Abstract
Bacterial toxins such as cholera toxin induce diarrhea by both direct epithelial cell generation of cyclic nucleotides as well as stimulation of the enteric nervous system (ENS). Agonists of the extracellular calcium-sensing receptor (CaSR) can reduce toxin-stimulated fluid secretion in ENS-absent colonic epithelial crypts by increasing phosphodiesterase-dependent cyclic-nucleotide degradation. Here we show that the CaSR is also highly expressed in tetrodotoxin (TTX)-sensitive neurons comprising the ENS, suggesting that CaSR agonists might also function through neuronal pathways. To test this hypothesis, rat colon segments containing intact ENS were isolated and mounted on Ussing chambers. Basal and cyclic nucleotide-stimulated electrolyte secretions were monitored by measuring changes in short-circuit current (Isc). CaSR was activated by R-568 and its effects were compared in the presence and absence of TTX. Consistent with active regulation of anion secretion by the ENS, a significant proportion of Isc in the proximal and distal colon was inhibited by serosal TTX, both at basal and under cyclic AMP-stimulated conditions. In the absence of TTX, activation of CaSR with R-568 significantly reduced basal Isc and cyclic AMP-stimulated Isc; it also completely reversed the cAMP-stimulated secretory responses if the drug was applied after the forskolin stimulation. Such inhibitory effects of R-568 were either absent or significantly reduced when serosal TTX was present, suggesting that this agonist exerts its antisecretory effect on the intestine by inhibiting ENS. The present results suggest a new model for regulating intestinal fluid transport in which neuronal and nonneuronal secretagogue actions are modulated by the inhibitory effects of CaSR on the ENS. The ability of a CaSR agonist to reduce secretagogue-stimulated Cl− secretion might provide a new therapeutic approach for secretory and other ENS-mediated diarrheal conditions.
Keywords: secretory diarrhea, short-circuit current
acute secretory diarrheal illnesses affect millions of children and adults each year, and morbidity and mortality from the large fluid and electrolyte losses remain high (2, 30). The extracellular calcium-sensing receptor (CaSR) (4) is an ancient G protein-coupled cell surface receptor that is expressed in diverse tissues in mammalian (5) and marine (32) species and is a key regulator of tissue responses required for calcium homeostasis (4), salt and water balance (34, 36), and osmotic regulation (32). The primary physiological ligand for the CaSR is extracellular ionized calcium (Cao2+), providing a mechanism for Cao2+ to function as a first messenger. The CaSR also functions as a more general sensor of the extracellular milieu due to allosteric modification of Cao2+ affinity by polyamines, l-amino acids, pH, and ionic strength (39).
Enteric mucosal cells along the entire small and large intestines express the CaSR (8, 10, 11, 20). Knowledge of the physiological function of CaSR in the gastrointestinal tract is limited (25). CaSR has been identified on both the apical and basolateral membranes of human (11, 38) and rat colonocytes (8, 11). Receptors localized to both membrane domains of this polarized epithelium are functionally active and can be activated by Cao2+ and other polycations such as spermine with similar potency and EC50 values (10, 11). Previous studies in colonic crypts showed that CaSR activation from either the mucosal or serosal side inhibited net fluid secretion (10, 11, 21) and cyclic nucleotide accumulation (21) induced by synthetic and natural secretagogues such as forskolin (37) and guanylin (16), which generate cyclic AMP and cyclic GMP, respectively. CaSR activation also blocks the effects of bacterial enterotoxins (21) such as cholera toxin (CTX) (19), a potent activator of membrane bound adenylyl cyclase leading to elevated intracellular levels of cyclic AMP. It also blocks the effects of the heat-stable Escherichia coli enterotoxin STa (18), which enhances cytosolic cGMP accumulation through the guanylyl cyclase C-type guanylin receptor.
Although the role of CaSR in intestinal epithelial cells has been studied, CaSR has also been localized to the enteric nervous system (ENS), including the myenteric plexus (Auerbach's plexus) and the submucosal plexus (Meissner's plexus) and thus could regulate neural responses (8). In rodents and humans, the ENS, mainly the submucosal plexus, secretes factors that regulate fluid secretion (see reviews in Refs. 14, 17, 27). There is evidence from in vivo experiments that the ENS modulates intestinal fluid secretion induced by bacterial enterotoxins (CTX, STa) (6, 17), as well as by viral enterotoxins (rotaviral NSP4) (26, 28). For example, CTX-induced fluid secretion is blocked by tetrodotoxin (TTX), an inhibitor of neurotransmission (6, 17). On this basis, a dual-pathway model for fluid secretion in intestine has been proposed: 1) a nonneuronal fluid secretory response due to the binding of enterotoxins directly to enterocytes, leading to generation of cyclic nucleotides, which is TTX insensitive; 2) a neuronal secretory response that is mediated by stimulation of the ENS, which is TTX sensitive. We have previously demonstrated that CaSR agonists can inhibit toxin-stimulated fluid secretion through direct enhancement of cyclic nucleotide degradation in colonic epithelial crypts in the absence of the ENS (10, 11, 21). We now show that CaSR agonists can also exert their antisecretory action indirectly by activating the CaSR in the ENS.
MATERIALS AND METHODS
Animals
Experiments were performed with young adult male Sprague-Dawley rats (5–10 wk old with body weight of 100–300 g) obtained from Charles River Laboratories and Taconic Farms. The use of rats as well as the protocol for isolating colon tissues was approved by the Institutional Animal Care and Use Committee (IACUC no. FG0008) at the Veterans Affairs Connecticut Healthcare System. Animals were fed and maintained on regular chow (PMI Nutrition International) with free access to water but fasted overnight before the investigation. Rats were euthanized with standard CO2 inhalation and by cervical dislocation before removing the colon.
Immunolocalization of CaSR Within the ENS
The colons from male Sprague-Dawley rats (weighing 200–250 g) were isolated, cut open along the mesenteric border, and fixed in PLP (periodate-lysine-paraformaldehyde) followed by 12.5% sucrose in PBS solution. Tissues were kept overnight at 4°C in 30% sucrose in PBS. Tissues were then embedded in Tissue Tek Optimum Cutting Temperature (Sakura Finetek USA, Torrance, CA) and frozen in isopentane cooled on dry ice. Five-micrometer frozen sections were cut with a Leica CM3050 cryostat and mounted on Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA).
Immunostaining was carried out as described (11). Briefly, cryosections were antigen retrieved by using Citra Microwave Solution (Biogenex, San Ramon, CA) followed by treatment with 1% SDS in PBS for 5 min to expose antigenic sites. After SDS treatment, slides were rinsed three times with PBS and incubated 30 min with 1% BSA-PBS followed by three 5-min washes in PBS. Slides were then incubated with 4% Seablock for 1 h at room temperature followed by overnight or over-weekend incubation with primary affinity-purified CaSR monoclonal antibody (5C10, ADD, 1:100 dilution, Thermo Scientific, Rockford, IL). Following three 5-min washes in PBS, the slides were incubated with Alexa 594 goat anti-mouse IgG followed by three 5-min washes in PBS. Slides were then mounted with Vectashield (Vector Laboratories) and were examined with a Nikon Eclipse 800 Research microscope equipped with a charge-coupled device camera. Immunofluorescence photomicrographs were processed using Adobe Photoshop 5.0 or CS3 software. To demonstrate the CaSR specificity of the antibody labeling, a control experiment was performed in which the primary antibody was preabsorbed overnight with an excess amount of the immunizing peptide (1 μg/ml) before applied for labeling. To differentiate the antibody labeling signals from background autofluorescence, control experiments in which the CaSR primary and/or secondary antibodies were omitted were also performed. All incubations were done at room temperature except those with primary antibodies were done at 4°C.
To localize the neuronal tissues within the ENS, an antibody that recognized the neuron-specific class III β-tubulin was used (TUJ1, mouse monoclonal, 1:1,500; Covance, Princeton, NJ). Immunofluorescence labeling was carried out by the same technique as described above except that no antigen-retrieval treatments were applied and the secondary antibody was Alexa 488 goat anti-mouse IgG (Molecular Probes, Eugene, OR; 1:5,000 diluted in 1% BSA/PBS).
To further establish that CaSR colocalizes with the ENS, double immunofluorescence labeling experiments were performed by using a modified protocol for a mouse-on-mouse immunodetection described by Tornehave et al. (40). Briefly, tissues were first stained with the CaSR primary and secondary antibodies, followed by microwaving in citrate buffer for 5 min × 3 before they were subjected to second staining with the class-III β-tubulin antibodies. Immunofluorescence signals from red and green channels were acquired and stored separately, and overlay images were generated with Adobe Photoshop CS3. Previous studies (40) as well as our control studies confirmed that microwaving in between the staining cycles does not elute antibodies but prevents their reactions with subsequently applied reagents.
