Abstract
An efficient BiVO4 thin film electrode for overall water splitting was prepared by dipping an F-doped SnO2 (FTO) substrate electrode in an aqueous nitric acid solution of Bi(NO3)3 and NH4VO3, and subsequently calcining it. X-ray diffraction of the BiVO4 thin film revealed that a photocatalytically active phase of scheelite-monoclinic BiVO4 was obtained. Scanning electron microscopy images showed that the surface of an FTO substrate was uniformly coated with the BiVO4 film with 300–400 nm of the thickness. The BiVO4 thin film electrode gave an excellent anodic photocurrent with 73% of an IPCE at 420 nm at 1.0 V vs. Ag/AgCl. Modification with CoO on the BiVO4 electrode improved the photoelectrochemical property. A photoelectrochemical cell consisting of the BiVO4 thin film electrode with and without CoO, and a Pt counter electrode was constructed for water splitting under visible light irradiation and simulated sunlight irradiation. Photocurrent due to water splitting to form H2 and O2 was confirmed with applying an external bias smaller than 1.23 V that is a theoretical voltage for electrolysis of water. Water splitting without applying external bias under visible light irradiation was demonstrated using a SrTiO3∶Rh photocathode and the BiVO4 photoanode.
Keywords: hydrogen production, solar energy conversion, visible light response
The development of powdered photocatalysts and semiconductor photoelectrodes for water splitting have been studied extensively in view of utilization of solar energy, since the report of the Honda-Fujishima effect (1). There are advantageous and disadvantageous points for water splitting using the powdered photocatalysts and photoelectrochemical cells. Although a powdered photocatalyst system is simple, H2 and O2 are produced as a mixture. In contrast to it, photoelectrochemical cells give H2 separately from O2 gas. Moreover, even if powdered photocatalysts do not possess band potentials suitable for water splitting (the bottom of the conduction band < 0 V and the top of the valence band > 1.23 V vs. NHE at pH 0), water splitting may be achieved by application of these powdered photocatalysts to photoelectrodes with applying some external bias. However, the electrical conductivity of semiconductor electrode is indispensable, resulting in that the number of the photoelectrode materials is limited.
It has been reported that many metal oxides are active photocatalysts for water splitting into H2 and O2 stoichiometrically under UV light irradiation (2). Photoelectrodes, such as TiO2 (1, 3, 4), SrTiO3 (5, 6), BaTiO3 (7), and KTaO3 (8) have been reported for photoelectrochemical water splitting under UV light irradiation. Development of a photoelectrode material with visible light response has been sought for efficient utilization of solar energy. It has been reported that Fe2O3 (9–11), WO3 (12–14), BiVO4 (15–24), and SrTiO3∶Rh (25) of metal oxide electrodes respond to visible light. Recently, some (oxy) nitride materials such as TaON (26, 27), Ta3N5 (27, 28), SrNbO2N (29), and Ta0.9Co0.1Nx (30) have also been found to be visible light responsive photoelectrodes for water splitting.
BiVO4 has three crystal systems: Scheelite structure with monoclinic (s-m) and tetragonal (s-t) phases, and zircon structure with tetragonal (z-t) phase (31–34). Among them, the scheelite monoclinic BiVO4 shows the highest photocatalytic activity for O2 evolution from an aqueous AgNO3 solution under visible light irradiation (19, 34–36). However, BiVO4-powdered photocatalyst is inactive for overall water splitting into H2 and O2, because the bottom of conduction band is more positive than the reduction potential of H2O to form H2. BiVO4, with an n-type semiconductor character, shows an excellent photoelectrochemical property (15). The photoelectrochemical cell consisting of BiVO4 can split water under visible light irradiation (16, 19). There are mainly two methods for the preparation of BiVO4 electrodes: BiVO4 powder-loaded electrodes and BiVO4-coated electrodes using metal organic precursors. The BiVO4 photoelectrode prepared by the metal-organic decomposition method gives 20% of IPCE at 420 nm with applying 0.6 V vs. Ag/AgCl for water splitting (15). It also works with 1.6 V of an externally applied bias vs. a counter electrode (C.E.) under visible light irradiation (16). A BiVO4 photocatalyst electrode that is readily prepared by pasting BiVO4 nanoparticle prepared in an acetic acid onto an FTO substrate can split water with an externally applied bias smaller than 1.23 V vs. a Pt counter electrode under simulated sunlight irradiation (19). It is important to develop a simple and large-scale producible preparation method for the photoelectrode.
Surface modification is a useful method to improve photoelectrochemical properties. Modification with Co3O4 by precipitation method increases the photocurrent of BiVO4 electrode (18). Recently, cobalt phosphate (CoPi) prepared by an electro-deposition has been reported as an oxygen evolving electrocatalyst (37). Photoelectrochemical properties of Si (37, 38), Fe2O3 (39–41), WO3 (42), BiVO4 (43, 44), and BiVO4 doped with Mo and W (45, 46) electrodes are improved with the surface modification with the CoPi cocatalyst. Modification with FeOOH is also effective (47).
