PEARLS
Hirayama disease is characterized by progressive, yet self-limited, neurogenic wasting and weakness of the distal upper extremities.
Its pathogenesis is unclear. We present the case of a typical young man with Hirayama disease in which CT venography (CTV) of the neck in attempted neck flexion showed engorgement of the epidural venous plexus, anterior displacement of the dura, and posterior compression of the cord even when MRI did not.
CASE REPORT
An 18-year-old, left-handed North Indian man presented with a 2-year history of wasting and weakness of the left more than right hands. He was unable to lift heavy objects, but he could button his clothes and hold cups. His medical history was significant only for a remote motor vehicle accident, without head trauma or subsequent neck pain. There was no family history of neurologic disease.
On examination, tone was normal. There was moderate atrophy and weakness of the intrinsic hand (interossei worse than abductor pollicis brevis) and forearm muscles, but the bulk of the brachioradialis muscles was preserved bilaterally. The left-sided muscles were more severely affected. Muscle strength was normal in all upper extremity muscle groups proximal to the elbows and the lower extremities. The left triceps and finger flexor reflexes were absent, but all other reflexes were normal and symmetric, and plantar reflexes were flexor. Pain and vibration sensation were intact throughout. Coordination testing was normal.
The results for CSF immunoglobulin G index and oligoclonal bands, CSF Venereal Disease Research Laboratory, antinuclear antibodies, neuromyelitis optica antibody, SSA/SSB antibodies, rheumatoid factor, and erythrocyte sedimentation rate were unremarkable. GM1 antibodies were not checked. EMG of the bilateral upper and left lower extremities showed evidence of severe, subacute, partial denervation of muscles innervated by bilateral C7, C8, and T1 nerve roots. MRI of the spinal cord revealed T2-hyperintense lesions at T1–T2 and C4–C5 (figure, A and B). Additional images in attempted neck flexion revealed no dynamic changes of the spinal cord (not shown), but the degree of flexion was limited by the head coil.
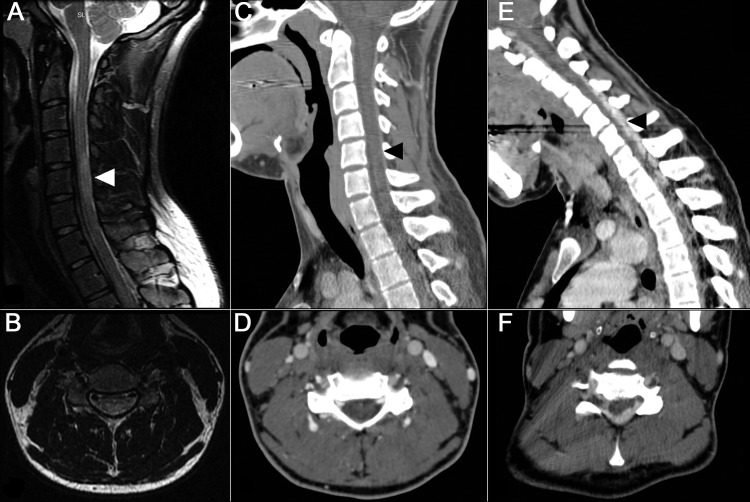
Figure. MRI and CT of the cervical spine.
(A) Sagittal and (B) axial T2-weighted MRI of the cervical spine in neutral position demonstrates central cord lesion at C4–C5 and T1–T2. (C) Sagittal and (D) axial CT venogram of the cervical spine in neutral position, at the level of C5. (E) Sagittal and (F) axial CT venogram of the cervical spine in flexed position, demonstrating enlargement of the epidural venous plexus, anterior displacement of the dura, and posterior compression of the cervical cord. Arrowheads on sagittal images indicate the spinal level of the corresponding axial image.
In an effort to assess vascular and anatomic changes with full neck flexion, CTV was performed with the neck in both neutral and maximally flexed positions. For each scan, helical axial acquisition from the skull base to the T8 level in the late arterial/venous phase was performed following bolus IV injection of 70 mL Omnipaque-350 contrast. In between scans, he was allowed to recover and ambulate. Multiple sagittal and coronal reformations were performed.
CTV was normal in the neutral position (figure, C and D). In the maximally flexed position, however, there was anterior displacement of the cord and thecal sac along with engorgement of intraspinal epidural veins from C4 to T2 (figure, E and F). In both flexed and neutral position, the right jugular vein was diminutive, with no visualized filling between the jugular bulb and C2–C3 (not shown). The dominant left internal jugular vein demonstrated moderate focal narrowing at the level of the mid sternocleidomastoid muscle, which worsened with flexion. The epidural venous system was noted to communicate with posterior muscular veins via the foramina of the lower cervical spine.
In an attempt to limit disease progression, the patient was told to wear a soft collar at night to limit the extent of neck flexion. Over the past year, his muscle weakness and atrophy have remained stable.
DISCUSSION
Hirayama disease, also known as juvenile muscular atrophy or monomelic amyotrophy, is a rare syndrome with the following features: unilateral or sometimes asymmetric bilateral weakness and atrophy of the upper extremities, jerking movements (polyminimyoclonus) in outstretched fingers, cold paresis, and absence of pyramidal signs or sensory loss. The disease predominantly affects males during the late second or early third decade of life. The disease progresses slowly for 1 to 5 years, with eventual spontaneous arrest but not recovery.