Ussing Chamber Isc Measurements from Colonic Segments
Male Sprague-Dawley rats weighing 100–300 g were euthanized and segments of colons were quickly isolated. Segments were cut open along the mesenteric border into a flat sheet and flushed with ice-cold basal HEPES-Ringer solution containing 0.5 mM Ca2+ and Mg2+. The intact full-thickness segments containing all the layers of the proximal and distal colons were used. The intestinal sheets were mounted between two halves of a modified Ussing chamber (Physiologic Instruments, San Diego, CA) and short circuited by a voltage clamp (VCC MC6, Physiologic Instruments) with correction for solution resistance. The exposure area was 0.3 cm2. Unless otherwise indicated, the mucosal and serosal surfaces of the tissue were bathed in reservoirs with 3–5 ml HEPES-Ringer solution containing 110 mM NaCl, 0.5 mM CaCl2, 0.5 mM MgCl2, 5 mM KCl, 10 mM glucose, and 22 mM HEPES, pH 7.4, maintained at 37°C and continuously bubbled with 100% O2. On average, an interval of 10 min elapsed between euthanizing the animal and mounting the tissue in the chamber. Tissues were allowed a minimum of 15-min stabilization and basal recording period before test reagents or vehicles were added to the mucosal and/or serosal sides of the intestine. Short-circuit current, Isc (μA/cm2), was monitored continuously and data were acquired via DATAQ instruments and were stored in a PC and processed using the program Acquire & Analyze. The Isc is defined as the current flow through the tissue when the tissue is short circuited (i.e., when the voltage across the tissue is zero). Magnitudes (μA/cm2) of change in Isc were determined before and after additions of test reagents or vehicle. Significance of changes was tested by using the paired Student's t-test.
For measurements of TTX-sensitive Isc, 2 μM TTX was applied to the serosal solution, and the differences in stable Isc before and after TTX treatment were compared and calculated. According to a pilot experiment in which a dose-response effect on Isc by TTX was sought, this dose of TTX was the lowest concentration that maximally blocked the activity of the ENS without directly affecting the function of the epithelium (21).
To determine whether the basal and forskolin-stimulated Isc reflected Cl− secretion, some experiments were performed in Cl−-free Ringer solutions. In those experiments, tissues were bathed initially in normal Cl− Ringer solutions and then in Cl−-free Ringer solutions. Isc were recorded and corrected for junction potentials before used for calculations.
Solutions
Since calcium bicarbonate could precipitate in calcium-containing bicarbonate-buffered solutions, HEPES-buffered Ringer solutions were used in the present study. In preliminary experiments using HEPES-buffered Ringer solutions, we established that bath Ca2+ remains constant. Unless otherwise indicated, a HEPES-Ringer solution containing 110 mM NaCl, 0.5 mM CaCl2, 0.5 mM MgCl2, 5 mM KCl, 10 mM glucose, and 22 mM HEPES, pH 7.4, was used throughout the entire study. A Ringer solution with reduced concentrations of divalent cations was used because it minimizes background stimulation of CaSR by extracellular Ca2+ and Mg2+ at basal state while maintaining tissue viability and integrity (10, 11, 21). However, similar inhibitory effects of R-568 were observed in a Ringer solution with 1.25 mM Ca2+ and Mg2+, although R-568 seemed to produce a slightly less pronounced effect in the latter solution (see Fig. 6 below). A Cl−-free HEPES-Ringer solution was used to determine the contribution of Cl− to the basal and stimulated Isc. For a Cl−-free HEPES-Ringer, 110 mM Na+ gluconate replaced NaCl, 0.5 mM magnesium gluconate replaced MgCl2, and 0.5 mM CaCl2 was replaced by calcium gluconate (2.5 mM calcium gluconate was added to offset the calcium-chelating effects of gluconate as determined by Ca2+ electrode measurements). All solutions were adjusted to pH of 7.4 at 37°C and osmolarity of 300 mosM.
Fig. 6.
R-568 produces similar inhibitory effects on Isc in the colon at 1.25 mM extracellular ionized calcium (Cao2+). Rat distal colons were treated the same way by forskolin and R-568 as in the legend of Fig. 5 except that Cao2+ of 1.25 mM was used. A: Isc responses to forskolin and R-568 in the absence of TTX. B: Isc responses to forskolin and R-568 in the presence of TTX. C summarizes the ΔIsc induced by R-568 in the absence and presence of TTX.
Chemicals
Forskolin, carbachol, and bumetanide were obtained from Sigma, and stock solutions were prepared in dimethyl sulfoxide (DMSO) (forskolin and bumetanide) or in water (carbachol). TTX was purchased from Enzo Life Science (Plymouth Meeting, PA), and 2 mM stock solutions were prepared in 10 mM acetic acid. R-568 was purchased from Tocris Bioscience (Ellisville, MI), and 10 mM stock solutions were prepared in DMSO.
Statistical Analysis
Values are given as means ± SE of n experiments. Statistical comparisons between two means were performed by Student's t-test, whereas comparisons among multiple means were by one-way ANOVA. Both tests were performed by using either Microsoft Excel 07 for Windows or GraphPad Prism version 3 for Windows (GraphPad Software, San Diego, CA). P < 0.05 was considered significant.
RESULTS
CaSR is Highly Expressed in the ENS
Although a previous study has suggested the presence of CaSR in the regions of the myenteric plexus (Auerbach's plexus) and the submucosal plexus (Meissner's plexus) of the ENS of the gastrointestinal tract (8), there remains uncertainty as to CaSR expression in the neurite projections and nerve fibers that extend from the enteric plexuses into the periphery. Thus in the present study we performed both single and double immunofluorescent labeling studies with special attention to its localization in the peripherally projecting neurites and nerve endings.
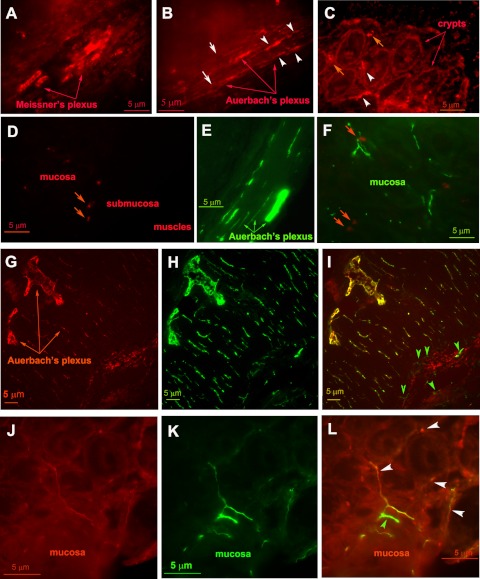
Figure 1, A–D, shows representative images of CaSR immunofluorescence labeling in rat distal colon. The CaSR antibody is an affinity-purified monoclonal antibody raised against a synthetic peptide corresponding to the extracellular domain (residues 214–235) of the human CaSR. Its cross-reactivity with rodents, specificity, and applications have been described (1, 22, 41, 42). Intense CaSR immunoreactivity (red fluorescence) was noted both in the submucosal Meissner's plexus (Fig. 1A) and in the region of the myenteric Auerbach's plexus (Fig. 1B). The peripherally projecting neurites and nerve fibers were labeled, both within the muscle layers (Fig. 1B, white arrowheads) and in the mucosa layer (Fig. 1C, white arrowheads). The pattern of the labeling for CaSR antibody was similar to that of class III β-tubulin, a specific marker of neural tissues (Fig. 1, E and F, green fluorescence). Consistent with our previous study (11), labeling on apical and basolateral membranes of the epithelium was also observed (Fig. 1C) and was weaker than in the ENS. No specific signal for CaSR was observed when the CaSR primary antibody was preabsorbed with an excess amount of the immunizing peptide (Fig. 1D) or when the CaSR “antigen retrieval” pretreatment was omitted (not shown). Note that the large red fluorescent structures between the crypts (Fig. 1, C and D, red arrows) were interstitial cells that exhibit autofluorescence because these were also seen in Fig. 1F (red arrows), in which the CaSR primary and secondary antibodies were absent. Thus by immunofluorescence CaSR was detected in the colon consistent with its presence in the ENS.
Fig. 1.
