Abstract
In the treatment of advanced bladder cancer (BC), attention has recently focused on small molecule therapy concerning EGFR and the downstream Akt signalling pathway. Cellular deregulation processes are poorly understood, and biological determinants for the selection of therapy and monitoring are currently not available. The proteins PTEN, p-Akt and p27Kip1 are suggested to be potentially significant biomarkers of Akt signalling. In this study, we investigated the expression of these proteins in advanced BC. PTEN, p-Akt and p27Kip1 expression was determined immunohistochemically in 86 T2-4 BC specimens using a tissue microarray technique. Staining was documented with regard to intensity, cellular frequency and a multiplied staining score. Staining characteristics of the three proteins were correlated by regression analysis with the parameters of tumour stage and grade. A positive correlation was observed in the expression scores of PTEN and p-Akt, p-Akt and p27Kip1 as well as PTEN and p27Kip1 (p<0.02 for all combinations). The positive correlation between PTEN and p-Akt resulted mainly due to the strong correlation of PTEN intensity with p-Akt (p=0.0003 and p=0.0006 to p-Akt frequency and intensity, respectively). A positive correlation between p-Akt and p27Kip1 was noted for p-Akt frequency as well as intensity (p<0.05 for all combinations). The positive correlation between PTEN and p27Kip1 resulted due to the correlation of PTEN intensity alone with p27Kip1 (p<0.03 for p27Kip1 frequency and intensity), whereas no significance was noted for PTEN frequency. No correlation was found between T or G and expression of the proteins. However, activation of Akt in BC is known to occur independently of PTEN protein loss and appears not to cause a decrease of p27Kip1. However, a direct regulatory impact of PTEN on p27Kip1 was found. PTEN intensity, rather than frequency, appears to be a superior biomarker. These results may provide information to support research into protein profiling-predicted targeted therapy for BC. Correlations to benign urothelium, superficial BC specimens and follow-up data remain to be investigated.
Keywords: bladder cancer, PTEN, Akt, p27Kip1, targeted therapy, biomarker, tissue microarray
Introduction
In 2002, almost 360,000 men and women were newly diagnosed with bladder cancer (BC) worldwide, with a total mortality exceeding 145,000 patients (1). Only limited therapeutic options exist for the treatment of advanced BC (>pT1), including radical cystectomy, which is used for the majority of patients. After cystectomy, the overall survival rate is reported to be 66% at 5 years and 43% at 10 years after surgery (2). As with other tumour entities, attention in advanced BC is increasingly focusing on targeting intracellular signalling pathways involved in cell survival, the cell cycle, angiogenesis and invasion (3). Previous studies identified the phosphatidylinositol-3-kinase (PI3K)-protein kinase B (PKB/Akt) signalling pathway as being a significant component of the regulatory pathways affecting cancer (4,5).
Activated Akt protein is reported to play a central role in an outermost complex network of cell growth modulation affecting protein biosynthesis, cell cycle arrest and apoptosis. The enzyme phosphatase and tensin homologue (PTEN) is reported to inhibit Akt activation (phosphorylation). Activated Akt affects various downstream molecules causing increased cell proliferation and acts as a transcriptional activator, an inhibitor of cell cycle arrest or a promoter of protein synthesis. The protein p27Kip1 belongs to these molecules and is reported to cause cell arrest in the G1 phase via a cascade of other mediators (6). Deregulation of Akt signalling appears to play a key role in the pathogenesis of cancer. Understanding the effect of the individual up- and downstream molecules and presumably existing deregulation of their cascades in tumour cells may shed light on prognosis, suggesting different therapeutic options and possibly targeted therapy depending on the individual changes in the signalling network (3,7).
Understanding the exact individual changes in the pattern of up- and downstream molecules may allow for the prediction of the effect of molecular inhibitors of tyrosine protein kinase as shown for treatment with trastuzumab (Herceptin®) in patients presenting with breast cancer tissue overexpressing HER2 (8). Such findings facilitate the use of tumour-specific as well as cost-effective treatment. Similarly, studies on various tumour entities were carried out to investigate the role of PKB/Akt signalling in prostate (9) and renal cell cancer (10). Findings of these studies showed that the prevalence and extent of phosphorylation, i.e., activation of Akt, its downstream target p27Kip1 and its inhibitory protein PTEN were determined. However, the findings of these studies were controversial (11,12).
