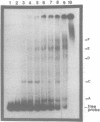
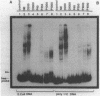
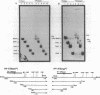
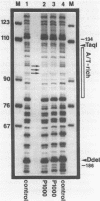
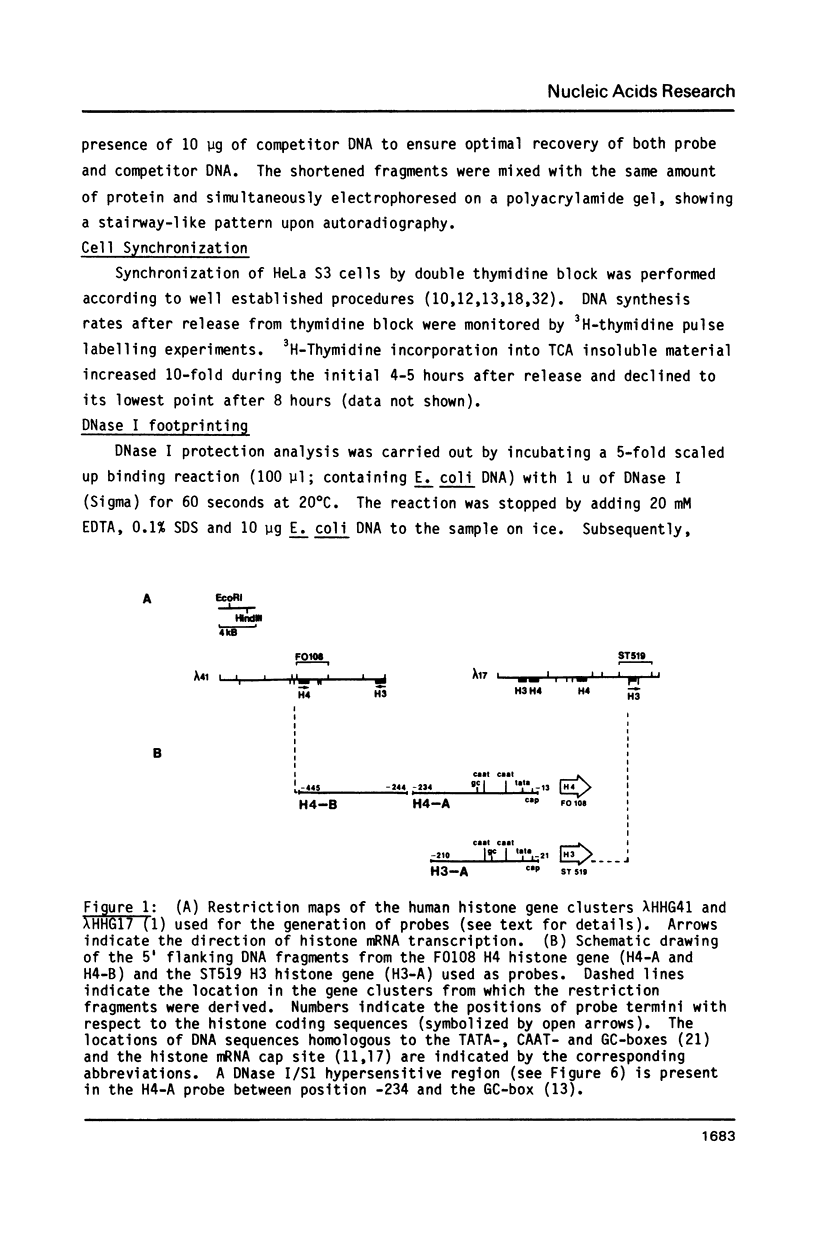
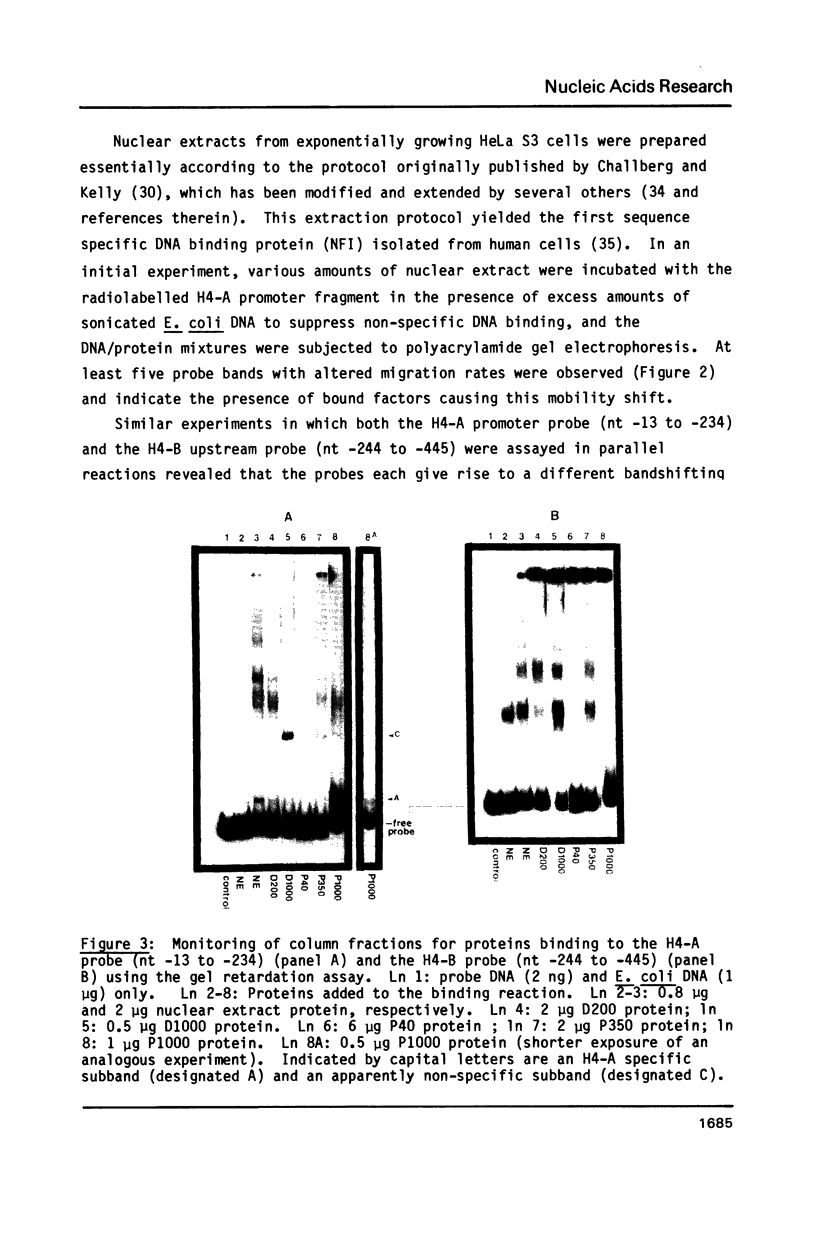
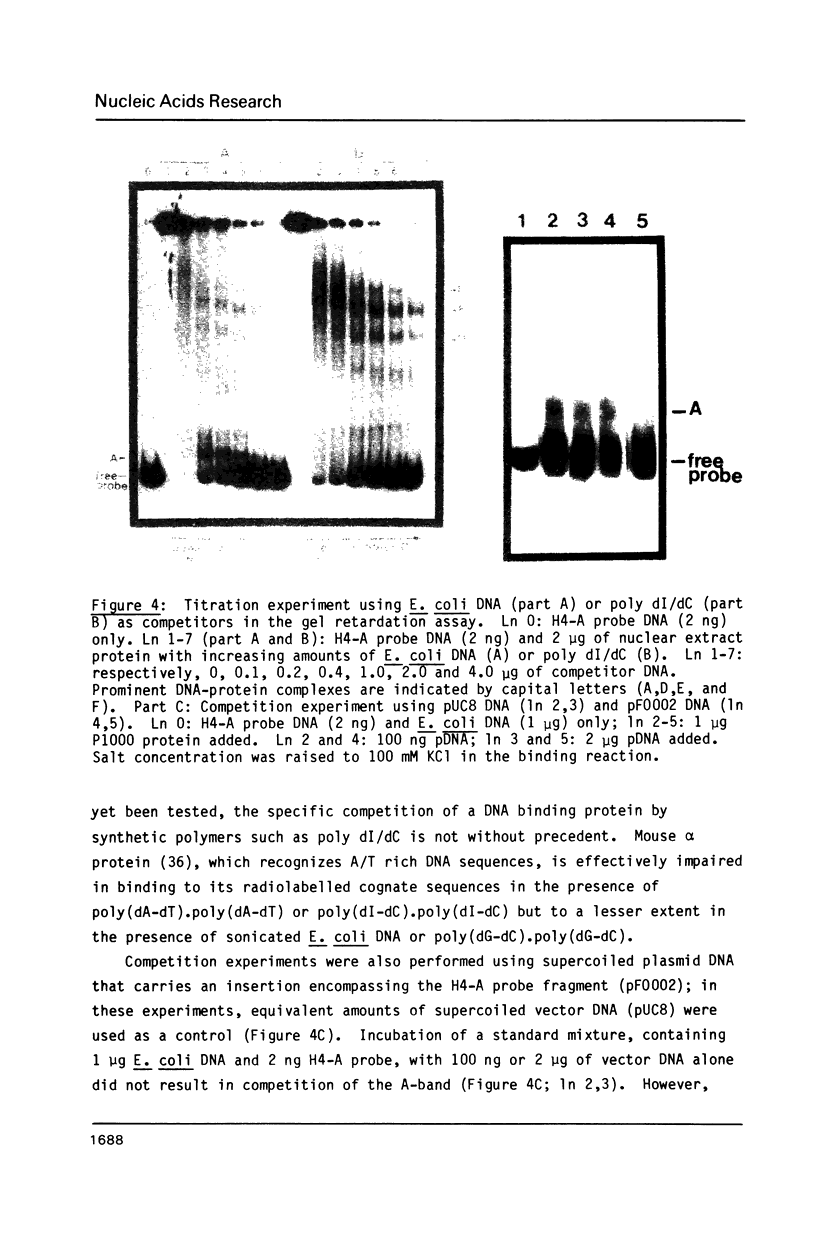
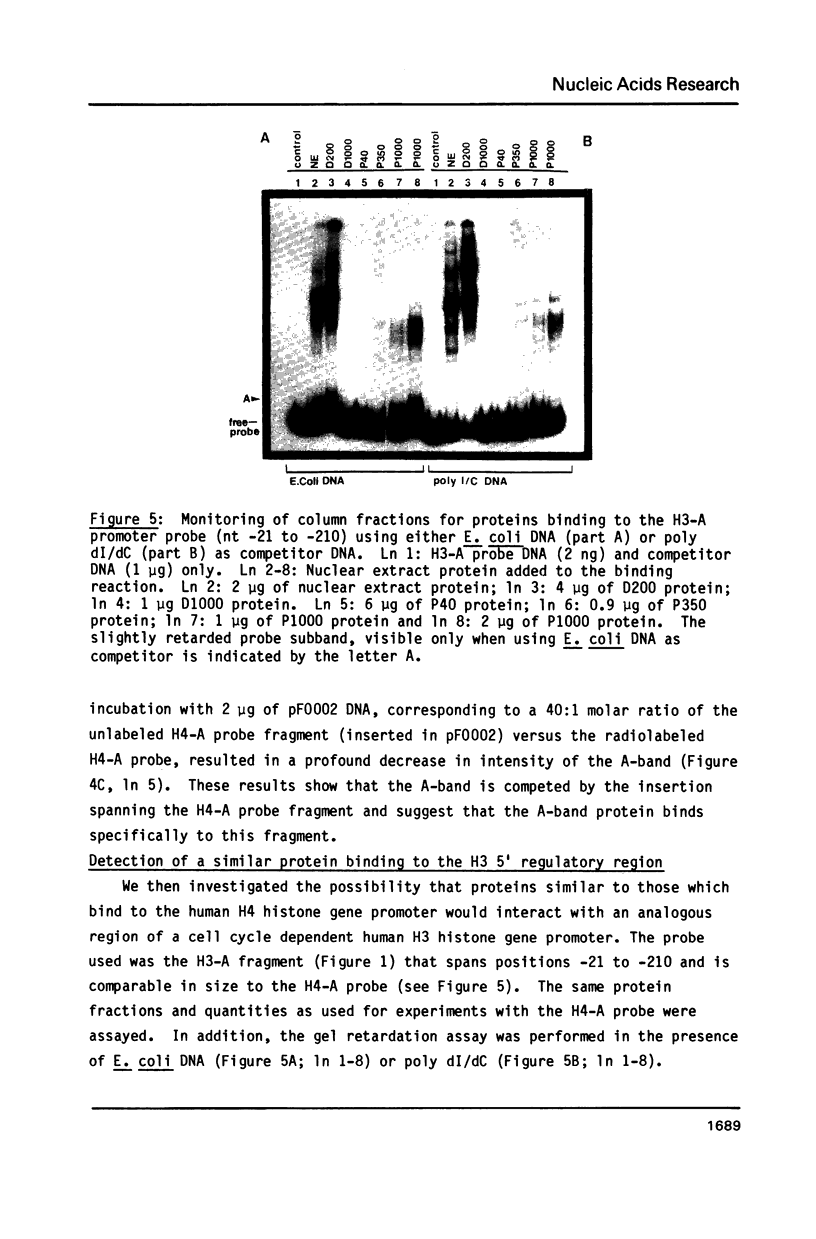
Abstract
A nuclear protein with affinity for the 5' flanking region of a cell cycle dependent human H4 histone gene has been partially purified from nuclear extracts of human HeLa S3 cells. The region involved in the binding of the protein has been localized to an upstream DNA segment using an electrophoretic mobility shift assay. This DNA segment is devoid of RNA polymerase II consensus sequences and contains both homopurinic and A/T rich tracts. Analogous experiments have identified a similar, and perhaps identical, factor that has affinity for a cell cycle dependent human H3 histone gene promoter. This protein appears to bind to a DNA segment containing A/T rich sequences that bear homology with the binding region of the H4 histone promoter. Cell synchronization experiments have shown that the overall affinity of the protein(s) for