Calcium-sensing receptor (CaSR) is highly expressed in the enteric nervous system (ENS) of the colon. Shown are representative images of single (A–F) or double (G–L) labeling immunofluorescence studies. Rat distal colon tissues were stained with anti-CaSR (red, A–D), anti-class III β-tubulin (green, E and F), or both (G–L). Note that intense CaSR staining is evident in the nerve fibers and neurite projections (white arrowheads) and somas (white arrows) of the submucosal Meissner's plexus (A) and the myenteric Auerbach's plexus (B), the pattern similar to that of class III β-tubulin (green, E and F). Consistent with our previous study (11), labeling on apical and basolateral membranes of the crypt epithelium was also noted (C). No signal similar to the CaSR was seen when the CaSR primary antibody was preabsorbed with an excess amount (1 μg/ml) of the immunizing peptide (D). Note that the large red fluorescent structures noted in the mucosa layer (orange arrows, D; also seen in C) were interstitial cells that exhibit autofluorescence; these latter were also seen in F (orange arrows), in which the CaSR primary and secondary antibodies were absent. G–L: representative immunofluorescence images with double labeling. Rat distal colon tissues were probed with both anti-CaSR and anti-class III β-tubulin as described in materials and methods. G and J: staining of nerve fibers and somas of the ENS was observed with anti-CaSR (red). H and K: staining against class III β-tubulin to identify neural tissues (green). I and L: merged images with labeling against CaSR (red) and the class III β-tubulin (green) demonstrating expression of both proteins in the same neutral tissues (yellow), including the nerve terminals innervating the crypt epithelium (L, white arrowheads). Note that, although labeling overlap is seen in most neuronal tissues, structures labeled by anti-class III β-tubulin alone but not by anti-CaSR are also noted (I and L, green arrowheads), suggesting that CaSR is present in most, but not all, neuronal tissues of the ENS. A similar labeling pattern of CaSR was noted in proximal colon (not shown).
To confirm the identity of the ENS, double labeling of CaSR and class III β-tubulin was performed (Fig. 1, G–L). Figure 1, G and J, shows CaSR immunofluorescence (red), Fig. 1, H and K, shows class III β-tubulin labeling (green), whereas Fig. 1, I and L are overlay images. CaSR was present in the majority of the neural tissues. The peripherally projecting neurites and nerve fibers were labeled both within the muscle layers (Fig. 1, G–I) and in the mucosa and submucosa layers (Fig. 1, J–L), including the nerve terminals innervating the epithelium (Fig. 1L, white arrowheads).
Activation of CaSR in the ENS Modulates Intestinal Fluid Secretion
Characterization of Isc responses of the colon in the presence and absence of TTX.
After verifying that CaSR protein was present in the ENS, we next determined whether the receptor is functionally active. The ENS is known to actively modulate the Cl− secretion by the intestinal epithelium (see reviews in Refs. 17, 27). The changes in Cl− secretion can be assessed by measuring Isc by the Ussing chamber technique. Thus, to assess the functional activity of the neuronal CaSR, rat colon segments with intact ENS were isolated, mounted on the Ussing chamber, and perfused. Basal and cyclic nucleotide-stimulated Isc responses to the CaSR agonist R-568 were measured. In this series of experiments, R-568 was added to the serosal compartment of the Ussing chamber to activate the neuronal CaSR. We have shown that CaSR is also expressed in the basolateral membrane of the colonic epithelial cells and modulates the fluid secretion by this epithelium in the isolated perfused colonic crypt that is devoid of ENS (10, 11, 21). Thus, in this setting, the serosal R-568 might activate both neuronal and nonneuronal CaSRs. To determine the contribution of neural pathways, TTX was added to the serosal side to inactivate the activity of the ENS.
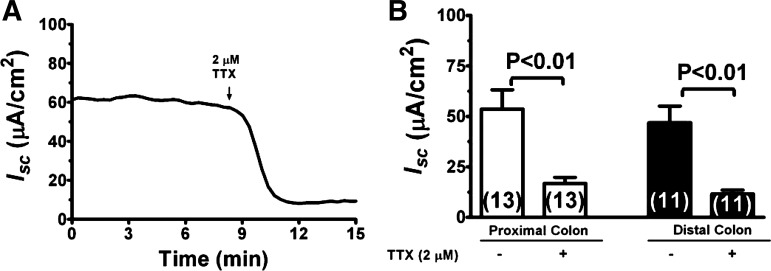
Under our experimental conditions, a relatively high basal Isc was observed in both the proximal and distal colon (Table 1). Ion substitution studies indicate that this basal Isc represented primarily Cl− secretion as substitution of bath Cl− ion by gluconate nearly completely eliminated Isc (Table 1; also see below in Fig. 8A). Figure 2 shows Isc responses to TTX at basal state. Addition of 2 μM TTX to serosal solution significantly reduced basal Isc by 69% in proximal colon and 75% in distal colon (Fig. 2: P < 0.01). In contrast, addition of TTX to mucosal solution did not elicit any significant changes in Isc, either in the proximal or distal colons (not shown). The results demonstrate that TTX-sensitive Isc was present at basal conditions. The differential effects of mucosal vs. serosal TTX on Isc are consistent with prior observations that the TTX inhibition of Isc is the result of inhibition of the ENS.
Table 1.
Basal bioelectric parameters in the rat proximal and distal colon
| Normal Ringer |
Cl−-Free Ringer |
|||
|---|---|---|---|---|
| Proximal | Distal | Proximal | Distal | |
| Isc, μA/cm2 | 44 ± 7 (35) | 43 ± 5 (35) | 1.4 ± 2.4 (10)* | 1.7 ± 1.7 (10)* |
| R, Ω/cm2 | 127 ± 13 (35) | 142 ± 12 (35) | 380 ± 35 (10)* | 423 ± 17 (10)* |
| PD, mV | 6.0 ± 0.7 (35) | 7.9 ± 0.9 (35) | 1.2 ± 0.2 (10)* | 1.1 ± 0.2 (10)* |
Data shown are means ± SE with number of intestinal segments shown in parentheses. Potential difference (PD) is lumen negative. No polarity is assigned to the short-circuit current (Isc), but it is in the same direction as PD. R, resistance.
P < 0.01 vs. normal Ringer.
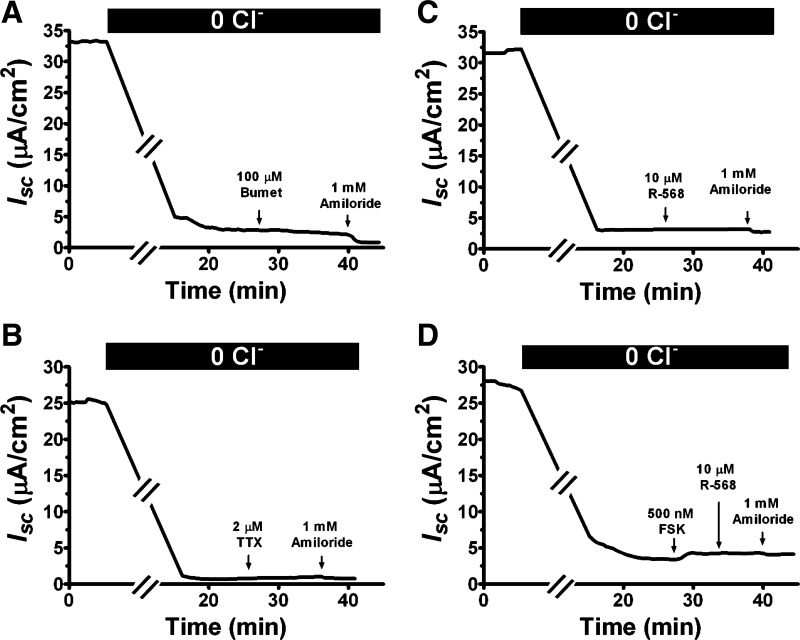
Fig. 8.
Removal of Cl− ion from bath solutions eliminates both basal and cAMP-stimulated Isc as well as their responses to TTX and R-568. Shown are representatives of 2–6 recordings of Isc responses of distal colon to bilateral Cl− ion substitution and subsequent additions of serosal bumetanide (A), serosal TTX (B), bilateral forskolin (D), mucosal amiloride (A–D), and serosal R-568 with (D) or without (C) prior forskolin stimulation. Similar responses were noted in the proximal colon as well.
Fig. 2.
Inhibition of ENS by TTX reduces basal short-circuit current (Isc) in the colon. A: representative recording of Isc responses in a rat distal colon to addition of 2 μM TTX to serosal solution. B: quantitation of Isc responses to TTX in proximal and distal colons.
