Abstract
Platelet activating factor (PAF; 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine) is a phospholipid mediator released from activated macrophages, mast cells, and basophils that promotes pathophysiologic inflammation. Eosinophil responses to PAF are complex and incompletely elucidated. We show here that PAF and its 2-deacetylated metabolite, lysoPAF, promote degranulation (release of eosinophil peroxidase), via a mechanism that is independent of the characterized PAF receptor (PAFR). Specifically, we demonstrate that receptor antagonists CV-3988 and WEB-2086, and pertussis toxin have no impact on PAF- or lysoPAF-mediated degranulation. Furthermore, cultured mouse eosinophils from PAFR−/− bone marrow progenitors degranulate in response to PAF and lysoPAF in a manner indistinguishable from their wild-type counterparts. In addition to PAF and lysoPAF, human eosinophils degranulate in response to lysophosphatidylcholine, but not phosphatidylcholine, lysophosphatidylethanolamine or phosphatidylethanolamine, demonstrating selective responses to phospholipids with a choline head-group and minimal substitution at the sn-2 hydroxyl. Human eosinophils release preformed cytokines in response to PAF, but not lysoPAF, also via a PAFR-independent mechanism. Mouse eosinophils do not release cytokines in response to PAF or lysoPAF, but are capable of doing so in response to IL-6. Overall, our work provides the first direct evidence for a role for PAF in activating and inducing degranulation of mouse eosinophils, a crucial feature for the interpretation of mouse models of PAF-mediated asthma and anaphylaxis. Likewise, we document and define PAF and lysoPAF-mediated activities that are not dependent on signaling via PAFR, suggesting the existence of other, as yet to be explored, molecular signaling pathways mediating responses from PAF, lysoPAF and closely-related phospholipid mediators. [250 words]
Introduction
Platelet activating factor (PAF; 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine) is a unique phospholipid secretory mediator, originally identified as an agent released from IgE-sensitized basophils that promotes platelet aggregation (1, 2). Since its discovery, numerous proinflammatory roles have been attributed to PAF, and it has been explored extensively as a crucial pathophysiologic mediator in asthma, anaphylaxis and other inflammatory states [reviewed in (3-5)]. Recently, heightened interest in the biology of PAF has emerged from the work of Tsujimura and colleagues (6, 7), who have implicated PAF as the primary modulator of systemic anaphylaxis resulting from IgG1-activated mouse basophils. Additionally, Vadas and colleagues (8) presented a positive correlation between serum PAF and the clinical severity of anaphylaxis in human subjects.
In addition to basophils, PAF is synthesized by mast cells and macrophages, and is the end-product and presumed sole active metabolite of a biosynthetic-degradation pathway involving the enzymes PAF-acetyltransferase and PAF-acetylhydrolase, respectively (9, 10). The precursor and product of these enzymatic reactions, the 2-hydroxy-deacetylated derivative of PAF known as lysoPAF, is generally perceived as inactive, or in some cases antagonistic to the biological activities of PAF, although the precise biochemical nature of this functional antagonism remains unclear.
Platelets are only one of many cell populations that respond to PAF [reviewed in (11)]. Human eosinophils are prominent among this group, and have numerous characterized PAF-mediated responses, including chemotaxis, superoxide production, adhesion, and release of cationic granule proteins (12-19). In contrast, mouse eosinophil responses to PAF have not been characterized directly, which is of significant concern, as asthma and anaphylaxis studies focusing on PAF are routinely carried out in mouse model systems (20-22). Human and mouse eosinophils are known for their profound dissimilarities (23), and mouse eosinophils are relatively resistant to stimuli that induce activation and degranulation in human eosinophils both in vitro and in models of inflammation and disease (24-26).
The receptor for PAF (PAFR) is a single seven-trans-membrane G-protein coupled receptor which has been identified and characterized (27-29), along with numerous biochemical receptor antagonists (30-32). PAFR has been detected in human eosinophils (33, 34) and in the eosinophil-related cell line EoL-1 (35). Only one PAF receptor has been identified, yet a number of complexities in receptor-mediated signaling have emerged. Most notable among these is the differential pertussis toxin (PTX) sensitivity for eosinophil chemotactic versus degranulation responses, suggesting the possibility of multiple receptors, multiple receptor conformations, and/or differential association with specific G proteins [reviewed in (36)].
Here, we examine PAF-mediated responses from another perspective, as we explore release of the granule protein, eosinophil peroxidase (EPO) and, for the first time, release of proinflammatory cytokines in response to both PAF and lysoPAF. In doing so, we have identified a distinct and somewhat unanticipated PAF-response pathway that functions independently of the characterized G-protein coupled PAF receptor.
Materials and Methods
Mice
Six to eight-week old wild-type BALB/c and C57BL/6 mice were purchased from Taconic Farms (Rockville, MD). Interleukin-5 transgenic (IL-5Tg) mice on the BALB/c background (37) are maintained on site. Platelet activating factor receptor gene-deleted (PAFR−/−) mice (C57BL/6 background (38)) were graciously provided by Dr. Peter Murray and Dr. Elaine Tuomanen (St. Jude’s Children’s Research Hospital, Memphis, TN); genotype was confirmed prior to use in this study. This study was reviewed and approved via NIAID Animal Study Proposal LAD-7E.
Reagents
All reagents unless otherwise specified were purchased from Sigma-Aldrich. Stock solutions of platelet activating factor (C16-PAF; P4904, 1-Hexadecyl-2-acetyl-sn-glycero-3-phosphocholine; hereafter referred to as PAF), C16-lysoPAF (L5016, 1-Hexadecyl-sn-glycero-3-phosphocholine; hereafter referred to as lysoPAF), C18-PAF (P6537, 1-O-Octadecyl-2-acetyl-sn-glycero-3-phosphocholine) were prepared at 1 or 10 mM in dimethyl-sulfoxide (DMSO); cytochalasin B at 10 mg/mL in DMSO; f-MLF at 50 μg/mL in dimethyl-formamide (DMF). Phosphatidylcholine (PC; 850355P, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine), lysophosphatidylcholine (lysoPC; 855675P, 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphocholine), phosphatidylethanolamine (PE; 856705P, 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine), lysophosphatidylethanolamine (lysoPE; 850705P, 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphoethanolamine) and C18-lysoPAF (878120P, 1-O-octadecyl-2-hydroxy-sn-glycero-3-phosphocholine) were purchased from Avanti Polar Lipids (Albaster, Alabama) and were prepared at 10 mM in DMSO. All subsequent dilutions were prepared in RPMI-1640 without phenol red. Cytokines used to challenge eosinophils were purchased from R&D Systems (Minneapolis, MN); stock solutions were prepared in PBS/0.1% BSA and further diluted in RPMI-1640 without phenol red. Stock solutions of PAF receptor antagonists WEB-2086 and CV-3988 (Enzo Life Sciences, Plymouth Meeting, PA) were prepared at 1 mM in ethanol and diluted in RPMI 1640 without phenol red for use in degranulation and cytokine release assays. Pertussis toxin (PTX) was purchased from Calbiochem and diluted to 100 μg/mL in dH2O. Cycloheximide was purchased as a 100 mg/mL solution and further diluted in RPMI-1640 without phenol red.
