Abstract
Background and Purpose
The effects of nicotine on cerebral metabolism and its influence on smoking behavior is poorly understood. An understanding of the chronic effects of nicotine on excitatory and inhibitory metabolic demand, and corresponding neurotransmission may provide clues for designing strategies for the optimal smoking cessation intervention. The objective of the current study was to investigate neuronal and astroglial metabolism in mice exposed to nicotine (0.5 and 2.0 mg/kg, sc) three times in a day for 4 weeks.
Experimental Approach/Principal Findings
Metabolic measurements were carried out by co-infusing [U-13C6]glucose and [2-13C]acetate, and monitoring 13C labeling of amino acids in brain tissue extract using 1H-[13C] and 13C-[1H]-NMR spectroscopy. Concentration of 13C-labeled glutamate-C4 was increased significantly from glucose and acetate with chronic nicotine treatment indicating an increase in glucose oxidation by glutamatergic neurons in all brain regions and glutamate-glutamine neurotransmitter cycle in cortical and subcortical regions. However, chronic nicotine treatment led to increased labeling of GABA-C2 from glucose only in the cortical region. Further, increased labeling of glutamine-C4 from [2-13C]acetate is suggestive of increased astroglial activity in subcortical and cerebellum regions of brain with chronic nicotine treatment.
Conclusions and Significance
Chronic nicotine exposure enhanced excitatory activity in the majority of brain regions while inhibitory and astroglial functions were enhanced only in selected brain regions.
Introduction
Nicotine addiction, a psychiatric disorder that underlies the widespread use of tobacco products, contributes to a large number of chronic illnesses and is a leading cause of deaths globally [1]. Although dysfunction of the brain reward circuitry involving the dopaminergic system has been implicated in nicotine addiction [2], impairment in cortical glutamatergic neurotransmission is also crucial for long term addictions [3]. Nicotine enhances the release of neurotransmitters glutamate, GABA, dopamine and serotonin by binding to nicotinic acetylcholine receptors (nAChRs) [4], [5], [6], [7]. Glutamate and GABA are the major excitatory and inhibitory neurotransmitters, respectively in the matured mammalian central nervous system which play major roles in glucose and energy metabolism, cortical excitability and cognitive function [8], [9], [10], [11]. 13C NMR studies have shown that the glutamate-glutamine cycle accounts for a major fraction of glutamine synthesis [12], and most importantly has a metabolic rate similar to neuronal glucose oxidation [13], [14], [15], [16]. Moreover, the rate of neurotransmitter cycle and neuronal mitochondrial glucose oxidation increased proportionately with a near 1∶1 slope [8], [16], [17], indicating that neurotransmitter energetics is supported by neuronal oxidative glucose metabolism. Alterations in glutamate and GABA pathways are associated with many neurological and neuropsychiatric disorders [18], [19], [20], [21].
Brain imaging studies in human have shown that nicotine increases the cerebral metabolic rate of glucose consumption in different regions of brain [22] along with regional cerebral blood flow in occipital cortex and cerebellum [23]. Animal studies have revealed an increase in local cerebral glucose utilization [24], cerebral blood flow and oxygen consumption during acute nicotine administration [25]. Acute and chronic nicotine exposure increased the expression of enzymes involved in glycolysis and Kreb’s cycle [26]. However, the implication of increased enzymatic activity upon nicotine exposure on the cerebral metabolism remains to be evaluated. Most of these studies have investigated the acute effects of nicotine on cerebral metabolism in human and rats. The effects of chronic nicotine on neuronal (glutamatergic and GABAergic) and astroglial metabolism in mice and rats are poorly understood.
Exposure to nicotine leads to activation, desensitization and up-regulation of nAChRs [27]. A single exposure to nicotine has been shown to transiently increase GABAergic transmission, which is followed by a persistent depression of these inhibitory inputs due to desensitization of nAChRs [6], [28], [29]. Simultaneously, nicotine enhances the glutamatergic transmission through nAChRs that desensitize less than that of GABA neurons [30]. We hypothesize that chronic exposure to nicotine will enhance the glutamatergic, excitatory neurotransmission. In the current study, we have used an approach of co-infusion of [U-13C6]glucose and [2-13C]acetate along with 1H-[13C]-NMR and 13C-[1H]-NMR spectroscopy to investigate the effects of chronic nicotine exposure on neuronal and astroglial metabolism in mice. Difference in isotopomers of amino acids from [U-13C6]glucose and [2-13C]acetate has been utilized to measure neuronal and astroglial metabolism simultaneously. 13C Labeling of amino acids from glucose and acetate were used to evaluate the neuronal and astroglial glucose oxidation and neurotransmitter cycle. Our findings indicate that chronic nicotine treatment led to an increase in glucose oxidation and neurotransmitter cycling associated with glutamatergic neurons. The preliminary findings of this study have been presented at the Annual Meeting of the International Society for Magnetic Resonance in Medicine, Stockholm [31].
Materials and Methods
Animal Preparation
All animal experiments were performed under protocols approved by the Institutional Animal Ethics Committee of the Centre for Cellular and Molecular Biology,Hyderabad, India. One month old male C57BL/6 mice were used for the study. Mice were maintained at 22°C and 60% relative humidity with a 12/12 h light and dark cycle and received standard chow and water ad libitum. Mice were divided into three groups: Group (i) mice treated with normal saline (0.9% NaCl) (n = 6), Group (ii) mice treated with 0.5 mg/kg nicotine (n = 6, sc), Group (iii) mice treated with 2.0 mg/kg nicotine (n = 6, sc). Nicotine dose was selected based on previous studies where nicotine, 0.1 to 4 mg/kg, has been used in different experimental paradigms [25], [26], [32], [33], [34], [35], [36]. Mice in Group (ii) and (iii) received nicotine hydrogen tartrate (SIGMA, 0.1 ml, sc) at a dose of 1.4 and 5.7 mg/kg body weight, which is equivalent to 0.5 and 2 mg/kg nicotine free base, 3 times a day every 8 h for 4 weeks. The control mice received 0.1 ml normal saline (0.9% NaCl, sc) for the same period. Metabolic measurements were carried out 2 days after the last treatment to minimize post traumatic shock with the nicotine injection.
Infusion of [U-13C6]Glucose and [2-13C]Acetate
For metabolic measurements, overnight (12–14 h) fasted mice were anesthetized with urethane (1.5 g/kg, ip) and the tail vein was cannulated for the infusion of 13C labeled substrates. Body temperature was maintained around 37°C using a heating pad connected to a temperature-regulated circulating water bath. The respiration rate was monitored using a Biopack device. Solution of [U-13C6]glucose (0.225 mol/L) and [2-13C]acetate (0.8 mol/L) (99 atom %; Cambridge Isotopes, Andover, MA, USA) was administered 45 min after injection of urethane, using a bolus-variable rate infusion for 20 min as described previously [37], [38]. Blood was collected from the retro-orbital sinus artery by using a fine capillary during the last minute of the infusion. Plasma was obtained by centrifugation and stored at −80°C. At the end of the infusion, the anesthetized mice head were frozen in situ using liquid nitrogen [37], [39].
Extraction of Metabolites from Brain Tissue
Brain was dissected in cryostat maintained at −20°C to isolate cortex, subcortex (hippocampus, striatum, thalamus and sub-thalamus regions of brain), cerebellum and olfactory bulb (OB), and stored at −80°C till further analysis. Metabolites were extracted from frozen brain tissues as described previously [40]. Tissues were powdered with 0.1 mol/L HCl (1∶2 v/w) in methanol using a glass homogenizer maintained in a dry-ice-ethanol bath. Following transfer to a wet ice-bath, a known quantity of [2-13C]glycine (0.2 µmol) was added as an internal concentration reference. The suspension was homogenized with phosphate buffered ice-cold ethanol (90%) until no visible pieces of tissue remained. The homogenate was centrifuged at 14,000 g for 30 min at 4°C. The supernatant was passed through a chelex-100 resin column (200–400 mesh, Bio-Rad Laboratories, Hercules, CA, USA), eluted with de-ionized water and lyophilized. The dried powder was dissolved in buffered (phosphate = 25 mmol/L, pH = 7) deuterium oxide containing 0.25 mmol/L sodium 3-trimethylsilyl [2, 2, 3, 3-D4]-propionate (TSP) for NMR analysis.