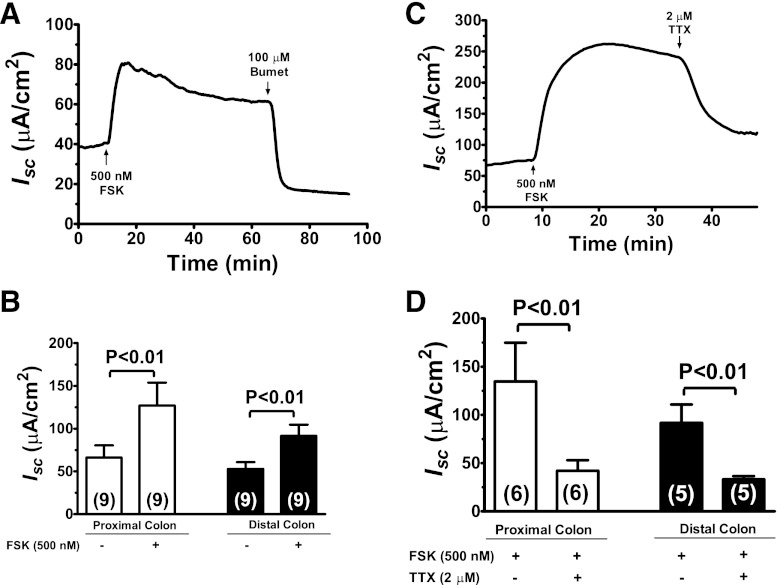
We next examined the effects of TTX on cyclic AMP-stimulated secretory responses. According to the present model, the fluid secretion in the intestine is primarily driven by Cl− secretion. In the rat colon, Cl− enters the epithelial cells via the basolateral bumetanide-sensitive Na+/K+/2Cl− cotransporter and exits into the lumen via the apical Cl− conductance; this process is stimulated by increases in intracellular cyclic AMP. Consistent with this model, we showed in Fig. 3A that stimulation of cyclic AMP by forskolin in the colon increased Isc; this increase in Isc was completely abolished by serosal bumetanide (Fig. 3A) or replacement of Cl− ion (see below in Fig. 8D), suggesting that the forskolin-stimulated increase in Isc was primarily due to Cl− secretion. The changes in Isc by forskolin in proximal and distal colons are shown in Fig. 3B. Forskolin significantly increased the Isc in both the proximal and distal colon; the increases in Isc induced by forskolin were similar in proximal and distal colons.
Fig. 3.
Inhibition of ENS by TTX reduces cAMP-stimulated Isc in the colon. A: representative recording of Isc responses to forskolin (FSK) and bumetanide (Bumet) in a rat distal colon segment. Following additions of 500 nM forskolin to mucosal and serosal solutions, 100 μM of bumetanide was added to serosal solution to inhibit the Na+/K+/2Cl− cotransporter, as indicated. B: quantitation of forskolin-stimulated Isc responses in proximal and distal colons. C: representative tracing of Isc responses in a rat distal colon segment to TTX under forskolin-stimulated condition. Following additions of 500 nM forskolin to mucosal and serosal solutions, 2 μM TTX was added to serosal solution, as indicated. D: quantitation of Isc responses to TTX under forskolin-stimulated condition in the proximal and distal colons.
Isc responses to TTX under forskolin-stimulated state are shown in Fig. 3, C and D. As illustrated in Fig. 3C, stimulation with forskolin increased Isc; subsequent addition of 2 μM TTX significantly reduced Isc responses induced by forskolin. This TTX effect was observed in both proximal and distal colon with an inhibition of 69% (P < 0.01) in the proximal colon and 64% (P < 0.01) in the distal colon (Fig. 3D). The results are consistent with a dual-pathway model of intestinal cyclic AMP-stimulated electrolyte secretion (17, 27) with both direct epithelial stimulation of Cl− secretion and indirect epithelial stimulation through activation of the ENS.
Effect of R-568 on Isc responses in the presence and absence of TTX.
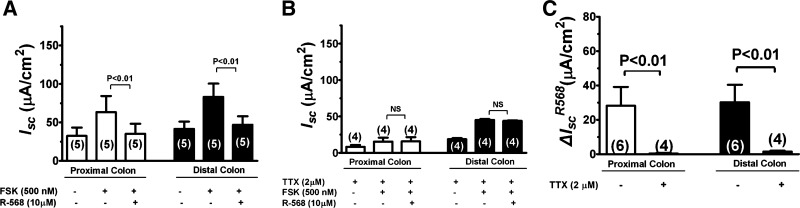
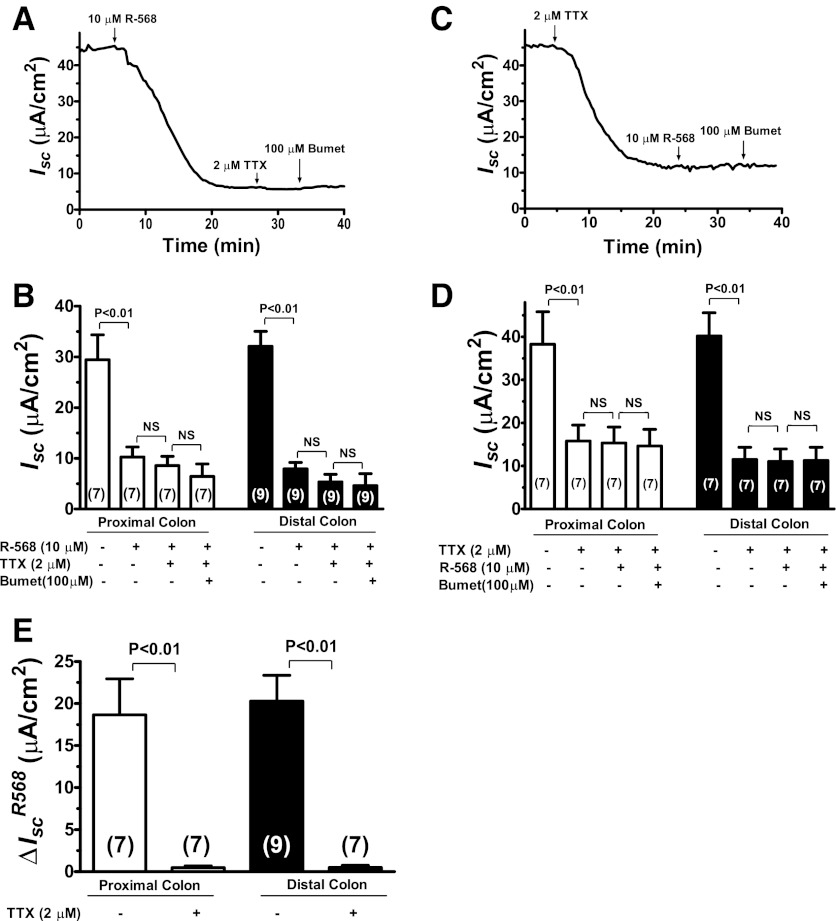
To assess the role of CaSR in mediating intestinal ion secretion, the effects of R-568, a specific CaSR agonist, were examined under basal (Fig. 4; also Table 2) and cyclic AMP-stimulated conditions (Fig. 5; also Table 3). The specificity of R-568 to activate CaSR has been characterized in prior studies (21, 24). Figure 4A shows a representative tracing and Fig. 4B summarizes changes of Isc responses to sequential additions of R-568, TTX, and bumetanide. Activation of CaSR by addition of 10 μM R-568 to serosal solution significantly reduced the basal Isc in both the proximal and distal colon with percent inhibition of 61% in proximal colon and 68% in distal colon. Following R-568 addition, subsequent additions of TTX and/or bumetanide had no or minimal additional effect on Isc, suggesting that R-568-mediated inhibition of Isc is achieved primarily through inhibition of TTX-sensitive “neuronal” Isc. In agreement with this, prior treatment with TTX completely abolished the effect of R-568 alone or together with bumetanide to reduce the Isc (Fig. 4, C and D). Similar inhibition was also observed with prior treatment with bumetanide (not shown) or by removal of Cl− anion from the bath (see Fig. 8 below). The latter suggests that the R-568-inhibitable, TTX-sensitive Isc represents Cl−-mediated secretion. Figure 4E summarizes the ΔIsc changes induced by R-568 in the absence and presence of TTX. In the absence of TTX, activation of CaSR with R-568 significantly reduced the Isc in both the proximal and distal colon; such inhibitory effects of R-568 were completely abolished when serosal TTX was present, suggesting that the effect of the agonist is mediated by inhibiting ENS. Tissue viability was confirmed by eliciting an evoked transient Isc increase with 100 μM carbachol (not shown).
Fig. 4.
