Abstract
Glycosphingolipids (GSLs) are present on cell surface membranes and are particularly abundant in the brain. Since over 300–400 GSLs are synthesized from glucosylceramide (GlcCer), GlcCer is believed to only serve as the source of most GSLs, including sialic acid-containing GSLs or gangliosides, in the brain. Recent studies, however, suggest that GlcCer itself plays a role in the heat stress response, as it functions as a glucose donor for the synthesis of cholesterylglucoside, a lipid mediator in heat stress responses in animals. GlcCer in adipose tissues is also thought to be involved in mechanisms that regulate energy (sugar and lipid) metabolism. Our extensive structural study revealed an additional novel glucosylated membrane lipid, called phosphatidylglucoside, in developing rodent brains and human neutrophils. These lipids, all modified with glucose, are enriched in lipid rafts and play important roles in basic cellular processes. Here, I summarize the recent progress regarding these glucosylated lipids and their biosynthesis and regulation in the central nervous system (CNS).
Keywords: sphingolipid, glycolipid, glucosylceramide, L-serine, Purkinje neuron, lipid raft
1. Introduction: From complexity to simplicity
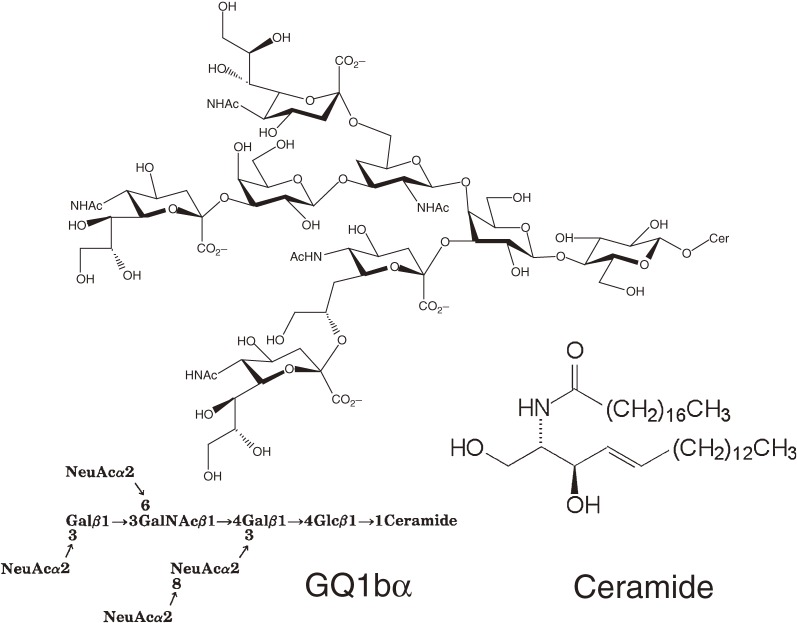
Sphingolipids have a common hydrophobic tail called ceramide, which is composed of a long chain base and a fatty acid.1) Ceramide is metabolically converted to glycosphingolipids (GSLs) and sphingomyelin, both of which are abundant in neural membranes. Over 300–400 GSL species exist, each with different sugar chain structures, in the cell membranes of organisms. Sphingolipids are critical components for cell survival and homeostasis. Our extensive structural analysis of brain complex GSLs has identified a novel type of ganglioside with o- and α-series core structure (cholinergic gangliosides) as minor components2–5) (Fig. 1).
Figure 1.
Cholinergic gangliosides are one the most complex type of gangliosides in the CNS. Q1bα, which was first isolated from bovine brain,5) is expressed in cholinergic neurons. Recent studies indicate that Q1bα may play a role in pathophysiological processes leading to Alzheimer’s disease.92)
Importantly, all complex gangliosides including those that are in the minor components category are synthesized from GlcCer through a ceramide glucosylation reaction.6,7) Thus, the study of GlcCer and its synthetic enzyme, ceramide glucosyltransferase (GlcT-1/GCS), is essential for understanding the basic roles of GSLs in the CNS. In 1994, we established a mouse melanoma cell line, termed GM-95, that has no endogenous GSLs due to a deficiency in GlcT-1 activity.8) Surprisingly, GM-95 cells survive and proliferate without GSLs, even in GSL-free culture medium. Soon thereafter, a human cDNA encoding GlcT-1 was cloned by complementation cloning using GM-95.9) Genomic analysis revealed that GlcT-1 is phylogenetically conserved, underscoring its biological significance. Discovery of the GlcCer-deficient cells and subsequent success in the molecular cloning of the GlcCer synthase gene triggered my research interest to re-focus metabolism and functions of common, basic, and simple sphingolipids instead of studying complex GSLs.
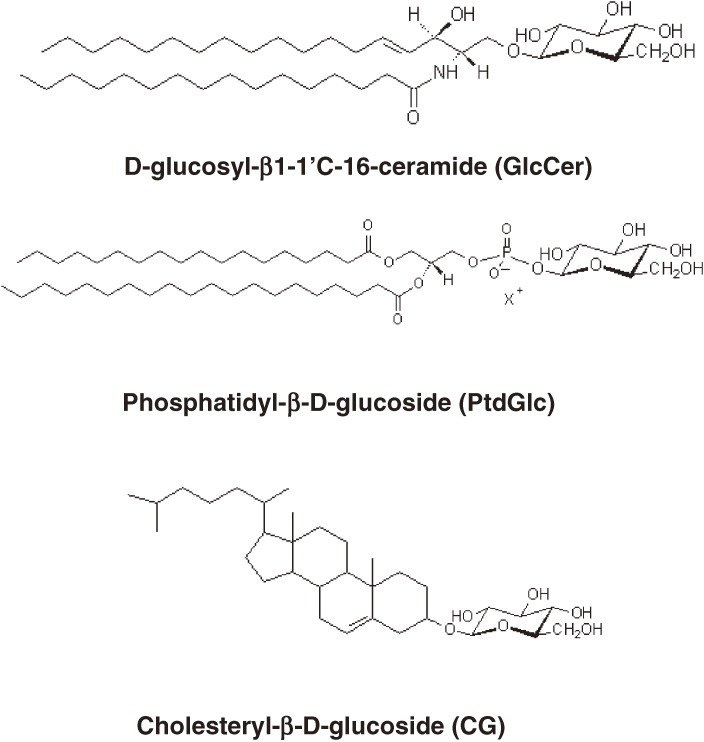
This review presents an overview of three critical synthetic enzymes required for sphingolipid synthesis—phosphoglycerate dehydrogenase (Phgdh), serine palmitoyltransferase (SPT), and glucosylceramide synthase (GlcT-1/GCS)—and their cellular functions. While re-examining simple glycosylated lipids in the brain, we discovered in developing rodent brains a novel glucose-modified lipid called phosphatidylglucoside (PtdGlc). Although most membrane lipids contain a variety of fatty acyl chains, PtdGlc is composed exclusively of double saturated fatty acids (C18:0/C20:0) (Fig. 2). Because of its unique fatty acid composition, PtdGlc has physical properties similar to those of GlcCer, which in turn can lead to the formation of lipid microdomains or lipid rafts. The biological functions of this new glycolipid are also discussed in this review.
Figure 2.
Three lipids modified with glucose present in mammalian membranes.
2. L-Serine synthesis and sphingolipids in the brain
2.1. Brain Phgdh—a key enzyme for L-serine synthesis.
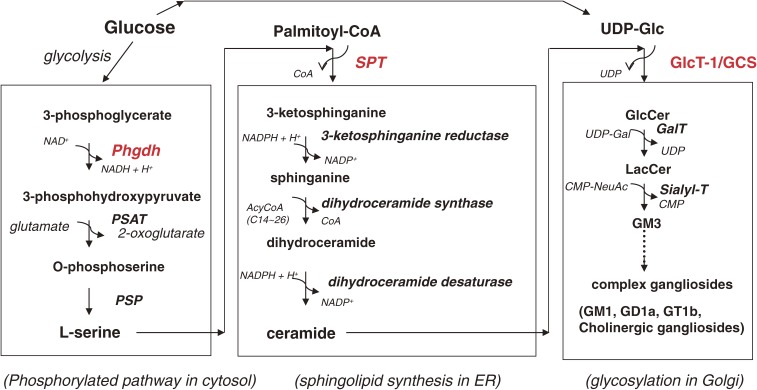
The water-soluble amino acid L-serine is a critical precursor amino acid for the synthesis of a variety of biomolecules, including membrane lipids. Sphingolipid biosynthesis starts with a condensation reaction between L-serine and hydrophobic C16:0-CoA to form 3-ketosphinganine (Fig. 2).1) This reaction is catalyzed by SPT in endoplasmic reticulum (ER) membranes.
Since L-serine is a non-essential amino acid, the importance of endogenous L-serine synthesis has been largely ignored. In 1998, however, Mitoma et al. reported that the survival and morphological development of cultured hippocampal pyramidal neurons depended on L-serine, which is actively synthesized and released from astrocytes. Thus, this non-essential, sweet amino acid is essential for neurons.10) Cerebellar Purkinje neurons also require L-serine supplied from Bergmann glia cells.11) Addition of L-serine dramatically improves dendritic development and synapse formation in mixed glia-neuron (Granular and Purkinje) cultures.
