Background: Animal modeling experiments suggest additional SH2 effectors bind to the erythropoietin receptor (EPO-R) Tyr(P)-343.
Results: SH2B1 constitutively associates with the EPO-R and binds to EPO-R Tyr(P)-343/Tyr(P)-401 upon stimulation. SH2B1 knockdown results in enhanced phosphorylation of EPO-R, JAK2, and ERK.
Conclusion: SH2B1 plays a negative role in EPO-mediated signal transduction.
Significance: The SH2B family plays an important role in negative regulation of cytokine signaling.
Keywords: Adaptor Proteins, Erythropoietin, JAK Kinase, Signal Transduction, siRNA, SH2B1
Abstract
Gene targeting experiments have shown that the cytokine erythropoietin (EPO), its cognate erythropoietin receptor (EPO-R), and associated Janus tyrosine kinase, JAK2, are all essential for erythropoiesis. Structural-functional and murine knock-in experiments have suggested that EPO-R Tyr-343 is important in EPO-mediated mitogenesis. Although Stat5 binds to EPO-R phosphotyrosine 343, the initial Stat5-deficient mice did not have profound erythroid abnormalities suggesting that additional Src homology 2 (SH2) domain-containing effectors may bind to EPO-R Tyr-343 and couple to downstream signaling pathways. We have utilized cloning of ligand target (COLT) screening to demonstrate that EPO-R Tyr(P)-343 and Tyr(P)-401 bind to the SH2 domain-containing adaptor protein SH2B1β. Immunoprecipitation and in vitro mixing experiments reveal that EPO-R binds to SH2B1 in an SH2 domain-dependent manner and that the sequence that confers SH2B1 binding to the EPO-R is pYXXL. Previous studies have shown that SH2B1 binds directly to JAK2, but we show that in hematopoietic cells, SH2B1β preferentially associates with the EPO-R. SH2B1 is capable of constitutive association with EPO-R, which is necessary for its optimal SH2-dependent recruitment to EPO-R-Tyr(P)-343/Tyr(P)-401. We also demonstrate that SH2B1 is responsive to EPO stimulation and becomes phosphorylated, most likely on serines/threonines, in an EPO dose- and time-dependent manner. In the absence of SH2B1, we observe enhanced activation of signaling pathways downstream of the EPO-R, indicating that SH2B1 is a negative regulator of EPO signaling.
Introduction
Erythropoietin (EPO)4 is the primary cytokine required for the development of red blood cells, specifically driving definitive erythropoiesis (1). The EPO receptor (EPO-R) (2), a 66-kDa transmembrane protein, belongs to the cytokine receptor superfamily. The EPO-R is dependent on a constitutively associated Janus tyrosine kinase, JAK2, to mediate its downstream signaling. Upon EPO binding, the pre-formed EPO-R homodimer undergoes a conformational change leading to activation of JAK2 (3). The activated JAK2 phosphorylates tyrosine residues of the EPO-R, generating docking sites for SH2 effector proteins (reviewed in Ref. 4).
Gene targeting studies have elucidated the critical role of EPO (1), EPO-R (1, 5, 6), and JAK2 (7, 8) in erythropoiesis as loss of any of these genes leads to embryonic lethality due to defective definitive erythropoiesis. These findings suggest that EPO-R and/or JAK2 deliver signals crucial to EPO-dependent proliferation, differentiation, and cell survival.
Initial structural-functional studies identified the membrane proximal region of the EPO-R, specifically Tyr-343, to play an important role in EPO signaling and colony forming unit-erythroid formation (9, 10). The role of EPO-R cytoplasmic tyrosines was examined in greater detail through the generation of knock-in mice. Although mice expressing EPO-R H (truncated EPO-R possessing only Tyr-343) and EPO-R HM (truncated EPO-R with a Y343F mutation) are both viable (11), the ability of the EPO-R HM mice to respond to the stress agent phenylhydrazine is impaired (12). EPO-R HM mice also have elevated serum EPO (13), decreased reticulocyte production (14), defective erythroid repopulation (14), and increased apoptosis of erythroid progenitors (12). These studies illustrate that although EPO-R Tyr-343 may not be required for viability, it plays an essential role in signal amplification contributing to erythroid survival and mitogenesis, especially in response to erythroid stress.
The JAK-Stat pathway is one of the major pathways activated downstream of the EPO-R. Stat5a and Stat5b, which are largely redundant, are recruited to Tyr(P)-343 of the EPO-R via their SH2 domains (15–17). Studies utilizing Stat5-deficient EPO-R-H erythroid progenitors indicated that the phenotypes observed in EPO-R HM mice may be due to the inability of EPO-R HM to activate Stat5a/b(11). Interestingly, the embryos from the initial Stat5a/b-deficient mice (Stat5a/bΔN/ΔN) were anemic (11, 18), but in the adult mice there was only a subtle decrease in erythroid survival (19, 20). However, these data must be interpreted with caution, as it is now known that Stat5a/b ΔN alleles are hypomorphic (21, 22). Conditional targeting of Stat5a/b in the erythroid lineage has demonstrated that Stat5a/b plays a critical role in the regulation of several erythroid genes, including transferrin receptor-1 and iron regulatory protein-2 (23). We hypothesized that there are additional SH2 effectors that bind to EPO-R Tyr-343 and play a role in earlier steps of erythroid development.
We performed an expression screen utilizing a tyrosine-phosphorylated EPO-R Tyr-343 peptide to address this question, and we identified the adaptor protein SH2B1 (SH2-B/PSM) as an EPO-R Tyr-343-binding partner. SH2B1 is a member of the SH2B family of adaptor proteins, whose other members include SH2B2 (APS) and SH2B3 (Lnk). All three members contain N-terminal pleckstrin homology (PH) and dimerization domains, a C-terminal Src homology 2 (SH2) domain, and several proline-rich regions (24). SH2B and SH2B3 are capable of modulating EPO signaling (25, 26), and SH2B1 has been shown to regulate JAK2 activity in the context of growth hormone and leptin signaling (27, 28). In this study, we characterize the interaction between SH2B1 and EPO-R. We show that SH2B1 becomes phosphorylated in response to EPO and acts as a negative regulator of signaling downstream of the EPO-R.
EXPERIMENTAL PROCEDURES
Cloning of Ligand Target (COLT) Expression Screen
A day 16 murine embryo phage expression library (Novagen, Madison, WI) was diluted at 4 × 104 pfu/plate, and protein expression was induced as per manufacturer's instructions. The nitrocellulose filters containing immobilized proteins were washed in 20 mm Tris-HCl (pH 8.0), 150 mm NaCl, 0.05% Tween 20 (TBST) and blocked overnight in TBST containing 2% (w/v) BSA, 1 mm dithiothreitol (DTT) (Buffer A). Biotinylated EPO-R peptides were briefly incubated with streptavidin-alkaline phosphatase, conjugated, and mixed with Buffer A. Blocked filters were incubated in blocking buffer at room temperature for 3 h with biotinylated peptides (25 pmol/ml). Filters were washed five times with TBST, and detection was performed with a colorimetric nitro blue tetrazolium/5-bromo-4-chloro-3′indolyl phosphate p-toluidine (ThermoScientific, Rockford, IL) reaction. Plaques interacting with EPO-R Tyr(P)-343 were back-screened with a nonphosphorylated EPO-R Tyr-343 peptide. Plaques that specifically interacted with EPO-R Tyr(P)-343 were purified, and plasmid DNA was isolated from the phage according to the manufacturer's directions, and inserts were sequenced.
Cell Lines and Culture
Ba/F3 cells stably expressing wild-type or mutant EPO-R were maintained in RPMI 1640 medium, 10% (v/v) fetal calf serum, 100 units of penicillin/ml, 100 μg of streptomycin/ml, and 50 μm β-mercaptoethanol (RPMI 1640 complete medium) supplemented with 0.1 ng/ml recombinant mouse IL-3 in the presence of 1 mg/ml G418 (29, 30). DA-3-EPO-R cells were maintained in RPMI 1640 complete medium and 0.5 units/ml human recombinant EPO (29). HCD-57 cells were cultured in Iscove's modified Dulbecco's medium supplemented with 20% fetal calf serum, 50 μm β-mercaptoethanol, and 0.5 units/ml human recombinant EPO (29).
Various EPO-R constructs were electroporated into Ba/F3 cells (29, 30). EPO-R deletion mutants were generated as reported previously (29, 30). Individual G418-resistant subclones were isolated by limiting dilution. The expression of the EPO-R was confirmed by Western blotting, and the EPO-dependent growth characteristics of each subclone were examined by performing an XTT assay as described (31).
293T cells were maintained in Dulbecco's modified Eagle's medium (DMEM) H-21, 10% FBS. 293T cells were transiently transfected with EPO-R and SH2B1 constructs using Lipofectamine 2000 (Invitrogen) as per the manufacturer's instructions. The SH2B1 constructs were described previously (32).
siRNA-mediated Knockdown of SH2B1
SH2B1-specific ON-TARGET plus pooled siRNA was purchased from Dharmacon RNAi Technologies (Lafayette, CO). ON-TARGETplus siCONTROL Nontargeting Pool was used as controls in the knockdown experiments. Ba/F3-EPO-R cells were transfected with siRNA via electroporation (33). Ba/F3-EPO-R cells were maintained in their log phase of growth prior to transfection. The cells were washed once with RPMI 1640 medium and resuspended at 107 cells/200 μl. The cells were incubated with 1 μg of siRNA in a 0.4-cm electroporation cuvette for 10 min at room temperature. The cells were pulsed once at 300 V, 450 microfarads using the Bio-Rad Gene Pulser II electroporator. The cells were then resuspended in 5 ml of RPMI 1640 medium/IL3/G418.
