Abstract
B cells are selected by the binding of antigen to clonally distributed B cell receptors (BCRs), triggering signalling cascades that result in B cell activation. With the recent application of high-resolution live-cell imaging, we are gaining an understanding of the events that initiate BCR signalling within seconds of its engagement with antigen. These observations are providing a molecular explanation for fundamental aspects of B cell responses, including antigen affinity discrimination and the value of class switching, as well as insights into the underlying causes of B cell tumorigenesis. Advances in our understanding of the earliest molecular events that follow antigen binding to the BCR may provide a general framework for the initiation of signalling in the adaptive immune system.
With the cooperation of helper T cells and the involvement of cells of the innate immune system, antigen has the potential to drive naive B cells to proliferate and differentiate into memory B cells and antibody-secreting plasma cells through processes that involve somatic hypermutation and class switching1. These events occur in specialized compartments in which B cells must compete for limited niches and resources in order to survive2. B cell receptors (BCRs) are clonally distributed and the life or death process for B cells begins with the clonal selection of specific B cells by antigen binding to BCRs on their cell surfaces3.
The BCR comprises a membrane-bound immunoglobulin and a disulphide-linked heterodimer composed of Igα and Igβ4,5 (FIG. 1). Igα and Igβ are transmembrane proteins with extracellular domains that, on the basis of their amino acid sequence, are predicted to have immunoglobulin-like folds, and intracellular domains that each contain an immunoreceptor tyrosine-based activation motif (ITAM). Upon antigen binding to mature B cells, the BCR is phosphorylated on its ITAM tyrosines by the first kinase in the BCR signalling pathway, primarily LYN, and then SYK (spleen tyrosine kinase) is recruited through its SH2 domain to the phosphorylated Igα–Igβ heterodimer, resulting in the triggering of at least four different signalling cascades6,7.
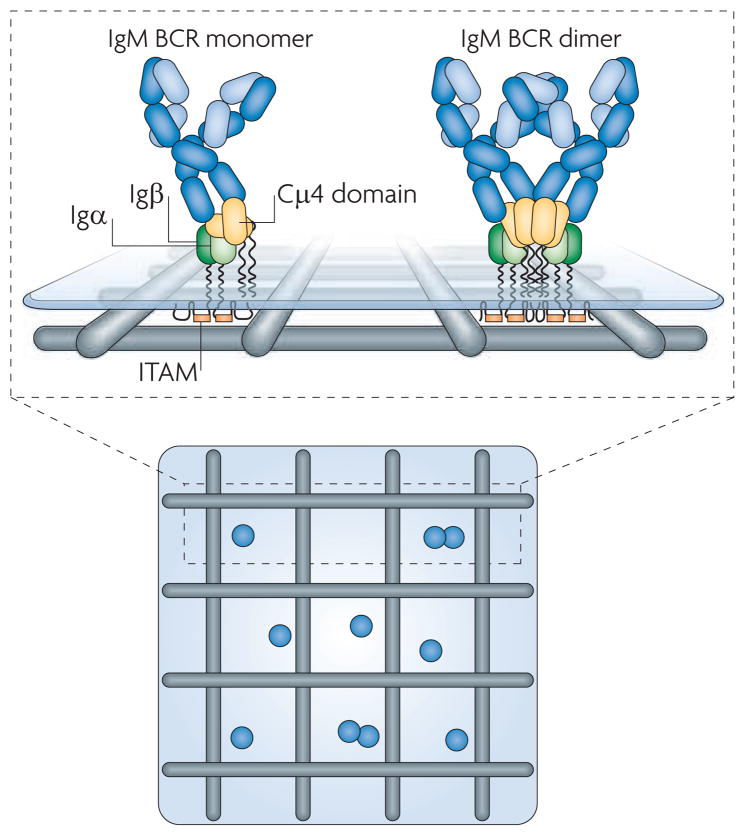
Figure 1. The structural organization of the B cell receptor.
The four-chain structure of the IgM B cell receptor (BCR), composed of the membrane form of IgM associated with a covalently linked heterodimer of Igα and Igβ, is depicted. Cell surface BCRs are shown in a compartmentalized plasma membrane such as that proposed by Kusumi and colleagues (BOX 2). This schematic is based on observations from single particle tracking experiments from live-cell imaging11 showing that the BCRs exist mainly as highly mobile monomers on resting B cells, with only ~20% comprising less-mobile dimers or more highly ordered oligomers. The membrane-proximal Cμ4 portion of the membrane-bound IgM (or the Cγ3 portion of membrane-bound IgG, not shown) was determined to be required and sufficient to induce BCR oligomerization and immobilization upon membrane-bound monomeric antigen binding11. The immunoreceptor tyrosine-based activation motif (ITAM)-containing cytoplasmic tails of the BCR extend through the cytoskeleton into the cytoplasm.
Over the past several years, biochemical and genetic approaches have elucidated a great deal about the nature of the complex signalling cascades that are triggered by antigen binding to the BCR6,7. However, it was not until the advent of live-cell imaging, particularly at the single-molecule level, that the field acquired tools (BOX 1) that could provide the temporal and spatial resolution necessary to begin to understand how BCRs perceive that antigen has bound to their ectodomains and how this information is translated across the membrane to trigger signalling cascades.
Box 1. Application of imaging technologies to the study of B cell activation.
Scanning electron microscopy
Scanning electron microscopy (SEM)73 uses a high-energy electron beam to scan the surface of samples with nanometre-scale resolution, providing information on three-dimensional characteristics. However, because SEM cannot be used for live-cell imaging, it cannot provide kinetic data with high temporal resolution.
Confocal laser-scanning microscopy
Confocal laser-scanning microscopy (CLSM)74 is one of the most widely used live-cell imaging techniques. CLSM uses a focused beam of laser light to excite fluorophores in a sample at a selected depth. Emitted light that is out of focus is prevented from reaching the detectors by the use of a pinhole device. CLSM generally produces spatial resolution of several hundred nanometres, approaching the limit of light diffraction (200 nm) and temporal resolution of several hundred milliseconds in living cells. However, CLSM does have limitations — it cannot produce quality images of samples thicker than 80 μm, and the depth of optical sections in CLSM is relatively thick, typically greater than 0.6 μm, making imaging thin specimen planes, such as the plasma membrane, difficult owing to unwanted signals reaching the detector.
Two-photon laser-scanning fluorescence microscopy
Two-photon laser-scanning fluorescence microscopy (2PM)75 allows imaging of living tissues up to a depth of approximately 1 millimetre. Typically, 2PM uses a long-wave infrared laser to excite a fluorophore that requires the absorption of two infrared photons to emit a single photon. Thus, 2PM produces the desired optical sectioning without the assistance of a pinhole device. The long-wavelength infrared photons can deeply penetrate tissues more efficiently than short-wavelength lights owing to limited scattering.
Total internal reflection fluorescence microscopy
Total internal reflection fluorescence microscopy (TIRFM)76 allows fluorophores to be visualized with a superior signal-to-noise ratio because the depth of optical section in TIRFM is limited to a 100 nm space above the cover slip on which a cell is placed. Typically, the glass cover slip has antigen absorbed onto it or supports a fluid lipid bilayer into which antigen is incorporated. A laser pointed at an angle to the cover slip generates an exponentially decaying evanescent light at the glass–cell interface. Single fluorophores can be individually resolved, as long as they are separated by a distance greater than the light diffraction limit (~200 nm). In addition, the application of particle-tracking methods can further improve the resolution to a sub-diffraction limit level. Typically, the temporal resolution of TIRFM can reach tens of milliseconds. These features make TIRFM an ideal tool for studying protein diffusion dynamics at or near the plasma membrane. TIRFM is especially useful when integrated with other fluorescence-based imaging techniques, described below. TIRFM cannot be applied to the study of B cells interacting with antigen-presenting cells and is limited in that regard.
Fluorescence resonance energy transfer
Fluorescence resonance energy transfer (FRET)77,78 is the transfer of energy from a donor fluorophore to an acceptor fluorophore within a limited distance (10 nm). Because of the extreme sensitivity of FRET efficiencies to the distance between FRET donor and acceptor, FRET has proved to be a highly sensitive molecular ruler with which to measure the interactions between proteins or proteins and lipids. In combination with TIRFM, FRET imaging allows the detection of molecular interactions of membrane-associated proteins at the contact interface of cells with glass or glass-supported membranes.
Single particle tracking
Single particle tracking (SPT)79 is an imaging technique that is widely used to examine the diffusion mobility of molecules. Multicolour imaging with SPT can provide information concerning the spatial and temporal relationship between receptors of interest or between receptors and important cellular structures. For single molecules to be resolved and tracked, they must be fluorescently labelled at a low level that is usually determined by the fluorescence intensity profile and bleaching characteristics. When labelling the target molecules, traditional organic dyes (such as Cy3) are widely used, however their application in long-term SPT (many tens of trajectory steps) is limited owing to photobleaching80.
