Abstract
Paternally inherited inactivating mutations of the GNAS gene have been associated with a rare and disabling genetic disorder, progressive osseous heteroplasia, in which heterotopic ossification occurs within extraskeletal soft tissues, such as skin, subcutaneous fat, and skeletal muscle. This ectopic bone formation is hypothesized to be caused by dysregulated mesenchymal progenitor cell differentiation that affects a bipotential osteogenic-adipogenic lineage cell fate switch. Interestingly, patients with paternally inherited inactivating mutations of GNAS are uniformly lean. Using a mouse model of Gsα-specific exon 1 disruption, we examined whether heterozygous inactivation of Gnas affects adipogenic differentiation of mesenchymal precursor cells from subcutaneous adipose tissues (fat pad). We found that paternally inherited Gsα inactivation (Gsα+/p−) impairs adipogenic differentiation of adipose-derived stromal cells (ASCs). The Gsα+/p− mutation in ASCs also decreased expression of the adipogenic factors CCAAT-enhancer-binding protein (C/EBP)β, C/EBPα, peroxisome proliferator-activated receptor gamma, and adipocyte protein 2. Impaired adipocyte differentiation was rescued by an adenylyl cyclase activator, forskolin, and provided evidence that Gsα-cAMP signals are necessary in early stages of this process. Supporting a role for Gnas in adipogenesis in vivo, fat tissue weight and expression of adipogenic genes from multiple types of adipose tissues from Gsα+/p− mice were significantly decreased. Interestingly, the inhibition of adipogenesis by paternally inherited Gsα mutation also enhances expression of the osteogenic factors, msh homeobox 2, runt-related transcription factor 2, and osteocalcin. These data support the hypothesis that Gsα plays a critical role in regulating the balance between fat and bone determination in soft tissues, a finding that has important implications for a wide variety of disorders of osteogenesis and adipogenesis.
Keywords: GNAS, Progressive osseous heteroplasia, Heterotopic ossification, Adipogenesis, Differentiation, Stem cells
Introduction
Progressive osseous heteroplasia (POH) is a rare disorder of bone formation characterized by heterotopic ossification (HO) that forms in skin and subcutaneous tissues with subsequent progression into deep connective tissues, such as skeletal muscles, tendons, and ligaments [1, 2]. Considerable evidence supports the existence of bipotential progenitor cells that can give rise to osteoblasts and adipocytes [3–9]. In POH patients, intramembranous bone formation is frequently observed to initiate within the subcutaneous fat tissue suggesting a close, perhaps reciprocal, relationship between adipogenesis and osteogenesis in peripheral tissues that is mediated by a common connective tissue progenitor cell [10, 11].
Heterozygous inactivating mutations in the GNAS gene have been identified as a cause of POH [11]. The GNAS locus encodes multiple mRNAs [2, 12–14] with distinct first exons that splice into a set of common downstream exons (exon 2–13). Transcripts include those for Gsα, a subunit of the heterotrimeric G-protein that couples heptahelical receptors for many hormones and neurotransmitters to adenylyl cyclase activation and cAMP production. Heterotrimeric G-proteins, composed of α, β, and γ subunits, couple extracellular signals from specific cell surface receptors to intracellular effectors [12, 15]. G-proteins bind guanine nucleotides and are defined by the α-subunit of the complex. In addition to Gsα, GNAS encodes XLαs, which functions similarly as Gsα [16], and the chromogranin-like protein, NESP55 [17] as well as noncoding transcripts. Importantly, the GNAS locus is imprinted, resulting in differential RNA expression patterns that are determined by the parent from whom a GNAS allele is inherited [12–14].
Experimental evidence supports that GNAS mutations in POH occur on the paternally inherited GNAS allele [11]. Our previous investigation of human disorders of GNAS-associated HO noted that individuals with paternally inherited inactivating mutations of GNAS were uniformly lean [18], suggesting that fat stores may be regulated by specific parental allele expression of GNAS in humans [18, 19]. Such a parent-of-origin effect is reflected by the distinctly different clinical disorders that are caused by maternally inherited inactivating mutations of GNAS (e.g., pseudohypoparathyroidism 1A) [18, 20] that are associated with obesity. These observations further support that adipocyte differentiation and function might be regulated by allele-specific expression of GNAS.
Adipose tissues contain multipotential progenitor cells that can differentiate into adipocytes and osteoblasts under appropriate conditions [21, 22], and the distribution of HO in POH suggests that progenitor cells in subcutaneous, dermal, and intramuscular fat differentiate preferentially along an osteogenic lineage in response to inactivating mutations of the paternally inherited GNAS allele [23]. Previously, we have demonstrated that mesenchymal progenitor cells from adipose tissue with GNAS paternal allele inactivation show enhanced osteogenesis [23]. In this study, we examine whether paternally inherited inactivation of GNAS impairs adipogenesis and the formation of fat tissue in vivo.
Materials and Methods
Patients/Human Subjects
The charts of 42 individuals who presented to the University of Pennsylvania Orthopaedic Surgery Outpatient Clinic for evaluation of nontraumatic HO of the skin and subcutaneous tissues who met diagnostic criteria for POH [18] were reviewed for documented birth weight and confirmed GNAS mutations. The study was approved by the Institutional Review Board of the University of Pennsylvania.
Animals
Male mice carrying a heterozygous deletion in exon 1 of Gnas (Gsα+/p−; [24, 25]) were bred to CD1 female mice to maintain the deletion mutation on the paternally inherited allele. All studies were performed in 3-month-old male mice. (No data support that a paternally inherited mutation is reflected as different phenotypes in male and female progeny; however, a single gender was used to minimize any potential variability.) Animal experiments were approved by the Institutional Animal Care and Use Committee, University of Pennsylvania.
Isolation and Culture of Adipose Soft Tissue Stromal Cells
Adipose-derived stromal cells (ASCs) from fat pads that overlie the pelvis and proximal femurs (subcutaneous adipose tissue; Supporting Information Fig. S2) from wild-type (WT) and Gsα+/p− mice were isolated as previously described [23]. Briefly, fat pads were excised, washed with 1× phosphate buffered saline (PBS) and minced into small pieces. Minced fat tissue was digested with type II collagenase (Sigma, St. Louis, MO, www.sigmaaldrich.com) for 1 hour with shaking in 37°C. After digestion, Dulbecco’s modified Eagle’s medium (DMEM)/F12 (with 10% fetal calf serum (FCS) and antibiotics) was added to neutralize the enzymatic activity of type II collagenase. The cell suspension was filtered through a 100-μM cell strainer (BD Biosciences, Franklin Lakes, NJ, www.bdbiosciences.com), recovered by centrifugation at 300g for 10 minutes, and then plated in growth medium containing DMEM/F12, 15%–20% FCS, and antibiotics during the first 2 days. After expansion, the cells were maintained in DMEM/F12 containing 10% FCS and antibiotics. ASCs from abdominal white adipose tissue (WAT) and interscapular brown adipose tissue (BAT) were isolated (Supporting Information Fig. S2) and processed as above, except the time for collagenase digestion was reduced to 20–30 minutes. Adherent cell strains were established from individual animals; cell strains from each single mouse were used at passage 3 or lower for all experiments and analyzed in triplicate; data in each experiment were from at least three individual mouse cell strains. The ability of ASCs to differentiate along adipogenic and osteogenic lineages was confirmed.
