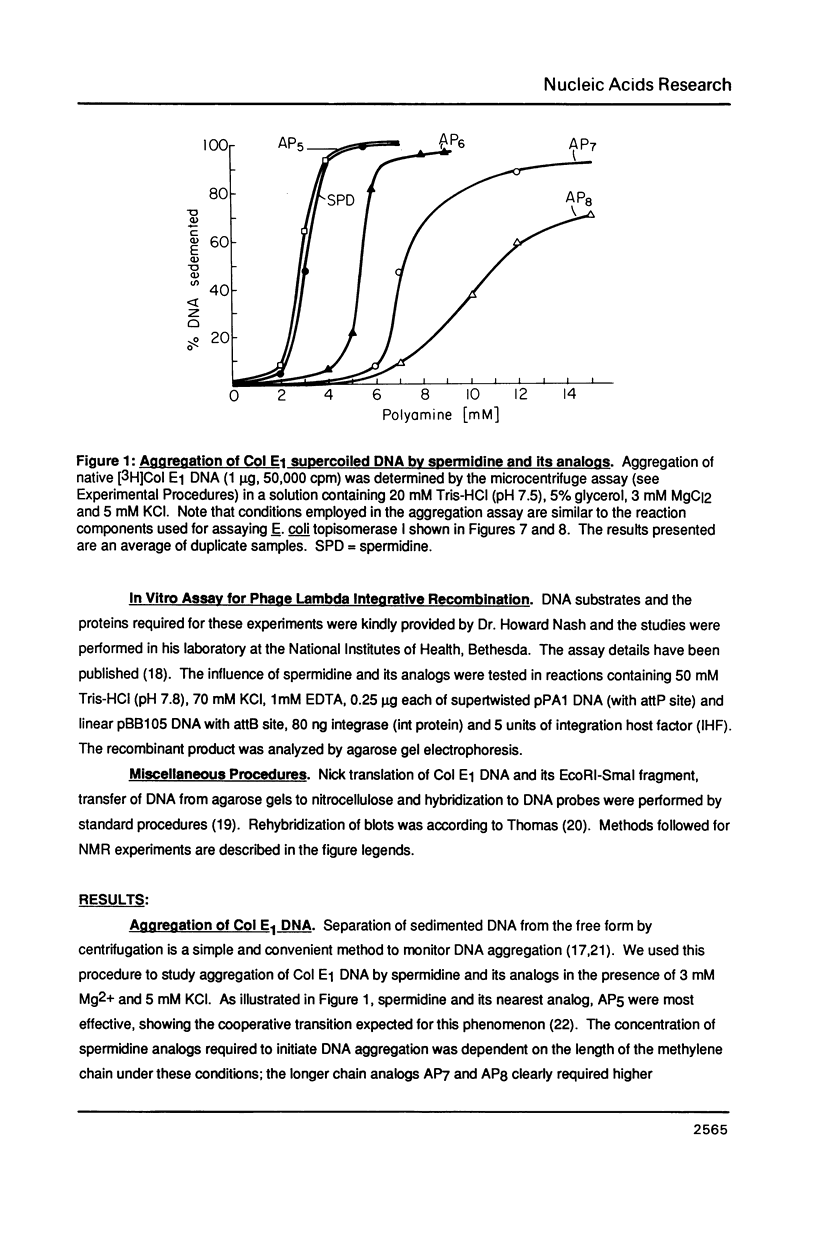
Abstract
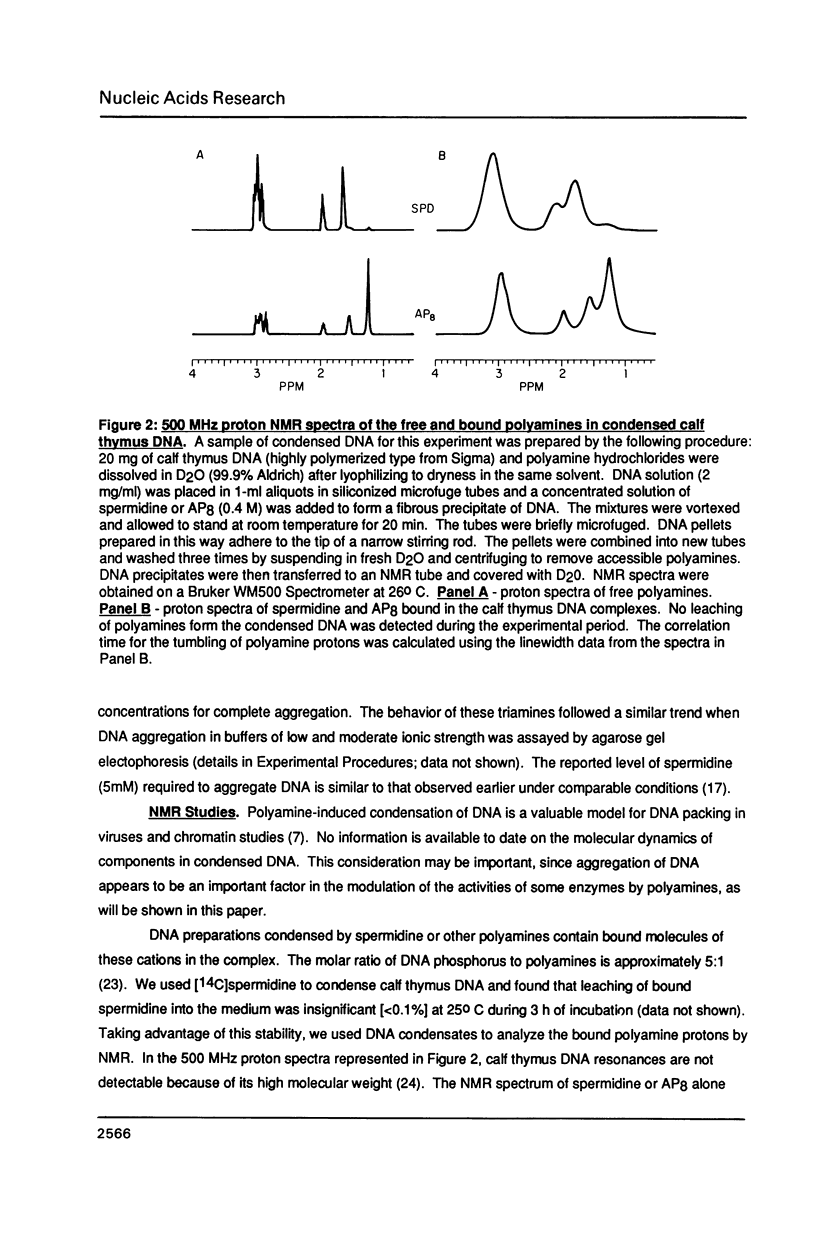
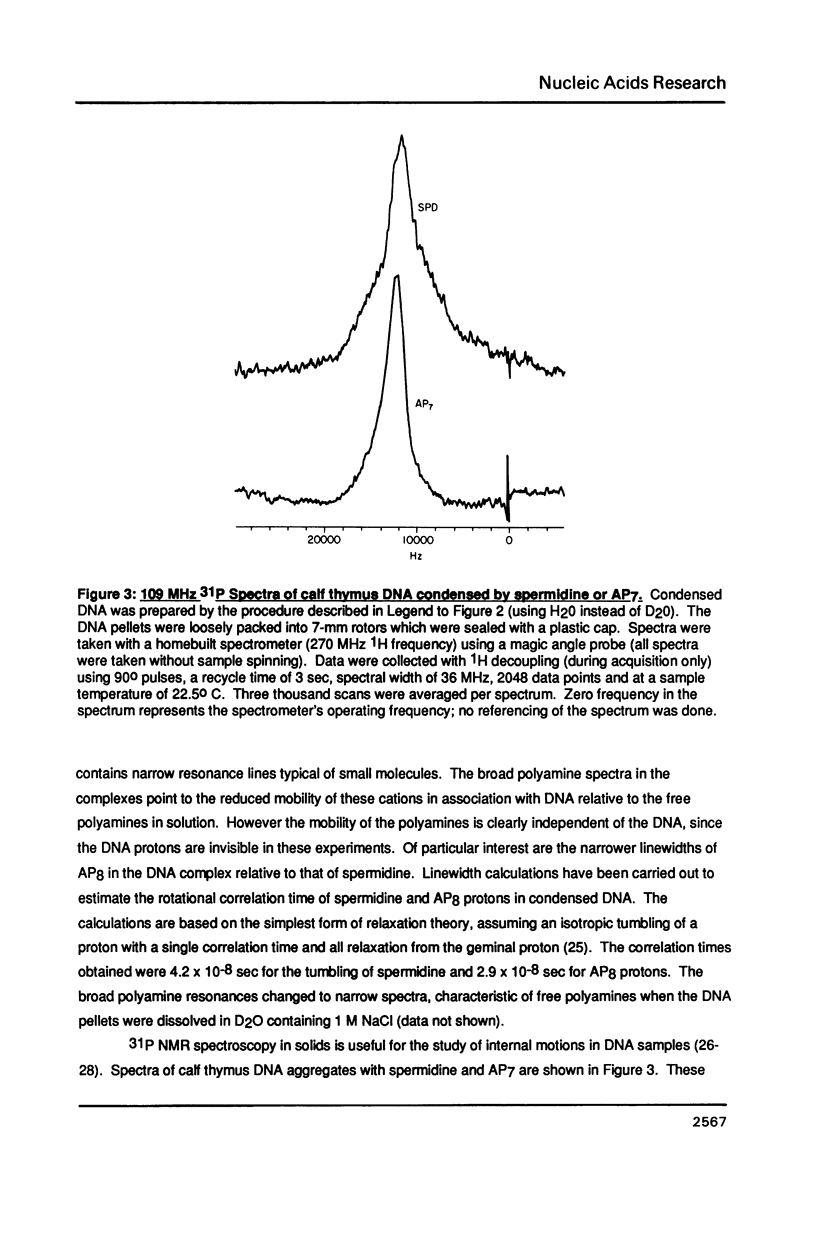
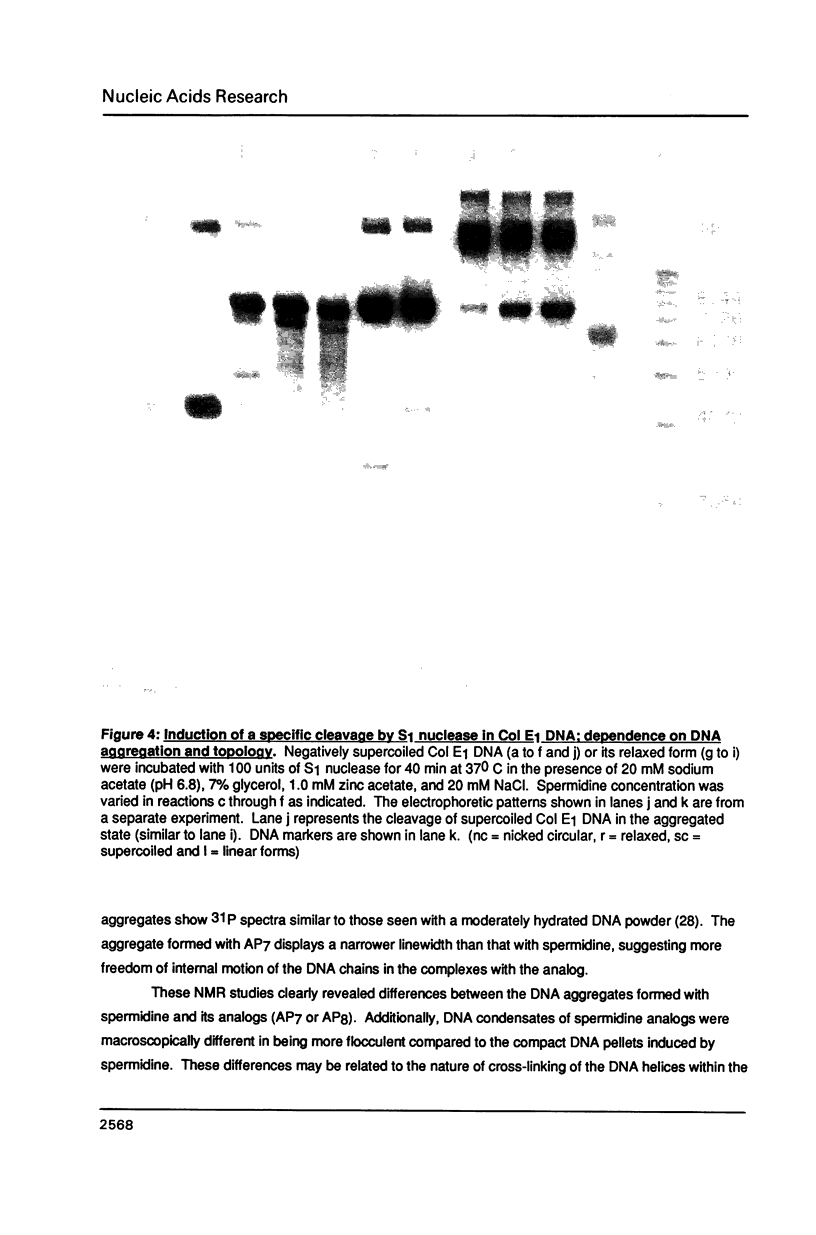
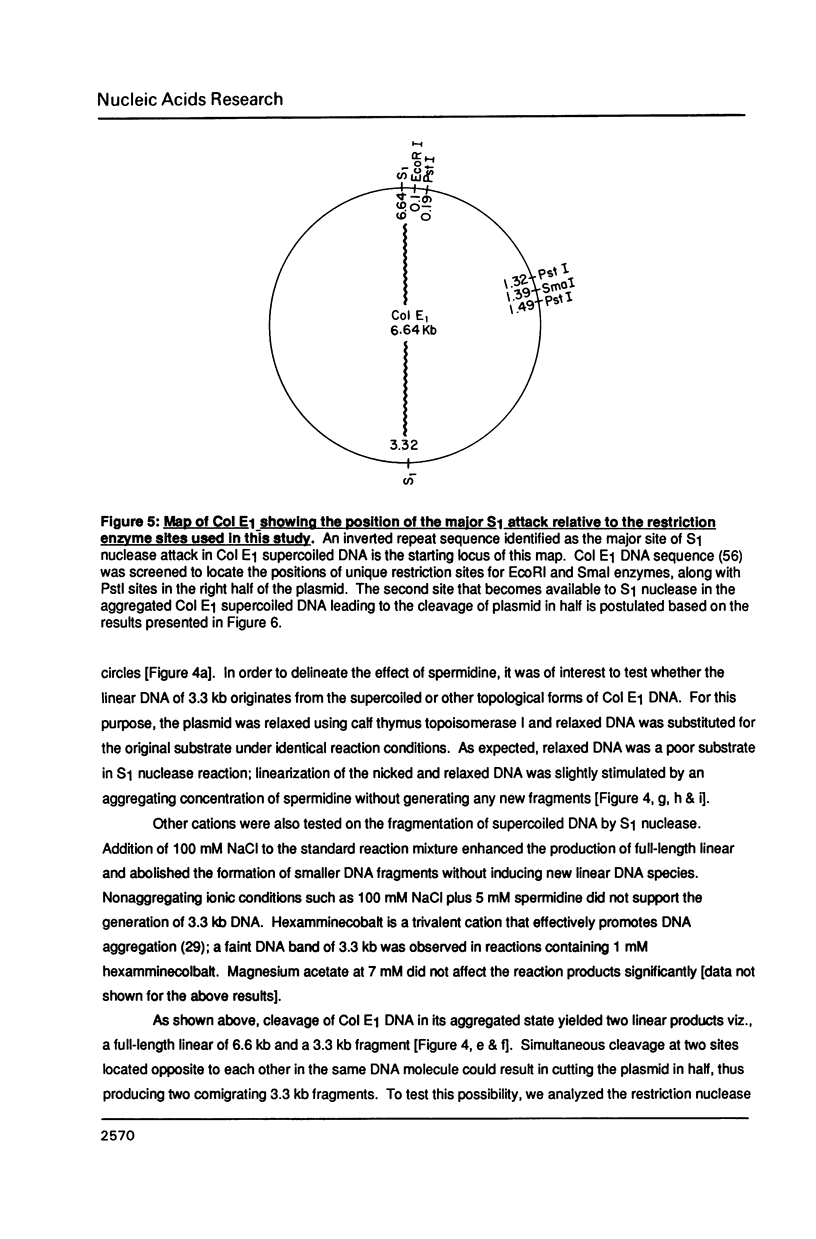
A homologous series of spermidine analogs, with defined abilities to replace the natural polyamine in supporting cell growth, was examined for its influence on the structure of supercoiled, aggregated DNA and on the ability of the DNA aggregates to act as substrates for various enzymes. The concentration of amine necessary to aggregate negatively supercoiled Col E1 DNA was progressively increased as the diaminobutane moiety of spermidine was extended beyond 5 methylene groups. 1H- and 31P-NMR spectroscopy suggested that less rigid DNA aggregates were formed by spermidine analogs than by spermidine itself. Spermidine and its analogs differentially modulated the activities of bacterial and mammalian type I topoisomerases and EcoRI restriction endonuclease on aggregated DNA in a manner reminiscent of the abilities of the amines to stimulate cell growth. When DNA was not aggregated, the influence of the various amines on these reactions was almost identical. These results are discussed in relation to the structures of the DNA aggregates in the presence of the various triamines.