Abstract
Circadian rhythms, which are found in most eukaryotes, are defined as rhythms that persist under constant conditions with a periodicity close to 24 h. One central key characteristic of all circadian rhythms is “temperature compensation”, which allows organisms to maintain robust rhythms with a period close to a diel cycle over a broad range of physiological temperatures. To better understand the response of the circadian clock in corals to temperature elevation, photosynthesis as an output process of the circadian clock was studied both in Stylophora pistillata corals and in cultured Symbiodinium algae. The time period of photosynthesis was not affected by temperature elevation in the cultured algae or in the corals harbouring Symbiodinium. However, the photosynthetic system responded to temperature elevations by adjusting the photosynthetic apparatus. These findings suggest that the endogenous algal circadian clock regulates the photosynthetic rhythm and compensates for temperature elevations that occur in the natural environment.
Circadian rhythms are generally characterised as free-running and maintain a periodicity of ∼24 h under constant stimuli (e.g., at constant light (LL), constant darkness (DD), or constant temperature) or in the absence of external cues. Thus, the oscillator will persist autonomously until the circadian clock phases out as a result of the absence of external signals1. Amongst the most conserved properties of these clocks is their ability to be entrained by daily light changes2. An important feature of circadian clocks is that they appear to run at approximately the same rate throughout a broad range of physiological temperatures3. This “temperature compensation” corrects for the natural tendency of biochemical reaction rates to change with temperature and thus permits the clock mechanism to have the necessary flexibility to accurately maintain time under changing environmental conditions. Generally, most biochemical reaction rates speed up progressively with increasing temperature, with a Q10 (ratio of rate at T°C to up to T- 10°C above ambient) value range of 2–34,5. By contrast, the rhythmicity of the circadian clock varies little with increasing temperature, and the Q10 values are limited to the range 0.9–1.32,6. This observation indicates that the circadian machinery is not independent of temperature but is compensated by it. This temperature compensation is efficient within a certain range of temperatures. In model organisms such as Drosophila7, Chlamydomonas8 and Neurospora9, genetic tools have been used to elucidate the mechanism of temperature compensation. Mutants with different daily rhythm lengths were studied to determine whether the temperature compensation mechanism was intact. The mutation did not affect the ability to compensate for temperature in Chlamydomonas, whereas minor changes in the Q10 value were found in the Drosophila mutant. The Neurospora mutant exhibited temperature compensation within certain temperature ranges. The Q10 value at lower temperatures demonstrated adequate compensation (Q10 = 0.95), whereas the compensation mechanism was more strongly affected (Q10 = 1.2–1.3) at higher temperatures10.
The cyanobacterium Synechococcus displays temperature compensation for cell division11 and amino acid uptake12. The Q10 for rhythm frequency in the thermophilic cyanobacterium Thermosynechococcus was estimated to have an almost constant value of 1 at temperatures of 35–55°C. This finding suggested the presence of a very robust temperature compensation apparatus in extremely hot environments with large temperature fluctuations5. In the dinoflagellate Gonyaulax, the circadian machinery displayed faster rhythms at low temperatures than at high temperatures. The Q10 value was less than 1 at lower temperatures, indicating a phenomenon known as “over-compensation”13.
A common feature of the circadian clock under temperature fluctuations is that high temperatures are interpreted as daylight and low temperatures are interpreted as darkness. These responses suggest that light and temperature signals are interpreted through common entrainment pathways14. The molecular component of the input pathway entrained by temperature has not been defined because changes in temperature affect nearly every chemical and physical process in the cell. Therefore, it may be impossible to define a single “temperature receptor” or a single input pathway. Hastings and Sweeny13 described a similar effect of temperature on two reactions that have antagonistic effects on the time period of the clock; these antagonistic effects result in temperature compensation. This idea was the basis for the antagonist balance model later proposed by Ruoff15 and Ruoff and Rensing16. This model is consistent with the findings of Gould et al.17 and provides an explanation for the temperature compensation phenomenon.
Coral reefs that harbour endosymbiotic dinoflagellate algae of the genus Symbiodinium are more susceptible than land plants to temperature elevations. Symbiotic corals evolved and live in tropical marine environments that are characterised by temperature stability. Strong temperature anomalies can therefore lead to drastic responses, including a breakdown of the coral (host)/algae symbiosis, known as bleaching18. Coral bleaching, in which corals lose their colouration because of the expulsion of symbiotic algae, results from many physical and biological factors, including high or low sea surface temperatures (SSTs), UV irradiation, bacterial infection, lowered salinity and pollution19(and references within). Mass bleaching events resulting from SST changes are thought to originate from acute photosynthetic damage to Symbiodinium18,20,21,22.
Since photosynthesis in dinoflagellates is a clock-regulated process23,24, the efficiency of temperature compensation (an important property of all circadian rhythms) may allow Symbiodinium to acclimate to temperature elevations in a manner similar to that of photoacclimation25. Because the circadian clock machinery controls numerous biochemical and physiological pathways, the present study was designed to elucidate the basic mechanism of the Symbiodinium clock machinery as manifested in Figure 1 (A–D), under the growth temperature of 24°C and the ways in which SST fluctuations (above 24°C) affect the clock apparatus, which eventually can lead to a mismatch in photosynthetic efficiency or, in extreme cases, to the breakdown of the coral/algae symbiosis. The mechanisms of the circadian clocks in Symbiodinium have not been systematically investigated, and the level of circadian control in this genus is still unknown. Temperature can entrain, reset and phase-shift the circadian rhythm, indicating that the clock recognises and responds to changes in temperature at several levels2.