the H3 and H4 histone 5' flanking regions in vitro is not dramatically altered during the cell cycle. Although the rate of histone gene transcription is modulated during early S phase, transcription occurs throughout the cell cycle. Hence, the protein(s) we have detected here may play a role in the basal expression of these genes.
Full text
PDF







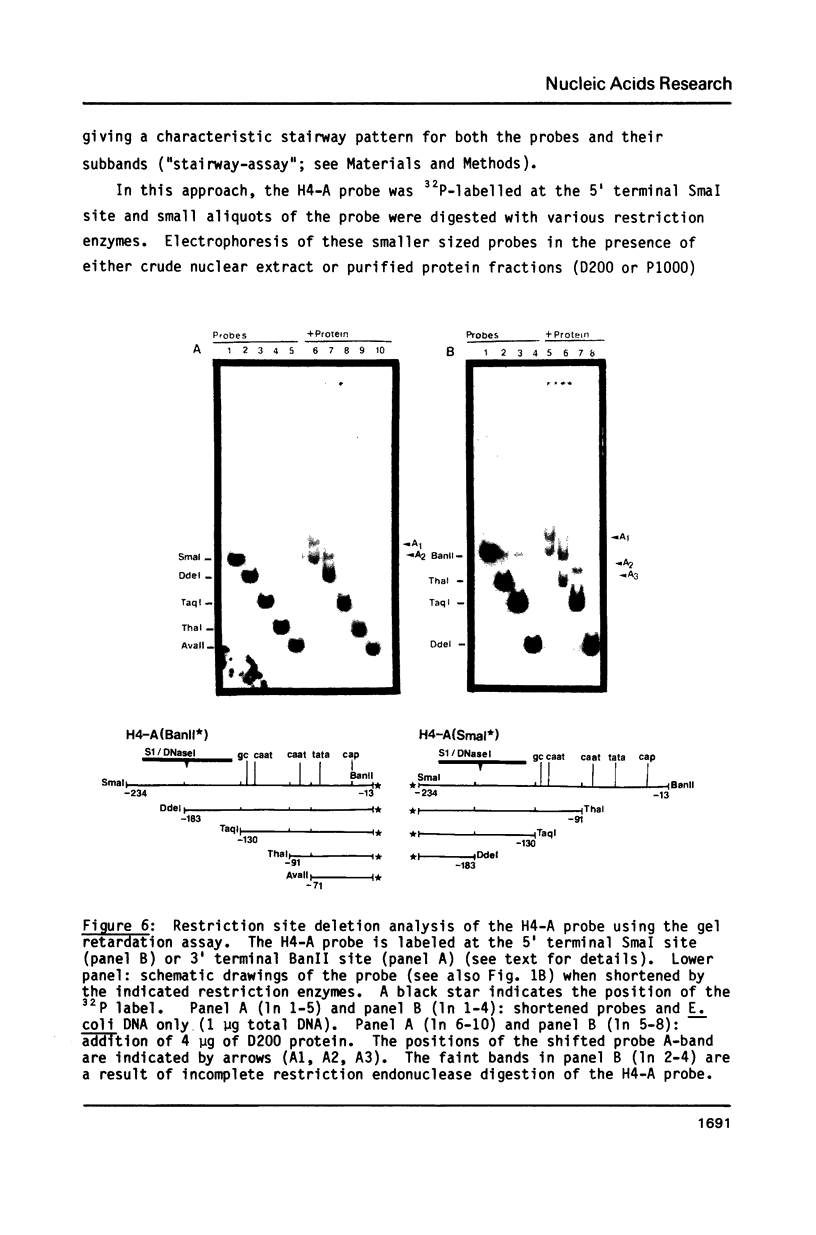

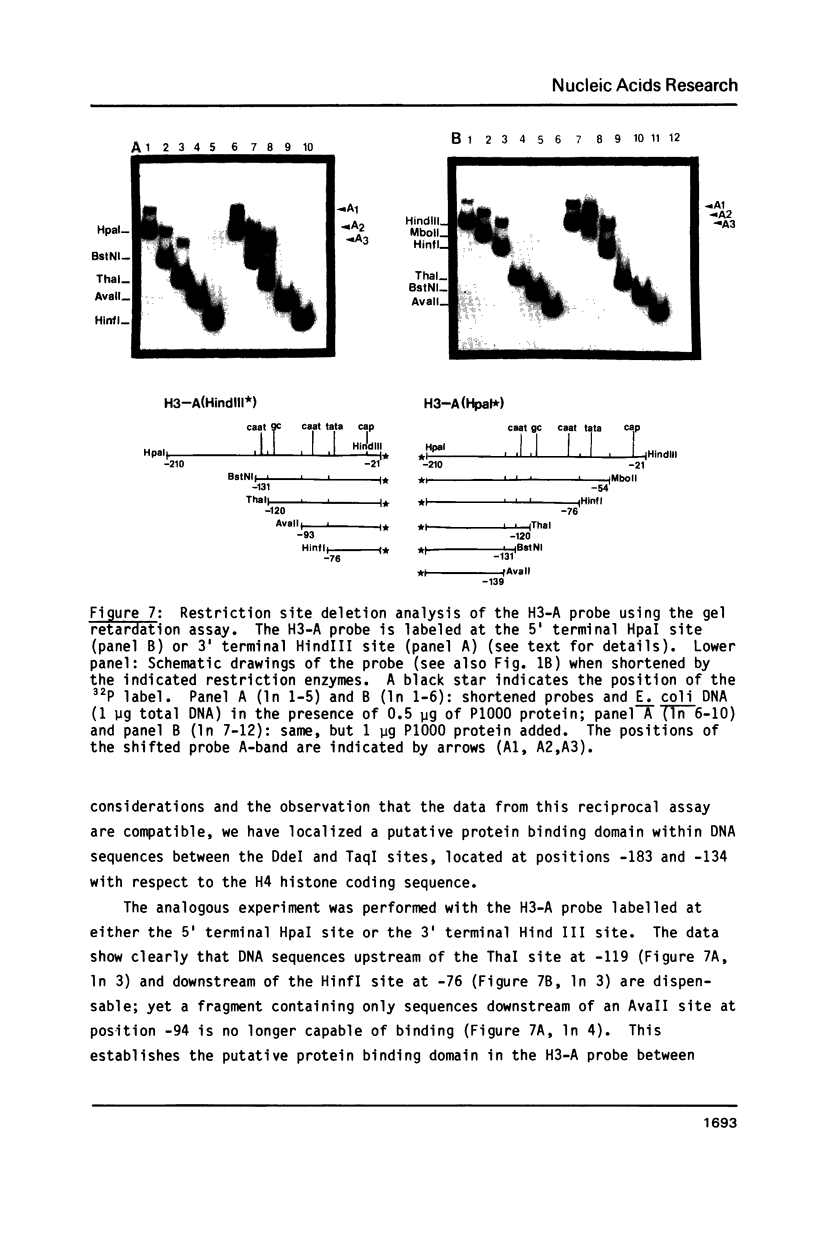

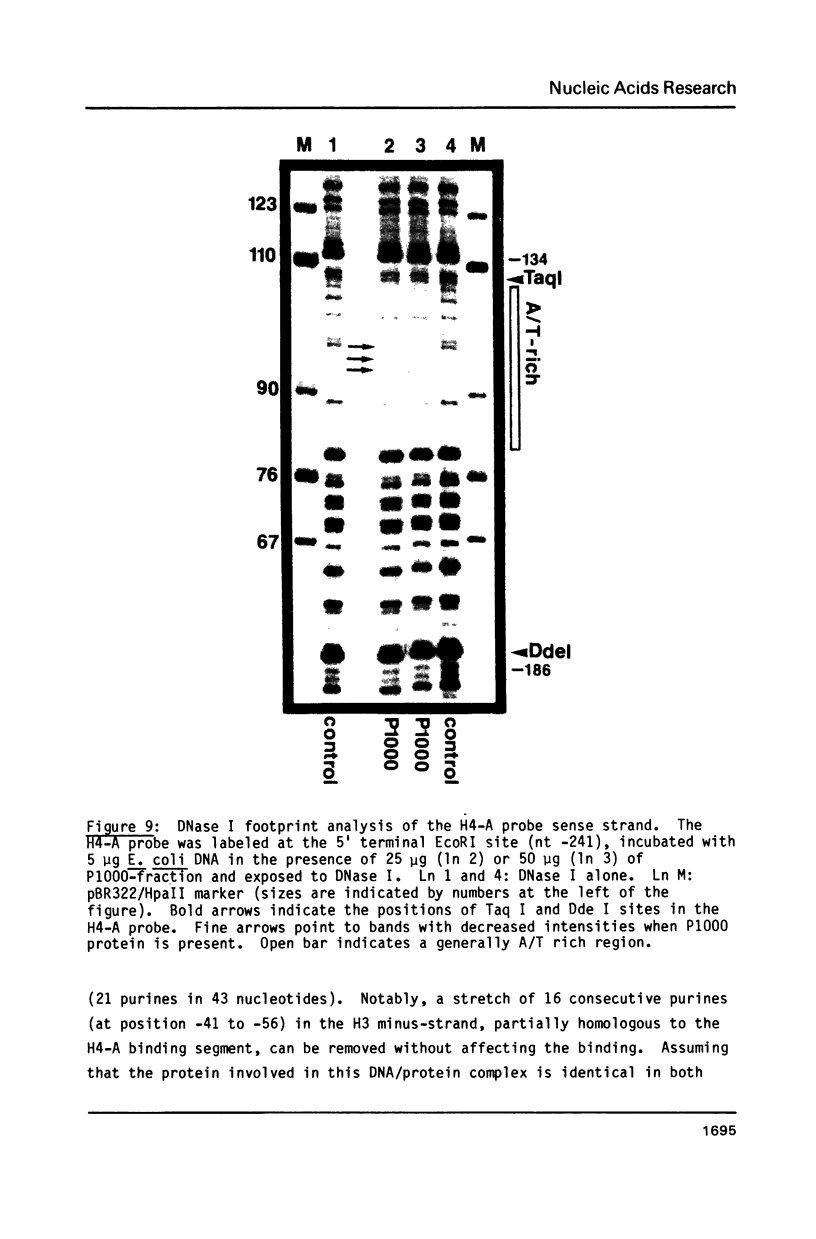



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Capasso O., Heintz N. Regulated expression of mammalian histone H4 genes in vivo requires a trans-acting transcription factor. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5622–5626. doi: 10.1073/pnas.82.17.5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carozzi N., Marashi F., Plumb M., Zimmerman S., Zimmerman A., Coles L. S., Wells J. R., Stein G., Stein J. Clustering of human H1 and core histone genes. Science. 1984 Jun 8;224(4653):1115–1117. doi: 10.1126/science.6719136. [DOI] [PubMed] [Google Scholar]
