Abstract
Objective:
Idiopathic REM sleep behavior disorder is a parasomnia characterized by dream enactment and is commonly a prediagnostic sign of parkinsonism and dementia. Since risk factors have not been defined, we initiated a multicenter case-control study to assess environmental and lifestyle risk factors for REM sleep behavior disorder.
Methods:
Cases were patients with idiopathic REM sleep behavior disorder who were free of dementia and parkinsonism, recruited from 13 International REM Sleep Behavior Disorder Study Group centers. Controls were matched according to age and sex. Potential environmental and lifestyle risk factors were assessed via standardized questionnaire. Unconditional logistic regression adjusting for age, sex, and center was conducted to investigate the environmental factors.
Results:
A total of 694 participants (347 patients, 347 controls) were recruited. Among cases, mean age was 67.7 ± 9.6 years and 81.0% were male. Cases were more likely to smoke (ever smokers = 64.0% vs 55.5%, adjusted odds ratio [OR] = 1.43, p = 0.028). Caffeine and alcohol use were not different between cases and controls. Cases were more likely to report previous head injury (19.3% vs 12.7%, OR = 1.59, p = 0.037). Cases had fewer years of formal schooling (11.1 ± 4.4 years vs 12.7 ± 4.3, p < 0.001), and were more likely to report having worked as farmers (19.7% vs 12.5% OR = 1.67, p = 0.022) with borderline increase in welding (17.8% vs 12.1%, OR = 1.53, p = 0.063). Previous occupational pesticide exposure was more prevalent in cases than controls (11.8% vs 6.1%, OR = 2.16, p = 0.008).
Conclusions:
Smoking, head injury, pesticide exposure, and farming are potential risk factors for idiopathic REM sleep behavior disorder.
REM sleep behavior disorder (RBD) is parasomnia with unknown prevalence, characterized by loss of the normal muscle atonia that accompanies REM sleep.1–3 Idiopathic RBD is most importantly a potential preclinical sign of synuclein-mediated neurodegenerative disease, including Parkinson disease (PD), dementia with Lewy bodies (DLB), and multiple system atrophy.4–6 However, the relationship between RBD and associated neurodegenerative diseases is complex. For example, only 35%–50% of patients with PD have associated RBD,2,7 and RBD in PD may mark a specific subtype of disease, characterized by more autonomic dysfunction, hallucinations, cognitive impairment, and akinetic rigid subtype.8–11 Pathologic studies have suggested that DLB manifestations also differ according to RBD status; patients with RBD have earlier onset of parkinsonism and hallucinations, earlier mortality, and fewer Alzheimer changes on neuropathology.12,13
If RBD is associated with both dementia and parkinsonism, and may be associated with subtypes of these diseases, one may hypothesize that risk factors for RBD may be similar to PD, to dementia, or could be unique. Except for male sex and age,3 risk factors for RBD are unknown. RBD is a relatively rare condition in sleep clinics (the largest reported cohort of idiopathic RBD included only 93 patients6), which has prevented risk factor studies from being performed. To address the sample size limitations, the international REM Sleep Behavior Disorder Study Group (RBDSG) was launched in 2008. The goal of the RBDSG was to combine resources and patient populations between centers, so that larger scale studies could be performed. This study describes a multicenter case-control study of risk factors for idiopathic RBD using member centers of the RBDSG.
METHODS
Standard protocol approvals, registrations, and patient consent.
Ethics approval was obtained from the research ethics board of each participating center. All patients gave informed consent according to the Declaration of Helsinki.
Cases and controls.
Cases were patients with polysomnography-confirmed idiopathic RBD recruited from 13 RBDSG centers from 2008 to 2011. All cases met International Classification of Sleep Disorders–2 criteria for RBD (i.e., enhanced REM muscle tone on polysomnography with a history of dream enactment or complex behaviors during REM sleep on polysomnography14) (convenience sampling was conducted, maximal recruitment each center). All cases had neurologic examination confirming the absence of dementia (as defined as Mini-Mental State Examination <24 with functional impairment due to cognitive decline15) and parkinsonism (according to UK Brain Bank criteria16). Each center also recruited controls, frequency-matched 1:1 on age (within 5 years) and sex (10% tolerance outside perfect matching was allowed). There were 2 groups of controls: patients referred to the sleep center for other sleep problems (e.g., apnea, restless legs, narcolepsy, insomnia, hypersomnia) and normal volunteers. Since the main drawback with using sleep center controls is the possibility that other sleep disorders may be associated with our assessed risk factors (e.g., obstructive sleep apnea may be associated with atherosclerotic risk factors16), we ensured recruitment of diverse diagnoses among diagnoses, such that no more than 35% of controls could have any single sleep disorder. All sleep center controls had polysomnogram documenting absence of RBD (as well as 67/129 volunteer controls). All participants provided written informed consent to participate, and the research ethics board of each center gave approval for the study.
