Abstract
Accumulating evidence suggests that abnormalities in neural circuitry and timing associated with the cerebellum may play a role in the pathophysiology of schizophrenia. Schizotypal personality disorder (SPD) may be genetically linked to schizophrenia, but individuals with SPD are freer from potential research confounds and may therefore offer insight into psychophysiological correlates of schizophrenia. The present study employed a delay eyeblink conditioning (EBC) procedure to examine cerebellar-dependent learning in schizophrenia, SPD, and healthy control subjects (n = 18 per group) who were matched for age and gender. The conditioned stimulus was a 400-ms tone that coterminated with a 50 ms unconditioned stimulus air puff. Cognitive performance on the Picture Completion, Digit Symbol Coding, Similarities, and Digit Span subscales of the Wechsler Adult Intelligence Scale—Third Edition was also investigated. The schizophrenia and SPD groups demonstrated robust EBC impairment relative to the control subjects; they had significantly fewer conditioned responses (CRs), as well as smaller CR amplitudes. Schizophrenia subjects showed cognitive impairment across subscales compared with SPD and control subjects; SPD subjects showed intermediate performance to schizophrenia and control subjects and performed significantly worse than controls on Picture Completion. Impaired EBC was significantly related to decreased processing speed in schizophrenia spectrum subjects. These findings support the role of altered cortico-cerebellar-thalamic-cortical circuitry in the pathophysiology of schizophrenia spectrum disorders.
Keywords: schizophrenia, schizotypal personality disorder, eyeblink conditioning, cerebellum, learning
Introduction
The clinical presentation of schizophrenia is widely understood to be heterogeneous in nature, consisting of positive (eg, hallucinations and delusions), negative (eg, blunted affect), cognitive (eg, impaired memory and executive function), and motor symptoms that vary from patient to patient. Although research into the biological basis of the disorder has made great progress in recent decades, much remains to be understood about the neural substrates that underlie its diverse presentation. One influential theory posits an important role for the cerebellum in schizophrenia through the cortico-cerebellar-thalamic-cortical (CCTC) circuit. In the “cognitive dysmetria” hypothesis of schizophrenia, disrupted cerebellar function within this circuit is thought to contribute to impaired coordinated processing of sensorimotor and cognitive information and consequently the broader symptoms of schizophrenia.1
Although the cerebellum has been traditionally viewed as responsible for coordination in motor function, compelling evidence suggests that it plays a role in broader psychological function including cognition. Patients with localized cerebellar lesions display a constellation of nonmotor deficits, including impaired attention, language, and executive function,2–4 and healthy subjects show cerebellar activation during cognitive activity.1 Indeed, a review of 275 functional imaging studies concluded that of the subcortical structures commonly investigated, only the cerebellum was consistently activated across various cognitive processes.5 This is consistent with findings of distinct cerebellar output channels that project through the thalamus not only to the primary motor and ventral premotor cortices but also to the areas of the prefrontal cortex involved in cognition.6,7 Thus, converging evidence suggests that the cerebellum may contribute to broader psychological function through the CCTC circuit.
Emerging evidence also suggests an important role for the cerebellum in the pathophysiology of schizophrenia. Abnormalities have been found at structural, cellular, and functional levels and correlate with clinical measures. Postmortem and imaging studies found reduced volume of the cerebellar vermis and lobules in chronic,8–11 neuroleptic-naive,12 and childhood-onset schizophrenia,13 as well as reduced bilateral hemispheric volume in first-episode schizophrenia,14 although not all studies reported reduced cerebellar volume.15,16 Postmortem studies also reported reduced size and density of Purkinje cells in schizophrenia.17–19 In addition, functional imaging studies reported abnormal cerebellar blood flow at rest20–23 and during activation for cognitive tasks.24–26 Finally, cerebellar abnormalities correlated with clinical symptoms, cognitive deficits, and outcome measures in schizophrenia.9,12,27–29 Thus, multiple lines of evidence indicate cerebellar pathology in schizophrenia.
Despite accumulating evidence for cerebellar abnormalities in schizophrenia, relatively few studies have assessed the functional integrity of the cerebellum in schizophrenia. Delay eyeblink conditioning (EBC) provides a well-validated method to investigate cerebellar function. In EBC, a conditioned stimulus (CS; ie, a tone) becomes associated with an unconditioned stimulus (US; ie, an airpuff). Subjects demonstrate learning when an eyeblink [the conditioned response (CR)] occurs prior to the onset of the US. The neural circuits that underlie delay EBC—where onset of the CS precedes that of the US, but the CS and US coterminate—have been well characterized, and extensive evidence indicates that the cerebellum is essential to both the development and the manifestation of the eyeblink CR.30,31 Specifically, information regarding the CS and US is conveyed to the cerebellar cortex and interpositus nucleus via mossy and climbing fibers, respectively. Learning-related activity in the anterior interpositus is thought to drive the somatic CR, whereas cerebellar cortex activity is thought to alter the gain (eg, blink magnitude) and timing of the interpositus’ activity.31 EBC therefore presents a useful method to assess the functional integrity of the cerebellum and related brain stem circuits.
Studies of EBC in schizophrenia, however, have yielded inconsistent findings. Some found impaired EBC in schizophrenia patients relative to healthy control subjects,32–34 whereas others found facilitated conditioning (to auditory CSs,35 to visual CSs36) or no difference between patients and controls.37 A recent review38 argued that these inconsistencies may result from differences in medication status; however, differences in testing parameters and methodological issues may be more likely explanations. For example, at least 1 study reported increased spontaneous blink rates in schizophrenia patients,36 which can artificially inflate CR estimates, and Sears et al35 did not address this possibility in their report of facilitated EBC in schizophrenia, despite increased CRs in patients within the first trials of the conditioning session. Small sample sizes37 and unreliable diagnostic criteria predating Diagnostic and Statistical Manual of Mental Disorders, Third Edition 36 are additional issues. Importantly, the largest study to date on EBC in schizophrenia (n = 62 per group) found robust EBC deficits in schizophrenia patients compared with controls, including in a subgroup of 12 patients who were unmedicated at time of testing.39 However, as chronic psychotropic medication may have lasting neural effects, further studies are necessary to confirm EBC impairment in schizophrenia and to rule out possible contributions of other illness-related confounds.