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison S. A., Herr J. C., Schurr J. M. Structure of viral phi 29 DNA condensed by simple triamines: a light-scattering and electron-microscopy study. Biopolymers. 1981 Mar;20(3):469–488. doi: 10.1002/bip.1981.360200305. [DOI] [PubMed] [Google Scholar]
- Ashikawa I., Furuno T., Kinosita K., Jr, Ikegami A., Takahashi H., Akutsu H. Internal motion of DNA in bacteriophages. J Biol Chem. 1984 Jul 10;259(13):8338–8344. [PubMed] [Google Scholar]
- Burton D. R., Forsén S., Reimarsson P. The interaction of polyamines with DNA: a 23Na NMR study. Nucleic Acids Res. 1981 Mar 11;9(5):1219–1228. doi: 10.1093/nar/9.5.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P. T., Ohmori H., Tomizawa J., Lebowitz J. Nucleotide sequence and gene organization of ColE1 DNA. J Biol Chem. 1985 Jul 25;260(15):8925–8935. [PubMed] [Google Scholar]
- Eickbush T. H., Moudrianakis E. N. The compaction of DNA helices into either continuous supercoils or folded-fiber rods and toroids. Cell. 1978 Feb;13(2):295–306. doi: 10.1016/0092-8674(78)90198-8. [DOI] [PubMed] [Google Scholar]
- Fujiwara T., Shindo H. Phosphorus-31 nuclear magnetic resonance of highly oriented DNA fibers. 2. Molecular motions in hydrated DNA. Biochemistry. 1985 Feb 12;24(4):896–902. doi: 10.1021/bi00325a013. [DOI] [PubMed] [Google Scholar]