Questionnaire administration.
A structured questionnaire assessing the presence (or a history of) a variety of risk factors was designed in English (appendix e-1 on the Neurology® Web site at www.neurology.org). This questionnaire was then translated into French, Spanish, Italian, German, Danish, Japanese, and Czech by medical translators. The questionnaire was designed to be self-administered, with participation by spouses/caregivers encouraged. If literacy was poor, centers could administer the questionnaire in person.
A diverse set of lifestyle risk factors was assessed, focusing on risk factors previously shown to be associated with PD and dementia (see appendix e-1 for questions used). Coffee and tea intake were assessed with questionnaires assessing current and historical intake, with variables that included ever use, duration of use, cessation of intake, and intake amount per week. Alcohol intake was assessed with questionnaire of current and past use frequency (on a 0–6 Likert scale from never [0] to every day [6]), habitual quantity, and binge drinking frequency (defined as >5 drinks on 1 occasion, also on a 0–6 Likert scale). Smoking was assessed for cigarettes, cigars, and pipes, with variables defined as ever smoker (>100 cigarettes over lifetime), ever regular smoker, ex-smoker, amount of habitual intake, duration of smoking, and total pack-years. Passive smoking (living with smokers) was also assessed, including duration and quantity of exposure.
Occupation was determined via open-ended questions, with additional specific questioning for history of farming, welding, mining, health care, and teaching. Occupational and nonoccupational exposure to pesticides (herbicides and insecticides), including type of pesticide and lifetime use frequency (on a 1–4 Likert scale, 1 = once or twice, 2 = 3–10 times, 3 = 10–50 times, 4 = >50 times), was assessed. Years