Schizotypal personality disorder (SPD) is phenomenologically and genetically related to schizophrenia and is considered part of the schizophrenia spectrum.40 SPD is characterized by positive and negative symptoms, such as perceptual distortions, magical thinking, and social deficits, as well as cognitive deficits, such as impaired working memory and verbal learning.41,42 Family and adoption studies indicate a genetic basis for SPD, with increased prevalence of schizotypal personality features in relatives of individuals with schizophrenia.43,44 Furthermore, individuals with SPD show psychophysiological abnormalities similar to those in schizophrenia, including reduced prepulse inhibition,45 smooth pursuit eye movement deficits,46 and event-related potential abnormalities.47 However, whereas chronic schizophrenia is associated with severe impairment across a broad variety of domains, impairments in SPD are milder and less pervasive. Individuals with SPD are therefore less affected by potential confounding effects of overt psychosis, chronic medication, and prolonged functional impairment and, accordingly, offer a unique and powerful strategy for clarifying biobehavioral abnormalities within schizophrenia spectrum disorders.
We investigated delay EBC in individuals diagnosed with SPD and schizophrenia and in healthy control subjects. To our knowledge, this is the first study to investigate EBC in SPD. Cognitive function was also investigated using 4 subscales of the Wechsler Adult Intelligence Scale—Third Edition (WAIS-III). The primary hypothesis was that schizophrenia and schizotypal subjects would show impaired EBC. A secondary hypothesis was that deficits in EBC would be associated with impaired cognitive performance.
Methods
Participants
Three groups of 18 age-matched subjects (8 women per group) participated in the current study: schizophrenia patients, individuals with SPD, and nonpsychiatric healthy controls. Inclusion criteria for all subjects were completion of grade school, normal or corrected hearing and vision, no history of significant head injury or cardiovascular or neurological disease, and no current alcohol or drug dependence.
Schizophrenia Subjects (N = 18).
Patients with schizophrenia were recruited from local inpatient and outpatient services. Diagnostic status was determined using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) Axis I Disorders (SCID-I) sections for mood, psychotic, and substance abuse disorders and chart review where available.
Schizotypal Subjects (N = 18).
Schizotypal subjects responded to newspaper advertisements for individuals with special abilities such as extrasensory perception, sixth sense, perception of auras, or clairvoyance. Diagnostic status was determined using the above noted sections of the SCID-I, as well as the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II) for Cluster A personality disorders and antisocial personality disorder. One schizotypal subject reported a first-degree relative with schizophrenia.
Control Subjects (N = 18).
Control subjects were recruited through newspaper advertisements. Diagnostics status was determined using the sections of the SCID-I and SCID-II noted above. Control subjects were additionally screened and negative for history of schizophrenia in first-degree relatives.
Seventeen subjects in each group completed the Picture Completion, Digit Symbol Coding, Similarities, and Digit Span subscales of the WAIS-III to represent each of the Perceptual Organization, Processing Speed, Verbal Comprehension, and Working Memory Indices, respectively.48 A subgroup of schizophrenia (n = 13) and schizotypal subjects (n = 16) also completed the Trail Making Test A.49 EBC data from the schizophrenia subjects and 15 control subjects have been previously reported as part of a larger study39 (Author’s note: We examined the effect of removing these schizophrenia subjects and their age-matched controls from our previously published data set to ensure that the patients used in the current study were not, by chance, particularly impaired. The remaining schizophrenia subjects showed the same deficit in percent CRs relative to control subjects). Study procedures were approved by the Indiana University’s Human Subjects Institutional Review Board, and written informed consent was obtained from all subjects.
As expected given the age-matching procedure, schizophrenia (M = 37.67, SD = 9.43), schizotypal (M = 38.06, SD = 9.87), and control (M = 37.89, SD = 9.85) subjects did not differ in age, F(2,51) = 0.007, P = ns. Three schizophrenia subjects were off psychotropic medication at time of testing. Of the remaining schizophrenia subjects, 14 were on antipsychotics, 6 were on antidepressants, 2 were on mood stabilizers, 3 were on anticholinergic drugs, and 1 was on a benzodiazepine. Three schizotypal subjects were taking antidepressants; the remaining schizotypal subjects and all control subjects were free of psychotropic medication.
EBC Procedure
Participants completed a single-cue tone delay EBC task following previously published procedures.32,39 The CS was a 400 ms, 1000 Hz (80 dB sound pressure level) tone, which, on paired trials, coterminated with a 50 ms air puff, the US. Subjects were presented with 8 US-alone trials (intertrial interval = 15 s), followed by 10 blocks of conditioning trials (mean intertrial interval = 15 s; range = 10–20 s). Each trial block consisted of 9 CS/US paired trials and 1 CS-alone trial. To maintain the participants’ attention throughout the experiment, neutral photographs selected from the International Affective Picture System50 were presented (2-s duration) between each trial and participants rated the pleasantness of the images by pressing a response pad button. Participants were observed via a closed circuit monitor to ensure that their eyes remained open. The experiment was briefly suspended if signs of fatigue were observed so that the examiner could interact with the participant.
Procedure
Bipolar electromyographic (EMG) electrodes (4 mm Ag/Ag–Cl), placed directly below the left eye and centered below the pupil, 1 cm from the eyelid and 1 cm apart were used to record eyeblinks from the orbicularis palpebrarum muscle. A ground electrode was placed on the forehead. The left eye was presented with a US air puff (50 ms, 10 lb psi at source) delivered from an air regulator, through 120″ of plastic tubing, to 1/16″ copper tubing fused on the rim of lensless glasses such that the air was released 1 cm from the inner canthus. The CS tone was delivered via ear inserts (E-A-RLINK—Aearo Company Auditory Systems). EMG recordings were made continuously (2.5 KHz A/D rate; high-pass filter = 1 Hz; low-pass filter = 500 Hz; gain = 1000) throughout the experiment and stored offline.
Data Analysis
Continuous data files for each subject were divided into 1086 ms epochs starting 500 ms prior to CS onset. After a 10 Hz (6 dB/octave) high-pass filter was applied, the data were rectified and smoothed using a 41-point Gaussian-weighted moving average. Data were entered into DataMunch, a Matlab program written for EBC data analysis (D. A. T. King, Ph.D., and J. Tracy, Ph.D., unpublished data) for further analysis. Alpha responses, which are reflexive nonassociative orienting EMG responses to the tone CS, were assessed between 25 and 100 ms after CS onset. On a subject-by-subject and trial-by-trial basis, responses were recorded as blinks if the amplitude exceeded 5 SD above baseline (baseline window for each trial = 125 ms prior to CS onset). CRs were recorded if the blink occurred between 100 and 350 ms after CS onset, which corresponded to a period beginning 250 ms before US onset. CR peak latency was calculated as the time to maximal value of the CR. Trials in which spontaneous blinks occurred between 75 ms before and 25 ms after CS onset were designated “bad trials” and excluded from further analysis because they occurred too early to be considered tone or conditioning related and could interfere with the subsequent execution of a CR. However, as number of bad trials is also an index of spontaneous blink rate, it is important to determine if group differences exist on this measure; one-way ANOVA revealed no group difference, F(2,51) = 0.20, P = ns.