In the present study, we developed a facile fabrication of an efficient BiVO4 thin film coated on FTO electrode using inorganic salts of bismuth and vanadium. The formation mechanism of the present BiVO4 thin film electrode was discussed. Photoelectrochemical property of the obtained BiVO4 thin film electrode with and without surface modification with cobalt oxide was examined. Water splitting was also demonstrated using photoelectrochemical cells consisting of a Pt counter electrode or a SrTiO3∶Rh photocathode under visible light and simulated sunlight irradiation.
Results and Discussion
Preparation of BiVO4 Thin Film Electrode.
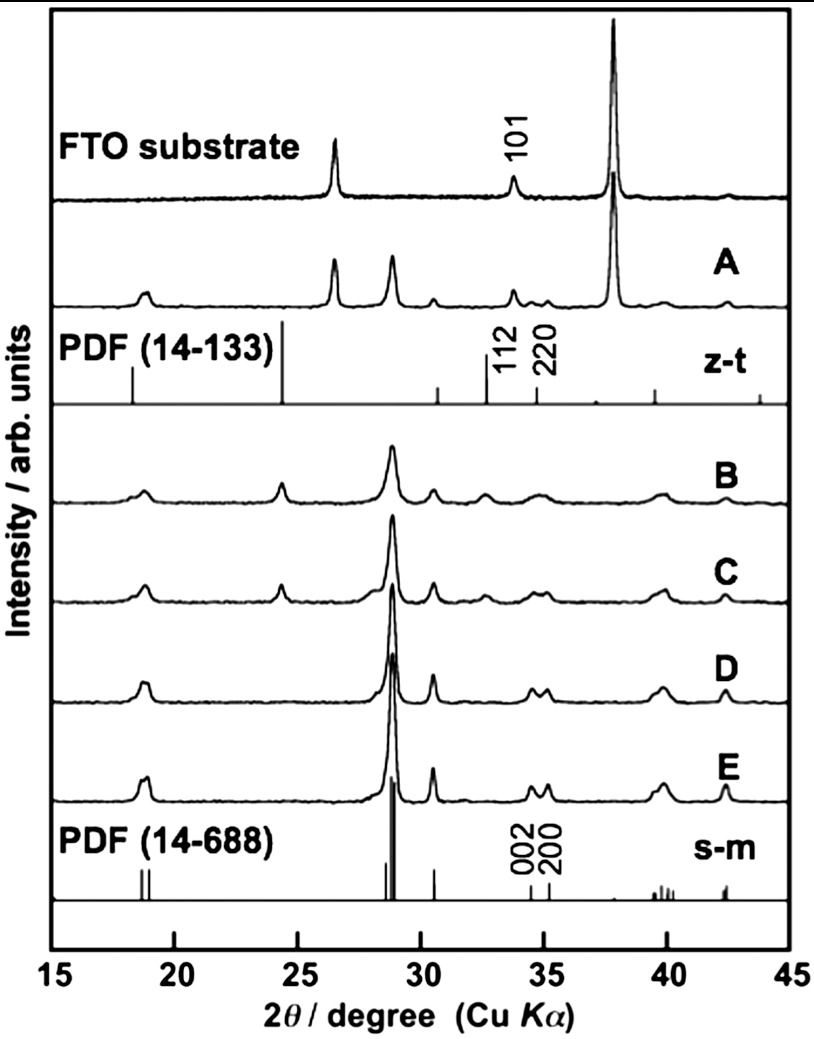
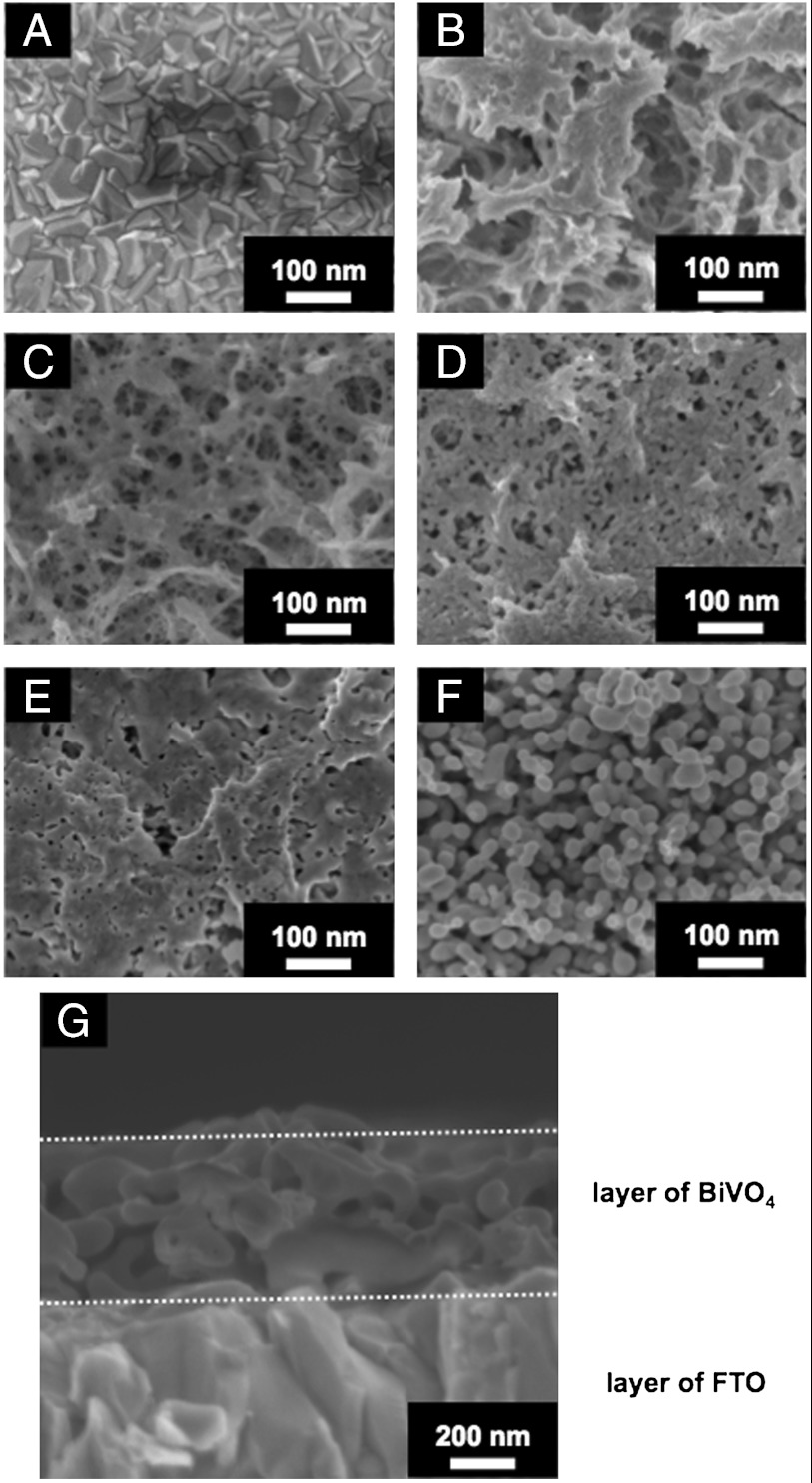
Fig. 1 shows XRD patterns of the obtained BiVO4 thin film electrodes prepared under different condition. The diffraction pattern of BiVO4 (s-m) was observed for the sample calcined at 673 K for 2 h after drying an FTO substrate treated with an aqueous nitric acid precursor solution dissolving Bi(NO3)3 and NH4VO3, in addition to the XRD pattern of SnO2 derived from the FTO substrate (Fig. 1A). Thus, BiVO4 thin film with scheelite structure with monoclinic phase (s-m) that showed high photocatalytic activity was obtained by this simple preparation method. The crystal structure of BiVO4 thin films prepared at various calcination temperatures was examined (Fig. 1 B–E). A soda-lime glass was used as a substrate for these XRD measurements, because the (002) diffraction peak of BiVO4 was overlapped with the (101) diffraction peak of FTO. BiVO4 (s-m) was obtained by calcination at 573 and 623 K as a major component, in addition to a minor product of zircon structure tetragonal phase BiVO4 (z-t). A single phase of BiVO4 (s-m) was obtained by calcination at 673 and 723 K. Fig. 2 shows SEM images of surface and cross-section of BiVO4 thin films prepared at various calcination temperatures. An FTO substrate without the BiVO4 