Data regarding Akt signalling in advanced BC are limited (3). This study aimed to determine the prevalence and inter-action of the three signalling molecules in advanced BC.
Materials and methods
Patients
For this study, paraffin-embedded tissue samples obtained from 86 patients suffering from muscle-invasive BC were used. Tissue samples were obtained from patients undergoing radical cystectomy between May 1993 and December 2002 at the Department of Urology, University of Tuebingen, Germany. The study was approved by the local ethics committee (no.383/2010BO2). TNM classification was re-validated by the Institute of Pathology at Tuebingen University Hospital in order to guarantee that pure tissue samples were obtained with muscle-invasive BC. Tumour stage was described according to the 2002 UICC TNM classification system. Tumour grading was determined using the WHO grading system of 1973. Table I shows the characteristics of the patients included in this study.
Table I.
Patient characteristics.
| Variable | No. of patients (%) |
|---|---|
| Gender | |
| Male | 58 (67.4) |
| Female | 28 (32.6) |
| Age (years) | |
| Median | 71 |
| Range | 45–91 |
| Pathological stage | |
| pT2 | 51 (59.3) |
| pT3/pT4 | 35 (40.7) |
| Tumour grade | |
| G1 | 1 (1.2) |
| G2 | 34 (39.5) |
| G3 | 51 (59.3) |
| Lymph node metastasis | |
| N0 | 51 (59.3) |
| N+ | 32 (37.2) |
| Nx | 3 (3.5) |
| Distant metastasis | |
| M0 | 61 (70.9) |
| M+ | 21 (24.4) |
| Mx | 4 (4.7) |
| N0/M0 | 49 (57.0) |
Tissue microarray
In order to obtain representative tumour cores for tissue microarray (TMA) construction, the specimens obtained were stained with H&E and suitable areas were selected for TMA. TMA preparation was conducted as described by Kallioniemi et al (13). Two cores from each patient were integrated into the TMA. In total, the TMA contained 172 tissue cores.
Staining
Specific staining was performed with commercially available antibodies against p-Akt, PTEN and p27Kip1 (PTEN monoclonal mouse, dilution 1:100; Cell Signaling Technology, Inc., Beverly, MA, USA; p-Akt polyclonal rabbit, dilution1:50; Cell Signaling; p27Kip1 monoclonal mouse, dilution1:200; Dako Cytomation, Glostrup, Denmark). Tissue slides were incubated overnight at 4°C with corresponding antibody solutions.
Tissue samples were then washed in TBS and incubated with a secondary biotinylated antibody (Vectastatin Elite ABC kit; Vector Laboratories, Inc., Burlingame, CA, USA) for 60min. Visualisation was achieved using the DAB method (Vector Laboratories), according to the manufacturer’s instructions. After brief rinsing, counterstaining was performed with Mayer’s haematoxylin and slides were finally mounted. Breast cancer tissue was used as a positive control for all three primary antibodies. For the negative control, the primary antibody was omitted.
For evaluation, two investigators blinded for patient data and antibody entity evaluated the staining intensity of each core independently of each other using a semi-quantitative scale (0, no staining at all; 1, sparse staining; 2, limited, but considerable intense staining; and 3, strong staining). Additionally, staining frequency of the cells was obtained from the percentage of stained tumour cells and intensity, and the frequency resulted in a staining score described by Theodorescu etal (14).
Statistical analysis
Using linear regression analysis staining intensity, the frequency and score of each protein was correlated to detect possible positive or negative correlations. Staining characteristics were correlated with tumour characteristics, including stage, grade and synchronous distant metastases, using the Wilcoxon-Kruskal-Wallis test. Statistical tests were performed using JMP software (SAS Inc., Cary, NC, USA). P<0.05 was defined as statistically significant.