L-Serine is biosynthesized from a glycolytic intermediate, 3-phosphoglycerate, through three sequential catalytic reactions (Fig. 3). First, 3-phosphoglycerate is oxidized by a dehydrogenase (PHGDH) using NAD+ to form 3-phosphohydroxypyruvate, which in turn is converted to phosphoserine by a transamination reaction catalyzed by 3-phosphohydroxypyruvate aminotransferase (PSAT). Finally, phosphoserine is dephosphorylated by 3-phosphoserine phosphatase (PSP), yielding L-serine. Importantly, the PSAT reaction requires the excitatory amino acid glutamate as a nitrogen donor to produce 2-ketoglutarate. Thus, the nitrogen in the sphingosine base originally derives from glutamate. Phgdh mRNA is abundantly expressed in astrocytes, and its expression is barely detectable in a certain type of CNS neuron.11) The mRNA of two other enzymes, PSAT and PSP, are expressed by both astrocytes and neurons, although the level of PSP mRNA in astrocytes is lower than that in mature neurons.
Figure 3.
Sphingolipids and sphingoglycolipids are synthesized from glucose, amino acid, and fatty acid. The ceramide molecule contains a nitrogen atom that is derived from the amino acid L-serine. To synthesize the precursor lipid for GSLs, glucosylceramide (GlcCer), glucose (UDP-Glc), L-serine, and palmitoyl-CoA are required. Thus, it is understandable that GlcCer and its related compounds are involved in the regulation of energy metabolism and in the pathophysiology of diabetes mellitus.
2.2. Phgdh knockout mice as a model for human neurological diseases and other diseases.
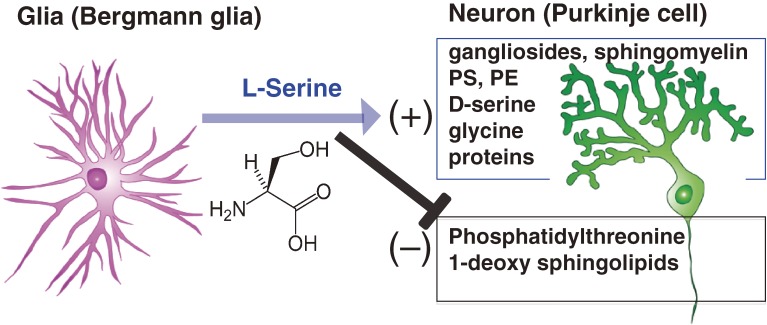
A reciprocal relationship between glia and neurons is vital for overall brain development and for the maintenance and proper functioning of neurons. Our L-serine studies revealed that, in the developing and mature brain, L-serine is a key mediator of neurotrophic/survival support provided by radial glia/astrocytes.12) To test whether L-serine synthesis is a fundamental system for normal brain development and function in vivo, we generated mice with a tissue-specific mutation of the Phgdh gene.13,14) The mice with a global knockout (KO) die at embryonic day 13.5 (E13.5). Moreover, in these KO mice normal cell cycle progression and subsequent neurogenesis involving radial glia are severely impaired in the spinal cord.15) As expected, all brain L-serine-derived lipids such as phosphatidylserine, phosphatidylethanolamine, sphingomyelin, and GD3 ganglioside are greatly reduced in Phgdh knockout mice. Of special note was the appearance of phosphatidylthreonine, which is barely detected with normal intracellular L-serine levels. It is unclear whether this newly synthesized lipid affects neuronal survival and function (Fig. 4).10) This issue will be discussed in the next section.
Figure 4.
Metabolic relationship between neurons and glia in cerebellum mediated through L-serine. Phgdh, a key enzyme for L-serine synthesis, is upregulated in Bergmann glia cells but downregulated in Purkinje neurons. Thus, Purkinje neurons are dependent on glial L-serine. L-serine stimulates the synthesis of membrane lipids such as sphingolipids. Importantly, L-serine inhibits the synthesis of phosphatidylthreonine and toxic lipids such as 1-deoxsphinganine.
Brain-specific Phgdh knockout mice may serve as a useful animal model for studying human genetic diseases characterized by PHGDH deficiency. PHGDH-deficient patients have lower levels of L-serine in blood plasma and cerebrospinal fluid. These patients exhibit severe neurological symptoms including congenital microcephaly, epilepsy, psychomotor retardation, and dysmyelination.16) It would be interesting to determine whether the serine deficiency in the brain of adult knockout mice causes similar morphological and/or functional alterations as well as changes in membrane lipid composition.
2.3. Phgdh and cancer.
PHGDH gene expression is also upregulated in cancer cells and tissues,17–19) indicating that L-serine promotes cancer cell proliferation and survival. Very recently, Possemato et al. reported that certain breast cancer cells (estrogen receptor-negative cells) overexpress the PHGDH gene.20) In these cells, L-serine synthesis through the phosphorylated pathway produces α-ketoglutarate from glutamate (Fig. 2), which contributes to 50% of the anaplerotic flux of glutamate into the tricarboxylic acid (TCA) cycle. This is one of the major reasons why L-serine synthesis is advantageous for cancer cell survival in terms of energy metabolism. As a result of these findings, PHGDH is now expected to be a target gene not only for the treatment of serine-deficient patients but also for patients with cancer (breast cancer, melanoma). It remains unknown whether high Phgdh expression levels in neural stem cells and radial glia cells are also related to energy metabolism.
3. Sphingolipid synthesis
3.1. SPT—the key enzyme for sphingolipid synthesis.
Mammalian SPT is composed of two subunits, SPTLC1 and either SPTLC2 or SPTLC3.21) Two additional factors, the small subunits SSSPTA and SSSPTB, are also associated with the complex.22) The composition of the complex determines substrate preference: The SPTLC1-SPTLC2 complex shows a strong preference for C16-CoA substrate, while the SPTLC1-SPTLC3 isozyme uses both C14-CoA and C16-CoA as substrates, with a slight preference for C14-CoA. The SPTLC1-SPTLC2-SSSPTB complex shows a strong preference for C18-CoA substrate, while the SPTLC1-SPTLC3-SSSPTB isozyme displays an ability to use a broader range of acyl-CoAs, without apparent preference.
More recently, ER membrane proteins of the orosomucoid (ORM) family have been shown to bind to the SPT complex and to inhibit SPT activities.23,24) ORM genes are a potential risk factor for childhood asthma. Orm proteins are now regarded to be key regulatory factors of sphingolipid biosynthesis. It would be interesting to know how Orm activities are regulated25) and how they are involved in the development of childhood asthma.
3.2. SPT knockout mice.
Gene-targeting technology has allowed us to define the in vivo roles of sphingolipid synthesis. Since both SPTLC1 and SPTLC2 are essential for SPT catalytic activity, two varieties of SPT knockout mice die during embryonic development.26) Physiological role of SPTLC3 remains to be elucidated. Since the Sptlc2 knockout causes embryonic lethality, Sptlc3 is either not functional or is absent at least during early developmental stages. Specifically when the embryos die and why endogenous sphingolipid synthesis is essential for embryo survival have not been examined carefully. Certain cell populations in a conditional Sptlc2 KO (inducible cKO) mouse showed an absolute requirement for sphingolipid synthesis. In these mice, single cell necrosis in the epithelia of crypts in the small and large intestines was observed as early as 24 h after inducing the knockout.27) Seventy-two hours after induction, body weight, spleen and thymus weights, and numbers of reticulocytes and lymphocytes decreased in the cKO mice. Thus, SPT is of particular importance in the proliferation and maintenance of mucosal epithelial cells.
3.3. Human disease, HSAN1, and novel sphingolipid metabolites.
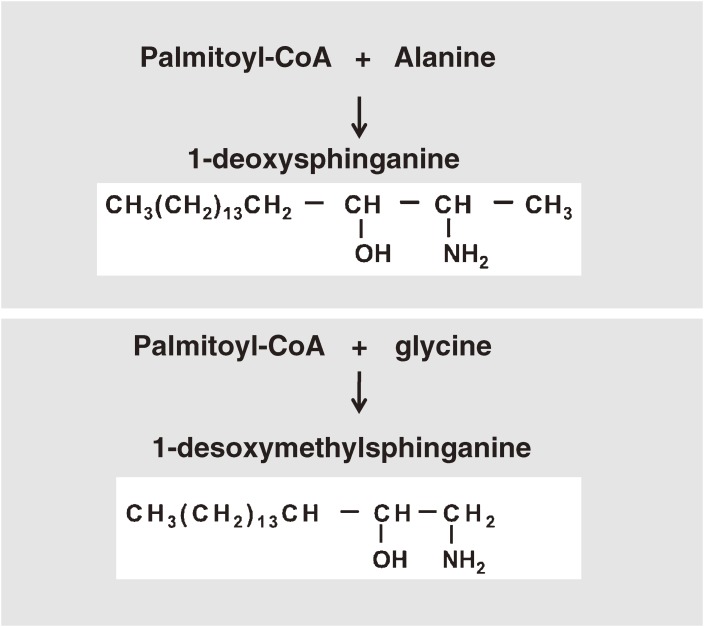
Defects in SPTLC1 cause hereditary sensory and autonomic neuropathy type 1A (HSAN1A). HSAN1A is an autosomal dominant axonal neuropathy. Initial symptoms are loss of pain, touch, heat, and cold sensation over the feet, followed by distal muscle wasting and weakness. It is largely unknown why this mutation causes defects in peripheral neurons not in central neurons. Interestingly, in HSAN1A the mutated SPT produces two novel sphingolipids, 1-deoxysphinganine and 1-desoxymethylsphinganine (Fig. 5).
Figure 5.