Cytokine Deprivation and Stimulation
Ba/F3, DA-3, and HCD-57 cells were washed three times in 10 mm HEPES (pH 7.4), Hanks' balanced salts, incubated in RPMI 1640 medium supplemented with 10% fetal calf serum and 50 μm β-mercaptoethanol for 4 h at 37 °C and then stimulated with 10 ng/ml murine recombinant IL-3, 50 units/ml murine recombinant IL-2, human recombinant EPO, or vehicle for 10 min at 37 °C. 293T cells were washed three times with PBS and starved in DME H-21 supplemented with 2.5 mg/ml BSA overnight at 37 °C. The cells were stimulated with 5 units/ml human recombinant EPO or vehicle for 10 min at 37 °C.
The cells were washed once in 10 mm HEPES (pH 7.4), Hanks' balanced salts containing 10 mm sodium pyrophosphate, 10 mm sodium fluoride, 10 mm EDTA, and 1 mm sodium orthovanadate, and lysed in ice-cold lysis buffer containing 1% Triton X-100, 50 mm Tris-HCl (pH 8.0), 150 mm NaCl, 10 mm sodium pyrophosphate, 10 mm sodium fluoride, 10 mm EDTA, 1 mm sodium orthovanadate, 1 mm phenylmethylsulfonyl fluoride, 2 mg/ml aprotinin, 2 mg/ml leupeptin, and 1 mg/ml pepstatin A. After 5 min on ice, the lysates were centrifuged at 10,000 × g for 5 min at 4 °C.
Antibodies
The polyclonal SH2B1 antibody that recognizes amino acids 527–670 of SH2B1β was produced by Antibodies Inc. (Davis, CA). Purified GST-SH2B1 fusion protein (corresponding to amino acids 527–670) was used to immunize rabbits. Protein-A-Sepharose columns were used to purify anti-SH2B1 antibodies from the sera of the immunized rabbits. Purified anti-SH2B1 antibodies were used in immunoprecipitation experiments. The polyclonal anti-Tyr(P)-343-EPO-R (34) and the antibody targeting the N terminus of the EPO-R were produced in-house. The anti-phosphotyrosine monoclonal antibody, 4G10, and the total JAK2 antibody were purchased from Upstate Biotechnology, Inc. (Lake Placid, NY). The anti-Tyr(P)-479 EPO-R, anti-EPO-R, monoclonal anti-GST and phospho-ERK1/2 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). SH2B1 specific antibodies used for immunoblotting were graciously provided by Dr. Liangyou Rui (University of Michigan).
Immunoprecipitations
Antibodies along with a 50-μl volume of protein A-Sepharose 4B beads (Amersham Biosciences) were added to 2 mg of lysates for an overnight incubation. The beads were washed three times in ice-cold lysis buffer. The immune complexes were eluted by boiling in Laemmli sample buffer containing 100 mm DTT. Samples were resolved by SDS-PAGE and transferred to a polyvinylidene fluoride (PVDF) membrane for Western blotting.
GST Fusion Protein Binding Experiments
Two mg of cell lysate were incubated for 1 h at 4 °C with GST fusion proteins expressing the SH2 domain of SH2B1β immobilized on glutathione-Sepharose 4B beads (Amersham Biosciences). The beads were washed three times in ice-cold lysis buffer, and precipitated complexes were eluted by boiling in Laemmli sample buffer with 100 mm DTT. Samples were resolved by SDS-PAGE and analyzed by Western blotting.
Western Blotting
Following the electrophoretic transfer of proteins to PVDF membrane (PerkinElmer Life Sciences), the membranes were blocked at room temperature with 2.5% BSA in Tris-buffered saline (50 mm Tris (pH 8.0) and 150 mm NaCl) for 1 h. Membranes were then incubated with an optimal concentration of the primary antibody in TBST for 1 h at room temperature or overnight at 4 °C, washed four times in TBST, and incubated with the relevant HRP-conjugated secondary antibody for 30–60 min. Membranes were washed four times in TBST and visualized by enhanced chemiluminescence with autoradiographic film (ECL, Amersham Biosciences). For reprobing, membranes were stripped in 62.5 mm Tris-HCl (pH 6.8), 2% SDS, and 0.1 m β-mercaptoethanol for 30 min at 50 °C, rinsed twice in TBST, and blocked in 2.5% BSA in Tris-buffered saline prior to primary antibody incubation. Western blots were scanned using film exposures in the linear range and quantified using ImageJ software.
Calf Intestinal Alkaline Phosphatase Treatment of SH2B1 Immunoprecipitations
Following SH2B1 immunoprecipitation, the Sepharose beads were washed twice in dephosphorylation buffer (50 mm HEPES, 1 mm MgCl2 (pH 7.5)). The immunoprecipitates were resuspended in dephosphorylation buffer, and 30 units of calf intestinal alkaline phosphatase was added to the beads and incubated at 30 °C. To terminate the reaction, SDS-PAGE sample buffer was added to the beads, and the sample was boiled to elute off the bound protein.
Preparation of Primary Erythroblasts
C57/Bl6 mice (8–12 weeks old) were injected intraperitoneally on days 1 and 2 with a sterile solution of phenylhydrazine (6 mg/ml) in PBS solution (final dose, 60 mg/kg) (15). After the mice were sacrificed on day 5, a single spleen cell suspension was prepared. Cells were washed once in 10 mm HEPES (pH 7.4) and Hanks' balanced salts and starved in Iscove's media containing 2% fetal calf serum and 50 μm β-mercaptoethanol for 4 h at 37 °C. Cells were then incubated with either no factor or 50 units/ml human recombinant EPO for 10 min at 37 °C. Cells were washed once in 10 mm HEPES (pH 7.4) and Hanks' balanced salts containing 10 mm sodium pyrophosphate, 10 mm sodium fluoride, 10 mm EDTA, and 1 mm sodium orthovanadate. Lysates were prepared as described above. Studies were approved by the Animal Care Committee at the Ontario Cancer Institute, University Health Network, Toronto, Ontario, Canada.
RESULTS
COLT Screening Identifies SH2B1β Interacting with EPO-R Tyr-343
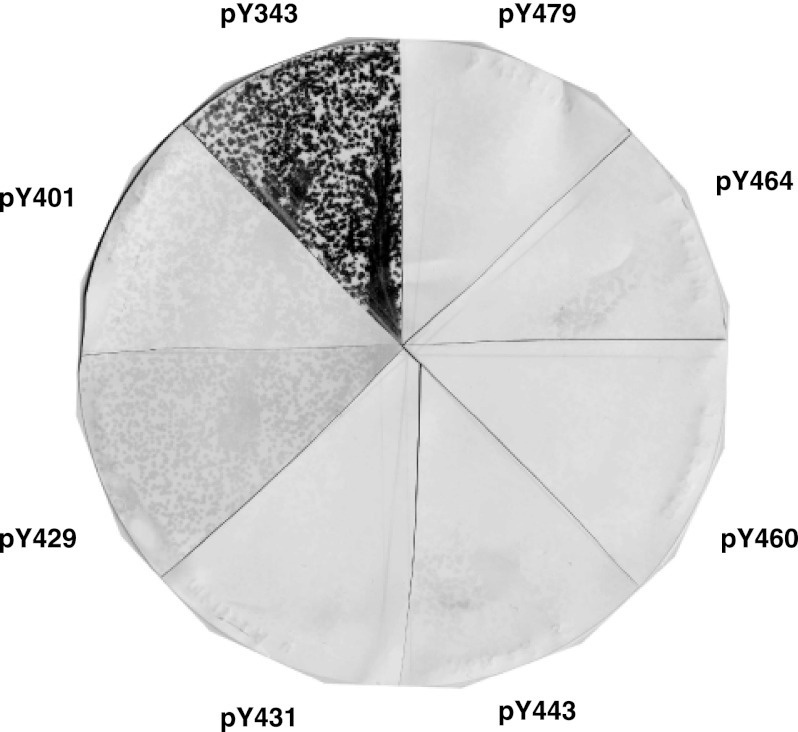
We hypothesized that in addition to Stat5, EPO-R Tyr-343 may recruit other SH2 signaling effectors. SH2 effectors that bind to EPO-R Tyr-343 were identified in an unbiased fashion via COLT screening (35, 36). A biotinylated EPO-R Tyr(P)-343 phosphopeptide was utilized to screen a murine day 16 embryonic library. Positive clones were back-screened with an identical EPO-R Tyr-343 nonphosphorylated peptide. Clones that met this specificity selection were sequenced. All were found to contain an SH2 domain. One of the clones isolated corresponded to the SH2 adaptor protein SH2B1β (27). To examine the specificity of the EPO-R/SH2B1β interaction, isolated SH2B1β plaques were incubated with specific biotinylated EPO-R phosphopeptides corresponding to the sequences of the eight EPO-R cytoplasmic tyrosines (Fig. 1). In addition to EPO-R Tyr(P)-343, EPO-R Tyr(P)-401 and EPO-R Tyr(P)-429 were also found to interact with SH2B1β, although to a lower extent. All three EPO-R sequences possess a common pYXXL motif, which is known to be recognized by several SH2 domains (37, 38), including the SH2 domain of SH2B1 (39). Considering that SH2B1 was characterized as a JAK2-binding partner (27), we were interested in determining the role of SH2B1 in EPO-mediated signaling pathways.
