Abstract
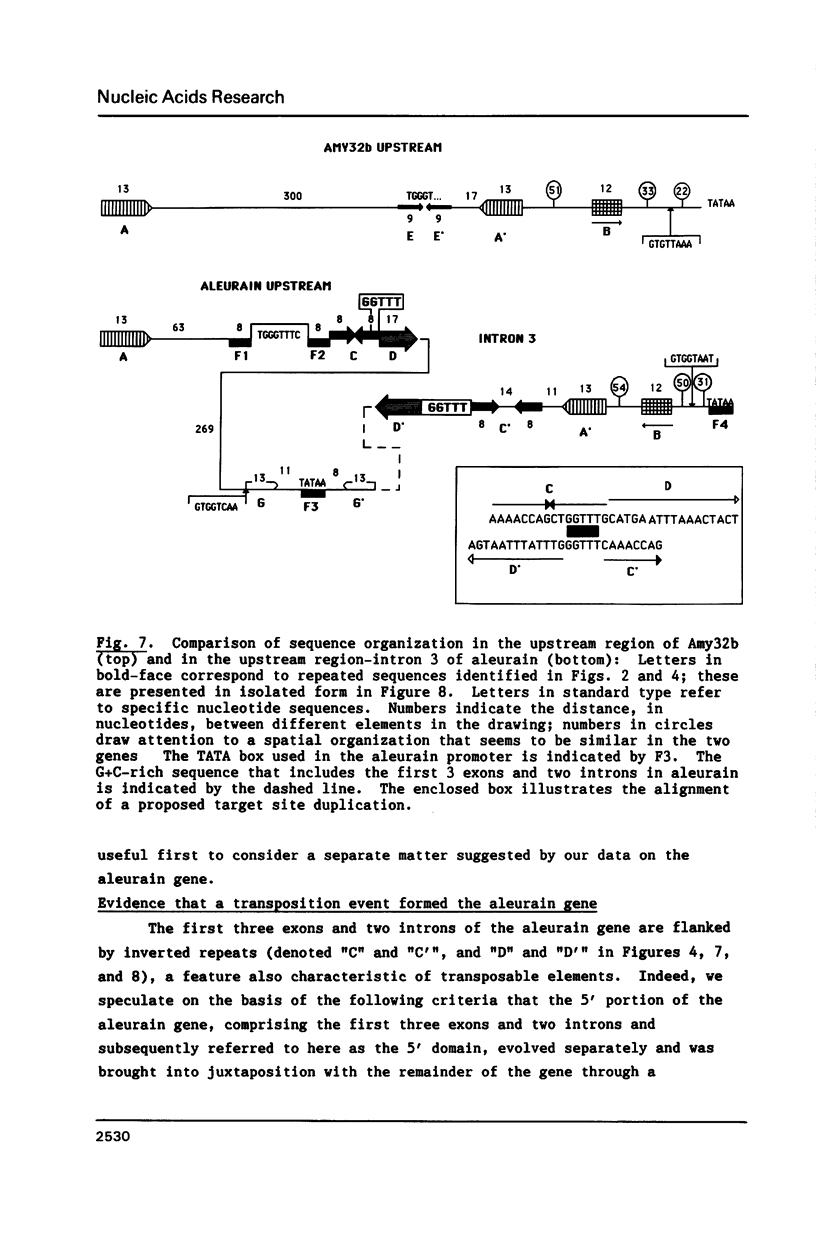
We have determined the nucleotide sequences of Amy32b, a type A alpha-amylase gene, and of the gene for aleurain, a thiol protease closely related to mammalian cathepsin H. Both are expressed in barley aleurone cells under control of the plant hormones gibberellic acid and abscisic acid, but only aleurain is expressed at high levels in other barley tissues. Sequence analysis indicates that the 5' end of the aleurain gene, comprising 3 exons and 2 introns, may have become associated with the remainder of the gene, encoding the protease domain of the protein, by some sort of recombination event. This 5' domain of the gene is very G + C-rich and is flanked by inverted repetitive sequences. We found two different groups of homologous sequence elements. The first group consists of four blocks of sequences conserved in the same spatial arrangement in both genes; these are arranged at similar intervals upstream from the Amy32b TATA box and from a TATA box present in intron 3 of aleurain, outside of the 5' domain and upstream from the protease domain. A part of two of these conserved sequences is similar to the core sequence of certain enhancer elements characterized from mammalian cells. The second group of homologous elements is present in the upstream region of both genes. We speculate that these conserved sets of sequences may have some role in either the tissue specificity of expression of the genes or in some part of the hormonal regulation imposed on them.