- Geiger L. E., Morris D. R. Specificity of the spermidine requirement for the replication of phi X174 DNA be cell-free extracts of Escherichia coli. Biochim Biophys Acta. 1980 Sep 19;609(2):264–271. doi: 10.1016/0005-2787(80)90237-3. [DOI] [PubMed] [Google Scholar]
- Geiger L. E., Morris D. R. Stimulation of deoxyribonucleic acid replication fork movement by spermidine analogs in polyamine-deficient Escherichia coli. J Bacteriol. 1980 Mar;141(3):1192–1198. doi: 10.1128/jb.141.3.1192-1198.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Nash H. A. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
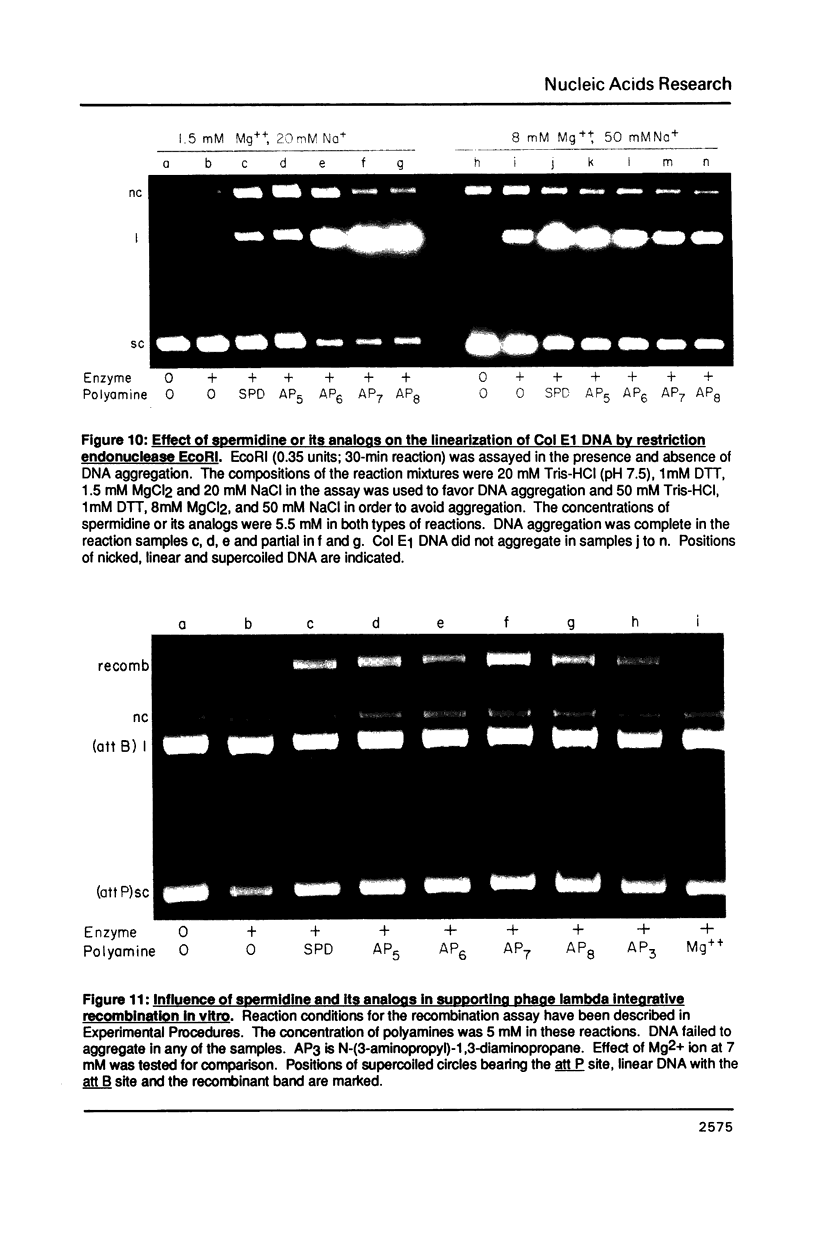
- Goppelt M., Langowski J., Pingoud A., Haupt W., Urbanke C., Mayer H., Maass G. The effect of several nucleic acid binding drugs on the cleavage of d(GGAATTCC) and pBR 322 by the Eco RI restriction endonuclease. Nucleic Acids Res. 1981 Nov 25;9(22):6115–6127. doi: 10.1093/nar/9.22.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosule L. C., Schellman J. A. DNA condensation with polyamines I. Spectroscopic studies. J Mol Biol. 1978 May 25;121(3):311–326. doi: 10.1016/0022-2836(78)90366-2. [DOI] [PubMed] [Google Scholar]
- Grosberg AYu, Zhestkov A. V. On the toroidal condensed state of closed circular DNA. J Biomol Struct Dyn. 1985 Dec;3(3):515–520. doi: 10.1080/07391102.1985.10508438. [DOI] [PubMed] [Google Scholar]
- Günther T. Functional compartmentation of intracellular magnesium. Magnesium. 1986;5(2):53–59. [PubMed] [Google Scholar]