FIGURE 1.
Specificity of SH2B1 for Tyr(P)-343, Tyr(P)-401, and Tyr(P)-429. Phage expressing SH2B1 were incubated with BL21 Escherichia coli cells and plated on 2× YT plates. Plaques were transferred onto nitrocellulose membranes and then cut into eight sections. Each section was incubated with a biotinylated phosphopeptide corresponding to one of the eight cytoplasmic tyrosines of EPO-R (as labeled). Positives were detected with colorimetric detection solution.
SH2B1 Associates with the EPO-R in Hematopoietic Cell Lines
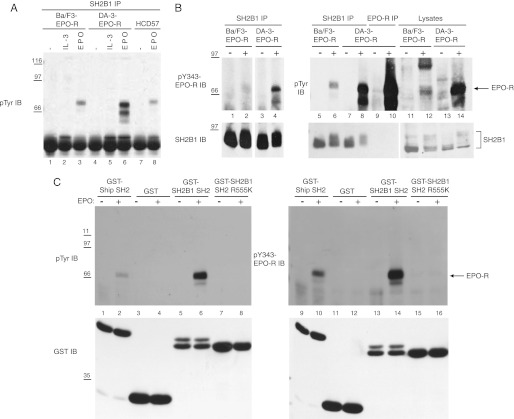
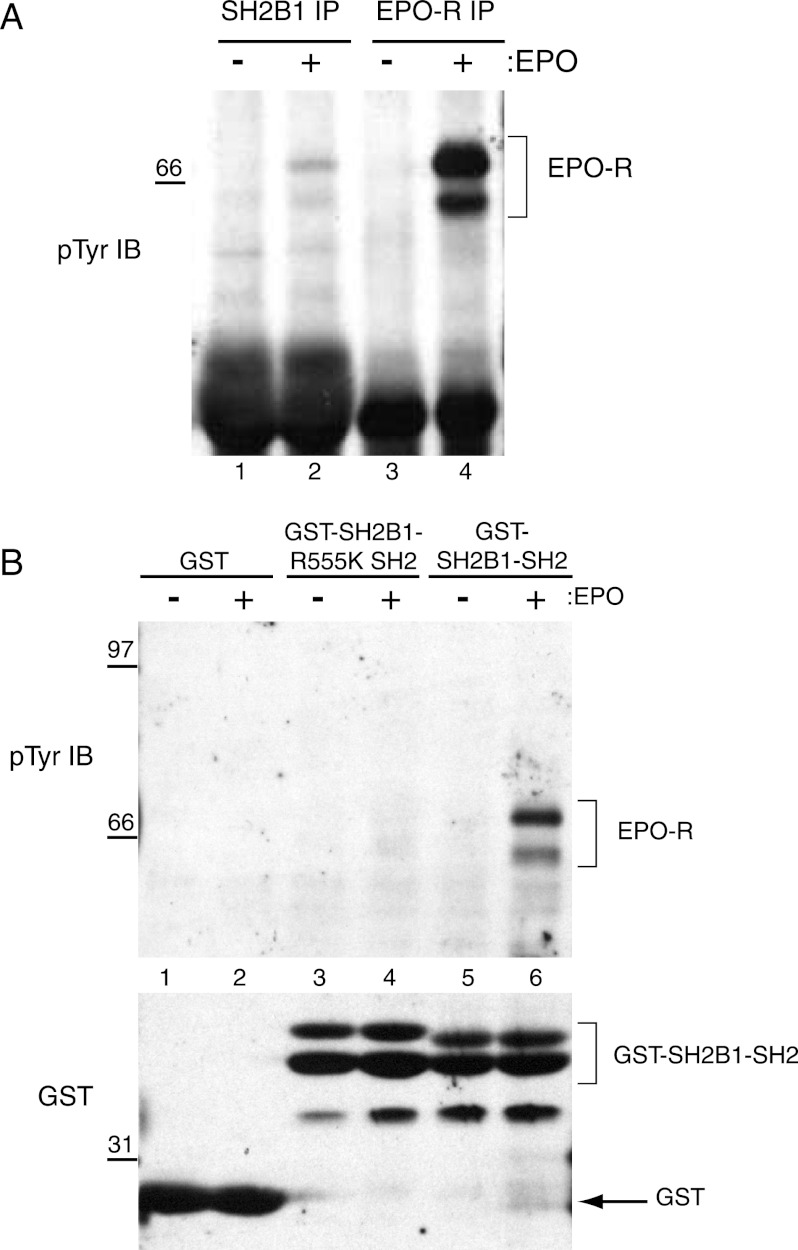
To investigate the role of SH2B1 in EPO signaling, we tested Ba/F3 and DA-3 hematopoietic cells, transfected to express EPO-R, as well as HCD-57 cells expressing endogenous EPO-R. These cells were depleted of cytokine and then incubated with no factor or with IL-3 or EPO. Immunoprecipitation experiments were performed using a polyclonal SH2B1 antibody, followed by Western blotting with the anti-phosphotyrosine antibody 4G10. Upon EPO stimulation, SH2B1 co-immunoprecipitated a phosphoprotein with a molecular weight corresponding to EPO-R in all cell lines (Fig. 2A, lanes 3, 6, and 8). To show that the phosphoprotein that co-immunoprecipitates with SH2B1 is EPO-R, a Tyr(P)-343-EPO-R specific antibody was also used for immunoblotting. Fig. 2B confirms that the phosphoprotein that co-IPs with SH2B1 (lanes 6 and 8) is indeed phosphorylated EPO-R (lanes 2 and 4).
FIGURE 2.
SH2B1 co-immunoprecipitates with phosphorylated EPO-R in hematopoietic cell lines via its SH2 domain. A, Ba/F3-EPO-R (lanes 1–3), DA-3-EPO-R (lanes 4–6), and HCD-57 (lanes 7 and 8) cells were depleted of cytokine for 4 h and incubated with no factor, 10 ng/ml murine IL-3, or 50 units/ml human recombinant EPO for 10 min at 37 °C. Immunoprecipitation (IP) was performed with an anti-SH2B1 antibody, and the immunoblot (IB) was probed with the anti-phosphotyrosine antibody 4G10. Molecular mass standards are indicated in kilodaltons. B, Ba/F3-EPO-R and DA-3-EPO-R cells were processed as in A, and the immunoblot was probed with Tyr(P)-343-EPO-R antibody (upper panel). The immunoblot was stripped and reprobed for SH2B1. Lanes 11–14 (SH2B1 IB) show a lighter exposure than lanes 1–10. C, Ba/F3-EPO-R cells were cytokine-depleted then either left unstimulated or were stimulated with EPO. Lysates were incubated with GST, GST-SH2B1 SH2, or GST-SH2B1 SH2-R555K. The immunoblots were probed with the anti-phosphotyrosine 4G10 or Tyr(P)-343-EPO-R antibody and then stripped and reprobed for GST.
COLT screening suggested that SH2B1 bound to EPO-R in an SH2 domain-dependent manner. In vitro mixing experiments were performed utilizing the following fusion proteins: GST-SH2B1 SH2, GST-SH2B1 SH2 R555K (an inactive SH2 domain mutant), and GST-Ship SH2 as a positive control (29). Bound proteins from Ba/F3-EPO-R lysates were detected using an anti-phosphotyrosine antibody (Fig. 2C, lanes 1–8) and the Tyr(P)-343-EPO-R-specific antibody (Fig. 2C, lanes 9–16). The GST-SH2B1 SH2 fusion protein associated with phosphorylated EPO-R upon EPO stimulation (Fig. 2C, lanes 6 and 14), and the SH2-inactivating R555K mutation (Fig. 2C, lanes 8 and 16) abolished this interaction. These results indicate that SH2B1 interacts with tyrosine-phosphorylated EPO-R via its SH2 domain.