Adipogenic Differentiation In Vitro
For adipogenic differentiation, ASCs from fad pads, WAT, or BAT were plated at a density of 20,000 per square centimeter and allowed to attach overnight in growth media. The cells from fat pads and WAT were grown to confluence and then induced to adipocyte differentiation with medium containing DMEM/F12 growth media supplemented with 10 μg/ml insulin, 10 ng/ml 3′,3′,5′ triiodo-L-thyronine (T3), 1 μM dexamethasone (all from Sigma), and 0.2 μM (fat pads) or 0.5 μM (WAT) rosiglitazone (Cayman Chemical, Ann Arbor, MI, www.caymanchem.com). Cells were harvested at indicated time points for total RNA isolation to quantify the expression of lipid marker genes and for lipid detection. ASCs from interscapular BAT were pretreated with 100 ng/ml rhBMP7 (R&D Systems, Minneapolis, MN, www.rndsystems.com) for 3–4 days upon confluence, then induced to adipocyte differentiation in DMEM/F12 media containing 10% FCS supplemented with 10 μg/ml insulin, 10 ng/ml T3, 1 μM dexamethasone, and 0.2 μM rosiglitazone for an additional 7 days. To activate adenylyl cyclase, ASCs from fat pads were treated with forskolin (10 ng/ml in dimethyl sulfoxide (DMSO)) as indicated in Figure 3.
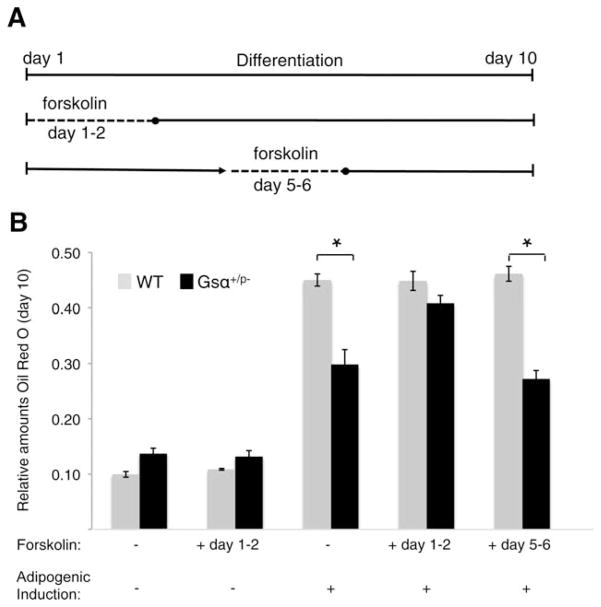
Figure 3.
Adenylyl cyclase activation rescues the adipogenic impairment of Gsα+/p− adipose stromal cells (ASCs) during an early stage of adipogenesis. (A): Schematic of treatment with forskolin, an activator of adenylyl cyclase. (B): On day 10 of adipogenic treatment, ASCs were fixed, stained with oil red O, and quantified at OD500. Di-methyl sulfoxide was added to cells without forskolin treatment as a solvent control. *, p < .05. Two independent experiments used cells from three WT and three mutant mice (analyzed individually in triplicate) for each experiment. Abbreviation: WT, wild type.
RNA Isolation and Relative Quantitative Reverse-Transcriptase Polymerase Chain Reaction
Quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) analysis used standard methods. Total RNA was extracted from cells at specific time points using the TRIzol Reagent (Invitrogen, Grand Island, NY, http://www.invitrogen.com) and MiniRNeasy (Qiagen, Valencia, CA, http://www1.qiagen.com), according to the manufacture’s instructions. Complementary DNA synthesis was carried out on 2–5 μg of total RNA per sample using the Superscript III RT kit (Invitrogen) following the manufacture’s instructions. Transcripts were amplified and their levels were quantified using gene-specific primers (primer pairs listed in Supporting Information Table S1) and Fast SYBR Green Master Mix (Applied Biosystems, Carlsbad, CA, www.appliedbiosystems.com) on the ABI 7500 Fast Real Time PCR System (Applied Biosystems). At least three biological replicates were performed for each transcript and measurements were made in triplicate per each sample; no-template samples served as negative controls. Gene expression was normalized to TATA box binding protein, β-actin, and/or β2-microglobulin as an internal standard, and the average of WT day 0 without induction was set to 1 (n = 3 per group).
Oil Red O Staining
Following adipogenic induction, cells were fixed in 10% formalin for 1 hour and stained with 60% oil red O (Sigma) for 10 minutes. For quantification, oil red O was solubilized in 100% isopropanol and the optical absorbance measured at 500 nm using a Bio-Rad microplate reader. Results were normalized to total protein content (BCA protein assay; Pierce, Rockford, IL, www.piercenet.com).
Anthropomorphic and Gross Tissue Measurements
Upon sacrifice, 3-month-old mice (that had been maintained on a standard chow diet; NIH-07, 5% fat by weight) were weighed, and their length measured as the distance from nose to the base of the tail. Body mass index (BMI) was used to evaluate adiposity [26] and calculated according to the formula BMI = mass (g)/(length [cm])2. WAT was dissected from the intra-abdominal region, BAT from the interscapular region, and fat pads from the subcutaneous areas that overlie the proximal femurs. The excised tissues were weighed immediately upon dissection.
Histology and Histomorphometry
Fat pads, BAT, and WAT were frozen at −40°C in tissue freezing medium (Triangle Biomedical Sciences, Durham, NC, www.trianglebiomedical.com). Frozen tissue sections were cut at the following thicknesses: 6 μm (brown fat), 8 μm (fat pads), and 12 μm (white fat). Sections were stained with hematoxylin and eosin by standard techniques. ImageJ software (Rasband, W.S., U.S. National Institutes of Health, Bethesda, MD, http://rsb.info.nih.gov/ij/) was used to analyze the mean adipocyte size (AS) and percent stroma. Briefly, images of stained sections were converted into tricolor images using Adobe Photoshop, with stroma, nuclei, and lipid (approximated as total cell contents with nuclei excluded) represented as different colors. After normalizing ImageJ and microscopy unit scales, areas representing each of the three morphologic regions were quantified by ImageJ within randomly selected regions of interest (ROIs) and the number of cells within each ROI was counted. Average AS (Avg. AS) within ROIs was calculated as: Avg. AS = (Area of cell contents [lipid] + nuclear area)/total number of adipocytes. Stroma area (StrA) was calculated as: StrA = ROI area − area occupied by adipocytes. The percent stroma (% Str) was calculated as: % Str = (Area occupied by stroma/total ROI area) × 100.