Analysis of Plasma Glucose and Acetate Enrichment
Plasma (100 µl) was mixed with deuterium oxide (450 µl) containing TSP (0.25 mmol/L), and passed through a centrifugal filter (10-kDa cut off) to remove macromolecules prior to NMR analysis. Isotopic 13C enrichment of glucose and acetate was determined in the plasma using 1H NMR spectroscopy at 600 MHz (Bruker AVANCE II) spectrometer. The percent 13C enrichment of glucose-C1α and acetate-C2 was calculated by dividing the intensity of the two 13C satellites by the total (12C+13C) intensity at 5.23 and 1.91 ppm, respectively.
NMR Analysis of Brain Tissue Extract
1H-[13C]-NMR spectra of the brain tissue extracts were acquired at 600 MHz NMR spectrometer (Bruker AVANCE) [13], [37]. Concentration of metabolites was determined relative to [2-13C]glycine. The 13C enrichment of cerebral metabolites was calculated from the ratio of the area in the 1H-[13C]-NMR difference (13C-labeled spectra) spectrum to that of the non-edited (12C+13C) spectrum. In addition, 1H decoupled 13C NMR (13C-[1H]-NMR) spectra of the brain tissue extracts were also acquired at 150 MHz. The isotopomer Intensity of Glu4, GABA2 and Gln4 was determined by using a peak fitting method provided in Topspin software (Bruker Biospin).
Metabolism of [U-13C6]Glucose and [2-13C]Acetate in the Brain
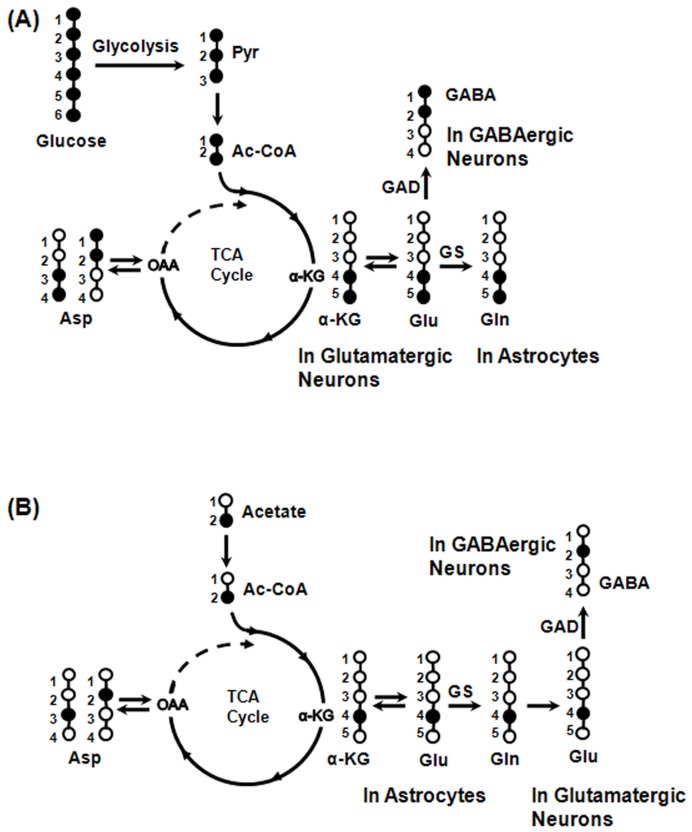
Metabolism of labeled glucose and acetate in the brain is depicted in Figure 1. While [U-13C6]glucose is mostly metabolized by neurons, [2-13C]acetate is selectively transported to astroglia [41] and gets metabolized there. Metabolism of [U-13C6]glucose via glutamatergic and GABAergic TCA cycles labels Glu4,5 which is de-carboxylated to GABA1,2 in GABAergic neurons [42]. Release of Glu4,5 and GABA1,2 from the respective neurons, followed by uptake and metabolism in astroglia via glutamate-glutamine and GABA-glutamine cycling pathways labels Gln4,5. Further metabolism via TCA cycle leads to labeling of [3-13C]Glu/Gln/GABA, [4-13C]GABA, [1,2,-13C2]Glu/Gln, [3,4,5-13C3]Glu/Gln, [1,2,4,5-13C4]Glu/Gln, [1,2,3-13C3]GABA and [1,2,4-13C3]GABA. [U-13C6]Glucose metabolism in astrocytes via pyruvate carboxylase pathway incorporates label into Gln2,3 and Glu2,3 (GABA3,4).
Figure 1. Depiction of 13C labeling of cerebral metabolites from labeled glucose and acetate.
(A) Metabolism of [U-13C6]glucose via glutamatergic and GABAergic TCA cycles labels Glu4,5 which is de-carboxylated to GABA1,2 in GABAergic neurons by GAD. Labeling of Gln4,5 occurs from Glu4,5 and GABA1,2 via glutamate-glutamine and GABA-glutamine cycle. (B) As transporters of acetate are present on astroglia only, [2-13C]acetate is selectively transported and metabolized in these cells and labels Gln4 by the combined action of astroglial TCA cycle and glutamine synthetase. Neurotransmitters Glu4 and GABA2 are labeled from Gln4 via glutamate-glutamine and GABA-glutamine substrates cycling between astroglia and neurons. Isotopomers of Glu4, GABA2 and Gln4 derived from [U-13C6]glucose and [2-13C]acetate are different. Open and filled circles represent 12C and 13C atoms, respectively.
[2-13C]Acetate metabolism in astrocyte labels Gln4 by a combined action of astrocytic TCA cycle and glutamine synthetase [43]. Neuronal Glu4 and GABA2 are labeled from Gln4 via glutamate-glutamine and GABA-glutamine substrates cycling between astroglia and neurons, respectively. Further metabolism in TCA cycle incorporates label into [2-13C]Glu/Gln, [4-13C]GABA, [3-13C]Glu/Gln/GABA, [3,4-13C2]Glu/Gln, [2,4-13C2]Glu/Gln/GABA and [2,3-13C2]GABA. Isotopomers of amino acids derived from [U-13C6]glucose and [2-13C]acetate via the first turn of TCA cycle are distinct. However, the isotopic differences in the labeling of amino acids from [U-13C6]glucose and [2-13C]acetate are lost upon their further metabolism by TCA cycle.
Changes in labeling of Glu4,5, GABA1,2 and Gln4,5 from [U-13C6]glucose will indicate an alteration in glutamatergic, GABAergic TCA cycle and total neurotransmitter cycle fluxes, respectively upon chronic nicotine exposure. Similarly, an alteration in the labeling of Glu4, GABA2 and Gln4 from [2-13C]acetate will signify changes in the rate of glutamate-glutamine, GABA-glutamine neurotransmitter cycle and oxidation of acetate by astroglia with nicotine treatment.
Contribution of Glucose and Acetate for Amino Acids Labeling
The fractional contribution of glucose and acetate for the labeling of Glu-C4 was determined from the isotopomer as following:
| (1) |
| (2) |
where Glu4,5, Glu3,4,5, Glu4 and Glu3,4 represent intensity of [4,5-13C2]-, [3,4,5-13C3]-, [4-13C]- and [3,4-13C2]glutamate, respectively, in 13C NMR spectrum.
Similarly, the contribution of glucose and acetate to GABA-C2 and Gln-C4 labeling was also determined. 13C Labeling of amino acids from [U-13C6]glucose and [2-13C]acetate was calculated by multiplying the percent enrichment of the amino acids (obtained from the 1H-[13C]-NMR spectrum) with the fractional contribution of the corresponding substrates (obtained using Eq. 1 or 2). In addition to [2-13C]acetate, Glu4/Gln4/GABA2 resonance are also contributed by 13C natural abundance (1.1%). Hence, the contribution of [2-13C]acetate to amino acids labeling (Glu-C4, GABA-C2 and Gln-C4) was corrected for the natural abundance by subtracting 1.1% from the deconvolved 13C labeling. The deconvolved enrichment of amino acids from [U-13C6]glucose and [2-13C]acetate was normalized with corresponding 13C labeling of precursors in plasma. The normalized enrichment of amino acids was multiplied to the corresponding concentration to determine concentration of 13C labeled amino acids during 20 min of infusion.