Hirayama disease is diagnosed clinically with support from electrodiagnostic testing. The differential diagnosis includes various compressive, inflammatory, and infectious cervical myelopathies as well as amyotrophic lateral sclerosis. To date, no laboratory studies or genotyping tests correlate with Hirayama disease, and EMG studies demonstrate only nonspecific denervation of muscles supplied by C7–T1 nerve roots.
The pathogenesis of Hirayama disease is unknown. Theories include repetitive mechanical trauma from anterior displacement of the cervical dural sac or venous obstruction. Each possibility could cause focal cervical microischemia and anterior horn cell loss. An intrinsic motor neuron disease is also possible.
Previous MRI studies have suggested that posterior compression of the cord is caused by anomalous anterior displacement of the posterior dura upon cervical flexion.1–3 In patients with Hirayama disease, displacement of the dura may be accompanied by dilated epidural veins when the neck is flexed. The engorged epidural venous plexus may compress and damage the cord, though a recent interventional study using a venous microcatheter showed no change in venous epidural pressure in the flexed position, suggesting that venous enlargement may represent a passive process.4 The sensitivity and specificity of these MRI findings in Hirayama disease are not known. Also, dynamic changes on MRI with neck flexion have not been observed in some patients.5–7
The proper management of Hirayama disease is controversial. Conservative management includes the use of a cervical collar to limit neck flexion. Both anterior and posterior spinal decompressions and spinal fusions have been reported to improve strength in patients with Hirayama disease.8 However, given the self-limited nature of this disease without any interventions and limited understanding of pathogenesis, the indications for surgical spinal decompressions are debatable.
In our case, CTV but not MRI successfully demonstrated 2 potentially characteristic features of this disease: 1) anterior displacement of the thecal sac and 2) engorgement of the epidural venous plexus upon flexion. The MRI coil restricted the degree of neck flexion, which probably explains why these findings were not seen on the MRI scans. CT allowed easier flexion of the neck although it is associated with radiation exposure. Although Hirayama disease remains a clinical diagnosis, CTV may be helpful in evaluating patients in whom this diagnosis is considered, and may aid in future studies of the pathogenesis of this interesting neuromuscular disorder.
ACKNOWLEDGMENT
The authors thank Dr. Robert Layzer from the University of California, San Francisco, for discussions regarding this manuscript.
AUTHOR CONTRIBUTIONS
Maggie W. Waung, Aaron W. Grossman, S. Andrew Josephson, William P. Dillon, and Jeffrey W. Ralph were involved in drafting and revising the manuscript. They were also involved in analyzing the clinical data. Sami J. Barmada, MD, analyzed the clinical data.
DISCLOSURE
M. Waung, A. Grossman, and S. Barmada report no disclosures. S.A. Josephson receives compensation as Associate Editor for Annals of Neurology and Editor-In-Chief of Journal Watch Neurology. W. Dillon receives compensation from Coaxia, Inc., for directing a core laboratory. J. Ralph receives support from the NIH R01 NS045686-01A2 and NIH 5 U54 RR19482-03 grants. He has also has received grant support from The Neuropathy Association. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Chen CJ, Chen CM, Wu CL, Ro LS, Chen ST, Lee TH. Hirayama disease: MR diagnosis. AJNR Am J Neuroradiol 1998; 19: 365– 368 [PMC free article] [PubMed] [Google Scholar]
- 2. Pradhan S, Gupta RK. Magnetic resonance imaging in juvenile asymmetric segmental spinal muscular atrophy. J Neurol Sci 1997; 146: 133– 138 [DOI] [PubMed] [Google Scholar]
- 3. Hirayama K, Tokumaru Y. Cervical dural sac and spinal cord in juvenile muscular atrophy of distal upper extremity. Neurology 2000; 54: 1922– 1926 [DOI] [PubMed] [Google Scholar]
- 4. Patel TR, Chiocca EA, Freimer ML, Christoforidis GA. Lack of epidural pressure change with neck flexion in a patient with Hirayama disease: case report. Neurosurgery 2009; 64: E1196– E1197 [DOI] [PubMed] [Google Scholar]
- 5. Schroder R, Keller E, Flacke S, et al. MRI findings in Hirayama's disease: flexion-induced cervical myelopathy or intrinsic motor neuron disease? J Neurol 1999; 246: 1069– 1074 [DOI] [PubMed] [Google Scholar]
- 6. Willeit J, Kiechl S, Kiechl-Kohlendorfer U, Golaszewski S, Peer S, Poewe W. Juvenile asymmetric segmental spinal muscular atrophy (Hirayama's disease): three cases without evidence of “flexion myelopathy.” Acta Neurol Scand 2001; 104: 320– 322 [DOI] [PubMed] [Google Scholar]
- 7. Ammendola A, Gallo A, Iannaccone T, Tedeschi G. Hirayama disease: three cases assessed by F wave, somatosensory and motor evoked potentials and magnetic resonance imaging not supporting flexion myelopathy. Neurol Sci 2008; 29: 303– 311 [DOI] [PubMed] [Google Scholar]
- 8. Watanabe K, Hasegawa K, et al. Anterior spinal decompression and fusion for cervical flexion myelopathy in young patients. J Neurosurg Spine 2005; 3: 86– 91 [DOI] [PubMed] [Google Scholar]