Statistical Analysis
The t test (Student’s t test, two-sided, and paired) was used to determine whether the mean value for relative transcript expression in cells differed significantly between groups with and without adipogenic differentiation, between WT and mutant mice upon adipogenic differentiation and between groups with or without forskolin treatment. The t test was also used to determine whether weight, length, BMI, and weights of gross adipose tissues significantly differed between WT and Gsα+/p− animals and to determine whether the average AS or percent stroma from ROIs in brown fat, white fat, and fat pads were significantly different between WT and Gsα+/p− animals. Significance was set to *, p < .05; **, p < .01; and ***, p < .001. All statistical calculations were performed using Microsoft Excel. Unless otherwise indicated, data are shown as mean ± SE of the mean (n = 3 per group).
Results
POH Patients Have Decreased Adiposity and Low Birth Weights
Paternally inherited inactivating GNAS mutations have been identified in patients with POH [11, 27]. We reviewed the available birth weights of 13 patients with POH and found that three of four males and seven of nine females had measurements at or below the fifth percentile compared to sex-matched normative data (Supporting Information Fig. S1). All patients had POH by clinical diagnostic criteria, were lean at the time of initial presentation, and all but one had confirmed inactivating GNAS mutations.
Paternally Inherited Gsα Inactivating Mutation Impairs Adipogenic Differentiation In Vitro
The low birth weights in patients with POH prompted us to investigate the effects on adipogenesis of reduced Gsα expression that results from paternal allele inactivation. During normal adipose development, adipocytes arise from precursor cells in the vascular stroma of fat tissue [28]. Subcutaneous adipose tissue is a frequent location of HO initiation in POH patients; therefore, we isolated murine ASCs from subcutaneous fat pad depots (Supporting Information Fig. S2A) of heterozygous knockout mice with a paternally inherited inactivating mutation of the Gsα transcript-specific exon 1 in Gnas (Gsα+/p−) [23, 29].
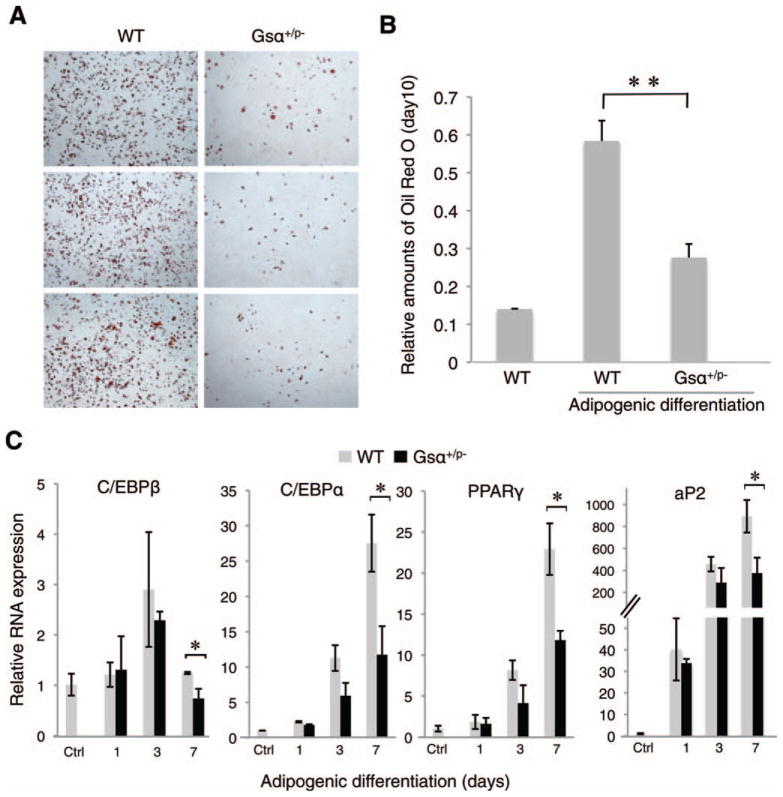
Under conditions of adipogenic induction, ASC cells derived from subcutaneous fat pads of WT littermates efficiently differentiated into adipocytes (Fig. 1A, 1B) as indicated by lipid detection with oil red O staining to quantify neural triglycerides. Gsα+/p− ASCs had significantly less lipid accumulation compared to WT ASCs (Fig. 1A, 1B), indicating impaired adipocyte differentiation. In our previous studies [23], we did not detect any difference in the proliferation rates between Gsα+/p− and WT ASCs by BrdU pulse labeling, excluding that impaired adipogenesis in Gsα+/p− cells results from reduced numbers of precursor cells.
Figure 1.
Paternally inherited Gsα mutation impairs adipogenesis in vitro. (A, B): Adipose stromal cells (ASCs) from subcutaneous fat pads from Gsα+/p− or WT mice were cultured under adipogenic conditions for 7 days then stained with oil red O and quantified at OD500. **, p < .01. (C): Quantitative reverse-transcriptase polymerase chain reaction analysis of adipogenic markers in ASCs from fad pads on days 1, 3, and 7 after adipogenic induction. *, p < .05. Four independent experiments used cells from three WT and three mutant mice (analyzed individually, in triplicate) for each experiment. Abbreviations: aP2, adipocyte protein 2; C/EBP, CCAAT-enhancer-binding protein; PPAR, peroxisome proliferator-activated receptor; WT, wild type.
Adipogenesis is regulated through multiple transcription factors, including peroxisome proliferator-activated receptor gamma (PPARγ) and CCAAT-enhancer-binding proteins (C/EBPs) [30]. We examined the expression of C/EBPβ, C/EBPα, and PPARγ as well as adipocyte protein 2 (aP2, also called fatty acid binding protein 4), a marker for mature adipocytes, in WT and Gsα+/p− ASCs by qRT-PCR. All of these transcripts were induced upon adipocyte differentiation of WT and Gsα+/p− ASCs; however, the mutant cells showed a lower level of upregulated expression (Fig. 1C) supporting that inactivation of the paternally inherited Gsα allele impairs adipogenic differentiation.
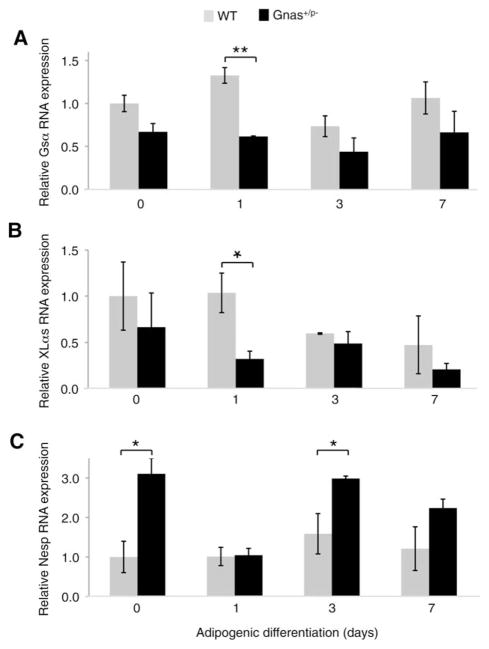
Gnas Transcripts are Differentially Expressed During Adipocyte Differentiation and in Response to Paternally Inherited Gsα Mutation
In both mouse and humans, Gnas/GNAS is a complex gene locus that encodes several transcripts including the biallelically expressed Gsα, paternal allele-specific XLαs, and maternal allele-specific Nesp (known as NESP55 in human). We quantified the levels of three transcripts (Gsα, XLαs, and Nesp) during adipogenesis of mouse ASCs and found that during initial stages of differentiation, Gsα and XLαs transcripts were reduced in Gsα+/p− ASCs compared to WT ASCs (Fig. 2A, 2B). By contrast, the Nesp transcript was detected at a statistically significant higher level in Gsα+/p− mutant ASCs prior to adipogenic differentiation (Fig. 2C). These data support that in addition to decreased expression of Gsα in cells from a Gsα+/p− exon 1-specific knockout, inactivation of Gsα in this model also influences the expression of other transcripts within the Gnas locus—reducing the paternal allele-specific XLαs transcript early after differentiation and increasing the maternal allele-specific Nesp transcript at baseline.