Results
Staining description
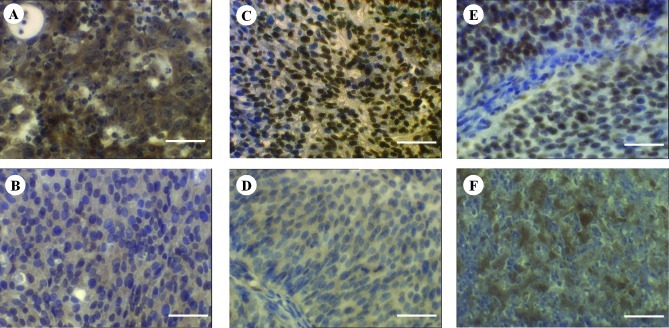
Staining of PTEN was mainly cytoplasmic. However, the intensely stained tissues showed additional nuclear localisation of ~20% of the stained cells. p-Akt and p27Kip1 expression was located predominantly in the nuclei, whereas a partial cytoplasmic expression was observed. Cytoplasmic expression was often ubiquitious in the case of PTEN and p-Akt, whereas that of p27Kip1 revealed a spotted distribution. Fig. 1 shows the representative staining results. There was an absence of staining in the negative controls, whereas the positive controls revealed intense staining reactions (data not shown).
Figure 1.
Representative results of intense and low expression of PTEN, p-Akt and p27Kip1 in muscle-invasive bladder cancer. (A) Intense and (B) low expression of PTEN, (C) intense and (D) low expression of p-Akt, (E) intense and (F) low expression of p27Kip1. Magnification, ×160; bar, 50 μm.
Correlation of the staining characteristics of PTEN, p-Akt and p27Kip1
Table II shows the calculated levels of significance correlating the staining characteristics of PTEN, p-Akt and p27Kip1 antibodies. A positive correlation was observed in the expression scores for PTEN and p-Akt, p-Akt and p27Kip1, as well as PTEN and p27Kip1. Each pair demonstrated a significant correlation (p<0.02 each, Table II).
Table II.
Levels of significance of the staining characteristics and their correlations.
| p-Akt | Intensity | Frequency | Score |
|---|---|---|---|
| PTEN | |||
| Intensity | 0.0006 | 0.0003 | 0.0330 |
| Frequency | 0.2400 | 0.0361 | 0.2033 |
| Score | 0.0044 | 0.0068 | 0.0059 |
|
| |||
| p27Kip1 | Intensity | Frequency | Score |
|
| |||
| p-Akt | |||
| Intensity | 0.0120 | 0.0378 | 0.0086 |
| Frequency | 0.0116 | 0.0231 | 0.0533 |
| Score | 0.0314 | 0.0760 | 0.0085 |
|
| |||
| p27Kip1 | Intensity | Frequency | Score |
|
| |||
| PTEN | |||
| Intensity | 0.0127 | 0.0179 | 0.0114 |
| Frequency | 0.0797 | 0.5045 | 0.0414 |
| Score | 0.0275 | 0.0734 | 0.0104 |
Bold, significant correlations.
The positive correlation between PTEN and p-Akt resulted mainly due to the strong correlation between PTEN intensity and p-Akt parameters. Highly significant correlations were observed between PTEN intensity and p-Akt intensity (p<0.0006), as well as between PTEN intensity and p-Akt frequency (p=0.0003, Table II). Positive correlations between the two staining characteristics of p-Akt and p27Kip1 were found for the three combinations, with each combination showing a significance of p<0.05 (Table II). Furthermore, positive correlations were observed between PTEN and p27Kip1 characteristics; these were based on positive correlations between PTEN intensity and the p27Kip1 characteristics with significance levels of p=0.0127 for p27Kip1 intensity and p=0.0179 for frequency. However, no significant correlation was observed between PTEN frequency and p27Kip1 attributes (Table II).
No significant correlation was observed between T and Gstage, and lymphatic and distant metastases and PTEN, p-Akt or p27Kip1 characteristics. However, a trend was observed in patients with synchronous distant metastases of a lower expression of p27Kip1 (mean scores 141 and 116, respectively; p=0.33) mainly resulting from a lower intensity (mean 1.7 and 1.3, respectively; p=0.16).