Occurrence of unusual sphingolipids: 1-deoxysphinganine and 1-desoxymethylsphinganine
HSAN1-associated mutant SPT has a reduced preference for L-serine and an increased activity toward amino acid substrates (specifically, alanine and glycine), which ultimately lead to the production of deoxy-type sphingosine bases. Unexpectedly, very recent studies demonstrated that oral administration of L-serine reduces the formation of 1-deoxy sphingolipids in mice and humans with HSAN1.28) L-Serine is abundantly synthesized in the CNS as noted above. Peripheral neurons may be particularly sensitive to the HSAN1 mutation possibly because peripheral neurons have low levels of serine synthase activities, resulting in a high alanine-to-serine ratio. Unusual sphingolipid metabolites have also been detected in the blood plasma of diabetic patients.29) Thus, oral L-serine treatment is expected not only to help patients with HSAN1 but also those with diabetic neuropathy.
4. Glucosylceramide
4.1. Glucosylceramide synthase (GlcT-1/GCS/UGCG).
GlcCer is a key component for the synthesis of almost all GSLs in the brain (Fig. 4). In 1979, the complete structural determination of Gaucher GlcCer was achieved through 13C-nuclear magnetic resonance (13C-NMR) analysis.30) GlcCer formation is catalyzed by the enzyme ceramide glucosyltransferase (GlcT-1/GCS/UGCG). Since catalytic activity is detectable when human GlcT-1 protein is expressed in E. coli, no proteinaceous factor is thought to be required for the expression of its activity. However, little is known about how GlcT-1 activity is regulated and how its protein synthesis and degradation are controlled.31) C-fos specifically activates GlcCer synthesis in PC-12 cells.32) However, the precise mechanisms underlying this activation remain unclear.
Ceramide glucosylation occurs in the cytosolic side of the cis-Golgi membranes of mammals. In Drosophila, GlcT-1 protein is also present in the ER membranes of the eyes33) and fat body.34) Based on its primary sequence, GlcT-1 is a type Ib (III) membrane protein. GlcT-1 protein has never been isolated in pure form, since it is a hydrophobic protein and is a very minor component. Since GlcT-1 is now expected to be a drug target for diabetes35,36) and sphingolipidoses,37,38) it is important to resolve its three-dimensional structure in order to develop new types of GlcT-1 inhibitors.
4.2. Glucosylceramide synthase knockout animals.
We have previously cloned cDNAs for human GlcT-1 (hGlcT-1, UGCG);9) mouse GlcT-1 (mGlcT-1);39) Drosophila GlcT-1 (dGlcT-1); and C. elegans GlcT-1 (CGT).40,41) The fact that GlcT-1 is evolutionarily conserved and that GlcCer is distributed widely in eukaryotes suggests that GlcCer synthesis plays some fundamental role in the cellular machinery (Table 1). Next, I discuss examples demonstrating the importance of GlcCer synthesis in vivo.
Table 1.
Organisms with genetically modified glucosylceramide synthase
| Organism | Targeted tissues and constructs | Phenotype | Biochemistry | References |
|---|---|---|---|---|
| Mouse | whole | embryonic death (E7.5) | loss of GSLs | Yamashita et al., 199942) |
| neural cells (Nestin-Cre) | case 1: dysfunction of cerebellum | reduction of gangliosides in brain | Yamashita et al., 200543) | |
| case 2: die after birth | Reduction of GSLs | Jennemann et al., 200544) | ||
| Purkinje neurons (L7-Cre) | axonal degeneration | loss of GSLs in Purkinje neurons | Watanabe et al., 201045) | |
| oligodendrocytes (Cnp-Cre) | normal myelin | normal GSL content | Saadat et al., 200946) | |
| epidermis (K14-Cre) | die after birth (loss of water-barrier function) | accumulation of ω-hydroxy ceramide | Jennemann et al., 200793) | |
| liver (Alb-Cre) | no abnormality | deletion of GlcCer in liver, no biochemical abnormality in plasma | Jennemann et al., 201094) | |
| C. elegans | RNAi (cgt-1 and-3) | death at L1 larval stage | reduction of GlcCer/GlcT-1 activity | Nomura et al., 201141) |
| RNAi (cgt-3) with rrf-1 strain | abnormal oocytes and early embryonic cell division | Nomura et al., 201141) | ||
| RNAi (cgt-1 and -3) | defects in digestive tract | Marza et al., 200950) | ||
| RNAi (cgt-1 and -3) | polarity abnormalities | Zhang, et al., 201152) | ||
| cgt-3 deletion allele tm504 | abnormal oocytes and early embryonic cell divison | reduction of GlcCer/GlcT-1 activity | Nomura et al., 201141) | |
| Drosophila | whole | lethality | reduction of GlcCer | Kohyama et al., 200433) |
| Fat body | lipid accumulation | changes in triacylglycerol content | Kohyama et al., 201134) |
GSL, glycosphingolipids; GlcCer, glucosylceramide.
4.3. Knockout mice.
Embryonic lethality of Ugcg knockout mice demonstrates that GlcCer synthesis is essential for development.42) In Ugcg knockout mice, enhanced apoptotic cell death is observed in ectodermal cells at the gastrulation stage (E7.5). It remains unclear, however, why eliminating the Ugcg gene enhances cell death selectively in ectoderm. Two research groups working independently disrupted Ugcg specifically in neural cells using the nestin-Cre/LoxP system (Table 1).43,44) Here, Cre recombinase protein driven by a nestin promoter was specifically expressed in neuronal and glial cell precursors during embryonic development. In Proia and associates’ knockout, the mice exhibited abnormal behaviors and a significant loss of Purkinje neurons. On the other hand, the knockout mice produced by Gröne and co-workers exhibited much more severe phenotypes, dying shortly after birth. The use of different nestin-Cre transgene mouse lines could explain these disparate findings.
To understand how GlcCer synthesis in the CNS contributes to post-mitotic neuronal survival and functional maintenance at the molecular level, we generated Purkinje neuron-specific knockout mice (L7-GlcT-1KO mice) using L7Cre transgenic mice.45) Although we intended to target only Purkinje neurons, we noticed that myelin had significant structural abnormalities. Their doubly myelinated axons were enveloped by an additional concentric myelin sheath around the original sheath. Our data showed that axonal GlcCer-derived GSLs are essential for correct myelin sheath formation and maintenance.
Unlike the Purkinje neuron-specific knockout mice, oligodendrocyte-specific knockout mice, which were generated by using Cnp-Cre transgenic mice, do not show any abnormal myelin structures or functions.46) GSLs and GalCer synthesis in myelin are not essential for myelin structure. In oligodendrocytes, myelin-associated glycoprotein (MAG) is known to be the binding partner of axonal GSLs.47) MAG is a type 1 transmembrane glycoprotein found in periaxonal oligodendrocyte membranes. MAG-null mice also make multiple myelin sheaths48) similar to those observed in L7-GlcT-1KO mice, suggesting that GSLs binding to MAG regulate the myelin configuration and maintenance of axo-glial interactions. Schnaar and his associates reported that the α-series gangliosides GQ1b α and GD1 α are the most potent gangliosides that support MAG-mediated cell adhesion.49) Since GD1 α is enriched in Purkinje neurons,3) the loss of adhesion mediated by interactions between MAG and GD1 α may underlie the abnormal myelin morphology seen in GlcT-1 KO mice.
4.4. Drosophila and C. elegans.
In Drosophila, GlcCer synthesis is essential for embryo survival: Loss of GlcT-1 function causes excessive cell death in part due to caspase-dependent apoptosis.33) Functions of GlcCer synthesis are tissue specific, since down-regulation of GlcT-1 in the fat body, the fly equivalent of mammalian adipose tissue, does not cause any cell death.34) Interestingly, GlcCer is the sole GSL of the fat body, and GlcCer itself may play a regulatory role in energy (lipid and glucose) homeostasis.
Although most organisms contain only one GlcCer synthase gene, C. elegans exceptionally possesses three distinct human Ugcg orthologs, cgt-1, cgt-2, and cgt-3 (cgt is not ceramide galactosyltransferase gene in mammals).40,41) Moreover, all three have GlcT-1 activities and cgt-3 shows the highest activities among the three.40,41) RNAi of cgt causes defects in a subset of cells in the digestive tract; these defects impair larval feeding, resulting in starvation-induced growth arrest.50) However, GSLs are dispensable in most cells in C. elegans, including those of the nervous system.50) More recently, Nomura et al.41) demonstrated, however, that the Ugcg gene is indispensable in oocyte formation and early embryonic cell division. This corroborates the initial proposal of De Smedt et al.,51) who used Xenopus oocytes. Further studies are necessary to fully understand regulatory mechanisms mediated by GlcCer synthesis in oocyte meiosis.
Zhang et al. have demonstrated that CGT product—GlcCer with a C17 branched-chain sphingosine and a saturated fatty acyl chain C22:0—is a critical determinant of in vivo cell polarity and morphogenesis of the intestines.52)
These recent studies examining the in vivo functions of GSLs suggest that GlcCer is not merely a precursor lipid needed for the synthesis of complex GSLs; rather, GlcCer itself exerts biological activities through unrecognized mechanisms. It is also extremely important to address the key questions: Why is GlcCer formed in the cytosolic face of Golgi membranes and translocated into the luminal part of the membranes, and how does this occur?53) Please see the review of Graham et al.53) for an additional discussion of these issues.
4.5. Glucosylceramide and human diseases.
Recent pharmaceutical studies demonstrated that GlcCer synthesis is possibly involved in the pathogenesis of diabetes mellitus and atherosclerosis.35,36,54,55) As discussed by Summers and others, “ceramide” is a key player in energy metabolism regulation and in metabolic syndrome and diabetes pathogenesis.56–58) The regulation of energy metabolism developed during evolution. Moreover, its mechanisms are extremely complicated, since energy homeostasis is maintained by highly integrated systems existing across cell types and various tissues, including pancreatic β–cells, brain, liver, muscle, macrophages, and adipocytes.