Figure 2.
Expression of Gnas transcripts during adipocyte differentiation. Quantitative reverse-transcriptase polymerase chain reaction analysis of Gnas transcripts Gsα (A), XLαs (B), and Nesp (C) in fat pad-derived adipose stromal cells from Gsα+/p− and WT mice on days 1, 3, and 7 after induction of adipogenesis. **, p < .01 and *, p < .05. Three independent experiments used cells from three WT and three mutant mice (analyzed individually, in triplicate) for each experiment. Abbreviations: Gsα, guanine nucleotide-binding protein G(s) subunit alpha; Nesp, neuroendocrine secretory protein of mol. wt. 55,000; WT, wild-type; XLαs, guanine nucleotide-binding protein G(s) subunit alpha extralarge isoform.
Adenylyl Cyclase Activation at Early Stages of Differentiation Rescues the Impaired Adipogenesis of Gsα Inactivating Mutation
Activation of heterotrimeric G-proteins promotes the binding of GTP to their α subunits (Gsα), and subsequently activate downstream intracellular effectors, including second messenger enzyme (adenylyl cyclase), protein kinases (PKA and PKC), and ion channels [12, 19]. Adipogenesis has previously been shown to be regulated by Gsα and cAMP signaling [31–34]. To investigate whether the impaired adipogenesis by Gsα+/p− ASCs is dependent on decreased cAMP signaling, cells were treated with an adenylyl cyclase activator, forskolin. Subcutaneous fat pad ASCs were induced with adipogenic media and treated with forskolin at an early differentiation stage (days 1 and 2 postinduction) or at a later stage (days 5 and 6 postinduction) and then assessed by oil red O staining on day 10 (Fig. 3). Forskolin treatment during days 5 and 6 had little effect on Gsα+/p− impaired adipogenesis; however, treatment during days 1 and 2 restored the adipogenic deficiency in Gsα+/p− ASCs (Fig. 3). Although we cannot exclude the possibility that another activation pathway, in addition to or as an alternative to cAMP, is involved in this process, these data support that Gsα enhances adipogenesis through generation of cAMP during early stages of adipogenic differentiation. Forskolin cannot induce adipocyte differentiation in the absence of adipogenic inducers, indicating that other factors are required in addition to cAMP. Our data support that early factors coordinate adipogenic differentiation and that Gsα-cAMP signals are necessary for this differentiation process.
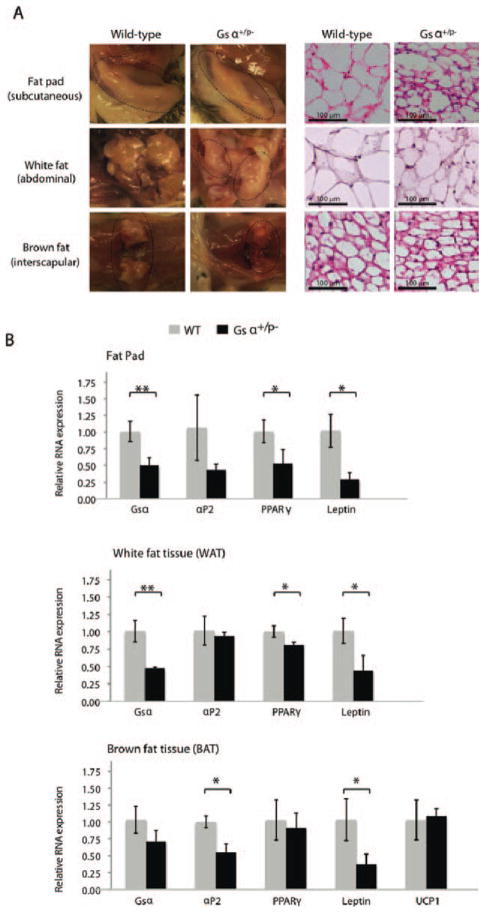
Inactivating Gsα Mutation Reduces Abundance of Adipose Tissues In Vivo
To examine the effects of Gsα inactivation on adipogenesis in vivo, we examined three sources of adipose tissues: subcutaneous fat pad, intra-abdominal WAT (visceral WAT), and interscapular BAT (Supporting Information Fig. S2A). We found all three sources of fat tissues reduced in size in Gsα+/p− mice compared to control littermates (Fig. 4A; Supporting Information Fig. S2B). Adipose tissue weights from the subcutaneous fat pads, intra-abdominal WAT, and interscapular BAT were reduced in Gsα+/p− mice by 50.0%, 45.0%, and 59.8%, respectively (Table 1). This amount of fat in Gsα+/p− mice is disproportionately lower compared to measured decreases in total body weight and body length that were reduced by 19.7% and 6.4%, respectively, in mutant mice compared to control littermates (Table 1). This is reflected by calculated BMI (weight/length2) that was not statistically significantly different between Gsα+/p− and control littermates, suggesting that the reduced fat content in Gsα+/p− mice is not simply a reflection of proportionally reduced body mass. Although the weights of the fat tissues were reduced in Gsα+/p− mice, histomorphologic examination of the fat tissues showed statistically significant increases in total adipocyte number per unit area (Table 2) and a smaller AS in Gsα+/p− mice (Table 2 and Fig. 4A).
Figure 4.
Inactivating Gsα mutation reduces adipogenic tissues in vivo. (A): Adipose tissues from subcutaneous fat pads, abdominal white fat, and interscapular brown fat from Gsα+/p− (n = 3) and WT (n = 3) mice (left panels) were examined histologically with hematoxylin and eosin staining (right panels). (Supporting Information Fig. 2). (B): Expression of adipose markers was quantified using reverse-transcriptase polymerase chain reaction. *, p < .05; **, p < .01. Three independent experiments used cells from three WT and three mutant mice (analyzed individually in triplicate) for each experiment. Abbreviations: aP2, adipocyte protein 2; PPAR, peroxisome proliferator-activated receptor; UCP1, uncoupling protein 1; WT, wild type.
Table 1.