- Hafner E. W., Tabor C. W., Tabor H. Mutants of Escherichia coli that do not contain 1,4-diaminobutane (putrescine) or spermidine. J Biol Chem. 1979 Dec 25;254(24):12419–12426. [PubMed] [Google Scholar]
- Hogan M. E., Jardetzky O. Internal motions in deoxyribonucleic acid II. Biochemistry. 1980 Jul 22;19(15):3460–3468. doi: 10.1021/bi00556a009. [DOI] [PubMed] [Google Scholar]
- Holt G. R., Davis W. E., Ailor E. I., Warren A. H., Elyassi H. Massive airway hemorrhage after transtracheal aspiration. South Med J. 1978 Mar;71(3):325–327. doi: 10.1097/00007611-197803000-00031. [DOI] [PubMed] [Google Scholar]
- Hoopes B. C., McClure W. R. Studies on the selectivity of DNA precipitation by spermine. Nucleic Acids Res. 1981 Oct 24;9(20):5493–5504. doi: 10.1093/nar/9.20.5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi K., Kashiwagi K., Hamasaki H., Miura A., Kakegawa T., Hirose S., Matsuzaki S. Formation of a compensatory polyamine by Escherichia coli polyamine-requiring mutants during growth in the absence of polyamines. J Bacteriol. 1986 Apr;166(1):128–134. doi: 10.1128/jb.166.1.128-134.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorstad C. M., Harada J. J., Morris D. R. Structural specificity of the spermidine requirement of an Escherichia coli auxotroph. J Bacteriol. 1980 Feb;141(2):456–463. doi: 10.1128/jb.141.2.456-463.