SH2B1 Binds Specifically to Tyr(P)-343 and Tyr(P)-401 of the EPO-R upon EPO Stimulation
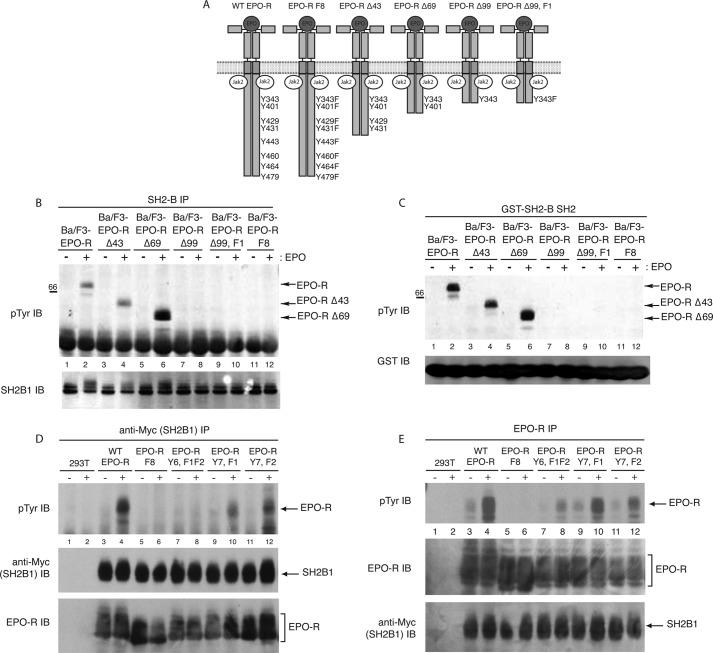
To confirm the COLT screen results that SH2B1 binds specifically to Tyr(P)-343 and/or Tyr(P)-401 of the EPO-R, we examined the interaction of SH2B1 with a panel of EPO-R deletion mutants. Ba/F3 cells expressing wild-type EPO-R, EPO-RΔ43 (containing Tyr-343, Tyr-401, Tyr-429, and Tyr-431), EPO-RΔ69 (containing Tyr-343 and Tyr-401), EPO-RΔ99 (containing Tyr-343), and EPO-RΔ99,F1 (containing a Y343F mutation) (Fig. 3A) were depleted of cytokine and stimulated with EPO or left unstimulated. SH2B1 was immunoprecipitated, and Western blotting using an anti-phosphotyrosine antibody was performed (Fig. 3B). EPO stimulation resulted in co-immunoprecipitation of SH2B1 with the tyrosine-phosphorylated EPO-R in Ba/F3-cells expressing full-length EPO-R (Fig. 3B, lane 2), EPO-RΔ43 (lane 4), and EPO-RΔ69 (lane 6), indicating that SH2B1β is specifically recruited to Tyr(P)-343 and/or Tyr(P)-401 of the EPO-R. To examine whether the truncated EPO-R mutants associate with the SH2 domain of SH2B1, in vitro mixing experiments were performed (Fig. 3C). Lysates were incubated with GST-SH2B1 SH2, and tyrosine-phosphorylated proteins were detected via anti-phosphotyrosine Western blotting. Upon EPO stimulation, association of the full-length EPO-R (Fig. 3B, lane 2), EPO-RΔ43 (lane 4), and EPO-RΔ69 (lane 6) with the SH2B1 SH2 domain was detected.
FIGURE 3.
Co-immunoprecipitation with EPO-R truncation mutants confirms SH2B1 binds specifically to Tyr(P)-343 and Tyr(P)-401 of the EPO-R. A, panel of EPO-R truncation mutants used. Deletion mutant numbers correspond to the number of amino acids deleted from the cytoplasmic tail of the EPO-R. B, SH2B1 immunoprecipitations (IP) were performed on lysates from EPO-stimulated and -unstimulated Ba/F3 cells expressing the various EPO-R mutants, followed by phosphotyrosine immunoblotting (IB). The membrane was reprobed with anti-SH2B1. C, GST-SH2B1 SH2 pulldowns were performed on lysates collected from EPO-stimulated and -unstimulated Ba/F3 cells expressing the EPO-R truncation mutant panel. Pulled down complexes were analyzed via phosphotyrosine immunoblot. Lysates were probed with anti-GST. D, 293T cells were transfected to express Myc-tagged SH2B1 and the indicated EPO-R tyrosine mutants. Transfected cells were depleted of cytokines and either left unstimulated or stimulated with 5 units/ml EPO for 10 min. Immunoprecipitation was performed with an anti-Myc antibody, and the membranes were probed with anti-Tyr(P) and reprobed with anti-EPO-R and anti-Myc (to detect myc-SH2B1β). E, from identical lysates as shown in D, immunoprecipitations were performed with an EPO-R antibody, followed by phosphotyrosine immunoblotting and reprobing with anti-EPO-R and anti-Myc.
To determine whether the binding of SH2B1 to phosphorylated EPO-R was dependent on Tyr(P)-343 or Tyr(P)-401 or both EPO-R tyrosines, we tested several mutant EPO-R (Fig. 3D). SH2B1 co-immunoprecipitated EPO-R-Y7, F1 lacking Tyr-343 (Fig. 3D, lane 10), and EPO-R-Y7, F2 lacking Tyr-401 (lane 12), but the interaction was abolished upon the expression of EPO-R-Y6, F1F2 lacking both Tyr-343 and Tyr-401 (lane 8). Tyrosine phosphorylation of the EPO-R was also examined in this panel of EPO-R receptor mutants (Fig. 3E). EPO-R (Fig. 3E, lane 4), EPO-R Y6, F1F2 (lane 8), EPO-R Y7,F1 (lane 10), and EPO-R Y7,F2 (lane 12) are tyrosine-phosphorylated in response to EPO-R. The lack of SH2B1 binding to EPO-R Y6,F1F2 was not due to lack of phosphorylation. These results suggest that upon EPO stimulation SH2B1, via its SH2 domain, specifically binds to phospho-Tyr-343 and phospho-Tyr-401 of the EPO-R.
SH2B1 Associates with Unphosphorylated EPO-R
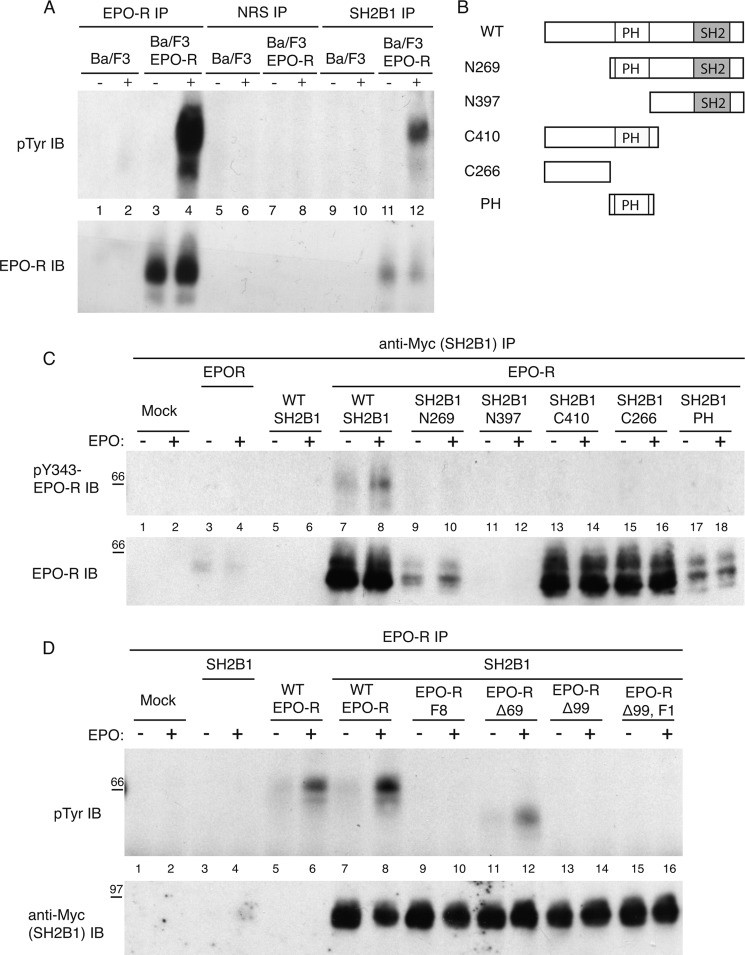
Stripping SH2B1 immunoprecipitation blots and reprobing for total EPO-R revealed that SH2B1 co-immunoprecipitated with unphosphorylated EPO-R in unstimulated samples (Fig. 4A). This indicates that SH2B1 may associate with the EPO-R prior to EPO stimulation, and upon EPO-R phosphorylation, it binds to Tyr(P)-343/Tyr(P)-401 of the EPO-R via its SH2 domain. To determine which region of SH2B1 was responsible for associating with unphosphorylated EPO-R, the EPO-R was co-expressed with a panel of Myc-tagged SH2B1 truncation mutants (Fig. 4B and supplemental Fig. 1A) (32). The cytokine-deprived cells were stimulated with EPO or left unstimulated, and myc-SH2B1 was immunoprecipitated using a Myc antibody. Probing with an EPO-R antibody revealed that SH2B1 full-length, C410, C266, and to a lesser extent N269 and PH truncation mutants associate with unphosphorylated EPO-R (Fig. 4C). However, phosphotyrosine immunoblotting indicated that only full-length SH2B1 was capable of associating with both unphosphorylated and phosphorylated EPO-R. These results indicate that both the PH domain and the region encompassing amino acids 1–266 are required for maximal association of SH2B1 with unphosphorylated EPO-R.
FIGURE 4.
PH domain and amino acids 1–266 of SH2B1 mediate a constitutive association with the membrane-proximal region of the EPO-R. A, lysates from Ba/F3 and Ba/F3-EPO-R cells stimulated or unstimulated with EPO were subjected to immunoprecipitation (IP) using anti-EPO-R, nonreactive serum (NRS), and anti-SH2B1 antibodies. Immunoblotting was performed using anti-phosphotyrosine antibody and anti-EPO-R antibody. B, panel of Myc-tagged SH2B1 truncation mutants used. C, 293T cells were transfected to express WT EPO-R and SH2B1 truncation mutants. The cells were cytokine-deprived overnight and then stimulated with 10 units/ml EPO for 10 min. Myc-SH2B1 was immunoprecipitated using an anti-Myc antibody. Immunoblot (IB) was performed with anti-phosphotyrosine and anti-EPO-R antibodies. D, 293T cells were transfected to express WT SH2B1 and EPO-R truncation mutants. The cells were cytokine-deprived overnight and then stimulated with 10 units/ml EPO for 10 min. EPO-R IPs were followed by anti-Tyr(P) and anti-Myc immunoblotting analysis.