Statistical Analysis
Statistical analysis was carried out by using the Data Analysis Tool package of Microsoft Excel 2007. Two factor ANOVA was performed to determine the global difference in the concentration and labeling of amino acids between nicotine-treated and control mice within the same region of the brain. To correct for the multiple comparisons incurred by different metabolites and 13C concentrations, the α value was set to 0.005 and 0.0083, respectively so as to minimize the possibility of Type I errors. Further, differences at individual amino acid level were evaluated by a single factor ANOVA. All results are presented as mean ± SEM.
Results
Behavior and Physiology with Nicotine Treatment
Mice appeared immobile for 10 to 15 min after nicotine administration in the first couple of days of the treatment. The time of immobilization reduced to zero after a week of the treatment. There was no symptom of seizures/convulsions even at a dose of 2.0 mg/kg. Nicotine-treated animals appeared hyperactive during the latter half of the treatment. There was a mild reduction in the weight of mice during the first week of nicotine treatment which regained the weight in the latter part of the treatment such that there was not much difference in the weight of control and nicotine treated mice after 4 weeks. The respiration rate (Control: 241±18 breaths/min; Nicotine (0.5 mg/kg): 257±11 breaths/min; Nicotine (2.0 mg/kg): 246±11 breaths/min) during [U-13C6]glucose and [2-13C]acetate infusion (under urethane anesthesia) was similar (p>0.1) in nicotine-treated and control mice. Plasma urethane level was not significantly different (p>0.13, single Factor ANOVA) among control (32.4±3.2 mM, n = 5) and nicotine-treated (nicotine 0.5 mg/kg, 28.6±1.8 mM, (n = 6); nicotine 2.0 mg/kg, 26.6±2.4 mM, n = 6) mice, suggesting that chronic nicotine treatment did not alter the metabolism of urethane.
Plasma Glucose and Acetate Enrichment
The 13C enrichment of glucose and acetate was measured using 1H NMR spectrum of plasma obtained at the last minute of [U-13C6]glucose and [2-13C]acetate infusion. 13C Labeling of glucose-C1 and acetate-C2 in control mice were 33.5±1.3% and 88.1±0.7%, respectively (Table 1). There was no significant (p = 0.11) difference in the plasma labeling of [U-13C6]glucose and [2-13C]acetate following chronic nicotine treatment.
Table 1. 13C Enrichment of glucose and acetate, and concentration of urethane in plasma of control and nicotine treated mice.
| Substrate | Control | Nicotine | |
| Saline (n = 6) | 0.5 mg/kg(n = 6) | 2 mg/kg(n = 6) | |
| [1-13C]Glucose (%) | 33.5±1.3 | 39.4±2.3 | 30.7±1.6 |
| [2-13C]Acetate (%) | 88.1±0.7 | 85.0±1.0 | 87.7±0.6 |
| Urethane (mM) | 32.4±3.2 | 28.6±1.8 | 26.4±2.4 |
Effect of Chronic Nicotine Treatment on Cerebral Metabolism
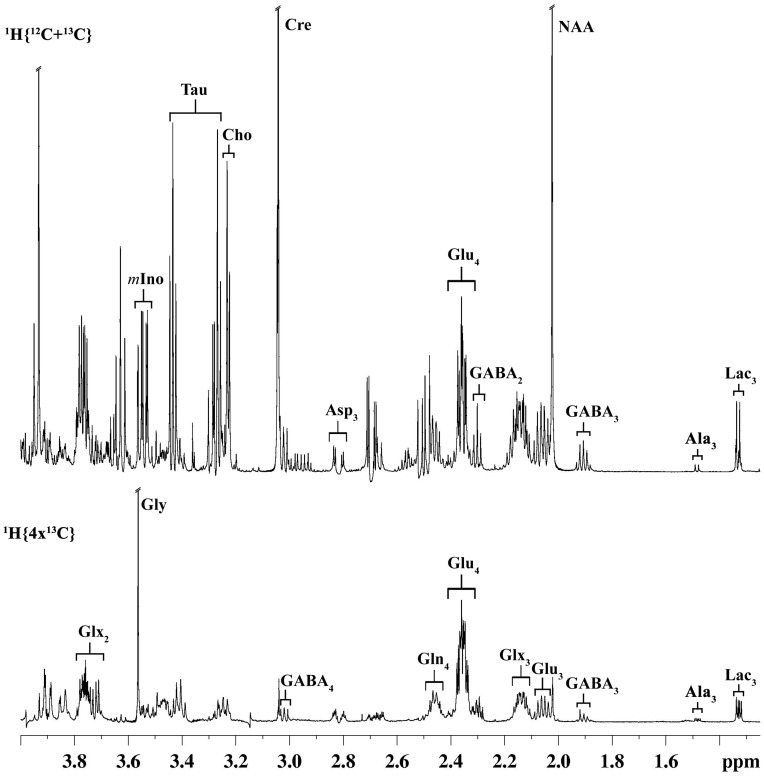
Cerebral metabolism was investigated by monitoring 13C labeling of brain metabolites using 1H-[13C]-NMR spectrum (Figure 2). Labeling of glutamate, glutamine, GABA and aspartate could be seen at different carbon positions (lower spectrum). Chronic nicotine (0.5 mg/kg) treatment led to a significant (F[1], [10] = 13.0, p = 0.0048, n = 6,6) increase in the percent enrichment of cortical GABA2 when compared with control, while no change was seen in the labeling of other amino acids (Table 2). An increase in the Glu3/Glu4 ratio from 0.35±0.01 for control to 0.42±0.01 for nicotine (F[1], [10] = 14.18, p = 0.0037, n = 6,6) indicates increase in cortical metabolism [37] in mice treated with nicotine (0.5 mg/kg). Nicotine (2.0 mg/kg) treatment resulted in an increase (p<0.01) in the labeling of all cortical metabolites except Gln4. Moreover, the Glu3/Glu4 ratio was also increased from 0.35±0.01 for control to 0.43±0.01 for nicotine (F[1], [10] = 23.3, p = 0.0007, n = 6,6), indicating that an increase in the rate of glutamatergic TCA cycle in cortex of mice exposed to 2.0 mg/kg nicotine. Increase in rate of glutamatergic TCA cycle with chronic nicotine (2.0 mg/kg) treatment was also observed in the cerebellum and sub-cortical regions (Table 2). Olfactory bulb showed a different activation pattern upon nicotine treatment. Nicotine exposure (0.5 mg/kg) increased the labeling of GABA3 (F[1], [10] = 6.37, p = 0.03, n = 6,6) with no significant (p>0.2) change in the labeling of other amino acids. Exposure to 2.0 mg/kg nicotine increased (p<0.02) percent labeling of Glu3, GABA2 and GABA3. In summary, glutamatergic metabolism was increased in the cortex, subcortex, cerebellum and OB with chronic nicotine exposure (Table 2).
Figure 2. Representative 1H-[13C]-NMR spectrum of cortical tissue extract of nicotine-treated mouse.
The upper spectrum (1H{12C+13C}) depicts total concentration of metabolites, while the lower spectrum (1H{4×13C}) represents the 13C labeled metabolites. Peak labels are: Ala3, alanine-C3; GABA, γ-amino butyric acid; Gln4, glutamine-C4; Glu4, glutamate-C4; Glu3, 0.5 × glutamate-C3; Glx3, (0.5 × glutamate + glutamine)-C3; Asp3, aspartate-C3; Cre, creatine; NAA, N-acetyl aspartate; Cho, choline, m-Ino, myo-inositol; Tau, taurine.