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnow M. A., Cozzarelli N. R. Catenation of DNA rings by topoisomerases. Mechanism of control by spermidine. J Biol Chem. 1982 Mar 10;257(5):2687–2693. [PubMed] [Google Scholar]
- Lilley D. M. DNA: sequence, structure and supercoiling. The twentieth Colworth medal lecture. Biochem Soc Trans. 1984 Apr;12(2):127–140. doi: 10.1042/bst0120127. [DOI] [PubMed] [Google Scholar]
- Lilley D. M. The inverted repeat as a recognizable structural feature in supercoiled DNA molecules. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6468–6472. doi: 10.1073/pnas.77.11.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. F. DNA topoisomerases--enzymes that catalyse the breaking and rejoining of DNA. CRC Crit Rev Biochem. 1983;15(1):1–24. doi: 10.3109/10409238309102799. [DOI] [PubMed] [Google Scholar]
- Manning G. S. The molecular theory of polyelectrolyte solutions with applications to the electrostatic properties of polynucleotides. Q Rev Biophys. 1978 May;11(2):179–246. doi: 10.1017/s0033583500002031. [DOI] [PubMed] [Google Scholar]
- Marx K. A., Reynolds T. C. Ion competition and micrococcal nuclease digestion studies of spermidine-condensed calf thymus DNA. Evidence for torus organization by circumferential DNA wrapping. Biochim Biophys Acta. 1983 Dec 22;741(3):279–287. doi: 10.1016/0167-4781(83)90146-x. [DOI] [PubMed] [Google Scholar]