Anthromorphometric and gross adipose tissue measurements
| Genotype | Total mouse weight (g) | Length (cm) | BMI (g/cm2) | Fat pads (g) | WAT (g) | BAT (g) |
|---|---|---|---|---|---|---|
| WT (n = 27) | 36.91 ± 1.98 | 9.55 ± 0.24 | 0.41 ± 0.02 | 0.88 ± 0.24 | 0.87 ± 0.22 | 0.20 ± 0.03 |
| KO (n = 16) | 29.66 ± 1.25*** | 8.94 ± 0.24*** | 0.37 ± 0.03 (N.S.) | 0.44 ± 0.08*** | 0.35 ± 0.08*** | 0.11 ± 0.03*** |
All values are average ± SD.
p < .001, two-sided t test Gsα+/p− (KO) versus WT mice.
Abbreviations: BAT, brown adipose tissue; BMI, body mass index; KO, knockout; N.S., not significant; WAT, white adipose tissue; WT, wild type.
Table 2.
Histomorphometric analysis of adipose tissues
| N (no. cells counted) | Mean cell size (μm2) | % stroma | Adipocyte number per 10,000 μm2 | |
|---|---|---|---|---|
| FP | ||||
| WT | 254 | 1704 ± 425 | 28 ± 3.8 | 4 ± 1.1 |
| KO | 544 | 642 ± 87.9*** | 36 ± 3.3** | 10 ± 1.8*** |
| WAT | ||||
| WT | 212 | 2853 ± 512 | 31 ± 3.6 | 2.4 ± 0.53 |
| KO | 635 | 1883 ± 332*** | 31 ± 5.8 | 3.7 ± 0.78*** |
| BAT | ||||
| WT | 784 | 319 ± 53 | 44 ± 7.2 | 17 ± 2.8 |
| KO | 435 | 259 ± 25.8* | 45 ± 5.4 | 21 ± 3.3* |
All values are average ± SD.
p < .05;
p < .01;
p < .001; two-sided t test Gsα+/p− (KO) versus WT mice.
Abbreviations: BAT, brown adipose tissue; FP, fat pads; KO, knockout; WAT, white adipose tissue; WT, wild type.
The expression of adipose marker genes in fat tissues was examined by qRT-PCR analysis. Consistent with in vitro data showing decreased levels of adipogenic markers in Gsα+/p− ASCs isolated from fat pads (Fig. 1), subcutaneous fat pad adipose tissue from Gsα+/p− mice showed decreased PPARγ and leptin (a WAT marker) (Fig. 4B). Expression of PPARγ and leptin was also reduced in abdominal WAT tissue from Gsα+/p− mice (Fig. 4B). Interscapular BAT tissue showed decreased leptin and aP2; however, uncoupling protein 1, a BAT-specific marker, was unchanged in the brown fat from Gsα+/p− mice (Fig. 4B). Thus, the paternally inherited Gsα mutation suppressed the expression of WAT-associated transcripts in vivo. Similarly to ASCs from subcutaneous fat pads, Gsα+/p− ASCs derived from abdominal WAT or from interscapular BAT showed reductions in expression of markers of adipogenesis compared to WT ASCs (data not shown), indicating that Gsα+/p− inactivating mutation impairs adipogenesis in multiple types of fat tissues.
Paternally Inherited Gsα Mutation Potentiates Osteogenesis Over Adipogenesis
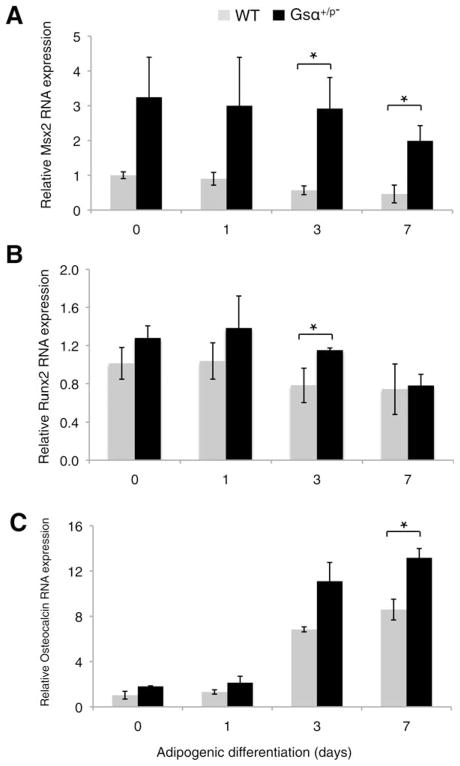
The formation of heterotopic intramembranous ossification frequently initiates within subcutaneous fat in patients with POH [1, 2], suggesting that adipocyte precursor cells could aberrantly differentiate along an osteogenic lineage. We examined the expression of osteogenic markers in ASCs of subcutaneous fat pads from Gsα+/p− mice under conditions of adipogenic differentiation. Msh homeobox 2, an early osteogenic marker, runt-related transcription factor 2, an osteogenic transcription factor, and osteocalcin, a marker of terminal osteogenic differentiation were all expressed at higher levels in Gsα+/p− ASCs during adipogenic induction compared to WT ASCs (Fig. 5). Neither mutant nor WT cells showed positive Alizarin red staining after 14 days (data not shown) indicating that these adipogenic conditions cannot support mineralization even in the presence of the Gsα+/p− mutation. These data suggest that inhibition of adipogenesis by paternally inherited Gsα mutation enhances osteogenic potential by directly or indirectly promoting osteoblastogenesis, even in the presence of adipogenic inducting factors.
Figure 5.
Paternally inherited Gsα mutation potentiates osteogenesis during adipogenic induction. Expression of the osteogenic markers, Msx2 (A), Runx2 (B), and Osteocalcin (C) were detected by quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) in adipose stromal cells from fat pads of Gsα+/p− and WT mice grown under adipogenic differentiation conditions. Samples were analyzed by qRT-PCR on days 0, 1, 3, and 7. *, p < .05. Three independent experiments used cells from three WT and three mutant mice (analyzed individually in triplicate) for each experiment. Abbreviations: Msx2, msh homeobox 2; Runx2, runt-related transcription factor 2; WT, wild type.
Discussion
In a Gnas knockout mouse model of Gsα-specific exon 1 disruption, we found that paternally inherited Gsα inactivating mutation (Gsα+/p−) impairs adipogenic differentiation of ASCs from subcutaneous fat tissue by decreasing the expression of adipogenic transcription factors C/EBPβ, C/EBPα, and PPARγ. Rescue of impaired adipocyte differentiation by an adenylyl cyclase activator indicates that Gsα-cAMP signals are necessary in the early stages of this process. In vivo, we observed a significant decrease in fat tissues and in expression of adipogenic genes from multiple adipose sources in Gsα+/p− mice, suggesting that Gsα may be an obesity risk factor. Importantly, inhibition of adipogenesis by a paternally inherited Gsα mutation also potentiates osteogenesis by enhancing expression of osteogenic factors. Thus, Gsα plays a critical role in regulating lineage determination in soft tissue progenitor cells between adipogenic and osteogenic fates.
We previously identified paternally inherited inactivating mutations in the human GNAS gene as a cause of POH [11]. The main product of the GNAS gene is the α-subunit of the stimulatory G-protein (Gsα). However, multiple RNAs are transcribed from different promoters at the GNAS locus and the GNAS gene locus is imprinted, showing differential expression patterns of these transcripts from maternal versus paternal alleles [12–14]. Our previous studies of GNAS-based human disorders of HO demonstrated that POH patients with paternally inherited inactivating GNAS mutations were never obese [18], suggesting that leanness may be related to parental allele expression of GNAS.