thin film was treated with 2 mmol L-1 of an aqueous nitric acid solution and subsequently calcined at 673 K for 2 h as a reference sample (Fig. 2A). It was observed that an FTO substrate was dense aggregates of crystal with bumpy surface. The morphologies of the BiVO4 thin films changed with calcination temperature. The thin film without calcination was sponge-like and orange. Calcination at 573 K gave network structure for the thin film. The thin film began to sinter by calcination at 623 K and became dense and porous by calcination at 673 K. The cross-sectional image revealed that the thickness of the BiVO4 porous film was several hundred nanometers. This morphology was quite different from that of powder-loaded electrodes (19). These network and porous structures were considered to be formed by volatilization of water and HNO3, and decomposition of some by-products, during the calcination as mentioned later. The thin film considerably sintered to form necked nano-particles by calcination at 723 K. Thus, this simple preparation method gave a porous BiVO4 (s-m) thin film well attached on the FTO substrate. UV-vis. transmission spectra of BiVO4 thin films showed that the thickness was controllable by changing the concentration (20–300 mmol L-1) of Bi(NO3)3 and NH4VO3 dissolving in an aqueous nitric acid precursor solution as shown in Fig. S1. The wavy spectra observed in the wavelength range of 500–800 nm was due to light interference.
Fig. 1.
X-ray diffraction patterns of BiVO4 thin films formed on (A) FTO and (B–E) soda-lime glass substrates. Preparation conditions, calcined at (B) 573 K, (C) 623 K, (A, D) 673 K, and (E) 723 K for 2 h.
Fig. 2.
Scanning electron microscope images of (A) FTO substrate, (B–F) surface and (G) cross-section of BiVO4 thin film electrodes. Preparation conditions, (B) dried and calcined at (C) 573 K, (D) 623 K, (E, G) 673 K, and (F) 723 K for 2 h.
Mechanism of Formation of BiVO4 Thin Film.