Discussion
The positive correlation noted between PTEN and p27Kip1 may be interpreted in two ways. Firstly, it is well known that a positive correlation of PTEN and p27Kip1 results from a decreased expression of p27Kip1 following the loss of PTEN expression. By inhibiting PIP3, PTEN appears to inhibit the phosphorylation of Akt, thus preventing the inhibitory effect of p-Akt on p27Kip1 (15). Notably, loss of the protective effect of PTEN, i.e., inhibition of Akt activation, results in a decrease of p27Kip1. On the other hand, this correlation may be interpreted as an increase in p27Kip1 expression being dependent on higher levels of PTEN expression, as has already been reported for renal cell carcinoma (26) and breast cancer by Chiarle et al (16). However, the present data suggest a reduction of p27Kip1 in M1 patients, suggesting a loss of BC progression, as reported for other malignancies (9,17).
However, data have indicated an association of PTEN and p27Kip1 with the Akt protein itself. Considering this correlation, a positive correlation between the inhibitory protein PTEN and the phosphorylated (activated) form of Akt was observed in the present study. Furthermore, a considerable expression of p27Kip1 was observed in the presence of activated Akt, indicating a decrease in p27Kip1. Similar results have already been observed in studies of ovarian cancer (12). Another example that contradicts this linear tumourigenic pathway is the study of Panigrahi etal (18) in which no correlation was found between PTEN or p-Akt and survival in breast cancer.
A number of conceivable principal mechanisms may explain these findings. Cantley and Neel reviewed several inherited and spontaneous tumours with PTEN deficiency or loss (19). Additionally, numerous studies on various tumour entities, such as colon and breast cancer, support this mechanism (20,21). However, the inhibitory effect of PTEN on the phosphorylation of Akt may be impaired without a reduction in protein expression. Possible reasons for this impairment include a structural alteration of the inhibitor itself, the presence of a PTEN antagonist or an alternative Pi3K-independent activation of Akt. Yoganathan etal (22) reported integrin-linked kinase (ILK) 1-associated Akt phosphorylation at Ser-473. Persad etal (23) also observed the phosphorylation of Akt at Thr-308 and Ser-473 by ILK in prostate carcinoma cell lines PC-3 and LNCaP. Widenmaier etal (24) reported that glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) activate Akt independently of PI3K. Later processing, i.e., inactivation of p-Akt in the nucleus, as observed by Trotman etal (25), explains the simultaneous staining of PTEN and (cytoplasmic) p-Akt. Local separation of the inhibitor and effector may also explain the simultaneous presence and positive correlation of the inhibitor and its downstream target. These explanations are also possible regarding the positive correlation between p-Akt and p27Kip1 observed in this study. Various authors have reported the nuclear export of p27Kip1 (26,27) preventing its effect on cell cycle regulation. Considering in particular a local separation as a possible explanation, even high levels of p27Kip1 would be incapable of inducing a G1 arrest if p27Kip1 was localised in the cytoplasm. This is also supported by studies observing an impaired nuclear import of p27Kip1 with a consecutive cytoplasmic accumulation (28,29).
Another principal mechanism may be an alternative, yet-unknown activation of p27Kip1 as a compensatory attempt to inhibit an accelerated cell cycle in tumour cells. Additionally, an overexpression of PTEN may be conceived as a compensatory attempt to antagonise an alternatively activated Akt. Loss of PTEN, which was previously correlated with poor prognosis by other authors (30,31), may be understood as a loss of this compensatory means during cancer progression in the sense of a late event.
Accumulating evidence suggests that inter-individual molecular differences within the Akt-mTOR signalling of clinically comparable tumours affect the individual prognosis and response to different tumour therapies. Several studies support this observation. Pantuk et al (31) and Azim etal (7) studied the mTOR pathway and its components and correlated this pathway significantly with pathological findings and survival in renal cell carcinoma. These authors also stressed the importance of individual assessment of distinct molecular markers to predict individual benefit of targeted therapy. This type of observation results in specifying individual molecular alterations in cancer.