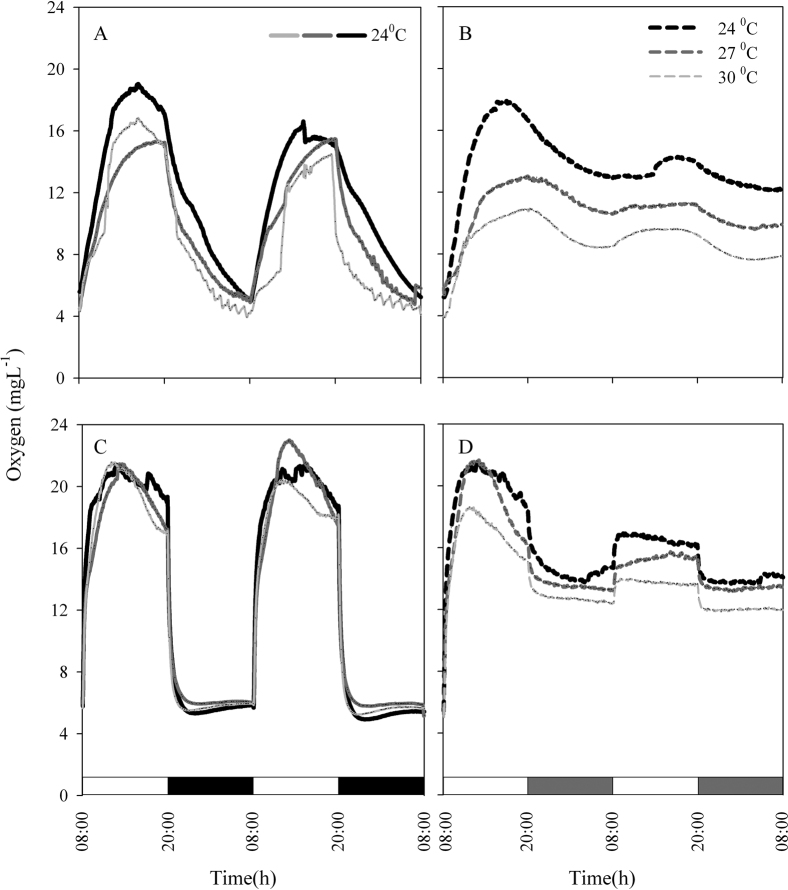
Figure 1. Oxygen evolution of cultured Symbiodinium clades (A, D) during 2 LD cycles at 24°C as presented in panels A and C (continues lines), followed by 2 cycles at 3 elevated temperatures (24°C, 27°C, 30°C), as presented in panels B and D (dashed lines).
White bars: light period; black bars: dark period; grey bars: subjective darkness. (A) clade A under LD; (B) clade A under LL; (C) clade C under LD; (D) clade C under LL. For each temperature, N = 4.
The specific aim of this study was to characterise the effects of elevated temperature on the circadian rhythm of photosynthesis under constant LL. We monitored oxygen evolution during photosynthesis in cultured Symbiodinium clade A and the clade D that is considered as temperature-tolerant26,27,28,29,30 and in the symbiotic Stylophora pistillata coral possess clades A and C31. In addition to the measurement of oxygen oscillation, an array of photosynthetic fluorescence parameters (Y(II), NPQ and ETR) were examined during a diel cycle under various physiological temperatures with constant light conditions.
Results
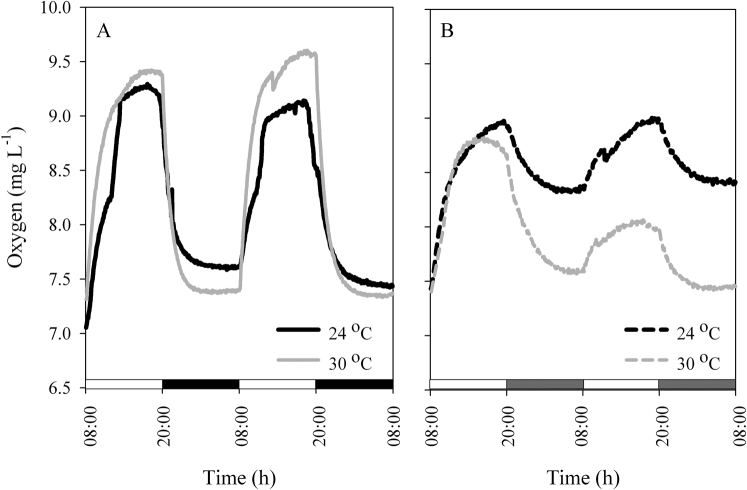
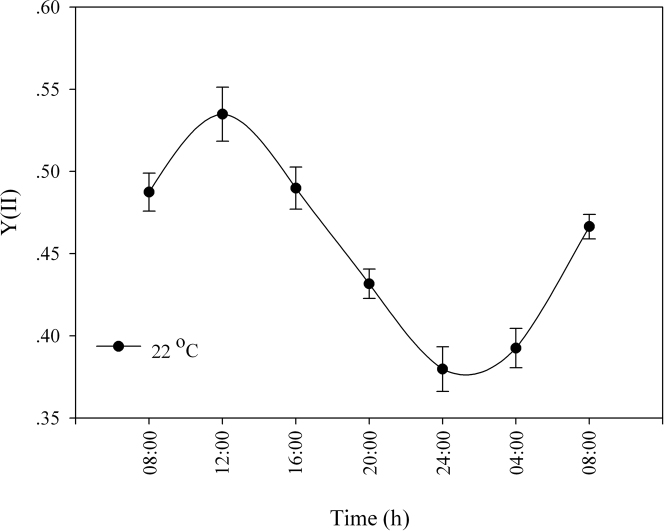
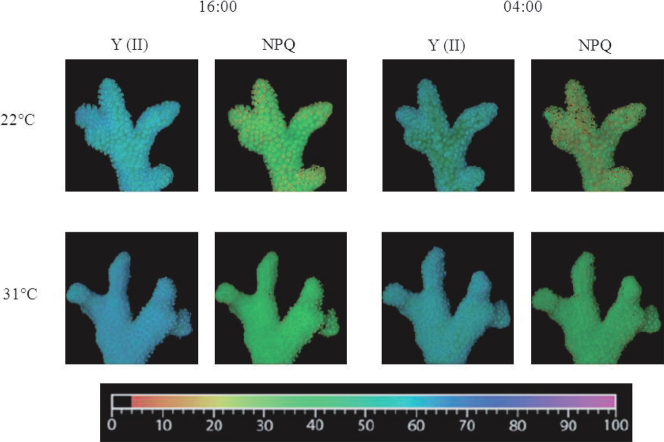
Temperature compensation effects were investigated in Symbiodinium cultures and in S. pistillata corals. Cultures were grown under LD cycles with a light intensity of 100 µmol quanta m−2 s−1 at 24°C and then transferred to LL conditions with elevated temperatures. The Q10 value was calculated with the formula Q10 = R2/R110/(T2-T1), where R2 is the rate of the process under the elevated temperature (T2) and R1 is the rate under the reference temperature (T1) under LD cycles. Q10 was found to be 1 for the ambient temperature (24°C) under constant light, 1.11 ± 0.08 for 27°C and 1.05±0.02 for 30°C (Figure 1A, C). When tested at a temperature of 6°C above the ambient temperature, clades A and D, exhibited the same periodicity behaviour when the Q10 values were calculated (paired t-test, P > 0.05). Both clades compensated for the temperature elevation and maintained a photosynthetic rhythm. However, oxygen production was lower under conditions of elevated temperature than at the ambient temperature, more dramatically so in clade A than in clade D (Figure 1). The decline in oxygen production was 23.5% in clade A and 16.9% in clade D, corresponding to the average of the maximal value. Corals were tested at the ambient temperature with a light intensity of 100 µmol quanta m−2 s−1 and compared with fragments subjected to an elevated temperature of 30°C. The calculated Q10 values were 1.02±0.01 for the corals grown at ambient temperature and 1.03 ± 0.005 for those grown at 30°C (Figure 2). Increasing the temperature by 6°C did not cause any significant difference in the diel pattern (paired t-test, P>0.05), and the circadian clock system maintained a circadian rhythm under this constant condition of 24.35±0.02, compared with a rhythm of 24.3±0.15 at ambient temperature. Fluorescence measurements were performed on S. pistillata fragments to test their ability to conserve a diel rhythm under an LD cycle (12∶12). These fragments showed a clear diel pattern in the effective PSII quantum yield Y(II), peaking between 08:00–16:00, then decreasing until 04:00, when an increase in the yield was observed