Quantum dots
Quantum dots (Qdots)80 are semiconductor materials with excellent fluorescence and photostability. In combination with TIRFM, molecules of interest labelled with Qdots can be tracked at the single-molecule level for long periods of time71,72. Qdots are especially useful in multicolour SPT TIRFM.
We think of these very early events that follow antigen binding to the cell surface BCR as ‘tipping points’: the crucial events that propel the BCRs towards triggering signalling cascades and all the sequelae that follow. In this Review, we describe the tipping points of antigen-induced initiation of BCR signalling that have been revealed by live-cell imaging. We suggest that very small differences in these early events result in big differences in the quality of the resulting humoral immune response.
We begin with a description of the BCR in resting cells and briefly review how and where B cells ‘see’ antigen. We describe the response to antigen at the whole-cell level, then zoom in on individual BCRs and a view of the molecular events that occur within seconds of antigen binding to the BCR, including the initial oligomerization of the receptor and the subsequent growth of BCR microclusters. We detail how these early BCR-intrinsic events can account for antigen affinity discrimination and explain the value of class switching. Mechanisms are proposed to explain how BCR oligomerization triggers signalling, and the regulation of early events in the initiation of BCR signalling and their implications for B cell tumorigenesis and autoimmunity are discussed. Finally, the BCR is a member of a family of multichain immune-recognition receptors (MIRRs) that includes the T cell receptor (TCR) and the high-affinity receptor for IgE (FcεR1). We attempt to put the advances in our understanding of molecular events that follow antigen binding to the BCR into a general framework for understanding the initiation of signalling by MIRRs in the adaptive immune system.
The BCR in resting cells
Although in the absence of antigen B cells are referred to as resting cells, they are not quiescent and are probably constantly hovering near a tipping point of activation. The expression of the BCR is essential for the survival of B cells at all developmental stages, leading to the hypothesis that the BCR must provide constitutive low-level ‘tonic’ signals that promote survival, either spontaneously or on interaction with ligands in the environment8,9. Recent genetic and biochemical analyses have shed light on the nature of the tonic signalling pathway, suggesting that the initiation of tonic signalling may not be identical to that of antigen-induced signalling, and may bypass the requirement for the phosphorylation of BCR ITAMs. Evidence was provided that phosphoinositide 3-kinase (PI3K) signalling is crucial for the survival of mature B cells and that the PI3K pathway might be connected to the BCR in resting cells by the GTPase TC21 (also known as RRAS2), which directly recruits the catalytic subunit of PI3K to the unphosphorylated ITAM of the BCR8,10.
However, regardless of the biochemical nature of the tonic signalling pathway, the mechanism by which the BCR initiates tonic signalling is not fully understood. Recent high-resolution imaging in live B cells has provided insights into the behaviour of BCRs in resting cells that have implications for how tonic signalling might be initiated in mature B cells. Live-cell total internal reflection fluorescence microscopy (TIRFM) in conjunction with analyses of fluorescence resonance energy transfer (FRET) (BOX 1) between BCRs containing FRET donor and acceptor fluorescent proteins indicated that in resting B cells most BCRs were not in close molecular proximity, but instead were monomeric5. In addition, tracking individual BCR molecules in resting B cells by single particle tracking (SPT) (BOX 1) in the absence of antigen showed the short-range movement of most BCRs to be consistent with free diffusion11,12. In the context of the ‘picket fence’ model of plasma–membrane organization (BOX 2) — in which the actin cytoskeleton (the fence) and trans-membrane proteins (pickets) compartmentalize both proteins and lipids — we interpret these observations to mean that, in resting B cells, most BCRs are freely diffusing and highly mobile as monomers within, and hop relatively easily between, compartments created by the actin cytoskeleton. These highly mobile BCR monomers seem to exist on the B cell surfaces with less mobile dimers or more highly ordered BCR oligomers (FIG. 1). We observed that approximately 20% of BCRs were immobile in the membrane of resting B cells5, which suggests that these BCRs may have formed either spontaneous or ligand-induced oligomers, and undergone ‘oligomerization-induced trapping’ (BOX 2), which decreases the long-range diffusion of membrane molecules (FIG. 1; Supplementary information S1 (figure)). Because antigen-induced signalling-active BCR oligomers show similar behaviour (see below), an in-depth analysis to determine whether these immobile BCRs are the source of tonic signals and the relationship, in mature B cells, between these immobile microclusters and PI3K and TC21, the proposed mediators of tonic signals, should be of interest.
Box 2. A contemporary view of the plasma membrane.
An appreciation of the interpretation of much of the data produced by live-cell imaging requires an understanding of the organization and function of the plasma membrane. Current evidence indicates that the plasma membrane is not simply a fluid mosaic structure of freely diffusing proteins in a phospholipid bilayer, as proposed by Singer and Nicholson nearly 40 years ago81. Current data better fit a model in which the plasma membrane is partitioned into highly dynamic compartments that range in size from tens to hundreds of nanometres, depending on the cell type31,32. The current view of the compartmentalized plasma membrane accounts for an important observation made using single particle tracking (SPT) techniques — namely that the mobility of proteins, as well as lipids, is considerably slower in the plasma membranes of cells than in artificial lipid bilayers31,82. These data suggest that proteins and lipids are in some way confined in the plasma membrane and cannot freely diffuse.
Of great importance to understanding the early events in B cell receptor signalling is the observation that the formation of oligomers of membrane proteins formed by crosslinking resulted in dramatically reduced motility in the plasma membranes of cells, a behaviour not observed for protein oligomers in model membranes. The nature of the confinement became clear when Kusumi and colleagues applied extremely high-resolution microscopy at the microsecond scale to describe the movement of membrane proteins82. These remarkable images showed that, in the short term, proteins and lipids diffused freely within confined areas of the plasma membrane, and in the longer term hopped between these compartments. Electron tomography produced a three-dimensional structure of the membrane cytoskeleton showing that the entire cytoplasmic surface of the plasma membrane is covered by a meshwork of the actin cytoskeleton that forms boundaries of a size that correlated well with the size of the membrane compartments determined by SPT83. Kusumi and colleagues proposed that membrane proteins and lipids are confined in their movement by actin cytoskeleton ‘fences’ and actin-anchored protein ‘pickets’; termed the picket fence model31. According to this model, monomeric proteins can readily hop between compartments, whereas larger oligomers are less able to hop and are consequently ‘trapped’ in the compartment in which the oligomers first formed.
As recently reviewed, there is considerable experimental support for Kusumi’s picket fence model32. The model implies that cells have evolved mechanisms to control the long-range diffusion of membrane molecules, allowing compartmentalization of receptors and ‘oligomerization-induced trapping’ of receptor complexes. The immobilization of receptor oligomers could have an important role in facilitating the assembly of signalling complexes on the cytoplasmic domain of the receptors. In addition, the trapping of receptors at the site of antigen encounter may be important in orienting cell polarization.
Recently, Yang and Reth13 provided evidence for the existence of antigen-independent BCR dimers on the surface of cells using bifluorescence complementation (BiFC) in conjunction with confocal microscopy. They observed surface fluorescence in S2 Drosophila Schneider cells expressing membrane-bound immunoglobulin heavy and light chains and Igβ, and two types of Igα molecules that contained either the amino- terminal or carboxy-terminal half-domains of yellow fluorescent protein (YFP) such that each BCR had either an Igα–NYFP or an Igα–CYFP. Upon BCR dimerization a complete YFP forms, resulting in a fluorescent signal (the BiFC method). Confocal microscopy revealed YFP fluorescence on the cell surface, indicating the presence of dimers. However, the authors did not determine what percentage of BCRs on the surface of the living cells were in dimers, and only quantified the dimers in cell lysates. An important question is whether the percentage of dimerized BCRs in resting B cells that was detected by BiFC in this study was similar to the percentage detected by SPT (which was ~20%). The authors concluded that the observed dimers were signalling inactive because mutations that decreased dimerization increased signalling in response to antigen. The authors also speculated that the signalling-inactive dimers were in equilibrium with signalling-active monomers that provide tonic signals (see below).
Using two-colour SPT (BOX 1), Treanor et al.12 recently directly visualized the behaviour of individual BCRs and the actin cytoskeleton in mature resting B cells. They observed two distinct BCR populations: BCRs with high diffusion coefficients were present in actin-poor regions of the membrane, whereas BCRs with low diffusion coefficients that were essentially immobile were in actin- and erzin-rich regions. Confinement in actin-rich regions seemed to be mediated through the cytoplasmic domain of Igβ. Alterations introduced into the actin network by drugs that either stabilized or disassembled the actin cytoskeleton had the dramatic effect of fully activating the B cell in the absence of antigen. These authors suggested that in resting B cells, tonic signalling is maintained by the dynamic organization of the actin cytoskeleton, either by concentrating activators of tonic signalling or by excluding inhibitors of tonic signalling, and that alterations in the actin cytoskeleton disrupt this steady state and result in full BCR signalling. It is also possible that, in addition to actin, other proteins that form extracellular matrixes and have the ability to organize membrane receptors, such as galectins, have a role in controlling BCR tonic signalling14. Although speculative, these SPT studies suggest that tonic signalling might result from the transient trapping of highly dynamic BCR oligomers, promoting the assembly of signalling complexes.