Table 2. 13C Enrichment of cerebral amino acids from [U-13C6]glucose and [2-13C]acetate in different brain regions.
| Brain Regions | Treatment Groups | 13C Enrichment (%) | ||||||
| Glu4 | GABA2 | Gln4 | Glu3 | GABA3 | Glu3/Glu4 | GABA3/GABA2 | ||
| Cortex | Control (n = 6) | 29.8±1.2 | 17.5±0.5 | 23.5±1.5 | 10.6±0.8 | 9.0±0.5 | 0.35±0.01 | 0.51±0.02 |
| Nic (0.5 mg, n = 6) | 31.0±1.0 | 20.7±0.7** | 24.2±1.5 | 13.1±0.5 | 11.0±0.5 | 0.42±0.01** | 0.53±0.01 | |
| Nic (2.0 mg, n = 6) | 34.8±1.0** | 22.1±0.6** | 27.7±1.6 | 14.9±0.7** | 12.2±0.5** | 0.43±0.01** | 0.55±0.02 | |
| Subcortex | Control (n = 6) | 30.5±1.3 | 16.6±0.8 | 27.2±1.3 | 10.8±0.8 | 8.0±0.5 | 0.35±0.01 | 0.48±0.01 |
| Nic (0.5 mg, n = 6) | 31.5±0.9 | 18.5±0.4 | 26.6±1.7 | 13.3±0.4* | 9.5±0.2* | 0.42±0.01** | 0.51±0.01 | |
| Nic (2.0 mg, n = 6) | 35.8±1.2* | 20.5±0.8** | 33.2±1.8* | 15.4±0.5** | 10.8±0.5** | 0.43±0.01** | 0.53±0.01* | |
| Cerebellum | Control (n = 6) | 27.3±1.1 | 20.9±1.1 | 22.7±1.5 | 9.2±0.7 | 10.3±1.0 | 0.34±0.01 | 0.49±0.02 |
| Nic (0.5 mg, n = 6) | 29.4±1.0 | 24.3±0.8* | 23.1±1.3 | 12.0±0.5* | 14.2±0.9* | 0.41±0.01** | 0.58±0.02* | |
| Nic (2.0 mg, n = 6) | 32.5±1.0** | 26.5±1.2** | 28.0±1.3* | 12.8±0.6** | 15.1±0.7** | 0.39±0.01** | 0.57±0.01** | |
| Olfactory Bulb | Control (n = 6) | 36.6±1.5 | 13.9±0.5 | 28.7±1.8 | 14.9±1.0 | 6.5±0.4 | 0.41±0.02 | 0.46±0.01 |
| Nic (0.5 mg, n = 6) | 36.1±1.9 | 14.8±0.5 | 26.9±1.9 | 16.3±0.8 | 8.4±0.4* | 0.45±0.01* | 0.54±0.02** | |
| Nic (2.0 mg, n = 6) | 42.0±3.1 | 17.5±0.8** | 34.4±2.1 | 19.7±1.4* | 9.1±0.5** | 0.47±0.01** | 0.52±0.01** | |
Percent 13C enrichment was measured in brain tissue extract using 1H-[13C]-NMR spectroscopy. Values represent mean±SEM.
p<0.05,
p<0.01 indicate significance of differences when compared to respective control.
Effect of Chronic Nicotine on Glutamatergic and GABAergic Pathways
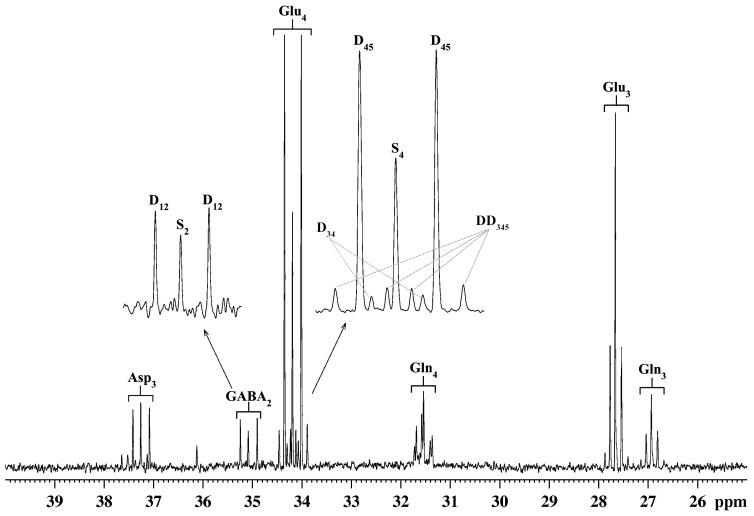
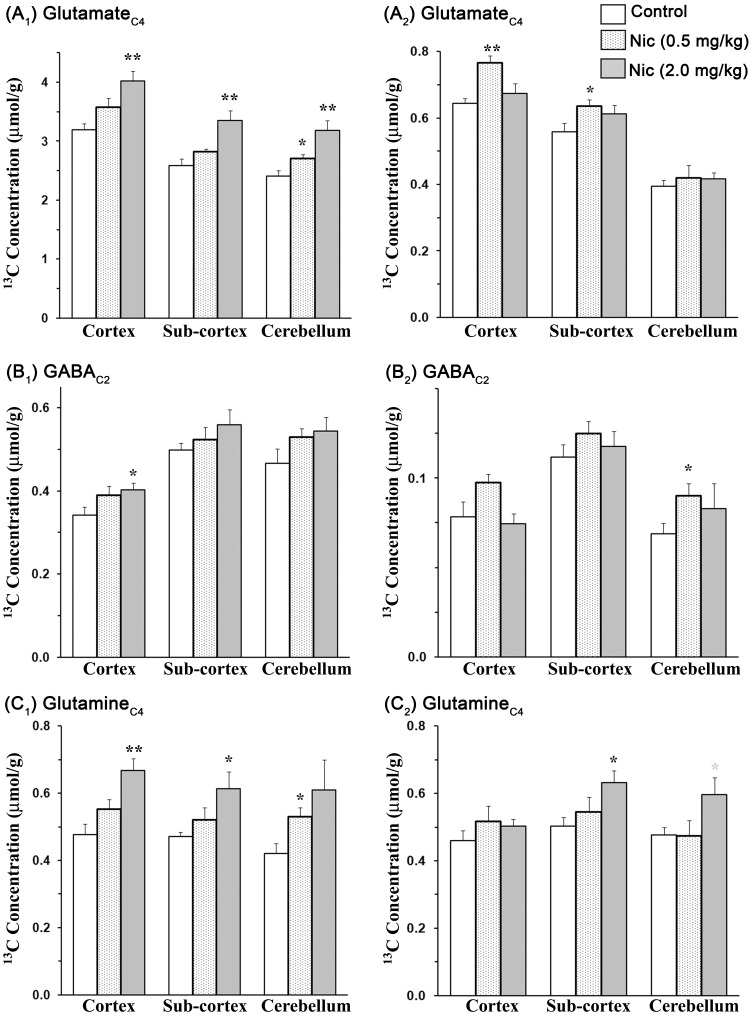
Isotopomers of the amino acids were measured from the 13C-[1H]-NMR spectrum (Figure 3). The 1H-[13C]-NMR measured enrichment of GluC4 was de-convolved for its contribution from [U-13C6]glucose and [2-13C]acetate using Eq. 1 and 2, respectively (Table 3). 13C Concentration of labeled amino acids from [U-13C6]glucose and [2-13C]acetate in nicotine- and normal saline (0.9% NaCl)-treated mice is presented in Figure 4. [4-13C]Glutamate labeling from [U-13C6]glucose was increased significantly (p<0.05) in the cortical, subcortical and cerebellum regions in mice exposed to nicotine (2.0 mg/kg). In addition, [4-13C]glutamine labeling from [U-13C6]glucose was also enhanced significantly (p<0.02, n = 6,6) in the cortex and subcortex upon nicotine treatment (2.0 mg/kg). Furthermore, [4-13C]glutamate labeling from [2-13C]acetate was found to be increased significantly (p<0.02, n = 6,6) in the cortical (Control 0.64±0.02 µmol/g; Nicotine 0.76±0.02 µmol/g) and subcortical (Control 0.56±0.03 µmol/g; Nicotine 0.63±0.02 µmol/g) regions of the brain in mice treated with 0.5 mg/kg nicotine (Figure 4, Table S1). Although there was a significant increase (p<0.05) in the percent 13C labeling of GABA2 with nicotine treatment in different regions of the brain (Table 2 and 3), the total level of GABA was reduced slightly with chronic nicotine exposure (data not shown). As a result, the concentration of newly synthesized [2-13C]GABA (total concentration×13C enrichment/100) was similar among different treatment groups in various regions of the brain except an elevation of [2-13C]GABA with nicotine treatment (2.0 mg/kg) (p<0.05) in the cortical region. Furthermore, concentration of labeled glutamine-C4 from [2-13C]acetate was increased (p<0.05) in the subcortex and cerebellum regions of the brain with chronic nicotine exposure, suggesting that astroglial activity is enhanced in these brain regions (Table S1). In summary, increased [4-13C]glutamate labeling from glucose and acetate with chronic nicotine treatment indicates an increase in glucose oxidation by glutamatergic neurons in all regions of the brain and glutamate-glutamine neurotransmitter cycle in the cortical and subcortical regions. The finding of increased [4-13C]glutamine from [U-13C6]glucose in nicotine treated mice further corroborates with the increased glutamate-glutamine cycle with chronic nicotine exposure. GABAergic and astroglial function are found to enhanced only in selected brain regions.