before the light came on (paired t-test, P = 0.014, N = 5) (Figure 3). Several photosynthetic parameters of the corals were measured under LL conditions: the effective PSII quantum yield Y(II), non-photochemical quenching (NPQ) and the electron transport rate (ETR). In all of the experiments, the coral fragments were subjected to an LD cycle of 12∶12 with an ambient sea-water temperature of 22°C and a light intensity of 100 µmol quanta m−2 s−1. The fragments were subsequently transferred to LL conditions and subjected to a temperature of 22°C (control) or elevated temperatures of 26°C, 29°C and 31°C. The S. pistillata fragments were analysed with the Imaging-PAM device under constant light conditions in the absence of dark adaptation to avoid the influence of a darkening period that could potentially entrain the circadian rhythm. Similar patterns were expected for each of the temperatures tested. The temporal diel measurements of effective quantum yield Y(II) showed increasing values toward midday (12:00) and late afternoon (16:00). The quantum yield subsequently decreased during the dark period. Three hours before the onset of the light period (at 04:00), the Y(II) value began to increase. The maximal Y(II) value (at 16:00) (but not the minimal value at 04:00) under the highest temperature was significantly different only from the value at ambient temperature (one-way ANOVA followed by Tukey's HSD test, P < 0.05, N = 4), whereas there was a significant difference between the 16:00 to 04:00 Y(II) values at all temperatures tested (paired t-test, P < 0.01, N = 4 per temperature) (Figure 4A; Figure 5). The estimated non-photochemical quenching (NPQ) measured at the light level (501, photosynthetic active radiation (PAR)) at which the curve reached a plateau revealed a diel cycle for each temperature tested (Figure 4B; Figure 5). The NPQ values at the lowest temperature were significantly different both at 16:00 and at 04:00 when compared with the respective values at higher temperatures (one-way ANOVA followed by Tukey's HSD test, P < 0.01, N = 4). At the highest temperature (31°C), no reduction was observed at 16:00 (8 h after the light was turned on), and the system maintained a high value for this time point (Figure 4B). The final fluorescence parameter measured was the electron transport rate (ETR) at 16:00 and 04:00 (Figure 6A, B). The rate was faster at lower temperatures (22°C, 26°C) than at higher temperatures (29°C, 31°C). A significant difference was observed between the values at 16:00 and 04:00 (paired t-test, P < 0.01, N = 4 per temperature). The ETR decreased dramatically (by 20–30%) between these times. There is a correlation between the ETR and oxygen production in the photosynthetic process. The maximal value of oxygen evolution occurred at noon, and the minimal value occurred at midnight, when the electron transport rate was lowest.
Figure 2. Oxygen evolution of Stylophora pistillata corals during 2 LD cycles at 24°C (continues lines) followed by 2 cycles at 2 elevated temperatures 24°C and 30°C, (dashed lines).
White bars: light period; black bars: dark period; grey bars: subjective darkness. For each time point, N = 4. (A) corals under LD; (B) corals under LL.
Figure 3. Effective PSII quantum yield Y(II) of Stylophora pistillata corals under an LD cycle at 22°C (ambient temperature).