The BCR response to antigen
Where B cells encounter antigen
B cells can respond to multivalent antigens in solution by BCR clustering and capping, and, ultimately, provided with the appropriate help, by proliferating and differentiating into antibody-secreting cells. Indeed, much of what we know about the nature of the BCR signalling pathways has come from biochemical studies of B cells activated by multivalent antigens in solution.
However, results of recent two-photon laser-scanning fluorescence microscopy (BOX 1) indicate that B cells recognize antigen on the surfaces of antigen-presenting cells (APCs) and that such recognition may be highly relevant for B cell activation in vivo15–20. Batista and colleagues21 first showed that the recognition by B cells of antigens on APCs in vitro was highly efficient and resulted in the formation of an immune synapse, a process similar to that described for T cells. Using antigen-containing fluid lipid bilayers as surrogate APCs, Fleire et al.22 provided evidence that the response of B cells to antigen encountered on membranes was much more active and dynamic than that predicted from the response of B cells to antigen in solution. They observed that B cells first engaged antigen through membrane protrusions, inducing the BCRs to form microclusters that resulted in B cell spreading over the lipid bilayer, forming additional microclusters in the peripheral lamellipodia of the cell. The microclusters then moved to the centre of the contact area through mechanisms that require the BCR to associate with the actin cytoskeleton and, following maximal spreading of the B cell, contracted to form an immune synapse. The B cell-spreading response to membrane-bound antigens was dependent on cooperation between phospholipase Cγ2 and the guanine-nucleotide exchange factor VAV, and on the small GTPase RAC2, and was regulated by Rap GTPases23–25.
Thus, B cells encounter and respond to antigen both in solution and on the surfaces of APCs. An important unanswered question is: are the B cell responses to antigen in solution and on APCs qualitatively and quantitatively similar? Also, will the rules established by the study of B cell responses to antigen in solution apply to B cells that are encountering antigen on APCs?
The formation of BCR oligomers: a tipping point
That the encounter of B cells with antigen (whether in solution or on the surface of APCs) leads to the formation of microclusters suggests that microclusters may be the elementary signalling unit for B cells and raises the questions: how does antigen drive the formation of microclusters, and are these mechanisms the same for antigen in solution and on APC surfaces? It is commonly observed that B cell activation by antigen in solution requires that the antigen be multivalent. Monovalent antigen does not induce the characteristic clustering and capping of BCRs that is observed after engagement of multivalent antigen. The striking difference between the effects of monovalent and multivalent antigen led to the conclusion that B cell activation required the physical cross-linking of the BCR by antigen26. Physical crosslinking is a common mechanism for the activation of various growth factor and chemokine receptors27,28. For these receptors, crosslinking by their growth factor or chemokine ligands brings the receptors into a spatially well-defined orientation in which kinases associated with the intracellular domains are in close molecular proximity, allowing for cross-phosphorylation of the receptor and the initiation of signalling29,30. By contrast, BCRs respond to a universe of foreign antigens of all sizes and shapes, and of spatial arrays of their antigenic epitopes. It is difficult to imagine how these diverse antigens can each succeed in bringing the crosslinked BCRs into spatially well-defined oligomers that can recruit LYN.
We recently provided the unexpected evidence that physical crosslinking of BCRs is, in fact, not required for B cell responses to antigens on membrane surfaces and that monovalent engagement of BCRs by membrane-bound antigens triggers signalling11. We followed individual BCRs by SPT TIRFM (BOX 1) as B cells first engaged fully mobile monovalent or multivalent antigens incorporated into a fluid lipid bilayer. Antigen binding greatly slowed the diffusion of the BCRs in the B cell plasma membrane, such that they were essentially immobile (Supplementary information S1 (figure)). This ‘stopping’ behaviour of the BCRs was indistinguishable between B cells responding to monovalent antigens and those responding to multivalent antigens, as long as the antigens were associated with planar lipid bilayers. The interpretation of the stopping behaviour in the context of the picket fence model of plasma-membrane organization (BOX 2) is that the receptors oligomerized after antigen binding and were trapped in membrane compartments formed by actin ‘fences’ and membrane protein ‘pickets’ and were unable to freely diffuse31,32.
BCR immobilization was highly dependent on the concentration of the antigen in the fluid lipid bilayer11, and antigen-bound BCRs did not stop when the concentration of antigen in the fluid lipid bilayer was low, decreasing the probability of random bumping between antigen-bound BCRs. We also observed that antigen-bound BCRs were much more likely to stop when encountering pre-existing BCR microclusters11. Through extensive analyses of BCRs with mutations — which resulted in the elimination of the Igα–Igβ heterodimer, alterations in the transmembrane domain of membrane-bound immunoglobulin or deletions of portions of the membrane-bound immunoglobulin ectodomains — it was determined that oligomerization and immobilization was a BCR-intrinsic event that did not require the signalling apparatus of the BCR, and that the membrane-proximal Cμ4 portion of the ectodomain of the membrane-bound IgM of the BCR was both necessary and sufficient for BCR oligomerization and signalling (FIG. 2). Similar results were obtained for the Cγ3 membrane-proximal domain of membrane-bound IgG.
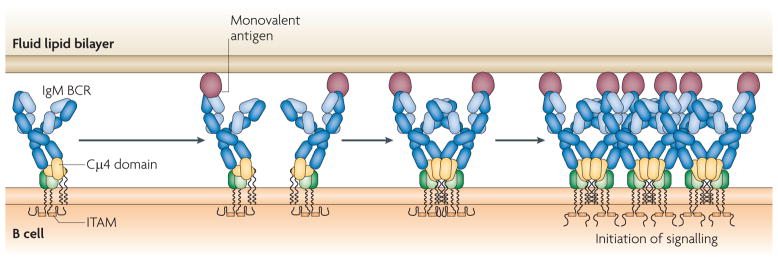
Figure 2. The ‘conformation-induced oligomerization model’ for B cell receptor microcluster formation.
Depicted is an IgM B cell receptor (BCR) in the absence of antigen and in a conformation that is not receptive to oligomerization. The binding of monovalent antigen in a fluid lipid bilayer that cannot physically crosslink the BCR exerts a force that brings the BCR into an oligomerization-receptive form. The subsequent random bumping of antigen-bound BCRs results in their oligomerization through the membrane-proximal Cμ4 domain and in the initiation of signalling. ITAM, immunoreceptor tyrosine-based activation motif.
Taken together, these results support a model for the initiation of BCR signalling that we termed the ‘conformation-induced oligomerization model’33 (FIG. 2). In this model, it is proposed that in the absence of antigen, the Cμ4 portion of the BCR ectodomain is not accessible for oligomerization and that the binding of the BCR to a monovalent antigen in an opposing membrane exerts a force that brings the Cμ4 domain into an oligomerization-receptive orientation. The exertion of this force did not require that the BCR be attached to the actin cytoskeleton because Cμ4-dependent oligomerization of the BCR was observed for BCRs that lacked the Igα–Igβ heterodimer and so had no means of associating with the actin cytoskeleton11. The antigen-bound BCR is still mobile and it is not until the BCR encounters another antigen-bound BCR that oligomerization occurs and the oligomerized BCRs are immobilized (FIGS 2,3). This model is attractive because it provides a mechanism by which the universe of foreign, structurally distinct antigens can bring the BCRs into a precise signalling-active oligomer. But we suspect that this model does not apply to B cells responding to multivalent antigens that physically crosslink BCRs. BCRs that lack the membrane-proximal domains of membrane-bound immunoglobulin are readily immobilized by multivalent antigen presented on membranes11. We suggest below that physically crosslinked BCRs might mimic oligomerized BCRs at later points during the process of signal initiation.