Parent-of-origin metabolic effects of Gnas mutations have been examined in mouse Gnas knockout models [24, 35–37]. Mice with mutations of Gnas exon 2 (E2), an exon common to all protein-coding Gnas transcripts including Gsα and XLαs, showed reciprocal changes in energy metabolism depending on the presence of mutations in either the paternal (E2+/p−) or maternal (E2m−/+) allele [36, 37]. E2+/p− mice had a severely lean phenotype with strikingly increased glucose tolerance, insulin sensitivity, and sympathetic nervous system activity. In contrast, E2m−/+ mice developed obesity with increased lipid accumulation in BAT and WAT and associated with increased serum leptin level but lowered energy expenditure.
Similarly to the phenotype of mice, we observed global losses in fat from multiple adipose tissues in Gsα+/p− mice with the accumulated fat from each source of adipose tissue in Gsα+/p− mice markedly reduced compared to that derived from WT mice. Gsα+/p− mice had a greater number of adipocytes per unit area, although these adipocytes were smaller in size. The defect in adipogenic differentiation by Gsα+/p− ASCs is thus reflected in both the decreased amount of fat tissue and in the inability of adipocytes to accumulate lipid.
Other studies in Gsα-specific knockout mice in which Gsα exon 1 was deleted (E1−) have confirmed that adiposity is related to Gsα mutation from the paternal allele [24, 35]. Consistent with our findings, Germain-Lee et al. reported that Gsα+/p− mice had a lean phenotype compared to Gsαm−/+ mice [24] and observed normal hormonal responsiveness in Gsα+/p− mice, consistent with our clinical findings in POH patients [18]. Interestingly, Chen et al., using a different exon 1 deletion, described their E1+/p− mice as mildly obese and insulin resistant [18, 35]. Compared to the E1+/p− mice model, the more limited exon 1 disruption in Gsα+/p− mice used in our and the Germain-Lee studies recapitulates the leanness observed in POH patients and implicates Gnas imprinting as a requirement for metabolic consequences of paternally inherited Gsα exon 1 mutations.
Of interest, we observed that subcutaneous fat pad ASCs from Gsα+/p− mice not only have decreased Gsα mRNA expression, as expected, but also show altered expression of other Gnas transcripts, an unexpected finding since the deletion only includes the Gsα-specific exon 1. Mice with paternal allele deletion of XLαs (Gnasxl+/p−) have a phenotype similar to E2+/p− mice, with a lean phenotype and reduced lipid accumulation in adipose tissues [38, 39]. During osteogenesis [23] and during adipogenesis (this study), ASCs from Gsα+/p− mice expressed reduced levels of XLαs and Gsα mRNAs relative to WT ASCs undergoing differentiation, suggesting that the altered regulation of adipogenic and osteogenic differentiation in these mice could be a combined effect of both of these G-protein subunit isoforms.
The role of cAMP signaling in adipogenesis has been well established to directly induce the expression of CEBPα and PPARγ, central transcriptional regulators of adipogenesis [30–34]. Consistently, we found that induction of adipogenesis requires activation of adenylyl cyclase and that deficits in adipogenic differentiation in ASCs from Gsα+/p− mice can be rescued by an adenylyl cyclase activator.
The effect of cAMP activation/inhibition in osteogenesis has also been examined, with most studies reporting a requirement for increased cAMP during osteogenesis [40–46]. However, these studies were conducted in preosteoblastic cells that were already committed to the osteoblast lineage. By contrast, our previous studies as well as other reports [23, 47–50] indicate that low cAMP levels and low GNAS/Gnas expression may be required to commit a progenitor cell to the osteogenic lineage.
Recently, Gsα+/p− mice have been reported to form subcutaneous heterotopic bone [23, 29]. Although the specific cell targets involved in HO and leanness in POH are unknown, investigation of bipotential osteoblast/adipocyte progenitors (such as ASCs) with inactivating Gnas mutations has direct implications for understanding this reciprocal phenotype in POH. We report here that ASCs from murine subcutaneous fat pads (adipose tissue intimately associated with cutaneous tissue and underlying fascia) show enhanced osteogenic differentiation, even in the presence of adipogenic induction factors, and impaired adipogenic differentiation when derived from Gsα+/p− mice, suggesting that they are relevant target cells that contribute to ectopic osteogenesis in POH patients. Our data show that cells with a paternally inherited Gnas mutation express osteogenic markers even in the presence of adipogenic factors, perhaps reflecting the tendency of mutant progenitor cells in POH patients to undergo ectopic osteogenesis. The levels of osteogenic marker expression was comparable to levels induced by ASCs under osteogenic conditions [23]; however, the cells did not mineralize under our adipogenic culture conditions. This suggests that the Gsα+/p− mutation may promote but not be sufficient to induce osteogenesis and may be reflected in the mosaic distribution of areas of HO in POH.
Extraskeletal bone formation in POH patients often arises within subcutaneous fat, suggesting a close, perhaps reciprocal, relationship between bone and fat cell differentiation. Taken together with existing reports, our data support the hypothesis that heterozygous inactivation of the GNAS gene alters cellular signaling in ASCs leading not only to ectopic osteogenesis but also to dramatically impaired adipogenesis. This study indicates that GNAS-regulated signaling directs bipotent osteoblast and adipocyte differentiation, demonstrates the complex phenotypic consequences of inactivating GNAS mutations that are regulated by maternal/paternal inheritance of GNAS, and suggests that lineage switching between osteogenic and adipogenic fates in fat tissue may be a therapeutic target in POH.
Summary
Paternally inherited heterozygous inactivation of Gnas impairs adipogenic differentiation of mesenchymal progenitor cells from subcutaneous adipose tissues by early dysregulation of Gsα-cAMP signaling. Deficits in adipogenic differentiation result in a significant reduction of fat depots from multiple adipose tissues in Gsα+/p− mice. Gsα appears to be an important determinant of the balance between fat and bone determination in soft tissues.
Acknowledgments
We acknowledge Michael Levine and Emily Germain-Lee for the generous gift of Gnas+/− mice. We also thank the members of our research laboratory, especially Meiqi Xu, Shengliang Zhang, Robert Caron, and Josef Kaplan for their thoughtful comments, assistance, and suggestions during the course of this work. This study was supported by the National Institutes of Health (NIH R01-AR046831 and ARRA R01-AR046831-S1), the Progressive Osseous Heteroplasia Association (POHA), the Italian POH Association, the International Fibrodysplasia Ossificans Progressiva Association (IFOPA), the University of Pennsylvania Center for Research in FOP and Related Disorders, the Penn Center for Musculoskeletal Disorders (NIH P30-AR050950), and the Isaac and Rose Nassau Professorship of Orthopaedic Molecular Medicine (F.S.K.).