EBC variables were assessed using 3 (Group) × 10 (Block) repeated measures ANOVA. Mauchly’s test of sphericity was violated on all EBC ANOVA except for that assessing percent CRs; P values for effects involving block on these ANOVA are reported using the Huynh-Feldt correction. For clarity, noncorrected degrees of freedom are reported. Tests of simple effects and Least Significant Difference (LSD) post hoc tests were used for follow-up analyses when effects involving group were significant. Results for dependent variables are reported with corresponding effect sizes using partial eta square ( ).51 To further elucidate the effects of medication, ANOVA were rerun on the 15 unmedicated schizotypal subjects and their age-matched controls for CR variables.
Outlier analyses were conducted for all neuropsychological tests and revealed no extreme outliers in any group on any measure as indicated by the SPSS statistical package. A 3 (Group) × 4 (Subscale) MANOVA was used to assess group differences in scaled WAIS scores, followed by tests of simple effects and LSD post hoc tests. Bivariate correlation analyses were conducted for schizophrenia spectrum subjects grouped together, and control subjects separately, to investigate the relationship between average percent CRs and WAIS scores. To account for the potential contribution of motor speed to the relationship between EBC and Digit Symbol performance in schizophrenia spectrum subjects, Trails A time was used to control for graphomotor speed in a subsequent partial correlational analysis.52 An alpha level of 0.05 was used to determine significance for all statistical analyses.
Results
Conditioned Responses
Percent CRs.
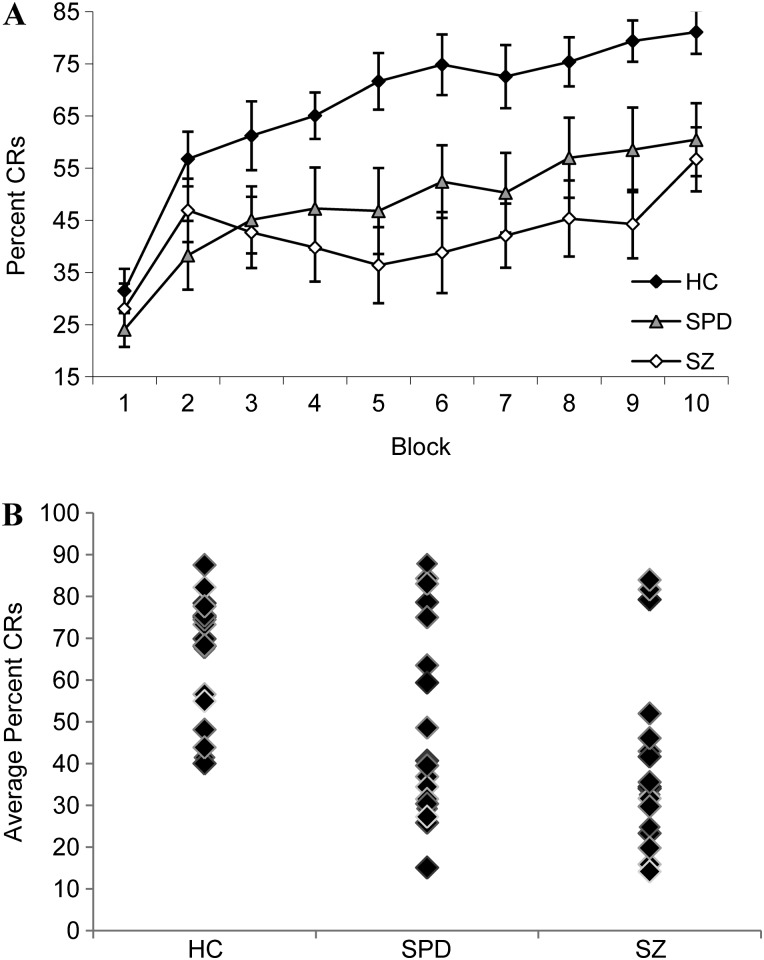
Although all groups showed learning as evidenced by increased percent CRs across successive trial blocks, schizophrenia and schizotypal subjects demonstrated poorer conditioning performance relative to control subjects (figure 1). Thus, there were significant effects of Block, F(9,459) = 16.03, P < .001, ηPp 2 = 0.24, and Group, F(2,51) = 7.29 P = .002, = 0.22, with control subjects showing greater CRs than schizophrenia, P = .001, and schizotypal subjects, P = .02, as well as a significant Group × Block interaction, F(18,459) = 1.81, P = .02, = 0.07. Follow-up one-way ANOVA revealed significant group effects on blocks 4 through 10. Control subjects showed greater CRs compared with schizophrenia subjects on blocks 4, 6, and 8 and to schizophrenia and schizotypal subjects on blocks 5, 7, 9, and 10.
Fig. 1.
(A) Mean ± SE Percent Conditioned Responses (CRs) Across Blocks for Healthy Control (HC), Schizotypal Personality Disorder (SPD), and Schizophrenia (SZ) Groups and (B) Distribution of Average Percent CRs at the Individual Level Within Group.
CR Peak Latency.
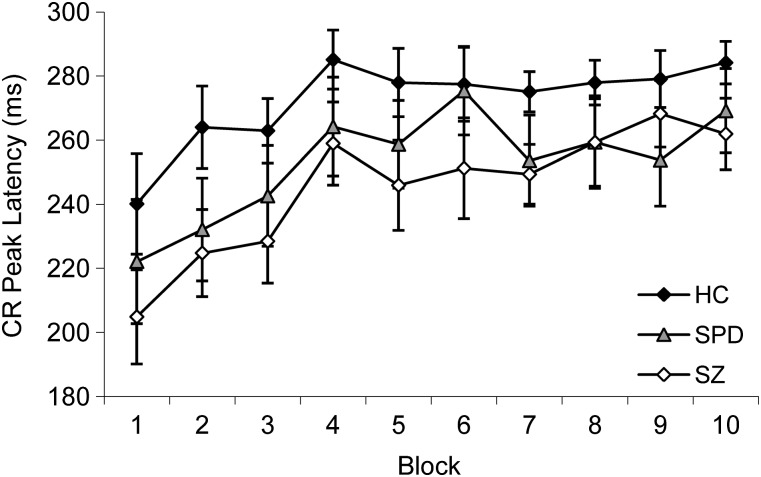
As learning occurs over the conditioning session, timing of the CR should shift to better anticipate the onset of the air puff; ie, CRs that occur shortly after CS onset are less adaptive than those that occur just prior to US onset. All groups showed learning as evidenced by an overall shift toward later CR peak latencies. Thus, there was a significant main effect of Block, F(9,459) = 5.52, P < .001, = 0.10. Schizophrenia and schizotypal subjects appeared to show shorter, or less adaptive, CR latencies than controls (figure 2); however, this only reached a trend level of significance, F(2,51) = 2.89, P = .07, = 0.10. The Group × Block interaction was not significant, F(18,459) = 0.31, P = ns.