of formal schooling was also queried. Rural living (defined as living in a community with fewer than 3,000 people) was assessed, as well as exposure to well water (including time and duration of exposure). History of traumatic head injury resulting in loss of consciousness was queried, including duration of unconsciousness, need for hospitalization, and date of trauma.
Statistical analysis.
Analysis was performed by R.P. and C.W. using PSAW statistics 18. Unadjusted odds ratios [OR] for each potential risk factor were calculated according to RBD status (case or control). The principal statistical analysis was unconditional logistic regression, adjusting for age, sex, and center. Additional covariates were entered into the model for selected variables, based upon known potential confounders (these are described in Results). To account for potential influence of the type of control, we conducted a sensitivity analysis that separately analyzed risk factors according to normal volunteer vs other sleep diagnosis, and with removing all sleep diagnoses with >20% prevalence (i.e., sleep apnea).
RESULTS
Demographics.
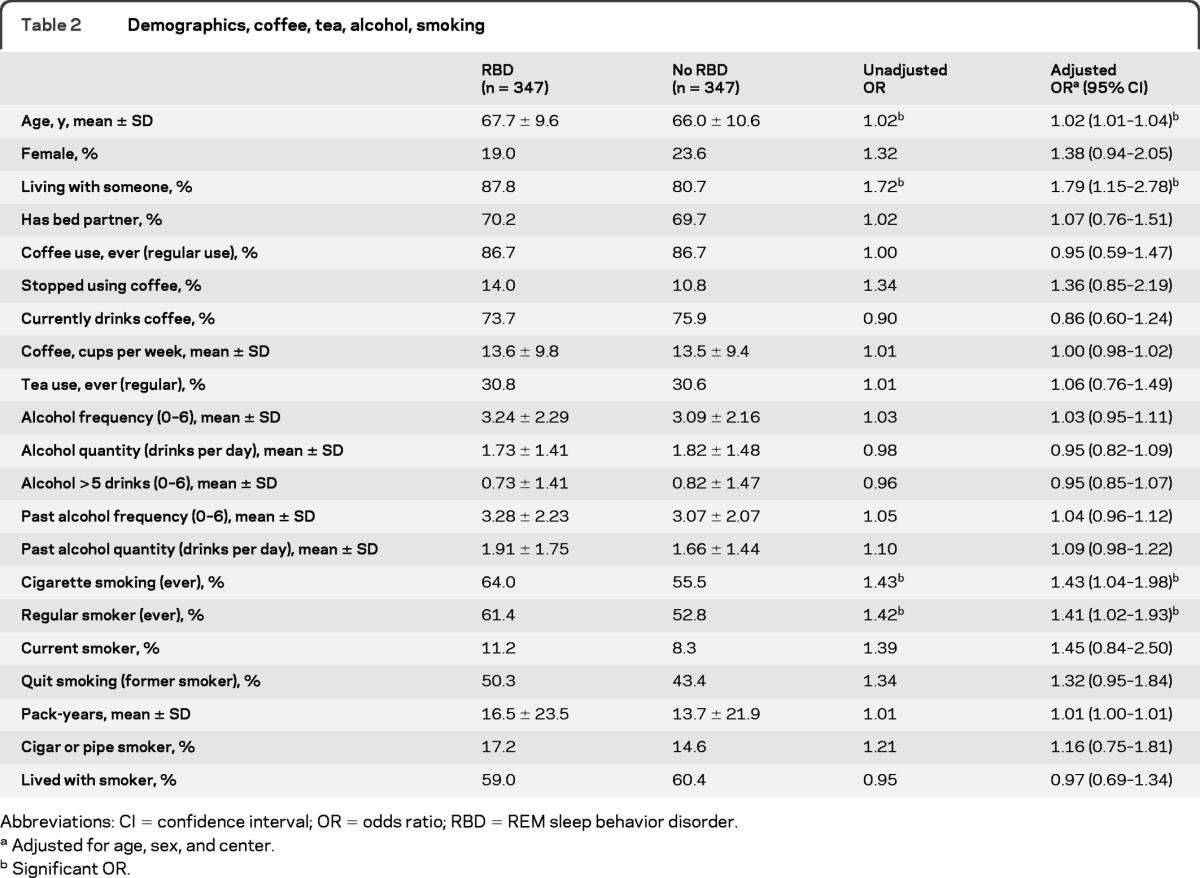
A total of 347 cases with idiopathic RBD were recruited from 13 centers in 10 countries. Table 1 presents the number of cases recruited in each center. The same number of controls were recruited: 129 were normal volunteers, and 218 had other sleep problems. The only sleep diagnosis that made up more than 20% of the sample was sleep apnea (n = 91, 26%). The remainder of the diagnoses were restless legs = 47 (14% of entire sample), insomnia = 38 (11%), somnolence = 7 (2%), and other = 35 (11%). Cases were slightly older than controls (67.7 vs 66.0 years, p = 0.029) (table 2). A total of 81.0% of cases were male, compared to 76.4% of controls (p = 0.15). Cases were slightly more likely to be living with someone (87.8% vs 80.7%, p = 0.009), but were not more likely to have a bed partner.