For each time point, N = 5; SE indicated by a bar.
Figure 4. Photosynthetic parameters of Stylophora pistillata corals under an LL cycle at 4 different temperatures (22°C, 26°C, 29°C, 31°C).
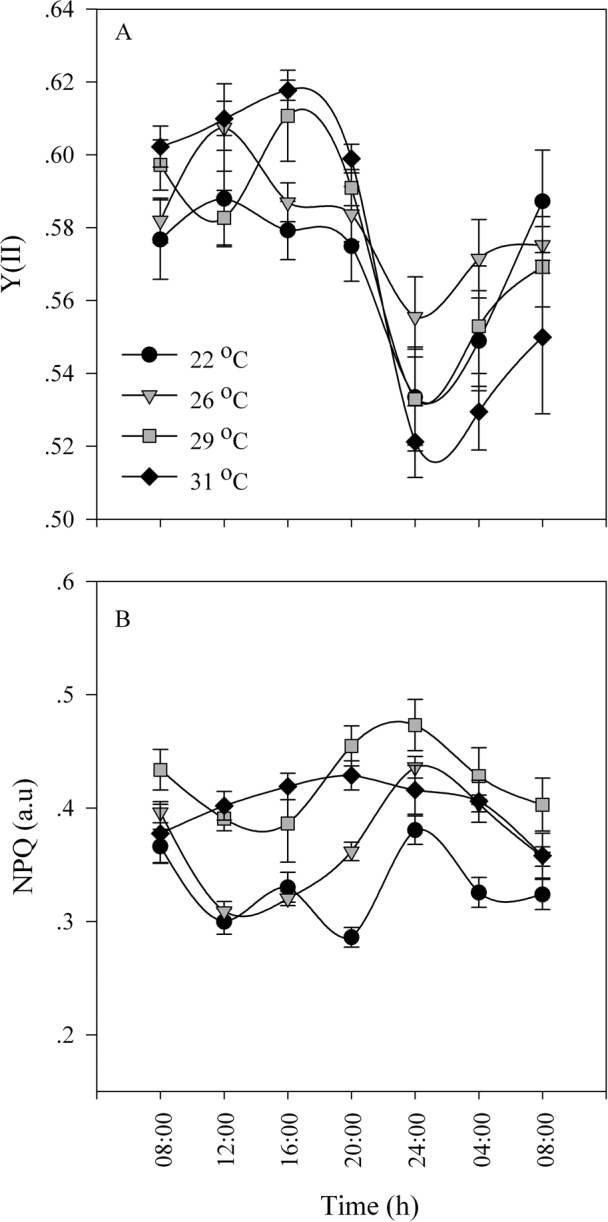
(A) effective PSII quantum yield Y(II). (B) NPQ (non-photochemical quenching). White bars: light period; black bars: dark period; grey bars: subjective darkness. For each time point, N = 4; SE indicated by a bar.
Figure 5. Representative images of effective PSII quantum yield Y(II) and non-photochemical quenching (NPQ) for Stylophora pistillata corals at 22°C and 31°C at two time points (16:00 and 04:00).
The relative values of fluorescence parameters, ranging from 0 to 100, are displayed with an identical false colour scale.
Figure 6. Electron transport rate (ETR) of Stylophora pistillata corals under an LL cycle at 4 different temperatures (22°C, 26°C, 29°C, 31°C).
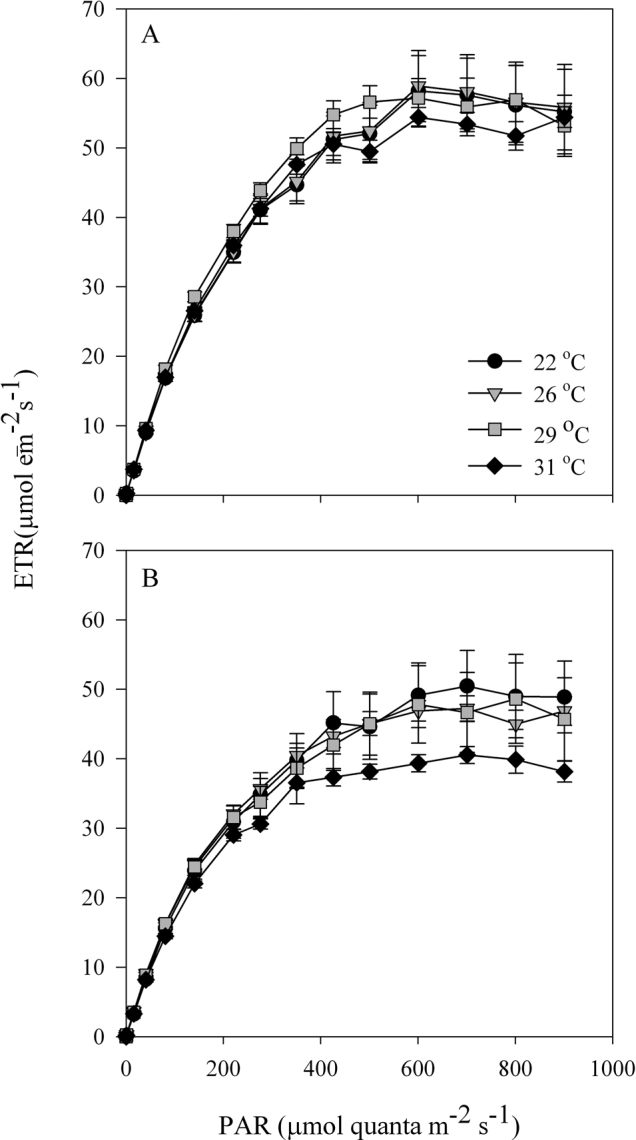
(A) 16:00 (8 h after the light was turned on). (B) 04:00 (20 h after the light was turned on). For each time point, N = 4; SE indicated by a bar.