Eosinophils from mouse bone marrow progenitor cultures
The method for eosinophil production from unselected bone marrow progenitors was as previously described (39). Briefly, bone marrow cells were suspended in RPMI-1640 (Invitrogen) and seeded at 106/mL in media containing RPMI-1640 with 20% fetal bovine serum (Cambrex), 100 IU/mL penicillin and 10 μg/mL streptomycin (Cellgro), 2 mM glutamine (Invitrogen), 25 mM HEPES, 1x non-essential amino acids, 1 mM sodium pyruvate (Gibco), 50 μM β-mercaptoethanol (Sigma-Aldrich) supplemented with 100 ng/mL stem-cell factor (SCF; PeproTech) and 100 ng/mL FLT3-Ligand (FLT3-L; PeproTech) and maintained in this medium from day 0 to day 4. On day 4, the medium containing SCF and FLT3-L was replaced with fresh medium containing 10 ng/mL recombinant mouse interleukin-5 (rmIL-5; R&D Systems). Cultures were utilized for experimental studies on day 10 through day 16, at >90-100% eosinophils as determined by visual inspection of Diff-Quik stained cytospin preparations.
Isolation of eosinophils from IL-5 transgenic mice
Single cell suspensions were obtained by cutting the spleen into small pieces in Hanks’ Buffered saline solution with 1% fetal bovine serum and 10 mM HEPES. Fragments were passed through a 100 μm strainer followed by a 21 gauge needle. After red blood cell lysis, T and B cells were removed by binding to Miltenyi CD90.2 and CD45R/B220 conjugated magnetic beads on a CS column as per manufacturer’s directions. Purity was assessed at ~80% visual inspection of Diff-Quik stained cytospin preparations, and viability, assessed by trypan blue exclusion, was >99%.
Isolation of human peripheral neutrophils and eosinophils
Human eosinophils were isolated from heparinized whole blood collected from normal volunteers (protocol #NIAID 09-I-0049) using Miltenyi human eosinophil isolation kit (Auburn, CA) as previously described (40). Purity was assessed by visual inspection of Diff-Quik stained cytospin preparations and was routinely >95%, with viability at >99%, as determined by trypan blue exclusion. Initially, eosinophils were maintained in 25 ng/mL recombinant human IL-5 for 18-24 hours at 37oC prior to performing the degranulation assay to detect release of eosinophil peroxidase (EPO). Cytokine release was assayed immediately after eosinophil isolation (i.e. cells were not exposed to IL5) and additional EPO release experiments were performed on freshly isolated eosinophils in the absence of IL5. Neutrophils were obtained by lysis of red blood cells following separation from whole blood using lymphocyte separation medium (MP Biomedicals, Solon, OH). Neutrophils purity was assessed by visual inspection of Diff-Quik stained cytospin preparations and was routinely >90%, with viability at >99%, as determined by trypan blue exclusion.
Degranulation of eosinophil peroxidase (EPO)
Detection of EPO released in response to challenge with platelet-activating factor (PAF) was essentially as described (41). Cells were collected by centrifugation and resuspended in RPMI-1640 without phenol red at 250,000 cells /mL, 100 μL was used per well unless otherwise indicated. One μL of secretagogue or vehicle control was added to achieve the indicated concentrations, and cells were then incubated at 37°C, 5% CO2 for 30 minutes. The assay was developed using 100 μL o-phenylene-diamine (OPD) reagent (800 μL 5mM OPD in 4 mL 1M Tris (pH8.0), 5.2 mL H2O and 1.25 μL 30% H2O2). The reaction was terminated by the addition of 100 μL of 4M H2SO4 to each well and read at 492 nm. In addition to the wells containing the secretagogue to be evaluated, each plate contains a set of cells that remain untreated and a set of wells in which the cells lysed in 0.2 % sodium dodecyl sulfate (SDS; KD Medical) in order to determine the total EPO content. Data is reported as percent of total EPO [(absorbance of stimulated sample – no treatment) × 100/total EPO from SDS-lysed cells]. All data are presented as mean ± SEM. Each experiment represents data from at least three different subjects. PAF receptor antagonists WEB-2086 and CV-3988 were introduced at 10 μM final concentration and cells were pre-incubated for 30 minutes at 37oC prior to the addition of PAF or lysoPAF. In experiments using PTX, the cells were incubated for one hour with 100 ng/mL PTX prior to the addition of PAF.
Cytokine release
Cells were collected by centrifugation and resuspended in RPMI-1640 without phenol red at 106 cells / mL; 100 μL were used per well. One μL of PAF, lysoPAF or vehicle control was added to achieve the 5 μM concentrations. Cells were incubated at 37°C, 5% CO2 for 60 minutes, and the cell free supernatant was stored at −80°C until assayed for cytokine content. Cytokines were assayed by using either the Milliplex (Millipore) or the Bioplex (BioRAD) multibead cytokine assays or by ELISA (R&D Systems) according to the manufacturers’ directions. Human peripheral blood eosinophils were isolated from normal donors; each isolate was assayed in triplicate and the data obtained from each donor are reported separately. The data collected from mouse eosinophils was averaged from 3 different experiments with each trial performed in at least triplicate. Human cytokines assayed include: IL-1β, IL-1 receptor antagonist, IL-2, IL-4, -5, -6, -7, -8, -9, and -10, IL-12(p70), IL-13, IL-15, IL-17, Eotaxin/CCL11, basic FGF, G-CSF, GM-CSF, IFNγ, IP-10, MCP-1, MIP-1α, MIP-1β, PDGF-bb, RANTES/CCL5, TNFα and VEGF. Mouse cytokines assayed included: IL-1α, IL-1β, IL-2, -3, -4, -5, -6, -7, -8, -9, and -10, IL-12(p40), IL-12(p70), IL-13, IL-15, IL-17, Eotaxin/CCL11, G-CSF, GM-CSF, IFNγ, IP-10, KC, MCP-1, MIP-1α, MIP-1β, RANTES/CCL5 and TNFα. The PAF receptor antagonists CV-3988 and WEB-2086 were introduced at 10 μM for 30 minutes at 37oC prior to the addition of PAF to stimulate cytokine release. Cycloheximide was introduced at 5 μg/mL immediately prior to the addition of PAF to inhibit de novo protein synthesis. Recombinant IL6 and Eotaxin (R&D Systems) were used at 20 ng/mL to stimulate degranulation from mouse bmEos.
