Abstract
MicroRNAs (miRNAs) regulate gene expression mainly by post-transcriptional gene silencing (PTGS) and in some cases by transcriptional genes silencing (TGS). miRNAs play critical roles in developmental processes, nutrient homeostasis, abiotic stress and pathogen responses of plants. In contrast to the large number of miRNAs predicted in cereal model plant rice, only 148 miRNAs were predicted in sorghum till date (miRBase release 17). This suggested that miRNAs identified in sorghum is far from saturation. Hence, we developed a bioinformatics pipeline using an in-house PERL script and publicly available structure prediction tools to identify miRNAs and their target genes from publically available Expressed Sequence Tags (EST) and Genomic Survey Sequence (GSS). About 1,379 known and unique plant miRNAs from 33 different crops were used to predict new miRNAs in sorghum. We identified 31 new miRNAs belonging to 10 different miRNA families. We predicted 72 potential target genes for 31 miRNAs, and most of these target genes are predicted to be involved in plant growth and development. These newly identified miRNAs add to the growing database of miRNA and lay the foundation for further understanding of miRNA function in sorghum plant development.
Keywords: miRNA, miRNA cluster, sorghum, target genes
Introduction
Sorghum (Sorghum bicolor L.) is an important cereal crop that is highly resistant to drought and heat stress, and is used for food, fodder, and as a raw materials for the production of starch, alcohol and biofuels.1–3 The extensive agricultural use of sorghum and the emerging demand of sorghum for biofuel production necessitates development of cultivars with higher yields, altered stem reserves, and improved resistance to biotic and abiotic stresses. MicroRNAs (miRNAs) play important roles in development, nutrient acquisition and use, and tolerance to abiotic and biotic stresses.4 miRNAs are small non-coding RNAs of approximately 21 nucleotides (nt) in length that act mainly in PTGS and in some cases TGS to regulate the expression of their target genes. miRNAsare widespread in all eukaryotes including unicellular green alga.5The genes encoding miRNAs, MIR genes, are transcribed by RNA polymerase II to produce a primary transcript (pri-miRNA). The stem-loop structure of the pri-miRNA is processed Dicer-Like (DCL) RNase III enzymes (mainly DCL1 in Arabidopsis) to produce ~21 nucleotide long miRNA-miRNA* duplex. The DCL1 catalyzed processing of pri-miRNAs to pre-miRNAs also requires two additional dsRNA-binding proteins namely HYPONASTIC LEAVES1 (HYL1) and SERRATE (SE) in Arabidopsis.6,7 The HEN1, a methyltransferase, catalyzes 2’-O-methylation of the 3′ termini nucleotide in the miRNA-miRNA* duplex. The miRNAs are exported in to cytosol with the help of HASTY (exportin 5). In cytosol, miRNA is loaded into AGO1 containing RISC which catalyzes PTGS.8,9 Plant miRNAs negatively regulate the transcripts levels of their target genes, and play important roles in plant growth, organ development, cell differentiation and proliferation, cell death, signal transduction, and stress response.8–11Identification of miRNAs and their target genes therefore is an important step toward understanding the biological functions of miRNAs. Recently, computational approaches are used wildly as rapid, accurate, and affordable method to identify miRNAs.The computational approaches have been very effective in plants, where miRNA and its target mRNA have often nearly perfectly complementary.12–14 The earliest miRNAs from plant kingdom were discovered in Arabidopsis thaliana in 2002,15,16 and subsequent miRNAs have been identified in several plants by computational and experimental approaches.8,17 Conserved nature of mature miRNAs among different species and the unique secondary structure of pri-miRNAs,15,16,18–21 facilitate miRNA prediction using bioinformatics approaches.22 A comparative genomics approach for the prediction of novel miRNAs and their targets was developed by Jones-Rhoades and Bartel.13 Identification of several miRNAs from Arabidopsis,23 rice,24 corn,25 cotton,26 Medicago truncatula,27 soybean,4 citrus,28 mustard,29 wheat,30 potato,31 tomato,32 switchgrass,33 and sorghum34 by computational approaches have been reported. The database of Genomic Survey Sequences (GSS) and Expressed Sequenced Tag (EST) are the major resources for identification of miRNAs in most of the plants. Using this approach, more than 700 miRNAs have been identified in plants.25,35,36 Most research groups prefer to use expressed sequence tags (ESTs) over genome sequence as ESTs provide direct evidence for miRNA expression.37,38 Several homology based tools (e.g., MIRcheck;13 miRU39) are available for identification of potential miRNA target genes. The microRNAs registry database40,41 (Release 17) include 232, 491, 234 and 170 miRNAs from Arabidopsis thaliana,12,42–45 Oryza sativa,24,46–49 Populus trichocarpa,50,51 and Zea mays,52 respectively. The number of miRNAs reported in sorghum in miRbase version-17 is only 148. This indicates potential for identification of additional miRNAs in sorghum as currently only less number of miRNAs are reported in sorghum as compared with other plant species. In this study, we used computational pipeline to predict novel miRNAs and their target genes in sorghum. Researchers can further validate these newly predicted miRNAs by using direct sequencing of small RNA libraries or by northern blotting.53 Validation of functions these miRNAs in sorghum will help understand development and stress responses of sorghum.
Results
Prediction of miRNAs
Plant miRNAs exhibit high degree of conservation within plant kingdom.54,55 Hence, known miRNAs from one plant species can be used to identify the conserved miRNAs in target species. A total of 2,728 plant miRNAs belong to 33 different plant species were downloaded from microRNA repository miRBase, version 17. From this data set, we omitted previously reported sorghum miRNAs to avoid prediction of previously identified sorghum miRNAs. Multiple sequence alignment was performed to eliminate miRNA with same sequence, and finally we obtained 1,379 non-redundant reference miRNAs belonging to 643 different miRNAs families. These reference miRNAs were used to identify miRNAs from ESTs (240161 sequence) and GSS (799,504 sequences) in sorghum by using an in-house PERL script. Sorghum GSS and ESTs that perfectly matched with reference miRNAs were considered as possible precursors of miRNAs (pri-miRNAs) in this study. Initially, we identified 375 pri-miRNAs (326 from GSS and 51 from ESTs).
Minimization of false positives
Removal of false positive is an essential step in computational prediction of miRNAs. We applied a number of initial filters to new potential pri-miRNAs as suggested by Zhang et al.56 and Bonnet et al.57 We aligned initially predicted 375 miRNAs with previously reported 148 miRNAs in sorghum (miRBase, version 17) and eliminated if found. To analyze, whether the predicted putative pri-miRNAs are non protein coding RNAs, BLASTX was performed against NCBI non-redundant protein database and excluded if putative pri-miRNAs with protein coding potential. The candidate miRNA precursors were also aligned with known non-coding RNAs such as tRNA, rRNA, snRNA or snoRNA and discarded if found similar. The candidate miRNA precursors were also aligned with plastid or mitochondrial genomes to eliminate precursors with similarity to these genomes. These primary filtering strategies reduced the number of predicted miRNA precursors in sorghum, and thus we obtained 33 and 53 valid new miRNA precursors from EST and GSS sequences, respectively.
Pri-miRNA structural filter
The 86 putative sorghum miRNA precursors were carefully examined to make sure that they qualify for the updated plant miRNA annotation criteria.53,58 One important feature that distinguishes miRNAs from other endogenous small RNAs is that pri-miRNA transcript adopts a stem-loop structure and the miRNA is derived from the stem-arm. The miRNA precursors with 250 nt upstream and downstream to the mature miRNA sequence were analyzed for their ability to fold into a stem-loop hairpin structure using the RNAFold program59,60 and those that fulfilled the hairpin structure criteria described by Jones-Rhoades et al.,8 were selected as potential candidate precursors for miRNA. Among the 86 putative miRNA precursors screened, only 54 passed initial filters for positional overlaps, secondary structure and orientation of mature miRNA sequence within the respective stem-loop structures. As a result, a total of 54 new miRNAs, nine from EST and 45 from GSS sequence, were predicted in the stem-arm of the stem-loop hairpin structures. All 54 sorghum miRNAs were considered as valid candidates after satisfying the empirical formula for biogenesis and expression of the miRNAs as suggested by Ambroset al.58 Additionally, we mapped all these 54 miRNA precursors on sorghum genome to eliminate overlapping miRNAs. As a result, we obtained 31 new miRNA precursors mapped to unique genome loci and listed in Table 1. The predicted miRNA precursor sequences and hairpin structures are shown in Figure S1.
Table 1. List of 31 sorghum miRNAs identified by comparative genomics and secondary structure analysis.