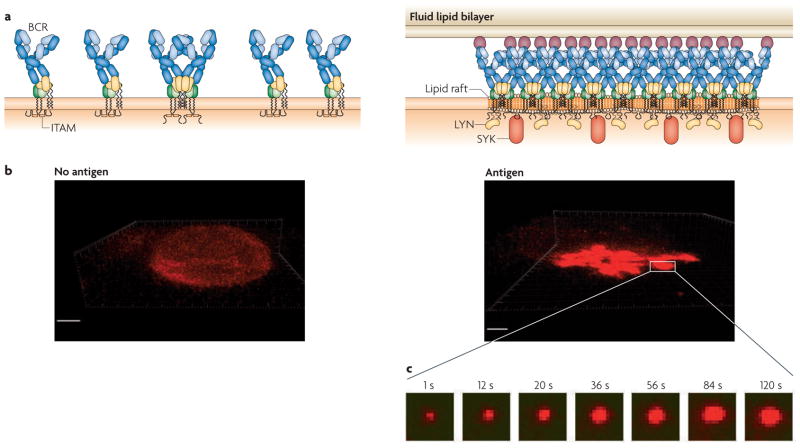
Figure 3. B cell microclusters grow with time after antigen binding.
a | B cell receptors (BCRs) in the absence of antigen (left) and in the presence of antigen (right). b | Confocal images showing the spatial distribution of BCRs on the surface of a B cell placed on a fluid lipid bilayer in the absence of antigen (left), showing the BCRs covering the entire B cell surface, and (right) on an antigen-containing fluid lipid bilayer, showing the BCRs accumulating at the interface of the B cell with the bilayer. c | Total internal reflection fluorescence microscopy images demonstrating the growth, over time, of the microcluster within the area indicated by the white box. SYK, spleen tyrosine kinase.
As mentioned above, Yang and Reth recently proposed an alternative model for BCR clustering, termed the ‘dissociation activation model’, in which most BCRs exist on the B cell surface in signalling-inactive ‘closed’ dimers in equilibrium with a small number of signalling-active ‘open’ BCR monomers13. This model is based, in part, on the results from BiFC studies (see above) in which the number of BCR dimers on living B cells was not directly quantified. In the dissociation activation model, multivalent antigens disrupt the closed dimers, resulting in open monomers that are then clustered by multivalent antigen in a manner that prevents the formation of inactive closed dimers within the cluster. An advantage of this model is that it accounts for the ability of structurally different antigens to activate B cells, because in this model antigen keeps BCRs apart rather than bringing them together into well-ordered oligomers. However, this is not a unique advantage, because the data supporting the conformation-induced oligomerization model for BCR clustering, which show that monovalent antigens presented on membranes induce BCR oligomerization, also account for the ability of structurally distinct antigens to oligomerize BCRs. The dissociation activation model does not accommodate the data from SPT and FRET analyses, which indicate that only a small fraction of BCRs in resting cells exist in dimers and that antigen binding results in a marked increase in FRET between BCRs in clusters, indicating the formation of dimers or oligomers. Neither does this model accommodate the observation that physical crosslinking of BCRs by multivalent antigens is not required for BCR oligomerization and clustering when antigens are presented to the B cell on an opposing membrane.
The growth of BCR microclusters
During TIRF imaging of B cells on antigen-containing fluid lipid bilayers, BCR microclusters can be seen to grow with time, providing a mechanism to amplify signalling34 (FIG. 3). Microclusters, although stationary, grew both in terms of the number of BCRs in the microcluster and of the size of the microcluster34. The SPT results described above, in which individual antigen-bound BCRs were immobilized as they entered pre-existing microclusters, suggest that the growth of microclusters may be driven by oligomerization-induced trapping (BOX 2), as proposed by the picket fence model of plasma-membrane organization. By pre-treating B cells with inhibitors of Src family kinases and of SYK, it was shown that the early growth of the BCR microcluster, during the first 60 seconds, was a BCR-intrinsic event, similar to the initial oligomerization itself34. However, growth of the microcluster beyond a certain size at later stages required LYN and SYK activity, and presumably BCR signalling components further downstream. Early microcluster growth may reflect a mechanism by which B cells count the number of antigens captured. The recruitment of BCRs to the microcluster is BCR intrinsic until a sufficient number of BCRs is trapped in the microcluster, which serves as a tipping point for the recruitment of LYN and the triggering of the signalling cascades. Subsequent signalling-dependent microcluster growth may allow the initial signal to be amplified in response to increased antigen. The mechanisms that underlie signalling-dependent microcluster growth are not known but are of interest.
Affinity maturation and class switching
Two hallmarks of humoral immune responses are affinity maturation and class switching35. As antibody responses to immunization mature, and after secondary immunization, antigen-specific antibodies become of increasingly higher affinity and predominantly of the IgG isotypes through the linked molecular processes of somatic hypermutation and class switching. Because the humoral immune response functions through clonal selection, the affinity maturation and class switching of antibody responses presumably reflect an advantage of B cells expressing high-affinity, class-switched BCRs in the selection process. The question is: how early in this process are high-affinity, class-switched BCRs an advantage?
Several important studies have provided strong evidence that high-affinity B cell clones out-compete low-affinity B cell clones for survival in adoptive transfer experiments, presumably reflecting a competition at the clonal level for limiting quantities of antigen, T cell help or appropriate niches36–42. At the level of individual B cells there is evidence that signalling through high-affinity, as opposed to low-affinity, BCRs is qualitatively different43 and, most recently, the affinity of the BCR for antigen was shown to dictate the degree to which B cells spread over antigen-containing fluid lipid bilayers, allowing increased accumulation of antigen and enhanced responses22. Similarly, several seminal studies showed that class-switched B cells out-competed unswitched B cells for survival in vivo and that this survival advantage could be attributed to the cytoplasmic tail of membrane-bound IgG44,45.
Both membrane-bound IgM and IgD expressed by unswitched B cells have short, three-amino-acid tails in contrast to all membrane-bound IgG isotypes, which have highly conserved cytoplasmic domains of 28 amino acids44,45. The membrane-bound IgG tail has been demonstrated to enhance B cell proliferative responses to antigen and to trigger signalling cascades that are qualitatively different from those triggered by membrane-bound IgM46–49. Engels et al.49 recently showed that enhanced signalling through membrane-bound IgG was dependent on the phosphorylation of a tyrosine in the IgG tail that served to recruit growth-factor-receptor-bound protein 2 (GRB2), resulting in sustained kinase activation and generation of second messengers culminating in enhanced B cell proliferation.
Using high-speed, high-resolution SPT TIRFM imaging, we recently provided evidence that the earliest events that occur following antigen binding to the BCR are highly sensitive to both the affinity of the BCR for antigen and the isotype of the BCR34,50. These results place the ability to discriminate antigen affinity and class switching at the tipping point in the initiation of BCR signalling, namely BCR-intrinsic oligomerization. Using an experimental system in which the affinity of BCRs for antigen differed by 50-fold, it was shown that high-affinity BCRs more readily formed immobile BCR microclusters that grew more rapidly, thereby resulting in larger microclusters that recruited more SYK and signalled for more robust calcium responses, than did low-affinity BCRs34. The robust signalling that is triggered by high-affinity BCRs should allow high-affinity B cells to respond to the low levels of antigen that may be presented by APCs.
Using SPT, we also discovered that BCRs of the IgG isotype were dramatically enhanced in their ability to oligomerize and in the growth of the resulting BCR microclusters, ultimately resulting in the increased recruitment of SYK and in more robust calcium responses compared with BCRs of the same affinity for antigen but of the IgM isotype50. Through an extensive domain swapping and mutagenesis analysis it was determined that the 12 membrane-proximal residues of the IgG tail conferred enhanced oligomerization50. This region of the tail does not contain the tyrosine residue that is phosphorylated following IgG-containing BCR crosslinking and that recruits GRB2 (REF. 49). Thus, these studies revealed a new function for the membrane-proximal region of the IgG tail, which was not previously appreciated to have a role in IgG-containing BCR signalling. We speculate that this region of the IgG tail binds to an adaptor protein of some sort or interacts with the inner leaflet of the plasma membrane in such a way as to promote the stabilization of the BCR oligomer and growth of the BCR microcluster.
These results place both affinity maturation and class switching at the earliest step in the initiation of BCR signalling: the point of BCR oligomerization. At this tipping point, relatively small differences in affinity and a small portion of the immunoglobulin tail make a big difference during B cell activation.
How BCR oligomerization triggers signalling
The studies reviewed thus far indicate that BCRs oligomerize and form microclusters in a BCR-intrinsic fashion, raising the important question: how do BCR oligomers recruit LYN and trigger the signalling cascade? One simple explanation is that the structure of the cytoplasmic domain of the BCR monomer is distinct from that of the oligomer and that LYN can only phosphorylate the oligomeric form (FIG. 4a). In this model it might be predicted that LYN is constitutively associated with the cytoplasmic domains of some monomeric BCRs but only phosphorylates BCRs in trans when the BCRs are clustered. If this model is correct, oligomerization should be sufficient to trigger signalling.