Measurement of intracellular Ca2+ mobilization
Purified human neutrophils and eosinophils were loaded into Biocoat poly-lysine 96 well plates (BD) at 1.3 × 105 cell/well and centrifuged at 100g for 5minutes to form a monolayer. Intracellular Ca2+ mobilization was measured using FLIPR calcium 3 assay kit (Molecular Devices) according to manufactory’s guide. Briefly, the cells were loaded with fluorescence dye for 50 minutes at 37°C in 5% CO2. The PAFR inhibitors WEB-2086 and CV-3988 were gently added to a final concentration of 10 μM to the appropriate wells and incubated for additional 10 minutes. Serial dilutions of PAF and lysoPAF were prepared separately at 10-fold concentrations and were added automatically during the calcium detection by FLEX Station II (Molecular Devices) instrument. Relative Fluorescence Units (RFUs) were recorded for 180 seconds, and maximum fluorescence signal minus basal minimum signal after agonist addition was used as the response to that agonist. Results are reported as percent of control due to the variability in response between the 3 donors.
Statistical analysis
All analysis was performed in GraphPad Prism 5 (GraphPad Software, La Jolla, CA). Each data set was examined ANOVA followed by either Bonferroni’s or Tukey’s post-test.
Results
Human and mouse eosinophils degranulate in response to platelet activating factor (PAF) and lysoPAF
PAF has been characterized as a chemotactic agent and secretagogue for human eosinophils [reviewed in (36)]. Here we demonstrate that human eosinophils degranulate in response to PAF and to its de-acetylated metabolite, lysoPAF; each promotes dose-dependent release of up to 60% of the total cellular content of the granule protein eosinophil peroxidase (EPO) [Figure 1A]. Mouse eosinophils are typically resistant to agents that are known to activate human eosinophils [(24, 25); Supplemental Figure 1]; however, eosinophils cultured from wild-type mouse bone marrow progenitors (bmEos) and splenic eosinophils isolated from IL-5 transgenic mice degranulate in response to the same concentrations of PAF and lysoPAF that activate human eosinophils [Figures 1B and 1C, respectively]. Similar to what has been reported for human eosinophils (42), mouse eosinophil degranulation in response to PAF is augmented by the fungal metabolite, cytochalasin B, which disrupts microfilament formation and facilitates release of granule proteins [Figure 2A]; cytochalasin B also results in augmented degranulation in response to lysoPAF [Figure 2B]. In contrast, treatment with brefeldin A, which disrupts the Golgi apparatus and results in diminished release of IL-4 from human eosinophil granules (43), has no impact on PAF or lysoPAF-mediated EPO release from mouse eosinophils.
Figure 1. Eosinophils degranulate in response to challenge with platelet activating factor (PAF) and lysoPAF.
Eosinophil peroxidase (EPO) release from (A) human peripheral blood eosinophils, (B) mouse eosinophils cultured from bone marrow progenitors; bmEos (39), (C) splenic eosinophils from IL-5Tg mice.
Figure 2. Eosinophil degranulation in response to PAF and lysoPAF is augmented by cytochalasin B, but is unaffected by brefeldin A.

Mouse bmEos challenged with increasing concentrations of (A) PAF or (B) lysoPAF in the presence of 5 μg/mL cytochalasin B or 1 μg/mL brefeldin A. ***p < 0.001, EPO release in the presence vs. the absence of cytochalasin B.
PAF and lysoPAF promote degranulation via PAF receptor-independent mechanism(s)
The seven-transmembrane G protein-coupled PAF receptor (PAFR) has been detected on human eosinophils (33, 34) but its existence on mouse eosinophils has only been inferred from in vivo studies. Transcript encoding PAFR was detected in cultured bmEos by qPCR (data not shown). The synthetic PAFR antagonists WEB-2086 and CV-3988, both characterized as blocking human eosinophil effector functions in vivo as well as in vitro (44-46) had no impact on PAF- or lysoPAF-mediated release of EPO from human eosinophils [Figures 3A and 3B]. Likewise, neither antagonist had any effect on degranulation of mouse bmEos, nor on eosinophils from IL-5 transgenic mice, either in response PAF or lysoPAF [Figures 3C – 3F]. Consistent with earlier findings (36), we observe PAF-induced calcium mobilization in human eosinophils, and also in neutrophils [Figure 4A and B, respectively]. However, calcium mobilization was inhibited by 10 μM WEB-2086 in neutrophils only. Pretreatment for 1 hour with PTX has no impact on EPO release from human eosinophils in response to PAF or lysoPAF [Figure 5]. Finally, bmEos generated from PAFR gene-deleted mice (PAFR−/−) responded to both PAF and lysoPAF [Figure 6A and 6B, respectively] releasing EPO in a manner that was indistinguishable from that of bmEos derived from PAFR-sufficient, wild-type mice (WT).
Figure 3. PAF receptor antagonists have no impact on eosinophil degranulation in response to PAF or lysoPAF. (A, B).
Human eosinophils, (C, D) mouse bmEos and (E, F) eosinophils from IL-5tg mice challenged with increasing concentrations of PAF (A, C, E) or lysoPAF (B, D, F) in the absence or presence of 10 μM PAF receptor antagonists CV-3988 or WEB-2086.
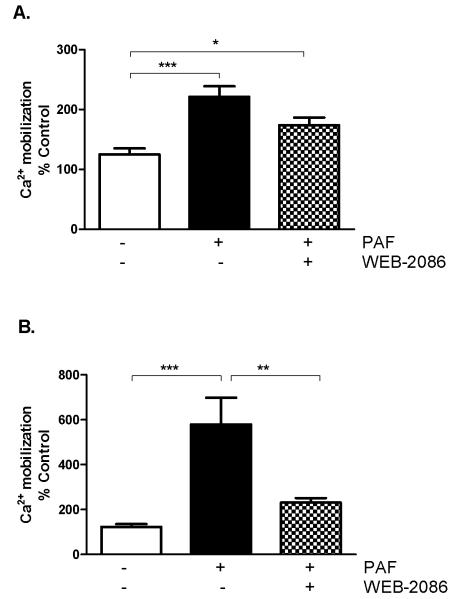
Figure 4. WEB-2086 antagonizes PAF-mediated activation of human neutrophils but not eosinophils.
Ca+2 mobilization in (A) eosinophils and (B) neutrophils stimulated with 5 μM PAF in the presence of the PAFR antagonist WEB-2086 (10 μM). Data are pooled from three separate experiments using different donors and presented as percent Ca+2 mobilization in untreated cells. *p < 0.05, **p < 0.01, ***p < 0.001
Figure 5. Eosinophil degranulation in response to PAF is not sensitive to PTX.

Release of EPO from human eosinophils in response to 5 μM PAF or lysoPAF following pretreatment for one hour with 100 ng/mL pertussis toxin (PTX).