- Carthew R. W., Chodosh L. A., Sharp P. A. An RNA polymerase II transcription factor binds to an upstream element in the adenovirus major late promoter. Cell. 1985 Dec;43(2 Pt 1):439–448. doi: 10.1016/0092-8674(85)90174-6. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J., Jr Adenovirus DNA replication in vitro. Proc Natl Acad Sci U S A. 1979 Feb;76(2):655–659. doi: 10.1073/pnas.76.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrysogelos S., Riley D. E., Stein G., Stein J. A human histone H4 gene exhibits cell cycle-dependent changes in chromatin structure that correlate with its expression. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7535–7539. doi: 10.1073/pnas.82.22.7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. 1985 Aug 29-Sep 4Nature. 316(6031):774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Isolation of transcription factors that discriminate between different promoters recognized by RNA polymerase II. Cell. 1983 Mar;32(3):669–680. doi: 10.1016/0092-8674(83)90053-3. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L., Schlaffer I., Wright K., Moreno M. L., Berand D., Hager G., Stein J., Stein G. Cell cycle-dependent expression of a stable episomal human histone gene in a mouse cell. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2315–2319. doi: 10.1073/pnas.83.8.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L., Van Antwerpen R., Stein J., Stein G., Tripputi P., Emanuel B., Selden J., Croce C. A major human histone gene cluster on the long arm of chromosome 1. Science. 1984 Nov 16;226(4676):838–840. doi: 10.1126/science.6494913. [DOI] [PubMed] [Google Scholar]
- Hanly S. M., Bleecker G. C., Heintz N. Identification of promoter elements necessary for transcriptional regulation of a human histone H4 gene in vitro. Mol Cell Biol. 1985 Feb;5(2):380–389. doi: 10.1128/mcb.5.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz N., Roeder R. G. Transcription of human histone genes in extracts from synchronized HeLa cells. Proc Natl Acad Sci U S A. 1984 May;81(9):2713–2717. doi: 10.1073/pnas.81.9.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz N., Zernik M., Roeder R. G. The structure of the human histone genes: clustered but not tandemly repeated. Cell. 1981 Jun;24(3):661–668. doi: 10.1016/0092-8674(81)90092-1. [DOI] [PubMed] [Google Scholar]