Activation of CaSR by R-568 abolishes the effect of TTX to reduce basal Isc in the colon. A: representative recording of Isc responses in a rat distal colon segment to sequential additions of R-568, TTX, and bumetanide. All drugs were added to serosal solution with the concentrations indicated. B: quantitative changes in Isc following treatment with R-568 ± TTX ± bumetanide in the proximal and distal colons. NS, not significant. C: representative recording of Isc responses in a rat distal colon segment to sequential additions of TTX, R-568, and bumetanide. All drugs were added to serosal solution with the concentrations indicated. D: quantitative changes in Isc following treatment with TTX ± R-568 ± bumetanide in the proximal and distal colons. E summarizes the ΔIsc induced by R-568 in the absence and presence of TTX.
Table 2.
Effect of R-568 on basal Isc in the colon
|
Isc, μA/cm2 |
||||
|---|---|---|---|---|
| Absence of TTX |
Presence of TTX |
|||
| Proximal | Distal | Proximal | Distal | |
| Control | 31 ± 5 (5) | 30 ± 3 (7) | 16 ± 4 (7)† | 12 ± 3 (7)‡ |
| R-568 | 12 ± 2 (5)* | 9 ± 1 (7)* | 15 ± 4 (7)nsNS | 11 ± 3 (7)nsNS |
Data shown are means ± SE (n).
P < 0.01 vs. control; ns, P > 0.05 vs. control.
P < 0.01 vs. absence of TTX;
P < 0.05 vs. absence of TTX; NS, P > 0.05 vs. absence of TTX.
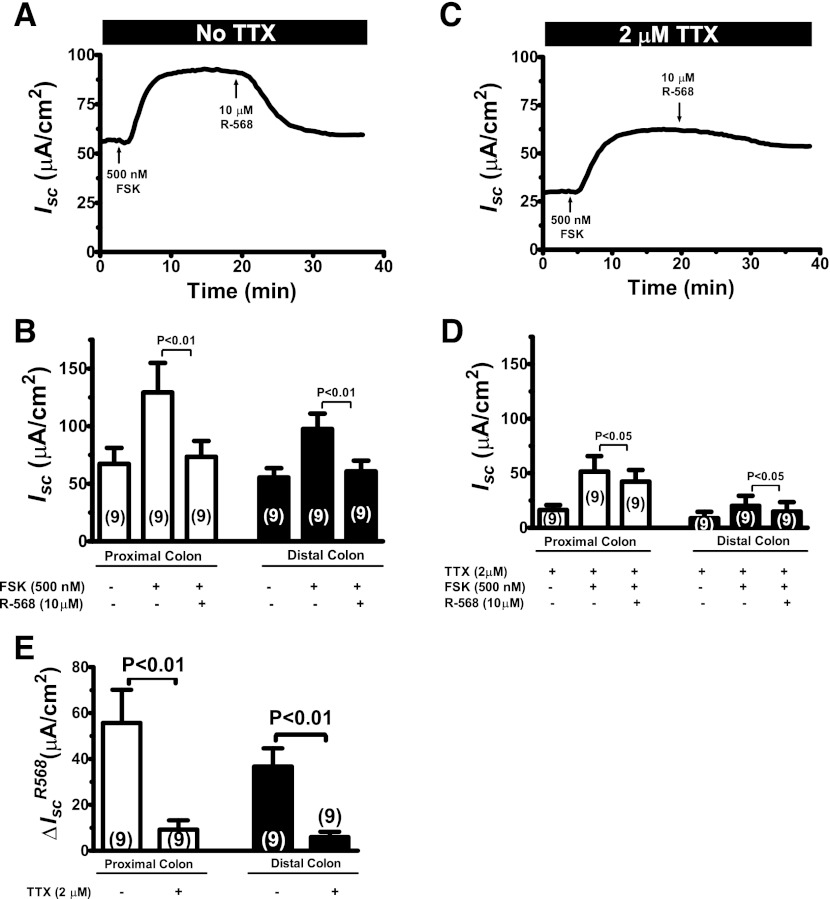
Fig. 5.
Activation of CaSR by R-568 attenuates the effect of TTX to reduce cAMP-stimulated Isc in the colon. A: representative recording of Isc responses to sequential additions of forskolin and R-568 in the absence of TTX in a rat distal colon segment. Following additions of 500 nM forskolin to mucosal and serosal solutions, 10 μM of R-568 was added to serosal solution to activate CaSR activity. B: quantitative changes in Isc following treatment with forskolin ± R-568 in the proximal and distal colons. C: representative recording of Isc responses to sequential additions of forskolin and R-568 in the presence of TTX in a rat distal colon segment. Following additions of 500 nM forskolin to mucosal and serosal solutions, 10 μM of R-568 was added to serosal solution to activate CaSR activity. D: quantitative changes in Isc following treatment with forskolin ± R-568 in the proximal and distal colons. E summarizes the ΔIsc induced by R-568 in the absence and presence of TTX.
Table 3.
Effect of R-568 in reducing forskolin-induced ΔIsc in the colon
| ΔIscFSK, μA/cm2 |
||||
|---|---|---|---|---|
| Absence of TTX |
Presence of TTX |
|||
| Proximal | Distal | Proximal | Distal | |
| Control | 59 ± 16 (9) | 37 ± 8 (9) | 33 ± 9 (8)† | 13 ± 5 (8)† |
| R-568 | 6 ± 5 (9)* | 5 ± 2 (9)* | 27 ± 7 (8)nsNS | 6 ± 4 (8)nsNS |
Data shown are means ± SE (n). ΔIscFSK, forskolin-induced ΔIsc.
P < 0.01 vs. control; ns P > 0.05 vs. control;
P < 0.05 vs. absence of TTX. NS, P > 0.05 vs. absence of TTX.
Figure 5 shows effects of R-568 on Isc under forskolin-stimulated condition. In the absence of TTX, forskolin increased Isc; the increases in Isc by forskolin were completely reversed by subsequent addition of R-568 (Fig. 5, A and B). In the presence of TTX, forskolin also increased Isc, but to a lesser extent. The ΔIsc changes induced by forskolin in the absence vs. presence of 2 μM TTX are shown in Table 3. TTX pretreatment significantly reduced forskolin-stimulated secretory responses by 41.7% in proximal colon and 67.8% in distal colon (P < 0.001). Under the TTX inhibitory condition, further addition of R-568 only partially but significantly reduced the increases in Isc induced by forskolin (Fig. 5, C and D). Figure 5E summarizes the ΔIsc changes induced by R-568 in the absence and presence of TTX. When TTX was present, the ability of R-568 to inhibit the forskolin stimulation of Isc was significantly attenuated (Fig. 5E). Similar effects of R-568 on forskolin-stimulated Isc were observed in a Ringer solution with 1.25 mM Ca2+ and Mg2+, although R-568 seemed to produce slightly less pronounced effects in the latter solution (Fig. 6; also see Table 4). Taken together, the results suggested that the effect of basolateral R-568 to inhibit cyclic AMP stimulation of Isc in the colon is achieved by at least two pathways: TTX-sensitive neuronal pathway and TTX-insensitive nonneuronal pathway.
Table 4.
Levels of R-568-induced ΔIsc in forskolin-stimulated colon at different Cao2+ conditions
| ΔIsR-568, μA/cm2 |
||||
|---|---|---|---|---|
| Absence of TTX |
Presence of TTX |
|||
| Proximal | Distal | Proximal | Distal | |
| Cao2+ = 0.5 mM | 56 ± 14 (9) | 37 ± 8 (9) | 9 ± 4 (8) | 6 ± 2 (8) |
| Cao2+ = 1.25 mM | 28 ± 11 (6)ns | 30 ± 10 (6)ns | 1 ± 1 (4)ns | 2 ± 1 (4)ns |
Data shown are means ± SE (n). ΔIscR-568, R-568-induced ΔIsc. ns, P > 0.05 vs. Cao2+ = 0.5 mM.