| miR Family |
miRNA mature Sequence |
L* |
Chr* |
Precursor |
PL* |
MM* |
Arm* |
Strand* |
MFE |
AMFE |
MFEI |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Start | End | kcal/ mol | ||||||||||
| sbi-MIR156j |
UGACAGAAGAGAGUGAGCACA |
21 |
3 |
3473047 |
3473132 |
86 |
2 |
5′ |
- |
54.9 |
63.84 |
1.25 |
| sbi-MIR156k |
UGACAGAAGAGAGUGAGCACA |
21 |
3 |
3473329 |
3473501 |
173 |
1 |
5′ |
- |
88 |
50.87 |
1.04 |
| sbi-MIR156l |
UGACAGAAGAGAGUGAGCACA |
21 |
4 |
5373507 |
5373664 |
158 |
3 |
5′ |
- |
79.5 |
50.32 |
0.92 |
| sbi-MIR156m |
UGCUCUCUGCUCUCACUGUCAUC |
23 |
2 |
62836711 |
62836860 |
150 |
3 |
3′ |
- |
81.8 |
54.53 |
0.91 |
| sbi-MIR166l |
GGAAUGUUGUCUGGUUCAAGG |
21 |
1 |
17295173 |
17295276 |
104 |
3 |
5′ |
- |
46.7 |
44.9 |
1.09 |
| sbi-MIR166m |
UCGGACCAGGCUUCAUUCC |
19 |
1 |
7426516 |
7426597 |
82 |
2 |
3′ |
+ |
38.6 |
47.07 |
0.73 |
| sbi-MIR166n |
UCGGACCAGGCUUCAUUCC |
19 |
1 |
69265255 |
69265358 |
104 |
3 |
3′ |
- |
59.1 |
56.83 |
1.02 |
| sbi-MIR166o |
UCGGACCAGGCUUCAUUCC |
19 |
1 |
17295173 |
17295276 |
104 |
3 |
3′ |
- |
46.7 |
44.9 |
1.09 |
| sbi-MIR166p |
UCGGACCAGGCUUCAUUCCCC |
21 |
1 |
17295156 |
17295297 |
142 |
4 |
3′ |
- |
66.7 |
46.97 |
1.13 |
| sbi-MIR166q |
UCGGACCAGGCUUCAUUCCCC |
21 |
1 |
69265255 |
69265360 |
106 |
3 |
3′ |
- |
60.7 |
57.26 |
1.05 |
| sbi-MIR166r |
UCGGACCAGGCUUCAUUCCCC |
21 |
1 |
7426516 |
7426597 |
82 |
2 |
3′ |
+ |
38.6 |
47.07 |
0.73 |
| sbi-MIR166s |
UCGGACCAGGCUUCAUUCCCCC |
22 |
1 |
69265255 |
69265358 |
104 |
4 |
3′ |
- |
59.1 |
56.83 |
1.02 |
| sbi-MIR166t |
UCGGACCAGGCUUCAUUCCCCU |
22 |
1 |
17295156 |
17295297 |
142 |
4 |
3′ |
- |
66.7 |
46.97 |
1.13 |
| sbi-MIR167j |
GAUCGUGCUGCGCAGUUUCACC |
22 |
3 |
64088363 |
64088485 |
123 |
2 |
3′ |
- |
61.9 |
50.33 |
1.11 |
| sbi-MIR167k |
UGAAGCUGCCAGCAUGAUCUGA |
22 |
3 |
64088363 |
64088485 |
123 |
1 |
5′ |
- |
61.9 |
50.33 |
1.11 |
| sbi-MIR168b |
CCCGCCUUGCACCAAGUGAA |
20 |
4 |
2246312 |
2246408 |
97 |
3 |
3′ |
- |
56.1 |
57.84 |
0.84 |
| sbi-MIR168c |
GAUCCCGCCUUGCACCAAGUGAAU |
24 |
4 |
2246328 |
2246408 |
81 |
5 |
3′ |
- |
52.9 |
65.31 |
0.98 |
| sbi-MIR171l |
UUGAGCCGUGCCAAUAUCAC |
20 |
7 |
7609102 |
7609232 |
131 |
1 |
3′ |
+ |
74.1 |
56.56 |
0.89 |
| sbi-MIR171m |
UUGAGCCGUGCCAAUAUCACG |
21 |
7 |
7609102 |
7609232 |
131 |
1 |
3′ |
+ |
74.1 |
56.56 |
0.89 |
| sbi-MIR390b |
CGCUAUCUAUCCUGAGCUCCA |
21 |
1 |
2870964 |
2871206 |
243 |
2 |
3′ |
+ |
115.5 |
47.54 |
1.03 |
| sbi-MIR396f |
GUUCAAGAAAGCUGUGGAAGA |
21 |
4 |
66092395 |
66092515 |
121 |
2 |
5′ |
- |
55.1 |
45.55 |
0.92 |
| sbi-MIR396 g |
GUUCAAUAAAGCUGUGGGAAA |
21 |
4 |
66092521 |
66092630 |
110 |
2 |
3′ |
- |
42 |
38.18 |
0.75 |
| sbi-MIR396h |
UCCACAGGCUUUCUUGAACUG |
21 |
4 |
67655115 |
67655256 |
142 |
2 |
5′ |
- |
61.8 |
43.52 |
0.86 |
| sbi-MIR396i |
UCCCACAGCUUUAUUGAACUG |
21 |
4 |
66092395 |
66092515 |
121 |
2 |
5′ |
- |
37.5 |
30.99 |
0.65 |
| sbi-MIR396j |
UCCCACAGCUUUAUUGAACUG |
21 |
4 |
66092515 |
66092635 |
121 |
2 |
3′ |
+ |
55.1 |
45.55 |
0.92 |
| sbi-MIR396k |
UCUCCACAGGCUUUCUUGAACU |
22 |
4 |
67655122 |
67655251 |
130 |
3 |
5′ |
- |
65.6 |
50.46 |
0.99 |
| sbi-MIR398b |
GGGGCGGACUGGGAACACAUG |
21 |
2 |
15190815 |
15190961 |
147 |
2 |
5′ |
- |
69.6 |
47.35 |
0.81 |
| sbi-MIR399l |
GGGCAACUUCUCCUUUGGCAGA |
22 |
9 |
55688233 |
55688348 |
116 |
3 |
5′ |
+ |
49.3 |
42.5 |
0.74 |
| sbi-MIR444a |
UGCAGUUGUUGUCUCAAGCUU |
21 |
4 |
53723200 |
53728533 |
126 |
1 |
3′ |
- |
75.2 |
59.68 |
1.27 |
| sbi-MIR444b |
UGUUGUCUCAAGCUUGCUGCC |
21 |
4 |
53723200 |
53728533 |
126 |
3 |
3′ |
- |
75.2 |
59.68 |
1.27 |
| sbi-MIR444c | UUGUGGCUUUCUUGCAAGUUG | 21 | 4 | 59021719 | 59021792 | 74 | 1 | 3′ | + | 22.5 | 30.4 | 0.64 |
L, length of mature miRNAs; *PL, precursor length; *Arm, location of mature miRNAs on secondary stem-loop structures of pre miRNA sequences; *Strand, miRNAs existence in sense (+) and antisense (-) strand.
Sorghum miRNAs family
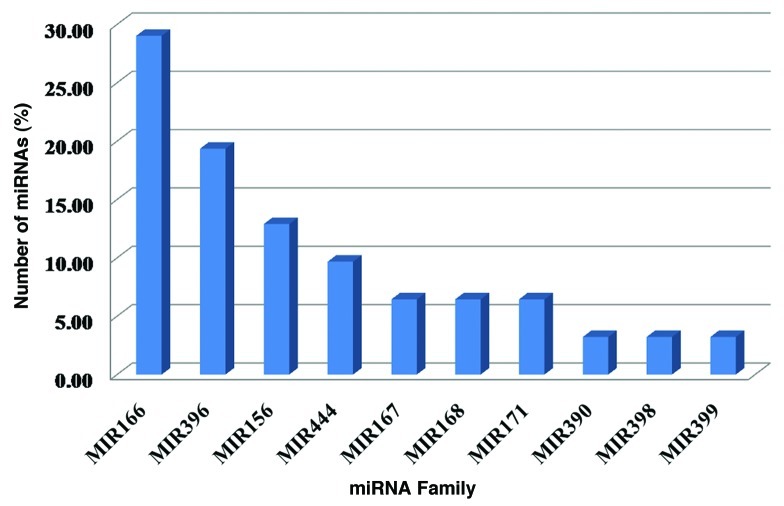
The newly identified miRNAs in this study were assigned to different families of miRNA in sorghum. The family assignment was based on sequence similarity between newly predicted miRNAs and already known miRNAs in other plants including known sorghum miRNAs in the miRBase. To determine the sequence similarity, multiple sequence alignment was performed by using ClustalW61 and based on cluster analysis (data not shown), predicted 31 miRNAs were assigned to respective MIR family. In this study, 31 newly identified miRNAs were assigned to 10 diverse MIR families namely miR156, miR166, miR167, miR168, miR171, miR390, miR396, miR398, miR399 and miR444 in sorghum (Fig. 1). Typically each MIR loci produced single precursor but a few MIR loci produced two or more precursors, probably due to exon shuffling.
Figure 1. Distribution of 31 newly predicted miRNAs under diverse 10 miRNA families.
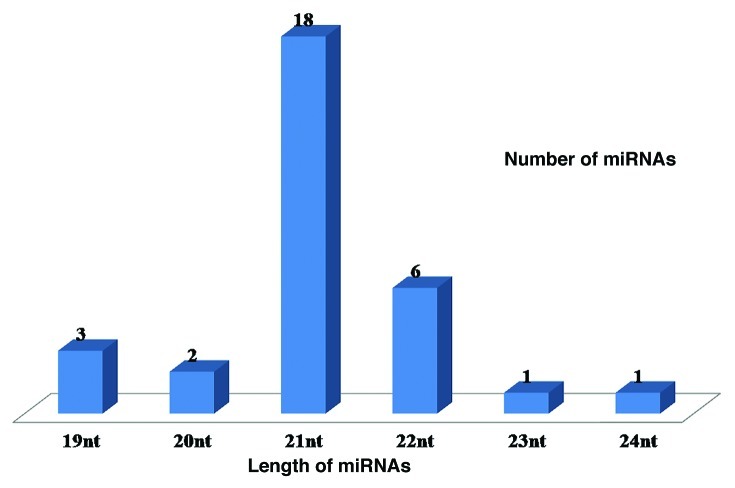
Sequence characteristics of new miRNAs
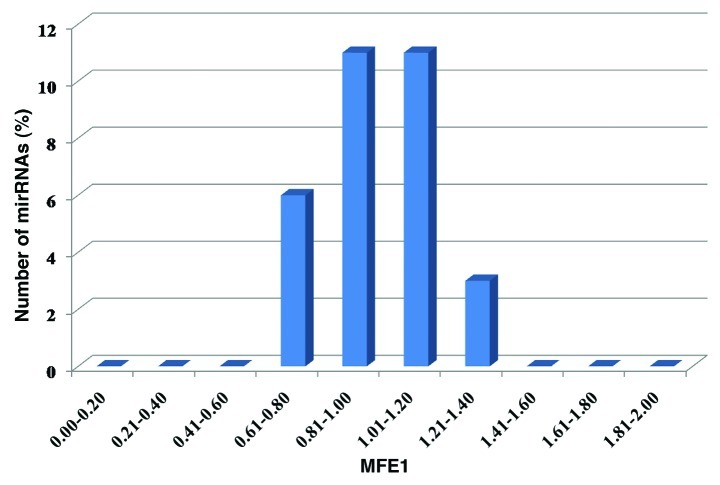
Among the newly predicted miRNAs, the largest number, i.e., nine miRNAs were assigned to miR166 family followed by six miRNAs assigned to miR396 family. The nucleotide length of these newly identified miRNAs varied from 19 to 24 nt, with an average of 20.91 ± 1.18 nt (Fig. 2). The nucleotide length of sorghum pre-miRNA (stem-loop) varied from 74 to 243 nt, with an average of 122.58 ± 32.78 nt.The length distribution of miRNAs and their precursor sequences are similar to the previous reports in other plant species.4,25,26,35 Out of 31 miRNAs, 22 (70.97%) began with a 5′ uridine, a characteristic feature of miRNAs. Mature miRNA sequences have been shown to be located on the stem-arm of the secondary stem-loop hairpin structure of the potential pre-miRNA. Out of 31 miRNAs identified, 11 (35.48%) were found to be located on the 5′ arm of the stem-loop hairpin structure, while 20 (64.52%) resided on the 3′ arm. It is previously reported that microRNA precursors, unlike other non-coding RNAs, have lower folding free energy than random sequence.14 Minimal folding free energy has been considered as one of important feature in previously described methods for miRNAs identification.62,63All newly identified sorghum miRNA precursors have negative minimal folding free energies (MFE), ranging from −22.5 to −115.5 kcal mol−1 with an average of −61.05 ± 17.82 kcal mol−1 (Table 1). MFEs are strongly and positively correlated with their sequence length.4 To normalize the potential effect of sequence length on MFE and to differentiate miRNAs from other RNAs,64 we used two energy measurementsnamely MFE (AMFE) and minimal folding free energy index (MFEI), and demonstrated that a candidate RNA sequence is more likely to be an miRNA when the MFEI is greater than 0.85. The newly identified sorghum pri-miRNAs had a high MFEI (0.64–1.27), with an average of about 0.96 (Fig. 3) which is significantly higher than that for tRNAs (0.64), rRNAs (0.59), and mRNAs (0.62–0.66).65
Figure 2. Length distribution of mature miRNAs in sorghum.