Discussion
The photosynthetic apparatus is very sensitive to thermal stress, and even moderate changes in temperature disrupt the system32. Many studies have addressed the effects of thermal stress on coral photosynthesis and the associations between the host and the symbiotic algae18,33,34,35. Other studies have focussed on photosynthetic photochemical efficiency measurements with chlorophyll fluorescence techniques and have identified diurnal changes in the efficiency of dinoflagellates in the host coral tissues36,37,38. The goal of the above studies was mainly to understand the impacts of temperature and light synergism on the bleaching phenomenon, which is lethal for corals. We did not find any previous study that characterised the relationships among the circadian clock, photosynthesis and thermal stress by testing the temperature compensation potential in symbiotic corals.
Compensation for temperature fluctuations (the Q10 factor) is the backbone of the circadian clock system machinery. Photosynthesis is a clock-regulated process in Symbiodinium (as shown in Figure 1 (A–D), under the growth temperature of 24°C) and can compensate for temperature changes. This ability was confirmed in our temperature experiments, which demonstrated oxygen production with a cycle close to 24 h (24°C = 24.3, 27°C = 23.55°C and 30°C = 23.5 h) under constant light (Figure 1A–D) and a cycle of 24.3 h under LD. The calculated Q10 values for 27°C and 30°C were close to 1 (1.1 and 1.05, respectively). Oxygen evolution decreased when temperature increased in both clades. The decline in oxygen evolution as the temperature increased from 24°C and 30°C was 16.9% for clade D and 23.5% for clade A. Thus, the decline was much more significant for clade A. Many researchers26,27,28,29,30 have claimed that clade D is more thermal-resistant than other clades, while other researchers have argued that the thermal sensitivity of Symbiodinium is not clade-specific but rather is dependent on physiological-biochemical features such as the thylakoid lipid composition, which determines the sensitivity of photoinhibition to heat stress22. Differences in the sensitivity of photoinhibition to thermal stress were reported between two cultured species of Symbiodinium clade A39. The effects of short-term exposure of Symbiodinium to elevated temperatures included an increase in oxygen evolution33; however, our longer exposure experiment revealed a reduction in photosynthetic oxygen evolution, as was also found in freshly isolated Symbiodinium from Caribbean corals20. Our findings revealed a clear difference between the photosynthetic performances (based on oxygen production) of clades A and D and demonstrated the robustness of clade D under temperature elevation. No significant difference was observed in the ability of the two clades to compensate for temperature changes. An increase in temperature from 24°C to 30°C revealed a Q10 value of 1.05, indicating the presence of a robust compensation system for the Symbiodinium endogenous clock under constant conditions in the absence of external stimuli (in the dark), (Figure 1), even when the organisms are part of the coral host tissue (Figure 2). A similar reduction in oxygen evolution was found for cultured algae as was noted in “hospite” Symbiodinium between the two temperatures. The circadian clock system successfully compensated for temperature changes in both Symbiodinium systems (as free-living organisms and as part of the coral tissue), even though the second system included corals, which have an endogenous clock in addition to the algal clock.
The sensitivity of the photosynthetic machinery to elevated temperatures is primarily attributed to photoinhibition of PSII35. Under elevated temperatures, Symbiodinium in its symbiotic form with corals displayed photoinhibition through inhibition of the PSII repair process; therefore bleaching tolerance is a consequence of high rates of repair relative to the photodamage, rather than differential degrees of photodamage process18 as suggested by earlier works35,45.
Diurnal patterns in steady-state quantum yield have been documented in corals that harbour endosymbiotic dinoflagellate algae of the genus Symbiodinium, suggesting a photoprotective function pattern that is linked to the ambient light course in shallow water environments36,37,38. Diel cycles were observed under LD conditions of artificial light (100 µmol quanta m−2 s−1); Y(II) values during the day revealed an increase towards noon, followed by a decline until 4:00, when Y(II) began to increase (Figure 3). The diel fluorescence measurements under constant dim light demonstrated that Y(II) values increased from the time when the lights came on until noon and then dropped during the afternoon. The increasing values of Y(II) at 4:00 before the appearance of the first light indicate anticipation by the photosynthetic apparatus, which prepares the system for the coming “dramatic event” of a change from dark to light (Figure 3, 4A).
The cycles under both LD and LL reveal a similar pattern and demonstrate the endogenous circadian rhythm of the photosynthetic apparatus. In this experiment, we used dim light to avoid the synergistic effect of high light levels and temperature elevation, which can mask the circadian rhythm. Because of the low-light conditions and constant temperature, the temporal patterns of Y(II) were different from those of other studies that used higher or ambient light intensities18,40. Nevertheless, the steady state of the quantum yield in S. pistillata was circadian under LL conditions at all four temperatures to which the corals were exposed. The dissipation of the light via non-photochemical pathways, which are known to be constitute an efficient mechanism in symbiotic corals41, also showed diel oscillations (Figure 4B) for temperatures of 22°C (ambient), 26°C and 29°C. The maximum NPQ values occurred at midnight (24:00), and lower values were recorded between 12:00–16:00 under constant light. At the highest temperature (31°C), no reduction was observed in NPQ during the afternoon (12:00–16:00), most likely indicating thermal stress. NPQ values usually increase in association with temperature changes (Figure 4B). In comparison with natural daily LD cycles41, the diel cycle of NPQ under LL exhibits an opposite trend during the day but a converse correlation with the Y(II) temporal pattern. The enhanced NPQ values may indicate the development of photoprotective processes that are related to the inner ratio of the carotenoid pool42,43. Fluctuations in the xanthophyll cycle that are correlated with exposure to intense light have a photoprotective role for the cycle in the coral Goniastrea aspera44 and play a protective role under elevated temperatures35. This may be the mechanism by which the system maintains a circadian rhythm in other parameters by dissipating excitation energy.
