulated by p53 and results in decreased SIRT1 protein, is elevated in the livers of ob/ob and streptozotocin (STZ)-induced diabetic mice (34, 58).
On the basis of our findings, one could hypothesize that when hepatic p53 abundance is increased in conditions of obesity or fatty liver, it could play a role in diminished SIRT1 and AMPK activity, which would further propagate the diseased state. Thus the ability of metformin to lower p53 abundance could represent a novel additional therapeutic pathway that contributes to its beneficial metabolic effects. Whether metformin lowers p53 abundance in in vivo models of hepatosteatosis remains to be determined. Importantly, metformin has been shown to inhibit cellular proliferation and decrease cancer risk in diabetic patients (30); thus any decrease in p53 abundance that it produces in vivo would not likely increase the propensity towards tumorigenesis.
GRANTS
This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (F30 DK-082136 to L. E. Nelson; RO1 DK-19514 and DK-067509 to N. B. Ruderman; T32 DK-07201 to J. M. Cacicedo; and T32 HL-70024 to R. J. Valentine). M.-S. Gauthier was supported by a postdoctoral research fellowship from Fonds de la Recherche en Santé du Québec.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
L.E.N., J.M.C., Y.I., and N.B.R. conception and design of the research; L.E.N., R.J.V., M.-S.G., and Y.I. performed the experiments; L.E.N. and R.J.V. analyzed the data; L.E.N., R.J.V., J.M.C., Y.I., and N.B.R. interpreted the results of the experiments; L.E.N., R.J.V., and M.-S.G. prepared the figures; L.E.N. drafted the manuscript; L.E.N., R.J.V., J.M.C., M.-S.G., Y.I., and N.B.R. approved the final version of the manuscript; R.J.V., J.M.C., M.-S.G., Y.I., and N.B.R. edited and revised the manuscript.
ACKNOWLEDGMENTS
We thank Pere Puigserver for experimental suggestions and for reviewing the manuscript.
Present address of M.-S. Gauthier: Montreal Diabetes Research Center, University of Montreal, Montreal, QC, Canada H1W 4A4.
Footnotes
This article is the topic of an Editorial Focus by Mary C. Sugden and Mark J. Holness (49a).
REFERENCES
- 1. Barroso E, Rodriguez-Calvo R, Serrano-Marco L, Astudillo AM, Balsinde J, Palomer X, Vazquez-Carrera M. The PPARbeta/delta activator GW501516 prevents the down-regulation of AMPK caused by a high-fat diet in liver and amplifies the PGC-1alpha-Lipin 1-PPARalpha pathway leading to increased fatty acid oxidation. Endocrinology 152: 1848–1859, 2011 [DOI] [PubMed] [Google Scholar]
- 2. Brady CA, Attardi LD. p53 at a glance. J Cell Sci 123: 2527–2532, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bressac B, Galvin KM, Liang TJ, Isselbacher KJ, Wands JR, Ozturk M. Abnormal structure and expression of p53 gene in human hepatocellular carcinoma. Proc Natl Acad Sci USA 87: 1973–1977, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cacicedo JM, Yagihashi N, Keaney JF, Jr, Ruderman NB, Ido Y. AMPK inhibits fatty acid-induced increases in NF-kappaB transactivation in cultured human umbilical vein endothelial cells. Biochem Biophys Res Commun 324: 1204–1209, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458: 1056–1060, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Canto C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab 11: 213–219, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Caton PW, Nayuni NK, Kieswich J, Khan NQ, Yaqoob MM, Corder R. Metformin suppresses hepatic gluconeogenesis through induction of SIRT1 and GCN5. J Endocrinol 205: 97–106, 2010 [DOI] [PubMed] [Google Scholar]
- 8. Cool B, Zinker B, Chiou W, Kifle L, Cao N, Perham M, Dickinson R, Adler A, Gagne G, Iyengar R, Zhao G, Marsh K, Kym P, Jung P, Camp HS, Frevert E. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab 3: 403–416, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Costford SR, Bajpeyi S, Pasarica M, Albarado DC, Thomas SC, Xie H, Church TS, Jubrias SA, Conley KE, Smith SR. Skeletal muscle NAMPT is induced by exercise in humans. Am J Physiol Endocrinol Metab 298: E117–E126, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Deng XQ, Chen LL, Li NX. The expression of SIRT1 in nonalcoholic fatty liver disease induced by high-fat diet in rats. Liver Int 27: 708–715, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Eid AA, Ford BM, Block K, Kasinath BS, Gorin Y, Ghosh-Choudhury G, Barnes JL, Abboud HE. AMP-activated protein kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes. J Biol Chem 285: 37503–37512, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ, Auwerx J. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab 8: 347–358, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature 460: 587–591, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell 14: 661–673, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gao Z, Zhang J, Kheterpal I, Kennedy N, Davis RJ, Ye J. Sirtuin 1 (SIRT1) protein degradation in response to persistent c-Jun N-terminal kinase 1 (JNK1) activation contributes to hepatic steatosis in obesity. J Biol Chem 286: 22227–22234, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gauthier MS, Miyoshi H, Souza SC, Cacicedo JM, Saha AK, Greenberg AS, Ruderman NB. AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte. J Biol Chem 283: 16514–16524, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gauthier MS, Miyoshi H, Souza SC, Cacicedo JM, Saha AK, Greenberg AS, Ruderman NB. AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte: potential mechanism and physiological relevance. J Biol Chem 283: 16514–16524, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ha SK, Kim J, Chae C. Role of AMP-activated protein kinase and adiponectin during development of hepatic steatosis in high-fat diet-induced obesity in rats. J Comp Pathol 145: 88–94, 2011 [DOI] [PubMed] [Google Scholar]
- 19. Hardie DG, Carling D. The AMP-activated protein kinase–fuel gauge of the mammalian cell? Eur J Biochem 246: 259–273, 1997 [DOI] [PubMed] [Google Scholar]
- 20. Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature 387: 296–299, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Hosono S, Lee CS, Chou MJ, Yang CS, Shih CH. Molecular analysis of the p53 alleles in primary hepatocellular carcinomas and cell lines. Oncogene 6: 237–243, 1991 [PubMed] [Google Scholar]
- 22. Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren TJ, Cohen RA, Zang M. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem 283: 20015–20026, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hsu IC, Tokiwa T, Bennett W, Metcalf RA, Welsh JA, Sun T, Harris CC. p53 gene mutation and integrated hepatitis B viral DNA sequences in human liver cancer cell lines. Carcinogenesis 14: 987–992, 1993 [DOI] [PubMed] [Google Scholar]
- 24. Huber BE, Dearfield KL, Williams JR, Heilman CA, Thorgeirsson SS. Tumorigenicity and transcriptional modulation of c-myc and N-ras oncogenes in a human hepatoma cell line. Cancer Res 45: 4322–4329, 1985 [PubMed] [Google Scholar]
- 25. Imai Si, Guarente L. Ten years of NAD-dependent SIR2 family deacetylases: implications for metabolic diseases. Trends Pharmacol Sci 31: 212–220, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115: 577–590, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell 18: 283–293, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Kim MJ, Park IJ, Yun H, Kang I, Choe W, Kim SS, Ha J. AMP-activated protein kinase antagonizes pro-apoptotic extracellular signal-regulated kinase activation by inducing dual-specificity protein phosphatases in response to glucose deprivation in HCT116 carcinoma. J Biol Chem 285: 14617–14627, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Knowles BB, Howe CC, Aden DP. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science 209: 497–499, 1980 [DOI] [PubMed] [Google Scholar]
- 30. Kourelis TV, Siegel RD. Metformin and cancer: new applications for an old drug. Med Oncol 29: 1314–1327, 2011 [DOI] [PubMed] [Google Scholar]
- 31. Lai KP, Leong WF, Chau JF, Jia D, Zeng L, Liu H, He L, Hao A, Zhang H, Meek D, Velagapudi C, Habib SL, Li B. S6K1 is a multifaceted regulator of Mdm2 that connects nutrient status and DNA damage response. EMBO J 29: 2994–3006, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem 283: 27628–27635, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li M, Luo J, Brooks CL, Gu W. Acetylation of p53 inhibits its ubiquitination by Mdm2. J Biol Chem 277: 50607–50611, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Li S, Chen X, Zhang H, Liang X, Xiang Y, Yu C, Zen K, Li Y, Zhang CY. Differential expression of microRNAs in mouse liver under aberrant energy metabolic status. J Lipid Res 50: 1756–1765, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, Park O, Luo Z, Lefai E, Shyy John YJ, Gao B, Wierzbicki M, Verbeuren Tony J, Shaw Reuben J, Cohen Richard A, Zang M. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab 13: 376–388, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell 107: 137–148, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Meek DW, Hupp TR. The regulation of MDM2 by multisite phosphorylation–opportunities for molecular-based intervention to target tumours? Semin Cancer Biol 20: 19–28, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 450: 712–716, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Minamino T, Orimo M, Shimizu I, Kunieda T, Yokoyama M, Ito T, Nojima A, Nabetani A, Oike Y, Matsubara H, Ishikawa F, Komuro I. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med 15: 1082–1087, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell 7: 1085–1094, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Nagata D, Mogi M, Walsh K. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J Biol Chem 278: 31000–31006, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Nakamura S, Roth JA, Mukhopadhyay T. Multiple lysine mutations in the C-terminal domain of p53 interfere with MDM2-dependent protein degradation and ubiquitination. Mol Cell Biol 20: 9391–9398, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nemoto S, Fergusson MM, Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science 306: 2105–2108, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B, Qiu X, Stockman B, Thanabal V, Varghese A, Ward J, Withka J, Ahn K. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem 285: 8340–8351, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Piwkowska A, Rogacka D, Jankowski M, Dominiczak MH, Stepinski JK, Angielski S. Metformin induces suppression of NAD(P)H oxidase activity in podocytes. Biochem Biophys Res Commun 393: 268–273, 2010 [DOI] [PubMed] [Google Scholar]