As we discussed above, small animal models such as C. elegans and Drosophila can provide new clues to better understand the fundamental roles of GlcCer and sphingolipids in the regulation of metabolism and in related diseases. GlcCer is also reported to play an important role in polycystic kidney disease,59) Parkinson’s disease,60) and Alzheimer’s disease.61,62)
5. From simplicity to diversity: the diversity of lipids modified with glucose
The lipid raft hypothesis has had a wide impact not only on the field of membrane lipid biology but also more generally on the field of cell biology. This concept has lead to profound insight into membrane dynamics, especially lipid dynamics in living cells. Membrane lipid rafts are thought to serve as molecular assembly and sorting platforms for cell-cell interactions or signal transduction and to modulate multiple cellular processes, such as axonal growth and guidance of neuronal growth cones, cytokine/growth factor receptor signaling, neuron-glia interactions, neuronal survival, and death. Since there is enormous molecular diversity among membrane lipids, the existence of membrane lipid microdomains other than sphingolipid-rich domains is quite possible.63) In fact, recent studies have revealed two additional lipids modified with glucose, cholesterylglucoside and phosphatidylglucoside. Both of these lipids are possibly enriched in lipid rafts or membrane microdomains distinct from sphingolipid-rich domains.
5.1. Cholesterylglucoside.
Murofushi and co-workers first isolated mammalian glucosylated cholesterol (1-O-cholesteryl-β–D-glucopyranoside, CG) in cultured human cells, TIG-3 fibroblasts,64) and gastric mucosa65) (Fig. 4). Interestingly, heat shock rapidly induces CG synthesis followed by HSF1 activation and HSP70 induction in human fibroblasts.66) To decipher the molecular mechanisms of HSP70-induction by CG, it is essential to identify the enzyme involved in CG synthesis that is sensitive to heat stress. Akiyama et al.67) proposed that sterol glucosyltransferase transfers glucose from GlcCer to cholesterol. Supporting this notion is the finding that GM-95 cells deficient in GlcCer synthase, GlcT-1, are unable to synthesize CG without the addition of exogenous GlcCer. It is interesting to note that ceramide generated from sphingomyelin is also a molecule important in stress responses.68,69) However, the relationship between heat shock responses and the ceramide signaling cascade is unclear.70)
Recently it was observed that dynamic changes in the cholesterol-rich domain structure induced by heat stress or benzyl alcohol (membrane fluidizer) treatment is responsible for the activation of HSF1 and HSP70.71) Lipidomic approaches demonstrate that heat shock causes metabolic changes in membrane lipid-raft-related molecules (ceramide, cholesterol, and saturated-fatty acid chain species of glycerol lipids).72) Thus, it is an intriguing idea that the production of CG may serve as a heat-associated signaling platform or a heat-signal molecule for HSP gene activation.
6. Phosphatidylglucoside (PtdGlc)
6.1. Chemical structure of PtdGlc.
In 2001, Nagatsuka et al.73) isolated an unusual glucose-containing lipid in human umbilical cord erythrocytes as a differentiation-associated antigen and proposed the tentative structure as phosphatidylglucose. Although the biological significance of this glycolipid puzzled us at that time, we decided to continue with the structural and functional analyses of the lipid. Because this lipid contained “glucose,” we expected it to be biologically very important. Four years later, a similar lipid was isolated in detergent-insoluble membrane (DIM) fractions from human promyelocytic leukemia cells (HL-60). The structure of this lipid was “phosphatidylglucoside (PtdGlc).” During the biochemical work on this novel type of lipid, we learned that it was a minor component and that it was labile under alkaline conditions. Thus, it was extremely difficult to purify and to obtain in a pure form.
To detect PtdGlc with high sensitivity and specificity, we tried to produce a PtdGlc-specific monoclonal antibody by immunizing mice with DIM isolated from HL60 cells.74) This work was motivated by the report that DIM fractions act as an effective immunogen.75,76) We successfully isolated about 100 clones that react with lipids and proteins present in the DIM. One of the clones, termed DIM21, specifically recognized PtdGlc.77,78) By using DIM21, we were able to successfully isolate pure PtdGlc from fetal rodent brains. Nuclear magnetic resonance (NMR) analyses together with the results of mass spectroscopy and gas chromatography analyses allowed us to determine the chemical structure of PtdGlc in fetal rat brain: It was 1-stearyl-2-arachidoyl-sn-glycerol-3-phosphoryl-β-D-glucopyranoside (Fig. 2).79) In addition, acetylated PtdGlc, 1-stearyl-2-arachidoyl-sn-glycerol-3-phosphoryl-β-D-(6-O-acetyl)glucopyranoside, exists as an isolated DIM21 antigen from fetal rat brain.
By comparing NMR data on acetylated PtdGlc from a natural source to that of chemically synthesized 6-O-acetyl PtdGlc, Greimel et al.78) confirmed that acetylation occurs at position 6 of the hexose ring of PtdGlc.
Most unexpected was the finding that PtdGlc isolated from fetal rat brain has only one fatty acid combination: That is, the sn-1 and sn-2 chains are exclusively stearic acid (C18:0) and arachidic acid (C20:0), respectively. A single molecular species rarely occurs in natural phospholipids. Also, very few natural lipids have the C20:0 acyl chain as a major component. In addition, PtdGlc from rat brain contained a stereoisomer, 1-sn-phosphatidylglucoside. How this isomer is biosynthesized is unknown.
PtdGlc is also present in human neutrophils, where it has a mixed fatty acid composition comprising C18:0/C18:0 and C18:0/C20:0.80)
Similar to GlcCer, PtdGlc exhibits a high main phase transition temperature in differential scanning calorimetry (DSC).81) However, DSC analysis showed that PtdGlc is not miscible with sphingomyelin even though GlcCer is. In addition, DSC and small-angle X-ray scattering (SAXS) experiments revealed that PtdGlc is poorly miscible with phosphatidylcholine. These results suggest that the lack of tight intermolecular interactions exclude PtdGlc from other lipid domains on the plasma membrane. Immunoelectron microscopy with DIM21 using the SDS-digested freeze fracture replica labeling method demonstrated that PtdGlc forms distinct lipid domains exclusively on the outer, not inner, leaflet of the plasma membrane of HL60 cells and A549 cells, a human alveolar epithelial cell line.81)
6.2. Expression of PtdGlc.
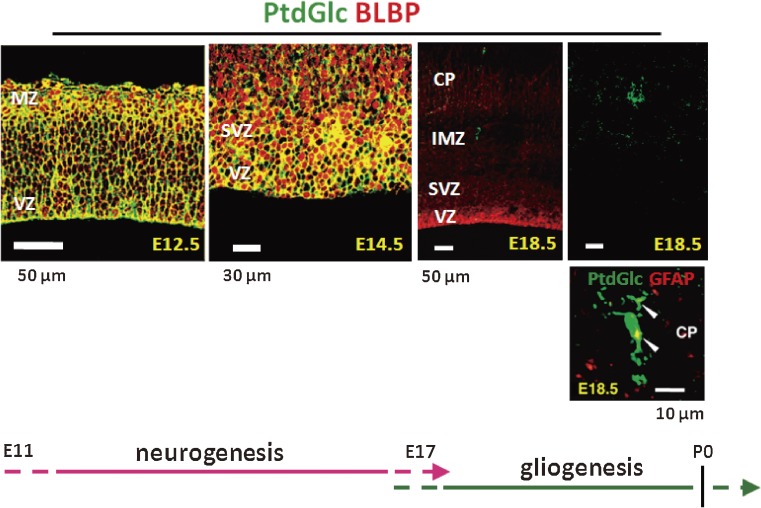
Expression of PtdGlc is developmentally regulated in the rodent brain (Fig. 6). A high level of PtdGlc expression is observed in radial glia and astrocytes in early developing rat brains (E12.5–14.5). At E18.5, PtdGlc expression is rapidly downregulated, and GFAP-positive astrocytes become DIM-21 positive. In adults, neural stem cells (Type B) in the subventricular zone continuously express PtdGlc.82) Thus, PtdGlc may serve also as a novel cell surface marker for stem cells.
Figure 6.
Developmental changes in PtdGlc expression in mouse cerebral cortex. In all images, cells labeled with DIM21 are green. Brain lipid-binding protein (BLBP) is a radial glia marker. DIM21-positive cells express the astrocyte marker GFAP (arrowheads) at E18.5.83)
6.3. Possible roles of PtdGlc.
The biological roles of PtdGlc are poorly understood. Since PtdGlc is enriched in astrocyte lineage cells in the developing mouse CNS, they may be potentially involved in astrogliogenesis in mouse cerebral cortex. This notion is supported by the finding that addition of DIM21 to neural progenitor cells prepared from fetal mouse telencephalon causes the recruitment of EGF receptors into lipid rafts, leading to the activation of EGF receptors.83)
PtdGlc has been found also in epithelial cells from various human organs.84) Oka et al.85) and Kina et al.80) demonstrated that PtdGlc is highly and specifically expressed on human neutrophils. In neutrophils, DIM21 treatment induces Fas clustering, leading to Fas-dependent apoptotic cell death,80) Moreover, these Fas clusters colocalize with PtdGlc to form large rafts.80) These findings underscore the importance of identifying natural PtdGlc ligands that mirror the effects of the artificial ligand DIM21.