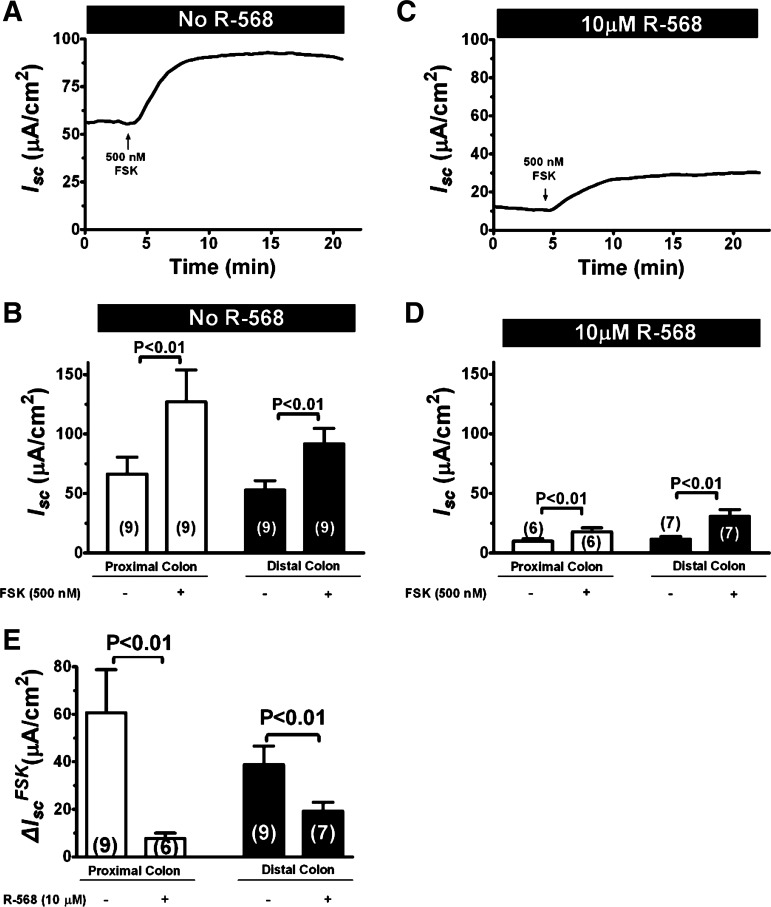
To provide further evidence in support of the conclusion that R-568 inhibits both basal and forskolin-stimulated Isc, additional experiments in which R-568 was present prior to the forskolin stimulation were performed, and the results are shown in Fig. 7. R-568 pretreatment reduced both basal (compare Fig. 7C vs. 7A; also see Fig. 4) and forskolin-stimulated Isc (compare Fig. 7, C and D vs. A and B). The ΔIsc changes induced by forskolin in the absence vs. presence of 10 μM R-568 are shown in Fig. 7E. R-568 pretreatment significantly reduced forskolin-stimulated Isc by 87.2% in proximal colon and 50.4% in distal colon (P < 0.001). Similar or slightly greater effects were observed when R-568 was added following forskolin stimulation (compare Fig. 5 vs. Fig. 7); under the latter condition, R-568 treatment significantly reduced forskolin-stimulated Isc by 90% in proximal colon and 87% in distal colon (P < 0.001). These results suggest that CaSR agonist may be useful not only for diarrhea “treatment” but also for diarrhea “prevention.” Together, the results suggest that the TTX-sensitive and the R-568-sensitive effects are likely mediated by the same neurons in the ENS.
Fig. 7.
Prior activation of CaSR by R-568 reduces cAMP-stimulated Isc in the colon. A: representative recording of Isc response to forskolin in the absence of R-568 in a rat distal colon segment. Forskolin (500 nM) was added to mucosal and serosal solutions. B: quantitative changes in Isc following treatment with forskolin in the proximal and distal colons in the absence of R-568. C: representative recording of Isc response to forskolin in the presence of R-568 in a rat distal colon segment. 10 μM R-568 was added to serosal solution 15 min before 500 nM forskolin was added to mucosal and serosal solutions. D: quantitative changes in Isc following treatment with forskolin in the proximal and distal colons in the presence of R-568. E summarizes the ΔIsc induced by forskolin in the absence and presence of R-568.
Effect of Cl− ion substitution.
To determine whether the basal and forskolin-stimulated Isc reflected Cl− secretion, dependence of these currents on the presence of Cl− ion was tested and is shown in Fig. 8. Bathing the tissue in serosal or bilateral Cl−-free Ringer solutions nearly eliminated the basal Isc completely (Fig. 8, A–D; also see Table 1). Under this Cl−-free condition, only a minimal residual current remained. The latter was reduced neither by serosal bumetanide (Fig. 8A) nor by mucosal amiloride (Fig. 8A), suggesting that basal Isc in the rat colon is not contributed by epithelial Na channel-mediated Na+ absorption but primarily mediated by Cl− secretion.
Similarly, removal of Cl− ion from bath solutions eliminated the ability of TTX (Fig. 8B) and R-568 (Fig. 8C) to alter Isc. Absence of Cl− also largely abolished the ability of forskolin to stimulate Isc in both proximal and distal colon [Fig. 8D; means ± SE (n) μA/cm2 absence vs. presence of Cl− ion: 0.2 ± 0.1 (4) and 0.7 ± 0.2 (4) vs. 59 ± 16 (9) and 37 ± 8 (9), P < 0.001]. Under Cl−-free condition, R-568 had no inhibition on basal (Fig. 8C) or forskolin-stimulated Isc (Fig. 8D). Together, these data suggested that both basal and forskolin-stimulated Isc and their responses to TTX and R-568 are dependent on Cl− secretion.
DISCUSSION
Our data demonstrate that CaSR is expressed and is functionally active in the ENS and that this neuronal CaSR is a modulator of the ENS function and a regulator of intestinal fluid transport. We and others have shown the presence of CaSR transcripts and protein in the ENS of the intestine (8). In the present study, we extend these observations by demonstrating that CaSR protein is highly expressed in the nerve extensions and neurite projections of the ENS, both within the circular and longitudinal muscle compartments and in the mucosa and submucosa layers, providing support for potential roles of the neural CaSR for modulation of ENS activity and gastrointestinal function. The functional relevance of this neural CaSR in colonic physiology and pathophysiology is suggested by the Isc studies (Figs. 4–7). In freshly isolated ENS-intact proximal and distal colon segments, activation of CaSR by serosal R-568 diminished both basal and cyclic AMP-stimulated Isc in the absence but not the presence of the neurotransmission inhibitor TTX. Thus the loss or decrease of ability of R-568 to reduce basal and cyclic AMP-stimulated Isc in the presence of TTX supports our conclusion that the inhibitory effect of R-568 on Isc was mediated primarily through activation of the CaSR from the ENS, and, to a lesser extent, via activation of the CaSR from the epithelial cells. The ability of CaSR, activated by either luminal or serosal R-568, to reduce both basal and cyclic AMP-stimulated secretory responses in ENS-absent colonic epithelial crypts (21) excludes the possibility that the abolition of R-568 effects by TTX was due to the requirement of ENS activity for the action of CaSR in the epithelium. Future studies using CaSR intestinal epithelium-specific knockout mice and mice lacking the ENS would be useful in further confirming these conclusions. Although this study was conducted primarily in a reduced concentration of Cao2+, under normal Cao2+ condition similar inhibitory effects of R-568 were observed, albeit with a slightly less pronounced effect (Fig. 6). As a result, the present results suggest that the calcimimetic R-568 may have clinical utility in the treatment of diarrhea. Indeed, this same class of drug has been employed successfully to inhibit parathyroid hormone secretion in hyperparathyroid patients, where serum Ca2+ can be either <1.0 mM (secondary hyperparathyroidism) or >1.5 mM (primary hyperparathyroidism) (see review in Ref. 39).