Figure 6. Eosinophils from PAFR gene deleted (PAFR−/−) mice degranulate in response to PAF and lysoPAF.

Wild-type and PAFR−/− bmEos challenged with increasing concentrations of (A) PAF or (B) lysoPAF.
Human eosinophils degranulate in response to a specific subset of phospholipid mediators
In an effort to characterize a potentially novel receptor or pattern recognition molecule mediating responses to both PAF and lysoPAF, we challenged human eosinophils with a series of structurally related phospholipid mediators [Figure 7]. In addition to PAF and lysoPAF, eosinophils released EPO in response to C18-lysoPAF and lysoPC over the same range of concentrations (1 – 5 μM); no degranulation was observed in response to PE, lysoPE PC, or C18-PAF. These results suggest that eosinophils respond selectively to phospholipids with a quaternary saturated amine (ie…choline) head-group and no (lysoPC, lysoPAF, C18-lysoPAF) or minimal substitution (PAF) at the sn-2 hydroxyl (see http://www.avantilipids.com for detailed structures). Additional characterization will be necessary in order to explain why C18-PAF is inactive in this assay.
Figure 7. Human eosinophil degranulation in response to a select subset of phospholipid mediators.

Release of EPO in response to increasing concentrations of PAF, lysoPAF, C18-PAF, C18-lysoPAF, PE, lysoPE, PC, and lysoPC. Pooled data from experiments performed in triplicate with eosinophils from 3 – 4 normal donors.
Human eosinophils experienced no loss of viability in response to challenge with any of the oxidized phospholipids evaluated [PAF and lysoPAF in Supplemental Figure 2A; others, data not shown]. In contrast, the viability of mouse bmEos (both wild-type and PAFR−/−) was reduced to ~10% in response to PAF challenge in experiments carried out using 25,000 cells per 100 μL. Loss of viability in response to PAF or lysoPAF was less profound when cells were maintained at concentrations of 50,000 or 100,000 cells per 100 μL, and the extent of PAF-mediated degranulation of cultured mouse eosinophils increased with enhanced viability [Supplemental Figure 2B]. However, degranulation was not merely a function of loss of viability, as cells at 25,000 per 100 μL challenged with lysoPAF did not undergo substantial loss of viability and the extent of degranulation in response to PAF and lysoPAF at this cell density was indistinguishable [Figure 1].
Human eosinophils release cytokines in response to PAF but not lysoPAF
Numerous cytokines have been localized to the specific granules of human eosinophils [reviewed in (47, 48)]; recent work has indicated that they are mobilized and released via distinct intracellular signaling mechanisms (49-52). Among the cytokines released in response to PAF are IL-13, IL-1 receptor antagonist, eotaxin/CCL11, basic fibroblast growth factor, RANTES/CCL5, IL-9 and platelet-derived growth factor [Figure 8A-8G]. No significant increase in cytokine release over vehicle control was observed from human eosinophils in response to lysoPAF with the exception of one donor who released a small amount of RANTES/CCL5 in response to this mediator[Figure 8G]. In a further exploration using eosinophils from 9 – 12 independent donors, we found that PAF consistently induced the release of RANTES/CCL5 while lysoPAF did not. Receptor antagonists CV-3988 and WEB-2086 had no impact on PAF-mediated RANTES/CCL5 release [Figure 8H]. Additionally, treatment with cycloheximide had no effect on PAF-stimulated RANTES release, consistent with the interpretation that PAF is inducing cytokine release from pre-formed granule stores rather than inducing synthesis de novo [Figure 8I]. In contrast, mouse bmEos do not release any cytokines in response to PAF, and released minimal amounts of IL-9 only in response to lysoPAF (data not shown). However, these cells are capable of cytokine release in response to other mediators [Figure 9]. BmEos challenged with IL-6 released significant quantities of IL-1β, IL-9, IL-12(p70), IFNγ, TNFα, and CCL2/MCP-1. In contrast, little to no cytokine release was observed in response to challenge with eotaxin/CCL11.
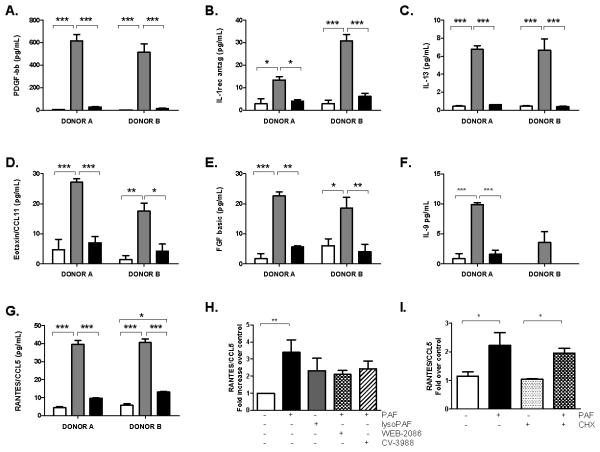
Figure 8. Cytokines are released from human eosinophils in response to PAF, but not lysoPAF.
Cytokines released in response to 5 μM PAF (gray bars) or lysoPAF (black bars) or DMSo control (white bars) include (A) platelet-derived growth factor-bb (B) interleukin-1 receptor antagonist (C) interleukin-13 (D) eotaxin-1 (E) fibroblast growth factor basic (FGF-basic), (F) interleukin-9 (IL-9) (F), (G) RANTES / CCL5. Data collected from two independent donors, each assayed in triplicate. (H) RANTES / CCL5 release in response to 5 μM PAF or lysoPAF in absence and presence of 10 μM PAFR antagonists, pooled data from 9-12 separate donors. (I) RANTES / CCL5 release in response to 5 μM PAF in the presence or absence of 5 μg/mL cycloheximide. Significance determined by 1-way ANOVA followed by Tukey’s multiple comparison; * p<0.05, ** p<0.01, *** p<0.001.
Figure 9. Cytokines are released from mouse eosinophils in response to proinflammatory mediators other than PAF and lysoPAF.
Cytokine release in response to 20 ng/mL IL-6 (black bars), eotaxin (hatched bars) or no treatment control (white bars) was assessed in three independent experiments, each including bmEos generated from 3 different mice. Significance determined by 1-way ANOVA followed by Tukey’s multiple comparison; * p<0.05, ** p<0.01, *** p<0.001.