Fig. 2.
Mean ± SE Conditioned Response (CR) Peak Latency (Millisecond) Across Blocks for Healthy Control (HC), Schizotypal Personality Disorder (SPD), and Schizophrenia (SZ) Groups.
CR Amplitude.
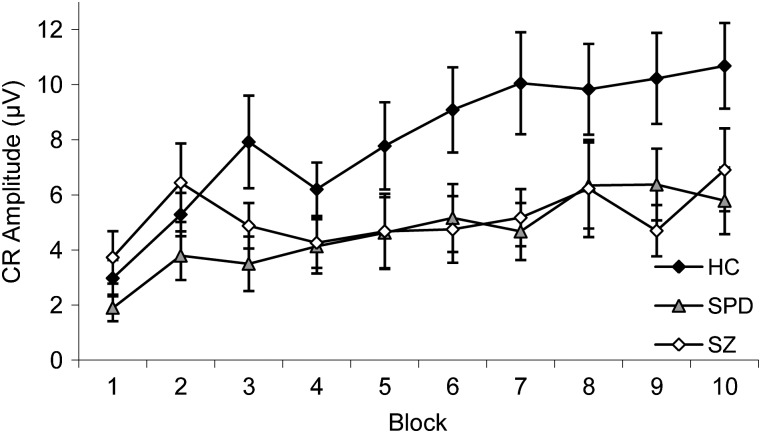
Overall, CR amplitude increased across the experiment; however, schizophrenia and schizotypal subjects demonstrated smaller CRs relative to controls, particularly on later trials (figure 3). There was a significant main effect of Block, F(9,459) = 8.76, P < .001, = 0.07, but not Group, F(2,51) = 2.23, P = ns; however, there was a significant Group × Block interaction, F(18,459) = 1.96, P = .03, = 0.07. Follow-up one-way ANOVA revealed significant Group effects for blocks 7 and 9. Control subjects showed larger CR amplitude compared with schizophrenia subjects on block 9 and to schizophrenia and schizotypal subjects on block 7.
Fig. 3.
Mean ± SE Conditioned Response (CR) Amplitude (μV) Across Blocks for Healthy Control (HC), Schizotypal Personality Disorder (SPD), and Schizophrenia (SZ) Groups.
Unconditioned Responses
Unconditioned Response Peak Amplitude.
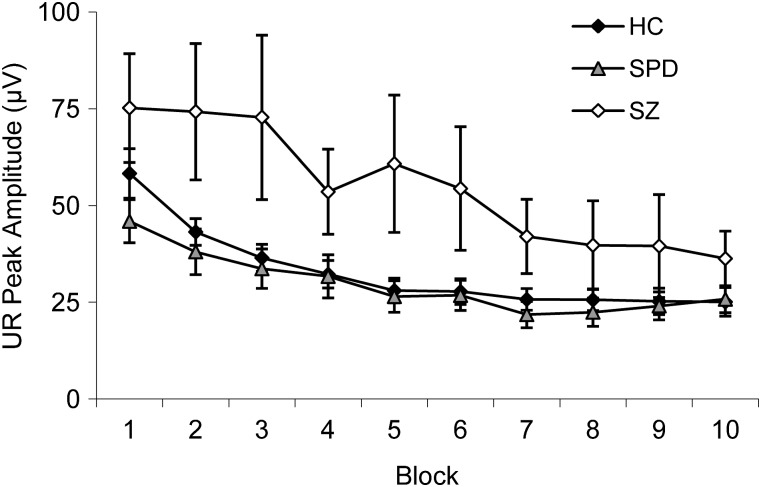
Unconditioned response (UR) amplitude decreased for all groups as the experiment progressed as evidenced by a significant effect of Block, F(9,459) = 16.90, P < .001, = 0.24. There was also a significant Group effect, F(2,51) = 3.72, P = .03, = 0.13. Follow-up analyses revealed that schizophrenia subjects had larger UR responses than schizotypal, P = .02, and control subjects, P = .03. There was no Group × Block interaction, F(18,459) = 1.78, P = ns (figure 4).
Fig. 4.
Mean ± SE Unconditioned Response (UR) Amplitude (μV) Across Blocks for Healthy Control (HC), Schizotypal Personality Disorder (SPD), and Schizophrenia (SZ) Groups.
UR Peak Latency.
Groups did not significantly differ on UR peak latency, F(2,51) = 3.07, P = ns. There was no effect of Block, F(9,459) = 1.45, P = ns, nor Group × Block interaction, F(18,459) = 1.17, P = ns (figure 5).
Fig. 5.
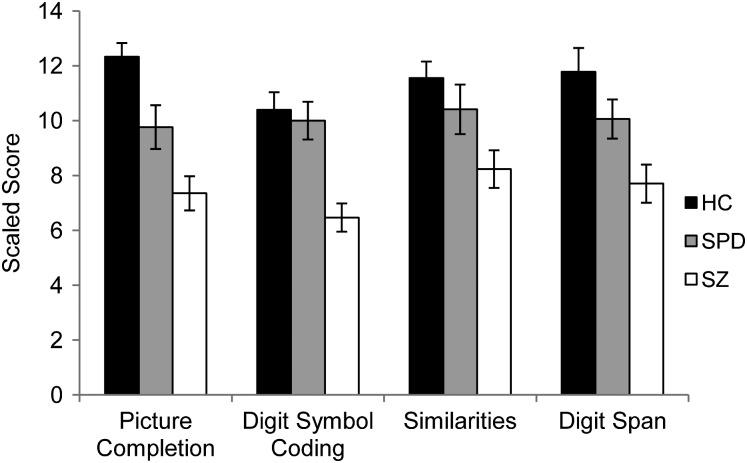
Mean ± SE Picture Completion, Digit Symbol Coding, Similarities, and Digit Span Scales Scores for Healthy Control (HC), Schizotypal Personality Disorder (SPD), and Schizophrenia (SZ) Groups.
CRs in Unmedicated Schizotypal Participants
Comparisons of unmedicated schizotypal subjects to matched controls on CR variables yielded a similar pattern of results. With reduced group sizes, schizotypal subjects showed significantly fewer percent CRs, F(1,28) = 4.70, P = .04, = 0.14, nonsignificantly earlier CR peak latencies, F(1,28) = 1.58, P = .22, = 0.05 and nonsignificantly reduced CR amplitudes, F(1,28) = 1.67, P = .21, = 0.06. There were no significant Group × Block interactions.