- 46. Ponugoti B, Kim DH, Xiao Z, Smith Z, Miao J, Zang M, Wu SY, Chiang CM, Veenstra TD, Kemper JK. SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. J Biol Chem 285: 33959–33970, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rodriguez MS, Desterro JM, Lain S, Lane DP, Hay RT. Multiple C-terminal lysine residues target p53 for ubiquitin-proteasome-mediated degradation. Mol Cell Biol 20: 8458–8467, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ruderman NB, Xu XJ, Nelson L, Cacicedo JM, Saha AK, Lan F, Ido Y. AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab 298: E751–E760, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Suchankova G, Nelson LE, Gerhart-Hines Z, Kelly M, Gauthier MS, Saha AK, Ido Y, Puigserver P, Ruderman NB. Concurrent regulation of AMP-activated protein kinase and SIRT1 in mammalian cells. Biochem Biophys Res Commun 378: 836–841, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49a. Sugden MC, Holness MJ. Metformin, metabolic stress, and mitochondria. Focus on “A novel inverse relationship between metformin-triggered AMPK-SIRT1 signaling and p53 protein abundance in high glucose-exposed HepG2 cells.” Am J Physiol Cell Physiol (March 21, 2012). doi:10.1152/ajpcell.00090.2012 [DOI] [PubMed] [Google Scholar]
- 50. Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, Fotouhi N, Liu EA. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 303: 844–848, 2004 [DOI] [PubMed] [Google Scholar]
- 51. Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 107: 149–159, 2001 [DOI] [PubMed] [Google Scholar]
- 52. Vigneron A, Vousden KH. p53, ROS and senescence in the control of aging. Aging (Albany NY) 2: 471–474, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang P, Xu TY, Guan YF, Tian WW, Viollet B, Rui YC, Zhai QW, Su DF, Miao CY. Nicotinamide phosphoribosyltransferase protects against ischemic stroke through SIRT1-dependent adenosine monophosphate-activated kinase pathway. Ann Neurol 69: 360–374, 2011 [DOI] [PubMed] [Google Scholar]
- 54. Wang S, Zhang M, Liang B, Xu J, Xie Z, Liu C, Viollet B, Yan D, Zou MH. AMPKalpha2 deletion causes aberrant expression and activation of NAD(P)H oxidase and consequent endothelial dysfunction in vivo: role of 26S proteasomes. Circ Res 106: 1117–1128, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang X, Taplick J, Geva N, Oren M. Inhibition of p53 degradation by Mdm2 acetylation. FEBS Lett 561: 195–201, 2004 [DOI] [PubMed] [Google Scholar]
- 56. Winder WW, Thomson DM. Cellular energy sensing and signaling by AMP-activated protein kinase. Cell Biochem Biophys 47: 332–347, 2007 [DOI] [PubMed] [Google Scholar]
- 57. Yahagi N, Shimano H, Matsuzaka T, Sekiya M, Najima Y, Okazaki S, Okazaki H, Tamura Y, Iizuka Y, Inoue N, Nakagawa Y, Takeuchi Y, Ohashi K, Harada K, Gotoda T, Nagai R, Kadowaki T, Ishibashi S, Osuga J, Yamada N. p53 involvement in the pathogenesis of fatty liver disease. J Biol Chem 279: 20571–20575, 2004 [DOI] [PubMed] [Google Scholar]
- 58. Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci USA 105: 13421–13426, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yu X, McCorkle S, Wang M, Lee Y, Li J, Saha AK, Unger RH, Ruderman NB. Leptinomimetic effects of the AMP kinase activator AICAR in leptin-resistant rats: prevention of diabetes and ectopic lipid deposition. Diabetologia 47: 2012–2021, 2004 [DOI] [PubMed] [Google Scholar]
- 60. Zang M, Zuccollo A, Hou X, Nagata D, Walsh K, Herscovitz H, Brecher P, Ruderman NB, Cohen RA. AMP-activated protein kinase is required for the lipid-lowering effect of metformin in insulin-resistant human HepG2 cells. J Biol Chem 279: 47898–47905, 2004 [DOI] [PubMed] [Google Scholar]
- 61. Zhai WR, Paronetto F. Relationship between c-myc gene protein, nucleic acids and hepatitis B virus expression in hepatoma cell lines and their corresponding tumors in nude mice. J Exp Pathol 4: 213–225, 1989 [PubMed] [Google Scholar]
- 62. Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 108: 1167–1174, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zou MH, Hou XY, Shi CM, Nagata D, Walsh K, Cohen RA. Modulation by peroxynitrite of Akt- and AMP-activated kinase-dependent Ser1179 phosphorylation of endothelial nitric oxide synthase. J Biol Chem 277: 32552–32557, 2002 [DOI] [PubMed] [Google Scholar]