Discussion
Given the recent findings implicating PAF in the pathophysiology of acute anaphylaxis (8, 20), the biological impact of this mediator has come under renewed scrutiny (4, 53). PAF is a phospholipid secretory mediator synthesized by basophils, mast cells and macrophages, and is the presumed sole active metabolite of a biosynthetic and degradation pathway involving the enzymes PAF-acetyltransferase and PAF-acetylhydrolase, respectively (9, 10). The precursor and product of these enzymatic reactions, the 2-hydroxy-deacetylated derivative of PAF known as lysoPAF, is generally considered inactive or in some cases antagonistic to the activities of PAF, although the precise biochemical nature of this antagonism remains unclear. Recently, Welch and colleagues (54) demonstrated that the opposing actions of PAF and lysoPAF on neutrophils are clearly not classic single receptor-based competitive antagonism, as neither classic biochemical antagonists nor PAFR gene-deletion had any impact on the antagonistic, or opposing actions carried out by lysoPAF. In eosinophils, we find that PAF and lysoPAF both promote dose-dependent degranulation responses, and both responses occurred independently of biochemical antagonists or PAFR gene-deletion. The fact that eosinophils degranulate equally effectively to challenge with either PAF and lysoPAF may explain some of the clinically unsatisfying results achieved with cloned PAF-acetylhydrolase, an agent intended to hydrolyze PAF to lysoPAF systemically, and explored for its effectiveness as an anti-asthma therapeutic (55).
In addition to PAF and lysoPAF, human eosinophils degranulate in response to C18-lysoPAF and lysoPC, phospholipids with a choline head group and either minimal or no fatty acid substitution at the sn-2 hydroxyl. Phospholipids without the saturated quaternary amine (ie…ethanolamine in place of choline) or with extensive sn-2 substitution do not promote degranulation. The identity of this receptor or pattern response molecule is not immediately apparent. PAFR is the only known receptor that responds to PAF, although the family of lipid receptors is large, and there are quite a few orphan receptors that remain unexplored and incompletely characterized (reviewed in (53)). The G2A receptor was initially characterized as responding to lysoPC, but this finding has recently been reconsidered (56). Likewise, several groups have reported that the actions of lysoPC are dependent on the presence of the receptor GPR4 (57, 58) yet the receptor itself has been characterized alternatively as ligand-independent (59) or proton sensing (60). However, it is interesting to note that the PAF biosynthetic enzyme acetyl-CoA:LYSO-PAF acetyltransferase can acetylate both lysoPAF and lysoPC (61); this suggests the possibility that there may be one or more phospholipid receptors with a similarly flexible ligand/substrate specificity.
We have demonstrated for the first time that PAF promotes the release of cytokines from human eosinophils; the subset released includes IL-13, IL-1 receptor antagonist, eotaxin-1, basic fibroblast growth factor, RANTES, IL-9 and platelet-derived growth factor. Most of these cytokines have been identified previously as components of human eosinophils (47, 48). Of those that have not, IL-1 receptor antagonist has been associated with eosinophilia (62) but it has not previously been identified directly as an eosinophil component. This is the first report of basic fibroblast growth factor as an endogenous eosinophil secretory mediator. Interestingly, we detected no release of IL-4 or IFNγ in response to PAF, which are both among the most prominent of the cytokines present in human eosinophil granules (63). Although many different cytokines have been localized to the eosinophil granule, they are not all released in response to a single given stimulus. The current model for cytokine release involves receptor sorting and differential mobilization of specific cytokine components in response to individual eosinophil-active agents; this has been described in detail for the release of IL-4 in response to eotaxin (49-52, 63, 64), and is the molecular basis for the electron-microscopic description of “piecemeal degranulation” (51). Given the role implicated for PAF in the pathogenesis of asthma (4, 65), one might envision shared PAF-induced signals that might serve to mobilize basic fibroblast growth factor, IL-13, and PDGF, which are all implicated in fibrosis and tissue remodeling (66-68). Similarly, eotaxin and RANTES are eosinophil chemoattractants (69, 70), which could be mobilized in response to PAF, and then serve to promote further eosinophil recruitment toward sites in need of remodeling and repair.
It is intriguing and important to note that, while EPO release is more or less equivalent and indistinguishable when comparing human and mouse eosinophils, cytokine release in response to PAF is not observed in mouse eosinophils – a substantial caveat for researchers studying mouse models of eosinophil pathobiology, and of particular importance with respect to mouse models invoking PAF in the pathogenesis of asthma (4, 65). There are many differences between human and mouse eosinophils (reviewed in (23)); among these differences, the evolutionary divergence of the human and mouse eosinophil secretory ribonucleases and cell surface Siglec proteins, the absence of the prominent galectin-10 protein and high affinity IgE receptor in the mouse eosinophils, and distinct responses to chemotactic cytokines and agents stimulating degranulation. Differential cytokine release in response to PAF can now be recognized as among the prominent differences between human and mouse eosinophils.
In summary, both human and mouse eosinophils degranulate, releasing EPO in a dose dependent fashion in response to PAF and also to its de-acetylated metabolite, lysoPAF. Studies with receptor antagonists, signaling pathway inhibitors, and gene-deleted mice clearly document that eosinophil degranulation is distinct from other PAF-mediated responses, and is not dependent on signaling via the characterized G-protein coupled PAFR. In addition to PAF and PAF-derivatives, human eosinophils also degranulate in response to lysoPC but do not degranulate in response to other closely related phospholipid mediators, including PC or PE and its derivatives. Taken together, our results suggest that eosinophil degranulation in response to PAF and related phospholipid ligands is specific and selective, involving an as-yet-to-be-characterized receptor or pattern recognition molecule.
Supplementary Material
Acknowledgements
The authors thank Dr. Peter Murray and Dr. Elaine Tuomanen of St. Jude’s Children’s Research Hospital for providing the PAFR−/− mice, and Dr. Geli Gao for the genotyping protocol. We thank Ms. Daly Cantave and Dr. Todd Wilson for their assistance in arranging for normal blood donors.