7. Perspectives
It is surprising to have identified a novel glycolipid molecule, PtdGlc, in mammalian tissues, even in this century. All glucose-modified lipids should have very important biological functions, since evolutionarily glucose is a very old sugar and most organisms use it for their survival. Synthesis of GlcCer requires glucose, palmitoyl CoA, and L-serine from glucose. Thus, it is quite understandable that GlcCer, sphingolipids, and sphingoglycolipids are heavily involved in energy metabolism for our whole body. Further studies are necessary to understand the molecular mechanisms by which energy homeostasis is tightly regulated. Model animals such as Drosophila and C. elegans provide valuable tools to solve these issues.34,86–88)
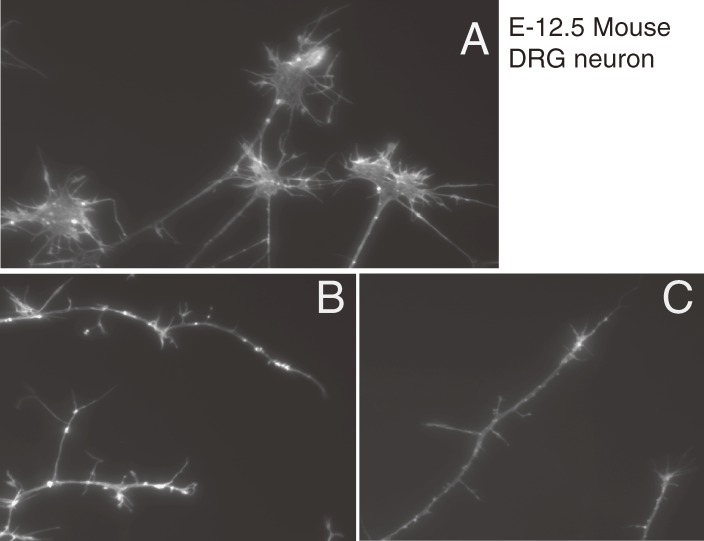
In contrast to GlcCer, the hydrophobic acyl-chains of PtdGlc are easily hydrolyzed by phospholipases.89) Indeed, the addition of a tiny amount of lyso-PtdGlc causes growth cones to collapse in developing rat DRG neurons in vitro (Fig. 7),90) suggesting that this lyso-glycolipid is a novel bioactive lipid mediating axonal guidance. Rapid progress of technologies in mass spectrometry enables us to discover the unique lipid molecule in the developing CNS.91) Little is known how PtdGlc is biosynthesized and what the molecular mechanism is for the collapse activities of lyso-PtdGlc. We believe that this lyso-lipid plays important roles in a variety of biological processes in both nervous systems and non-nervous systems. To understand the precise roles of PtdGlc and its metabolites, it is crucial to identify the enzyme involved in the glucosylation of PtdGlc and the gene that encodes it.
Figure 7.
Exogenous addition of lyso-phosphatidylglucoside induces growth cone collapse of rat DRG neurons. DRG neurons were isolated from an E12.5 rat. Actin filaments were visualized with rhodamine-labeled phalloidin (M. Yamazaki et al., unpublished data). A, Control; B, LPA (100 µM); C, lyso-PtdGlc (10 µM). The lyso form of PtGlc showed more potent activity for growth cone collapse.
Although monoglucosylated lipids are structurally very simple—just one sugar attached to each lipid—they have diverse biological functions. The glycolipids identified thus far are commonly enriched within lipid microdomains or lipid rafts. A variety of molecules, especially glycosylated and acylated membrane proteins, are partitioned into these lipid rafts, where they perform diverse biological functions in a cooperative manner with glycolipids.
Further investigations are still need to determine how lipids interact with functional proteins and how they contribute to membrane structure-function relationships. Additional research is also needed in order to fully understand how lipids mediate dynamic interactions between organelles and between cells, and how lipids organize intracellular multiple signaling networks.
Acknowledgements
I thank all people involved in our project on glycolipid and lipid biology: Professors Toshihide Kobayashi, Yukishige Ito, Makoto Ito, Kazuya Nomura, Ichiro Miyoshi, Tadashi Yamashita, Keiko Kato, Tsutomu Hashikawa, Kojiro Tohyama, Kimiko Murakami-Murofushi, and Masayuki Miura. Also, I would like to express special thanks to people who have worked in my laboratory: Professors Shigeki Furuya, Shinichi Ichikawa, and Hideyoshi Higashi, and Drs. Yasuko Nagatsuka, Ayako Kohyama, Masakazu Yamazaki, Takuji Nabetani, Peter Greimel, Shun Watanabe, Jyunya Mitoma, Yasuhiro Horibata, Yohei Ishibashi, Masami Kinoshita, Yeon-Jeong Kim, and Takamitsu Sano.
I thank Dr. Yoko Ohashi, for kind advice about MS analysis. I wish to express special thanks to Professor Yoshitaka Nagai for his valuable advice and encouragement.
Abbreviations
- CG
1-O-cholesteryl-β-D-glucopyranoside
- DIM
detergent-insoluble membrane
- GlcCer
glucosylceramide
- GlcT-1/GCS/UGCG
glucosylceramide synthase (ceramide glucosyltransferase)
- GSLs
glycosphingolipids
- MAG
myelin-associated glycoprotein
- Phgdh
phosphoglycerate dehydrogenase
- PSAT
3-phosphohydroxypyruvate aminotransferase
- PSP
3-phosphoserine phosphatase
- PtdGlc
phosphatidylglucoside
- SPT
serine palmitoyltransferase
Profile
In 1979–82, after studying biochemistry and completing his Ph.D. in Biochemistry in Shizuoka (Prof. Makoto Matsumoto), he studied glycolipid-activator protein in the Department of Biochemistry at Tulane University, U.S.A. (Prof. Yu-Teh Li’s Laboratory). Soon thereafter, he became a Lecturer in the Department of Biochemistry, School of Pharmaceutical Sciences, at the University of Shizuoka, Japan. In 1989, he traveled to the University of California at San Diego, U.S.A. (Prof. John O’Brien) to study the molecular biology of the activator protein (saposin) responsible for sphingolipid hydrolysis. In 1990, he was appointed as team leader of the Laboratory for Cellular Glycobiology, for the Glycobiology Project of the RIKEN Frontier Research Program (Group Director, Prof. Yoshitaka Nagai). During the project, the glucosylceramide synthase gene was successfully cloned. In 1999, he moved to RIKEN Brain Science Institute (Group Director, Prof. Masao Ito) and now is the senior team leader of the Molecular Membrane Neuroscience Laboratory. His research interests include neuron-glia interactions through glucose metabolites, such as L-serine for neuronal glycolipid/sphingolipid synthesis and long-term survival. He is currently interested in elucidating the biological functions of novel neural membrane components—phosphatidylglucoside and BOSS/GPRC5B, a N-linked glycoprotein—involved in neuronal networks, memory formation, and energy metabolism.