Figure 3. Representative 13C-[1H]-NMR spectrum of cortical tissue extract of nicotine-treated mouse.
The inset depicts the resonances for Glu4 and GABA2. In Glu4, D45 and DD345 represent [4,5-13C2]glutamate and [3,4,5-13C3]glutamate, and are the product of glucose metabolism in the first and second turn of the TCA cycle, respectively. S4 and D34 indicate [4-13C]glutamate and [3,4-13C2]glutamate which are labeled from [4-13C]glutamine and [3,4-13C2]glutamine, respectively. [4-13C]Glutamine and [3,4-13C2]glutamine are the products of [2-13C]acetate metabolism in the first and second turn of the astrocytic TCA cycle, respectively. In GABA2, D1,2 represents [1,2-13C2]GABA which is the product of direct metabolism of [U-13C6]glucose in the GABAergic TCA cycle, while S2 ([2-13C]GABA) is the result of the labeling from [2-13C]acetate via astrocytic TCA cycle followed by trafficking into GABAergic neurons.
Table 3. Deconvolved 13C labeling of cerebral amino acids from [U-13C6]glucose and [2-13C]acetate in different brain regions.
| Brain Region | Treatment | GluC4 | GABAC2 | GlnC4 | |||
| Glucose | Acetate | Glucose | Acetate | Glucose | Acetate | ||
| Cortex | Control (n = 6) | 22.2±1.1 | 4.5±0.1 | 12.2±0.3 | 2.8±0.2 | 9.6±0.6 | 9.2±0.5 |
| Nic (0.5 mg) (n = 6) | 23.9±0.7 | 5.2±0.2* | 15.1±0.5** | 3.8±0.2* | 11.1±0.4 | 10.5±1.0 | |
| Nic (2.0 mg) (n = 6) | 26.6±0.8** | 4.5±0.2 | 16.0±0.6** | 3.0±0.2 | 12.3±0.7* | 9.3±0.4 | |
| Subcortex | Control (n = 6) | 22.5±1.0 | 4.8±0.1 | 11.6±0.6 | 2.6±0.1 | 10.6±0.5 | 11.2±0.5 |
| Nic (0.5 mg) (n = 6) | 24.2±0.5 | 5.4±0.2* | 13.6±0.3* | 3.2±0.1** | 11.6±0.8 | 12.2±1.0 | |
| Nic (2.0 mg) (n = 6) | 26.9±0.8** | 4.9±0.1 | 14.3±0.6** | 3.0±0.1* | 12.6±0.8 | 13.0±0.2* | |
| Cerebellum | Control (n = 6) | 21.1±1.0 | 3.5±0.2 | 16.1±0.9 | 2.4±0.2 | 8.4±0.7 | 9.5±0.4 |
| Nic (0.5 mg) (n = 6) | 24.0±0.9 | 3.7±0.3 | 19.3±0.7* | 3.3±0.3* | 11.0±0.8* | 9.6±0.6 | |
| Nic (2.0 mg) (n = 6) | 25.7±0.8** | 3.4±0.2 | 20.2±1.1* | 3.1±0.5 | 10.8±1.4 | 10.7±0.8 | |
The 1H-[13C]-NMR measured percent labeling was deconvolved for [U-13C6]glucose and [2-13C]acetate by using the isotopomer information obtained in 13C-[13H]-NMR and normalized with the percent 13C labeling of precursors (glucose and acetate) in plasma. Values represent mean±SEM.
p<0.05,
p<0.01 indicate significance of differences when compared to respective control.
Figure 4. Concentration of 13C labeled (A) glutamate-C4, (B) GABA-C2 and (C) glutamine-C4 from [U-13C6]glucose and [2-13C]acetate.
The subscripts 1 and 2 with alphabet A, B and C represent labeling from [U-13C6]glucose and [2-13C]acetate, respectively. 1H-[13C]-NMR measured 13C enrichment was deconvolved for the contribution of [U-13C6]glucose and [2-13C]acetate using Eq. 1 and 2, respectively. 13C Concentrations of amino acids were obtained by multiplying the normalized deconvolved labeling with the total concentration. Values represent mean±SEM. *p<0.05, **p<0.01 indicate significance of differences when compared to the respective controls.
Discussion
Although the acute effects of nicotine on cerebral blood flow and glucose utilization have been investigated in rats and human brain, there is scarcity of data pertaining to the chronic effect of nicotine on cerebral function. To the best of our knowledge, this is the first study which has evaluated the effect of chronic nicotine on glutamatergic, GABAergic and astroglial glucose oxidation and neurotransmitter cycle in different regions of the mouse brain. Using an approach of co-infusion of [U-13C6]glucose and [2-13C]acetate together with 13C NMR spectroscopy, we found that chronic treatment of nicotine increased excitatory activity in all regions of the brain, while inhibitory function was enhanced in the cerebral cortex only.
Nicotine and Cerebral Glucose Utilization
The effects of acute nicotine treatment on cerebral metabolism have been studied in rats and human brain. Autoradiographic investigations have shown increased utilization of glucose in rats treated with nicotine ranging from 0.1 to 1.75 mg/kg [34]. The highest stimulation in the most brain areas was obtained with 0.3 mg/kg nicotine. Intravenous injection of nicotine (4 mg/kg, every 30 min) has been shown to increase cortical glucose oxidation and cerebral blood flow in rats anesthetized with morphine [25]. Effect of nicotine on regional cerebral glucose metabolism and blood flow has been investigated by nasal spray of nicotine in humans [23]. Increase in cerebral glucose consumption was reported in the cerebral left inferior frontal gyrus, left posterior cingulate gyrus and right thalamus [22]. Positron emission tomography study have suggested an increase in blood flow in the visual cortex and cerebellum, and a decrease in the right hippocampus and ventral striatum including the nucleus accumbens in overnight abstinent smokers followed by nasal spray [44]. However, a very recent study conducted in awake rats indicated a significant reduction in neuronal glucose oxidation and neurotransmitter cycle flux with 0.7 mg/kg nicotine exposure [45]. The effects of nicotine on the activity of various glycolytic and TCA cycle enzymes have been studied under acute and chronic nicotine treatments in frontoparietal regions and subcortical nuclei of the rat brain [26]. Both acute as well as chronic exposure of nicotine increases enzymatic activities in the frontoparietal cortex but deeper layers of the cortex, substantia nigra, caudate-putamen, nucleus accumbens or nucleus basalis magnocellularis did not exhibit increase in activity as high as seen in the frontoparietal cortex. It is noteworthy that most of these studies have investigated effects of acute nicotine exposure on brain energy metabolism and the consequences of chronic nicotine exposure on glutamatergic, GABAergic and astroglial function is still an open problem. Furthermore, with the exception to findings of Wang et al [45], most of the studies conducted in rats and human have indicated a stimulatory role for nicotine. Our findings of increased energy metabolism associated with glutamatergic neurons in the cortex, subcortex and cerebellum in mice under chronic nicotine exposure is consistent with increased glucose consumption observed under acute nicotine in autoradiography [34], PET [44] and NMR [25] studies as well as with reported increase in enzymatic activity under acute and chronic nicotine exposure [26].
Effect of Chronic Nicotine Treatment on Glutamatergic and GABAergic Activity
Nicotine exposure leads to activation, desensitization and up-regulation of nAChRs [27]. A single nicotine exposure increases dopamine levels in the mesolimbic reward system for hours. Nicotine exposure has been shown to increase GABAergic transmission transiently [6], [29], which is followed by a persistent depression of these inhibitory inputs due to desensitization of nAChRs. Simultaneously, nicotine enhances the glutamatergic transmission through activation of nAChRs that desensitizes lesser than GABAergic neurons [28], [30]. The net effect of these modulatory changes is in a shift towards greater excitability of dopaminergic neurons in ventral tegmental area (VTA). Thus, both activation and desensitization of nAChRs contribute to nicotine’s effect on the excitability of dopaminergic neurons [46]. Although, activation and desensitization of GABAergic neurons has been reported under acute condition, desensitization of GABAergic neurons might also be expected with chronic nicotine treatment. The data from the current study suggest an increase in glucose oxidation and neurotransmitter cycling by glutamatergic neurons in the cortex, subcortex and cerebellum upon chronic nicotine exposure. However, GABAergic metabolism was found to increase in the cortex and remain unperturbed in the subcortical and cerebellum regions. It may be possible that local increase in GABAergic metabolism in the subcortex and cerebellum upon chronic nicotine exposure might have been nullified due to measurement carried out in macroscopic brain volume.