Footnotes
The Eosinophil Biology Section is supported by funding from the NIAID Division of Intramural Research (NIAID AI00941-06) to HFR
References
- 1.Benveniste J, Henson PM, Cochrane CG. Leukocyte-dependent histamine release from rabbit platelets. The role of IgE, basophils, and a platelet-activating factor. J Exp Med. 1972;136:1356–1377. doi: 10.1084/jem.136.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanahan DJ. Platelet activating factor: a biologically active phosphoglyceride. Annu Rev Biochem. 1986;55:483–509. doi: 10.1146/annurev.bi.55.070186.002411. [DOI] [PubMed] [Google Scholar]
- 3.Finkelman FD. Anaphylaxis: lessons from mouse models. J Allergy Clin Immunol. 2007;120:506–515. doi: 10.1016/j.jaci.2007.07.033. quiz 516-507. [DOI] [PubMed] [Google Scholar]
- 4.Kasperska-Zajac A, Brzoza Z, Rogala B. Platelet activating factor as a mediator and therapeutic approach in bronchial asthma. Inflammation. 2008;31:112–120. doi: 10.1007/s10753-007-9056-9. [DOI] [PubMed] [Google Scholar]
- 5.Montrucchio G, Alloatti G, Camussi G. Role of platelet-activating factor in cardiovascular pathophysiology. Physiol Rev. 2000;80:1669–1699. doi: 10.1152/physrev.2000.80.4.1669. [DOI] [PubMed] [Google Scholar]
- 6.Karasuyama H, Mukai K, Tsujimura Y, Obata K. Newly discovered roles for basophils: a neglected minority gains new respect. Nat Rev Immunol. 2009;9:9–13. doi: 10.1038/nri2458. [DOI] [PubMed] [Google Scholar]
- 7.Tsujimura Y, Obata K, Mukai K, Shindou H, Yoshida M, Nishikado H, Kawano Y, Minegishi Y, Shimizu T, Karasuyama H. Basophils play a pivotal role in immunoglobulin-G-mediated but not immunoglobulin-E-mediated systemic anaphylaxis. Immunity. 2008;28:581–589. doi: 10.1016/j.immuni.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Vadas P, Gold M, Perelman B, Liss GM, Lack G, Blyth T, Simons FE, Simons KJ, Cass D, Yeung J. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med. 2008;358:28–35. doi: 10.1056/NEJMoa070030. [DOI] [PubMed] [Google Scholar]
- 9.Karabina SA, Ninio E. Plasma PAF-acetylhydrolase: an unfulfilled promise? Biochim Biophys Acta. 2006;1761:1351–1358. doi: 10.1016/j.bbalip.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Snyder F. Platelet-activating factor: the biosynthetic and catabolic enzymes. Biochem J. 1995;305(Pt 3):689–705. doi: 10.1042/bj3050689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishii S, Nagase T, Shimizu T. Platelet-activating factor receptor. Prostaglandins Other Lipid Mediat. 2002;68-69:599–609. doi: 10.1016/s0090-6980(02)00058-8. [DOI] [PubMed] [Google Scholar]
- 12.Bankers-Fulbright JL, Kephart GM, Bartemes KR, Kita H, O’Grady SM. Platelet-activating factor stimulates cytoplasmic alkalinization and granule acidification in human eosinophils. J Cell Sci. 2004;117:5749–5757. doi: 10.1242/jcs.01498. [DOI] [PubMed] [Google Scholar]
- 13.Horie S, Kita H. CD11b/CD18 (Mac-1) is required for degranulation of human eosinophils induced by human recombinant granulocyte-macrophage colony-stimulating factor and platelet-activating factor. J Immunol. 1994;152:5457–5467. [PubMed] [Google Scholar]
- 14.Kroegel C, Yukawa T, Dent G, Venge P, Chung KF, Barnes PJ. Stimulation of degranulation from human eosinophils by platelet-activating factor. J Immunol. 1989;142:3518–3526. [PubMed] [Google Scholar]
- 15.Takizawa T, Kato M, Kimura H, Suzuki M, Tachibana A, Obinata H, Izumi T, Tokuyama K, Morikawa A. Inhibition of protein kinases A and C demonstrates dual modes of response in human eosinophils stimulated with platelet-activating factor. J Allergy Clin Immunol. 2002;110:241–248. doi: 10.1067/mai.2002.126303. [DOI] [PubMed] [Google Scholar]
- 16.Takizawa T, Kato M, Suzuki M, Tachibana A, Motegi Y, Fujiu T, Kimura H, Arakawa H, Mochizuki H, Tokuyama K, Morikawa A. Distinct isoforms of protein kinase C are involved in human eosinophil functions induced by platelet-activating factor. Int Arch Allergy Immunol. 2003;131(Suppl 1):15–19. doi: 10.1159/000070476. [DOI] [PubMed] [Google Scholar]
- 17.Wardlaw AJ, Moqbel R, Cromwell O, Kay AB. Platelet-activating factor. A potent chemotactic and chemokinetic factor for human eosinophils. J Clin Invest. 1986;78:1701–1706. doi: 10.1172/JCI112765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zoratti EM, Sedgwick JB, Vrtis RR, Busse WW. The effect of platelet-activating factor on the generation of superoxide anion in human eosinophils and neutrophils. J Allergy Clin Immunol. 1991;88:749–758. doi: 10.1016/0091-6749(91)90182-n. [DOI] [PubMed] [Google Scholar]
- 19.Kimani G, Tonnesen MG, Henson PM. Stimulation of eosinophil adherence to human vascular endothelial cells in vitro by platelet-activating factor. J Immunol. 1988;140:3161–3166. [PubMed] [Google Scholar]
- 20.Finkelman FD, Rothenberg ME, Brandt EB, Morris SC, Strait RT. Molecular mechanisms of anaphylaxis: lessons from studies with murine models. J Allergy Clin Immunol. 2005;115:449–457. doi: 10.1016/j.jaci.2004.12.1125. quiz 458. [DOI] [PubMed] [Google Scholar]
- 21.Henderson WR, Jr., Lu J, Poole KM, Dietsch GN, Chi EY. Recombinant human platelet-activating factor-acetylhydrolase inhibits airway inflammation and hyperreactivity in mouse asthma model. J Immunol. 2000;164:3360–3367. doi: 10.4049/jimmunol.164.6.3360. [DOI] [PubMed] [Google Scholar]
- 22.Ishii S, Nagase T, Shindou H, Takizawa H, Ouchi Y, Shimizu T. Platelet-activating factor receptor develops airway hyperresponsiveness independently of airway inflammation in a murine asthma model. J Immunol. 2004;172:7095–7102. doi: 10.4049/jimmunol.172.11.7095. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol. 2007;119:1303–1310. doi: 10.1016/j.jaci.2007.03.048. quiz 1311-1302. [DOI] [PubMed] [Google Scholar]
- 24.Clark K, Simson L, Newcombe N, Koskinen AM, Mattes J, Lee NA, Lee JJ, Dent LA, Matthaei KI, Foster PS. Eosinophil degranulation in the allergic lung of mice primarily occurs in the airway lumen. J Leukoc Biol. 2004;75:1001–1009. doi: 10.1189/jlb.0803391. [DOI] [PubMed] [Google Scholar]
- 25.Lee JJ, Lee NA. Eosinophil degranulation: an evolutionary vestige or a universally destructive effector function? Clin Exp Allergy. 2005;35:986–994. doi: 10.1111/j.1365-2222.2005.02302.x. [DOI] [PubMed] [Google Scholar]