WAIS Subscale Performance
Schizophrenia subjects were impaired across the 4 WAIS subscales relative to controls; schizotypal subjects demonstrated intermediate performance (figure 5). Thus, there was an overall Group effect, Λ = 4.63, P < .001, = 0.29, that was significant on follow-up tests for each subscale. Post hoc analyses revealed that schizophrenia subjects demonstrated significantly lower scores than controls across subscales and compared with schizotypal subjects on the Digit Symbol Coding, Picture Completion, and Digit Span subscales ( Ps < .05). Schizotypal subjects demonstrated significantly lower scores than controls only on Picture Completion, P = .01.
Correlations
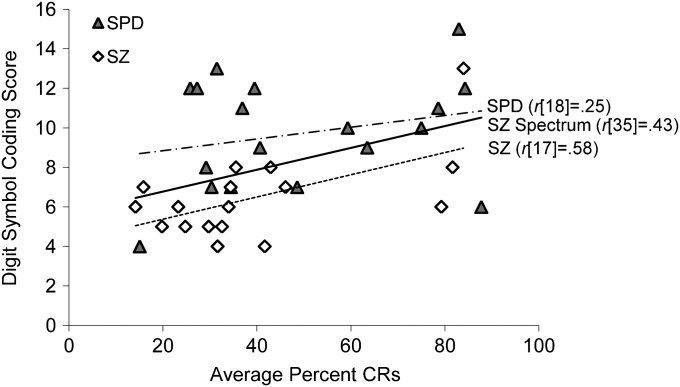
Bivariate correlation analyses for percent CRs and WAIS scores were conducted for schizophrenia and schizotypal subjects combined and controls separate, following evidence of reduced CRs in schizophrenia and schizotypal subjects relative to controls. This was done in order to avoid results that could be artifacts of schizophrenia spectrum subjects and controls being differentially distributed on these measures. There was a significant positive relationship between average percent CRs and Digit Symbol score for the schizophrenia spectrum group, r(35) = .43, P = .01 (figure 6); no other correlations were significant. This relationship between percent CRs and Digit Symbol score in the schizophrenia spectrum group remained significant after controlling for graphomotor speed using Trails A time, r = .37, P < .05. Exploratory analyses showed a significant positive relationship between percent CRs and Digit Symbol score for the schizophrenia group alone, r(17) = .58, P = .01. This relationship was not significant for SPD subjects alone, r(18) = .25, P = .32; however, the nature of the relationship held for both groups suggesting that this may be due to a loss of statistical power.
Fig. 6.
Correlation Between Digit Symbol Coding Scaled Score and Average Percent Conditioned Responses (CRs) for the Combined Schizophrenia and Schizotypal Group (SZ Spectrum) and for the Schizophrenia (SZ) and Schizotypal Personality Disorder (SPD) Groups Separate.
Discussion
The present study examined the functional integrity of the cerebellum in patients with schizophrenia and SPD using delay EBC methodology. The main finding was that schizophrenia and schizotypal subjects demonstrated impaired EBC as evidenced by a significant reduction in CRs compared with nonpsychiatric controls. In addition, schizophrenia and schizotypal subjects showed reduced CR amplitude and a trend toward earlier or less adaptively timed CRs. Schizophrenia subjects also performed worse than controls on tests of cognitive function; schizotypal subjects were intermediate, performing significantly better than schizophrenia subjects on Picture Completion, Digit Span, and Digit Symbol Coding, and significantly worse than control subjects on Picture Completion. Poorer EBC was associated with decreased processing speed in the combined schizophrenia spectrum group.
Multiple observations suggest that the current EBC impairment was not due to preexisting deficits in general motor or sensory processes. First, schizophrenia and schizotypal subjects exhibited similar CR rates as controls during early trial blocks; second, groups did not differ on an index of spontaneous blink rate; and third, schizotypal and control subjects did not differ in UR characteristics. Group differences therefore appear to reflect a failure of schizophrenia and schizotypal subjects to show the same robust increase in CRs as controls with successive CS-US pairings rather than deficits in motor or sensory function.
The current EBC impairment is consistent with previous studies showing impaired EBC in chronic32,33,39 and unmedicated39 schizophrenia patients during delay EBC and in chronic schizophrenia patients during a related conditional discrimination EBC paradigm.34 Numerous studies show that individuals with SPD exhibit psychophysiological abnormalities similar to those in schizophrenia, including impaired prepulse inhibition and P50 gating and reduced P300 amplitude.40 To our knowledge, this is the first investigation of EBC as a potential psychophysiological marker for schizophrenia using schizotypal subjects. However, our finding of impaired EBC in SPD is consistent with increased incidence of motor abnormalities, which were also linked to cerebellar dysfunction,53,54 in schizophrenia spectrum individuals,54 and in psychometrically53,55 and interview identified schizotypy.56 Thus, similar EBC impairment in schizophrenia and schizotypal subjects in the current study provides strong support for EBC impairment as a psychophysiological marker for schizophrenia that is related to core pathophysiological processes rather than to artifacts of overt psychosis, chronic medication, or functional impairment.
The neural mechanisms that underlie impaired EBC in schizophrenia may relate to aberrant functional connectivity in cerebellar microcircuitry. The cerebellum is a uniquely powerful information processing center that receives widespread input from the spinal cord, brain stem, and diverse regions of the cerebral cortex.57,58 Incoming information to the cerebellum arrives via climbing fibers that synapse with the deep nuclei and Purkinje cells and mossy fibers that synapse with the deep nuclei and granule cells. As the primary output neurons of the cerebellar cortex, Purkinje cells branch extensively in the molecular layer of the cortex to form synapses with granule cell axons before sending their inhibitory output to the deep nuclei that project to the rest of the brain. Lesioning, neural recording, and genetic knock out model studies indicate that during delay EBC, information regarding the CS and US is carried into the cerebellum by the mossy and climbing fibers, respectively, and that the interpositus deep nucleus and Purkinje cells both receive convergent CS-US input. Subsequent changes in firing patterns of anterior interpositus neurons drive the conditioned eyeblink via projections to brain stem neurons and changes in Purkinje cells alter the gain and timing of the interpositus’ activity.31
Importantly, postmortem studies of schizophrenia patients revealed abnormalities at key junctions of this circuitry, including impaired GABAergic modulation of granule cells by Golgi interneurons,59 reduced markers of granule cell synapses,60,61 and altered levels of key molecules regulating axonal guidance and synaptogenesis in the molecular layer where granule and Purkinje cells form synapses.62 Consistent with this, granule63 and Purkinje cells64 appear to show aberrant output in schizophrenia. Additional research is needed to further characterize the nature and extent of cerebellar abnormalities in schizophrenia spectrum disorders; however, aberrant connectivity at key junctions of cerebellar microcircuitry is a compelling mechanism for impaired cerebellar plasticity and may therefore contribute to the current finding of impaired EBC in schizophrenia and schizotypal subjects. Conversely, EBC may be a powerful assay of dysfunction at these key junctions in cerebellar circuitry in schizophrenia spectrum disorders.