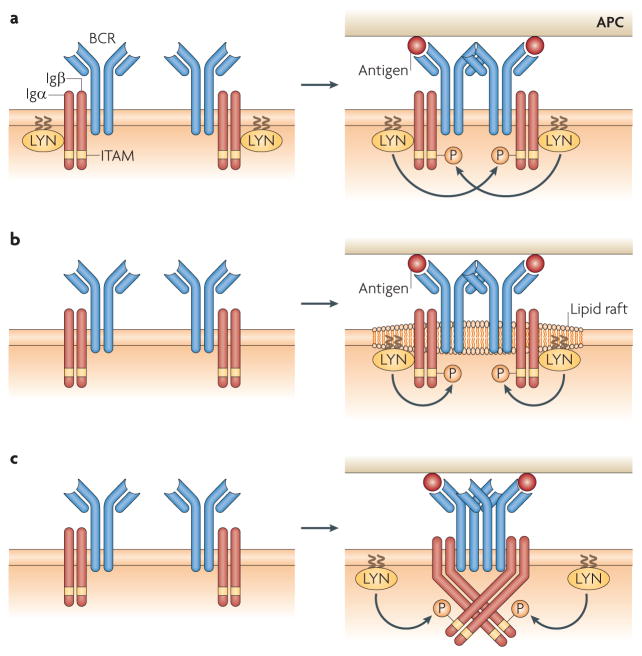
Figure 4. Models for how B cell receptor oligomers trigger signalling.
a | B cell receptor (BCR) oligomers may simply bring the BCR-associated tyrosine kinase LYN into close proximity with the Igα–Igβ heterodimer for trans-phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs). b | Alternatively, they may perturb the local lipid environment, causing the coalescence of lipid rafts and bringing the lipid-raft-associated LYN into close proximity with ITAMs of the Igα–Igβ heterodimer for phosphorylation. c | Yet another possibility is that BCR oligomers alter the association of membrane-bound immunoglobulin and Igα–Igβ, inducing changes in the cytoplasmic tails of Igα–Igβ and allowing LYN to access the ITAMs for phosphorylation. APC, antigen-presenting cell.
However, other studies suggest that the mechanisms for the recruitment of LYN to the BCR oligomers are more complicated. One model is based on the observation that the BCR oligomer perturbs the local lipid microenvironment, leading to the coalescing of lipid rafts51 around the BCR oligomers. Various other studies indicated that crosslinking of BCRs resulted in their association with detergent-insoluble lipid rafts52. More recently, we provided evidence that in living B cells, antigen-engaged BCR microclusters transiently associated with lipid rafts, and that this was followed by a more stable association of the BCR microcluster with lipid-raft-tethered LYN53. These results suggest that the recruitment of LYN to the BCR oligomers is a repercussion of the association of the BCR oligomers with lipid rafts (FIG. 4b). It is not known how the BCR microclusters perturb the local lipid environment but, as reviewed by Viola and Gupta54, it is possible that BCR microclusters signal for local dissolution of the membrane cytoskeleton, allowing the coalescence of lipid rafts with the BCR microclusters. Such perturbation of the local lipid microenvironment has been observed both for BCR oligomers formed in response to monovalent membrane-associated antigens and for BCRs physically crosslinked by multivalent antigens53,55. This suggests that the ability of BCR microclusters to perturb the local lipid environment, resulting in the recruitment of LYN, may provide a common mechanism by which BCRs responding to multivalent or monovalent membrane-associated antigens initiate signalling.
Lipid perturbation may have additional roles in transducing signals. Saturated raft lipids form thicker membranes56 and have the potential to induce a pronounced curvature in the membrane57 that could cause alterations in the BCR cytoplasmic domains, providing a docking site for LYN. Consistent with these possibilities are results from an analysis of BCRs that contained FRET donor and acceptor fluorescent proteins in their cytoplasmic domains5. After antigen binding, BCRs clustered and FRET occurred between the cytoplasmic domains, indicating that they were in close molecular proximity. However, the FRET signal then rapidly decreased, although the oligomers stayed intact, indicating a change in the distance between the cytoplasmic domains. This decrease in FRET was referred to as an ‘opening’ of the BCR cytoplasmic domain and this opening occurred simultaneously with the association of the BCR microcluster with LYN5,53. The finding mirrored the results of Wucherpfennig and colleagues58 showing that the cytoplasmic tail of the CD3ε chain of the TCR associated with the membrane through the deep insertion of two key tyrosines, resulting in their sequestration from Src kinases in the unligated receptor. In the ligated TCR these tyrosines were phosphorylated. Although the mechanism by which the CD3ε tail became accessible is not known, it is possible that perturbations in the local lipid environment induced the dissociation of the CD3ε tail from the membrane.
To fully understand the molecular basis of the antigen-induced initiation of BCR signalling it will be essential to determine the structure of the BCR and, ultimately, the entire structure as it exists in the membrane. An important step towards this goal was the recent solution of the structure of a disulphide-linked homodimer of Igβ, which allowed the modelling of an Igα–Igβ heterodimer with the existing structure of the Cα4 domain59 (Supplementary information S2 (figure)). Combined with solution- binding studies and microcluster mutagenesis analyses, these results predicted an extensive contact surface between membrane-bound immunoglobulin and both Igα and Igβ through multiple charged residues. The extensive interactions between membrane-bound immunoglobulin and the Igα–Igβ heterodimer suggest the possibility that changes in the membrane–proximal region of the membrane-bound immunoglobulin induced by binding to membrane-tethered antigens could alter the association of the membrane-bound immunoglobulin with Igα–Igβ. In turn, this could result in changes in the cytoplasmic domains of Igα–Igβ that recruit LYN (FIG. 4c). Although these are still early days, current evidence from live-cell imaging and from structural studies of the BCR suggest that the information that antigen has bound to the BCR ectodomain may be transduced across the membrane by perturbations of either the local lipid microenvironment or by the association of the ectodomain of the membrane-bound immunoglobulin with the Igα–Igβ heterodimer.
Regulation of the initiation of BCR signalling
B cell responses are highly regulated by various co-receptors, raising the question: are the earliest events in the initiation of BCR signalling targets of regulation? Although, to date, few studies have addressed this question, the early indication is that two key regulators of BCR signalling, the Fc receptor IIB for IgG (FcγRIIB) and CD19, influence early events.
FcγRIIB expressed by B cells is a potent inhibitor of BCR signalling60. FcγRIIB blocks BCR signalling at steps downstream of the early activation events by reversing the BCR signalling-induced activation of PI3K through the recruitment of the lipid phosphatase SH2-domain-containing inositol polyphosphate 5′ phosphatase (SHIP) to the co-ligated complex. In addition, when co-ligated to the BCR, FcγRIIB inhibits the formation of immobile BCR oligomers by an unexpected mechanism61. FRET analyses showed that FcγRIIB-mediated blocking of BCR oligomerization did not require molecular proximity of the cytoplasmic tails of the BCR and FcγRIIB, but instead appeared to be the repercussion of the ability of FcγRIIB to tip the balance of the association of the BCR with raft lipids by associating itself with raft lipids in a highly stable way. Consistent with this interpretation, a loss-of-function mutation in the transmembrane domain of FcγRIIB that inhibits the ability of ligated FcγRIIB to associate with lipid rafts62,63 blocked the ability of FcγRIIB to inhibit BCR oligomerization. The ability of the FcγRIIB to target two different points in the BCR signalling pathway through two entirely different mechanisms may account for its potent inhibitory function.
CD19 has also recently been shown to have an integral role in early BCR signalling events64. CD19 is an activating B cell co-receptor that is phosphorylated in response to BCR antigen binding and functions as a molecular adaptor that recruits VAV, PI3K and LYN to augment BCR signalling65. CD19 is expressed on the surface of B cells, both alone and as part of a complex containing CD21, and is the receptor for the Cd3 component of complement, allowing complement-coupled antigen to co-ligate the CD19–CD21 complex with the BCR. Batista and colleagues64 provided evidence that CD19 alone functions to regulate early events in BCR signalling. They showed that in response to antigen presented on fluid lipid bilayers, BCR microclusters transiently associated with CD19. This association seemed to be essential for BCR activation, because CD19-deficient B cells failed to form signalling-active BCR microclusters or to trigger the spreading response, suggesting that the BCR–CD19 complex may be a basic signalling unit.
Collectively, these studies indicate that the earliest events in the initiation of BCR signalling are regulated by co-receptors. Understanding which B cell-surface receptors influence these early events and the mechanisms underlying their effects may reveal new tipping points in the initiation of BCR signalling.