- 26.Ochkur SI, Jacobsen EA, Protheroe CA, Biechele TL, Pero RS, McGarry MP, Wang H, O’Neill KR, Colbert DC, Colby TV, Shen H, Blackburn MR, Irvin CC, Lee JJ, Lee NA. Coexpression of IL-5 and eotaxin-2 in mice creates an eosinophil-dependent model of respiratory inflammation with characteristics of severe asthma. J Immunol. 2007;178:7879–7889. doi: 10.4049/jimmunol.178.12.7879. [DOI] [PubMed] [Google Scholar]
- 27.Honda Z, Nakamura M, Miki I, Minami M, Watanabe T, Seyama Y, Okado H, Toh H, Ito K, Miyamoto T, et al. Cloning by functional expression of platelet-activating factor receptor from guinea-pig lung. Nature. 1991;349:342–346. doi: 10.1038/349342a0. [DOI] [PubMed] [Google Scholar]
- 28.Ishii S, Kuwaki T, Nagase T, Maki K, Tashiro F, Sunaga S, Cao WH, Kume K, Fukuchi Y, Ikuta K, Miyazaki J, Kumada M, Shimizu T. Impaired anaphylactic responses with intact sensitivity to endotoxin in mice lacking a platelet-activating factor receptor. J Exp Med. 1998;187:1779–1788. doi: 10.1084/jem.187.11.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishii S, Shimizu T. Platelet-activating factor (PAF) receptor and genetically engineered PAF receptor mutant mice. Prog Lipid Res. 2000;39:41–82. doi: 10.1016/s0163-7827(99)00016-8. [DOI] [PubMed] [Google Scholar]
- 30.Koltai M, Guinot P, Hosford D, Braquet PG. Platelet-activating factor antagonists: scientific background and possible clinical applications. Adv Pharmacol. 1994;28:81–167. [PubMed] [Google Scholar]
- 31.Negro Alvarez JM, Miralles Lopez JC, Ortiz Martinez JL, Abellan Aleman A, Rubio del Barrio R. Platelet-activating factor antagonists. Allergol Immunopathol (Madr) 1997;25:249–258. [PubMed] [Google Scholar]
- 32.Summers JB, Albert DH. Platelet activating factor antagonists. Adv Pharmacol. 1995;32:67–168. doi: 10.1016/s1054-3589(08)61012-1. [DOI] [PubMed] [Google Scholar]
- 33.Korth RM. Specific high affinity binding of platelet activating factor to intact human blood neutrophils and eosinophils. Int Arch Allergy Immunol. 1996;110:124–131. doi: 10.1159/000237276. [DOI] [PubMed] [Google Scholar]
- 34.Ukena D, Krogel C, Dent G, Yukawa T, Sybrecht G, Barnes PJ. PAF-receptors on eosinophils: identification with a novel ligand, [3H]WEB 2086. Biochem Pharmacol. 1989;38:1702–1705. doi: 10.1016/0006-2952(89)90322-5. [DOI] [PubMed] [Google Scholar]
- 35.Izumi T, Kishimoto S, Takano T, Nakamura M, Miyabe Y, Nakata M, Sakanaka C, Shimizu T. Expression of human platelet-activating factor receptor gene in EoL-1 cells following butyrate-induced differentiation. Biochem J. 1995;305(Pt 3):829–835. doi: 10.1042/bj3050829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kato M, Kita H, Tachibana A, Hayashi Y, Tsuchida Y, Kimura H. Dual signaling and effector pathways mediate human eosinophil activation by platelet-activating factor. Int Arch Allergy Immunol. 2004;134(Suppl 1):37–43. doi: 10.1159/000077791. [DOI] [PubMed] [Google Scholar]
- 37.Dent LA, Strath M, Mellor AL, Sanderson CJ. Eosinophilia in transgenic mice expressing interleukin 5. J Exp Med. 1990;172:1425–1431. doi: 10.1084/jem.172.5.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Radin JN, Orihuela CJ, Murti G, Guglielmo C, Murray PJ, Tuomanen EI. beta-Arrestin 1 participates in platelet-activating factor receptor-mediated endocytosis of Streptococcus pneumoniae. Infect Immun. 2005;73:7827–7835. doi: 10.1128/IAI.73.12.7827-7835.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dyer KD, Moser JM, Czapiga M, Siegel SJ, Percopo CM, Rosenberg HF. Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J Immunol. 2008;181:4004–4009. doi: 10.4049/jimmunol.181.6.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Percopo CM, Dyer KD, Killoran KE, Rosenberg HF. Isolation of human eosinophils: microbead method has no impact on IL-5 sustained viability. Exp Dermatol. 2009 doi: 10.1111/j.1600-0625.2009.00974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adamko DJ, Wu Y, Gleich GJ, Lacy P, Moqbel R. The induction of eosinophil peroxidase release: improved methods of measurement and stimulation. J Immunol Methods. 2004;291:101–108. doi: 10.1016/j.jim.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 42.Takafuji S, Tadokoro K, Ito K, Nakagawa T. Release of granule proteins from human eosinophils stimulated with mast-cell mediators. Allergy. 1998;53:951–956. doi: 10.1111/j.1398-9995.1998.tb03795.x. [DOI] [PubMed] [Google Scholar]
- 43.Bandeira-Melo C, Sugiyama K, Woods LJ, Weller PF. Cutting edge: eotaxin elicits rapid vesicular transport-mediated release of preformed IL-4 from human eosinophils. J Immunol. 2001;166:4813–4817. doi: 10.4049/jimmunol.166.8.4813. [DOI] [PubMed] [Google Scholar]
- 44.Miyagawa H, Nabe M, Hopp RJ, Okada C, Bewtra AK, Townley G. The effect of WEB 2086 on PAF-induced eosinophil chemotaxis and LTC4 production from eosinophils. Agents Actions. 1992;37:39–43. doi: 10.1007/BF01987888. [DOI] [PubMed] [Google Scholar]
- 45.Oshiro T, Kakuta Y, Shimura S, Nara M, Shirato K. Characterization of platelet-activating factor-induced cytosolic calcium mobilization in human eosinophils. Clin Exp Allergy. 2000;30:699–705. doi: 10.1046/j.1365-2222.2000.00786.x. [DOI] [PubMed] [Google Scholar]
- 46.Takizawa H, Ishii A, Suzuki S, Shiga J, Miyamoto T. Bronchoconstriction induced by platelet-activating factor in the guinea pig and its inhibition by CV-3988, a PAF antagonist: serial changes in findings of lung histology and bronchoalveolar lavage cell population. Int Arch Allergy Appl Immunol. 1988;86:375–382. doi: 10.1159/000234622. [DOI] [PubMed] [Google Scholar]
- 47.Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, Kay AB, Rothenberg ME. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008;38:709–750. doi: 10.1111/j.1365-2222.2008.02958.x. [DOI] [PubMed] [Google Scholar]
- 48.Lacy P, Moqbel R. Eosinophil cytokines. Chem Immunol. 2000;76:134–155. doi: 10.1159/000058782. [DOI] [PubMed] [Google Scholar]