Table 1.
Centers recruiting
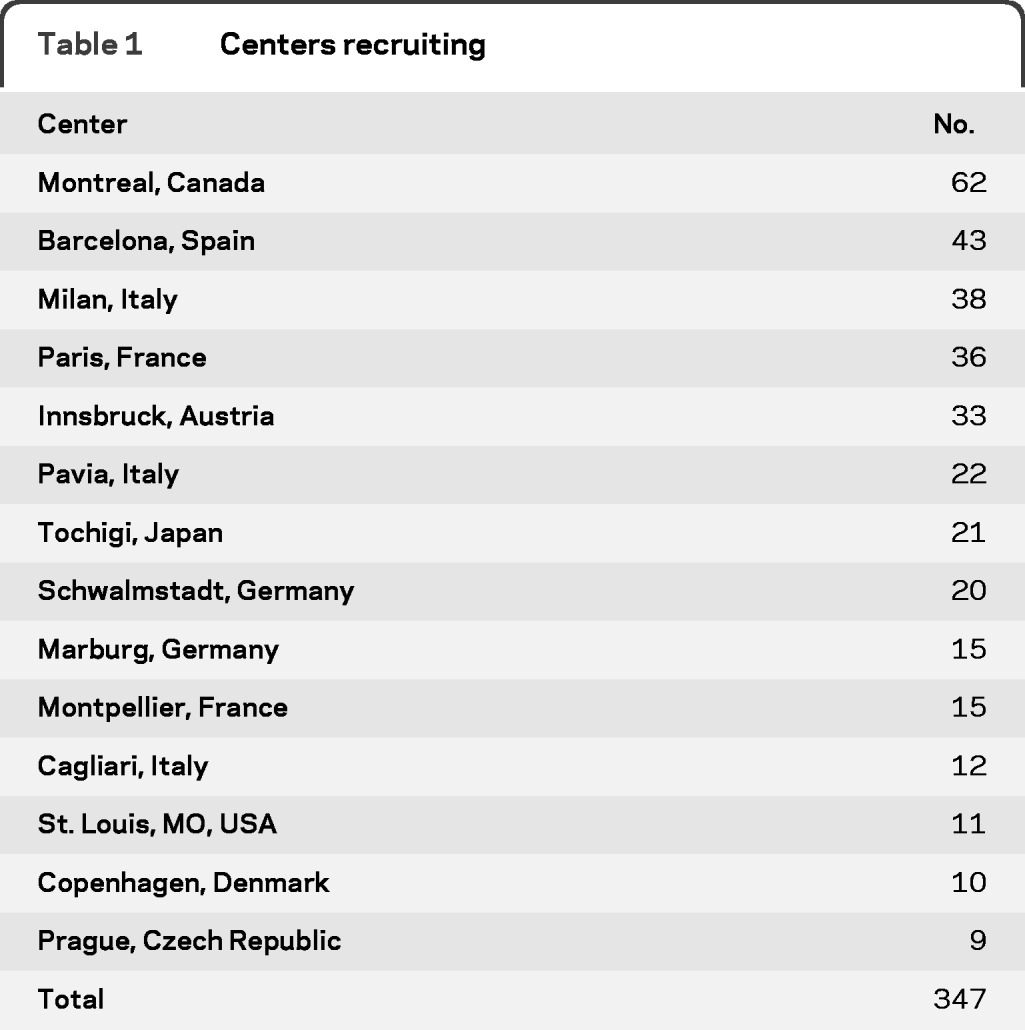
Table 2.
Demographics, coffee, tea, alcohol, smoking
Abbreviations: CI = confidence interval; OR = odds ratio; RBD = REM sleep behavior disorder.
Adjusted for age, sex, and center.
Significant OR.
Risk factors.
Coffee, tea, and alcohol.
Despite the consistent association found between caffeine nonuse and PD,17 we found no evidence of an association between coffee or tea intake and RBD (ever regular coffee use OR = 0.95, p = 0.77, habitual intake OR = 1.00, p = 0.85) (table 2). We assessed if cessation of caffeine use could be associated with preclinical disease (due to loss of effect or intolerance); however, cases were not more likely to have stopped using coffee than controls (OR = 1.36, p = 0.20). Similarly, we found no association between RBD and frequency of alcohol use, habitual alcohol quantity, or binge drinking.
Smoking.
Although smoking has been found to be a protective factor in studies of PD (with nonsmokers at higher risk),17 in the current study we found increased smoking among cases compared to controls (table 2). Cases were more likely than controls to have ever smoked more than 100 cigarettes (OR = 1.43 p = 0.028) and to have been regular smokers (OR = 1.41, p = 0.035). Current smoking was uncommon in both groups (11.2% vs 8.3%, OR = 1.45, p = 0.18). The point estimate of total pack-years was higher in the RBD group, though this did not reach statistical significance (16.5 ± 23.5 vs 13.7 ± 21.9, p = 0.12). Adding caffeine, alcohol, and smoking together in the regression model did not appreciably change OR or assessment of statistical significance. There was no statistically significant association found between RBD and cigar/pipe smoking or living with smokers (i.e., passive smoke exposure).
Head injury.