Alterations in early signalling events in disease
Understanding that the early BCR-intrinsic events in the initiation of BCR signalling are regulated raises the possibility that naturally occurring mutations affecting BCR oligomerization could result in spontaneous BCR clustering and hyperactivation of B cells, as occurs in systemic autoimmune disease or even tumorigenesis. In collaboration with Staudt and colleagues, we recently analysed the mobility of BCRs on activated B cell-like diffuse large B-cell lymphomas (DLBCLs) that a small interfering RNA screen had demonstrated were dependent on the BCR for survival66. Remarkably, we observed that the BCRs on these tumours were immobilized in microclusters. By contrast, BCRs on several B cell tumour types that were not dependent on the BCR for survival, including Burkitt’s lymphoma, mantle cell lymphoma and germinal centre B-cell-like DLBCL, were highly mobile. These findings are exciting because they raise the possibility that by understanding the molecular basis of constitutive oligomerization, therapies could be developed to selectively disrupt the BCR oligomers. It is of interest that constitutive oligomerization of the Epstein Barr virus (EBV) latency membrane protein 2A (LMP2A), which contains ITAMs in its cytoplasmic tail and functions to mimic BCR signalling, is required for the transformation of B cells by EBV67. It will be of interest to determine the activation state of BCRs in cells of individuals with systemic autoimmune disease, with a view towards targeting spontaneous oligomerization for therapy.
Similarities in immunoreceptor signalling
The BCR, TCR and FcεR1 are all members of the family of MIRRs, raising the question: do these receptors initiate signalling by similar mechanisms? The engagement of antigen by the BCR, TCR and FcεR1 induces receptor microclusters that are essential for productive signalling. Each receptor has also been shown to associate with detergent-insoluble lipid rafts after antigen engagement, suggesting similar mechanisms for signal transduction52. The TCRs engage antigenic peptides bound to MHC monomers on the surface of APCs, suggesting that physical crosslinking of the TCRs may not be required for activation. Two-colour SPT imaging in conjunction with confocal laser-scanning microscopy provided evidence that antigen engagement of the TCR resulted in the immobilization of the kinase LCK, CD2 (a TCR co-receptor) and linker for activation of T cells (LAT), which is an adaptor protein in the TCR microclusters68. Similarly, our recent studies showed that antigen engagement by the BCR resulted in the immobilization of SYK with BCR microclusters11. For FcεR1, McConnell and colleagues69,70 showed that monovalent antigen incorporated into a fluid lipid bilayer was sufficient to trigger the aggregation of IgE-loaded FcεR1 on mast cells, as well as their degranulation. These authors proposed that monovalent mobile antigen aggregated the FcεR1 by means of thermodynamic forces acting on a topologically constrained system, although they did not rule out a role for antigen-induced conformational changes in the FcεR1. In recent experiments, Lidke and colleagues71 used SPT to follow FcεR1 molecules that were labelled with quantum dots (BOX 1) in cells expressing fluorescent actin. They showed that the highly mobile FcεR1 molecules became immobilized within seconds of antigen binding in an actin-dependent manner at high antigen concentration. However, at low antigen concentration, small FcεR1 oligomers remained mobile and signalling active, suggesting that in mast cells the organization of the actin cytoskeleton might serve not to trap oligomers efficiently but to support mast cell degranulation72.
Collectively, these data suggest that the BCR, TCR and FcεR1 may share common mechanisms for the initiation of receptor signalling that involve immobilization of receptors and membrane perturbations. Future SPT imaging of TCRs and mutational studies of the TCR and FcεR1 to determine what features of the receptor are required for immobilization in signalling complexes should reveal just how similar these processes might be.
Conclusions and perspectives
High-resolution live-cell imaging has provided the first view of the events that occur within seconds of the encounter of BCRs with antigen and that ultimately lead to B cell activation. So far, this technology has been used to explore only a small area of B cell biology. It will be of interest to understand how BCRs behave on B cells at different developmental stages and in diseases that involve B cell hyper- or hypoactivation. Further advances in multi-colour SPT should provide the tools necessary to meet the next challenge of understanding the dynamic behaviour of the BCR in relation to important signalling molecules and cell structures. Ultimately, the goal of such studies is to understand the tipping points in B cell activation, with the long-term goal of applying this knowledge to the development of therapies for autoimmunity and B cell cancers and to the engineering of effective vaccines.
Supplementary Material
Acknowledgments
We thank J. Brzostowski for expert comments on live-cell imaging techniques. This work has been supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases.
Glossary
- Somatic hypermutation
A unique mutation mechanism that is targeted to the variable regions of rearranged immunoglobulin gene segments. Combined with selection for B cells that produce high-affinity antibodies, somatic hypermutation leads to affinity maturation of B cells in germinal centres
- Class switching
The somatic recombination process by which the class of immunoglobulin is switched from IgM to IgG, IgA or IgE
- Microclusters
Microscopic assemblies of receptor oligomers in the plasma membrane that recruit signalling molecules. They first form in the contact areas between the B cell and antigen-presenting cell, and ultimately form the central supramolecular activation cluster of the immune synapse
- BCR clustering and capping
The binding of multivalent ligands to B cell receptors (BCRs) induces the redistribution and aggregation of the bound receptors into clusters (clustering). Capping, which requires metabolic energy and cytoskeleton dynamics, represents the coalescence of clusters to form a single aggregate called a cap
- Immune synapse
The specialized contact area between a T or B cell and one or more antigen-presenting cells. The synapse is dynamic and shows lipid and protein segregation, signalling compartmentalization and bidirectional information exchange through soluble and membrane-bound transmitters
- Lamellipodia
Thin sheet-like processes that extend at the leading edge of moving cells. They are actin-rich zones formed in response to chemokine signals, and propel a migrating cell forward
- Lipid rafts
Cholesterol- and sphingolipid-rich membrane microdomains that provide ordered structure to the lipid bilayer and have the ability to include or exclude specific signalling molecules and complexes
- Small interfering RNA
Short double-stranded RNAs of 19–23 nucleotides that induce RNA interference, a post-transcriptional process that leads to gene silencing in a sequence-specific manner
Footnotes
Competing interests statement
The authors declare no competing financial interests.
FURTHER INFORMATION
Susan K. Pierce’s homepage: http://www.niaid.nih.gov/labsandresources/labs/aboutlabs/lig/lymphocyteactivationsection/Pages/pierce.aspx
See online article: S1 (figure) | S2 (figure)
References
- 1.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 2.Tarlinton D. B-cell memory: are subsets necessary? Nature Rev Immunol. 2006;6:785–790. doi: 10.1038/nri1938. [DOI] [PubMed] [Google Scholar]
- 3.Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- 4.Reth M, Wienands J. Initiation and processing of signals from the B cell antigen receptor. Annu Rev Immunol. 1997;15:453–479. doi: 10.1146/annurev.immunol.15.1.453. [DOI] [PubMed] [Google Scholar]
- 5.Tolar P, Sohn HW, Pierce SK. The initiation of antigen-induced B cell antigen receptor signaling viewed in living cells by fluorescence resonance energy transfer. Nature Immunol. 2005;6:1168–1176. doi: 10.1038/ni1262. [DOI] [PubMed] [Google Scholar]
- 6.DeFranco AL. The complexity of signaling pathways activated by the BCR. Curr Opin Immunol. 1997;9:296–308. doi: 10.1016/s0952-7915(97)80074-x. [DOI] [PubMed] [Google Scholar]
- 7.Dal Porto JM, et al. B cell antigen receptor signaling 101. Mol Immunol. 2004;41:599–613. doi: 10.1016/j.molimm.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Srinivasan L, et al. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139:573–586. doi: 10.1016/j.cell.2009.08.041. Using an elegant genetic approach, these authors demonstrated that BCR-deficient mature B cells can be rescued by PI3K signalling, suggesting the molecular nature of the survival signal delivered by BCRs in mature B cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grande SM, Bannish G, Fuentes-Panana EM, Katz E, Monroe JG. Tonic B-cell and viral ITAM signaling: context is everything. Immunol Rev. 2007;218:214–234. doi: 10.1111/j.1600-065X.2007.00535.x. [DOI] [PubMed] [Google Scholar]
- 10.Delgado P, et al. Essential function for the GTPase TC21 in homeostatic antigen receptor signaling. Nature Immunol. 2009;10:880–888. doi: 10.1038/ni.1749. [DOI] [PubMed] [Google Scholar]