However, data concerning this correlation with BC are limited. Chen et al (32) reported molecular data on Akt signalling and significant correlation with survival, whereas Shariatetal (33) associated an increased risk of disease progression and death with alterations of p27Kip1 in combination with p53, p21 and pRB.
The identification of molecular markers would allow for the calculation of the individual response rate, and the prediction of treatment modalities, as well as molecularly targeted and individually adjusted therapy. Determining the individual expression levels and the correlation of signalling molecules, as examined in this study, may result in a better understanding of transitional cell carcinoma and individualised targeted therapy. Mass etal (8) observed a benefit in treating metastatic breast cancer with trastuzumab in patients overexpressing HER2. Based on these findings, Laé et al (34) evaluated HER2 expression in muscle-invasive BC and observed HER2 gene amplification in 5.1% of the BCs. Another example of biomarkers in BC is found in the study by Havaleshkoetal (35), who combined microarray data and phosphoprotein profiling to predict lapatinib sensitivity in BC. In non-invasive BC, Miyakeetal (36) profiled the possible benefit of the tyrosine kinase inhibitor PD173074 by analysis of fibroblast growth factor receptor-3 mutations.
The results of the current study in muscle-invasive BC suggest that neither a reduced expression of PTEN nor p27Kip1 are reliable biomarkers for the activation of the Akt pathway. The staining intensity of PTEN appears to be superior to its frequency in terms of its potential use as a marker for targeted therapy or a predictor of progression. This finding may also indicate the various mechanisms of Akt activation within different tumour cell subpopulations that are partially independent of PTEN.
As a study predominantly addressing signalling, neither a reduced nor an increased expression of the parameters in BC tissue could be determined, nor could their role in the progression sequence be examined.
The semi-quantitative analysis appears to be a suitable method for this pilot study; however, a quantitative method is required to confirm the findings presented in this study. Consequently, future studies should involve benign bladder tissues as well as non-invasive BC and correlate patient follow-up with the expression pattern of parameters investigated in the present study. The semi-quantitative approach should be extended with quantitative methods, including Western blotting and RT-PCR, and analysis of cellular allocation should be conducted.
The findings of Akt signalling in BC observed in this study are predictable. However, unforeseen correlations of its mediators were additionally detected, suggesting a yet-unknown regulatory effect on Akt and p27Kip1 and mechanisms in cancer, bypassing cellular mechanisms of cell cycle restriction. Further studies are required to correlate the effect of different antiproliferative agents with the individual expression patterns of these signalling molecules.
References
- 1.Parkin M, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 3.Netto G, Epstein J. Theranostic and prognostic biomarkers: genomic applications in urological malignancies. Pathology. 2010;42:384–394. doi: 10.3109/00313021003779145. [DOI] [PubMed] [Google Scholar]
- 4.Altomare D, Testa J. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- 5.Samuels Y, Ericson K. Oncogenic PI3K and its role in cancer. Curr Opin Oncol. 2006;18:77–82. doi: 10.1097/01.cco.0000198021.99347.b9. [DOI] [PubMed] [Google Scholar]
- 6.Sherr C, Roberts J. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 7.Azim H, Azim H, Escudier B. Targeting mTOR in cancer: renal cell is just a beginning. Target Oncol. 2010;5:269–280. doi: 10.1007/s11523-010-0141-x. [DOI] [PubMed] [Google Scholar]