Thermal stress usually causes a decrease in the electron transport rate, as shown by Jones et al45. Our light-curve analysis revealed clear oscillations in the ETR parameter values. Differences between the rates at the elevated temperatures were demonstrated at two time points (16:00 and 04:00) under LL conditions (Figure 6A,B), and the electron transport rates were lower at lower temperatures (22°C, 26°C) than at higher temperatures (29°C, 31°C). The electron transport rate also decreased between 16:00 and 04:00 under constant light, similar to the patterns of the ETR curve under the natural daily cycle of corals36, in which the capacity for electron transport increases at noon and then decreases during sunset. The temporal circadian behaviour of the ETR reflects the photosynthetic machinery, which is governed by the Symbiodinium endogenous core clock oscillator. These findings are consistent with the oxygen production behaviour, i.e., higher photosynthetic production at noon and lower production during the subjective night under constant light (Figure 6).
Under elevated temperature and constant light, the photosynthetic parameters of the photosynthetic system exhibited a strongly diurnal behaviour. Similar results have been obtained in photosynthetic organisms such as macroalgae and, specifically, in two species of symbiotic corals40,41,46.
An asymmetric temporal response of photosynthesis was observed at similar light intensities between morning and afternoon; this response has been termed the “hysteresis effect”, the “afternoon nap” or the “afternoon depression” of photosynthesis47. The present results indicate that the hysteresis effect can be attributed to the endogenous circadian rhythm. In our experimental conditions, both temperature and light were constant. Therefore, a desynchronisation between those two physical factors, as was suggested by Vonshak et al.48, cannot explain the difference in the performance of the photosynthesis apparatus. In general, the circadian clock system responds to LL conditions but still maintains diel oscillations, as observed under LD conditions. Elevated temperature did not change the time period of the investigated photosynthetic parameters under LL conditions, but the photosynthetic system did respond to elevated temperature by altering the photosynthetic quantum yield and oxygen production. The circadian clock system thus successfully compensated for a temperature elevation of 6°C in free-living Symbiodinium as well as in corals (temperature elevation of 9°C) harbouring Symbiodinium. We did not attempt to determine the “breakpoint” temperature at which the circadian machinery no longer controls the rhythmicity of photosynthesis, leading to a cascade of biochemical and transcriptomic changes and, finally, to bleaching. We have successfully described the circadian clock capabilities for temperature compensation displayed by free-living Symbiodinium and the symbiotic coral/algae association. Future studies should consider integrating the relationships among thermal stress, temperature compensation, the circadian clock of Symbiodinium photosynthesis and the potential breakdown of the coral/algae symbiosis.
Methods
Collection and maintenance of corals and Symbiodinium cultures
The Symbiodinium cultures used in this study were from clade A (CCMP 2467) and clade D (CCMP 2556). All cultures were grown in 2 L Fernbach flasks containing 1 L of half-strength medium (f/2) without silica. Illumination was provided by lateral fluorescent lamps under a 12∶12 h LD cycle. The cultures were maintained in a culture room with a controlled temperature set to 24°C for all indoor experiments. The irradiance was measured by a quantum sensor (LI-COR, Lincoln, NE, USA) as 100 µmol quanta m−2 s−1. The S. pistillata corals tested were collected by scuba divers from a depth of 5 m in front of the Interuniversity Institute (IUI) for Marine Sciences, Eilat, Gulf of Aqaba, Red Sea, Israel. After collection, the corals were fragmented, placed in running seawater and then placed in 900 L of aquaria for a period of one week for acclimation.
Monitoring of oxygen evolution
For the cultured Symbiodinium, oxygen evolution was monitored with an OXY4- 4 channel oxygen meter coupled to 4 optods (optical oxygen sensors; PreSens, Germany) and connected to 4 dipping probes (DP- PSts). The optods continuously monitored the oxygen evolution every 5 min. The effects of temperature and compensation ability were tested in clades A and D. For each of the temperatures tested, N = 4 cultures were exposed to two LD cycles under an ambient temperature of 24°C followed by two cycles of LL with the temperature set at 24°C, 27°C or 30°C. Similar experiments were performed with corals at temperatures of 24°C and 30°C (For each temperature N = 4 corals were tested).
Imaging-PAM measurements
Photosynthetic efficiencies were measured in S. pistillata corals during an LD period (12∶12) with culture under a light intensity of 100 µmol quanta m−2 s−1. The corals were analysed every 4 h with Imaging-PAM (pulse amplitude modulation; Maxi-PAM, Walz Gmbh, Effeltrich, Germany). The resulting images were analysed with the Imaging-Win software program (v2.00 m, Walz Gmbh, Effeltrich, Germany) and recorded for each of the branches (N = 5). Light-response curves were measured with increasing illuminations of 20 s intervals (0, 1, 16, 41, 81, 141, 221, 276, 351, 426, 501, 601, 701, 801, and 901 µmol quanta m−2 s−1) under an ambient temperature of 22°C and analysed for the effective PSII quantum yield (Y(II)) (with no dark adaptation). Under an LL cycle, four ambient temperatures of 22°C, 26°C, 29°C and 31°C were examined (For each tested temperature N = 4). After irradiation, the following parameters of the coral fragments were analysed: effective PSII quantum yield (Y(II)) (with no dark adaptation), non-photochemical quenching (NPQ) and electron transport rate (ETR).