Footnotes
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
Author contributions: J.-J.L.: experimental design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, and final approval of manuscript; E.R.: collection and/or assembly of data, data analysis and interpretation, manuscript writing, and final approval of manuscript; D.Z.: provision of study materials and final approval of manuscript; F.S.K.: data analysis and interpretation, manuscript writing, and final approval of manuscript; R.J.P.: conception and design, financial support, collection and/or assembly of data, data analysis and interpretation, manuscript writing, and final approval of manuscript; E.M.S.: conception and design, financial support, data analysis and interpretation, manuscript writing, and final approval of manuscript. R.J.P. and E.M.S. contributed equally to this article.
References
- 1.Kaplan FS, Shore EM. Progressive osseous heteroplasia. J Bone Miner Res. 2000;15:2084–2094. doi: 10.1359/jbmr.2000.15.11.2084. [DOI] [PubMed] [Google Scholar]
- 2.Shore EM, Kaplan FS. Inherited human diseases of heterotopic bone formation. Nat Rev Rheumatol. 2010;6:518–527. doi: 10.1038/nrrheum.2010.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahdjoudj S, Lasmoles F, Oyajobi BO, et al. Reciprocal control of osteoblast/chondroblast and osteoblast/adipocyte differentiation of multipotential clonal human marrow stromal F/STRO-1(+) cells. J Cell Biochem. 2001;81:23–38. doi: 10.1002/1097-4644(20010401)81:1<23::aid-jcb1021>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 4.Davis LA, Zur Nieden NI. Mesodermal fate decisions of a stem cell: The Wnt switch. Cell Mol Life Sci. 2008;65:2658–2674. doi: 10.1007/s00018-008-8042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gori F, Thomas T, Hicok KC, et al. Differentiation of human marrow stromal precursor cells: Bone morphogenetic protein-2 increases OSF2/CBFA1, enhances osteoblast commitment, and inhibits late adipocyte maturation. J Bone Miner Res. 1999;14:1522–1535. doi: 10.1359/jbmr.1999.14.9.1522. [DOI] [PubMed] [Google Scholar]
- 6.Nuttall ME, Gimble JM. Is there a therapeutic opportunity to either prevent or treat osteopenic disorders by inhibiting marrow adipogenesis? Bone. 2000;27:177–184. doi: 10.1016/s8756-3282(00)00317-3. [DOI] [PubMed] [Google Scholar]
- 7.Nuttall ME, Patton AJ, Olivera DL, et al. Human trabecular bone cells are able to express both osteoblastic and adipocytic phenotype: Implications for osteopenic disorders. J Bone Miner Res. 1998;13:371–382. doi: 10.1359/jbmr.1998.13.3.371. [DOI] [PubMed] [Google Scholar]
- 8.Sabatakos G, Sims NA, Chen J, et al. Overexpression of DeltaFosB transcription factor(s) increases bone formation and inhibits adipogenesis. Nat Med. 2000;6:985–990. doi: 10.1038/79683. [DOI] [PubMed] [Google Scholar]
- 9.Spinella-Jaegle S, Rawadi G, Kawai S, et al. Sonic hedgehog increases the commitment of pluripotent mesenchymal cells into the osteoblastic lineage and abolishes adipocytic differentiation. J Cell Sci. 2001;114:2085–2094. doi: 10.1242/jcs.114.11.2085. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan FS, Craver R, MacEwen GD, et al. Progressive osseous heteroplasia: A distinct developmental disorder of heterotopic ossification. Two new case reports and follow-up of three previously reported cases. J Bone Joint Surg Am. 1994;76:425–436. [PubMed] [Google Scholar]
- 11.Shore EM, Ahn J, de Beur SJ, et al. Paternally inherited inactivating mutations of the GNAS1 gene in progressive osseous heteroplasia. N Eng J Med. 2002;346:99–106. doi: 10.1056/NEJMoa011262. [DOI] [PubMed] [Google Scholar]
- 12.Plagge A, Kelsey G, Germain-Lee EL. Physiological functions of the imprinted Gnas locus and its protein variants Galpha(s) and XLalpha(s) in human and mouse. J Endocrinol. 2008;196:193–214. doi: 10.1677/JOE-07-0544. [DOI] [PubMed] [Google Scholar]
- 13.Weinstein LS, Chen M, Xie T, et al. Genetic diseases associated with heterotrimeric G proteins. Trends Pharmacol Sci. 2006;27:260–266. doi: 10.1016/j.tips.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Weinstein LS, Xie T, Zhang Q-H, et al. Studies of the regulation and function of the Gs alpha gene Gnas using gene targeting technology. Pharmacol Ther. 2007;115:271–291. doi: 10.1016/j.pharmthera.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinstein LS, Xie T, Qasem A, et al. The role of GNAS and other imprinted genes in the development of obesity. Int J Obesity. 2010;34:6–17. doi: 10.1038/ijo.2009.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bastepe M, Gunes Y, Perez-Villamil B, et al. Receptor-mediated adenylyl cyclase activation through XLalpha(s), the extra-large variant of the stimulatory G protein alpha-subunit. Mol Endocrinol. 2002;16:1912–1919. doi: 10.1210/me.2002-0054. [DOI] [PubMed] [Google Scholar]
- 17.Ischia R, Lovisetti-Scamihorn P, Hogue-Angeletti R, et al. Molecular cloning and characterization of NESP55, a novel chromogranin-like precursor of a peptide with 5-HT1B receptor antagonist activity. J Biol Chem. 1997;272:11657–11662. doi: 10.1074/jbc.272.17.11657. [DOI] [PubMed] [Google Scholar]
- 18.Adegbite NS, Xu M, Kaplan FS, et al. Diagnostic and mutational spectrum of progressive osseous heteroplasia (POH) and other forms of GNAS-based heterotopic ossification. Am J Med Genet A. 2008;146:1788–1796. doi: 10.1002/ajmg.a.32346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinstein LS, Yu S, Warner DR, et al. Endocrine manifestations of stimulatory G protein alpha-subunit mutations and the role of genomic imprinting. Endocr Rev. 2001;22:675–705. doi: 10.1210/edrv.22.5.0439. [DOI] [PubMed] [Google Scholar]
- 20.Bastepe M, Juppner H. GNAS locus and pseudohypoparathyroidism. Horm Res. 2005;63:65–74. doi: 10.1159/000083895. [DOI] [PubMed] [Google Scholar]
- 21.Pignolo RJ, Kassem M. Circulating osteogenic cells: Implications for injury, repair, and regeneration. J Bone Miner Res. 2011;26:1685–1693. doi: 10.1002/jbmr.370. [DOI] [PubMed] [Google Scholar]
- 22.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pignolo RJ, Xu MQ, Russell E, et al. Heterozygous inactivation of Gnas in adipose-derived mesenchymal progenitor cells enhances osteoblast differentiation and promotes heterotopic ossification. J Bone Miner Res. 2011;26:2647–2655. doi: 10.1002/jbmr.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Germain-Lee EL, Schwindinger W, Crane JL, et al. A mouse model of albright hereditary osteodystrophy generated by targeted disruption of exon 1 of the Gnas gene. Endocrinology. 2005;146:4697–4709. doi: 10.1210/en.2005-0681. [DOI] [PubMed] [Google Scholar]