We were also interested to determine which region of the EPO-R was involved in the constitutive association with SH2B1. Therefore, EPO-R truncation mutants (Fig. 3A and supplemental Fig. 1B) were co-expressed with Myc-tagged wild-type SH2B1. SH2B1 co-immunoprecipitated with all EPO-R mutants (Fig. 4D), indicating that the membrane-proximal region of the EPO-R mediates constitutive association with SH2B1.
Because SH2B1 also associates with both inactive and active JAK2 (32, 40), we wanted to confirm that the constitutive association between SH2B1 and the EPO-R was independent of JAK2. We investigated whether SH2B1 could constitutively bind to EPO-R carrying the W282R point mutation, which abolishes EPO-R binding to JAK2 (41, 42). SH2B1 co-immunoprecipitated to a similar extent with WT EPO-R and EPO-R W282R (supplemental Fig. 2). Because EPO-R W282R is unable to bind JAK2, it is also not phosphorylated in response to EPO stimulation. Therefore, as anticipated, only the co-precipitating WT EPO-R and not the co-precipitating EPO-R W282R was detected using Tyr(P)-343 EPO-R antibody. SH2B1 also failed to co-immunoprecipitate with JAK2 in co-immunoprecipitation experiments (supplemental Fig. 3). These data demonstrate that the constitutive association between SH2B1 and EPO-R is not dependent on JAK2.
SH2B1 Is Phosphorylated in Response to EPO Stimulation
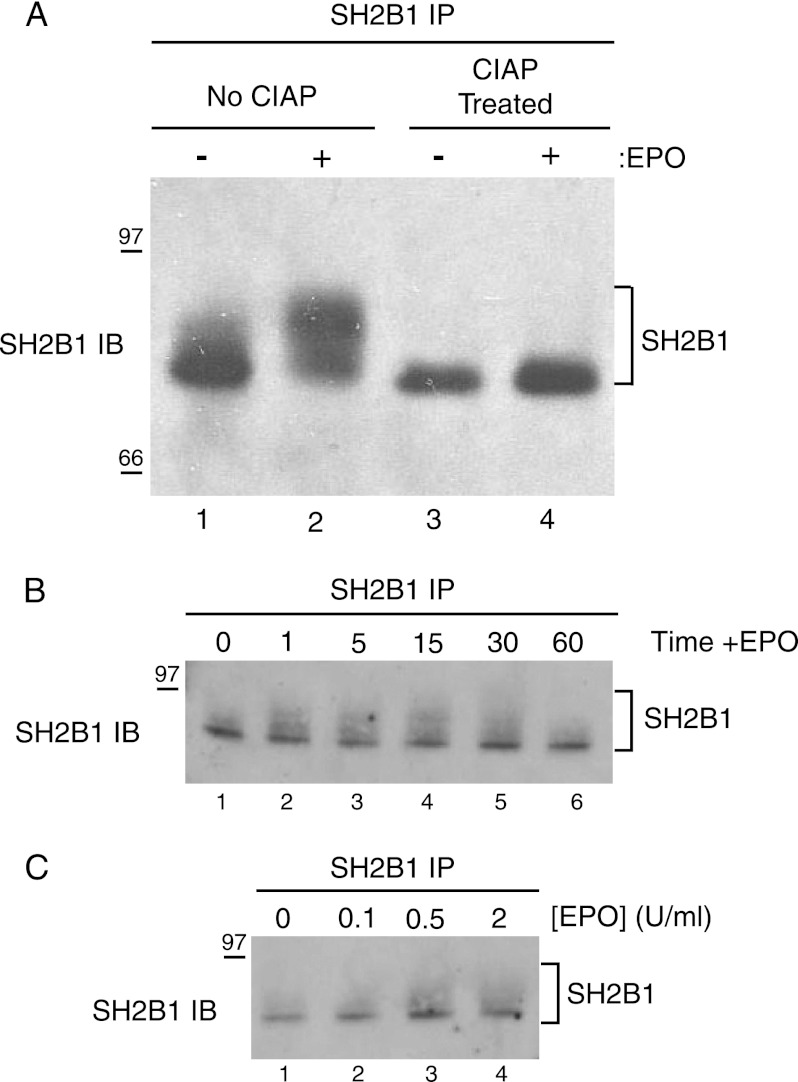
We observed that upon EPO stimulation, SH2B1 migrates as a broader, slower migrating band, consistent with SH2B1 phosphorylation (Fig. 2B, lower panel). To test whether this EPO-dependent upward mobility shift is due to increased phosphorylation of SH2B1, we treated immunoprecipitated SH2B1 with calf intestinal alkaline phosphatase, a nonspecific phosphatase. Calf intestinal alkaline phosphatase treatment results in the collapse of the broader SH2B1 band in the stimulated samples, generating a single dense band similar to that precipitated from the unstimulated samples (Fig. 5A). SH2B1 has been reported to undergo both tyrosine (21, 27) and serine/threonine (43) phosphorylation. Because no phosphotyrosine signal was observed at the molecular weight corresponding to SH2B1 (Fig. 2, A and B), it is likely that SH2B1 is becoming serine/threonine-phosphorylated in response to EPO. Although it is possible that the 4G10 phosphotyrosine antibody may not recognize the EPO-specific phosphorylated tyrosines in SH2B1, Rui et al. (27, 43, 44) have shown the 4G10 antibody recognizes tyrosine-phosphorylated SH2B1. A time course study (Fig. 5B) indicated that SH2B1 exhibits increased phosphorylation after 1 min of EPO stimulation and that phosphorylation returns to its basal state within 60 min post-EPO stimulation. SH2B1 phosphorylation was observed at the physiological dose of 0.5 units/ml of EPO, and it was further enhanced with increasing EPO dosage (Fig. 5C). These results indicate that SH2B1 becomes rapidly and transiently phosphorylated, most likely on serine and threonine residues, in response to physiological doses of EPO.
FIGURE 5.
EPO stimulation induces SH2B1 phosphorylation in a time- and dose-dependent manner. A, immunoprecipitations were performed on unstimulated and EPO-stimulated Ba/F3-EPO-R lysates. The immunoprecipitations (IPs) were treated with 30 units of calf intestinal alkaline phosphatase (CIAP) and immunoblotted (IB) with SH2B1-specific antibodies. Cytokine-deprived Ba/F3-EPO-R cells were stimulated with 50 units/ml EPO for the indicated times (B) or stimulated with indicated doses of EPO for 10 min (C). Cell lysates were immunoprecipitated and immunoblotted with a SH2B1 antibody.
SH2B1 Associates with the EPO-R in Primary Splenic Erythroblasts
Phenylhydrazine priming of mice results in the generation of EPO-responsive splenic erythroblasts (45), by inducing hemolytic anemia. The physiological response to the induced low hematocrit levels is an increase in hematopoiesis, whereby 4 days after the phenylhydrazine treatments, the spleen and bone marrow consist of greater than 90% primary erythroblasts.
To test whether SH2B1 binds to EPO-R in primary splenocytes, C57BL/6J mice were treated with phenylhydrazine, and after 4 days the splenocytes were harvested. The cells were depleted of cytokine for 4 h and stimulated with EPO or vehicle. SH2B1 and EPO-R immunoprecipitations were performed. Anti-phosphotyrosine Western blotting demonstrated that SH2B1 co-immunoprecipitated with tyrosine-phosphorylated EPO-R in primary splenic erythroblasts (Fig. 6A).
FIGURE 6.
SH2B1 associates with the EPO-R in primary erythroblasts. Splenocytes were collected from phenylhydrazine-treated C57/Bl6 mice and depleted of cytokine for 4 h. Cells were unstimulated or stimulated with 50 units/ml EPO for 10 min. A, following cell lysis, immunoprecipitations (IP) were performed with anti-SH2B1 or anti-EPO-R antibodies, followed by immunoblotting (IB) with an anti-phosphotyrosine antibody. B, same lysates used in A were incubated with GST, GST-SH2B1 SH2, or GST-SH2B1 SH2 R555K. Bound proteins were identified by immunoblotting with Tyr(P) antibody.
To determine whether the SH2 domain is required for the interaction of SH2B1 with activated EPO-R in the primary splenic erythroblasts as in the cultured cell systems, an in vitro mixing experiment was performed (Fig. 6B). Lysates were incubated with GST-SH2B1 SH2 and mutant GST-SH2B1 SH2 R555K fusion proteins, followed by anti-phosphotyrosine Western blotting. As shown previously for Ba/F3-EPO-R cells (Fig. 2C), in primary erythroblasts SH2B1 also associates with phosphorylated EPO-R in an SH2-dependent manner (Fig. 6B).