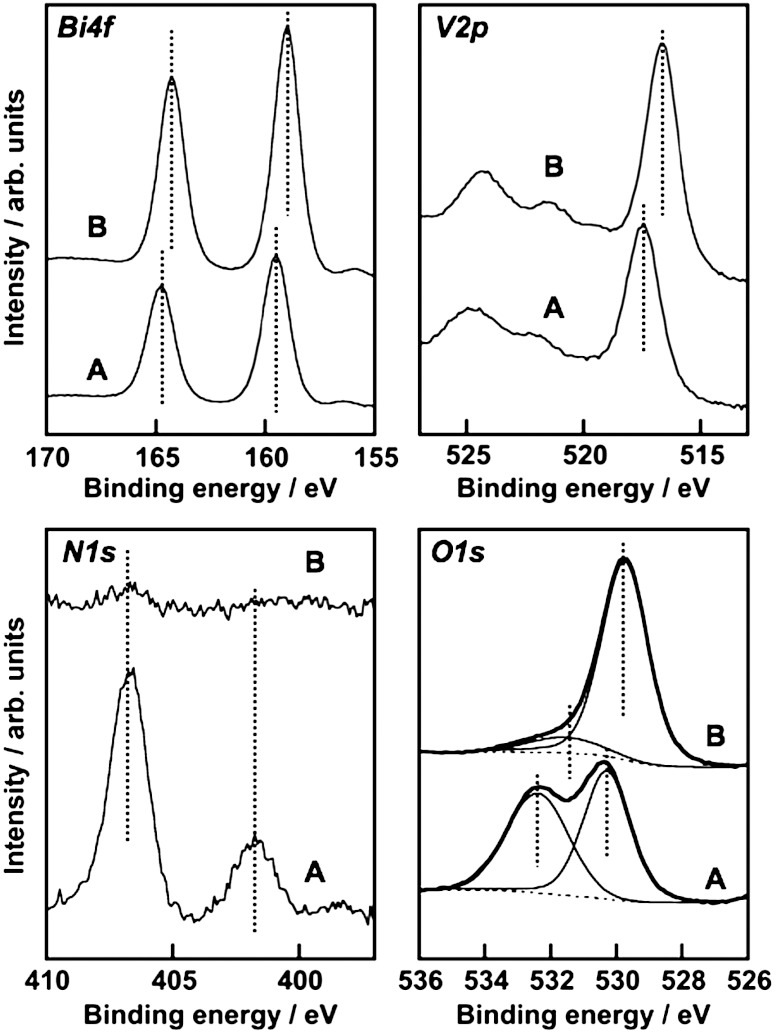
The formation process of BiVO4 thin films from an aqueous nitric acid precursor solution containing Bi(NO3)3 and NH4VO3 was examined with XPS and TG. The precursor solution covered on an FTO substrate became an orange film after drying, and a bright yellow film after calcination. The chemical state of the orange film was examined by XPS and TG-DTA. XPS spectra of each element of the orange film were different from those of the BiVO4 thin film as shown in Fig. 3. The binding energies of Bi4f7/2 (159.0 eV), V2p3/2 (516.7 eV), and O1s (529.7 eV) of the BiVO4 thin film calcined at 673 K were coincident with literature’s data of BiVO4 (s-m) (48, 49). The deconvoluted O1s peak at 531.6 eV was assigned to surface hydroxyl groups (50, 51). 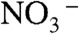 was almost decomposed by the calcination. Binding energies of Bi4f (159.5 eV) and V2p (517.5 eV) of the orange film without calcination were larger than those of the calcined BiVO4 thin film, whereas they were close to those of (BiO)2Cr2O7 at 159.8 eV (52) and NaVO3 at 517.0 eV (53), respectively. It suggested that Bi and V in the orange film took the chemical states similar to [BiO]+ and [VO3]- species, respectively. The XPS spectra of N1s at 401.8 eV and 406.7 eV were assigned to
was almost decomposed by the calcination. Binding energies of Bi4f (159.5 eV) and V2p (517.5 eV) of the orange film without calcination were larger than those of the calcined BiVO4 thin film, whereas they were close to those of (BiO)2Cr2O7 at 159.8 eV (52) and NaVO3 at 517.0 eV (53), respectively. It suggested that Bi and V in the orange film took the chemical states similar to [BiO]+ and [VO3]- species, respectively. The XPS spectra of N1s at 401.8 eV and 406.7 eV were assigned to 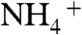 and
and 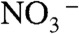 (54), respectively. The O1s peak at 532.4 eV well agreed with that of
(54), respectively. The O1s peak at 532.4 eV well agreed with that of 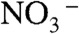 in NH4NO3 (532.5 eV) (54). These results indicated that [BiO]+,
in NH4NO3 (532.5 eV) (54). These results indicated that [BiO]+, 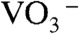 ,
, 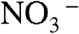 , and
, and 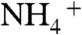 were contained in the orange film before calcination. TG-DTA measurement was carried out to examine the formation temperature of the BiVO4 thin film from these chemical species as shown in Fig. S2. Significant loss of weight was observed around 480 K accompanied with an exothermic process and a subsequent endothermic process. This exothermic process was probably due to the chemical reaction heat of [BiO]+ with
were contained in the orange film before calcination. TG-DTA measurement was carried out to examine the formation temperature of the BiVO4 thin film from these chemical species as shown in Fig. S2. Significant loss of weight was observed around 480 K accompanied with an exothermic process and a subsequent endothermic process. This exothermic process was probably due to the chemical reaction heat of [BiO]+ with 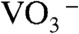 to form BiVO4. The loss of weight with the endothermic process was mainly caused by the decomposition of NH4NO3 to release N2O and NH3 gases (55). These results led the formation process of the BiVO4 thin film as shown in Fig. S3. When Bi(NO3)3 of a starting material was dissolved in an aqueous nitric acid solution, [BiO]+ and
to form BiVO4. The loss of weight with the endothermic process was mainly caused by the decomposition of NH4NO3 to release N2O and NH3 gases (55). These results led the formation process of the BiVO4 thin film as shown in Fig. S3. When Bi(NO3)3 of a starting material was dissolved in an aqueous nitric acid solution, [BiO]+ and 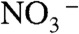 species formed (56). The bright yellow color of the precursor solution suggested the existence of
species formed (56). The bright yellow color of the precursor solution suggested the existence of 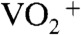 species that was formed from
species that was formed from  under a strongly acidic condition (57).