References
- 1).Smith W.L., Merrill A.H., Jr. (2002) Sphingolipid metabolism and signaling minireview series. J. Biol. Chem. 277 (29), 25841–25842 [DOI] [PubMed] [Google Scholar]
- 2).Ando S., Tanaka Y., Kobayashi S., Fukui F., Iwamoto M., Waki H., Tai T., Hirabayashi Y. (2004) Synaptic function of cholinergic-specific Chol-1α ganglioside. Neurochem. Res. 29 (4), 857–867 [DOI] [PubMed] [Google Scholar]
- 3).Furuya S., Irie F., Hashikawa T., Nakazawa K., Kozakai A., Hasegawa A., Sudo K., Hirabayashi Y. (1994) Ganglioside GD1α in cerebellar Purkinje cells. Its specific absence in mouse mutants with Purkinje cell abnormality and altered immunoreactivity in response to conjunctive stimuli causing longterm desensitization. J. Biol. Chem. 269 (51), 32418–32425 [PubMed] [Google Scholar]
- 4).Hidari K.I., Irie F., Suzuki M., Kon K., Ando S., Hirabayashi Y. (1993) A novel ganglioside with a free amino group in bovine brain. Biochem. J. 296 (Pt 1), 259–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Hirabayashi Y., Nakao T., Irie F., Whittaker V.P., Kon K., Ando S. (1992) Structural characterization of a novel cholinergic neuron-specific ganglioside in bovine brain. J. Biol. Chem. 267 (18), 12973–12978 [PubMed] [Google Scholar]
- 6).Nishimura K., Yamakawa T. (1968) Isolation of cerebroside containing glucose (Glucosyl ceramide) and its possible significance in ganglioside synthesis. Lipids 3, 262–266 [DOI] [PubMed] [Google Scholar]
- 7).Basu S., Kaufman B., Roseman S. (1973) Enzymatic synthesis of glucocerebroside by a glucosyltransferase from embryonic chicken brain. J. Biol. Chem. 248 (4), 1388–1394 [PubMed] [Google Scholar]
- 8).Ichikawa S., Nakajo N., Sakiyama H., Hirabayashi Y. (1994) A mouse B16 melanoma mutant deficient in glycolipids. Proc. Natl. Acad. Sci. U.S.A. 91 (7), 2703–2707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Ichikawa S., Sakiyama H., Suzuki G., Hidari K.I., Hirabayashi Y. (1996) Expression cloning of a cDNA for human ceramide glucosyltransferase that catalyzes the first glycosylation step of glycosphingolipid synthesis. Proc. Natl. Acad. Sci. U.S.A. 93 (10), 4638–4643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Mitoma J., Kasama T., Furuya S., Hirabayashi Y. (1998) Occurrence of an unusual phospholipid, phosphatidyl-L-threonine, in cultured hippocampal neurons. Exogenous L-serine is required for the synthesis of neuronal phosphatidyl-L-serine and sphingolipids. J. Biol. Chem. 273 (31), 19363–19366 [DOI] [PubMed] [Google Scholar]
- 11).Furuya S., Tabata T., Mitoma J., Yamada K., Yamasaki M., Makino A., Yamamoto T., Watanabe M., Kano M., Hirabayashi Y. (2000) L-serine and glycine serve as major astroglia-derived trophic factors for cerebellar Purkinje neurons. Proc. Natl. Acad. Sci. U.S.A. 97 (21), 11528–11533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Hirabayashi Y., Furuya S. (2008) Roles of L-serine and sphingolipid synthesis in brain development and neuronal survival. Prog. Lipid Res. 47 (3), 188–203 [DOI] [PubMed] [Google Scholar]
- 13).Yoshida K., Furuya S., Osuka S., Mitoma J., Shinoda Y., Watanabe M., Azuma N., Tanaka H., Hashikawa T., Itohara S., Hirabayashi Y. (2004) Targeted disruption of the mouse 3-phosphoglycerate dehydrogenase gene causes severe neurodevelopmental defects and results in embryonic lethality. J. Biol. Chem. 279 (5), 3573–3577 [DOI] [PubMed] [Google Scholar]
- 14).Yang J.H., Wada A., Yoshida K., Miyoshi Y., Sayano T., Esaki K., Kinoshita M.O., Tomonaga S., Azuma N., Watanabe M., Hamase K., Zaitsu K., Machida T., Messing A., Itohara S., Hirabayashi Y., Furuya S. (2010) Brain-specific Phgdh deletion reveals a pivotal role for L-serine biosynthesis in controlling the level of D-serine, an N-methyl-D-aspartate receptor co-agonist, in adult brain. J. Biol. Chem. 285 (53), 41380–41390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Kawakami Y., Yoshida K., Yang J.H., Suzuki T., Azuma N., Sakai K., Hashikawa T., Watanabe M., Yasuda K., Kuhara S., Hirabayashi Y., Furuya S. (2009) Impaired neurogenesis in embryonic spinal cord of Phgdh knockout mice, a serine deficiency disorder model. Neurosci. Res. 63 (3), 184–193 [DOI] [PubMed] [Google Scholar]
- 16).Klomp L.W., de Koning T.J., Malingré H.E., van Beurden E.A., Brink M., Opdam F.L., Duran M., Jaeken J., Pineda M., van Maldergem L., Poll-The B.T., van den Berg I.E., Berger R. (2000) Molecular characterization of 3-phosphoglycerate dehydrogenase deficiency--a neurometabolic disorder associated with reduced L-serine biosynthesis. Am. J. Hum. Genet. 67 (6), 1389–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Pollari S., Käkönen S.M., Edgren H., Wolf M., Kohonen P., Sara H., Guise T., Nees M., Kallioniemi O. (2010) Enhanced serine production by bone metastatic breast cancer cells stimulates osteoclastogenesis. Breast Cancer Res. Treat. 125 (2), 421–430 [DOI] [PubMed] [Google Scholar]
- 18).Mullarky E., Mattaini K.R., Vander Heiden M.G., Cantley L.C., Locasale J.W. (2011) PHGDH amplification and altered glucose metabolism in human melanoma. Pigment Cell Melanoma Res. 24 (6), 1112–1115 [DOI] [PubMed] [Google Scholar]
- 19).Locasale J.W., Grassian A.R., Melman T., Lyssiotis C.A., Mattaini K.R., Bass A.J., Heffron G., Metallo C.M., Muranen T., Sharfi H., Sasaki A.T., Anastasiou D., Mullarky E., Vokes N.I., Sasaki M., Beroukhim R., Stephanopoulos G., Ligon A.H., Meyerson M., Richardson A.L., Chin L., Wagner G., Asara J.M., Brugge J.S., Cantley L.C., Vander Heiden M.G. (2011) Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat. Genet. 43 (9), 869–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Possemato R., Marks K.M., Shaul Y.D., Pacold M.E., Kim D., Birsoy K., Sethumadhavan S., Woo H.K., Jang H.G., Jha A.K., Chen W.W., Barrett F.G., Stransky N., Tsun Z.Y., Cowley G.S., Barretina J., Kalaany N.Y., Hsu P.P., Ottina K., Chan A.M., Yuan B., Garraway L.A., Root D.E., Mino-Kenudson M., Brachtel E.F., Driggers E.M., Sabatini D.M. (2011) Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature 476 (7360), 346–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Hornemann T., Richard S., Rütti M.F., Wei Y., von Eckardstein A. (2006) Cloning and initial characterization of a new subunit for mammalian serine-palmitoyltransferase. J. Biol. Chem. 281 (49), 37275–37281 [DOI] [PubMed] [Google Scholar]
- 22).Hornemann T., Wei Y., von Eckardstein A. (2007) Is the mammalian serine palmitoyltransferase a high-molecular-mass complex? Biochem. J. 405 (1), 157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Han S., Lone M.A., Schneiter R., Chang A. (2010) Orm1 and Orm2 are conserved endoplasmic reticulum membrane proteins regulating lipid homeostasis and protein quality control. Proc. Natl. Acad. Sci. U.S.A. 107 (13), 5851–5856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Breslow D.K., Collins S.R., Bodenmiller B., Aebersold R., Simons K., Shevchenko A., Ejsing C.S., Weissman J.S. (2010) Orm family proteins mediate sphingolipid homeostasis. Nature 463 (7284), 1048–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Roelants F.M., Breslow D.K., Muir A., Weissman J.S., Thorner J. (2011) Protein kinase Ypk1 phosphorylates regulatory proteins Orm1 and Orm2 to control sphingolipid homeostasis in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U.S.A. 108 (48), 19222–19227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Hojjati M.R., Li Z., Jiang X.C. (2005) Serine palmitoyl-CoA transferase (SPT) deficiency and sphingolipid levels in mice. Biochim. Biophys. Acta 1737 (1), 44–51 [DOI] [PubMed] [Google Scholar]
- 27).Ohta E., Ohira T., Matsue K., Ikeda Y., Fujii K., Ohwaki K., Osuka S., Hirabayashi Y., Sasaki M. (2009) Analysis of development of lesions in mice with serine palmitoyltransferase (SPT) deficiency -Sptlc2 conditional knockout mice. Exp. Anim. 58 (5), 515–524 [DOI] [PubMed] [Google Scholar]
- 28).Garofalo K., Penno A., Schmidt B.P., Lee H.J., Frosch M.P., von Eckardstein A., Brown R.H., Hornemann T., Eichler F.S. (2011) Oral L-serine supplementation reduces production of neurotoxic deoxysphingolipids in mice and humans with hereditary sensory autonomic neuropathy type 1. J. Clin. Invest. 121 (12), 4735–4745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Bertea M., Rütti M.F., Othman A., Marti-Jaun J., Hersberger M., von Eckardstein A., Hornemann T. (2010) Deoxysphingoid bases as plasma markers in diabetes mellitus. Lipids Health Dis. 9, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Koerner T.A.W., Jr., Cary L.W., Li S.C., Li Y.T. (1979) Carbon 13 NMR spectroscopy of a cerebroside. Proof of the β-pyranosyl structure of D-glucosylceramide. J. Biol. Chem. 254 (7), 2326–2328 [PubMed] [Google Scholar]