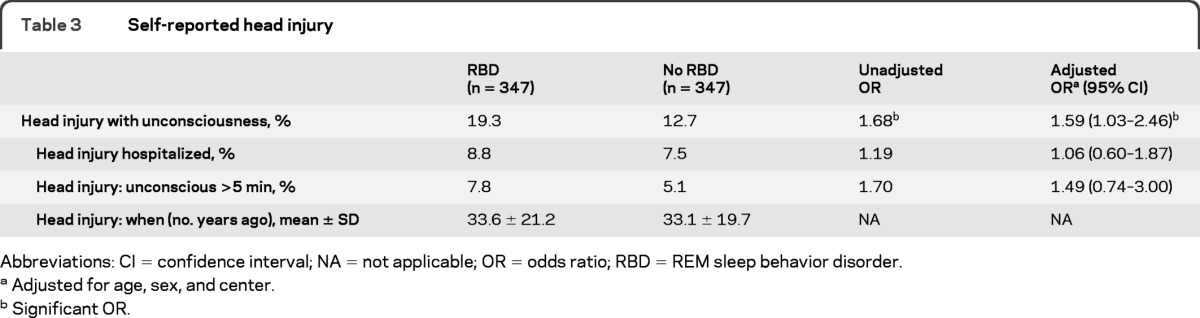
Cases were more likely to have reported having had a head injury than controls (OR = 1.59, p = 0.037) (table 3). ORs were similar between short duration and long duration unconsciousness (all head injury = 1.59, unconscious ≥5 minutes = 1.49). Because head injury can be related to preclinical neurodegeneration (i.e., increased risk of falls among individuals with preclinical neurodegeneration), we examined whether the reported head injuries occurred more recently in those with RBD than in controls. Head injuries occurred on average 33.5 years before questionnaire administration, and this timing was the same in the 2 groups (cases = 33.6 ± 21.2, controls = 33.1 ± 19.7, p = 0.71), suggesting reverse causality was an unlikely explanation of the effect.
Table 3.
Self-reported head injury
Abbreviations: CI = confidence interval; NA = not applicable; OR = odds ratio; RBD = REM sleep behavior disorder.
Adjusted for age, sex, and center.
Significant OR.
Education and occupation.
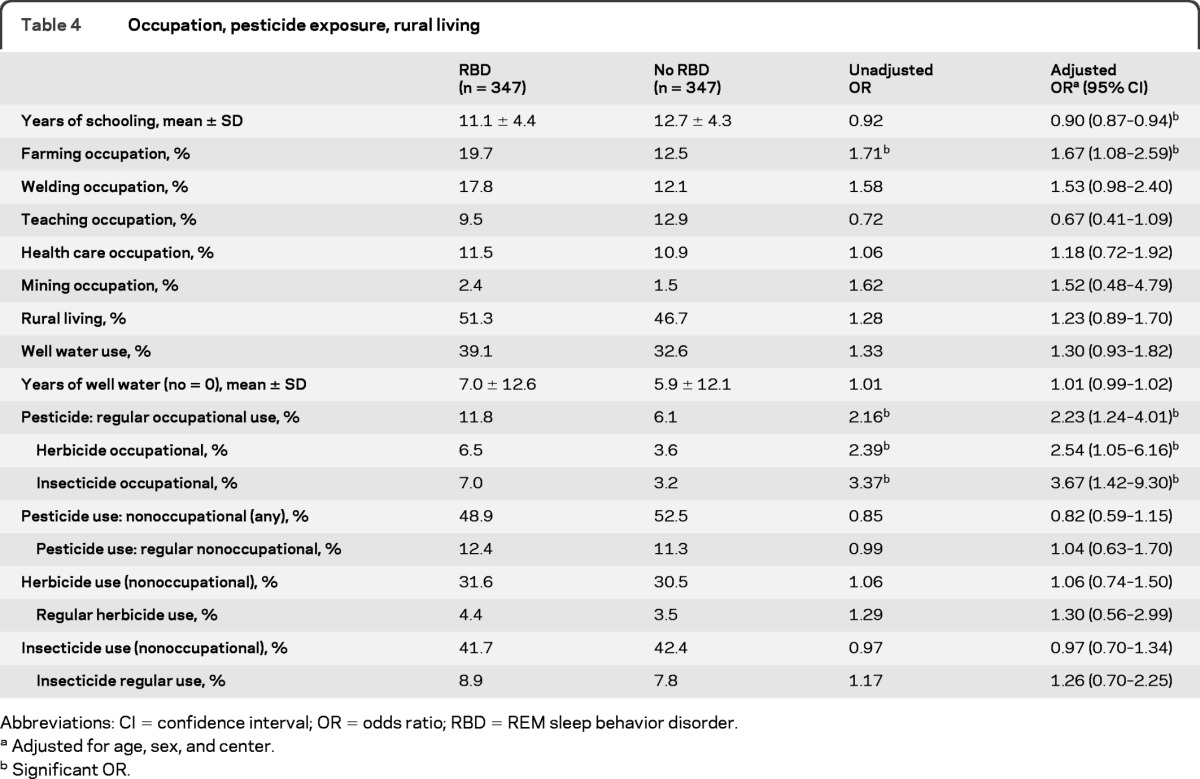
Since low education has been associated with an increased risk of dementia,18 we examined education as a risk factor in this analysis (table 4). In our sample, RBD cases had fewer years of schooling on average than did controls (11.1 ± 4.4 years, vs 12.7 ± 4.3, p < 0.001). In addition we examined various occupational groupings, including farming, welding, teaching, and health care, that have been linked to PD.19 We found that farming (OR = 1.67, p = 0.022) was associated with RBD, but the occupations of teaching, health care, and mining were not. A borderline relationship was found with welding (OR = 1.53, p = 0.063). Occupation could not be completely disentangled from education—upon addition of education to the regression model for occupation, the association between RBD and farming became of borderline statistical significance (OR = 1.44, p = 0.10), although education remained significant (OR = 0.91, p < 0.001).