The observations of high rate of glutamine labeling from [1-13C]glucose in in vivo 13C NMR studies indicate that glutamine is synthesized primarily from released neuronal glutamate [12], [47]. These studies have further established that the neurotransmitter cycle comprising of glutamate-glutamine accounts for major fraction (>80%) of glutamine synthesis [12], [15], [48]; importantly, it has a metabolic rate similar to neuronal glucose oxidation [16], [17]. High rate of the neurotransmitter cycle has been found in NMR studies of human cerebral cortex [49], [50], [51]. 13C NMR studies have further established that the rate of neurotransmitter cycle and neuronal mitochondrial glucose oxidation increased proportionately with a near 1∶1 slope [8], [16], [17], indicating that neurotransmitter energetics is supported by neuronal oxidative glucose metabolism. Therefore, increased glucose oxidation by glutamatergic and GABAergic neurons could be ascribed to increased glutamatergic and GABAergic neurotransmission upon chronic nicotine exposure.
Relevance to Nicotine Addiction
A thorough understanding of molecular processes of nicotine addiction is lacking. Nicotine addiction has been related to the up-regulation of nAChRs, desensitization and modulation of the dopaminergic and glutamatergic systems [52]. In the classical model, it is hypothesized that chronic nicotine exposure leads to up-regulation of nAChRs by increasing their half-life [53], [54]. nAChRs modulate the release of dopamine which is important for development of nicotine addiction. The up-regulation of nAChRs due to chronic nicotine exposure is followed by desensitization via a reversible reduction in response [55]. Level of nicotine present in smokers desensitizes as well as up-regulates α4β2 without having much effect on α7. There is an increased dopamine release in the nucleus accumbens and activation of dopaminergic neurons in the VTA by nicotine levels that are obtained by smoking [56]. Furthermore, chronic nicotine reduces basal extracellular dopamine levels [57]. Systemic administration of nicotine increases the activity of dopaminergic neurons in the VTA via N-methyl-D-aspartate (glutamate) receptors on dopaminergic cell bodies [58] which is mediated by α7 receptors present on the glutamatergic terminals facilitating glutamate release [30], [59], [60]. These studies point to a significant increase in glutamate release and desensitization of GABA in VTA during nicotine exposure [6]. Our findings, along with those of others [29], [61], indicate an increase in glutamatergic activity in the cortex, subcortex, cerebellum and OB, suggesting that nicotine-addiction related increase in glutamate release may not be limited to the VTA.
Limitations of the Study
In this study we have used an approach of co-infusion of [U-13C6]glucose and [2-13C]acetate together with 1H-[13C] and 13C-[1H]-NMR spectroscopy to study neuronal and astroglial metabolism in mice treated with nicotine. The low sensitivity of 13C-[1H]-NMR necessitates the use of concentrated sample i.e. more tissue for the measurement, hence compromised spatial resolution. Metabolic investigations at higher spatial resolution are difficult using the co-infusion approach. In fact, due to this limitation, we could not measure contribution of glucose and acetate to the total labeling of amino acids in the olfactory bulb. As we have combined all the subcortical (striatum, hippocampus, thalamus and hypothalamus) regions for the NMR analysis, it may be possible that the regional changes in GABA metabolism due to nicotine treatment might have subsided. Metabolic investigations at higher spatial resolution can be achieved by infusing labeled glucose or acetate individually and analyzing the 13C labeling of amino acids by inverse detection.
There may be a change in the expression of monocabroxylate transporters in mice treated with nicotine due to changes in the food intake. Although there was a decrease in the weight of animals during the first week of nicotine treatment, mice regained the weight in the latter part of the treatment such that there was not much difference in the weight of mice in different treatment groups at the time of study (not shown). These data suggest that food intake was similar in control and nicotine-treated mice at the time of metabolic analysis. Further, NMR analysis of plasma did not show significant presence of β-hydroxy butyrate, a marker for ketone bodies. Therefore, the possibility of increase in monocarboxylate transporters and increased utilization of ketogenic substrates in nicotine-treated mice is minimal. An increase utilization of ketone bodies may reduce the 13C labeling of acetylCoA, the precursor for TCA cycle, which will reduce the labeling of GluC4, and thus the TCA cycle flux evaluated using a single point measurement. Therefore, single point measurement with a short infusion of 13C labeled substrates provides qualitative information for the changes in the metabolism under chronic nicotine exposure. This approach has been utilized in many studies to gain insights in metabolism under different conditions [37], [62], [63]. The quantitative changes in metabolic rates associated with glutamatergic and GABAergic pathways could be obtained by constructing the time course of labeling of brain amino acids during infusion of labeled substrates for different time points and modeling the data by a three compartment metabolic model [15]. However, the modeling of 13C turnover data during co-infusion of [U-13C6]glucose and [2-13C]acetate is not fully understood and parameterized in different regions of brain. Hence, quantitative evaluation of the effects of chronic nicotine on metabolic rates was not attempted. The absolute changes in the value of glutamatergic and GABAergic neurotransmission and TCA cycle rates during chronic nicotine exposure is still an unanswered question, which can be addressed by modeling of the 13C turnover curve of amino acids from [1-13C]glucose as described by us earlier [15,64], and by Wang et al [45] under acute nicotine treatment. However, the directional changes in the metabolic fluxes between control and treated groups will remain the same, but the absolute values of the TCA cycle and neurotransmitter cycle may be different.
Statistical analysis of 13C enrichment of plasma [1-13C]glucose and [2-13C]acetate did not yield difference (p = 0.11) among different groups. Moreover, the data presented in Tables 2 & 3 and Figure 4 are normalized with the respective percent enrichment of precursors in the plasma. Hence, the differences in the 13C labeling of brain amino acids between different groups are due to changes in metabolism and not because of differences in the 13C enrichment of precursors in the plasma. Normalization with enrichment of precursors in the plasma does not rule out differential changes in the utilization of substrates used for the synthesis of acetylCoA. As mentioned above the possibility of fractional changes in the substrate utilized is minimum and will have a positive effect on metabolism.
In the present study metabolic analysis has been carried out under urethane anesthesia. It may be possible that chronic nicotine treatment stimulates liver enzymes so that urethane is metabolized with rate different in nicotine-treated mice from those in the control; therefore, the effect of anesthetic is different among the groups. However, the level of urethane in blood plasma at the time of substrates infusion was not significantly different (p>0.13, single Factor ANOVA) among control and nicotine (0.5 mg/kg and nicotine 2.0 mg/kg)-treated mice, suggesting chronic nicotine treatment did not alter urethane metabolism. Furthermore, the measured respiration rate during substrate infusion was similar (p>0.1) in control and nicotine-treated mice. Hence, anesthetic effect should be similar in both groups. As metabolic analysis was carried out 2 days after the last treatment of nicotine, the contribution of processes associated with withdrawal of nicotine on observed changes in metabolism could not be ruled out. This could be evaluated by studying the metabolism at different times after treatment of nicotine.
In conclusion, chronic nicotine exposure enhanced excitatory activity associated with glutamatergic neurons in majority of the brain regions. The inhibitory and astroglial functions were found to be perturbed in the selected brain regions following chronic nicotine treatment.
Supporting Information
The concentration of 13C labeled amino acids was calculated by multiplying the normalized deconvolved enrichment with the total concentration. Values represent mean±SEM. *p<0.05, **p<0.01 indicate significance of differences when compared to respective control.