- 31).Aida J., Higuchi S., Hasegawa Y., Nagano-Ito M., Hirabayashi Y., Banba A., Shimizu T., Kikuchi A., Saga M., Ichikawa S. (2011) Up-regulation of ceramide glucosyltransferase during the differentiation of U937 cells. J. Biochem. 150 (3), 303–310 [DOI] [PubMed] [Google Scholar]
- 32).Crespo P.M., Silvestre D.C., Gil G.A., Maccioni H.J., Daniotti J.L., Caputto B.L. (2008) c-Fos activates glucosylceramide synthase and glycolipid synthesis in PC12 cells. J. Biol. Chem. 283 (45), 31163–31171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Kohyama-Koganeya A., Sasamura T., Oshima E., Suzuki E., Nishihara S., Ueda R., Hirabayashi Y. (2004) Drosophila glucosylceramide synthase: a negative regulator of cell death mediated by proapoptotic factors. J. Biol. Chem. 279 (34), 35995–36002 [DOI] [PubMed] [Google Scholar]
- 34).Kohyama-Koganeya A., Nabetani T., Miura M., Hirabayashi Y. (2011) Glucosylceramide synthase in the fat body controls energy metabolism in Drosophila. J. Lipid Res. 52 (7), 1392–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Zhao H., Przybylska M., Wu I.H., Zhang J., Siegel C., Komarnitsky S., Yew N.S., Cheng S.H. (2007) Inhibiting glycosphingolipid synthesis improves glycemic control and insulin sensitivity in animal models of type 2 diabetes. Diabetes 56 (5), 1210–1218 [DOI] [PubMed] [Google Scholar]
- 36).Fox T.E., Han X., Kelly S., Merrill A.H., 2nd, Martin R.E., Anderson R.E., Gardner T.W., Kester M. (2006) Diabetes alters sphingolipid metabolism in the retina: a potential mechanism of cell death in diabetic retinopathy. Diabetes 55 (12), 3573–3580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Radin N.S. (1996) Treatment of Gaucher disease with an enzyme inhibitor. Glycoconj. J. 13 (2), 153–157 [DOI] [PubMed] [Google Scholar]
- 38).Cox T.M. (2010) Eliglustat tartrate, an orally active glucocerebroside synthase inhibitor for the potential treatment of Gaucher disease and other lysosomal storage diseases. Curr. Opin. Investig. Drugs 11 (10), 1169–1181 [PubMed] [Google Scholar]
- 39).Ichikawa S., Ozawa K., Hirabayashi Y. (1998) Molecular cloning and characterization of the mouse ceramide glucosyltransferase gene. Biochem. Biophys. Res. Commun. 253 (3), 707–711 [DOI] [PubMed] [Google Scholar]
- 40).Ichikawa S., Hirabayashi Y. (1998) Glucosylceramide synthase and glycosphingolipid synthesis. Trends Cell Biol. 8 (5), 198–202 [DOI] [PubMed] [Google Scholar]
- 41).Nomura K.H., Murata D., Hayashi Y., Dejima K., Mizuguchi S., Kage-Nakadai E., Gengyo-Ando K., Mitani S., Hirabayashi Y., Ito M., Nomura K. (2011) Ceramide glucosyltransferase of the nematode Caenorhabditis elegans is involved in oocyte formation and in early embryonic cell division. Glycobiology 21 (6), 834–848 [DOI] [PubMed] [Google Scholar]
- 42).Yamashita T., Wada R., Sasaki T., Deng C., Bierfreund U., Sandhoff K., Proia R.L. (1999) A vital role for glycosphingolipid synthesis during development and differentiation. Proc. Natl. Acad. Sci. U.S.A. 96 (16), 9142–9147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Yamashita T., Allende M.L., Kalkofen D.N., Werth N., Sandhoff K., Proia R.L. (2005) Conditional LoxP-flanked glucosylceramide synthase allele controlling glycosphingolipid synthesis. Genesis 43 (4), 175–180 [DOI] [PubMed] [Google Scholar]
- 44).Jennemann R., Sandhoff R., Wang S., Kiss E., Gretz N., Zuliani C., Martin-Villalba A., Jäger R., Schorle H., Kenzelmann M., Bonrouhi M., Wiegandt H., Gröne H.J. (2005) Cell-specific deletion of glucosylceramide synthase in brain leads to severe neural defects after birth. Proc. Natl. Acad. Sci. U.S.A. 102 (35), 12459–12464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Watanabe S., Endo S., Oshima E., Hoshi T., Higashi H., Yamada K., Tohyama K., Yamashita T., Hirabayashi Y. (2010) Glycosphingolipid synthesis in cerebellar Purkinje neurons: roles in myelin formation and axonal homeostasis. Glia 58 (10), 1197–1207 [DOI] [PubMed] [Google Scholar]
- 46).Saadat L., Dupree J.L., Kilkus J., Han X., Traka M., Proia R.L., Dawson G., Popko B. (2009) Absence of oligodendroglial glucosylceramide synthesis does not result in CNS myelin abnormalities or alter the dysmyelinating phenotype of CGT-deficient mice. Glia 58 (4), 391–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47).Yang L.J., Zeller C.B., Shaper N.L., Kiso M., Hasegawa A., Shapiro R.E., Schnaar R.L. (1996) Gangliosides are neuronal ligands for myelin-associated glycoprotein. Proc. Natl. Acad. Sci. U.S.A. 93 (2), 814–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48).Bartsch U., Montag D., Bartsch S., Schachner M. (1995) Multiple myelinated axons in the optic nerve of mice deficient for the myelin-associated glycoprotein. Glia 14 (2), 115–122 [DOI] [PubMed] [Google Scholar]
- 49).Ito H., Ishida H., Collins B.E., Fromholt S.E., Schnaar R.L., Kiso M. (2003) Systematic synthesis and MAG-binding activity of novel sulfated GM1b analogues as mimics of Chol-1 (α-series) gangliosides: highly active ligands for neural siglecs. Carbohydr. Res. 338 (16), 1621–1639 [DOI] [PubMed] [Google Scholar]
- 50).Marza E., Simonsen K.T., Faergeman N.J., Lesa G.M. (2009) Expression of ceramide glucosyltransferases, which are essential for glycosphingolipid synthesis, is only required in a small subset of C. elegans cells. J. Cell Sci. 122 (Pt 6), 822–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51).De Smedt V., Rime H., Jessus C., Ozon R. (1995) Inhibition of glycosphingolipid synthesis induces p34cdc2 activation in Xenopus oocyte. FEBS Lett. 375 (3), 249–253 [DOI] [PubMed] [Google Scholar]
- 52).Zhang H., Abraham N., Khan L.A., Hall D.H., Fleming J.T., Gobel V. (2011) Apicobasal domain identities of expanding tubular membranes depend on glycosphingolipid biosynthesis. Nat. Cell Biol. 13 (10), 1189–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53).Graham T.R., Burd C.G. (2011) Coordination of Golgi functions by phosphatidylinositol 4-kinases. Trends Cell Biol. 21 (2), 113–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54).Aerts J.M., Ottenhoff R., Powlson A.S., Grefhorst A., van Eijk M., Dubbelhuis P.F., Aten J., Kuipers F., Serlie M.J., Wennekes T., Sethi J.K., O'Rahilly S., Overkleeft H.S. (2007) Pharmacological inhibition of glucosylceramide synthase enhances insulin sensitivity. Diabetes 56 (5), 1341–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55).Bietrix F., Lombardo E., van Roomen C.P., Ottenhoff R., Vos M., Rensen P.C., Verhoeven A.J., Aerts J.M., Groen A.K. (2010) Inhibition of glycosphingolipid synthesis induces a profound reduction of plasma cholesterol and inhibits atherosclerosis development in APOE*3 Leiden and low-density lipoprotein receptor−/− mice. Arterioscler. Thromb. Vasc. Biol. 30 (5), 931–937 [DOI] [PubMed] [Google Scholar]
- 56).Bikman B.T., Summers S.A. (2011) Ceramides as modulators of cellular and whole-body metabolism. J. Clin. Invest. 121 (11), 4222–4230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57).Stratford S., Hoehn K.L., Liu F., Summers S.A. (2004) Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J. Biol. Chem. 279 (35), 36608–36615 [DOI] [PubMed] [Google Scholar]
- 58).Summers S.A. (2006) Ceramides in insulin resistance and lipotoxicity. Prog. Lipid Res. 45 (1), 42–72 [DOI] [PubMed] [Google Scholar]
- 59).Natoli T.A., Smith L.A., Rogers K.A., Wang B., Komarnitsky S., Budman Y., Belenky A., Bukanov N.O., Dackowski W.R., Husson H., Russo R.J., Shayman J.A., Ledbetter S.R., Leonard J.P., Ibraghimov-Beskrovnaya O. (2010) Inhibition of glucosylceramide accumulation results in effective blockade of polycystic kidney disease in mouse models. Nat. Med. 16 (7), 788–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60).Mazzulli J.R., Xu Y.H., Sun Y., Knight A.L., McLean P.J., Caldwell G.A., Sidransky E., Grabowski G.A., Krainc D. (2011) Gaucher disease glucocerebrosidase and α-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell 146 (1), 37–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61).Satoi H., Tomimoto H., Ohtani R., Kitano T., Kondo T., Watanabe M., Oka N., Akiguchi I., Furuya S., Hirabayashi Y., Okazaki T. (2005) Astroglial expression of ceramide in Alzheimer's disease brains: a role during neuronal apoptosis. Neuroscience 130 (3), 657–666 [DOI] [PubMed] [Google Scholar]
- 62).Marks N., Berg M.J., Saito M. (2008) Glucosylceramide synthase decrease in frontal cortex of Alzheimer brain correlates with abnormal increase in endogenous ceramides: consequences to morphology and viability on enzyme suppression in cultured primary neurons. Brain Res. 1191, 136–147 [DOI] [PubMed] [Google Scholar]
- 63).Kobayashi T., Takahashi M., Nagatsuka Y., Hirabayashi Y. (2006) Lipid rafts: new tools and a new component. Biol. Pharm. Bull. 29 (8), 1526–1531 [DOI] [PubMed] [Google Scholar]