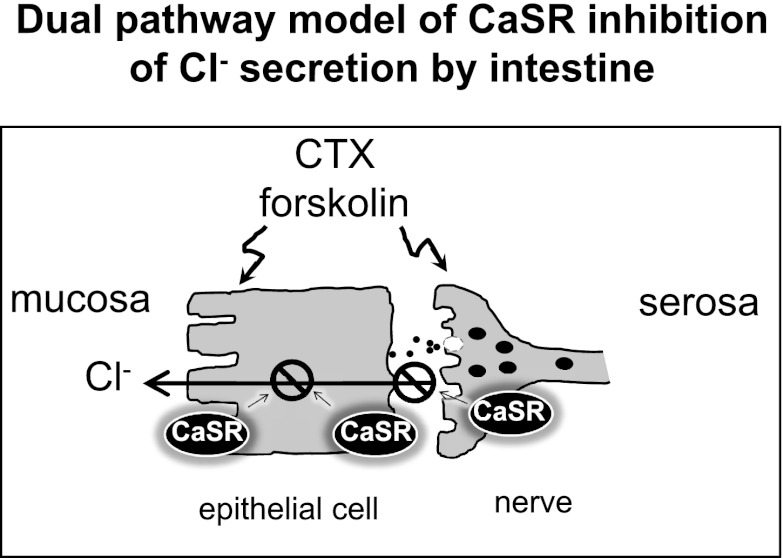
Fluid movement in the intestine is controlled by humoral factors as well as by the activity of the ENS, the myenteric plexus (Auerbach's plexus), and the submucosal plexus (Meissner's plexus). Both plexuses are actively involved in the control of fluid secretion by the intestine. During normal digestion, neurogenic and local hormonal factors regulate the production of second messengers such as cyclic AMP in intestinal epithelial cells to promote net fluid secretion. This enhanced secretion will lubricate the intestine, ensuring an appropriate mixing and flow of digest along its length and, once digestion is completed, return to baseline. Our studies show that CaSR is expressed in both the enterocyte and the ENS and inhibits intestinal fluid secretion (also see Refs. 10, 11, 21). Accordingly, local dietary/nutrient signals released as a result of digestion, such as calcium, amino acids, and polyamines, can activate the CaSR to function as a negative modulator of physiological neurogenic and local hormonal secretory actions and provide a mechanism for modulating net fluid movement during normal digestion. On the other hand, bacterial enterotoxins, such as CTX, and other secretagogues, such as forskolin, a synthetic adenylyl cyclase activator, enhance intestinal fluid secretion and cause severe diarrhea through both direct epithelial cell generation of cyclic nucleotides and indirectly by stimulating the ENS to release secretagogues such as vasoactive peptide. We show that both secretory pathways can be inactivated, respectively, by activation of epithelial cell CaSR (10, 11, 21) and by stimulation of CaSR associated with the ENS (present study). This negative regulatory action of the CaSR on intestinal fluid secretion and possibly other ENS-related functions could contribute to the known constipating effect of hypercalcemia and the antisecretory effect of increasing oral calcium intake on the severity of diarrhea resulting from enterotoxigenic E. coli infection in humans (3). The dual-pathway model for CaSR modulation of fluid secretion in intestine is depicted in Fig. 9.
Fig. 9.
Dual-pathway model for fluid secretion in intestine and sites of antisecretory action for CaSR. Secretagogues such as cholera toxin (CTX) and forskolin induce diarrhea through direct epithelial cell generation of cyclic nucleotides and indirectly via stimulation of ENS to release secretagogues such as vasoactive peptide. The CaSR is expressed in both cell types and upon its activation can inhibit both secretory pathways.
Normally, the ENS coordinates and relays information from the parasympathetic and sympathetic nervous system to the gastrointestinal tract. However, in the absence of extrinsic innervation, the ENS can still mediate intestinal secretion. Indeed, we showed that the freshly isolated proximal and distal colon segments not only had large TTX-sensitive Isc but also responded to agonists to stimulate Cl− secretion. The proportion of the TTX-sensitive Isc observed in the present preparations is comparable to those observed in vivo (28, 43) and in vitro (13, 23). Thus, in conjunction with the Ussing chamber technique, this freshly isolated ENS-intact perfused intestinal preparation constitutes an excellent ex vivo model to study regulation of ENS-mediated neural regulation of ion transport.
Consistent with prior observations made in single isolated colonic crypts (10, 11, 21), we show in the present study that cyclic AMP-stimulated TTX-insensitive nonneuronal Isc was inhibited by serosal R-568, although it was inhibited to a less extent (see Fig. 5, C and D). The latter might be related to the thickness of intestinal preparations used where the accessibility of the serosal R-568 to activate the basolateral CaSR of the epithelium could be limited due to the presence of diffusion barrier generated by thick layers of muscle and/or complex structures of submucosa (12, 15). The lack of the ability of R-568 to reduce TTX-insensitive Isc observed under basal unstimulated condition (Fig. 4, C–E) may be due to the insensitivity in detecting changes on this relatively small basal current. In addition, the epithelial cell effect of R-568 depends on degradation of intracellular cyclic nucleotides (21), which were low under basal conditions (21).
The mechanism by which CaSR inhibits the ENS activity remains unknown. The observations of intense CaSR immunoreactivity in the neurite projections and terminal nerve fibers of both the enteric neurons (see Fig. 1) and the brain (35) together with the abolition of TTX-sensitive Isc by R-568 (see Fig. 4) suggest that CaSR might be functioning as an inhibitor, negatively regulating the neurotransmission and neurotransmitter release of enteric neurons, as observed recently in the central nervous system (CNS) (9, 33). Alternatively, complex formation of the CaSR with the type B γ-aminobutyric acid (GABA) receptor has recently been shown in neuronal cells of the CNS (7). There is evidence that this GABA receptor is expressed in the ENS as well (31). Thus it is possible that this inhibitory GABA receptor thereby confers neural inhibition to the CaSR in the ENS. Clearly, further studies are needed to address these possibilities. Also, future experiments will be required to understand which types of neuron(s) in the ENS expresses and is affected by CaSR and to determine what the intracellular second messenger mechanism(s) is involved in this process.
Effectively reducing the life-threatening intestinal fluid loss in enterotoxin-induced diarrhea remains a major challenge. This novel pathway for modulating intestinal Cl− secretion through the colonic CaSR may lead to new pharmaconutritional therapies for prevention or treatment of certain clinical diarrheal diseases (i.e., cholera and other cyclic nucleotide-associated diarrheal diseases). Given that ENS-mediated secretory response is also critically implicated in many other forms of diarrhea, including viral [e.g., rotavirus (43)], neurogenic [e.g., irritable bowel disease (44)], and immunogenic/inflammatory diarrhea [e.g., inflammatory bowel disease (29)], the CaSR-mediated inhibition of the ENS-mediated secretory response observed in the present study might be of particular clinical importance in treating those forms of diarrhea as well.
In conclusion, the present study demonstrated that CaSR is highly expressed and functionally active in the ENS of rat colon. We further demonstrated that activation of this neural CaSR inhibits both basal and cyclic AMP-stimulated fluid transport in both proximal and distal colon via inhibiting the ENS activity. Our results suggest a paradigm for neural regulation of intestinal fluid transport where CaSR localized to the ENS modulates neurally and nonneurally stimulated epithelial secretion. The ability of CaSR agonists to reduce forskolin-induced fluid secretion by this neural mechanism suggests that this class of drugs may provide a unique therapy for secretory diarrhea and other ENS-mediated clinical conditions.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant T32 DK 007017 in Investigative Gastroenterology. The Training Program in Investigative Gastroenterology (DK007017) supported S. X. Cheng.
DISCLOSURES
There are no conflicts of interest, financial or otherwise, associated with this manuscript. There is no concern about ethical adherence in submitting this work.
ACKNOWLEDGMENTS
S. X. Cheng thanks Drs. Henry Binder, Judy Cho, Fred Gorelick, and Pramod Mistry, members of his T32 scholarship oversight committee, for mentorship, and critical review of this manuscript. Sue-Ann Mentone and Chris Shugrue provided technical support for immunostaining; Catherine Cheng, Max Stahl, and Ekaterina Petrova provided technical assistance for Ussing chamber recordings; and Thomas Kolodecik provided administrative support. S. X. Cheng also expresses thanks to dear friend, mentor, and colleague, the late Dr. Steven C. Hebert, who introduced him to the field of CaSR and was the inspiration to his current research area of CaSR in the enteric nervous system.