SH2B1 Is a Negative Regulator of Downstream EPO Signaling
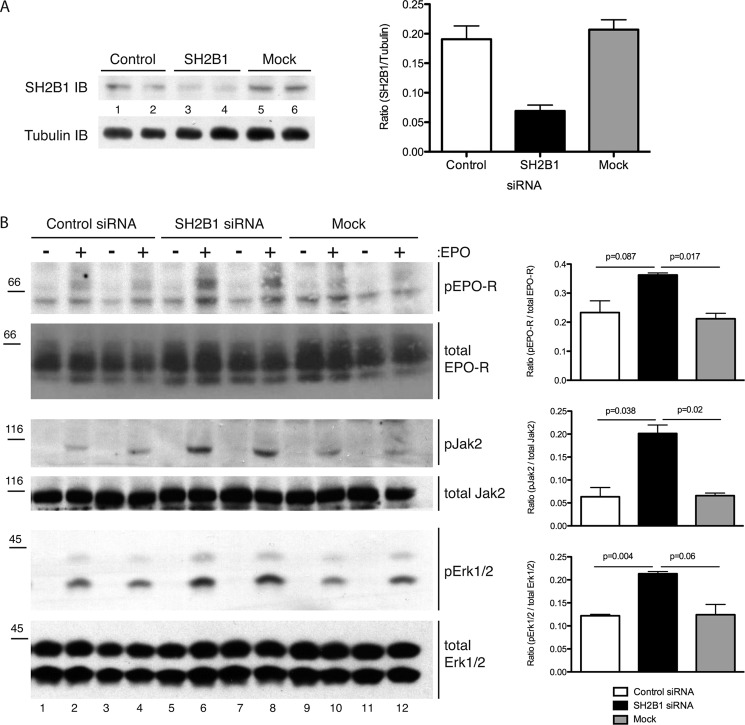
To determine the functional signaling consequences of the association of SH2B1 with EPO-R, siRNA was used to knock down (KD) SH2B1 in Ba/F3-EPO-R cells (Fig. 7A). Lysates from unstimulated or EPO-stimulated KD cells were probed for signaling effectors downstream of the EPO-R. When compared with mock and control siRNA-transfected cells, EPO stimulation of SH2B1 knockdown cells resulted in an increase in tyrosine phosphorylation of the EPO-R (detected by Tyr(P)-343 EPO-R (Fig. 7B) and Tyr(P)-479 EPO-R (data not shown) Western blotting), JAK2 (assessed by blotting with an antibody to the activatory Tyr(P)-1007/Tyr(P)-1008 of JAK2), and ERK1/2 phosphorylation (detected by an antibody to the activatory Tyr(P)-204 of ERK) (Fig. 7B). These results indicate that SH2B1 is a negative regulator of EPO signaling.
FIGURE 7.
Knockdown of SH2-B results in enhanced EPO-mediated signaling. A, indicated siRNAs were electroporated into Ba/F3-EPO-R cells. The resulting knockdown was assessed via SH2B1 immunoblotting (IB). Quantification of the knockdown is presented as a ratio of the density of the SH2B1 band to tubulin. B, Ba/F3-EPO-R cells were electroporated with the indicated siRNAs. The lysates from unstimulated or EPO stimulated cells were analyzed via phospho-JAK2, Tyr(P)-343-EPO-R, and phospho-ERK1/2 Western blotting. The blots were stripped and reprobed for amounts of total EPO-R, JAK2, and ERK1/2. Quantification of signaling blots is presented as the ratio of the density of the phospho band to the total band ± S.D. (n = 4).
DISCUSSION
Several structural-functional studies and the EPO-R H and EPO-R HM knock-in mice have demonstrated that tyrosine 343 of the EPO-R plays an important role in erythropoiesis, especially in response to erythropoietic stress (9–14). The phenotypes observed in EPO-R HM mice were attributed to the inability of the mutant receptor to activate Stat5a/b downstream of Tyr(P)-343 (11). Stat5a/b is not essential for erythroid differentiation (23); however, these transcription factors play a critical role in iron metabolism in erythroid progenitors as demonstrated by floxed alleles of Stat5a/b deleted in the erythroid lineage (23, 46). This raises the question of whether there are other effectors downstream of Tyr(P)-343 of the EPO-R, which may be playing an important role in earlier stages of erythroid development and erythroblast differentiation.
We utilized a COLT screen to identify additional SH2 effectors that bind to EPO-R Tyr(P)-343 to address this question in an unbiased fashion. We identified the adaptor protein SH2B1 as a novel EPO-R-binding protein. Because SH2B1 was originally identified as a JAK2-interacting protein, we were interested in examining the recruitment and role of SH2B1 in EPO-mediated signaling pathways in hematopoietic cells.
The major findings of this study include the following: (i) SH2B1 binding to tyrosine-phosphorylated EPO-R in an SH2-dependent manner in hematopoietic cell lines and primary erythroblasts; (ii) tyrosine-dependent binding is mediated via a membrane-proximal region of the EPO-R containing Tyr(P)-343 and Tyr(P)-401; (iii) SH2B1 and EPO-R also constitutively associate; (iv) SH2B1 preferentially associates with EPO-R rather than JAK2; (v) EPO enhances phosphorylation of SH2B1, and (vi) SH2B1 negatively regulates EPO signaling.
We have shown that SH2B1 interacts with EPO-R in several hematopoietic cell lines as well as in primary murine splenic erythroblasts. Detailed investigation of the SH2B1/EPO-R interaction revealed that SH2B1 binds Tyr(P)-343 and/or Tyr(P)-401 of the EPO-R in an SH2-dependent manner. SH2B1 has been reported to interact with JAK2 (24), and tyrosine 813 of JAK2 is the primary binding site of the SH2B1 SH2 domain (39). SH2B1 binds to inactive JAK2 via a region encompassing amino acids 410–555 (40, 47). Like JAK2 Tyr-813, EPO-R Tyr-343 and Tyr-401 are in a YXXL motif. We have also shown that SH2B1 binds to unphosphorylated EPO-R. However, the constitutive association of SH2B1 with EPO-R appears to be primarily mediated by the N-terminal region (amino acids 1–266) of SH2B1, binding to the membrane-proximal region of the EPO-R (amino acids 248–384). This N-terminal segment of SH2B1 contains the polybasic nuclear localization sequence, which is required for the recruitment of SH2B1 to the plasma membrane (48). These data raise the possibility that the localization of SH2B1 to the plasma membrane is required for the constitutive association with EPO-R. It is important to note that SH2B1 mutants (SH2B1-N269, SH2B1-N397) containing the SH2 domain, but with the EPO-R constitutive association region deleted, are unable to co-immunoprecipitate phosphorylated EPO-R (Fig. 4). This indicates that optimal constitutive association between SH2B1 and inactive EPO-R at the plasma membrane is required for the binding of the SH2 domain of SH2B1 to Tyr(P)-343/Tyr(P)-401 of EPO-R upon stimulation. Although there are a number of similarities between the SH2B1/JAK2 and SH2B1/EPO-R interactions, the constitutive association of SH2B1 with EPO-R appears to be independent of JAK2 binding to the erythropoietin receptor. This leads us to conclude that in context of signaling downstream of the EPO-R, SH2B1 preferentially binds to the EPO receptor and not to JAK2. This is a novel interaction for SH2B1, as this adaptor protein has been shown to preferentially bind to JAK2 downstream of both leptin (40) and growth hormone receptors (27) instead of the cognate receptors. It is possible that the dynamic interaction observed between SH2B1 and EPO-R serves a similar function as does the interaction between SH2B1 and JAK2, whereby constitutive association of SH2B1 with the EPO-R increases the local concentration of SH2B1 around the receptor, and upon EPO stimulation allows for rapid binding of the SH2 domain of SH2B1 with Tyr(P)-343 and/or Tyr(P)-401 of EPO-R. This would allow for robust recruitment of effectors downstream of SH2B1.
SH2B1 has 9 tyrosine, 82 serine, and 29 threonine residues and is capable of becoming phosphorylated at multiple sites. Specifically, NGF and PDGF have been shown to induce serine/threonine phosphorylation of SH2B1 (43, 44). In this study, we have shown that SH2B1 is responsive to EPO and upon EPO stimulation becomes phosphorylated. We believe that phosphorylation is most likely on serines/threonines because tyrosyl phosphorylation is not detected using 4G10 antibody, which recognizes the tyrosines in SH2B1β that are phosphorylated in response to growth hormone (27), NGF (43), and PDGF (44). This EPO-induced phosphorylation of SH2B1 is both dose- and time-dependent. SH2B1 becomes serine/threonine-phosphorylated by MEK or a downstream kinase in response to NGF stimulation (43). Considering that EPO activates the MAPK pathway (49), and a number of SH2B1 serine/threonine residues lie in the ERK1/2 consensus phosphorylation sites (43, 44), it is likely that in response to EPO, SH2-B becomes serine/threonine-phosphorylated by ERK1/2. EPO also activates protein kinase C (50) and could lead to phosphorylation of SH2B1 Ser-161 and Ser-165, as these sites are phosphorylated by protein kinase C. EPO-stimulated phosphorylation of SH2B1 could have very important functional consequences. SH2B1 phospho-sites can either recruit phosphoserine-binding proteins or cause conformational changes, which may lead to enhanced accessibility of proline-rich regions and recruitment of SH3-containing proteins, in both scenarios leading to recruitment of downstream effectors. Furthermore, phosphorylation of serine/threonine residues in the vicinity of the polybasic nuclear localization sequence region of SH2B1 causes the release of SH2B1 from the plasma membrane (48) and facilitates entry into the nucleus. Therefore, EPO-mediated phosphorylation of SH2B1 may be releasing SH2B1 from the plasma membrane to allow binding to phosphorylated EPO-R via the SH2 domain.
siRNA depletion of SH2B1 results in enhanced EPO-stimulated phosphorylation of EPO-R, JAK2, Stat5, and ERK1/2, indicating that SH2B1 is a negative regulator of EPO signaling. Increased phosphorylation of EPO-R Tyr(P)-343 and Tyr(P)-479 was observed. Whether altered expression of SH2B1 affects tyrosine phosphorylation of other EPO-R tyrosines remains to be resolved. SH2B1-dependent regulation of JAK2 has been well documented (24). In the absence of SH2B1, there is not only hyperactivation of JAK2 but also increased phosphorylation of the EPO-R and enhanced activation of several downstream pathways. These results suggest that SH2B1 mediates its inhibitory effect on EPO signaling by modulating JAK2 activity. This result was somewhat surprising, as SH2B1 has been shown to be a positive regulator of JAK2 activity downstream of growth hormone and leptin receptors (40, 51) and to enhance the kinase activity of the insulin receptor (52) and NGF receptor, TrkA (53).