- 64).Kunimoto S., Kobayashi T., Kobayashi S., Murakami-Murofushi K. (2000) Expression of cholesteryl glucoside by heat shock in human fibroblasts. Cell Stress Chaperones 5 (1), 3–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65).Kunimoto S., Murofushi W., Yamatsu I., Hasegawa Y., Sasaki N., Kobayashi S., Kobayashi T., Murofushi H., Murakami-Murofushi K. (2003) Cholesteryl glucoside-induced protection against gastric ulcer. Cell Struct. Funct. 28 (3), 179–186 [DOI] [PubMed] [Google Scholar]
- 66).Kunimoto S., Murofushi W., Kai H., Ishida Y., Uchiyama A., Kobayashi T., Kobayashi S., Murofushi H., Murakami-Murofushi K. (2002) Steryl glucoside is a lipid mediator in stress-responsive signal transduction. Cell Struct. Funct. 27 (3), 157–162 [DOI] [PubMed] [Google Scholar]
- 67).Akiyama H., Sasaki N., Hanazawa S., Gotoh M., Kobayashi S., Hirabayashi Y., Murakami-Murofushi K. (2011) Novel sterol glucosyltransferase in the animal tissue and cultured cells: evidence that glucosylceramide as glucose donor. Biochim. Biophys. Acta 1811 (5), 314–322 [DOI] [PubMed] [Google Scholar]
- 68).Hannun Y.A. (1996) Functions of ceramide in coordinating cellular responses to stress. Science 274 (5294), 1855–1859 [DOI] [PubMed] [Google Scholar]
- 69).Obeid L.M., Hannun Y.A. (1995) Ceramide: a stress signal and mediator of growth suppression and apoptosis. J. Cell. Biochem. 58 (2), 191–198 [DOI] [PubMed] [Google Scholar]
- 70).Kim H.J., Lee K.J. (2002) Heat shock and ceramide have different apoptotic pathways in radiation induced fibrosarcoma (RIF) cells. Mol. Cell. Biochem. 229 (1–2), 139–151 [DOI] [PubMed] [Google Scholar]
- 71).Nagy E., Balogi Z., Gombos I., Åkerfelt M., Björkbom A., Balogh G., Török Z., Maslyanko A., Fiszer-Kierzkowska A., Lisowska K., Slotte P.J., Sistonen L., Horváth I., Vígh L. (2007) Hyperfluidization-coupled membrane microdomain reorganization is linked to activation of the heat shock response in a murine melanoma cell line. Proc. Natl. Acad. Sci. U.S.A. 104 (19), 7945–7950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72).Balogh G., Péter M., Liebisch G., Horváth I., Török Z., Nagy E., Maslyanko A., Benkö S., Schmitz G., Harwood J.L., Vígh L. (2010) Lipidomics reveals membrane lipid remodelling and release of potential lipid mediators during early stress responses in a murine melanoma cell line. Biochim. Biophys. Acta 1801 (9), 1036–1047 [DOI] [PubMed] [Google Scholar]
- 73).Nagatsuka Y., Kasama T., Ohashi Y., Uzawa J., Ono Y., Shimizu K., Hirabayashi Y. (2001) A new phosphoglycerolipid, `phosphatidylglucose', found in human cord red cells by multi-reactive monoclonal anti-i cold agglutinin, mAb GL-1/GL-2. FEBS Lett. 497 (2–3), 141–147 [DOI] [PubMed] [Google Scholar]
- 74).Nagatsuka Y., Hara-Yokoyama M., Kasama T., Takekoshi M., Maeda F., Ihara S., Fujiwara S., Ohshima E., Ishii K., Kobayashi T., Shimizu K., Hirabayashi Y. (2003) Carbohydrate-dependent signaling from the phosphatidylglucoside-based microdomain induces granulocytic differentiation of HL60 cells. Proc. Natl. Acad. Sci. U.S.A. 100 (13), 7454–7459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75).Katagiri Y.U., Kiyokawa N., Fujimoto J. (2001) A role for lipid rafts in immune cell signaling. Microbiol. Immunol. 45 (1), 1–8 [DOI] [PubMed] [Google Scholar]
- 76).Katagiri Y.U., Ohmi K., Katagiri C., Sekino T., Nakajima H., Ebata T., Kiyokawa N., Fujimoto J. (2001) Prominent immunogenicity of monosialosyl galactosylgloboside, carrying a stage-specific embryonic antigen-4 (SSEA-4) epitope in the ACHN human renal tubular cell line-a simple method for producing monoclonal antibodies against detergent-insoluble microdomains/raft. Glycoconj. J. 18 (4), 347–353 [DOI] [PubMed] [Google Scholar]
- 77).Yamazaki Y., Nagatsuka Y., Oshima E., Suzuki Y., Hirabayashi Y., Hashikawa T. (2006) Comprehensive analysis of monoclonal antibodies against detergent-insoluble membrane/lipid rafts of HL60 cells. J. Immunol. Methods 311 (1–2), 106–116 [DOI] [PubMed] [Google Scholar]
- 78).Greimel P., Lapeyre M., Nagatsuka Y., Hirabayashi Y., Ito Y. (2008) Syntheses of phosphatidyl-β-D-glucoside analogues to probe antigen selectivity of monoclonal antibody `DIM21'. Bioorg. Med. Chem. 16 (15), 7210–7217 [DOI] [PubMed] [Google Scholar]
- 79).Nagatsuka Y., Horibata Y., Yamazaki Y., Kinoshita M., Shinoda Y., Hashikawa T., Koshino H., Nakamura T., Hirabayashi Y. (2006) Phosphatidylglucoside exists as a single molecular species with saturated fatty acyl chains in developing astroglial membranes. Biochemistry 45 (29), 8742–8750 [DOI] [PubMed] [Google Scholar]
- 80).Kina K., Masuda H., Nakayama H., Nagatsuka Y., Nabetani T., Hirabayashi Y., Takahashi Y., Shimada K., Daida H., Ogawa H., Takamori K., Iwabuchi K. (2011) The novel neutrophil differentiation marker phosphatidylglucoside mediates neutrophil apoptosis. J. Immunol. 186 (9), 5323–5332 [DOI] [PubMed] [Google Scholar]
- 81).Murate M., Hayakawa T., Ishii K., Inadome H., Greimel P., Watanabe M., Nagatsuka Y., Ito K., Ito Y., Takahashi H., Hirabayashi Y., Kobayashi T. (2010) Phosphatidylglucoside forms specific lipid domains on the outer leaflet of the plasma membrane. Biochemistry 49 (23), 4732–4739 [DOI] [PubMed] [Google Scholar]
- 82).Kaneko J., Kinoshita M.O., Machida T., Shinoda Y., Nagatsuka Y., Hirabayashi Y. (2011) Phosphatidylglucoside: a novel marker for adult neural stem cells. J. Neurochem. 116 (5), 840–844 [DOI] [PubMed] [Google Scholar]
- 83).Kinoshita M.O., Furuya S., Ito S., Shinoda Y., Yamazaki Y., Greimel P., Ito Y., Hashikawa T., Machida T., Nagatsuka Y., Hirabayashi Y. (2009) Lipid rafts enriched in phosphatidylglucoside direct astroglial differentiation by regulating tyrosine kinase activity of epidermal growth factor receptors. Biochem. J. 419 (3), 565–575 [DOI] [PubMed] [Google Scholar]
- 84).Kitamura Y., Okazaki T., Nagatsuka Y., Hirabayashi Y., Kato S., Hayashi K. (2007) Immunohistochemical distribution of phosphatidylglucoside using anti-phosphatidylglucoside monoclonal antibody (DIM21). Biochem. Biophys. Res. Commun. 362 (2), 252–255 [DOI] [PubMed] [Google Scholar]
- 85).Oka S., Nagatsuka Y., Kikuchi J., Yokote T., Hirabayashi Y., Hanafusa T., Ozawa K., Muroi K. (2009) Preferential expression of phosphatidylglucoside along neutrophil differentiation pathway. Leuk. Lymphoma 50 (7), 1190–1197 [DOI] [PubMed] [Google Scholar]
- 86).Lee J.H., Koh H., Kim M., Kim Y., Lee S.Y., Karess R.E., Lee S.H., Shong M., Kim J.M., Kim J., Chung J. (2007) Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature 447 (7147), 1017–1020 [DOI] [PubMed] [Google Scholar]
- 87).Kohyama-Koganeya A., Kim Y.J., Miura M., Hirabayashi Y. (2008) A Drosophila orphan G protein-coupled receptor BOSS functions as a glucose-responding receptor: loss of boss causes abnormal energy metabolism. Proc. Natl. Acad. Sci. U.S.A. 105 (40), 15328–15333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88).Kohyama-Koganeya A., Hirabayashi Y. (2010) The Drosophila 7-pass transmembrane glycoprotein BOSS and metabolic regulation: What Drosophila can teach us about human energy metabolism. Methods Enzymol. 480, 525–538 [DOI] [PubMed] [Google Scholar]
- 89).Horibata Y., Nagatsuka Y., Greimel P., Ito Y., Hirabayashi Y. (2007) Sensitivity of phosphatidylglucoside against phospholipases. Anal. Biochem. 365 (1), 149–151 [DOI] [PubMed] [Google Scholar]
- 90).Nagatsuka Y., Hirabayashi Y. (2008) Phosphatidylglucoside: a new marker for lipid rafts. Biochim. Biophys. Acta 1780 (3), 405–409 [DOI] [PubMed] [Google Scholar]
- 91).Ito S., Nabetani T., Shinoda Y., Nagatsuka Y., Hirabayashi Y. (2008) Quantitative analysis of a novel glucosylated phospholipid by liquid chromatography-mass spectrometry. Anal. Biochem. 376 (2), 252–257 [DOI] [PubMed] [Google Scholar]
- 92).Ariga T., Yanagisawa M., Wakade C., Ando S., Buccafusco J.J., McDonald M.P., Yu R.K. (2010) Ganglioside metabolism in a transgenic mouse model of Alzheimer's disease: expression of Chol-1α antigens in the brain. ASN Neuro 2 (4), e00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93).Jennemann R., Sandhoff R., Langbein L., Kaden S., Rothermel U., Gallala H., Sandhoff K., Wiegandt H., Gröne H.J. (2007) Integrity and barrier function of the epidermis critically depend on glucosylceramide synthesis. J. Biol. Chem. 282 (5), 3083–3094 [DOI] [PubMed] [Google Scholar]
- 94).Jennemann R., Rothermel U., Wang S., Sandhoff R., Kaden S., Out R., van Berkel T.J., Aerts J.M., Ghauharali K., Sticht C., Gröne H.J. (2010) Hepatic glycosphingolipid deficiency and liver function in mice. Hepatology 51 (5), 1799–1809 [DOI] [PubMed] [Google Scholar]