- 11.Tolar P, Hanna J, Krueger PD, Pierce SK. The constant region of the membrane immunoglobulin mediates B cell-receptor clustering and signaling in response to membrane antigens. Immunity. 2009;30:44–55. doi: 10.1016/j.immuni.2008.11.007. In this study, two-colour TIRFM SPT was used to demonstrate that monovalent membrane-associated antigen induced the formation of immobile BCR oligomers, a very early event in the initiation of BCR signalling, and that the oligomerization depended on the constant region of the BCR’s membrane immunoglobulin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Treanor B, et al. The membrane skeleton controls diffusion dynamics and signaling through the B cell receptor. Immunity. 2010;32:187–199. doi: 10.1016/j.immuni.2009.12.005. This study used two-colour TIRFM SPT to image BCRs and the membrane cytoskeleton, and provided evidence for the restriction of BCR mobility by the membrane cytoskeleton. The authors also showed that simply disrupting the membrane cytoskeleton network in resting B cells induced BCR signalling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang J, Reth M. Oligomeric organization of the B-cell antigen receptor on resting cells. Nature. 2010 Sep;5 doi: 10.1038/nature09357. [DOI] [PubMed] [Google Scholar]
- 14.Gauthier L, Rossi B, Roux F, Termine E, Schiff C. Galectin-1 is a stromal cell ligand of the pre-B cell receptor (BCR) implicated in synapse formation between pre-B and stromal cells and in pre-BCR triggering. Proc Natl Acad Sci USA. 2002;99:13014–13019. doi: 10.1073/pnas.202323999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carrasco YR, Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007;27:160–171. doi: 10.1016/j.immuni.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Junt T, et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- 17.Pape KA, Catron DM, Itano AA, Jenkins MK. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity. 2007;26:491–502. doi: 10.1016/j.immuni.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Phan TG, Grigorova I, Okada T, Cyster JG. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nature Immunol. 2007;8:992–1000. doi: 10.1038/ni1494. [DOI] [PubMed] [Google Scholar]
- 19.Qi H, Egen JG, Huang AY, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science. 2006;312:1672–1676. doi: 10.1126/science.1125703. [DOI] [PubMed] [Google Scholar]
- 20.Schwickert TA, et al. In vivo imaging of germinal centres reveals a dynamic open structure. Nature. 2007;446:83–87. doi: 10.1038/nature05573. [DOI] [PubMed] [Google Scholar]
- 21.Batista FD, Iber D, Neuberger MS. B cells acquire antigen from target cells after synapse formation. Nature. 2001;411:489–494. doi: 10.1038/35078099. [DOI] [PubMed] [Google Scholar]
- 22.Fleire SJ, et al. B cell ligand discrimination through a spreading and contraction response. Science. 2006;312:738–741. doi: 10.1126/science.1123940. Scanning electron microscopy (SEM) and confocal laser-scanning microscopy (CLSM) were used to describe the spreading and contraction responses of B cells encountering membrane-bound antigens, leading to B cell activation. [DOI] [PubMed] [Google Scholar]
- 23.Arana E, et al. Activation of the small GTPase Rac2 via the B cell receptor regulates B cell adhesion and immunological-synapse formation. Immunity. 2008;28:88–99. doi: 10.1016/j.immuni.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Lin KBL, et al. The Rap GTPases regulate B cell morphology, immune-synapse formation, and signaling by particulate B cell receptor ligands. Immunity. 2008;28:75–87. doi: 10.1016/j.immuni.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 25.Weber M, et al. Phospholipase C-γ2 and Vav cooperate within signaling microclusters to propagate B cell spreading in response to membrane-bound antigen. J Exp Med. 2008;205:853–868. doi: 10.1084/jem.20072619. References 23–25 report the identification of the molecular requirements for the B cell spreading and contraction response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metzger H. Transmembrane signaling: the joy of aggregation. J Immunol. 1992;149:1477–1487. [PubMed] [Google Scholar]
- 27.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 28.Hubbard SR, Miller WT. Receptor tyrosine kinases: mechanisms of activation and signaling. Curr Opin Cell Biol. 2007;19:117–123. doi: 10.1016/j.ceb.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ihle JN, Kerr IM. Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet. 1995;11:69–74. doi: 10.1016/s0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- 30.Burgess AW, et al. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol Cell. 2003;12:541–552. doi: 10.1016/s1097-2765(03)00350-2. [DOI] [PubMed] [Google Scholar]
- 31.Kusumi A, et al. Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annu Rev Biophys Biomol Struct. 2005;34:351–378. doi: 10.1146/annurev.biophys.34.040204.144637. [DOI] [PubMed] [Google Scholar]
- 32.Kusumi A, Shirai YM, Koyama-Honda I, Suzuki KG, Fujiwara TK. Hierarchical organization of the plasma membrane: investigations by single-molecule tracking vs. fluorescence correlation spectroscopy. FEBS Lett. 2010;584:1814–1823. doi: 10.1016/j.febslet.2010.02.047. [DOI] [PubMed] [Google Scholar]
- 33.Tolar P, Pierce SK. A conformation-induced oligomerization model for B cell receptor microclustering and signaling. Curr Top Microbiol Immunol. 2010;340:155–169. doi: 10.1007/978-3-642-03858-7_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu W, Meckel T, Tolar P, Sohn HW, Pierce SK. Antigen affinity discrimination is an intrinsic function of the B cell receptor. J Exp Med. 2010;207:1095–1111. doi: 10.1084/jem.20092123. In this study, the early BCR-intrinsic molecular events in the initiation of BCR signalling were determined to be sensitive to the affinity of the BCR for antigen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gray D. Immunological memory. Annu Rev Immunol. 1993;11:49–77. doi: 10.1146/annurev.iy.11.040193.000405. [DOI] [PubMed] [Google Scholar]
- 36.Brink R, Phan TG, Paus D, Chan TD. Visualizing the effects of antigen affinity on T-dependent B-cell differentiation. Immunol Cell Biol. 2008;86:31–39. doi: 10.1038/sj.icb.7100143. [DOI] [PubMed] [Google Scholar]
- 37.Dal Porto JM, Haberman AM, Kelsoe G, Shlomchik MJ. Very low affinity B cells form germinal centers, become memory B cells, and participate in secondary immune responses when higher affinity competition is reduced. J Exp Med. 2002;195:1215–1221. doi: 10.1084/jem.20011550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shih TA, Meffre E, Roederer M, Nussenzweig MC. Role of BCR affinity in T cell dependent antibody responses in vivo. Nature Immunol. 2002;3:570–575. doi: 10.1038/ni803. [DOI] [PubMed] [Google Scholar]
- 39.Shih TA, Roederer M, Nussenzweig MC. Role of antigen receptor affinity in T cell-independent antibody responses in vivo. Nature Immunol. 2002;3:399–406. doi: 10.1038/ni776. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi Y, Dutta PR, Cerasoli DM, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl V Affinity maturation develops in two stages of clonal selection. J Exp Med. 1998;187:885–895. doi: 10.1084/jem.187.6.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paus D, et al. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J Exp Med. 2006;203:1081–1091. doi: 10.1084/jem.20060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phan TG, et al. High affinity germinal center B cells are actively selected into the plasma cell compartment. J Exp Med. 2006;203:2419–2424. doi: 10.1084/jem.20061254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kouskoff V, et al. Antigens varying in affinity for the B cell receptor induce differential B lymphocyte responses. J Exp Med. 1998;188:1453–1464. doi: 10.1084/jem.188.8.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaisho T, Schwenk F, Rajewsky K. The roles of γ1 heavy chain membrane expression and cytoplasmic tail in IgG1 responses. Science. 1997;276:412–415. doi: 10.1126/science.276.5311.412. [DOI] [PubMed] [Google Scholar]
- 45.Martin SW, Goodnow CC. Burst-enhancing role of the IgG membrane tail as a molecular determinant of memory. Nature Immunol. 2002;3:182–188. doi: 10.1038/ni752. In references 44 and 45, the highly conserved cytoplasmic tail of membrane-bound IgG was demonstrated to be both necessary and sufficient for enhanced IgG memory antibody responses in vivo. [DOI] [PubMed] [Google Scholar]
- 46.Horikawa K, et al. Enhancement and suppression of signaling by the conserved tail of IgG memory-type B cell antigen receptors. J Exp Med. 2007;204:759–769. doi: 10.1084/jem.20061923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waisman A, et al. IgG1 B cell receptor signaling is inhibited by CD22 and promotes the development of B cells whose survival is less dependent on Igα/β. J Exp Med. 2007;204:747–758. doi: 10.1084/jem.20062024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wakabayashi C, Adachi T, Wienands J, Tsubata T. A distinct signaling pathway used by the IgG-containing B cell antigen receptor. Science. 2002;298:2392–2395. doi: 10.1126/science.1076963. [DOI] [PubMed] [Google Scholar]