under a strongly acidic condition (57). 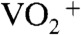 species changed to
species changed to 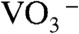 after drying, and these chemical species turned into NH4NO3 and [BiO]+[VO3]-. Calcination above 480 K caused the decomposition of NH4NO3 and the reaction of [BiO]+[VO3]- to form BiVO4. Porous structure of the thin film observed by SEM (Fig. 2C) formed accompanied with running out of gaseous products.
after drying, and these chemical species turned into NH4NO3 and [BiO]+[VO3]-. Calcination above 480 K caused the decomposition of NH4NO3 and the reaction of [BiO]+[VO3]- to form BiVO4. Porous structure of the thin film observed by SEM (Fig. 2C) formed accompanied with running out of gaseous products.
Fig. 3.
X-ray photoelectron spectra of BiVO4 thin film electrode. (A) Before and (B) after calcination at 673 K for 2 h.
Photoelectrochemical Property of BiVO4 Thin Film Electrode.
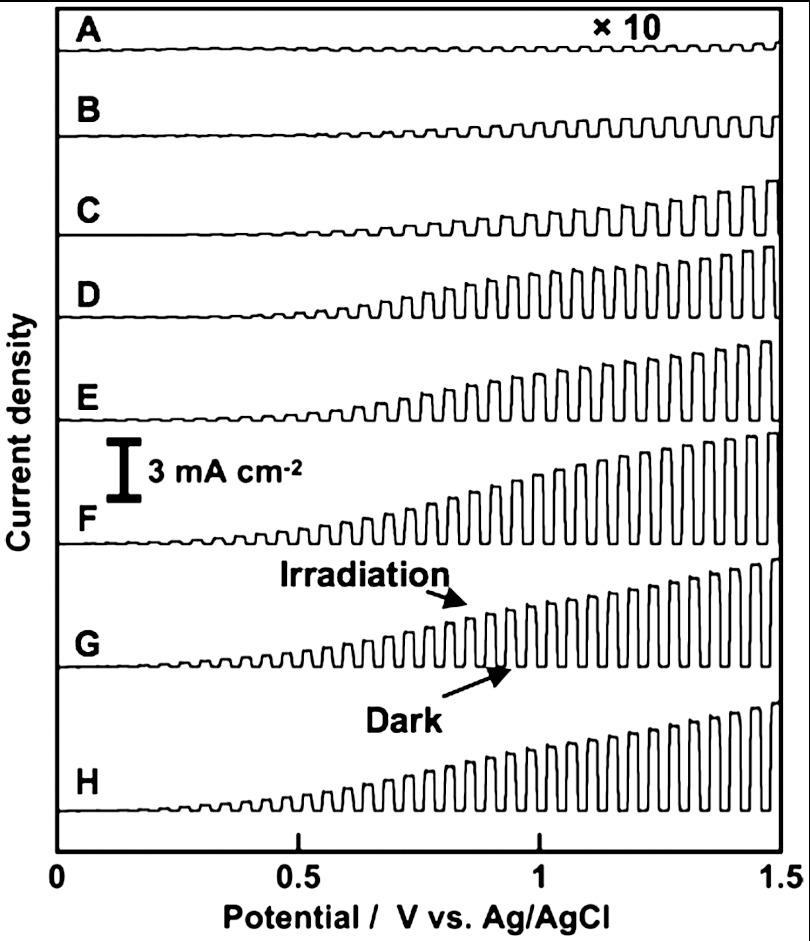
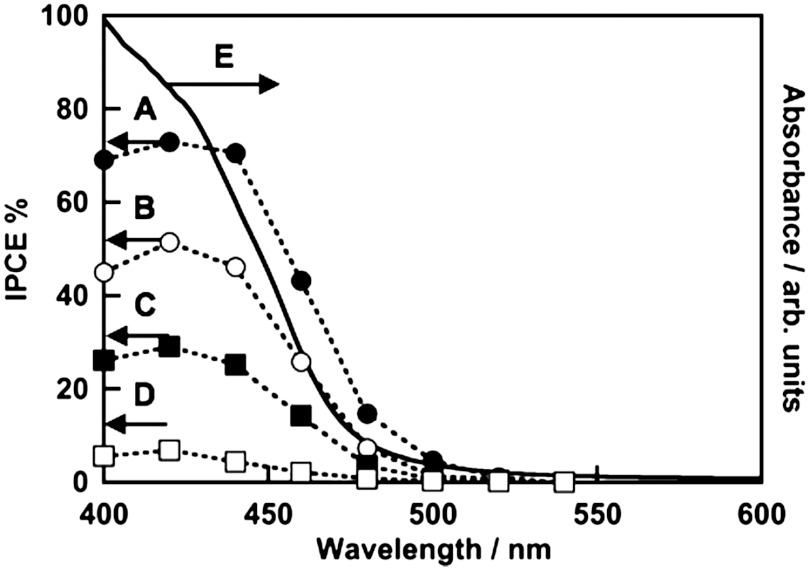
Effects of calcination temperature and film thickness on photoelectrochemical properties of BiVO4 thin films were examined as shown in Fig. 4. BiVO4 thin film electrodes calcined at different temperatures gave anodic photocurrents under visible light irradiation. The anodic photocurrent increased as the calcination temperature was raised. The photocatalytic activity of the BiVO4 (z-t) powder was lower than that of BiVO4 (s-m) for O2 evolution from an aqueous AgNO3 solution under visible light irradiation (35). The thin film containing BiVO4 (z-t) calcined at 573 and 623 K gave relatively small photocurrents corresponding to the photocatalytic property. The thin film electrodes with a single phase of BiVO4 (s-m) calcined at 673–723 K showed large photocurrents. The increase in the anodic photocurrent with the increase in the calcination temperature was due to the improvement of the denseness of the thin film as observed by SEM, and the purity and crystallinity of scheelite-monoclinic phase as confirmed by XRD. On the other hand, as the thickness of the BiVO4 film was increased, the anodic photocurrent became large. The BiVO4 thin film prepared using 200–300 mmol L-1 of precursor solutions gave an excellent photoelectrochemical property. Thicknesses of these thin films were sufficient for light absorption. These results concluded that the optimum