- 25.Schwindinger WF, Reese KJ, Lawler AM, et al. Targeted disruption of Gnas in embryonic stem cells. Endocrinology. 1997;138:4058–4063. doi: 10.1210/endo.138.10.5439. [DOI] [PubMed] [Google Scholar]
- 26.Ishikawa A, Tanahashi T, Kodama H. A proximal genomic region of mouse chromosome 10 contains quantitative trait loci affecting fatness. Anim Sci J. 2011;82:209–214. doi: 10.1111/j.1740-0929.2010.00842.x. [DOI] [PubMed] [Google Scholar]
- 27.Lebrun M, Richard N, Abeguile G, et al. Progressive osseous heteroplasia: A model for the imprinting effects of GNAS inactivating mutations in humans. J Clin Endocrinol Metab. 2010;95:3028–3038. doi: 10.1210/jc.2009-1451. [DOI] [PubMed] [Google Scholar]
- 28.Yu ZK, Wright JT, Hausman GJ. Preadipocyte recruitment in stromal vascular cultures after depletion of committed preadipocytes by immunocytotoxicity. Obes Res. 1997;5:9–15. doi: 10.1002/j.1550-8528.1997.tb00277.x. [DOI] [PubMed] [Google Scholar]
- 29.Huso DL, Edie S, Levine MA, et al. Heterotopic ossifications in a mouse model of albright hereditary osteodystrophy. PLoS One. 2011;6:e21755. doi: 10.1371/journal.pone.0021755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HB, Kim WH, Han KL, et al. cAMP-response element binding protein (CREB) positively regulates mouse adiponectin gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2010;391:634–639. doi: 10.1016/j.bbrc.2009.11.111. [DOI] [PubMed] [Google Scholar]
- 32.Tang QQ, Jiang MS, Lane MD. Repressive effect of Sp1 on the C/EBP alpha gene promoter: Role in adipocyte differentiation. Mol Cell Biol. 1999;19:4855–4865. doi: 10.1128/mcb.19.7.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang HY, Watkins DC, Malbon CC. Antisense oligonucleotices to G (s) protein alpha-subunit sequence accelerate differentiation of fibroblasts to adipocytes. Nature. 1992;358:334–337. doi: 10.1038/358334a0. [DOI] [PubMed] [Google Scholar]
- 34.Zhang L, Paddon C, Lewis MD, et al. Gs alpha signalling suppresses PPAR gamma 2 generation and inhibits 3T3L1 adipogenesis. J Endocrinol. 2009;202:207–215. doi: 10.1677/JOE-09-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen M, Gavrilova O, Liu J, et al. Alternative Gnas gene products have opposite effects on glucose and lipid metabolism. Proc Natl Acad Sci USA. 2005;102:7386–7391. doi: 10.1073/pnas.0408268102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu S, Castle A, Chen M, et al. Increased insulin sensitivity in Gsalpha knockout mice. J Biol Chem. 2001;276:19994–19998. doi: 10.1074/jbc.M010313200. [DOI] [PubMed] [Google Scholar]
- 37.Yu S, Gavrilova O, Chen H, et al. Paternal versus maternal transmission of a stimulatory G-protein alpha subunit knockout produces opposite effects on energy metabolism. J Clin Invest. 2000;105:615–623. doi: 10.1172/JCI8437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plagge A, Gordon E, Dean W, et al. The imprinted signaling protein XL alpha s is required for postnatal adaptation to feeding. Nat Genet. 2004;36:818–826. doi: 10.1038/ng1397. [DOI] [PubMed] [Google Scholar]
- 39.Xie T, Plagge A, Gavrilova O, et al. The alternative stimulatory G protein alpha-subunit XLalphas is a critical regulator of energy and glucose metabolism and sympathetic nerve activity in adult mice. J Biol Chem. 2006;281:18989–18999. doi: 10.1074/jbc.M511752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clark CA, Li TF, Kim KO, et al. Prostaglandin E2 inhibits BMP signaling and delays chondrocyte maturation. J Orthop Res. 2009;27:785–792. doi: 10.1002/jor.20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakao Y, Koike T, Ohta Y, et al. Parathyroid hormone enhances bone morphogenetic protein activity by increasing intracellular 3′, 5′-cyclic adenosine monophosphate accumulation in osteoblastic MC3T3-E1 cells. Bone. 2009;44:872–877. doi: 10.1016/j.bone.2009.01.370. [DOI] [PubMed] [Google Scholar]
- 42.Ohta Y, Nakagawa K, Imai Y, et al. Cyclic AMP enhances smad-mediated BMP signaling through PKA-CREB pathway. J Bone Min Metab. 2008;26:478–484. doi: 10.1007/s00774-008-0850-8. [DOI] [PubMed] [Google Scholar]
- 43.Siddappa R, Martens A, Doorn J, et al. cAMP/PKA pathway activation in human mesenchymal stem cells in vitro results in robust bone formation in vivo. Proc Natl Acad Sci USA. 2008;105:7281–7286. doi: 10.1073/pnas.0711190105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teplyuk NM, Galindo M, Teplyuk VI, et al. Runx2 regulates G protein-coupled signaling pathways to control growth of osteoblast progenitors. J Biol Chem. 2008;283:27585–27597. doi: 10.1074/jbc.M802453200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsutsumimoto T, Wakabayashi S, Kinoshita T, et al. A phosphodiesterase inhibitor, pentoxifylline, enhances the bone morphogenetic protein-4 (BMP-4)-dependent differentiation of osteoprogenitor cells. Bone. 2002;31:396–401. doi: 10.1016/s8756-3282(02)00839-6. [DOI] [PubMed] [Google Scholar]
- 46.Zhao L, Yang S, Zhou GQ, et al. Downregulation of cAMP-dependent protein kinase inhibitor gamma is required for BMP-2-induced osteoblastic differentiation. Int J Biochem Cell Biol. 2006;38:2064–2073. doi: 10.1016/j.biocel.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 47.Hong SHH, Lu XH, Nanes MS, et al. Regulation of osterix (Osx, Sp7) and the Osx promoter by parathyroid hormone in osteoblasts. J Mol Endocrinol. 2009;43:197–207. doi: 10.1677/JME-09-0012. [DOI] [PubMed] [Google Scholar]
- 48.Yang D-C, Tsay H-J, Lin S-Y, et al. cAMP/PKA regulates osteogenesis, adipogenesis and ratio of RANKL/OPG mRNA expression in mesenchymal stem cells by suppressing leptin. PLoS One. 2008;3:e1540. doi: 10.1371/journal.pone.0001540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang S, Xu M, Kaplan FS, et al. G protein-cAMP pathway regulates early stage embryonic stem cell-derived osteoblast differentiation. J Bone Miner Res. 2009;24:S115. [Google Scholar]
- 50.Zhao Y, Ding S. A high-throughput siRNA library screen identifies osteogenic suppressors in human mesenchymal stem cells. Proc Natl Acad Sci USA. 2007;104:9673–9678. doi: 10.1073/pnas.0703407104. [DOI] [PMC free article] [PubMed] [Google Scholar]