In light of the “cognitive dysmetria” hypothesis of schizophrenia, it is interesting that impaired functional integrity of the cerebellum (as measured by EBC) was significantly related to Digit Symbol Coding performance in schizophrenia spectrum subjects, even after controlling for motor speed. Digit Symbol Coding is a putative measure of processing speed that requires the fluid coordination of multiple sensory, motor, and cognitive processes.65 It is possible that the rapid coordination required for successful performance may be particularly sensitive to disruptions in the CCTC circuit, consistent with findings of impaired Digit Symbol Coding in patients with frontal and cerebellar lesions.66 In schizophrenia, impaired Digit Symbol Coding yields the largest effect size of commonly investigated cognitive tests and relates significantly to illness risk, severity, and outcome.67 Although further research is needed to elucidate mechanisms by which cerebellar dysfunction may relate to cognitive dysfunction in schizophrenia, this provides intriguing evidence for broader consequences of cerebellar abnormalities in the clinical presentation of schizophrenia.
There are some general limitations to the current study that should be noted. The group sample sizes were relatively small, although not unusual for initial biobehavioral studies using intermediate phenotype groups. As a result, we did not assess illness severity variables (eg, age of symptom onset, comorbidity issues, etc) and could not directly investigate medication effects on EBC performance. Future studies should examine EBC in larger samples of schizophrenia and schizotypal subjects to allow for more systematic study of these variables on EBC performance.
To our knowledge, this is the first study to demonstrate EBC impairment in individuals with SPD. Additional studies with schizophrenia patients and other spectrum populations such as first-degree relatives and high-risk or prodromal individuals are necessary to substantiate the role of EBC impairment as a potential biomarker in schizophrenia; however, the present results suggest that medication status and other effects related to chronic psychotic illness do not account for EBC impairment in schizophrenia. Imaging and translational animal studies would also be useful to better characterize the specific contributions of cerebellar dysfunction to EBC and broader impairment in schizophrenia. Nevertheless, the current results support the role of cerebellar dysfunction in the pathophysiology of schizophrenia and underscore the importance of additional studies to further elucidate the nature and consequences of CCTC circuit abnormalities in schizophrenia.
Funding
National Institute of Mental Health (MH074983-01 to W.P.H., MH091774-01 to B.F.O.); National Alliance for Research on Schizophrenia and Depression (Young Investigator Awards 2002–2004, 2004–2006 to W.P.H.).
Acknowledgments
We would like to thank the clinical research team at Larue D. Carter Memorial Hospital and the Indiana University Clinical and Cognitive Neuroscience Center for their support. We are especially grateful to Emily Lazar, Colleen Merrill, and Ashley Steffen for their assistance in data collection.The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Andreasen NC. A unitary model of schizophrenia: bleuler’s “fragmented phrene” as schizencephaly. Arch Gen Psychiatry. 1999;56:781–787. doi: 10.1001/archpsyc.56.9.781. [DOI] [PubMed] [Google Scholar]
- 2.Akshoomoff NA, Courchesne E. A new role for the cerebellum in cognitive operations. Behav Neurosci. 1992;106:731–738. doi: 10.1037//0735-7044.106.5.731. [DOI] [PubMed] [Google Scholar]
- 3.Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16:367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- 4.Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- 5.Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 6.Middleton FA, Strick PL. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266:458–461. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- 7.Middleton FA, Strick PL. Cerebellar output: motor and cognitive channels. Trends Cogn Sci. 1998;2:348–354. doi: 10.1016/s1364-6613(98)01220-0. [DOI] [PubMed] [Google Scholar]
- 8.Loeber RT, Cintron CMB, Yurgelun-Todd DA. Morphometry of individual cerebellar lobules in schizophrenia. Am J Psychiatry. 2001;158:952–954. doi: 10.1176/appi.ajp.158.6.952. [DOI] [PubMed] [Google Scholar]
- 9.Nopoulos PC, Ceilley JW, Gailis EA, Andreasen NC. An MRI study of cerebellar vermis morphology in patients with schizophrenia: evidence in support of the cognitive dysmetria concept. Biol Psychiatry. 1999;46:703–711. doi: 10.1016/s0006-3223(99)00093-1. [DOI] [PubMed] [Google Scholar]
- 10.Volz H-P, Gaser C, Sauer H. Supporting evidence for the model of cognitive dysmetria in schizophrenia—a structural magnetic resonance imaging study using deformation-based morphometry. Schizophr Res. 2000;46:45–56. doi: 10.1016/s0920-9964(99)00236-4. [DOI] [PubMed] [Google Scholar]
- 11.Weinberger DR, Kleinman JE, Luchins DJ, Bigelow LB, Wyatt RJ. Cerebellar pathology in schizophrenia: a controlled post-mortem study. Am J Psychiatry. 1980;137:359–361. doi: 10.1176/ajp.137.3.359. [DOI] [PubMed] [Google Scholar]
- 12.Ichiyama T, Okubo Y, Suhara T, Sudo Y. Reduced volume of the cerebellar vermis in neuroleptic-naïve schizophrenia. Biol Psychiatry. 2001;49:20–27. doi: 10.1016/s0006-3223(00)01081-7. [DOI] [PubMed] [Google Scholar]
- 13.Jacobsen LK, Giedd JN, Berquin PC, et al. Quantitative morphology of the cerebellum and fourth ventricle in childhood-onset schizophrenia. Am J Psychiatry. 1997;154:1663–1669. doi: 10.1176/ajp.154.12.1663. [DOI] [PubMed] [Google Scholar]