calcination temperature and the concentration of the precursor solution were 673–723 K and 200–300 mmol L-1, respectively. Fig. 5 shows action spectra of the optimized BiVO4 thin film electrode. The onset of the IPCE agreed well with that of an absorption edge of BiVO4. This result indicated that the anodic photocurrent attributed to the band gap transition from the valence band of hybrid Bi6s-O2p orbitals to the conduction band of V3d (35). IPCE increased with an increase in the electrode potential. The BiVO4 thin film electrode gave an excellent anodic photocurrent with 73% of an IPCE at 420 nm at 1 V vs. Ag/AgCl. Modification with cobalt oxide on the BiVO4 electrode improved the photoelectrochemical property as previously reported (43, 45, 46). The onset potential of the anodic photocurrent for the cobalt oxide-modified BiVO4 electrode was more negative than that for the non-modified electrode as shown in Fig. S4. This result indicated that the loaded cobalt oxide improved the photoelectrochemical performance of the BiVO4 thin film electrode. XPS was carried out to examine the chemical state of the cobalt oxide loaded on the BiVO4 thin film electrode. Fig. S5 shows the Co2p and P2p spectra of cobalt oxide-modified BiVO4 electrode before and after photoelectrochemical water splitting. The XPS spectra of Co2p at 780.1 eV accompanied with the satellite were assigned to Co2+ in CoO. The peak of P2p was not detected, suggesting that the cobalt compound should be CoO. An average ratio of Co to Bi was estimated to be about 1∶1 by XPS.
Fig. 4.
Current vs. potential curves of BiVO4 thin film electrodes under visible light irradiation. Concentration of precursor solution and calcination temperature, (A) 200 mmol L-1, 573 K, (B) 200 mmol L-1, 623 K, (C) 20 mmol L-1, 673 K, (D) 50 mmol L-1, 673 K, (E) 100 mmol L-1, 673 K, (F) 200 mmol L-1, 673 K, (G) 300 mmol L-1, 673 K, and (H) 200 mmol L-1, 723; calcination time, 2 h. Electrolyte, 0.1 mmol L-1 of an aqueous K2SO4 solution; sweep rate, 20 mV s-1; light source, 300 W Xe-lamp with a cut-off filter (λ > 420 nm).
Fig. 5.
(A–D) Action spectra and (E) a diffuse reflection spectrum of BiVO4 thin film electrode. Preparation condition, calcined at 673 K for 2 h. Electrolyte, 0.1 mmol L-1 of an aqueous K2SO4 solution; potential, (A) 1 V, (B) 0.8 V, (C) 0.6 V, and (D) 0.4 V vs. Ag/AgCl.
Photoelectrochemical Overall Water Splitting.