Table 4.
Occupation, pesticide exposure, rural living
Abbreviations: CI = confidence interval; OR = odds ratio; RBD = REM sleep behavior disorder.
Adjusted for age, sex, and center.
Significant OR.
Pesticide exposure.
Pesticide exposure is a well-established risk factor for PD19 and for this reason we included questions on occupational and nonoccupational exposure to pesticides in this study. We found that cases had a higher prevalence of occupational pesticide exposure (OR = 2.23, p = 0.008) than controls. ORs were slightly higher for self-reported insecticide exposure than for herbicide exposure (3.37, p = 0.006 vs 2.39, p = 0.039) but the confidence intervals overlapped. We found no difference in nonoccupational exposure to pesticides (48.9% vs 52.5%, p = 0.26). In interpreting these findings, however, it is important to note that among those reporting nonoccupational exposure, the magnitude of the exposure was low: only 12.7% of herbicide users (i.e., 4% of the total population) had used herbicides more than 10 times in their lives, and only 19.8% of insecticide users (8.2% of the total population) had used insecticides more than 10 times. Farming was correlated with reported occupational pesticide use: 66.7% of occupational pesticide users reported having been farmers in the past.
Rural living/well water.
Although cases more frequently reported both rural living and well water than controls (OR = 1.23, p = 0.20 for rural living and OR = 1.30, p = 0.12), we found no statistically significant association between rural living or well water use and RBD.
Sensitivity analysis: Control type.
As noted, 2 distinct types of controls were included and we conducted a sensitivity analysis to determine whether the results of analysis were different depending upon the control group used. Thus we conducted separate analyses reassessing selected risk factors as follows: comparing RBD cases to normal volunteers, to other sleep center patients, and to all controls excluding sleep apnea (table e-1). For smoking, head injury, farming, and welding, there was a somewhat larger impact on the magnitude of the ORs when cases were compared to normal volunteers than when cases were compared to other sleep center controls, although ORs were in the same direction for all control types. ORs for pesticide use were similar across all control types. Removal of sleep apnea patients did not appreciably change results.
DISCUSSION
In this multicenter case-controlled study of risk factors for idiopathic RBD, we found that head injury, occupational pesticide exposure, low education, farming, and cigarette smoking are potential risk factors for RBD.
Idiopathic RBD as a syndrome has an unusual status—it exists both as an independent sleep condition and as a prediagnostic marker of synuclein-mediated neurodegenerative disease. Also, within established neurodegenerative diseases, RBD may mark a unique subtype that may differ in pathophysiology and risk factors. The purpose of this study was to assess environmental risk factors in RBD, particularly those that have previously shown to be potential risk factors for PD or dementia. We found some similarity in risk factors between RBD and dementia and PD. Head injury, occupational pesticide exposure, and farming have been linked with PD.19 Low education, head injury, and smoking have been linked with dementia.18,20,21 Conversely, there were important differences. Caffeine nonuse has been strongly and consistently linked to PD in case control and prospective cohort studies, with relative risk in coffee drinkers approximating 0.6.17 However, we found no association between caffeine and RBD. Similarly, nonsmoking status has been consistently linked with risk of PD,17 yet we found the opposite relationship between smoking and RBD.