Data analysis
Oxygen data sampled in the time domain were transformed into the frequency domain via Fourier transform. For this purpose, we coded an application in the Python environment with the ‘scipy’ library function ‘fft’. From the output of the application, we extracted the dominant frequencies that characterised the examined rhythm. The frequency of each of the experiments was used in the formula for calculating Q10 = R2/R110/ (T2-T1). For statistical analysis, paired t-tests and a one-way ANOVA followed by Tukey's HSD test were used to assess the differences among the experimental treatments of oxygen periodicity and the fluorescence measurements. All statistical analyses were conducted with the SPSS 20.0 program (IBM, USA), and differences in the results were considered statistically significant at P < 0.05.
Author Contributions
M.S. and O.L. designed the research; M.S. performed the research; M.S. analysed the data; M.S. and O.L wrote the paper.
Acknowledgments
We thank Dr. M. Fine at the Interuniversity Institute for Marine Sciences in Eilat and also acknowledge the help of Mr. E. Rahav and Mr. M. Samuelson of the Faculty of Life Sciences, Bar-Ilan University, Israel and Dr. D.M. Charutz during this study. This study represents partial fulfilment of the requirements for a PhD thesis by M. Sorek at Bar-Ilan University. The study was funded in part by an Israeli Science Foundation (No. 243/10) grant to O. Levy.
References
- Kreps J. A. & Kay S. A. Coordination of plant metabolism and development by the circadian clock. Plant Cell 9, 1235–1244 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney B. M. & Hastings J. W. Effects of temperature upon diurnal rhythms. Cold Spring Harb. Symp. Quant. Biol. 25, 87–104 (1960). [DOI] [PubMed] [Google Scholar]
- Pittendrigh C. S. On the temperature independence in the clock system controlling emergence time in Drosophila. Proc. Natl. Acad. Sci. USA 40, 1018–1029 (1954). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C. H., Golden S. S. & Kondo T. Adaptive significance of circadian programs in cyanobacteria. Trends Microbiol. 6, 407–410 (1998). [DOI] [PubMed] [Google Scholar]
- Onai K. et al. Large-scale screening of Arabidopsis circadian clock mutants by a high-throughput real-time bioluminescence monitoring system. Plant J. 40, 1–11 (2004). [DOI] [PubMed] [Google Scholar]
- Bunning E. The Physiological Clock: Circadian Rhythms and Biological Chronometry, 3rd ed (English University Press, London, UK, 1973). [Google Scholar]
- Konopka R. J. Genetic dissection of the Drosophila circadian system. Fed. Proc. 38, 2602–2605 (1979). [PubMed] [Google Scholar]
- Bruce V. G. Mutants of the biological clock in Chlamydomonas reinhardi. Genetics 70, 537–548 (1972). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman J. F., Gardner G. F. & Denison R. in Biological Rhythms and Their Central Mechanism (edited by Suda M., Hayaishi O., & Nakagawa H. Elsevier/North Holland Biomedical Press, Amsterdam, The Netherlands, pp. 57–66, 1979). [Google Scholar]
- Sargent M. L., Briggs W. R. & Woodword D. O. The circadian nature of a rhythm expressed by an invertaseless strain of Neurospora crassa. Plant Physiol. 41, 1343–1349 (1966). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney B. M. & Borgese M. B. Circadian rhythm in cell division in a prokaryote, the cyanobacterium Synechococcus WH78031. J. Phycol. 25, 183–186 (1989). [Google Scholar]
- Chen T. H., Chen T. L., Hung L. M. & Huang T. C. Circadian rhythm in amino acid uptake by Synechococcus RF-1. Plant Physiol. 97, 55–59 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings J. W. & Sweeny B. M. On the mechanism of temperature independence in a biological clock. Proc. Natl. Acad. Sci. USA 43, 804 (1957). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosthwaite S. K., Loros J. J. & Dunlap J. C. Light-induced resetting of a circadian clock is mediated by a rapid increase in frequency transcript. Cell 81, 1003–1012 (1995). [DOI] [PubMed] [Google Scholar]
- Ruoff P. Introducing temperature compensation in any reaction kinetic oscillator model. J. Interdiscipl. Cycle Res. 23, 92–99 (1992). [Google Scholar]
- Ruoff P. & Rensing L. The temperature compensated Goodwin model simulates many circadian clock properties. J. Theor. Biol. 79, 275–285 (1996). [Google Scholar]
- Gould P. D. et al. The molecular basis of temperature compensation in the Arabidopsis circadian clock. Plant Cell 18, 1177–1187 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Nakamura T., Sakamizu M., van Woesik R. & Yamasaki H. Repair machinery of symbiotic photosynthesis as the primary target of heat stress for reef building corals. Plant Cell Physiol. 45, 251–255 (2004). [DOI] [PubMed] [Google Scholar]
- Stat M., Carter D. & Hoegh-Guldberg O. The evolutionary of Symbiodinium and scleractinian hosts – Symbiosis, diversity and the effect of climate change. Perspect. Plant Ecol. Evol. Syst. 8, 23–43 (2006). [Google Scholar]
- Fitt W. K. & Warner M. E. Bleaching patterns of four species of Caribbean reef corals. Biol. Bull. 189, 298–307 (1995). [DOI] [PubMed] [Google Scholar]
- Yakovleva I. M. & Hidaka M. Differential recovery of PSII function and electron transport rate in symbiotic dinoflagellates as a possible determinant of bleaching susceptibility of corals. Mar. Ecol. Prog. Ser. 268, 43–53 (2004). [Google Scholar]