- 8.Mass R, Press M, Anderson S, et al. Evaluation of clinical outcomes according to HER2 detection by fluorescence in situ hybridization in women with metastatic breast cancer treated with trastuzumab. Clin Breast Cancer. 2005;6:240–246. doi: 10.3816/CBC.2005.n.026. [DOI] [PubMed] [Google Scholar]
- 9.Graff JR, Konicek BW, McNulty AM, et al. Increased AKT activity contributes to prostate cancer progression by dramatically accelerating prostate tumor growth and diminishing p27Kip1 expression. J Biol Chem. 2000;275:24500–24505. doi: 10.1074/jbc.M003145200. [DOI] [PubMed] [Google Scholar]
- 10.Merseburger A, Hennenlotter J, Kuehs U, et al. Activation of PI3K is associated with reduced survival in renal cell carcinoma. Urol Int. 2008;80:372–377. doi: 10.1159/000132694. [DOI] [PubMed] [Google Scholar]
- 11.Hennenlotter J, Ohneseit P, Simon P, et al. PTEN and p27Kip1 are not downregulated in the majority of renal cell carcinomas – implications for Akt activation. Oncol Rep. 2008;19:1141–1147. [PubMed] [Google Scholar]
- 12.Kurose K, Zhou X-P, Araki T, Cannistra S, Maher E, Eng C. Frequent loss of PTEN expression is linked to elevated phosphorylated Akt levels, but not associated with p27 and cyclinD1 expression, in primary epithelial ovarian carcinomas. Am J Pathol. 2001;158:2097–2106. doi: 10.1016/S0002-9440(10)64681-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kallioniemi OP, Wagner U, Kononen J, Sauter G. Tissue microarray technology for high-throughput molecular profiling of cancer. Hum Mol Genet. 2001;10:657–662. doi: 10.1093/hmg/10.7.657. [DOI] [PubMed] [Google Scholar]
- 14.Theodorescu D, Broder SR, Boyd JC, Mills SE, Frierson HF. Cathepsin D and chromogranin A as predictors of long term disease specific survival after radical prostatectomy for localized carcinoma of the prostate. Cancer. 1997;80:2109–2119. [PubMed] [Google Scholar]
- 15.Blain S, Scher H, Cordon-Cardo C, Koff A. p27 as a target for cancer therapeutics. Cancer Cell. 2003;3:111–115. doi: 10.1016/s1535-6108(03)00026-6. [DOI] [PubMed] [Google Scholar]
- 16.Chiarle R, Pagano M, Inghirami G. The cyclin dependent kinase inhibitor p27 and its prognostic role in breast cancer. Breast Cancer Res. 2001;3:91–94. doi: 10.1186/bcr277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seki R, Ohshima K, Fujisaki T, et al. Prognostic significance of S-phase kinase-associated protein 2 and p27kip1 in patients with diffuse large B-cell lymphoma: effects of rituximab. Ann Oncol. 2010;21:833–841. doi: 10.1093/annonc/mdp481. [DOI] [PubMed] [Google Scholar]
- 18.Panigrahi AR, Pinder SE, Chan SY, Paish EC, Robertson JFR, Ellis IO. The role of PTEN and its signalling pathways, including AKT, in breast cancer; an assessment of relationships with other prognostic factors and with outcome. J Pathol. 2004;204:93–100. doi: 10.1002/path.1611. [DOI] [PubMed] [Google Scholar]
- 19.Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA. 1999;96:4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khaleghpour K, Li Y, Banville D, Yu Z, Shen SH. Involvement of the PI 3-kinase signaling pathway in progression of colon adenocarcinoma. Carcinogenesis. 2004;25:241–248. doi: 10.1093/carcin/bgg195. [DOI] [PubMed] [Google Scholar]
- 21.Shi W, Zhang X, Pintilie M, et al. Dysregulated PTEN-PKB and negative receptor status in human breast cancer. Int J Cancer. 2003;104:195–203. doi: 10.1002/ijc.10909. [DOI] [PubMed] [Google Scholar]
- 22.Yoganathan TN, Costello P, Chen X, et al. Integrin-linked kinase (ILK): a ‘hot’ therapeutic target. Biochem Pharmacol. 2000;60:1115–1119. doi: 10.1016/s0006-2952(00)00444-5. [DOI] [PubMed] [Google Scholar]