- Hromas R., Van Ness B. Nuclear factors bind to regulatory regions of the mouse kappa immunoglobulin gene. Nucleic Acids Res. 1986 Jun 25;14(12):4837–4848. doi: 10.1093/nar/14.12.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leegwater P. A., van Driel W., van der Vliet P. C. Recognition site of nuclear factor I, a sequence-specific DNA-binding protein from HeLa cells that stimulates adenovirus DNA replication. EMBO J. 1985 Jun;4(6):1515–1521. doi: 10.1002/j.1460-2075.1985.tb03811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leegwater P. A., van der Vliet P. C., Rupp R. A., Nowock J., Sippel A. E. Functional homology between the sequence-specific DNA-binding proteins nuclear factor I from HeLa cells and the TGGCA protein from chicken liver. EMBO J. 1986 Feb;5(2):381–386. doi: 10.1002/j.1460-2075.1986.tb04223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marashi F., Helms S., Shiels A., Silverstein S., Greenspan D. S., Stein G., Stein J. Enhancer-facilitated expression of prokaryotic and eukaryotic genes using human histone gene 5' regulatory sequences. Biochem Cell Biol. 1986 Apr;64(4):277–289. doi: 10.1139/o86-039. [DOI] [PubMed] [Google Scholar]
- Moreno M. L., Chrysogelos S. A., Stein G. S., Stein J. L. Reversible changes in the nucleosomal organization of a human H4 histone gene during the cell cycle. Biochemistry. 1986 Sep 23;25(19):5364–5370. doi: 10.1021/bi00367a003. [DOI] [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Enomoto T., Lichy J. H., Hurwitz J. Adenovirus DNA replication in vitro: identification of a host factor that stimulates synthesis of the preterminal protein-dCMP complex. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6438–6442. doi: 10.1073/pnas.79.21.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumb M., Stein J., Stein G. Coordinate regulation of multiple histone mRNAs during the cell cycle in HeLa cells. Nucleic Acids Res. 1983 Apr 25;11(8):2391–2410. doi: 10.1093/nar/11.8.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revzin A., Ceglarek J. A., Garner M. M. Comparison of nucleic acid-protein interactions in solution and in polyacrylamide gels. Anal Biochem. 1986 Feb 15;153(1):172–177. doi: 10.1016/0003-2697(86)90077-1. [DOI] [PubMed] [Google Scholar]
- Schneider R., Gander I., Müller U., Mertz R., Winnacker E. L. A sensitive and rapid gel retention assay for nuclear factor I and other DNA-binding proteins in crude nuclear extracts. Nucleic Acids Res. 1986 Feb 11;14(3):1303–1317. doi: 10.1093/nar/14.3.