Figure 3. Minimal folding free energy index of pre-miRNAs in sorghum.
MIR gene clusters in sorghum genome
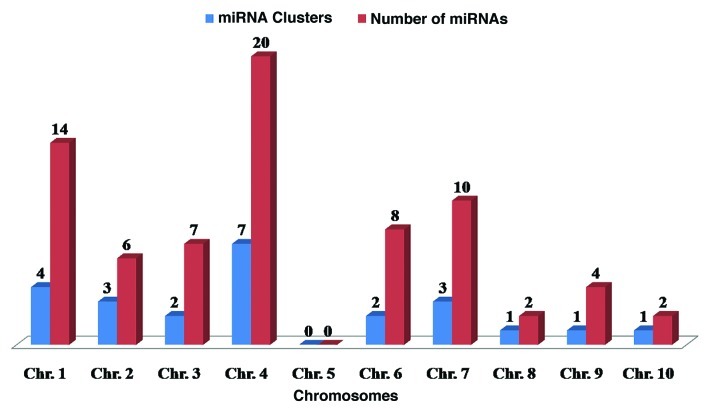
In general, miRNA gene clusters are found in both animal and plant genomes. Several studies revealed that some members of MIR gene family are physically clustered in plant genomes.18,66–70 Some clusters are so compact where multiple miRNAs are aligned in the same orientation and transcribed as a polycistronic transcript.68,71,72 Clusters are conserved across vertebrates: from teleost fish to human.73 However in plants, only few miRNA cluster have been found.13,26,56,68,70,74 We mapped all the previously registered 148 sorghum miRNAs and 31 miRNA predictedin this study to examine the potential clusters of MIR genes on the sorghum genome. As a result, 24 compact clusters were predicted for 73 sorghum miRNAs, having their genomic organization within 10 kb. The identified miRNA clusters belong to 12 different MIR gene families (Table 2). Our analysis revealed that chromosome 4 has seven MIR gene clusters, while chromosome 1 has four MIR geneclusters (Fig. 4). We also noticed that no cluster was detected on chromosome 2. This indicates MIR gene clusters are common in sorghum. This prediction is similar as previously predicted MIR gene clusters in human75 and soybean.4 Altuvia et al.75 confirmed that 42% miRNA genes are placed in clusters in the human genome using a 3 kb threshold or 48% if using 10 kb threshold between two miRNA genes. Zhang and colleges4 also observed that 16% of the total identified soybean miRNAs genes are arranged in clusters. The sorghum MIR gene clusters were found diverse in structure and varies in cluster length from 82 to 8,633 bp, with an average of 1689.54. Here, we observed that each cluster consists of miRNA genes strictly from same gene families. This is in support to the previously given hypothesis that plant MIR gene clusters are comprised of homologous members.13,56,68 Generally, miRNA members in a cluster share high sequence conservation; whereas a regular decrease in sequence similarity suggests that duplication events occurred at various time points. Compact clusters between two miRNAs have been found in various plant species. 25,54,70,76,77 In this article, we identified 31 new miRNAs. Most of which are arranged in compact cluster, and same miRNA family members with high sequence similarity formed a cluster.
Table 2. Sorghum miRNA gene clusters on different chromosomes.
| Cluster number | Cluster name | miRNAs members | Cluster length$ | Distance between miRNAs# | Chromosome |
|---|---|---|---|---|---|
| 1 |
MIR390 |
b*, a |
242 |
b-a: Overlap |
Chr.1 |
| 2 |
MIR166 |
m*, r*, b |
82 |
m-r: Overlap; r-b: Overlap |
Chr.1 |
| 3 |
MIR166 |
p*, t*, a, l*, o* |
142 |
p-t: Overlap; t-a: Overlap; a-l: Overlap; l-o: Overlap |
Chr.1 |
| 4 |
MIR166 |
n*, q*, s*, c |
106 |
n-q: overlap; q-s: Overlap; s-c: Overlap |
Chr.1 |
| 5 |
MIR398 |
b*, a |
147 |
b-a: Overlap |
Chr.2 |
| 6 |
MIR156 |
m*, d |
150 |
m-d: Overlap |
Chr.2 |
| 7 |
MIR169 |
f, g |
2984 |
f-g: 2686 |
Chr.2 |
| 8 |
MIR156 |
j*, b, k*, c |
454 |
j-b: Overlap; b-k: 198; k-c: Overlap |
Chr.3 |
| 9 |
MIR167 |
j*, k*, g |
124 |
j-k: Overlap; k-g: Overlap |
Chr.3 |
| 10 |
MIR168 |
b*, a, c* |
110 |
b-a: Overlap; a-c: Overlap |
Chr.4 |
| 11 |
MIR156 |
l*, a |
158 |
l-a: Overlap |
Chr.4 |
| 12 |
MIR399 |
j, k |
5412 |
j-k: 5258 |
Chr.4 |
| 13 |
MIR444 |
a*, b* |
126 |
a-b: Overlap |
Chr.4 |
| 14 |
MIR166 |
g, f |
407 |
g-f: 136 |
Chr.4 |
| 15 |
MIR396 |
c, f*, i*,a, j*, g* |
7351 |
c-f: 6947; f-i:Overlap; i-a; Overlap; a-j: Overlap; j-g: Overlap |
Chr.4 |
| 16 |
MIR396 |
h*, k*, d |
142 |
h-k: Overlap; k-d: Overlap |
Chr.4 |
| 17 |
MIR395 |
f, c, d, e |
805 |
f-c: 71, c-d: 246, g-h: 89 |
Chr.6 |
| 18 |
MIR395 |
a, b, g, h |
1014 |
a-b: 445, b-g: 75, g-h: 94 |
Chr.6 |
| 19 |
MIR395 |
i, j, k, l |
742 |
i-j: 87, j-k: 84, k-l: 227 |
Chr.7 |
| 20 |
MIR171 |
b, l*, m* |
133 |
b-l: Overlap; l-m: Overlap |
Chr.7 |
| 21 |
MIR169 |
l, m, n |
8633 |
l-m: 5291; m-n: 3063 |
Chr.7 |
| 22 |
MIR167 |
i, e |
2466 |
i-e: 2157 |
Chr.8 |
| 23 |
MIR399 |
c, e, g, l* |
6127 |
c-e: 1438; e-g: 4311; g-l: Overlap |
Chr.9 |
| 24 | MIR399 | f, h | 2492 | f-h: 2242 | Chr.10 |
Distance, Distance (nt) to previous miRNA gene in the cluster;
Cluster length, Total miRNAs occupied region in one cluster;
miRNA members, A star mark denote predicted sorghum miRNAs in this study.
Figure 4. Number of miRNA clusters and occupied miRNAs gene on sorghum chromosomes.
Potential target genes for newly predicted miRNAs
The putative target genes of sorghum miRNAs were identified by a perfect or near-perfect sequence complementarity between miRNA and its target transcript. We searched the potential miRNA targets for predicted 31 new miRNAs against mRNA sequence of sorghum by using plant miRNA analysis psRNA Target tool39 and UEA sRNA plant target prediction tool78 and as a results, we obtained 72 (of which 49 are unique) potential target genes for 31 newly predicted miRNAs belonging to 10 different miR families (Table 3). The sequence alignments of 31 putative miRNAs and their corresponding targets in sorghum are shown in Supplemental Figure 2. We observe that number of targets per miRNA varied and some miRNAs have multiple target genes. For example, miR396 has 13 target genes, whereas miR444 has eight target genes. We noticed that a miRNA family members target the same set of genes, suggesting a functional redundancy amongthe family members. For example, a few members of miR166 family (miR166m-s) target the mRNA of homeobox leucine zipper transcription factor gene (target accession no: CN140010). In contrast, some members of miRNA families (e.g., miR444) have specific target genes. For example, miR444a target to WD-40 repeat family protein (target accession no: CN125113), involved in signal transducer activity. Pathway analysis of predicted target genes revealed that 14 targets are metabolism-associated. Among these, six miRNA (e.g., miR444b, 166 min, 166o, 166p, 166q and 166r) targets genes involved in sulfur metabolism, whereas eight members of miR177 targets genes involved in riboflavin metabolism. Most of the predicted targets of newly identified mRNAs are transcription factors that may have potential role in plant growth and development (Table 3). Remaining miRNAs target genes are involved in a broad range of biological functions, such as hydrolase activity (miR156 target EH409419) oligopeptide transporter activity (miR167target CX614408), riboflavin synthase activity (all predicted targets of miR171), kinase activity (miR396), zinc and calcium ion binding (miR444), translation initiation factor activity (miR444) and signal transducer activity (miR444) (Table 3). We also observed that when miRNA has more than one target, the potential targets belonged to the same gene family. All predicted miRNA and their targets share high similarity to their orthologs in Arabidopsis thaliana and Zea mays.