The association between RBD and education is difficult to explain. Low education has been shown to be strongly linked with dementia risk in case control and prospective cohort studies.18 The mechanism for this effect is unclear—one frequent explanation is that patients with high levels of education can compensate better for mild dementia, and therefore present later. However, in this case, a prediagnostic marker of dementia is also associated with education. Since in this context, the compensatory explanation does not apply, our data suggest that the cognitive compensation explanation may be insufficient to fully explain the education/dementia link. Of course, there are other potential explanations for our finding, including artifact of presentation producing selection bias (e.g., education modulating dream content and therefore changing likelihood of presentation to clinics), low education predisposing to other nonassessed occupations that increase risk, and residual confounding from unmeasured variables.
Sensitivity analysis that stratified by control type found that some markers, namely smoking, head injury, farming, and welding, were more strongly associated with disease when comparing RBD to normal volunteers than when compared to other sleep center controls. This suggests that some of the observed effect of these risk factors can be explained by the healthy volunteer effect; that is, normal volunteers who agree to participate in medical studies are more likely to be healthy, be motivated, and have positive lifestyle choices. However, there are alternate explanations—for example, it is also possible that some sleep diagnoses have similar risk factor profiles to RBD, leading to overmatching when sleep controls are used.
Some limitations of the study should be noted. The current study is a cross-sectional study, and as with every cross-sectional case-control study, recall bias may be present. This may be of special importance in patients who may be in preclinical stages of dementia. We tried to mitigate this by requesting input of caregivers, but in many cases caregivers could not participate (e.g., 13% of our RBD sample lived alone). Although the sample was diverse (representing 10 countries), all RBD patients were selected from clinical sleep centers. There are certainly patients with RBD who never present to sleep centers, and these patients may have different risk factor profiles. To limit this potential selection bias, population-based sampling would be ideal; however, currently there are no population-based samples of idiopathic RBD available. Since the current analysis is exploratory (i.e., there has never been a case-control study of RBD), adjustment for multiple comparisons was not performed22; of course, some of the positive results could simply be due to chance. The questionnaire was translated into multiple languages, and test-retest reliability was not tested for original or translated questionnaires. We matched for age and sex in selecting our controls—this implies that we cannot confirm these as risk factors; however, we felt that these risk factors were well-established (at least in sleep clinics), and it was more important to assess unknown risk factors using a control sample properly matched to these important potential confounds. We noted differences in risk factor profiles between our patients with idiopathic RBD and known risk factors for PD—we have not assessed if risk factor profiles within PD or DLB differ according to RBD status, but this would be of considerable interest. Cases were chosen from sleep centers with subspecialty interest in sleep—results may incompletely generalize to RBD detected in the general population. Finally, although sample size was relatively large, there may have been insufficient power to find small differences in risk, particularly among exposures with low prevalence. There would be also insufficient power to assess risk factors in smaller subgroups, such as women or age <50.
Therefore, we found potential environmental/lifestyle risk factors for idiopathic RBD, including head injury, occupational pesticide exposure, low education, farming, and cigarette smoking. Although these partially resemble risk factors for PD and dementia, they also differ in important ways, suggesting that RBD may have an independent risk profile.
Supplementary Material
GLOSSARY
- DLB
dementia with Lewy bodies
- OR
odds ratio
- PD
Parkinson disease
- RBD
REM sleep behavior disorder
- RBDSG
REM Sleep Behavior Disorder Study Group
Footnotes
Editorial, page 402
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