However, both of the other members of the SH2B family of adaptor proteins, SH2B2 (Aps) and SH2B3 (Lnk), have been shown to be negative regulators of EPO signaling. Yoshimura and co-workers (25) have shown that SH2B2/Aps negatively regulates EPO signaling by recruiting Cbl to the EPO-R·JAK2 complex. Studies investigating the erythroid phenotype of SH2B3 (Lnk−/−) mice have shown that SH2B3/Lnk is also a negative regulator of erythropoiesis and EPO signaling (26). Interestingly, the erythroid compartment of Lnk−/− mice is normal at steady state (26), indicative of a potential compensatory mechanism, which may be fulfilled by SH2B1. One potential mechanism by which SH2B1 may be inhibiting EPO signaling is by recruiting negative regulators via its proline-rich regions or its EPO-stimulated phosphoserine sites and targeting them to JAK2. SH2B1 has been shown to be capable of heterodimerizing with SH2B2 (54). Therefore, another possible mechanism may involve heterodimerization with either SH2B2 or SH2B3 to form an inhibitory complex, which can then modulate the kinase activity of JAK2 downstream of the EPO-R.
In conclusion, our data suggest that SH2B1 binds constitutively to the EPO-R. Upon EPO stimulation, SH2B1 is phosphorylated, most likely on serine/threonines, releasing it from the plasma membrane and allowing SH2B1 to bind to EPO-R Tyr(P)-343 and Tyr(P)-401. This binding can cause conformational changes, which could lead to the recruitment of SH2B1 binding partners to EPO-R. We have also demonstrated that SH2B1 preferentially binds to the EPO-R and not JAK2. Similar to its other family members, SH2B1 is a negative regulator of EPO signaling. Assessment of the definitive role of SH2B1 in erythroid development will be best addressed through analysis of SH2B1 and compound SH2B family gene-targeted animals tested under conditions of erythroid stress.
Supplementary Material
Acknowledgments
We thank Jane McGlade and members of the Barber laboratory for assistance throughout this project.
This work was supported, in whole or in part, by National Institutes of Health Grant RO1-DK54222 (to C. C. S.). This work was also supported by Grant MOP-13612 (to D. L. B.) from Canadian Institutes of Health Research.

This article contains supplemental Figs. 1–3.
- EPO
- erythropoietin
- EPO-R
- EPO receptor
- SH2
- Src homology 2
- PH
- pleckstrin homology.
REFERENCES
- 1. Wu H., Liu X., Jaenisch R., Lodish H. F. (1995) Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell 83, 59–67 [DOI] [PubMed] [Google Scholar]
- 2. D'Andrea A. D., Lodish H. F., Wong G. G. (1989) Expression cloning of the murine erythropoietin receptor. Cell 57, 277–285 [DOI] [PubMed] [Google Scholar]
- 3. Livnah O., Stura E. A., Middleton S. A., Johnson D. L., Jolliffe L. K., Wilson I. A. (1999) Crystallographic evidence for preformed dimers of erythropoietin receptor before ligand activation. Science 283, 987–990 [DOI] [PubMed] [Google Scholar]
- 4. Richmond T. D., Chohan M., Barber D. L. (2005) Turning cells red. Signal transduction mediated by erythropoietin. Trends Cell Biol. 15, 146–155 [DOI] [PubMed] [Google Scholar]
- 5. Lin C. S., Lim S. K., D'Agati V., Costantini F. (1996) Differential effects of an erythropoietin receptor gene disruption on primitive and definitive erythropoiesis. Genes Dev. 10, 154–164 [DOI] [PubMed] [Google Scholar]
- 6. Kieran M. W., Perkins A. C., Orkin S. H., Zon L. I. (1996) Thrombopoietin rescues in vitro erythroid colony formation from mouse embryos lacking the erythropoietin receptor. Proc. Natl. Acad. Sci. U.S.A. 93, 9126–9131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Neubauer H., Cumano A., Müller M., Wu H., Huffstadt U., Pfeffer K. (1998) JAK2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell 93, 397–409 [DOI] [PubMed] [Google Scholar]
- 8. Parganas E., Wang D., Stravopodis D., Topham D. J., Marine J. C., Teglund S., Vanin E. F., Bodner S., Colamonici O. R., van Deursen J. M., Grosveld G., Ihle J. N. (1998) JAK2 is essential for signaling through a variety of cytokine receptors. Cell 93, 385–395 [DOI] [PubMed] [Google Scholar]
- 9. Socolovsky M., Dusanter-Fourt I., Lodish H. F. (1997) The prolactin receptor and severely truncated erythropoietin receptors support differentiation of erythroid progenitors. J. Biol. Chem. 272, 14009–14012 [DOI] [PubMed] [Google Scholar]
- 10. Miller C. P., Heilman D. W., Wojchowski D. M. (2002) Erythropoietin receptor-dependent erythroid colony-forming unit development. Capacities of Tyr-343 and phosphotyrosine-null receptor forms. Blood 99, 898–904 [DOI] [PubMed] [Google Scholar]
- 11. Zang H., Sato K., Nakajima H., McKay C., Ney P. A., Ihle J. N. (2001) The distal region and receptor tyrosines of the Epo receptor are nonessential for in vivo erythropoiesis. EMBO J. 20, 3156–3166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li K., Menon M. P., Karur V. G., Hegde S., Wojchowski D. M. (2003) Attenuated signaling by a phosphotyrosine-null Epo receptor form in primary erythroid progenitor cells. Blood 102, 3147–3153 [DOI] [PubMed] [Google Scholar]
- 13. Menon M. P., Fang J., Wojchowski D. M. (2006) Core erythropoietin receptor signals for late erythroblast development. Blood 107, 2662–2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Menon M. P., Karur V., Bogacheva O., Bogachev O., Cuetara B., Wojchowski D. M. (2006) Signals for stress erythropoiesis are integrated via an erythropoietin receptor-phosphotyrosine-343-Stat5 axis. J. Clin. Invest. 116, 683–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barber D. L., Beattie B. K., Mason J. M., Nguyen M. H., Yoakim M., Neel B. G., D'Andrea A. D., Frank D. A. (2001) A common epitope is shared by activated signal transducer and activator of transcription-5 (STAT5) and the phosphorylated erythropoietin receptor. Implications for the docking model of STAT activation. Blood 97, 2230–2237 [DOI] [PubMed] [Google Scholar]
- 16. Klingmüller U., Bergelson S., Hsiao J. G., Lodish H. F. (1996) Multiple tyrosine residues in the cytosolic domain of the erythropoietin receptor promote activation of STAT5. Proc. Natl. Acad. Sci. U.S.A. 93, 8324–8328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Damen J. E., Wakao H., Miyajima A., Krosl J., Humphries R. K., Cutler R. L., Krystal G. (1995) Tyrosine 343 in the erythropoietin receptor positively regulates erythropoietin-induced cell proliferation and Stat5 activation. EMBO J. 14, 5557–5568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Socolovsky M., Fallon A. E., Wang S., Brugnara C., Lodish H. F. (1999) Fetal anemia and apoptosis of red cell progenitors in Stat5a−/−5b−/− mice. A direct role for Stat5 in Bcl-XL. Cell 98, 181–191 [DOI] [PubMed] [Google Scholar]
- 19. Teglund S., McKay C., Schuetz E., van Deursen J. M., Stravopodis D., Wang D., Brown M., Bodner S., Grosveld G., Ihle J. N. (1998) Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell 93, 841–850 [DOI] [PubMed] [Google Scholar]
- 20. Socolovsky M., Nam H., Fleming M. D., Haase V. H., Brugnara C., Lodish H. F. (2001) Ineffective erythropoiesis in Stat5a−/−5b−/− mice due to decreased survival of early erythroblasts. Blood 98, 3261–3273 [DOI] [PubMed] [Google Scholar]
- 21. Yao Z., Cui Y., Watford W. T., Bream J. H., Yamaoka K., Hissong B. D., Li D., Durum S. K., Jiang Q., Bhandoola A., Hennighausen L., O'Shea J. J. (2006) Stat5a/b are essential for normal lymphoid development and differentiation. Proc. Natl. Acad. Sci. U.S.A. 103, 1000–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hoelbl A., Kovacic B., Kerenyi M. A., Simma O., Warsch W., Cui Y., Beug H., Hennighausen L., Moriggl R., Sexl V. (2006) Clarifying the role of Stat5 in lymphoid development and Abelson-induced transformation. Blood 107, 4898–4906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kerenyi M. A., Grebien F., Gehart H., Schifrer M., Artaker M., Kovacic B., Beug H., Moriggl R., Müllner E. W. (2008) Stat5 regulates cellular iron uptake of erythroid cells via IRP-2 and TfR-1. Blood 112, 3878–3888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maures T. J., Kurzer J. H., Carter-Su C. (2007) SH2B1 (SH2-B) and JAK2. A multifunctional adaptor protein and kinase made for each other. Trends Endocrinol. Metab. 18, 38–45 [DOI] [PubMed] [Google Scholar]