Table 3. Potential target genes and their predicted functions for 31 newly identified miRNAs in sorghum.
| miRNA Acc. | Target Gene Acc. | Gene Annotation | Target Function | KEGG Pathway | COG Function | EST Expression |
|---|---|---|---|---|---|---|
| sbi-MIR156j |
BG947367 |
Squamosa promoter-binding-like protein 16 |
DNA Binding Transcription Factor |
No Hits Found |
No Hits Found |
Panicle and Callus |
| sbi-MIR156j |
AW747167 |
SBP transcription factor |
DNA Binding Transcription Factor |
No Hits Found |
No Hits Found |
No Hits Found |
| sbi-MIR156j |
EH409419 |
Hydrolase, α/β fold family protein, expressed |
Hydrolase Activity |
No Hits Found |
Hydrolases or Acyltransferases (α/β hydrolase superfamily) |
Ovary and Root |
| sbi-MIR156j |
CF756128 |
Jumonji Domain Protein |
DNA Binding Transcription Factor |
No Hits Found |
No Hits Found |
Pollen |
| sbi-MIR156k |
BG947367 |
Squamosa promoter-binding-like protein 16 |
DNA Binding Transcription Factor |
No Hits Found |
No Hits Found |
Panicle and Callus |
| sbi-MIR156k |
AW747167 |
SBP transcription factor |
DNA Binding Transcription Factor |
No Hits Found |
No Hits Found |
No Hits Found |
| sbi-MIR156k |
EH409419 |
Hydrolase, α/β fold family protein, expressed |
Hydrolase Activity |
No Hits Found |
Hydrolases or Acyltransferases (α/β hydrolase superfamily) |
Ovary and Root |
| sbi-MIR156k |
CF756053 |
Jumonji Domain Protein |
DNA Binding Transcription Factor |
No Hits Found |
No Hits Found |
Pollen |
| sbi-MIR156l |
BG947367 |
Squamosa promoter-binding-like protein 16 |
DNA Binding Transcription Factor |
No Hits Found |
No Hits Found |
Panicle and Callus |
| sbi-MIR156l |
AW747167 |
SBP transcription factor |
DNA Binding Transcription Factor |
No Hits Found |
No Hits Found |
No Hits Found |
| sbi-MIR156l |
EH409419 |
Hydrolase, α/β fold family protein, expressed |
Hydrolase Activity |
No Hits Found |
Predicted Hydrolases or Acyltransferases (α/β hydrolase superfamily) |
Ovary and Root |
| sbi-MIR156l |
CF756128 |
Jumonji Domain Protein |
DNA Binding Transcription Factor |
No Hits Found |
No Hits Found |
Pollen |
| sbi-MIR156m |
BG948102 |
Jumonji Domain Protein |
Actin Filament Binding |
No Hits Found |
No Hits Found |
Panicle and Root |
| sbi-MIR156m |
BM325400 |
Phagocytosis and cell motility protein ELMO1-like |
Mysoin II Binding |
No Hits Found |
No Hits Found |
Ovary and Panicle |
| sbi-MIR166l |
CN126049 |
Calcium channel α-1 subunit |
Molecular Function Unknown |
No Hits Found |
No Hits Found |
No Hits Found |
| sbi-MIR166l |
CD461667 |
Catalytic Domain of Protein Kinases |
Kinase Activity |
No Hits Found |
Serine/threonine protein kinases |
Leaf |
| sbi-MIR166l |
CN135236 |
CAP22 protein |
Molecular Function Unknown |
No Hits Found |
No Hits Found |
No Hits Found |
| sbi-MIR166m |
CN140010 |
Homeobox-leucine zipper protein |
DNA Binding Transcription Factor |
No Hits Found |
No Hits Found |
No Hits Found |
| sbi-MIR166m |
CF074035 |
Diphosphonucleotide Phosphatase1 |
3′(2'),5′-Bisphosphate Nucleotidase activity / Inositol or Phosphatidylinositol Phosphataseactivity |
Sulfur Metabolism |
Inorganic ion Transport and Metabolism |
Shoot |
| sbi-MIR166n |
CN140010 |
Homeobox-leucine zipper protein |
DNA Binding Transcription Factor |
No Hits Found |
No Hits Found |
No Hits Found |
| sbi-MIR166n |
CD209789 |
Sodium- and Lithium-Tolerant 1 (SLT1) |
Molecular Function Unknown |
No Hits Found |
No Hits Found |
Callus, Leaf, Ovary, Panicle and Root |
| sbi-MIR166o |
CN140010 |
Homeobox-leucine zipper protein |
DNA Binding Transcription Factor |
No Hits Found |
No Hits Found |
No Hits Found |
| sbi-MIR166o |
CF074035 |
Diphosphonucleotide Phosphatase1 |
3′(2'),5′-Bisphosphate Nucleotidase activity / Inositol or Phosphatidylinositol Phosphataseactivity |
Sulfur Metabolism |
Inorganic ion Transport and Metabolism |
Shoot |
| sbi-MIR166o |
CD209789 |
Sodium- and Lithium-Tolerant 1 (SLT1) |
Molecular Function Unknown |
No Hits Found |
No Hits Found |
Callus, Leaf, Ovary, Panicle and Root |
| sbi-MIR166p |
CN140010 |
Homeobox-leucine zipper protein |
DNA Binding Transcription Factor |
No Hits Found |
No Hits Found |
No Hits Found |
| sbi-MIR166p |
CF074035 |
Diphosphonucleotide Phosphatase1 |
3′(2'),5′-Bisphosphate Nucleotidase activity / Inositol or Phosphatidylinositol Phosphataseactivity |
Sulfur Metabolism |
Inorganic ion Transport and Metabolism |
Shoot |
| sbi-MIR166p |
CN126049 |
Calcium channel α-1 subunit |
Molecular Function Unknown |
No Hits Found |
No Hits Found |
No Hits Found |
| sbi-MIR166q |
CN140010 |
Homeobox-leucine zipper protein |
DNA Binding Transcription Factor |
No Hits Found |
No Hits Found |
No Hits Found |
| sbi-MIR166q |
CF074035 |
Diphosphonucleotide Phosphatase1 |
3′(2'),5′-Bisphosphate Nucleotidase activity / Inositol or Phosphatidylinositol Phosphataseactivity |
Sulfur Metabolism |
Inorganic ion Transport and Metabolism |
Shoot |
| sbi-MIR166q |
CN126049 |
Calcium channel α-1 subunit |
Molecular Function Unknown |
No Hits Found |
No Hits Found |
No Hits Found |
| sbi-MIR166r |
CN140010 |
Homeobox-leucine zipper protein |
DNA Binding Transcription Factor |
No Hits Found |
No Hits Found |
No Hits Found |
| sbi-MIR166r |
CF074035 |
Diphosphonucleotide Phosphatase1 |
3′(2'),5′-Bisphosphate Nucleotidase activity / Inositol or Phosphatidylinositol Phosphataseactivity |
Sulfur Metabolism |
Inorganic ion Transport and Metabolism |
Shoot |
| sbi-MIR166r |
CN126049 |
Calcium channel α-1 subunit |
Molecular Function Unknown |
No Hits Found |
No Hits Found |
No Hits Found |
| sbi-MIR166r |
CN140010 |
Homeobox-leucine zipper protein |
DNA Binding Transcription Factor |
No Hits Found |
No Hits Found |
No Hits Found |
| sbi-MIR166r |
CN126049 |
Calcium channel α-1 subunit |
Molecular Function Unknown |
No Hits Found |
No Hits Found |
No Hits Found |
| sbi-MIR166s |
CN126049 |
Calcium channel α-1 subunit |
Molecular Function Unknown |
No Hits Found |
No Hits Found |
No Hits Found |
| sbi-MIR167j |
CX614408 |
Iron-Phytosiderophore Transporter Yellow Stripe |
Oligopeptide Transporter Activity |
No Hits Found |
No Hits Found |
Root and Shoot |
| sbi-MIR167k |
CD423596 |
Auxin Response Factor 9 |
DNA Binding Transcription Factor / Protein Dimerization Activity |
No Hits Found |
No Hits Found |
Ovary, Panicle and Root |
| sbi-MIR167k |
CN140701 |
Arv1-like protein |
Molecular Function Unknown |
No Hits Found |
No Hits Found |
No Hits Found |
| sbi-MIR168c |
CD209773 |
PWWP domain-containing protein |
Molecular Function Unknown |
No Hits Found |
No Hits Found |
Leaf |
| sbi-MIR171l |
CN129969 |
6,7-dimethyl-8-ribityllumazine synthase |
Riboflavin Synthase Activity |
Riboflavin Metabolism |
Riboflavin Synthase Beta-Chain |
Leaf, Ovary, Polen and Root |
| sbi-MIR171l |
CN142205 |
6,7-dimethyl-8-ribityllumazine synthase |
Riboflavin Synthase Activity |
Riboflavin Metabolism |
Riboflavin Synthase Beta-Chain |
Leaf, Ovary, Polen and Root |
| sbi-MIR171l |
AW747132 |
6,7-dimethyl-8-ribityllumazine synthase |
Riboflavin Synthase Activity |
Riboflavin Metabolism |
Riboflavin Synthase Beta-Chain |
Leaf, Ovary, Polen and Root |
| sbi-MIR171l |
CN131513 |
6,7-dimethyl-8-ribityllumazine synthase |
Riboflavin Synthase Activity |
Riboflavin Metabolism |
Riboflavin Synthase Beta-Chain |
Leaf, Ovary, Polen and Root |
| sbi-MIR171 |
CN131513 |
6,7-dimethyl-8-ribityllumazine synthase |
Riboflavin Synthase Activity |
Riboflavin Metabolism |
Riboflavin Synthase Beta-Chain |
Leaf, Ovary, Polen and Root |
| sbi-MIR171m |
CN129969 |
6,7-dimethyl-8-ribityllumazine synthase |
Riboflavin Synthase Activity |
Riboflavin Metabolism |
Riboflavin Synthase Beta-Chain |
Leaf, Ovary, Polen and Root |
| sbi-MIR171m |
BM317608 |
6,7-dimethyl-8-ribityllumazine synthase |
Riboflavin Synthase Activity |
Riboflavin Metabolism |
Riboflavin Synthase Beta-Chain |
Leaf, Ovary, Pollen and Root |
| sbi-MIR171m |
BE355399 |
6,7-dimethyl-8-ribityllumazine synthase |
Riboflavin Synthase Activity |
Riboflavin Metabolism |
Riboflavin Synthase Beta-Chain |
Leaf, Ovary, Pollen and Root |
| sbi-MIR390b |
BE363723 |
ARF-like GTPas |
Transcription factor |
No Hits Found |
No Hits Found |
Ovary |
| sbi-MIR396f |
CF480198 |
Exosome complex exonuclease RRP41 |
RNA Binding |
RNA Degradation |
RNase PH |
Leaf and pollen |
| sbi-MIR396 g |
CB927788 |
plant synaptotagmin |
Metal Ion Binding |
No Hits Found |
No Hits Found |
Leaf, Panicle and Root |
| sbi-MIR396 g |
CD211715 |
Ubiquitin carrier protein |
Ubiquitin-Protein Ligase Activity |
Ubiquitin Mediated Proteolysis |
No Hits Found |
Callus and Embryo |
| sbi-MIR396 g |
CD222706 |
Ubiquitin carrier protein |
Ubiquitin-Protein Ligase Activity |
Ubiquitin Mediated Proteolysis |
No Hits Found |
Callus and Embryo |
| sbi-MIR396h |
AF466199 |
Putative Receptor Protein Kinase |
Kinase Activity |
No Hits Found |
Serine/Threonine Protein Kinases |
Ovary and Panicle |
| sbi-MIR396h |
CF072738 |
Growth-regulating factor 1 |
Kinase Activity |
No Hits Found |
Serine/Threonine Protein Kinases |
No Hits Found |