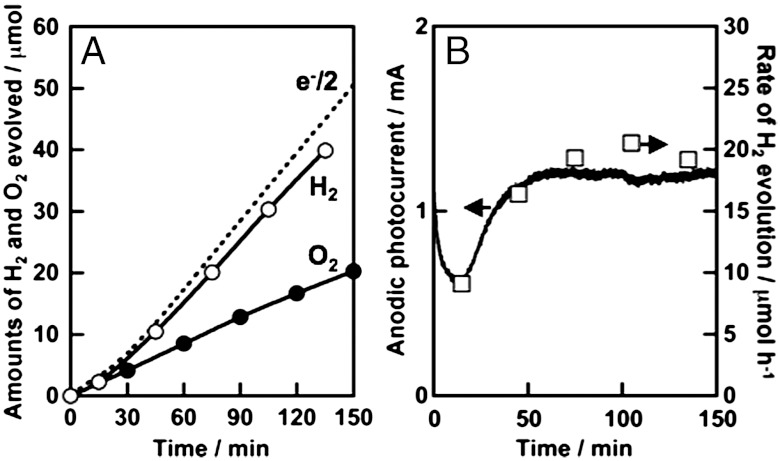
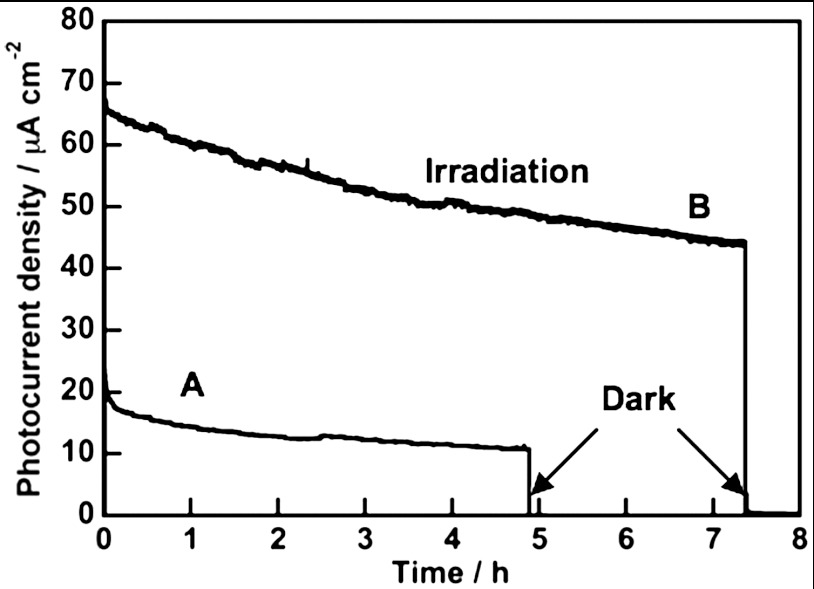
It is important to see the stability of photocurrent and to determine the amounts of evolved H2 and O2 for photoelectrochemical water splitting, because we cannot always guarantee that obtained photocurrent is due to water splitting. Fig. 6 shows photoelectrochemical water splitting using a BiVO4 thin film (1.5 cm2) with 1.0 V of an applied potential vs. Ag/AgCl. Steady photocurrent was obtained after 30 minutes and the rate of H2 evolution agreed well with the photocurrent (Fig. 6B). H2 and O2 evolved in a stoichiometric ratio from Pt and BiVO4 electrodes, respectively. The amount of H2 evolved was similar to the half of the amount of electron passing through the outer circuit (Fig. 6A). This result indicated that the observed photocurrent was due to water splitting. Photoelectrochemical water splitting was also examined using a solar simulator (AM1.5) with applying an external bias smaller than 1.23 V that was a theoretical voltage required for electrolysis of water in order to see if the present photoelectrochemical cell consisting of BiVO4 thin film and Pt electrodes works for solar energy conversion as shown in Fig. 7A. The solar energy conversion efficiency was estimated to be 0.005% with applying an external bias at 1 V vs. C.E. Large photocurrent was obtained for the CoO-modified BiVO4 thin film even applying a smaller external bias (0.6 V vs. C.E.) as shown in Fig. 7B. The solar energy conversion efficiency was estimated to be 0.04% with 0.6 V vs. C.E.
Fig. 6.
(A) Photoelectrochemical overall water splitting over BiVO4 thin film electrode and (B) stability of photocurrent using BiVO4 thin film electrode and Pt counter under visible light irradiation. Dashed line: the half of the amount of electron, open square: the rate of H2 evolution. Preparation condition, calcined at 673 K for 2 h. Electrolyte, 0.1 mmol L-1 of an aqueous K2SO4 solution; potential, 1 V vs. Ag/AgCl; electrode area, 1.5 cm2; light source, 300 W Xe-lamp with a cut-off filter (λ > 420 nm).
Fig. 7.
Stability of photocurrents of (A) BiVO4 and (B) CoO-modified BiVO4 thin film electrodes under simulated sunlight irradiation (AM1.5). Preparation condition, calcined at 673 K for 2 h. Electrolyte, 0.1 mmol L-1 of an aqueous K2SO4 solution (phosphate buffer, pH = 6.9); applied voltage, (A) 1 V, (B) 0.6 V vs. C.E.