- 49.Melo RC, Perez SA, Spencer LA, Dvorak AM, Weller PF. Intragranular vesiculotubular compartments are involved in piecemeal degranulation by activated human eosinophils. Traffic. 2005;6:866–879. doi: 10.1111/j.1600-0854.2005.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Melo RC, Spencer LA, Dvorak AM, Weller PF. Mechanisms of eosinophil secretion: large vesiculotubular carriers mediate transport and release of granule-derived cytokines and other proteins. J Leukoc Biol. 2008;83:229–236. doi: 10.1189/jlb.0707503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Melo RC, Spencer LA, Perez SA, Ghiran I, Dvorak AM, Weller PF. Human eosinophils secrete preformed, granule-stored interleukin-4 through distinct vesicular compartments. Traffic. 2005;6:1047–1057. doi: 10.1111/j.1600-0854.2005.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spencer LA, Melo RC, Perez SA, Bafford SP, Dvorak AM, Weller PF. Cytokine receptor-mediated trafficking of preformed IL-4 in eosinophils identifies an innate immune mechanism of cytokine secretion. Proc Natl Acad Sci U S A. 2006;103:3333–3338. doi: 10.1073/pnas.0508946103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shimizu T. Lipid mediators in health and disease: enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu Rev Pharmacol Toxicol. 2009;49:123–150. doi: 10.1146/annurev.pharmtox.011008.145616. [DOI] [PubMed] [Google Scholar]
- 54.Welch EJ, Naikawadi RP, Li Z, Lin P, Ishii S, Shimizu T, Tiruppathi C, Du X, Subbaiah PV, Ye RD. Opposing effects of platelet-activating factor and lyso-platelet-activating factor on neutrophil and platelet activation. Mol Pharmacol. 2009;75:227–234. doi: 10.1124/mol.108.051003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Henig NR, Aitken ML, Liu MC, Yu AS, Henderson WR., Jr. Effect of recombinant human platelet-activating factor-acetylhydrolase on allergen-induced asthmatic responses. Am J Respir Crit Care Med. 2000;162:523–527. doi: 10.1164/ajrccm.162.2.9911084. [DOI] [PubMed] [Google Scholar]
- 56.Bercher M, Hanson B, van Staden C, Wu K, Ng GY, Lee PH. Agonists of the orphan human G2A receptor identified from inducible G2A expression and beta-lactamase reporter screen. Assay Drug Dev Technol. 2009;7:133–142. doi: 10.1089/adt.2008.179. [DOI] [PubMed] [Google Scholar]
- 57.Meyer zu Heringdorf D, Jakobs KH. Lysophospholipid receptors: signalling, pharmacology and regulation by lysophospholipid metabolism. Biochim Biophys Acta. 2007;1768:923–940. doi: 10.1016/j.bbamem.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 58.Qiao J, Huang F, Naikawadi RP, Kim KS, Said T, Lum H. Lysophosphatidylcholine impairs endothelial barrier function through the G protein-coupled receptor GPR4. Am J Physiol Lung Cell Mol Physiol. 2006;291:L91–101. doi: 10.1152/ajplung.00508.2005. [DOI] [PubMed] [Google Scholar]
- 59.Bektas M, Barak LS, Jolly PS, Liu H, Lynch KR, Lacana E, Suhr KB, Milstien S, Spiegel S. The G protein-coupled receptor GPR4 suppresses ERK activation in a ligand-independent manner. Biochemistry. 2003;42:12181–12191. doi: 10.1021/bi035051y. [DOI] [PubMed] [Google Scholar]
- 60.Tobo M, Tomura H, Mogi C, Wang JQ, Liu JP, Komachi M, Damirin A, Kimura T, Murata N, Kurose H, Sato K, Okajima F. Previously postulated “ligand-independent” signaling of GPR4 is mediated through proton-sensing mechanisms. Cell Signal. 2007;19:1745–1753. doi: 10.1016/j.cellsig.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 61.Shindou H, Hishikawa D, Nakanishi H, Harayama T, Ishii S, Taguchi R, Shimizu T. A single enzyme catalyzes both platelet-activating factor production and membrane biogenesis of inflammatory cells. Cloning and characterization of acetyl-CoA:LYSO-PAF acetyltransferase. J Biol Chem. 2007;282:6532–6539. doi: 10.1074/jbc.M609641200. [DOI] [PubMed] [Google Scholar]
- 62.Allen JN, Liao Z, Wewers MD, Altenberger EA, Moore SA, Allen ED. Detection of IL-5 and IL-1 receptor antagonist in bronchoalveolar lavage fluid in acute eosinophilic pneumonia. J Allergy Clin Immunol. 1996;97:1366–1374. doi: 10.1016/s0091-6749(96)70206-3. [DOI] [PubMed] [Google Scholar]
- 63.Spencer LA, Szela CT, Perez SA, Kirchhoffer CL, Neves JS, Radke AL, Weller PF. Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J Leukoc Biol. 2009;85:117–123. doi: 10.1189/jlb.0108058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Melo RC, Spencer LA, Perez SA, Neves JS, Bafford SP, Morgan ES, Dvorak AM, Weller PF. Vesicle-mediated secretion of human eosinophil granule-derived major basic protein. Lab Invest. 2009;89:769–781. doi: 10.1038/labinvest.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ito S, Noguchi E, Shibasaki M, Yamakawa-Kobayashi K, Watanabe H, Arinami T. Evidence for an association between plasma platelet-activating factor acetylhydrolase deficiency and increased risk of childhood atopic asthma. J Hum Genet. 2002;47:99–101. doi: 10.1007/s100380200009. [DOI] [PubMed] [Google Scholar]
- 66.Bosse Y, Thompson C, Stankova J, Rola-Pleszczynski M. Fibroblast growth factor 2 and transforming growth factor beta1 synergism in human bronchial smooth muscle cell proliferation. Am J Respir Cell Mol Biol. 2006;34:746–753. doi: 10.1165/rcmb.2005-0309OC. [DOI] [PubMed] [Google Scholar]
- 67.Kumar RK, Herbert C, Yang M, Koskinen AM, McKenzie AN, Foster PS. Role of interleukin-13 in eosinophil accumulation and airway remodelling in a mouse model of chronic asthma. Clin Exp Allergy. 2002;32:1104–1111. doi: 10.1046/j.1365-2222.2002.01420.x. [DOI] [PubMed] [Google Scholar]
- 68.Simeone-Penney MC, Severgnini M, Rozo L, Takahashi S, Cochran BH, Simon AR. PDGF-induced human airway smooth muscle cell proliferation requires STAT3 and the small GTPase Rac1. Am J Physiol Lung Cell Mol Physiol. 2008;294:L698–704. doi: 10.1152/ajplung.00529.2007. [DOI] [PubMed] [Google Scholar]
- 69.Elsner J, Escher SE, Forssmann U. Chemokine receptor antagonists: a novel therapeutic approach in allergic diseases. Allergy. 2004;59:1243–1258. doi: 10.1111/j.1398-9995.2004.00710.x. [DOI] [PubMed] [Google Scholar]
- 70.Romagnani S. Cytokines and chemoattractants in allergic inflammation. Mol Immunol. 2002;38:881–885. doi: 10.1016/s0161-5890(02)00013-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.