Full text
PDF



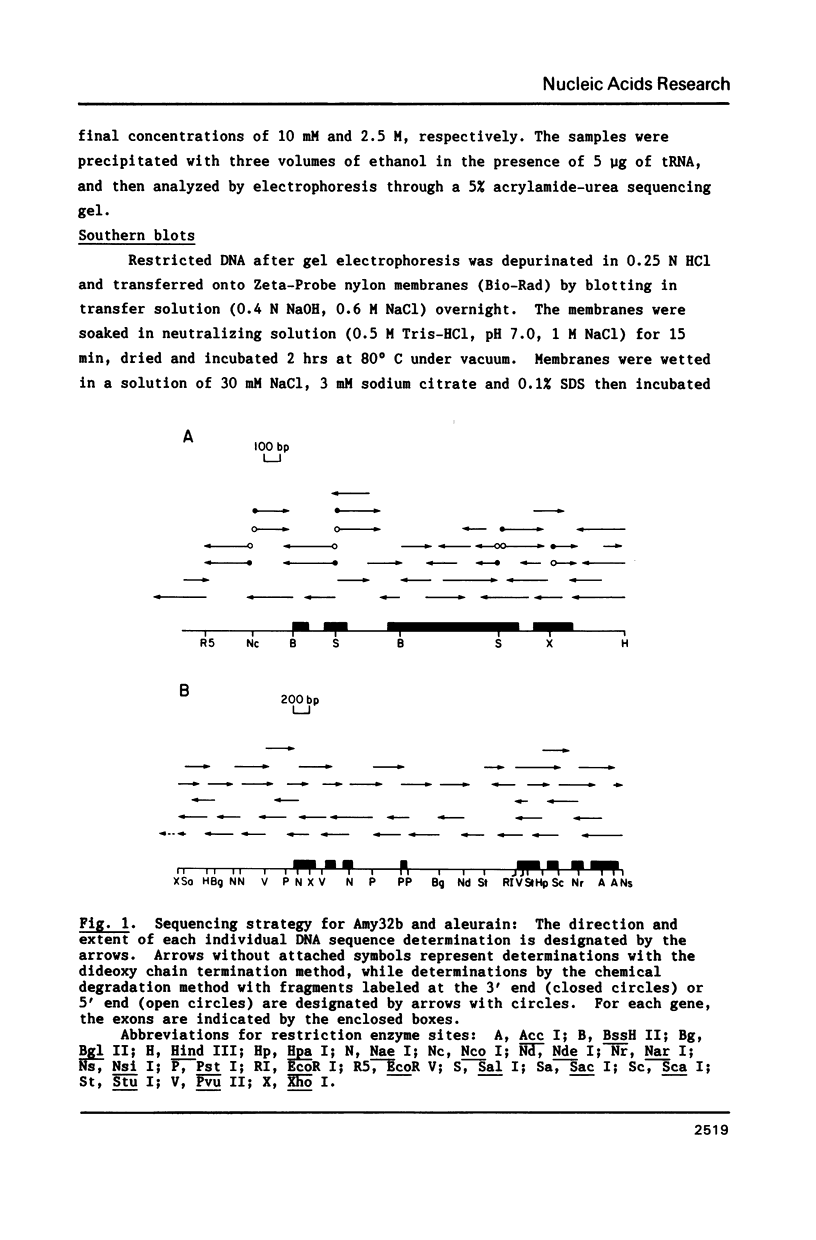















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker R. F., Thompson D. V., Talbot D. R., Swanson J., Bennetzen J. L. Nucleotide sequence of the maize transposable element Mul. Nucleic Acids Res. 1984 Aug 10;12(15):5955–5967. doi: 10.1093/nar/12.15.5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke J. F. High-sensitivity S1 mapping with single-stranded [32P]DNA probes synthesized from bacteriophage M13mp templates. Gene. 1984 Oct;30(1-3):63–68. doi: 10.1016/0378-1119(84)90105-7. [DOI] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Fluhr R., Kuhlemeier C., Nagy F., Chua N. H. Organ-specific and light-induced expression of plant genes. Science. 1986 May 30;232(4754):1106–1112. doi: 10.1126/science.232.4754.1106. [DOI] [PubMed] [Google Scholar]
- Gillies S. D., Morrison S. L., Oi V. T., Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983 Jul;33(3):717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Ho D. T. Response of barley aleurone layers to abscisic Acid. Plant Physiol. 1976 Feb;57(2):175–178. doi: 10.1104/pp.57.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaulen Hildegard, Schell Jeff, Kreuzaler Fritz. Light-induced expression of the chimeric chalcone synthase-NPTII gene in tobacco cells. EMBO J. 1986 Jan;5(1):1–8. doi: 10.1002/j.1460-2075.1986.tb04169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Moore D. D., Marks A. R., Buckley D. I., Kapler G., Payvar F., Goodman H. M. The first intron of the human growth hormone gene contains a binding site for glucocorticoid receptor. Proc Natl Acad Sci U S A. 1985 Feb;82(3):699–702. doi: 10.1073/pnas.82.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozer T. J. Control of protein synthesis in barley aleurone layers by the plant hormones gibberellic acid and abscisic acid. Cell. 1980 Jun;20(2):479–485. doi: 10.1016/0092-8674(80)90634-0. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Odell J. T., Nagy F., Chua N. H. Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. 1985 Feb 28-Mar 6Nature. 313(6005):810–812. doi: 10.1038/313810a0. [DOI] [PubMed] [Google Scholar]
- Rogers J. C., Dean D., Heck G. R. Aleurain: a barley thiol protease closely related to mammalian cathepsin H. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6512–6516. doi: 10.1073/pnas.82.19.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J. C., Milliman C. Coordinate increase in major transcripts from the high pI alpha-amylase multigene family in barley aleurone cells stimulated with gibberellic acid. J Biol Chem. 1984 Oct 10;259(19):12234–12240. [PubMed] [Google Scholar]
- Rogers J. C., Milliman C. Isolation and sequence analysis of a barley alpha-amylase cDNA clone. J Biol Chem. 1983 Jul 10;258(13):8169–8174. [PubMed] [Google Scholar]
- Rogers J. C. Two barley alpha-amylase gene families are regulated differently in aleurone cells. J Biol Chem. 1985 Mar 25;260(6):3731–3738. [PubMed] [Google Scholar]
- Shepherd N. S., Schwarz-Sommer Z., Blumberg vel Spalve J., Gupta M., Wienand U., Saedler H. Similarity of the Cin1 repetitive family of Zea mays to eukaryotic transposable elements. Nature. 1984 Jan 12;307(5947):185–187. doi: 10.1038/307185a0. [DOI] [PubMed] [Google Scholar]