- Nash H. A., Robertson C. A. Purification and properties of the Escherichia coli protein factor required for lambda integrative recombination. J Biol Chem. 1981 Sep 10;256(17):9246–9253. [PubMed] [Google Scholar]
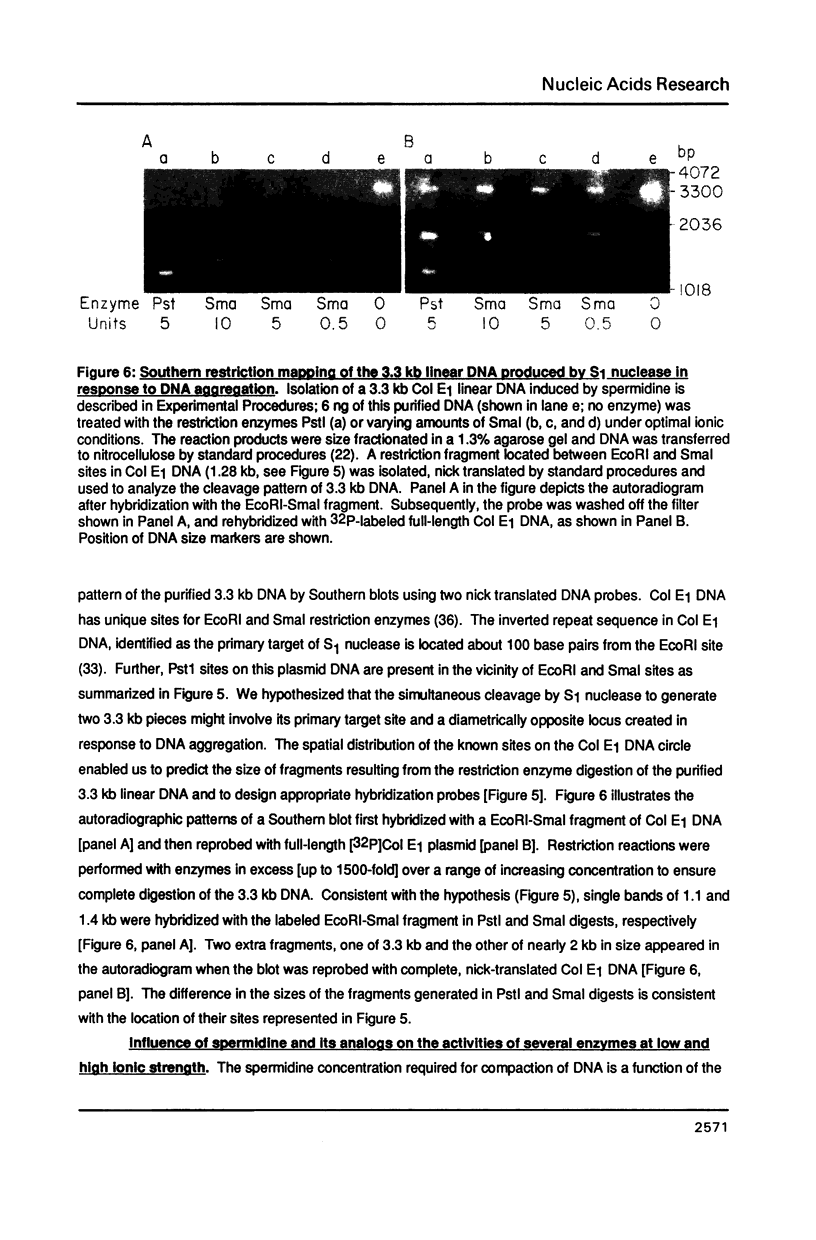
- Osland A., Kleppe K. Polyamine induced aggregation of DNA. Nucleic Acids Res. 1977 Mar;4(3):685–695. doi: 10.1093/nar/4.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pingoud A. Spermidine increases the accuracy of type II restriction endonucleases. Suppression of cleavage at degenerate, non-symmetrical sites. Eur J Biochem. 1985 Feb 15;147(1):105–109. doi: 10.1111/j.1432-1033.1985.tb08725.x. [DOI] [PubMed] [Google Scholar]
- Pingoud A., Urbanke C., Alves J., Ehbrecht H. J., Zabeau M., Gualerzi C. Effect of polyamines and basic proteins on cleavage of DNA by restriction endonucleases. Biochemistry. 1984 Nov 20;23(24):5697–5703. doi: 10.1021/bi00319a006. [DOI] [PubMed] [Google Scholar]
- Porschke D. Dynamics of DNA condensation. Biochemistry. 1984 Oct 9;23(21):4821–4828. doi: 10.1021/bi00316a002. [DOI] [PubMed] [Google Scholar]
- Porter C. W., Bergeron R. J. Spermidine requirement for cell proliferation in eukaryotic cells: structural specificity and quantitation. Science. 1983 Mar 4;219(4588):1083–1085. doi: 10.1126/science.6823570. [DOI] [PubMed] [Google Scholar]