- 14.Bottmer C, Bachmann S, Pantel J, et al. Reduced cerebellar volume and neurological soft signs in first-episode schizophrenia. Psychiatry Res. 2005;140:239–250. doi: 10.1016/j.pscychresns.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Cahn W, Hulshoff Pol HE, Bongers M, et al. Brain morphology in antipsychotic-naive schizophrenia: a study of multiple brain structures. Br J Psychiatry. 2002;181:s66–s72. doi: 10.1192/bjp.181.43.s66. [DOI] [PubMed] [Google Scholar]
- 16.Levitt JJ, McCarley RW, Nestor PG, et al. Quantitative volumetric MRI study of the cerebellum and vermis in schizophrenia: clinical and cognitive correlates. Am J Psychiatry. 1999;156:1105–1107. doi: 10.1176/ajp.156.7.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maloku E, Covelo IR, Hanbauer I, et al. Lower number of cerebellar Purkinje neurons in psychosis is associated with reduced reelin expression. Proc Natl Acad Sci U S A. 2010;107:4407–4411. doi: 10.1073/pnas.0914483107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reyes MG, Gordon A. Cerebellar vermis in schizophrenia. Lancet. 1981;318:700–701. doi: 10.1016/s0140-6736(81)91039-4. [DOI] [PubMed] [Google Scholar]
- 19.Tran KD, Smutzer GS, Doty RL, Arnold SE. Reduced Purkinje cell size in the cerebellar vermis of elderly patients with schizophrenia. Am J Psychiatry. 1998;155:1288–1290. doi: 10.1176/ajp.155.9.1288. [DOI] [PubMed] [Google Scholar]
- 20.Kim J-J, Mohamed S, Andreasen NC, et al. Regional neural dysfunctions in chronic schizophrenia studied with positron emission tomography. Am J Psychiatry. 2000;157:542–548. doi: 10.1176/appi.ajp.157.4.542. [DOI] [PubMed] [Google Scholar]
- 21.Loeber RT, Sherwood AR, Renshaw PF, Cohen BM, Yurgelun-Todd DA. Differences in cerebellar blood volume in schizophrenia and bipolar disorder. Schizophr Res. 1999;37:81–89. doi: 10.1016/s0920-9964(98)00137-6. [DOI] [PubMed] [Google Scholar]
- 22.Steinberg JL, Devous MD, Moeller FG, Paulman RG, Raese JD, Gregory RR. Cerebellar blood flow in schizophrenic patients and normal control subjects. Psychiatry Res. 1995;61:15–31. doi: 10.1016/0925-4927(95)02574-h. [DOI] [PubMed] [Google Scholar]
- 23.Volkow ND, Levy A, Brodie JD, et al. Low cerebellar metabolism in medicated patients with chronic schizophrenia. Am J Psychiatry. 1992;149:686–688. doi: 10.1176/ajp.149.5.686. [DOI] [PubMed] [Google Scholar]
- 24.Andreasen NC, O'Leary DS, Cizaldo T, et al. Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc Natl Acad Sci U S A. 1996;93:9985–9990. doi: 10.1073/pnas.93.18.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crespo-Facorro B, Paradiso S, Andreasen NC, et al. Recalling word lists reveals “Cognitive Dysmetria” in schizophrenia: a positron emission tomography study. Am J Psychiatry. 1999;156:386–392. doi: 10.1176/ajp.156.3.386. [DOI] [PubMed] [Google Scholar]
- 26.Riechemann S, Volz H-P, Stutzer P, Smesny S, Gaser C, Sauer H. Hypofrontality in neuroleptic-naïve schizophrenic patients during the Wisconsin Card Sorting Test—a fMRI study. Eur Arch Psychiatry Clin Neurosci. 2001;251:66–71. doi: 10.1007/s004060170055. [DOI] [PubMed] [Google Scholar]
- 27.Ho B-C, Mola C, Andreasen NC. Cerebellar dysfunction in neuroleptic naive schizophrenia patients: clinical, cognitive, and neuroanatomic correlates of cerebellar neurologic signs. Biol Psychiatry. 2004;55:1146–1153. doi: 10.1016/j.biopsych.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 28.Potkin SG, Alva G, Fleming K, et al. A PET study of the pathophysiology of negative symptoms in schizophrenia. Am J Psychiatry. 2002;159:227–237. doi: 10.1176/appi.ajp.159.2.227. [DOI] [PubMed] [Google Scholar]
- 29.Wassink TH, Andreasen NC, Nopoulos P, Flaum M. Cerebellar morphology as a predictor of symptom and psychosocial outcome in schizophrenia. Biol Psychiatry. 1999;45:41–48. doi: 10.1016/s0006-3223(98)00175-9. [DOI] [PubMed] [Google Scholar]
- 30.Kim JJ, Thompson RE. Cerebellar circuits and synaptic mechanisms involved in classical eyeblink conditioning. Trends Neurosci. 1997;20:177–181. doi: 10.1016/s0166-2236(96)10081-3. [DOI] [PubMed] [Google Scholar]
- 31.Steinmetz JE. Brain substrates of classical eyeblink conditioning: a highly localized but also distributed system. Behav Brain Res. 2000;110:13–24. doi: 10.1016/s0166-4328(99)00181-3. [DOI] [PubMed] [Google Scholar]
- 32.Brown SM, Kieffaber PD, Carroll CA, et al. Eyeblink conditioning deficits indicate timing and cerebellar abnormalities in schizophrenia. Brain Cogn. 2005;58:94–108. doi: 10.1016/j.bandc.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 33.Edwards CR, Newman S, Bismark A, et al. Cerebellum volume and eyeblink conditioning in schizophrenia. Psychiatry Res: Neuroimaging. 2008;162:185–194. doi: 10.1016/j.pscychresns.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hofer E, Doby D, Anderer P, Dantendorfer K. Impaired conditional discrimination learning in schizophrenia. Schizophr Res. 2001;51:127–136. doi: 10.1016/s0920-9964(00)00118-3. [DOI] [PubMed] [Google Scholar]
- 35.Sears LL, Andreasen NC, O'Leary DS. Cerebellar functional abnormalities in schizophrenia are suggested by classical eyeblink conditioning. Biol Psychiatry. 2000;48:204–209. doi: 10.1016/s0006-3223(00)00247-x. [DOI] [PubMed] [Google Scholar]
- 36.Spain B. Eyelid conditioning and arousal in schizophrenic and normal subjects. J Abnorm Psychol. 1966;71:260–266. doi: 10.1037/h0023596. [DOI] [PubMed] [Google Scholar]
- 37.Marenco S, Weinberger DR, Schreurs BG. Single-cue delay and trace classical conditioning in schizophrenia. Biol Psychiatry. 2003;53:390–402. doi: 10.1016/s0006-3223(02)01506-8. [DOI] [PubMed] [Google Scholar]
- 38.Lubow RE. Classical eyeblink conditioning and schizophrenia: a short review. Behav Brain Res. 2008;202:1–4. doi: 10.1016/j.bbr.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Bolbecker AR, Mehta CS, Edwards CR, Steinmetz JE, O'Donnell BF, Hetrick WP. Eye-blink conditioning deficits indicate temporal processing abnormalities in schizophrenia. Schizophr Res. 2009;111:182–191. doi: 10.1016/j.schres.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siever LJ, Davis KD. The pathophysiology of schizophrenia disorders: perspectives from the spectrum. Am J Psychiatry. 2004;163:398–413. doi: 10.1176/appi.ajp.161.3.398. [DOI] [PubMed] [Google Scholar]