REFERENCES
- 1. Alam Mu Kirton J, Wilkinson F, Towers E, Sinha S, Rouhi M, Vizard T, Sage A, Martin D, Ward D, Alexander M, Riccardi D, Canfield A. Calcification is associated with loss of functional calcium-sensing receptor in vascular smooth muscle cells. Cardiovasc Res 81: 260– 268, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Black R, Cousens S, Johnson H, Lawn J, Rudan I, Bassani D, Jha P, Campbell H, Walker C, Cibulskis R, Eisele T, Liu L, Mathers C; Child Health Epidemiology Reference Group of WHO and UNICEF Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 375: 1969– 1987, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Bovee-Oudenhoven IMJ, Lettink-Wissink MLG, Van Doesburg W, Witteman BJM, Van Der Meer R. Diarrhea caused by enterotoxigenic Escherichia coli infection of humans is inhibited by dietary calcium. Gastroenterology 125: 469– 476, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature 366: 575– 580, 1993 [DOI] [PubMed] [Google Scholar]
- 5. Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev 81: 239– 297, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Burleigh DE, Borman RA. Evidence for a nonneural electrogenic effect of cholera toxin on human isolated ileal mucosa. Dig Dis Sci 42: 1964– 1968, 1997 [DOI] [PubMed] [Google Scholar]
- 7. Chang W, Tu C, Cheng Z, Rodriguez L, Chen TH, Gassmann M, Bettler B, Margeta M, Jan L, Shoback D. Complex formation with the Type B gamma-aminobutyric acid receptor affects the expression and signal transduction of the extracellular calcium-sensing receptor. Studies with HEK-293 cells and neurons. J Biol Chem 282: 25030– 25040, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Chattopadhyay N, Cheng I, Rogers K, Riccardi D, Hall A, Diaz R, Hebert SC, Soybel DI, Brown EM. Identification and localization of extracellular Ca2+-sensing receptor in rat intestine. Am J Physiol Gastrointest Liver Physiol 274: G122– G130, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Chen W, Bergsman J, Wang X, Gilkey G, Pierpoint CR, Daniel E, Awumey E, Dauban P, Dodd R, Ruat M, Smith S. Presynaptic external calcium signaling involves the calcium-sensing receptor in neocortical nerve terminals. PLoS ONE 5: e8563, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cheng SX, Geibel J, Hebert S. Extracellular polyamines regulate fluid secretion in rat colonic crypts via the extracellular calcium-sensing receptor. Gastroenterology 126: 148– 158, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Cheng SX, Okuda M, Hall A, Geibel JP, Hebert SC. Expression of calcium-sensing receptor in rat colonic epithelium: evidence for modulation of fluid secretion. Am J Physiol Gastrointest Liver Physiol 283: G240– G250, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Clarke L. A guide to Ussing chamber studies of mouse intestine. Am J Physiol Gastrointest Liver Physiol 296: G1151– G1166, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cooke H, Wang YZ, Wray D, O'Dorisio MS, Woltering E, Coy D, Murphy W, Christofi F, Gosh P, O'Dorisio T. A multi-tyrosinated sst1/2 receptor preferring somatostatin agonist inhibits reflex and immune-mediated secretion in the guinea pig colon. Regul Pept 114: 51– 60, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Cooke HJ. Neurotransmitters in neuronal reflexes regulating intestinal secretion. Ann NY Acad Sci 915: 77– 80, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Cox HM, Cuthbert AW, Hkanson R, Wahlestedt C. The effect of neuropeptide Y and peptide YY on electrogenic ion transport in rat intestinal epithelia. J Physiol 398: 65– 80, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Currie MG, Fok KF, Kato J, Moore RJ, Hamra FK, Duffin KL, Smith CE. Guanylin: an endogenous activator of intestinal guanylate cyclase. Proc Natl Acad Sci USA 89: 947– 951, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Field M. Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest 111: 931– 943, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Field M, Graf LH, Laird WJ, Smith PL. Heat-stable enterotoxin of Escherichia coli: in vitro effects on guanylate cyclase activity, cyclic GMP concentration, and ion transport in small intestine. Proc Natl Acad Sci USA 75: 2800– 2804, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Flores J, Sharp G. The activation of adenylate cyclase by cholera toxin: possible interaction with the nucleotide regulatory site. Ciba Found Symp 42: 89– 108, 1976 [DOI] [PubMed] [Google Scholar]
- 20. Gama L, Baxendale-Cox LM, Breitwieser GE. Ca2+-sensing receptors in intestinal epithelium. Am J Physiol Cell Physiol 273: C1168– C1175, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Geibel J, Sritharan K, Geibel R, Geibel P, Persing JS, Seeger A, Roepke T, Deichstetter M, Prinz C, Cheng S, Martin D, Hebert S. Calcium-sensing receptor abrogates secretagogue- induced increases in intestinal net fluid secretion by enhancing cyclic nucleotide destruction. Proc Natl Acad Sci USA 103: 9390– 9397, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goldsmith PK, Fan G, Miller JL, Rogers KV, Spiegel AM. Monoclonal antibodies against synthetic peptides corresponding to the extracellular domain of the human Ca2+ receptor: characterization and use in studying concanavalin A inhibition. J Bone Miner Res 12: 1780– 1788, 1997 [DOI] [PubMed] [Google Scholar]
- 23. Guarino A, Albano F, Guandalini S. Oral rehydration: toward a real solution. J Pediatr Gastroenterol Nutr 33, Suppl 2: S2– S12, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Hammerland LG, Garrett JE, Hung BC, Levinthal C, Nemeth EF. Allosteric activation of the Ca2+ receptor expressed in Xenopus laevis oocytes by NPS 467 or NPS 568. Mol Pharmacol 53: 1083– 1088, 1998 [PubMed] [Google Scholar]
- 25. Hebert S, Cheng S, Geibel J. Functions and roles of the extracellular Ca2+sensing receptor in the gastrointestinal tract. Cell Calcium 35: 239– 247, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Lorrot M, Vasseur M. How do the rotavirus NSP4 and bacterial enterotoxins lead differently to diarrhea? Virol J 4: 31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lundgren O. Enteric nerves and diarrhoea. Pharmacol Toxicol 90: 109– 120, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Lundgren O, Peregrin AT, Persson K, Kordasti S, Uhnoo I, Svensson L. Role of the enteric nervous system in the fluid and electrolyte secretion of rotavirus diarrhea. Science 287: 491– 495, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Margolis KG, Stevanovic K, Karamooz N, Li ZS, Ahuja A, D'autreaux F, Saurman V, Chalazonitis A, Gershon MD. Enteric neuronal density contributes to the severity of intestinal inflammation. Gastroenterology 141: 588– 598, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moore S, Lima AAM, Guerrant R. Infection: preventing 5 million child deaths from diarrhea in the next 5 years. Nat Rev Gastroenterol Hepatol 8: 363– 364, 2011 [DOI] [PubMed] [Google Scholar]
- 31. Nakajima K, Tooyama I, Kuriyama K, Kimura H. Immunohistochemical demonstration of GABAB receptors in the rat gastrointestinal tract. Neurochem Res 21: 211– 215, 1996 [DOI] [PubMed] [Google Scholar]
- 32. Nearing J, Betka M, Quinn S, Hentschel H, Elger M, Baum M, Bai M, Chattopadyhay N, Brown EM, Hebert SC, Harris HW. Polyvalent cation receptor proteins (CaRs) are salinity sensors in fish. Proc Natl Acad Sci USA 99: 9231– 9236, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Phillips C, Harnett M, Chen W, Smith S. Calcium-sensing receptor activation depresses synaptic transmission. J Neurosci 28: 12062– 12070, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Riccardi D, Park J, Lee WS, Gamba G, Brown EM, Hebert SC. Cloning and functional expression of a rat kidney extracellular calcium/polyvalent cation-sensing receptor. Proc Natl Acad Sci USA 92: 131– 135, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ruat M, Molliver ME, Snowman AM, Snyder SH. Calcium sensing receptor: molecular cloning in rat and localization to nerve terminals. Proc Natl Acad Sci USA 92: 3161– 3165, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sands JM, Naruse M, Baum M, Jo I, Hebert SC, Brown EM, Harris HW. Apical extracellular calcium/polyvalent cation-sensing receptor regulates vasopressin-elicited water permeability in rat kidney inner medullary collecting duct. J Clin Invest 99: 1399– 1405, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seamon KB, Daly JW. Forskolin: a unique diterpene activator of cyclic AMP-generating systems. J Cyclic Nucleotide Res 7: 201– 224, 1981 [PubMed] [Google Scholar]
- 38. Sheinin Y, Kallay E, Wrba F, Kriwanek S, Peterlik M, Cross HS. Immunocytochemical localization of the extracellular calcium-sensing receptor in normal and malignant human large intestinal mucosa. J Histochem Cytochem 48: 595– 602, 2000 [DOI] [PubMed] [Google Scholar]
- 39. Tfelt-Hansen J, Brown E. The calcium-sensing receptor in normal physiology and pathophysiology: a review. Crit Rev Clin Lab Sci 42: 35– 70, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Tornehave D, Hougaard DM, Larsson L. Microwaving for double indirect immunofluorescence with primary antibodies from the same species and for staining of mouse tissues with mouse monoclonal antibodies. Histochem Cell Biol 113: 19– 23, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Wang M, Yao Y, Kuang D, Hampson D. Activation of family C G-protein-coupled receptors by the tripeptide glutathione. J Biol Chem 281: 8864– 8870, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Weston A, Absi M, Ward D, Ohanian J, Dodd R, Dauban P, Petrel C, Ruat M, Edwards G. Evidence in favor of a calcium-sensing receptor in arterial endothelial cells: studies with calindol and Calhex 231. Circ Res 97: 391– 398, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Wickelgren I. How rotavirus causes diarrhea. Science 287: 409, 411– 409, 411, 2000 [DOI] [PubMed] [Google Scholar]
- 44. Wood JD. Histamine, mast cells, and the enteric nervous system in the irritable bowel syndrome, enteritis, and food allergies. Gut 55: 445– 447, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]