- Rubin R. L. Spermidine-Deoxyribonucleic acid interaction in vitro and in Escherichia coli. J Bacteriol. 1977 Feb;129(2):916–925. doi: 10.1128/jb.129.2.916-925.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellman J. A., Parthasarathy N. X-ray diffraction studies on cation-collapsed DNA. J Mol Biol. 1984 May 25;175(3):313–329. doi: 10.1016/0022-2836(84)90351-6. [DOI] [PubMed] [Google Scholar]
- Shishido K. Relationship between S1 endonuclease-sensitivity and number of superhelical turns in a negatively-twisted DNA. FEBS Lett. 1980 Mar 10;111(2):333–336. doi: 10.1016/0014-5793(80)80821-0. [DOI] [PubMed] [Google Scholar]
- Singleton C. K., Wells R. D. Relationship between superhelical density and cruciform formation in plasmid pVH51. J Biol Chem. 1982 Jun 10;257(11):6292–6295. [PubMed] [Google Scholar]
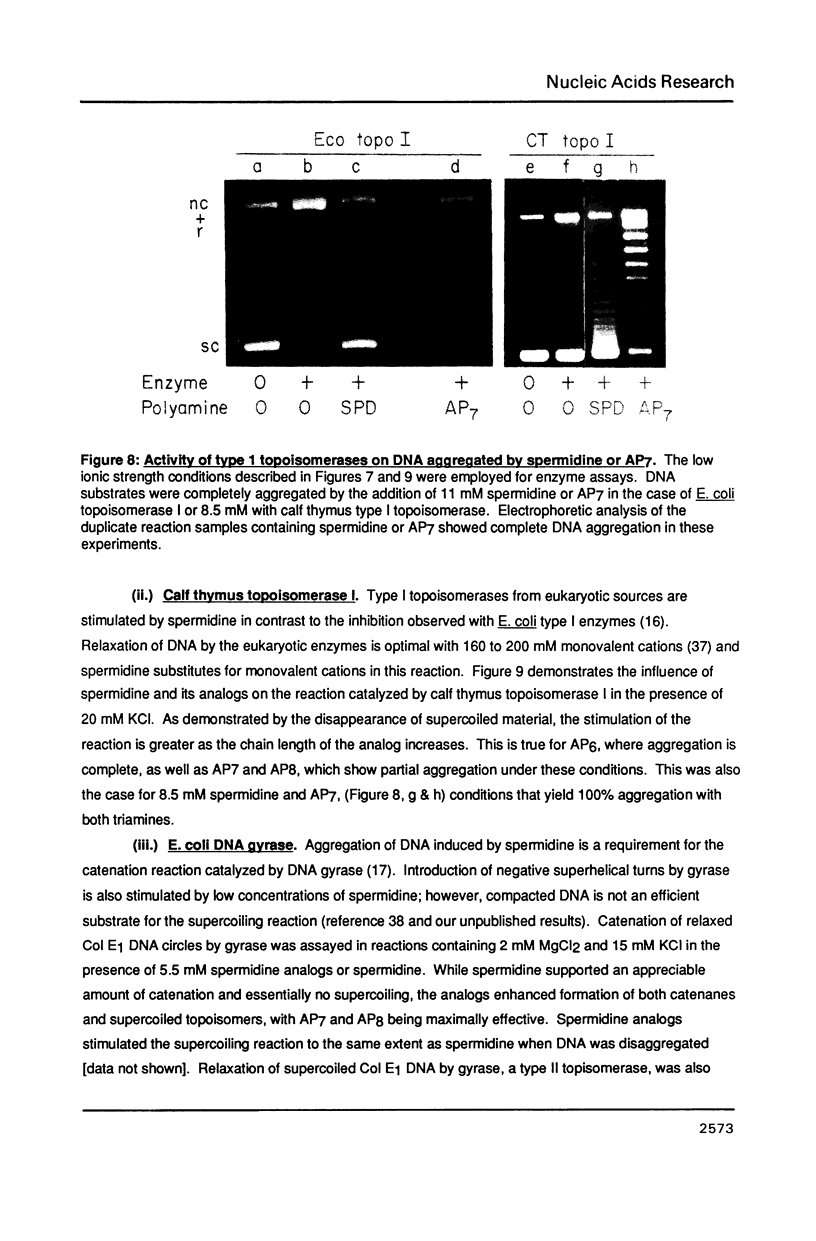
- Srivenugopal K. S., Morris D. R. Differential modulation by spermidine of reactions catalyzed by type 1 prokaryotic and eukaryotic topoisomerases. Biochemistry. 1985 Aug 27;24(18):4766–4771. doi: 10.1021/bi00339a009. [DOI] [PubMed] [Google Scholar]
- Srivenugopal K. S., Morris D. R. Modulation of the relaxing activity of Escherichia coli topoisomerase I by single-stranded DNA binding proteins. Biochem Biophys Res Commun. 1986 Jun 13;137(2):795–800. doi: 10.1016/0006-291x(86)91149-6. [DOI] [PubMed] [Google Scholar]