| sbi-MIR396h |
CD204209 |
Ankyrin-repeat containing protein |
Protein Binding, Protein Kinase Activity, Protein Self-Association and Ubiquitin-Protein Ligase Activity |
No Hits Found |
Ankyrin repeat proteins |
Leaf |
| sbi-MIR396i |
CD234788 |
Homeodomainleucine zipper protein 16 |
DNA-binding Transcription Factor Activity |
No Hits Found |
No Hits Found |
Callus, Embryo and Shoot |
| sbi-MIR396i |
CF427893 |
Homeodomainleucine zipper protein 16 |
DNA-binding Transcription Factor Activity |
No Hits Found |
No Hits Found |
Callus, Embryo and Shoot |
| sbi-MIR396j |
CD234788 |
Homeodomainleucine zipper protein 16 |
DNA-binding Transcription Factor Activity |
No Hits Found |
No Hits Found |
Callus, Embryo and Shoot |
| sbi-MIR396j |
CX612479 |
Homeodomainleucine zipper protein 16 |
DNA-binding Transcription Factor Activity |
No Hits Found |
No Hits Found |
Callus, Embryo and Shoot |
| sbi-MIR396k |
AW676947 |
Growth-regulating factor 1 |
Protein Binding |
No Hits Found |
No Hits Found |
Root |
| sbi-MIR396k |
AF466199 |
Putative Receptor Protein Kinase |
Kinase Activity |
No Hits Found |
Serine/Threonine Protein Kinases |
Ovary and Panicle |
| sbi-MIR396k |
BM329506 |
Peptidase family protein |
Peptidase Activity |
No Hits Found |
No Hits Found |
Leaf, Pollen and Root |
| sbi-MIR396k |
CD207048 |
C2 domain-containing protein |
Molecular Function Unknown |
No Hits Found |
No Hits Found |
Embryo, Leaf and Shoot |
| sbi-MIR398b |
CF480868 |
UDP-N-acetylglucosaminetransferase subunit ALG14 |
Transferase Activity |
N-Glycan Biosynthesis |
No Hits Found |
Pollen and Root |
| sbi-MIR398b |
CF487358 |
UDP-N-acetylglucosaminetransferase subunit ALG14 |
Transferase Activity |
N-Glycan Biosynthesis |
No Hits Found |
Pollen and Root |
| sbi-MIR398b |
BG158064 |
Ent-kaurene oxidase |
Oxidoreductase activity, |
No Hits Found |
Cytochrome P450 |
No Hits Found |
| sbi-MIR398b |
BM330737 |
Chloroplast 30S ribosomal protein S3 |
Structural Constituent of Ribosome |
Ribosome |
Ribosomal Protein L22 |
Leaf, Ovary and Panicle |
| sbi-MIR444a |
BM323459 |
MADS-box transcription factor 57 |
Transcription Factor Binding |
No Hits Found |
No Hits Found |
No Hits Found |
| sbi-MIR444a |
CD224118 |
Zinc finger (C3HC4-type RING finger) protein-like |
Zinc Ion Binding |
No Hits Found |
No Hits Found |
Callus and Ovary |
| sbi-MIR444a |
CD225619 |
MADS-box transcription factor 57 |
Calcium Ion Binding |
No Hits Found |
No Hits Found |
Callus and Embryo |
| sbi-MIR444a |
CN125113 |
WD-40 repeat family protein |
Signal Transducer Activity |
No Hits Found |
WD40 Repeat Protein |
Embryo, Ovary and Root |
| sbi-MIR444b |
BE596704 |
MADS-box transcription factor 57 |
Transcription Factor Binding |
No Hits Found |
No Hits Found |
No Hits Found |
| sbi-MIR444b |
BM330337 |
Putative far-red impaired response protein |
Zinc Ion Binding |
No Hits Found |
No Hits Found |
Leaf |
| sbi-MIR444b |
AW564049 |
Ferredoxin-sulfite reductase precursor |
Sulfite Reductase Activity |
Sulfur Metabolism |
Sulfite ReductaseHemoprotein Beta-Component |
Callus, Embryo, Leaf and Pollen |
| sbi-MIR444c |
BM323459 |
MADS-box transcription factor 57 |
Transcription Factor Binding |
No Hits Found |
No Hits Found |
No Hits Found |
| sbi-MIR444c | BM325378 | Eukaryotic translation initiation factor 3 subunit A | Translation Initiation Factor Activity | RNA Transport | Chromosome Segregation ATPases | Leaf and Ovary |
Discussion
Large numbers of miRNAs have been predicted in cereal model plant rice. However, in sorghum, only 148 miRNAs were reported till date (miRBase release 17). This suggested that miRNA identification in sorghum is far from saturation. We used ESTs and GSS to predict miRNAs and predicted 31 new miRNAs, in addition to 148 miRNAs in miRBase release 17. We also predicted 72 potential target genes for the newly identified 31 miRNAs. In consistent with earlier studies, members of a miRNA family target same set of genes, suggesting a functional redundancy of the miRNA family members. We mapped newly identified 31 miRNAs and previously known 148 miRNAs on sorghum genome, and found that several MIR genes are arranged in clusters in sorghum genome. Each cluster consists of MIR genes belonging to the same family.
Sorghum crop is highly tolerant to drought and heat stress, and the expansion of members of MIR gene families, specifically miR169 family, was suggested as one of the probable reasons for adaptation of sorghum to abiotic stresses.79 Besides miR169 family, expansion of other miRNA families may also contribute to the better adaptation of sorghum. The miR166 gene family and its role in leaf development are evolutionarily conserved in all land plants. The miR166 family regulates the expression of the HD-ZIP III (class-III homeodomain-leucin zipper) gene family that is necessary for proper specification of leaf polarity, in both Arabidopsis and maize.80 In this study, initially we predicted 26 candidate miRNAs, belong to miR166 family. Later, genome mapping of these miRNAs showed that only nine miRNAs mapped to unique genomic loci. The fact that the copies of Sb-miR166 family member do not vary during the evolution suggests its importance in sorghum. Previous studies also showed that many miRNAs are evolutionarily conserved across animals and plants.85–87 However, some miRNAs are species-specific.88 For instance, miR444 family is present in rice but not in Arabidopsis, suggesting that it might be restricted to monocots.89 Further studies revealed that miR444 family members are conserved only in monocot species (e.g., barley, maize, wheat, sorghum, Brachypodium and sugarcane) but not in dicot species (e.g., Arabidopsis and Populus).8,89,90 Here, we identified three members of miR444 family in sorghum namely miR444a, b and c which were identical to the previously reported miR444c.1, c.2 and d, respectively in rice.91 The precursors of these newly identified miR444a, b and c, had a high minimal folding free energy index of 1.27, 1.27 and 0.64, respectively. This is significantly higher than those reported for tRNAs (0.64), rRNAs (0.59), and mRNAs (0.62–0.66), suggesting that pri-miRNA folding of this family is less stable than that of others. It is previously reported that miR444 family members target MADS-box transcription factors that are involved in number of biological functions including developmental processes namely meristem identity, root development, fruit dehiscence, flowering time94–97 and tolerance to salt and cold stresses.92,93,98 We also observed that all three members of miR444 family target to MADS-box transcription factors (target accession no: BM323459 and BE596704) and other target genes with a role in calcium ion binding, translation initiation factor activity, DNA binding transcription factor, sulfite reducates activity and signal transducer activity. The lack of a miR444 homolog and their conserved target gene (MADS box) in dicot families such as Arabidopsis provided strong evidence that miRNA-mediated regulation of MADS box gene is conserved only in monocots and known as ‘monocot-specific’ family. Further molecular genetic analysis of miR444 family might be helpful to unravel the significance of this monocot-specific family.
EST mining from publically available database could provide evidence for the expression of miRNAs in different tissues. In this study, EST profiles were explored from UniGene database that revealed the expression patterns of miRNAs families in various tissues and at different development stages (Table S1). The differential expression pattern of miRNAs suggests their potential role in development of the respective tissues or process in these tissues. Thus, the newly identified miRNAs and their predicted roles form the basis for understanding their role in sorghum plant development and stress adaptation.
Materials and Methods
Reference sequences for miRNA prediction
To identify potential conserved miRNAs, a total 2,728 previously identified plant miRNA from 33 different plant species were obtained from miRBase database (release 17, April 2011) (www.mirbase.org/).40,41,99 This set include miRNA sequences from Chlamydomonas reinhardtii (50), Pinus taeda (37), Physcomitrella patens (229), Selaginella moellendorffii (58), Arabidopsis thaliana (232), Brassica napus (46), Brassica oleracea (6), Brassica rapa (19), Carica papaya (1), Glycine max (203), Lotus japonicus (3), Medicago truncatula (375), Phaseolus vulgaris (8), Vigna unguiculata (2), Gossypium arboreum (1), Gossypiumherbecium (1), Gossypium hirsutum (34), Gossypium raimondii (4), Aquilegia coerulea (45), Malusdo mestica (1), Citrus clementine (5), Citrus reticulata (4) Citrus sinensis (60), Citrus trifoliata (6), Populus euphratica (5), Populus trichocarpa (234), Solanum lycopersicum (36), Vitis vinifera (163), Brachypodium distachyon (139), Oryza sativa (491), Saccharum officinarum (16), Triticum aestivum (44) and Zea mays (170). After removal of the redundant sequences, 1379 miRNAs were used as reference set.