- 41.Cadenhead KS, Perry W, Shafer K, Braff DL. Cognitive functions in schizotypal personality disorder. Schizophr Res. 1999;37:123–132. doi: 10.1016/s0920-9964(98)00147-9. [DOI] [PubMed] [Google Scholar]
- 42.Voglmaier MM, Seidman LJ, Salisbury D, McCarley RW. Neuropsychological dysfunction in schizotypal personality disorder: a profile analysis. Biol Psychiatry. 1997;41:530–540. doi: 10.1016/s0006-3223(96)00056-x. [DOI] [PubMed] [Google Scholar]
- 43.Torgersen S. Relationship of schizotypal personality disorder to schizophrenia: genetics. Schizophr Bull. 1985;11:554–563. doi: 10.1093/schbul/11.4.554. [DOI] [PubMed] [Google Scholar]
- 44.Tsuang MT, Sloane WS, Faraone SV. Schizophrenia: a review of genetic studies. Harv Rev Psychiatry. 1999;7:185–207. [PubMed] [Google Scholar]
- 45.Cadenhead KS, Swerdlow NR, Shafer KM, Diaz M, Braff DL. Modulation of the startle response and startle latency in relatives of schizophrenia patients and in subjects with schizotypal personality disorder: evidence of inhibitory deficits. Am J Psychiatry. 2000;157:1660–1668. doi: 10.1176/appi.ajp.157.10.1660. [DOI] [PubMed] [Google Scholar]
- 46.Siever LJ, Keefe R, Bernstein DP, et al. Eye tracking impairment in clinically identified patients with schizotypal personality disorder. Am J Psychiatry. 1990;147:740–745. doi: 10.1176/ajp.147.6.740. [DOI] [PubMed] [Google Scholar]
- 47.Salisbury DF, Voglmaier MM, Seidman LJ, McCarley RW. Topographic abnormalities of P3 in schizotypal personality disorder. Biol Psychiatry. 1996;40:165–172. doi: 10.1016/0006-3223(95)00373-8. [DOI] [PubMed] [Google Scholar]
- 48.Wechsler D. Wechsler Adult Intelligence Scale—Third edition (WAIS-III) San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 49.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 50.Lang PJ, Greenwald MK. The International Affective Picture System Standardization Procedure and Initial Group Results for Affective Judgements: Technical Report 1A. Gainseville, FL: The Center for Research in Psychophysiology; 1988. [Google Scholar]
- 51.Cohen J. Eta-squared and partial eta-squared in fixed factor and ANOVA designs. Educ Psychol Meas. 1973;33:107–112. [Google Scholar]
- 52.Brebion G, David AS, Jones HM, Pilowsky LS. Working memory span and cognitive speed in schizophrenia. Cogn Behav Neurol. 2009;22:101–108. doi: 10.1097/WNN.0b013e3181a722a0. [DOI] [PubMed] [Google Scholar]
- 53.Kaczorowski JA, Barrantes-Vidal N, Kwapil TR. Neurological soft signs in psychometrically identified schizotypy. Schizophr Res. 2009;115:293–302. doi: 10.1016/j.schres.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 54.Schiffman J, Sorensen HJ, Maeda J, et al. Childhood motor coordination and adult schizophrenia spectrum disorders. Am J Psychiatry. 2009;166:1041–1047. doi: 10.1176/appi.ajp.2009.08091400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mechri A, Gassab L, Slama H, Gaha L, Saoud M, Krebs MO. Neurological soft signs and schizotypal dimensions in unaffected siblings of patients with schizophrenia. Psychiatry Res. 2010;175:22–36. doi: 10.1016/j.psychres.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 56.Bollini AM, Compton MT, Esterberg ML, Rutland J, Chien VH, Walker EF. Associations between schizotypal features and indicators of neurological and morphological abnormalities. Schizophr Res. 2007;92:32–49. doi: 10.1016/j.schres.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 57.Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci. 2006;7:511–522. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- 58.Huang C. Implications on cerebellar function from information coding. Cerebellum. 2008;7:314–331. doi: 10.1007/s12311-008-0032-1. [DOI] [PubMed] [Google Scholar]
- 59.Bullock WM, Cardon K, Bustillo J, Roberts RC, Perrone-Bizzozero NI. Altered expression of genes involved in GABAergic transmission and neuromodulation of granule cell activity in the cerebellum of schizophrenia patients. Am J Psychiatry. 2008;165:1594–1603. doi: 10.1176/appi.ajp.2008.07121845. [DOI] [PubMed] [Google Scholar]
- 60.Eastwood SL, Cotter D, Harrison PJ. Cerebellar synaptic protein expression in schizophrenia. Neuroscience. 2001;105:219–229. doi: 10.1016/s0306-4522(01)00141-5. [DOI] [PubMed] [Google Scholar]
- 61.Mukaetova EB, Hurt J, Honer WG, Harrington CR, Wischik CM. Loss of synaptic but not cytoskeletal proteins in the cerebellum of chronic schizophrenics. Neurosci Lett. 2002;317:161–165. doi: 10.1016/s0304-3940(01)02458-2. [DOI] [PubMed] [Google Scholar]
- 62.Eastwood SL, Law AJ, Everall IP, Harrison PJ. The axonal chemorepellant semaphorin 3A is increased in the cerebellum in schizophrenia and may contribute to its synaptic pathology. Mol Psychiatry. 2003;9:148–155. doi: 10.1038/sj.mp.4001233. [DOI] [PubMed] [Google Scholar]
- 63.Paz RD, Andreasen NC, Daoud SZ, et al. Increased expression of activity-dependent genes in cerebellar glutamatergic neurons of patients with schizophrenia. Am J Psychiatry. 2006;163:1829–1831. doi: 10.1176/ajp.2006.163.10.1829. [DOI] [PubMed] [Google Scholar]
- 64.Daskalakis ZJ, Christensen BK, Fitzgerald PB, Fountain SI, Chen R. Reduced cerebellar inhibition in schizophrenia: a preliminary study. Am J Psychiatry. 2005;162:1203–1205. doi: 10.1176/appi.ajp.162.6.1203. [DOI] [PubMed] [Google Scholar]
- 65.Joy S, Fein D, Kaplan E. Decoding digit symbol: speed, memory and visual scanning. Assessment. 2003;10:56–65. doi: 10.1177/0095399702250335. [DOI] [PubMed] [Google Scholar]
- 66.Casini L, Ivry RB. Effects of divided attention on temporal processing in patients with lesions of the cerebellum or frontal lobe. Neuropsychology. 1999;13:10–21. doi: 10.1037//0894-4105.13.1.10. [DOI] [PubMed] [Google Scholar]
- 67.Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry. 2007;64:532–542. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]