- Suwalsky M., Traub W., Shmueli U., Subirana J. A. An X-ray study of the interaction of DNA with spermine. J Mol Biol. 1969 Jun 14;42(2):363–373. doi: 10.1016/0022-2836(69)90049-7. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. J., Bloomfield V. A., Canellakis Z. N. Differential effects on the B-to-Z transition of poly(dG-me5dC).poly(dG-me5dC) produced by N1- and N8-acetyl spermidine. Biopolymers. 1985 Apr;24(4):725–729. doi: 10.1002/bip.360240411. [DOI] [PubMed] [Google Scholar]
- Thomas T. J., Bloomfield V. A. Ionic and structural effects on the thermal helix-coil transition of DNA complexed with natural and synthetic polyamines. Biopolymers. 1984 Jul;23(7):1295–1306. doi: 10.1002/bip.360230713. [DOI] [PubMed] [Google Scholar]
- Wemmer D. E., Srivenugopal K. S., Reid B. R., Morris D. R. Nuclear magnetic resonance studies of polyamine binding to a defined DNA sequence. J Mol Biol. 1985 Sep 20;185(2):457–459. doi: 10.1016/0022-2836(85)90418-8. [DOI] [PubMed] [Google Scholar]
- Widom J., Baldwin R. L. Cation-induced toroidal condensation of DNA studies with Co3+(NH3)6. J Mol Biol. 1980 Dec 25;144(4):431–453. doi: 10.1016/0022-2836(80)90330-7. [DOI] [PubMed] [Google Scholar]
- Wilson R. W., Bloomfield V. A. Counterion-induced condesation of deoxyribonucleic acid. a light-scattering study. Biochemistry. 1979 May 29;18(11):2192–2196. doi: 10.1021/bi00578a009. [DOI] [PubMed] [Google Scholar]