- 25. Wakioka T., Sasaki A., Mitsui K., Yokouchi M., Inoue A., Komiya S., Yoshimura A. (1999) APS, an adaptor protein containing Pleckstrin homology (PH) and Src homology-2 (SH2) domains, inhibits the JAK-STAT pathway in collaboration with c-Cbl. Leukemia 13, 760–767 [DOI] [PubMed] [Google Scholar]
- 26. Tong W., Zhang J., Lodish H. F. (2005) Lnk inhibits erythropoiesis and Epo-dependent JAK2 activation and downstream signaling pathways. Blood 105, 4604–4612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rui L., Mathews L. S., Hotta K., Gustafson T. A., Carter-Su C. (1997) Identification of SH2-Bβ as a substrate of the tyrosine kinase JAK2 involved in growth hormone signaling. Mol. Cell. Biol. 17, 6633–6644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Duan C., Li M., Rui L. (2004) SH2-B promotes insulin receptor substrate 1 (IRS1)- and IRS2-mediated activation of the phosphatidylinositol 3-kinase pathway in response to leptin. J. Biol. Chem. 279, 43684–43691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mason J. M., Beattie B. K., Liu Q., Dumont D. J., Barber D. L. (2000) The SH2 inositol 5-phosphatase Ship1 is recruited in an SH2-dependent manner to the erythropoietin receptor. J. Biol. Chem. 275, 4398–4406 [DOI] [PubMed] [Google Scholar]
- 30. Miller B. A., Barber D. L., Bell L. L., Beattie B. K., Zhang M. Y., Neel B. G., Yoakim M., Rothblum L. I., Cheung J. Y. (1999) Identification of the erythropoietin receptor domain required for calcium channel activation. J. Biol. Chem. 274, 20465–20472 [DOI] [PubMed] [Google Scholar]
- 31. Barber D. L., DeMartino J. C., Showers M. O., D'Andrea A. D. (1994) A dominant negative erythropoietin (EPO) receptor inhibits EPO-dependent growth and blocks F-gp55-dependent transformation. Mol. Cell. Biol. 14, 2257–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rui L., Gunter D. R., Herrington J., Carter-Su C. (2000) Differential binding to and regulation of JAK2 by the SH2 domain and N-terminal region of SH2-Bβ. Mol. Cell. Biol. 20, 3168–3177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Walters D. K., Stoffregen E. P., Heinrich M. C., Deininger M. W., Druker B. J. (2005) RNAi-induced down-regulation of FLT3 expression in AML cell lines increases sensitivity to MLN518. Blood 105, 2952–2954 [DOI] [PubMed] [Google Scholar]
- 34. Halupa A., Chohan M., Stickle N. H., Beattie B. K., Miller B. A., Barber D. L. (2005) Erythropoietin receptor Y479 couples to ERK1/2 activation via recruitment of phospholipase Cγ. Exp. Cell Res. 309, 1–11 [DOI] [PubMed] [Google Scholar]
- 35. Liu S. K., McGlade C. J. (1998) Gads is a novel SH2 and SH3 domain-containing adaptor protein that binds to tyrosine-phosphorylated Shc. Oncogene 17, 3073–3082 [DOI] [PubMed] [Google Scholar]
- 36. Pirozzi G., McConnell S. J., Uveges A. J., Carter J. M., Sparks A. B., Kay B. K., Fowlkes D. M. (1997) Identification of novel human WW domain-containing proteins by cloning of ligand targets. J. Biol. Chem. 272, 14611–14616 [DOI] [PubMed] [Google Scholar]
- 37. Songyang Z., Shoelson S. E., Chaudhuri M., Gish G., Pawson T., Haser W. G., King F., Roberts T., Ratnofsky S., Lechleider R. J., et al. (1993) SH2 domains recognize specific phosphopeptide sequences. Cell 72, 767–778 [DOI] [PubMed] [Google Scholar]
- 38. Songyang Z., Shoelson S. E., McGlade J., Olivier P., Pawson T., Bustelo X. R., Barbacid M., Sabe H., Hanafusa H., Yi T., et al. (1994) Specific motifs recognized by the SH2 domains of Csk, 3BP2, fps/fes, GRB-2, HCP, SHC, Syk, and Vav. Mol. Cell. Biol. 14, 2777–2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kurzer J. H., Argetsinger L. S., Zhou Y. J., Kouadio J. L., O'Shea J. J., Carter-Su C. (2004) Tyrosine 813 is a site of JAK2 autophosphorylation critical for activation of JAK2 by SH2-Bβ. Mol. Cell. Biol. 24, 4557–4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li Z., Zhou Y., Carter-Su C., Myers M. G., Jr., Rui L. (2007) SH2B1 enhances leptin signaling by both Janus kinase 2 Tyr-813 phosphorylation-dependent and -independent mechanisms. Mol. Endocrinol. 21, 2270–2281 [DOI] [PubMed] [Google Scholar]
- 41. Miura O., Nakamura N., Quelle F. W., Witthuhn B. A., Ihle J. N., Aoki N. (1994) Erythropoietin induces association of the JAK2 protein tyrosine kinase with the erythropoietin receptor in vivo. Blood 84, 1501–1507 [PubMed] [Google Scholar]
- 42. Huang L. J., Constantinescu S. N., Lodish H. F. (2001) The N-terminal domain of Janus kinase 2 is required for Golgi processing and cell surface expression of erythropoietin receptor. Mol. Cell 8, 1327–1338 [DOI] [PubMed] [Google Scholar]
- 43. Rui L., Herrington J., Carter-Su C. (1999) SH2-B, a membrane-associated adapter, is phosphorylated on multiple serines/threonines in response to nerve growth factor by kinases within the MEK/ERK cascade. J. Biol. Chem. 274, 26485–26492 [DOI] [PubMed] [Google Scholar]
- 44. Rui L., Carter-Su C. (1998) Platelet-derived growth factor (PDGF) stimulates the association of SH2-Bβ with PDGF receptor and phosphorylation of SH2-Bβ. J. Biol. Chem. 273, 21239–21245 [DOI] [PubMed] [Google Scholar]
- 45. Halupa A., Bailey M. L., Huang K., Iscove N. N., Levy D. E., Barber D. L. (2005) A novel role for STAT1 in regulating murine erythropoiesis. Deletion of STAT1 results in overall reduction of erythroid progenitors and alters their distribution. Blood 105, 552–561 [DOI] [PubMed] [Google Scholar]
- 46. Zhu B. M., McLaughlin S. K., Na R., Liu J., Cui Y., Martin C., Kimura A., Robinson G. W., Andrews N. C., Hennighausen L. (2008) Hematopoietic-specific Stat5-null mice display microcytic hypochromic anemia associated with reduced transferrin receptor gene expression. Blood 112, 2071–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kurzer J. H., Saharinen P., Silvennoinen O., Carter-Su C. (2006) Binding of SH2-B family members within a potential negative regulatory region maintains JAK2 in an active state. Mol. Cell. Biol. 26, 6381–6394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Maures T. J., Su H. W., Argetsinger L. S., Grinstein S., Carter-Su C. (2011) Phosphorylation controls a dual-function polybasic nuclear localization sequence in the adapter protein SH2B1β to regulate its cellular function and distribution. J. Cell Sci. 124, 1542–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Haq R., Halupa A., Beattie B. K., Mason J. M., Zanke B. W., Barber D. L. (2002) Regulation of erythropoietin-induced STAT serine phosphorylation by distinct mitogen-activated protein kinases. J. Biol. Chem. 277, 17359–17366 [DOI] [PubMed] [Google Scholar]
- 50. von Lindern M., Parren-van Amelsvoort M., van Dijk T., Deiner E., van den Akker E., van Emst-de Vries S., Willems P., Beug H., Löwenberg B. (2000) Protein kinase Cα controls erythropoietin receptor signaling. J. Biol. Chem. 275, 34719–34727 [DOI] [PubMed] [Google Scholar]
- 51. Rui L., Carter-Su C. (1999) Identification of SH2-Bβ as a potent cytoplasmic activator of the tyrosine kinase Janus kinase 2. Proc. Natl. Acad. Sci. U.S.A. 96, 7172–7177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang M., Deng Y., Tandon R., Bai C., Riedel H. (2008) Essential role of PSM/SH2-B variants in insulin receptor catalytic activation and the resulting cellular responses. J. Cell. Biochem. 103, 162–181 [DOI] [PubMed] [Google Scholar]
- 53. Qian X., Ginty D. D. (2001) SH2-B and APS are multimeric adapters that augment TrkA signaling. Mol. Cell. Biol. 21, 1613–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nishi M., Werner E. D., Oh B. C., Frantz J. D., Dhe-Paganon S., Hansen L., Lee J., Shoelson S. E. (2005) Kinase activation through dimerization by human SH2-B. Mol. Cell. Biol. 25, 2607–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.