(PDF)
Acknowledgments
We thank Dr. Robin A. de Graaf, Yale University for providing the 1H-[13C]-NMR sequence, Mr. Bhargidhar Babu for his assistance in animal study, Mr. Sheikh Nizamuddin for help with statistical analysis, Dr. T. Ramakrishna Murti for the proof reading of the manuscript and Mr. K. S. Varadarajan for his assistance in recording NMR experiments. All NMR experiments were performed at NMR Microimaging and Spectroscopy Facility, Centre for Cellular and Molecular Biology, Hyderabad, India.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by funding from Council of Scientific and Industrial Research, Government of India - Centre for Cellular and Molecular Biology, Hyderabad, India. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol. 2009;49:57–71. doi: 10.1146/annurev.pharmtox.48.113006.094742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paterson NE, Semenova S, Gasparini F, Markou A. The mGluR5 antagonist MPEP decreased nicotine self-administration in rats and mice. Psychopharmacology (Berl) 2003;167:257–264. doi: 10.1007/s00213-003-1432-z. [DOI] [PubMed] [Google Scholar]
- 3.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 4.Benowitz NL. Pharmacology of nicotine: addiction and therapeutics. Annu Rev Pharmacol Toxicol. 1996;36:597–613. doi: 10.1146/annurev.pa.36.040196.003121. [DOI] [PubMed] [Google Scholar]
- 5.MacDermott AB, Role LW, Siegelbaum SA. Presynaptic ionotropic receptors and the control of transmitter release. Annu Rev Neurosci. 1999;22:443–485. doi: 10.1146/annurev.neuro.22.1.443. [DOI] [PubMed] [Google Scholar]
- 6.Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 7.McGehee DS, Heath MJ, Gelber S, Devay P, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269:1692–1696. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- 8.Hyder F, Patel AB, Gjedde A, Rothman DL, Behar KL, et al. Neuronal-glial glucose oxidation and glutamatergic-GABAergic function. J Cereb Blood Flow Metab. 2006;26:865–877. doi: 10.1038/sj.jcbfm.9600263. [DOI] [PubMed] [Google Scholar]
- 9.Ottersen OP, Storm-Mathisen J. Excitatory amino acid pathways in the brain. Adv Exp Med Biol. 1986;203:263–284. doi: 10.1007/978-1-4684-7971-3_20. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt WJ, Bubser M, Hauber W. Behavioural pharmacology of glutamate in the basal ganglia. J Neural Transm. 1992. pp. 65–89. [PubMed]
- 11.Shulman RG, Rothman DL, Behar KL, Hyder F. Energetic basis of brain activity: implications for neuroimaging. Trends Neurosci. 2004;27:489–495. doi: 10.1016/j.tins.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Sibson NR, Dhankhar A, Mason GF, Behar KL, Rothman DL, et al. In vivo 13C NMR measurements of cerebral glutamine synthesis as evidence for glutamate-glutamine cycling. Proc Natl Acad Sci U S A. 1997;94:2699–2704. doi: 10.1073/pnas.94.6.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Graaf RA, Brown PB, Mason GF, Rothman DL, Behar KL. Detection of [1,6–13C2]-glucose metabolism in rat brain by in vivo 1H-[13C]-NMR spectroscopy. Magn Reson Med. 2003;49:37–46. doi: 10.1002/mrm.10348. [DOI] [PubMed] [Google Scholar]
- 14.de Graaf RA, Mason GF, Patel AB, Rothman DL, Behar KL. Regional glucose metabolism and glutamatergic neurotransmission in rat brain in vivo. Proc Natl Acad Sci U S A. 2004;101:12700–12705. doi: 10.1073/pnas.0405065101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel AB, de Graaf RA, Mason GF, Rothman DL, Shulman RG, et al. The contribution of GABA to glutamate/glutamine cycling and energy metabolism in the rat cortex in vivo. Proc Natl Acad Sci U S A. 2005;102:5588–5593. doi: 10.1073/pnas.0501703102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sibson NR, Dhankhar A, Mason GF, Rothman DL, Behar KL, et al. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci U S A. 1998;95:316–321. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel AB, de Graaf RA, Mason GF, Kanamatsu T, Rothman DL, et al. Glutamatergic neurotransmission and neuronal glucose oxidation are coupled during intense neuronal activation. J Cereb Blood Flow Metab. 2004;24:972–985. doi: 10.1097/01.WCB.0000126234.16188.71. [DOI] [PubMed] [Google Scholar]
- 18.Greenamyre JT, Maragos WF. Neurotransmitter receptors in Alzheimer disease. Cerebrovasc Brain Metab Rev. 1993;5:61–94. [PubMed] [Google Scholar]
- 19.Heath PR, Shaw PJ. Update on the glutamatergic neurotransmitter system and the role of excitotoxicity in amyotrophic lateral sclerosis. Muscle Nerve. 2002;26:438–458. doi: 10.1002/mus.10186. [DOI] [PubMed] [Google Scholar]
- 20.Pearl PL, Gibson KM. Clinical aspects of the disorders of GABA metabolism in children. Curr Opin Neurol. 2004;17:107–113. doi: 10.1097/00019052-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Sanacora G, Rothman DL, Mason G, Krystal JH. Clinical studies implementing glutamate neurotransmission in mood disorders. Ann N Y Acad Sci. 2003;1003:292–308. doi: 10.1196/annals.1300.018. [DOI] [PubMed] [Google Scholar]
- 22.Domino EF, Minoshima S, Guthrie SK, Ohl L, Ni L, et al. Effects of nicotine on regional cerebral glucose metabolism in awake resting tobacco smokers. Neuroscience. 2000;101:277–282. doi: 10.1016/s0306-4522(00)00357-2. [DOI] [PubMed] [Google Scholar]
- 23.Domino EF, Minoshima S, Guthrie S, Ohl L, Ni L, et al. Nicotine effects on regional cerebral blood flow in awake, resting tobacco smokers. Synapse. 2000;38:313–321. doi: 10.1002/1098-2396(20001201)38:3<313::AID-SYN10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 24.Marenco T, Bernstein S, Cumming P, Clarke PB. Effects of nicotine and chlorisondamine on cerebral glucose utilization in immobilized and freely-moving rats. Br J Pharmacol. 2000;129:147–155. doi: 10.1038/sj.bjp.0703005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hyder F, Kennan RP, Kida I, Mason GF, Behar KL, et al. Dependence of oxygen delivery on blood flow in rat brain: a 7 tesla nuclear magnetic resonance study. J Cereb Blood Flow Metab. 2000;20:485–498. doi: 10.1097/00004647-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Turegano L, Martinez-Rodriguez R, Alvarez MI, Gragera RR, Gomez de Segura A, et al. Histochemical study of acute and chronic intraperitoneal nicotine effects on several glycolytic and Krebs cycle dehydrogenase activities in the frontoparietal cortex and subcortical nuclei of the rat brain. J Neurosci Res. 2001;64:626–635. doi: 10.1002/jnr.1116. [DOI] [PubMed] [Google Scholar]
- 27.Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fagen ZM, Mansvelder HD, Keath JR, McGehee DS. Short- and long-term modulation of synaptic inputs to brain reward areas by nicotine. Ann N Y Acad Sci. 2003;1003:185–195. doi: 10.1196/annals.1300.011. [DOI] [PubMed] [Google Scholar]
- 29.Zhang TA, Tang J, Pidoplichko VI, Dani JA. Addictive nicotine alters local circuit inhibition during the induction of in vivo hippocampal synaptic potentiation. J Neurosci. 2010;30:6443–6453. doi: 10.1523/JNEUROSCI.0458-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27:349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- 31.Patel AB, Shameem M. NMR investigations of neuronal and astroglial metabolism in nicotine addiction. Proc Intl Soc Magn Reson Med. 2010;18:3356. [Google Scholar]
- 32.Grabus SD, Martin BR, Batman AM, Tyndale RF, Sellers E, et al. Nicotine physical dependence and tolerance in the mouse following chronic oral administration. Psychopharmacology (Berl) 2005;178:183–192. doi: 10.1007/s00213-004-2007-3. [DOI] [PubMed] [Google Scholar]