R.B. Postuma was responsible for drafting the manuscript, study concept, acquisition of data, and analysis and interpretation of data. C. Wolfson was responsible for study concept, interpretation of data, and revision of manuscript. A. Pelletier was responsible for interpretation of data and revision of manuscript. J.Y. Montplaisir and Y. Dauvilliers were responsible for acquisition of data, analysis of data, and revision of the manuscript. W. Oertel, A. Iranzo, L. Ferini-Strambi, I. Arnulf, B. Hogl, R. Manni, T. Miyamoto, G. Mayer, K. Stiasny-Kolster, M. Puligheddu, Y. Ju, P. Jennum, K. Sonka, J. Santamaria, M.L. Fantini, M. Zucconi, S. Leu-Semenescu, B. Frauscher, M. Miyamoto, M. Terzaghi, M. Miyamoto, M. Unger, and V. Cochen De Cock were responsible for acquisition of data and revision of the manuscript.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Schenck CH, Bundlie SR, Patterson AL, Mahowald MW. Rapid eye movement sleep behavior disorder: a treatable parasomnia affecting older adults. JAMA 1987; 257: 1786– 1789 [PubMed] [Google Scholar]
- 2.Gagnon JF, Postuma RB, Mazza S, Doyon J, Montplaisir J. Rapid-eye-movement sleep behaviour disorder and neurodegenerative diseases. Lancet Neurol 2006; 5: 424– 432 [DOI] [PubMed] [Google Scholar]
- 3.Boeve BF, Silber MH, Saper CB, et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain 2007; 130: 2770– 2788 [DOI] [PubMed] [Google Scholar]
- 4.Iranzo A, Molinuevo JL, Santamaria J, et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol 2006; 5: 572– 577 [DOI] [PubMed] [Google Scholar]
- 5.Schenck CH, Bundlie SR, Mahowald MW. Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder. Neurology 1996; 46: 388– 393 [DOI] [PubMed] [Google Scholar]
- 6.Postuma RB, Gagnon JF, Vendette M, Fantini ML, Massicotte-Marquez J, Montplaisir J. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology 2009; 72: 1296– 1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sixel-Döring F, Trautmann E, Mollenhauer B, Trenkwalder C. Associated factors for REM sleep behavior disorder in Parkinson disease. Neurology 2011; 77: 1048– 1054 [DOI] [PubMed] [Google Scholar]
- 8.Gagnon JF, Vendette M, Postuma RB, et al. Mild cognitive impairment in rapid eye movement sleep behavior disorder and Parkinson's disease. Ann Neurol 2009; 66: 39– 47 [DOI] [PubMed] [Google Scholar]
- 9.Postuma RB, Gagnon JF, Vendette M, Charland K, Montplaisir J. Manifestations of Parkinson disease differ in association with REM sleep behavior disorder. Mov Disord 2008; 23: 1665– 1672 [DOI] [PubMed] [Google Scholar]
- 10.Postuma RB, Gagnon JF, Vendette M, Massicotte-Marquez J, Charland K, Montplaisir J. REM sleep behavior disorder in Parkinson's disease is associated with specific motor features. J Neurol Neurosurg Psychiatry 2008; 79: 1117– 1121 [DOI] [PubMed] [Google Scholar]
- 11.Onofrj M, Bonanni L, Albani G, Mauro A, Bulla D, Thomas A. Visual hallucinations in Parkinson's disease: clues to separate origins. J Neurol Sci 2006; 248: 143– 150 [DOI] [PubMed] [Google Scholar]
- 12.Boeve BF, Silber MH, Parisi JE, et al. Synucleinopathy pathology and REM sleep behavior disorder plus dementia or parkinsonism. Neurology 2003; 61: 40– 45 [DOI] [PubMed] [Google Scholar]
- 13.Dugger BN, Boeve BF, Murray ME, et al. Rapid eye movement sleep behavior disorder and subtypes in autopsy-confirmed dementia with Lewy bodies. Mov Disord Epub. 2011 doi: 10.1002/mds.24003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. American Academy of Sleep Disorders. International Classification of Sleep Disorders–2. Westchester, IL: American Academy of Sleep Disorders; 2005 [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189– 198 [DOI] [PubMed] [Google Scholar]
- 16.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992; 55: 181– 184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernan MA, Takkouche B, Caamano-Isorna F, Gestal-Otero JJ. A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson's disease. Ann Neurol 2002; 52: 276– 284 [DOI] [PubMed] [Google Scholar]
- 18.Caamano-Isorna F, Corral M, Montes-Martinez A, Takkouche B. Education and dementia: a meta-analytic study. Neuroepidemiology 2006; 26: 226– 232 [DOI] [PubMed] [Google Scholar]
- 19.Lai BC, Marion SA, Teschke K, Tsui JK. Occupational and environmental risk factors for Parkinson's disease. Parkinsonism Relat Disord 2002; 8: 297– 309 [DOI] [PubMed] [Google Scholar]
- 20.Anstey KJ, von SC, Salim A, O'Kearney R. Smoking as a risk factor for dementia and cognitive decline: a meta-analysis of prospective studies. Am J Epidemiol 2007; 166: 367– 378 [DOI] [PubMed] [Google Scholar]
- 21.Van Den Heuvel C, Thornton E, Vink R. Traumatic brain injury and Alzheimer's disease: a review. Prog Brain Res 2007; 161: 303– 316 [DOI] [PubMed] [Google Scholar]
- 22.Bender R, Lange S. Adjusting for multiple testing: when and how? J Clin Epidemiol 2001; 54: 343– 349 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