- 23.Persad S, Attwell S, Gray V, et al. Inhibition of integrin-linked kinase (ILK) suppresses activation of protein kinase B/Akt and induces cell cycle arrest and apoptosis of PTEN-mutant prostate cancer cells. Proc Natl Acad Sci USA. 2000;97:3207–3212. doi: 10.1073/pnas.060579697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Widenmaier S, Sampaio A, Underhill M, McIntosh C. Noncanonical activation of Akt/protein kinase B in {beta}-cells by the incretin hormone glucose-dependent insulinotropic polypeptide. J Biol Chem. 2009;284:10764–10773. doi: 10.1074/jbc.M809116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trotman L, Alimonti A, Scaglioni PP, Koutcher J, Cordon-Cardo C, Pandolfi PP. Identification of a tumour suppressor network opposing nuclear Akt function. Nature. 2006;441:523–527. doi: 10.1038/nature04809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blain S, Massagué J. Breast cancer banishes p27 from nucleus. Nat Med. 2002;8:1076–1078. doi: 10.1038/nm1002-1076. [DOI] [PubMed] [Google Scholar]
- 27.Connor M, Kotchetkov R, Cariou S, et al. CRM1/Ran-mediated nuclear export of p27(Kip1) involves a nuclear export signal and links p27 export and proteolysis. Mol Biol Cell. 2003;14:201–213. doi: 10.1091/mbc.E02-06-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang J, Zubovitz J, Petrocelli T, et al. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med. 2002;8:1153–1160. doi: 10.1038/nm761. [DOI] [PubMed] [Google Scholar]
- 29.Shin I, Yakes M, Rojo F, et al. PKB/Akt mediates cell-cycle progression by phosphorylation of p27Kip1 at threonine 157 and modulation of its cellular localization. Nat Med. 2002;8:1145–1152. doi: 10.1038/nm759. [DOI] [PubMed] [Google Scholar]
- 30.Capodanno A, Camerini A, Orlandini C, et al. Dysregulated PI3K/Akt/PTEN pathway is a marker of a short disease-free survival in node-negative breast carcinoma. Hum Pathol. 2009;40:1408–1417. doi: 10.1016/j.humpath.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Pantuck A, Seligson D, Klatte T, et al. Prognostic relevance of the mTOR pathway in renal cell carcinoma. Cancer. 2007;109:2257–2267. doi: 10.1002/cncr.22677. [DOI] [PubMed] [Google Scholar]
- 32.Chen M, Gu J, Delclos G, et al. Genetic variations of the PI3K-AKT-mTOR pathway and clinical outcome in muscle invasive and metastatic bladder cancer patients. Carcinogenesis. 2010;31:1387–1391. doi: 10.1093/carcin/bgq110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shariat S, Ashfaq R, Sagalowsky A, Lotan Y. Predictive value of cell cycle biomarkers in nonmuscle invasive bladder transitional cell carcinoma. J Urol. 2007;177:481–487. doi: 10.1016/j.juro.2006.09.038. [DOI] [PubMed] [Google Scholar]
- 34.Laé M, Couturier J, Oudard S, Radvanyi F, Beuzeboc P, Vieillefond A. Assessing HER2 gene amplification as a potential target for therapy in invasive urothelial bladder cancer with a standardized methodology: results in 1005 patients. Ann Oncol. 2010;21:815–819. doi: 10.1093/annonc/mdp488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Havaleshko D, Smith SC, Cho H, et al. Comparison of global versus epidermal growth factor receptor pathway profiling for prediction of lapatinib sensitivity in bladder cancer. Neoplasia. 2009;11:1185–1193. doi: 10.1593/neo.09898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyake M, Ishii M, Koyama N, et al. 1-tert-butyl-3-[6-(3,5-dimethoxy-phenyl)-2-(4-diethylamino-butylamino)-pyrido[2,3-d]pyrimidin-7-yl]-urea (PD173074), a selective tyrosine kinase inhibitor of fibroblast growth factor receptor-3 (FGFR3), inhibits cell proliferation of bladder cancer carrying the FGFR3 gene mutation along with up-regulation of p27/Kip1 and G1/G0 arrest. J Pharmacol Exp Ther. 2010;332:795–802. doi: 10.1124/jpet.109.162768. [DOI] [PubMed] [Google Scholar]