Sorghum EST, GSS and WGS sequence data set
Sorghum expresses sequence tag (EST), genomic survey sequences (GSS) and whole genome sequence (WGS) were obtained from GenBank nucleotide database available at NCBI (www.ncbi.nlm.nih.gov/). This data set contains 240161 nucleotide sequence from EST and 799,504 nucleotide sequences from GSS (Till January 5, 2010).
Non-coding data set
Non coding data set of mRNA were used to discriminate between miRNA and other structural RNAs (e.g., tRNA, rRNA, snRNA and snoRNA). The BLASTN search was performed against pfam (http://rfam.sanger.ac.uk/) database100 to remove ESTs or GSS having similarity with structural RNAs. The filter for tRNA was also conducted by blast of possible miRNAs precursors against genomic tRNA database (v.2.4.2) (http://gtrnadb.ucsc.edu/blast.html).101 The parameters for BLAST alignment was fixed as Alignment Program: blastn; Expect: 0.01; Word Size; 11; Database All eukaryotic tRNA.The tRNA genomic data set contain tRNA gene sequences from Arabidopsis, soybean and rice. The snRNA and snoRNA sequences from plant kingdom were also retrieved at random basis from NCBI (www.ncbi.nlm.nih.gov) and mapped with predicted miRNA data set to exclude false positive miRNA precursors in sorghum.
Prediction of secondary structure
To make data non-redundant, including EST, GSS and reference miRNA sequence, multiple sequence alignment was performed by using locally installed ClustalX (version 2.0.12) and web based ClustalW61 (version 1.83) (www.genome.jp/tools/clustalw) with default parameters. The unique reference miRNA sequences were mapped on EST and GSS sequence by using an in-house PERL script (www.perl.org) and miRNAs with no mismatch were only retained for further analysis. Flanking region of 250 nt base pair upstream and downstream from miRNA sequence from EST and GSS sorghum sequences were extracted and folded using RNAFold version 1.8.4 from the Vienna RNA package60 (rna.tbi.univie.ac.at/) to find out minimum free energy containing structure. To predict real miRNA precursor triplet-SVM classifier102 program which is based on support vector machine was used (bioinfo.au.tsinghua.edu.cn/mirnasvm/). This software package needs third-party softwares namelyRNAfold and LibSVM packages. The minimal folding free energy Index (MFEI) was calculated using the following equation: MFEI = [(MFE*/length of the RNA sequence)*100]/(G+C) %. *MFE denotes the negative folding free energies (ΔG).
MicroRNAs target genes
The putative target sites of miRNAs were identified by aligning the miRNA sequences either perfectly or near-perfectly binding to complementary sites on their target mRNA sequences64 by using Plant Target Prediction Tool available on UEA sRNA ToolKit78 (srna-tools.cmp.uea.ac.uk/plant/cgi-bin/srna-tools.cgi) and psRNA Target server39 (http://plantgrn.noble.org/psRNATarget/) with default parameters; Maximum expectation: 3.0, length for complementarity scoring (hspsize): 20, Target accessibility-allowed maximum energy to unpair the target site (UPE): 25.0, Flanking length around target site for target accessibility analysis: 17 bp in upstream and 13 bp in downstream, Range of central mismatch leading to translation inhibition: 9–11 nt. The rules used in UEA sRNA Tool Kit for target prediction suggested by Allen et al.103 and Schwab et al.64 were as follows: (1) No more than four mismatches between the small RNA and the target (G-U bases count as 0.5 mismatches); (2) No more than two adjacent mismatches in the miRNA: target duplex; (3) No adjacent mismatches in positions 2–12 of the miRNA: target duplex (5′ of miRNA); (4) No mismatches in positions 10–11 of the miRNA: target duplex; (5) No more than 2.5 mismatches in positions 1–12 of the of the miRNA: target duplex (5′ of miRNA); (6) The minimum free energy (MFE) of the miRNA/target duplex should be ≥ 74% of the MFE of the miRNA bound to its perfect complement.
Functional analysis of target genes
The functional assignment of predicted target genes were annotated by COGnitor program that compare gene sequence against the Clusters of Orthologous Groups of proteins (COG) database104 (version 66) (www.ncbi.nih.gov/COG). AmiGO (version 1.8) (amigo.genontology.org) and KEGG (Kyoto Encyclopedia of Genes and Genomes) (www.genome.jp/kegg)pathway analyses were employed to further investigate the biological processes and corresponding metabolic networks regulated by potential miRNAs. All predicted target genes with an e value of 1e−30 were identified by BLASTX searching program105 against the GO protein and KEGG databases (version 58.0) (Released on April 1, 2011).
Conclusions and Prospective
In this study, we identified 31 new miRNAs in sorghum by analyzing ESTs and GSS. The study revealed that 73 diverse miRNAs (including miRBase, version 17 registered sorghum miRNAs) were arranged into 24 compact clusters on sorghum genome. We also found three members of monocot species-specific MIR444 family, widely involved in regulation of MADS-box transcription factor expression. About 72 potential target genes for 31 individual miRNAs belonging to nine different miRNA families were predicted. We noticed that majority of the predicted target genes were transcription factors, which are involved in the regulation of plant growth and development. The findings from this study will contribute to further understanding the miRNAs function and regulatory mechanisms in sorghum.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Indian Council of Agricultural Research (ICAR) for supporting this work through the ICAR-sponsored Network Project on Transgenics in Crops (NPTC).
Glossary
Abbreviations:
- GSS
genomic survey sequences
- EST
expressed sequenced tag
- WGS
whole genome sequence
- KEGG
Kyoto encyclopedia of genes and genomes
- COG
clusters of orthologous groups of proteins
- GO
gene ontology
- MFEI
minimal folding free energy index
Supplementary Material
Supplementary material may be found at: www.landesbioscience.com/journals/psb/article/18914
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/18914
References
- 1.Prasad PVV, Pisipati SR, Mutava RN, Tuinstra MR. Sensitivity of grain sorghum to high temperature stress during reproductive development. Crop Sci. 2008;48:1911–7. doi: 10.2135/cropsci2008.01.0036. [DOI] [Google Scholar]
- 2.Hattori T, Sonobe K, Araki H, Inanaga S, An P, Morita S. Silicon application by sorghum through the alleviation of stress-induced increase in hydraulic resistance. J Plant Nutr. 2008;31:1482–95. doi: 10.1080/01904160802208477. [DOI] [Google Scholar]
- 3.Mutegi E, Fabrice S, Moses M, Ben K, Bernard R, Caroline M, et al. Ecogeographical distribution of wild, weedy and cultivated Sorghum bicolor (L.) Moench in Kenya: implications for conservation and crop-to-wild gene flow. Genet Resour Crop Evol. 2010;57:243–53. doi: 10.1007/s10722-009-9466-7. [DOI] [Google Scholar]
- 4.Zhang B, Pan X, Stellwag EJ. Identification of soybean microRNAs and their targets. Planta. 2008;229:161–82. doi: 10.1007/s00425-008-0818-x. [DOI] [PubMed] [Google Scholar]
- 5.Zhao T, Li G, Mi S, Li S, Hannon GJ, Wang XJ, et al. A complex system of small RNAs in the unicellular green alga Chlamydomonas reinhardtii. Genes Dev. 2007;21:1190–203. doi: 10.1101/gad.1543507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiraguri A, Itoh R, Kondo N, Nomura Y, Aizawa D, Murai Y, et al. Specific interactions between Dicer-like proteins and HYL1/DRB-family dsRNA-binding proteins in Arabidopsis thaliana. Plant Mol Biol. 2005;57:173–88. doi: 10.1007/s11103-004-6853-5. [DOI] [PubMed] [Google Scholar]
- 7.Lu C, Fedoroff N. A mutation in the Arabidopsis HYL1 gene encoding a dsRNA binding protein affects responses to abscisic acid, auxin, and cytokinin. Plant Cell. 2000;12:2351–66. doi: 10.1105/tpc.12.12.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones-Rhoades MW, Bartel DP, Bartel B. MicroRNAS and their regulatory roles in plants. Annu Rev Plant Biol. 2006;57:19–53. doi: 10.1146/annurev.arplant.57.032905.105218. [DOI] [PubMed] [Google Scholar]
- 9.Chen R, Hu Z, Zhang H. Identification of microRNAs in wild soybean (Glycine soja) J Integr Plant Biol. 2009;51:1071–9. doi: 10.1111/j.1744-7909.2009.00887.x. [DOI] [PubMed] [Google Scholar]
- 10.Sunkar R, Chinnusamy V, Zhu J, Zhu JK. Small RNAs as big players in plant abiotic stress responses and nutrient deprivation. Trends Plant Sci. 2007;12:301–9. doi: 10.1016/j.tplants.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Voinnet O. Origin, biogenesis, and activity of plant microRNAs. Cell. 2009;136:669–87. doi: 10.1016/j.cell.2009.01.046. [DOI] [PubMed] [Google Scholar]
- 12.Rhoades MW, Reinhart BJ, Lim LP, Burge CB, Bartel B, Bartel DP. Prediction of plant microRNA targets. Cell. 2002;110:513–20. doi: 10.1016/S0092-8674(02)00863-2. [DOI] [PubMed] [Google Scholar]
- 13.Jones-Rhoades MW, Bartel DP. Computational identification of plant microRNAs and their targets, including a stress-induced miRNA. Mol Cell. 2004;14:787–99. doi: 10.1016/j.molcel.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 14.Bonnet E, Wuyts J, Rouze´ P, Van de Peer Y. Evidence that microRNA precursors, unlike other non-coding RNAs, have lower folding free energies than random sequences. Bioinformatics. 2004;20:2911–7. doi: 10.1093/bioinformatics/bth374. [DOI] [PubMed] [Google Scholar]