- 49.Engels N, et al. Recruitment of the cytoplasmic adaptor Grb2 to surface IgG and IgE provides antigen receptor-intrinsic costimulation to class-switched B cells. Nature Immunol. 2009;10:1018–1025. doi: 10.1038/ni.1764. This study showed that a conserved tyrosine in the cytoplasmic domain of membrane-bound IgG and membrane-bound IgE was phosphorylated upon BCR crosslinking. This recruited the adaptor molecule GRB2 to the BCR, resulting in enhanced calcium response and B cell proliferation. [DOI] [PubMed] [Google Scholar]
- 50.Liu W, Meckel T, Tolar P, Sohn HW, Pierce SK. Intrinsic properties of immunoglobulin IgG1 isotype-switched B cell receptors promote microclustering and the initiation of signaling. Immunity. 2010;32:778–789. doi: 10.1016/j.immuni.2010.06.006. In this study, the early BCR-intrinsic molecular events in the initiation of BCR signalling were found to be sensitive to the isotype of the BCR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simons K, Toomre D. Lipid rafts and signal transduction. Nature Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 52.Dykstra M, Cherukuri A, Sohn HW, Tzeng SJ, Pierce SK. Location is everything: lipid rafts and immune cell signaling. Annu Rev Immunol. 2003;21:457–481. doi: 10.1146/annurev.immunol.21.120601.141021. [DOI] [PubMed] [Google Scholar]
- 53.Sohn HW, Tolar P, Pierce SK. Membrane heterogeneities in the formation of B cell receptor-Lyn kinase microclusters and the immune synapse. J Cell Biol. 2008;182:367–379. doi: 10.1083/jcb.200802007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Viola A, Gupta N. Tether and trap: regulation of membrane-raft dynamics by actin-binding proteins. Nature Rev Immunol. 2007;7:889–896. doi: 10.1038/nri2193. [DOI] [PubMed] [Google Scholar]
- 55.Sohn HW, Tolar P, Jin T, Pierce SK. Fluorescence resonance energy transfer in living cells reveals dynamic membrane changes in the initiation of B cell signaling. Proc Natl Acad Sci USA. 2006;103:8143–8148. doi: 10.1073/pnas.0509858103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McIntosh TJ, Vidal A, Simon SA. Sorting of lipids and transmembrane peptides between detergent-soluble bilayers and detergent-resistant rafts. Biophys J. 2003;85:1656–1666. doi: 10.1016/S0006-3495(03)74595-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reynwar BJ, et al. Aggregation and vesiculation of membrane proteins by curvature-mediated interactions. Nature. 2007;447:461–464. doi: 10.1038/nature05840. [DOI] [PubMed] [Google Scholar]
- 58.Xu C, et al. Regulation of T cell receptor activation by dynamic membrane binding of the CD3ε cytoplasmic tyrosine-based motif. Cell. 2008;135:702–713. doi: 10.1016/j.cell.2008.09.044. FRET and nuclear magnetic resonance techniques were used to demonstrate that the tyrosine motif within the cytoplasmic domain of the TCR CD3ε chain is inserted into the plasma membrane and is not available for phosphorylation until the TCR binds its ligand. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Radaev S, et al. Structural and functional studies of Igαβ and its assembly with the B cell antigen receptor. Structure. 2010;18:934–943. doi: 10.1016/j.str.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nimmerjahn F, Ravetch JV. Fcγ receptors as regulators of immune responses. Nature Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 61.Liu W, Won Sohn H, Tolar P, Meckel T, Pierce SK. Antigen-induced oligomerization of the B cell receptor is an early target of FcγRIIB inhibition. J Immunol. 2010;184:1977–1989. doi: 10.4049/jimmunol.0902334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Floto RA, et al. Loss of function of a lupus-associated FcγRIIb polymorphism through exclusion from lipid rafts. Nature Med. 2005;11:1056–1058. doi: 10.1038/nm1288. [DOI] [PubMed] [Google Scholar]
- 63.Kono H, et al. FcγRIIB IIe232Thr transmembrane polymorphism associated with human systemic lupus erythematosus decreases affinity to lipid rafts and attenuates inhibitory effects on B cell receptor signaling. Hum Mol Genet. 2005;14:2881–2892. doi: 10.1093/hmg/ddi320. [DOI] [PubMed] [Google Scholar]
- 64.Depoil D, et al. CD19 is essential for B cell activation by promoting B cell receptor-antigen microcluster formation in response to membrane-bound ligand. Nature Immunol. 2008;9:63–72. doi: 10.1038/ni1547. This study showed the essential function of the BCR co-receptor CD19 in the initiation of BCR signalling in response to membrane-bound antigens. [DOI] [PubMed] [Google Scholar]
- 65.Fearon DT, Carroll MC. Regulation of B lymphocyte responses to foreign and self-antigens by the CD19/CD21 complex. Annu Rev Immunol. 2000;18:393–422. doi: 10.1146/annurev.immunol.18.1.393. [DOI] [PubMed] [Google Scholar]
- 66.Davis RE, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463:88–92. doi: 10.1038/nature08638. This study showed that the BCRs in B cell lymphomas that are dependent on BCRs for their survival spontaneously form prominent immobile clusters in the plasma membrane similar to antigen-stimulated BCRs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matskova L, Ernberg I, Pawson T, Winberg G. C-terminal domain of the Epstein-Barr virus LMP2A membrane protein contains a clustering signal. J Virol. 2001;75:10941–10949. doi: 10.1128/JVI.75.22.10941-10949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Douglass AD, Vale RD. Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell. 2005;121:937–950. doi: 10.1016/j.cell.2005.04.009. This study used two-colour SPT in conjunction with CLSM technique to show that antigen engagement of the TCR resulted in immobilization in TCR microclusters of LCK, CD2 and LAT molecules. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Balakrishnan K, Hsu FJ, Cooper AD, McConnell HM. Lipid hapten containing membrane targets can trigger specific immunoglobulin E-dependent degranulation of rat basophil leukemia cells. J Biol Chem. 1982;257:6427–6433. [PubMed] [Google Scholar]
- 70.Weis RM, Balakrishnan K, Smith BA, McConnell HM. Stimulation of fluorescence in a small contact region between rat basophil leukemia cells and planar lipid membrane targets by coherent evanescent radiation. J Biol Chem. 1982;257:6440–6445. [PubMed] [Google Scholar]
- 71.Andrews NL, et al. Actin restricts FcεRI diffusion and facilitates antigen-induced receptor immobilization. Nature Cell Biol. 2008;10:955–963. doi: 10.1038/ncb1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Andrews NL, et al. Small, mobile FcεRI receptor aggregates are signaling competent. Immunity. 2009;31:469–479. doi: 10.1016/j.immuni.2009.06.026. In references 71 and 72, the authors used a quantum-dot-based SPT technique to image FcεR1 mobility on ligand binding, and showed that highly mobile FcεR1 molecules became immobilized within seconds of antigen binding in an actin-dependent manner at high antigen concentration, whereas at low antigen concentration the FcεR1 aggregates were mobile and signalling active. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bajenoff M, Germain RN. Seeing is believing: a focus on the contribution of microscopic imaging to our understanding of immune system function. Eur J Immunol. 2007;37:S18–S33. doi: 10.1002/eji.200737663. [DOI] [PubMed] [Google Scholar]
- 74.Dehmelt L, Bastiaens PI. Spatial organization of intracellular communication: insights from imaging. Nature Rev Mol Cell Biol. 2010;11:440–452. doi: 10.1038/nrm2903. [DOI] [PubMed] [Google Scholar]
- 75.Coombes JL, Robey EA. Dynamic imaging of host–pathogen interactions in vivo. Nature Rev Immunol. 2010;10:353–364. doi: 10.1038/nri2746. [DOI] [PubMed] [Google Scholar]
- 76.Groves JT, Parthasarathy R, Forstner MB. Fluorescence imaging of membrane dynamics. Annu Rev Biomed Eng. 2008;10:311–338. doi: 10.1146/annurev.bioeng.10.061807.160431. [DOI] [PubMed] [Google Scholar]
- 77.Vogel SS, Thaler C, Koushik SV. Fanciful FRET. Sci STKE. 2006;2006:re2. doi: 10.1126/stke.3312006re2. [DOI] [PubMed] [Google Scholar]
- 78.Sohn HW, Tolar P, Brzostowski J, Pierce SK. A method for analyzing protein-protein interactions in the plasma membrane of live B cells by fluorescence resonance energy transfer imaging as acquired by total internal reflection fluorescence microscopy. Methods Mol Biol. 2010;591:159–183. doi: 10.1007/978-1-60761-404-3_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vale RD. Microscopes for fluorimeters: the era of single molecule measurements. Cell. 2008;135:779–785. doi: 10.1016/j.cell.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Resch-Genger U, Grabolle M, Cavaliere-Jaricot S, Nitschke R, Nann T. Quantum dots versus organic dyes as fluorescent labels. Nature Methods. 2008;5:763–775. doi: 10.1038/nmeth.1248. [DOI] [PubMed] [Google Scholar]
- 81.Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972;175:720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- 82.Fujiwara T, Ritchie K, Murakoshi H, Jacobson K, Kusumi A. Phospholipids undergo hop diffusion in compartmentalized cell membrane. J Cell Biol. 2002;157:1071–1081. doi: 10.1083/jcb.200202050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morone N, et al. Three-dimensional reconstruction of the membrane skeleton at the plasma membrane interface by electron tomography. J Cell Biol. 2006;174:851–862. doi: 10.1083/jcb.200606007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.