- Tchernov D. et al. Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching in corals. Proc. Natl. Acad. Sci. USA 101, 13531–13535 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prezelin B. B., Meeson B. W. & Sweeney B. M. Characterization of photosynthetic rhythms in marine dinoflagellates. 1. Pigmentation, photosynthetic capacity and respiration. Plant Physiol. 60, 384–387 (1977a). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prezelin B. B., Meeson B. W. & Sweeney B. M. Characterization of phytosynthetic rhythms in marine dinoflagellates. Plant Physiol. 60, 384–387 (1977b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison I. R. Enviromental effects on algal photosynthesis: temperature. J. Phycol. 27, 2–8 (1991). [Google Scholar]
- Jones A. M., Berkelmans R. A., van Oppen M. J. H., Mieog J. C. & Sinclair W. A community change in the algal endosymbionts of a scleractinian coral a following natural bleaching event: field evidence of acclimatization. Proc. R. Soc. B 275, 1359–1365 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkelmans R. & Van Oppen M. J. H. The role of zooxanthellae in the thermal tolerance of corals: a ‘nugget of hope’ for coral reefs in an era of climate change. Proc. R. Soc. B 273, 2305–2312 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker A. C. Flexibility and specificity in coral-algal symbiosis: diversity, ecology and biogeography of Symbiodinium. Ann. Rev. Ecol. Syst. 34, 661–689 (2003). [Google Scholar]
- Baker A. C. Diversity and Ecology of Symbiodinium on Coral Reefs and Its Relationship to Bleaching Resistance and Resilience (Springer-Verlag, New York/Berlin, 2004). [Google Scholar]
- Baker A. C., Starger C. J., McClanahan T. R. & Glynn P. W. Corals' adaptive response to climate change. Nature 430, 741 (2004). [DOI] [PubMed] [Google Scholar]
- Karako-Lampert S., Katcoff D. J., Achituv Y., Dubinsky Z. & Stambler N. Do clades of symbiotic dinoflagellates in scleractinian corals of the Gulf of Eilat (Red Sea) differ from those of other coral reefs? J. Exp. Mar. Biol. Ecol. 311, 301–314 (2004). [Google Scholar]
- Long S. P., Humphries S. & Falkowski P. G. Photoinhibition of photosynthesis in nature. Annu. Rev. Plant Physiol. Plant Mol. Biol. 45, 633–662 (1994). [Google Scholar]
- Iglesias-Prieto R., Matta J. L., Robins W. A. & Trench R. K. Photosynthesis respone to elevated temperature in the symbiotic dinoflagellate Symbiodinium microadriaticum in culture. Proc. Natl. Acad. Sci. USA 89, 10302–10305 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner M. E., Fitt W. K. & Schimidt G. W. The effect of elevated temperature on the photosynthetic efficiency of zooxanthellae in hospite from four different species of reef coral: a novel approach. Plant Cell Environ. 19, 291–299 (1996). [Google Scholar]
- Warner M. E., Fitt W. K. & Schimidt G. W. Damage to photosystem II in symbiotic dinoflagellates: a determinant of coral bleaching. Proc. Natl. Acad. Sci. USA 96, 8007–8012 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoegh-Guldberg O. & Jones R. J. Photoinhibition and photoprotection in symbiotic dinoflagellates from reef building corals. Mar. Ecol. Prog. Ser. 183, 73–86 (1999). [Google Scholar]
- Jones R. J. & Hoegh-Guldberg O. Diurnal changes in the photochemical efficiency of the symbiotic dinoflagellates (Dinophyceae) of corals: photoprotection, photoinactivation and relationship to coral bleaching. Plant Cell Environ. 24, 89–99 (2001). [Google Scholar]
- Brown B. E. et al. Diurnal changes in photochemical efficiency and xanthophyll concentrations in shallow water reef corals: evidence for photoinhibition and photoprotection. Coral Reefs 18, 99–105 (1999). [Google Scholar]
- Takahashi S., Whitney S. M. & Badger M. R. Different thermal sensitivity of the repair of photodamaged photosynthetic machinery in cultured Symbiodinium species. Proc. Natl. Acad. Sci. USA 106, 3237–3242 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy O. et al. Diurnal hysteresis in coral photosynthesis. Mar. Ecol. Prog. Ser. 268, 105–117 (2004). [Google Scholar]
- Gorbunov M. Y., Kolber Z. S., Lesser M. P. & Faikowski P. G. Photosynthesis and photoprotection in symbiotic corals. Limnol. Oceanog. 46, 75–87 (2001). [Google Scholar]
- Demmig- Adams B. & Adams W. W. I. The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci. 1, 21–26 (1996). [Google Scholar]
- Young A. J. & Frank H. A. Energy transfer reactions involving carotenoids- quenching of chlorophyll fluorescence. J. Photochem. Photobiol. 36, 3–15 (1996). [DOI] [PubMed] [Google Scholar]
- Ambarsari I., Brown B. E., Barlow R. G., Britton G. & Cummings D. Fluctuations in algal chlorophyll and carotenoid pigments during solarbleaching in the coral Goniastrea aspera at Phuket, Thailand. Mar. Ecol. Prog. Ser. 159, 303–307 (1997). [Google Scholar]
- Jones R. J., Hoegh-Guldberg O., Larkum A. W. D. & Schreiber U. Temperature induced bleaching of corals begins with impairment of the CO2 fixation mechanism in zooxanthellae. Plant Cell Environ. 21, 1219–1230 (1998). [Google Scholar]
- Lesser M. P. & Gorbunov M. Y. Diurnal and bathymetric changes in chlorophyll fluorescence yields of reef corals measured in situ with a fast repetition rate fluorometer. Mar. Ecol. Prog. Ser. 212, 69–77 (2001). [Google Scholar]
- Vollenweider R. A. Calculative models of photosynthesis – depth curves and some implications regarding day rate estimates in primary production measurments. Mem. Ist. Ital. Idrobiol. 18, 425–457 (1965). [Google Scholar]
- Vonshak A., Torzillo G., Masojidek J. & Boussiba S. Sub-optimal morning temperature induces photoinhibition in dense outdoor cultures of the alga Monodus subterraneus (Eustigmatophyta). Plant Cell Environ. 24, 1113–1118 (2001). [Google Scholar]