- 15.Park W, Li J, Song R, Messing J, Chen X. CARPEL FACTORY, a Dicer homolog, and HEN1, a novel protein, act in microRNA metabolism in Arabidopsis thaliana. Curr Biol. 2002;12:1484–95. doi: 10.1016/S0960-9822(02)01017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reinhart BJ, Weinstein EG, Rhoades MW, Bartel B, Bartel DP. MicroRNAs in plants. Genes Dev. 2002;16:1616–26. doi: 10.1101/gad.1004402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sunkar R, Zhu J-K. Novel and stress-regulated microRNAs and other small RNAs from Arabidopsis. Plant Cell. 2004;16:2001–19. doi: 10.1105/tpc.104.022830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–8. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 19.Lau NC, Lim LP, Weinstein EG, Bartel DP. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science. 2001;294:858–62. doi: 10.1126/science.1065062. [DOI] [PubMed] [Google Scholar]
- 20.Lee RC, Ambros V. An extensive class of small RNAs in Caenorhabditis elegans. Science. 2001;294:862–4. doi: 10.1126/science.1065329. [DOI] [PubMed] [Google Scholar]
- 21.Mourelatos Z, Dostie J, Paushkin S, Sharma A, Charroux B, Abel L, et al. miRNPs: a novel class of ribonucleoproteins containing numerous microRNAs. Genes Dev. 2002;16:720–8. doi: 10.1101/gad.974702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoon S, De Micheli G. Computational identification of microRNAs and their targets. Birth Defects Res C Embryo Today. 2006;78:118–28. doi: 10.1002/bdrc.20067. [DOI] [PubMed] [Google Scholar]
- 23.Adai A, Johnson C, Mlotshwa S, Archer-Evans S, Manocha V, Vance V, et al. Computational prediction of miRNAs in Arabidopsis thaliana. Genome Res. 2005;15:78–91. doi: 10.1101/gr.2908205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Li W, Jin YX. Computational identification of novel family members of microRNA genes in Arabidopsis thaliana and Oryza sativa. Acta Biochim Biophys Sin (Shanghai) 2005;37:75–87. doi: 10.1093/abbs/37.2.75. [DOI] [PubMed] [Google Scholar]
- 25.Zhang BH, Pan XP, Anderson TA. Identification of 188 conserved maize microRNAs and their targets. FEBS Lett. 2006;580:3753–62. doi: 10.1016/j.febslet.2006.05.063. [DOI] [PubMed] [Google Scholar]
- 26.Zhang BH, Wang QL, Wang KB, Pan XP, Liu F, Guo TL, et al. Identification of cotton microRNAs and their targets. Gene. 2007;397:26–37. doi: 10.1016/j.gene.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 27.Zhou ZS, Huang SQ, Yang ZM. Bioinformatic identification and expression analysis of new microRNAs from Medicago truncatula. Biochem Biophys Res Commun. 2008;374:538–42. doi: 10.1016/j.bbrc.2008.07.083. [DOI] [PubMed] [Google Scholar]
- 28.Song C, Jia Q, Fang J, Li F, Wang C, Zhang Z. Computational identification of citrus microRNAs and target analysis in citrus expressed sequence tags. Plant Biol (Stuttg) 2010;12:927–34. doi: 10.1111/j.1438-8677.2009.00300.x. [DOI] [PubMed] [Google Scholar]
- 29.Xie FL, Huang SQ, Guo K, Xiang AL, Zhu YY, Nie L, et al. Computational identification of novel microRNAs and targets in Brassica napus. FEBS Lett. 2007;581:1464–74. doi: 10.1016/j.febslet.2007.02.074. [DOI] [PubMed] [Google Scholar]
- 30.Jin WB, Li NN, Zhang B, Wu FL, Li WJ, Guo AG, et al. Identification and verification of microRNA in wheat (Triticum aestivum) J Plant Res. 2008;121:351–5. doi: 10.1007/s10265-007-0139-3. [DOI] [PubMed] [Google Scholar]
- 31.Zhang WW, Luo YP, Gong X, Zeng WH, Li SG. Computational identification of 48 potato microRNAs and their targets. Comput Biol Chem. 2009;33:84–93. doi: 10.1016/j.compbiolchem.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 32.Yin Z, Li C, Han X, Shen F. Identification of conserved microRNAs and their target genes in tomato (Lycopersicon esculentum) Gene. 2008;414:60–6. doi: 10.1016/j.gene.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Xie F, Frazier TP, Zhang B. Identification and characterization of microRNAs and their targets in the bioenergy plant switchgrass (Panicum virgatum) Planta. 2010;232:417–34. doi: 10.1007/s00425-010-1182-1. [DOI] [PubMed] [Google Scholar]
- 34.JiangFeng D, YongJun W, XiaoFeng F, JunXia C, Liang Z, ShiHeng T. Prediction of sorghum miRNAs and their targets with computationalMethods. Chin Sci Bull. 2010;55:1263–70. [Google Scholar]
- 35.Zhang BH, Pan XP, Wang QL, Cobb GP, Anderson TA. Identification and characterization of new plant microRNAs using EST analysis. Cell Res. 2005;15:336–60. doi: 10.1038/sj.cr.7290302. [DOI] [PubMed] [Google Scholar]
- 36.Pan XP, Zhang BH, San Francisco M, Cobb GP. Characterizing viral microRNAs and its application on identifying new microRNAs in viruses. J Cell Physiol. 2007;211:10–8. doi: 10.1002/jcp.20920. [DOI] [PubMed] [Google Scholar]
- 37.Adams MD, Kelley JM, Gocayne JD, Dubnick M, Polymeropoulos MH, Xiao H, et al. Complementary DNA sequencing: expressed sequence tags and human genome project. Science. 1991;252:1651–6. doi: 10.1126/science.2047873. [DOI] [PubMed] [Google Scholar]
- 38.Matukumalli LK, Grefenstette JJ, Sonstegard TS, Van Tassell CP. EST-PAGE–managing and analyzing EST data. Bioinformatics. 2004;20:286–8. doi: 10.1093/bioinformatics/btg411. [DOI] [PubMed] [Google Scholar]
- 39.Dai X, Zhao PX. psRNATarget: a plant small RNA target analysis server. Nucleic Acids Res. 2011;39(Web Server issue):W155-9. doi: 10.1093/nar/gkr319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Griffiths-Jones S. The microRNA Registry. Nucleic Acids Res. 2004;32(Database issue):D109–11. doi: 10.1093/nar/gkh023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34(Database issue):D140–4. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie Z, Allen E, Fahlgren N, Calamar A, Givan SA, Carrington JC. Expression of Arabidopsis MIRNA genes. Plant Physiol. 2005;138:2145–54. doi: 10.1104/pp.105.062943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu C, Kulkarni K, Souret FF, MuthuValliappan R, Tej SS, Poethig RS, et al. MicroRNAs and other small RNAs enriched in the Arabidopsis RNA-dependent RNA polymerase-2 mutant. Genome Res. 2006;16:1276–88. doi: 10.1101/gr.5530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajagopalan R, Vaucheret H, Trejo J, Bartel DP. A diverse and evolutionarily fluid set of microRNAs in Arabidopsis thaliana. Genes Dev. 2006;20:3407–25. doi: 10.1101/gad.1476406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moldovan D, Spriggs A, Yang J, Pogson BJ, Dennis ES, Wilson IW. Hypoxia-responsive microRNAs and trans-acting small interfering RNAs in Arabidopsis. J Exp Bot. 2010;61:165–77. doi: 10.1093/jxb/erp296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu QH, Spriggs A, Matthew L, Fan L, Kennedy G, Gubler F, et al. A diverse set of microRNAs and microRNA-like small RNAs in developing rice grains. Genome Res. 2008;18:1456–65. doi: 10.1101/gr.075572.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merchan F, Boualem A, Crespi M, Frugier F. Plant polycistronic precursors containing non-homologous microRNAs target transcripts encoding functionally related proteins. Genome Biol. 2009;10:R136. doi: 10.1186/gb-2009-10-12-r136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu L, Zhang Q, Zhou H, Ni F, Wu X, Qi Y. Rice MicroRNA effector complexes and targets. Plant Cell. 2009;21:3421–35. doi: 10.1105/tpc.109.070938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xue LJ, Zhang JJ, Xue HW. Characterization and expression profiles of miRNAs in rice seeds. Nucleic Acids Res. 2009;37:916–30. doi: 10.1093/nar/gkn998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu S, Sun YH, Chiang VL. Stress-responsive microRNAs in Populus. Plant J. 2008;55:131–51. doi: 10.1111/j.1365-313X.2008.03497.x. [DOI] [PubMed] [Google Scholar]
- 51.Lu S, Sun YH, Shi R, Clark C, Li L, Chiang VL. Novel and mechanical stress-responsive MicroRNAs in Populus trichocarpa that are absent from Arabidopsis. Plant Cell. 2005;17:2186–203. doi: 10.1105/tpc.105.033456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang L, Chia JM, Kumari S, Stein JC, Liu Z, Narechania A, et al. A genome-wide characterization of microRNA genes in maize. PLoS Genet. 2009;5:e1000716. doi: 10.1371/journal.pgen.1000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meyers BC, Axtell MJ, Bartel B, Bartel DP, Baulcombe D, Bowman JL, et al. Criteria for annotation of plant MicroRNAs. Plant Cell. 2008;20:3186–90. doi: 10.1105/tpc.108.064311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Axtell MJ, Bartel DP. Antiquity of microRNAs and their targets in land plants. Plant Cell. 2005;17:1658–73. doi: 10.1105/tpc.105.032185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fahlgren N, Howell MD, Kasschau KD, Chapman EJ, Sullivan CM, Cumbie JS, et al. High-throughput sequencing of Arabidopsis microRNAs: evidence for frequent birth and death of MIRNA genes. PLoS One. 2007;2:e219. doi: 10.1371/journal.pone.0000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang B, Pan X, Cannon CH, Cobb GP, Anderson TA. Conservation and divergence of plant microRNA genes. Plant J. 2006;46:243–59. doi: 10.1111/j.1365-313X.2006.02697.x. [DOI] [PubMed] [Google Scholar]