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels A., Marashi F., Stein G., Stein J. Enhancer-facilitated expression of a human H4 histone gene. Biochem Biophys Res Commun. 1985 Feb 28;127(1):239–246. doi: 10.1016/s0006-291x(85)80150-9. [DOI] [PubMed] [Google Scholar]
- Sierra F., Lichtler A., Marashi F., Rickles R., Van Dyke T., Clark S., Wells J., Stein G., Stein J. Organization of human histone genes. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1795–1799. doi: 10.1073/pnas.79.6.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra F., Stein G., Stein J. Structure and in vitro transcription of a human H4 histone gene. Nucleic Acids Res. 1983 Oct 25;11(20):7069–7086. doi: 10.1093/nar/11.20.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., Sen R., Baltimore D., Sharp P. A. A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature. 1986 Jan 9;319(6049):154–158. doi: 10.1038/319154a0. [DOI] [PubMed] [Google Scholar]
- Solomon M. J., Strauss F., Varshavsky A. A mammalian high mobility group protein recognizes any stretch of six A.T base pairs in duplex DNA. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1276–1280. doi: 10.1073/pnas.83.5.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein G. S., Borun T. W. The synthesis of acidic chromosomal proteins during the cell cycle of HeLa S-3 cells. I. The accelerated accumulation of acidic residual nuclear protein before the initiation of DNA replication. J Cell Biol. 1972 Feb;52(2):292–307. doi: 10.1083/jcb.52.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripputi P., Emanuel B. S., Croce C. M., Green L. G., Stein G. S., Stein J. L. Human histone genes map to multiple chromosomes. Proc Natl Acad Sci U S A. 1986 May;83(10):3185–3188. doi: 10.1073/pnas.83.10.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells D. E. Compilation analysis of histones and histone genes. Nucleic Acids Res. 1986;14 (Suppl):r119–r149. doi: 10.1093/nar/14.suppl.r119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwollo P., Stein G. S., Stein J. L. Variations in the organization of human genomic DNA segments containing H1 histone genes. Biochem Biophys Res Commun. 1984 Nov 14;124(3):988–993. doi: 10.1016/0006-291x(84)91055-6. [DOI] [PubMed] [Google Scholar]