- 33.Jackson KJ, Walters CL, Miles MF, Martin BR, Damaj MI. Characterization of pharmacological and behavioral differences to nicotine in C57Bl/6 and DBA/2 mice. Neuropharmacology. 2009;57:347–355. doi: 10.1016/j.neuropharm.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.London ED, Connolly RJ, Szikszay M, Wamsley JK, Dam M. Effects of nicotine on local cerebral glucose utilization in the rat. J Neurosci. 1988;8:3920–3928. doi: 10.1523/JNEUROSCI.08-10-03920.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marks MJ, Meinerz NM, Brown RW, Collins AC. 86Rb+ efflux mediated by alpha4beta2*-nicotinic acetylcholine receptors with high and low-sensitivity to stimulation by acetylcholine display similar agonist-induced desensitization. Biochem Pharmacol. 2010;80:1238–1251. doi: 10.1016/j.bcp.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, et al. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- 37.Bagga P, Patel AB. Regional cerebral metabolism in mouse under chronic manganese exposure: Implications for Manganism. Neurochem Int. 2012;60:177–185. doi: 10.1016/j.neuint.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 38.Fitzpatrick SM, Hetherington HP, Behar KL, Shulman RG. The flux from glucose to glutamate in the rat brain in vivo as determined by 1H-observed, 13C-edited NMR spectroscopy. J Cereb Blood Flow Metab. 1990;10:170–179. doi: 10.1038/jcbfm.1990.32. [DOI] [PubMed] [Google Scholar]
- 39.Tiwari V, Patel AB. Impaired Glutamatergic and GABAergic Function at Early Age in AbetaPPswe-PS1dE9 Mice: Implications for Alzheimer’s Disease. J Alzheimers Dis. 2012;28:765–769. doi: 10.3233/JAD-2011-111502. [DOI] [PubMed] [Google Scholar]
- 40.Patel AB, Rothman DL, Cline GW, Behar KL. Glutamine is the major precursor for GABA synthesis in rat neocortex in vivo following acute GABA-transaminase inhibition. Brain Res. 2001;919:207–220. doi: 10.1016/s0006-8993(01)03015-3. [DOI] [PubMed] [Google Scholar]
- 41.Waniewski RA, Martin DL. Preferential utilization of acetate by astrocytes is attributable to transport. J Neurosci. 1998;18:5225–5233. doi: 10.1523/JNEUROSCI.18-14-05225.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zwingmann C, Leibfritz D, Hazell AS. Energy metabolism in astrocytes and neurons treated with manganese: relation among cell-specific energy failure, glucose metabolism, and intercellular trafficking using multinuclear NMR-spectroscopic analysis. J Cereb Blood Flow Metab. 2003;23:756–771. doi: 10.1097/01.WCB.0000056062.25434.4D. [DOI] [PubMed] [Google Scholar]
- 43.Badar-Goffer RS, Bachelard HS, Morris PG. Cerebral metabolism of acetate and glucose studied by 13C NMR spectroscopy. A technique for investigating metabolic compartmentation in the brain. Biochem J. 1990;266:133–139. doi: 10.1042/bj2660133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zubieta J, Lombardi U, Minoshima S, Guthrie S, Ni L, et al. Regional cerebral blood flow effects of nicotine in overnight abstinent smokers. Biol Psychiatry. 2001;49:906–913. doi: 10.1016/s0006-3223(00)01070-2. [DOI] [PubMed] [Google Scholar]
- 45.Wang J, Jiang L, Jiang Y, Ma X, Chowdhury GM, et al. Regional metabolite levels and turnover in the awake rat brain under the influence of nicotine. J Neurochem. 2010;113:1447–1458. doi: 10.1111/j.1471-4159.2010.06684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mao D, McGehee DS. Nicotine and behavioral sensitization. J Mol Neurosci. 2010;40:154–163. doi: 10.1007/s12031-009-9230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gruetter R, Novotny EJ, Boulware SD, Mason GF, Rothman DL, et al. Localized 13C NMR spectroscopy in the human brain of amino acid labeling from D-[1–13C]glucose. J Neurochem. 1994;63:1377–1385. doi: 10.1046/j.1471-4159.1994.63041377.x. [DOI] [PubMed] [Google Scholar]
- 48.Patel AB, de Graaf RA, Rothman DL, Behar KL, Mason GF. Evaluation of cerebral acetate transport and metabolic rates in the rat brain in vivo using 1H-[13C]-NMR. J Cereb Blood Flow Metab. 2010;30:1200–1213. doi: 10.1038/jcbfm.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gruetter R, Seaquist ER, Ugurbil K. A mathematical model of compartmentalized neurotransmitter metabolism in the human brain. Am J Physiol Endocrinol Metab. 2001;281:E100–112. doi: 10.1152/ajpendo.2001.281.1.E100. [DOI] [PubMed] [Google Scholar]
- 50.Lebon V, Petersen KF, Cline GW, Shen J, Mason GF, et al. Astroglial contribution to brain energy metabolism in humans revealed by 13C nuclear magnetic resonance spectroscopy: elucidation of the dominant pathway for neurotransmitter glutamate repletion and measurement of astrocytic oxidative metabolism. J Neurosci. 2002;22:1523–1531. doi: 10.1523/JNEUROSCI.22-05-01523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shen J, Petersen KF, Behar KL, Brown P, Nixon TW, et al. Determination of the rate of the glutamate/glutamine cycle in the human brain by in vivo 13C NMR. Proc Natl Acad Sci U S A. 1999;96:8235–8240. doi: 10.1073/pnas.96.14.8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ortells MO, Barrantes GE. Tobacco addiction: a biochemical model of nicotine dependence. Med Hypotheses. 2010;74:884–894. doi: 10.1016/j.mehy.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 53.Kuryatov A, Luo J, Cooper J, Lindstrom J. Nicotine acts as a pharmacological chaperone to up-regulate human alpha4beta2 acetylcholine receptors. Mol Pharmacol. 2005;68:1839–1851. doi: 10.1124/mol.105.012419. [DOI] [PubMed] [Google Scholar]
- 54.Sallette J, Pons S, Devillers-Thiery A, Soudant M, Prado de Carvalho L, et al. Nicotine upregulates its own receptors through enhanced intracellular maturation. Neuron. 2005;46:595–607. doi: 10.1016/j.neuron.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 55.Giniatullin R, Nistri A, Yakel JL. Desensitization of nicotinic ACh receptors: shaping cholinergic signaling. Trends Neurosci. 2005;28:371–378. doi: 10.1016/j.tins.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 56.Laviolette SR, van der Kooy D. The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nat Rev Neurosci. 2004;5:55–65. doi: 10.1038/nrn1298. [DOI] [PubMed] [Google Scholar]
- 57.Rahman S, Zhang J, Engleman EA, Corrigall WA. Neuroadaptive changes in the mesoaccumbens dopamine system after chronic nicotine self-administration: a microdialysis study. Neuroscience. 2004;129:415–424. doi: 10.1016/j.neuroscience.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 58.Nisell M, Nomikos GG, Svensson TH. Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse. 1994;16:36–44. doi: 10.1002/syn.890160105. [DOI] [PubMed] [Google Scholar]
- 59.Marchi M, Risso F, Viola C, Cavazzani P, Raiteri M. Direct evidence that release-stimulating alpha7* nicotinic cholinergic receptors are localized on human and rat brain glutamatergic axon terminals. J Neurochem. 2002;80:1071–1078. doi: 10.1046/j.0022-3042.2002.00805.x. [DOI] [PubMed] [Google Scholar]
- 60.Schilstrom B, Fagerquist MV, Zhang X, Hertel P, Panagis G, et al. Putative role of presynaptic alpha7* nicotinic receptors in nicotine stimulated increases of extracellular levels of glutamate and aspartate in the ventral tegmental area. Synapse. 2000;38:375–383. doi: 10.1002/1098-2396(20001215)38:4<375::AID-SYN2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 61.Sharma G, Grybko M, Vijayaraghavan S. Action potential-independent and nicotinic receptor-mediated concerted release of multiple quanta at hippocampal CA3-mossy fiber synapses. J Neurosci. 2008;28:2563–2575. doi: 10.1523/JNEUROSCI.5407-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chowdhury GM, Behar KL, Cho W, Thomas MA, Rothman DL, et al. (1)H-[(13)C]-Nuclear Magnetic Resonance Spectroscopy Measures of Ketamine’s Effect on Amino Acid Neurotransmitter Metabolism. Biol Psychiatry. 2012;71:1022–1025. doi: 10.1016/j.biopsych.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meisingset TW, Risa O, Brenner M, Messing A, Sonnewald U. Alteration of glial-neuronal metabolic interactions in a mouse model of Alexander disease. Glia. 2010;58:1228–1234. doi: 10.1002/glia.21003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The concentration of 13C labeled amino acids was calculated by multiplying the normalized deconvolved enrichment with the total concentration. Values represent mean±SEM. *p<0.05, **p<0.01 indicate significance of differences when compared to respective control.
(PDF)