- 57.Bonnet E, Wuyts J, Rouze´ P, Van de Peer Y. Detection of 91 potential conserved plant microRNAs in Arabidopsis thaliana and Oryza sativa identifies important target genes. Proc Natl Acad Sci U S A. 2004;101:11511–6. doi: 10.1073/pnas.0404025101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ambros V, Bartel B, Bartel DP, Burge CB, Carrington JC, Chen X, et al. A uniform system for microRNA annotation. RNA. 2003;9:277–9. doi: 10.1261/rna.2183803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zuker M, Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981;9:133–48. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hofacker IL. Vienna RNA secondary structure server. Nucleic Acids Res. 2003;31:3429–31. doi: 10.1093/nar/gkg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–80. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang X, Zhang J, Li F, Gu J, He T, Zhang X, et al. MicroRNA identification based on sequence and structure alignment. Bioinformatics. 2005;21:3610–4. doi: 10.1093/bioinformatics/bti562. [DOI] [PubMed] [Google Scholar]
- 63.Jiang P, Wu H, Wang W, Ma W, Sun X, Lu Z. MiPred: classification of real and pseudo microRNA precursors using random forest prediction model with combined features. Nucleic Acids Res. 2007;35(Web Server issue):W339-44. doi: 10.1093/nar/gkm368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schwab R, Palatnik JF, Riester M, Schommer C, Schmid M, Weigel D. Specific effects of microRNAs on the plant transcriptome. Dev Cell. 2005;8:517–27. doi: 10.1016/j.devcel.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 65.Zhang BH, Pan XP, Cox SB, Cobb GP, Anderson TA. Evidence that miRNAs are different from other RNAs. Cell Mol Life Sci. 2006;63:246–54. doi: 10.1007/s00018-005-5467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee Y, Jeon K, Lee JT, Kim S, Kim VN. MicroRNA maturation: stepwise processing and subcellular localization. EMBO J. 2002;21:4663–70. doi: 10.1093/emboj/cdf476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lagos-Quintana M, Rauhut R, Meyer J, Borkhardt A, Tuschl T. New microRNAs from mouse and human. RNA. 2003;9:175–9. doi: 10.1261/rna.2146903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guddeti S, Zhang DC, Li AL, Leseberg CH, Kang H, Li XG, et al. Molecular evolution of the rice miR395 gene family. Cell Res. 2005;15:631–8. doi: 10.1038/sj.cr.7290333. [DOI] [PubMed] [Google Scholar]
- 69.Tanzer A, Amemiya CT, Kim CB, Stadler PF. Evolution of microRNAs located within Hox gene clusters. J Exp Zool B Mol Dev Evol. 2005;304:75–85. doi: 10.1002/jez.b.21021. [DOI] [PubMed] [Google Scholar]
- 70.Maher C, Stein L, Ware D. Evolution of Arabidopsis microRNA families through duplication events. Genome Res. 2006;16:510–9. doi: 10.1101/gr.4680506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bompfünewerer AF, Flamm C, Fried C, Fritzsch G, Hofacker IL, Lehmann J, et al. Evolutionary patterns of non-coding RNAs. Theory Biosci. 2005;123:301–69. doi: 10.1016/j.thbio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 72.Weber MJ. New human and mouse microRNA genes found by homology search. FEBS J. 2005;272:59–73. doi: 10.1111/j.1432-1033.2004.04389.x. [DOI] [PubMed] [Google Scholar]
- 73.Tanzer A, Stadler PF. Molecular evolution of a microRNA cluster. J Mol Biol. 2004;339:327–35. doi: 10.1016/j.jmb.2004.03.065. [DOI] [PubMed] [Google Scholar]
- 74.Talmor-Neiman M, Stav R, Frank W, Voss B, Arazi T. Novel micro-RNAs and intermediates of micro-RNA biogenesis from moss. Plant J. 2006;47:25–37. doi: 10.1111/j.1365-313X.2006.02768.x. [DOI] [PubMed] [Google Scholar]
- 75.Altuvia Y, Landgraf P, Lithwick G, Elefant N, Pfeffer S, Aravin A, et al. Clustering and conservation patterns of human microRNAs. Nucleic Acids Res. 2005;33:2697–706. doi: 10.1093/nar/gki567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maher C, Timmermans MC, Stein L, Ware D. Identifying microRNAs in plant genomes. Proc IEEE CSB 2004; 718–723. [Google Scholar]
- 77.Wang S, Zhu QH, Guo X, Gui Y, Bao J, Helliwell C, et al. Molecular evolution and selection of a gene encoding two tandem microRNAs in rice. FEBS Lett. 2007;581:4789–93. doi: 10.1016/j.febslet.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 78.Moxon S, Schwach F, Dalmay T, Maclean D, Studholme DJ, Moulton V. A toolkit for analysing large-scale plant small RNA datasets. Bioinformatics. 2008;24:2252–3. doi: 10.1093/bioinformatics/btn428. [DOI] [PubMed] [Google Scholar]
- 79.Paterson AH, Bowers JE, Bruggmann R, Dubchak I, Grimwood J, Gundlach H, et al. The Sorghum bicolor genome and the diversification of grasses. Nature. 2009;457:551–6. doi: 10.1038/nature07723. [DOI] [PubMed] [Google Scholar]
- 80.Juarez MT, Kui JS, Thomas J, Heller BA, Timmermans MC. microRNA-mediated repression of rolled leaf1 specifies maize leaf polarity. Nature. 2004;428:84–8. doi: 10.1038/nature02363. [DOI] [PubMed] [Google Scholar]
- 81.Floyd SK, Bowman JL. Gene regulation: ancient microRNA target sequences in plants. Nature. 2004;428:485–6. doi: 10.1038/428485a. [DOI] [PubMed] [Google Scholar]
- 82.Mallory AC, Reinhart BJ, Jones-Rhoades MW, Tang G, Zamore PD, Barton MK, et al. MicroRNA control of PHABULOSA in leaf development: importance of pairing to the microRNA 5′ region. EMBO J. 2004;23:3356–64. doi: 10.1038/sj.emboj.7600340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vaucheret H, Vazquez F, Cre´te´ P, Bartel DP. The action of ARGONAUTE1 in the miRNA pathway and its regulation by the miRNA pathway are crucial for plant development. Genes Dev. 2004;18:1187–97. doi: 10.1101/gad.1201404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guo HS, Xie Q, Fei JF, Chua NH. MicroRNA directs mRNA cleavage of the transcription factor NAC1 to downregulate auxin signals for arabidopsis lateral root development. Plant Cell. 2005;17:1376–86. doi: 10.1105/tpc.105.030841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–5. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 86.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 87.Pasquinelli AE, Reinhart BJ, Slack F, Martindale MQ, Kuroda MI, Maller B, et al. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature. 2000;408:86–9. doi: 10.1038/35040556. [DOI] [PubMed] [Google Scholar]
- 88.Ambros V, Lee RC, Lavanway A, Williams PT, Jewell D. MicroRNAs and other tiny endogenous RNAs in C. elegans. Curr Biol. 2003;13:807–18. doi: 10.1016/S0960-9822(03)00287-2. [DOI] [PubMed] [Google Scholar]
- 89.Sunkar R, Girke T, Zhu JK. Identification and characterization of endogenous small interfering RNAs from rice. Nucleic Acids Res. 2005;33:4443–54. doi: 10.1093/nar/gki758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sunkar R, Girke T, Jain PK, Zhu JK. Cloning and characterization of microRNAs from rice. Plant Cell. 2005;17:1397–411. doi: 10.1105/tpc.105.031682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sunkar R, Zhou X, Zheng Y, Zhang W, Zhu J-K. Identification of novel and candidate miRNAs in rice by high throughput sequencing. BMC Plant Biol. 2008;8:25. doi: 10.1186/1471-2229-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kuo MH, Nadeau ET, Grayhack EJ. Multiple phosphorylated forms of the Saccharomyces cerevisiae Mcm1 protein include an isoform induced in response to high salt concentrations. Mol Cell Biol. 1997;17:819–32. doi: 10.1128/mcb.17.2.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lozano R, Angosto T, Gomez P, Payan C, Capel J, Huijser P, et al. Tomato flower abnormalities induced by low temperatures are associated with changes of expression of MADS-Box genes. Plant Physiol. 1998;117:91–100. doi: 10.1104/pp.117.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Coen ES, Meyerowitz EM. The war of the whorls: genetic interactions controlling flower development. Nature. 1991;353:31–7. doi: 10.1038/353031a0. [DOI] [PubMed] [Google Scholar]
- 95.Weigel D, Meyerowitz EM. The ABCs of floral homeotic genes. Cell. 1994;78:203–9. doi: 10.1016/0092-8674(94)90291-7. [DOI] [PubMed] [Google Scholar]
- 96.Riechmann JL, Meyerowitz EM. MADS domain proteins in plant development. Biol Chem. 1997;378:1079–101. [PubMed] [Google Scholar]
- 97.Theissen G, Becker A, Di Rosa A, Kanno A, Kim JT, Münster T, et al. A short history of MADS-box genes in plants. Plant Mol Biol. 2000;42:115–49. doi: 10.1023/A:1006332105728. [DOI] [PubMed] [Google Scholar]
- 98.Ding Y, Chen Z, Zhu C. Microarray-based analysis of cadmium-responsive microRNAs in rice (Oryza sativa) J Exp Bot. 2011;62:3563–73. doi: 10.1093/jxb/err046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res. 2008;36(Database issue):D154–8. doi: 10.1093/nar/gkm952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gardner PP, Daub J, Tate J, Moore BL, Osuch IH, Griffiths-Jones S, et al. Rfam: Wikipedia, clans and the “decimal” release. Nucleic Acids Res. 2011;39(Database issue):D141–5. doi: 10.1093/nar/gkq1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chan PP, Lowe TM. GtRNAdb: a database of transfer RNA genes detected in genomic sequence. Nucleic Acids Res. 2009;37(Database issue):D93–7. doi: 10.1093/nar/gkn787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xue C, Li F, He T, Liu GP, Li Y, Zhang X. Classification of real and pseudo microRNA precursors using local structure-sequence features and support vector machine. BMC Bioinformatics. 2005;6:310. doi: 10.1186/1471-2105-6-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Allen E, Xie Z, Gustafson AM, Carrington JC. microRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell. 2005;121:207–21. doi: 10.1016/j.cell.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 104.Tatusov RL, Natale DA, Garkavtsev IV, Tatusova TA, Shankavaram UT, Rao BS, et al. The COG database: new developments in phylogenetic classification of proteins from complete genomes. Nucleic Acids Res. 2001;29:22–8. doi: 10.1093/nar/29.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.