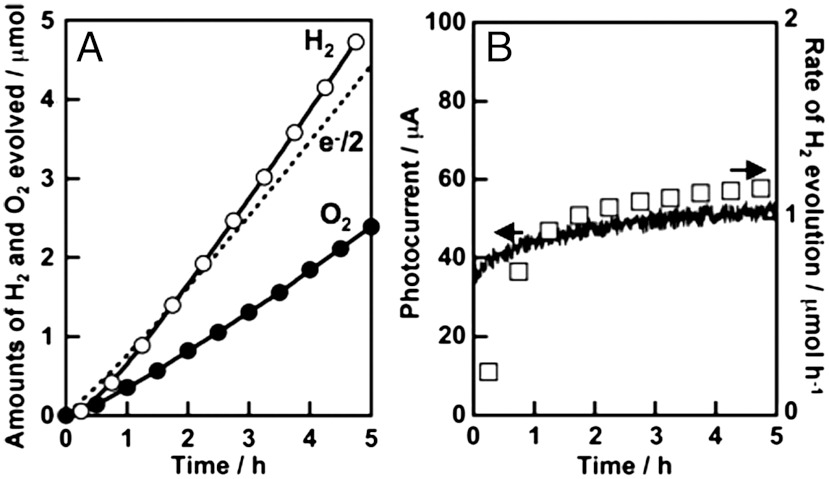
A photoelectrochemical cell consisting of a SrTiO3∶Rh (7%) photocathode (25) and the present BiVO4 thin film photoanode was constructed for water splitting. Photoelectrochemical water splitting proceeded under visible light irradiation with applying non-bias, as shown in Fig. 8. Steady photocurrent was obtained and the rate of H2 evolution agreed well with the photocurrent (Fig. 8B). H2 and O2 evolved in a stoichiometric ratio on the SrTiO3∶Rh and BiVO4 electrodes, respectively. The amount of H2 evolved was similar to the half of the amount of electron passing through the outer circuit (Fig. 8A). Thus, we succeeded in constructing a photoelectrochemical cell consisting of metal oxide materials for water splitting without applying an external bias.
Fig. 8.
(A) Photoelectrochemical overall water splitting and (B) stability of photocurrent using BiVO4 thin film electrode and SrTiO3∶Rh photocatalyst electrode under visible light irradiation. Dashed line: the half of the amount of electron, open square: the rate of H2 evolution. Electrolyte, 0.1 mmol L-1 of an aqueous K2SO4 solution; applied voltage, 0 V; electrode area, 4 cm2 each; light source, 300 W Xe-lamp with a cut-off filter (λ > 420 nm).
In summary, we have developed a facile fabrication of an efficient BiVO4 thin film electrode for overall water splitting from an aqueous nitric acid solution of Bi(NO3)3 and NH4VO3. It was revealed that the chemical species contained in the starting materials reacted with each other to form BiVO4 (s-m) via BiVO4 (z-t) by calcination. The BiVO4 (s-m) electrode showed a larger anodic photocurrent than BiVO4 (z-t). This was consistent with the order in an activity of powdered BiVO4 photocatalyst for O2 evolution from an aqueous AgNO3 solution under visible light irradiation. Anodic photocurrent of the BiVO4 thin film electrode depended on the concentration of a precursor solution and calcination temperature. The BiVO4 thin film electrode prepared at 673–723 K using 200–300 mmol L-1 of the precursor solution gave an excellent anodic photocurrent with 73% of an IPCE at 420 nm at 1 V vs. Ag/AgCl. Photoelectrochemical water splitting using the BiVO4 thin film proceeded with applying an external bias smaller than 1.23 V, which is a theoretical voltage required for electrolysis of water. The amount of H2 evolved was similar to the half of the amount of electron passing through the outer circuit, indicating that the photocurrent was due to water splitting. Modification with CoO on the BiVO4 electrode improved the photoelectrochemical property. The metal oxide photoelectrochemical system was constructed for water splitting under visible light irradiation with applying no external bias employing a SrTiO3∶Rh photocathode and the BiVO4 thin film photoanode.
Materials and Methods
Preparation of BiVO4 Thin Film and SrTiO3∶Rh Electrodes.
When 0.2–3 mmol of Bi(NO3)3 (Kanto Chemical; 99.9%) and NH4VO3 (Kanto Chemical; 99.0%) at an equal molar ratio was dissolved in 10 mL of an aqueous nitric acid solution (2 mol L-1), a yellow precursor solution was obtained. An FTO (F-doped SnO2) transparent substrate electrode (AGC Fabritech; A-110U80, sheet resistance: 20 Ω sq-1) was dipped in the precursor solution for 1–2 s. Then, the FTO substrate was covered with the yellow liquid of the precursor solution. After dryness at room temperature, an orange film was obtained on the FTO. Calcination of this film at 573–723 K for 2 h in air gave a yellow BiVO4 thin film. Cobalt oxide cocatalyst was loaded on the BiVO4 film with an area of 2.5 cm2 by an impregnation method with calcination at 673 K for 1 h in air using an aqueous solution (5 μL, 80 mmol L-1) of Co(NO3)2 (Wako Pure Chemical; 99.5%), if necessary (17, 18).
SrTiO3 powder doped with 7 mol% of Rh to a Ti site was prepared by a solid state reaction in order to obtain a photocathode for the construction of a photoelectrochemical cell (25). Starting materials, SrCO3 (Kanto Chemical; 99.9%), TiO2 (Soekawa Chemical; 99.9%), and Rh2O3 (Wako Pure Chemical) were mixed with the ratio of Sr∶Ti∶Rh = 1.07∶0.93∶0.07. The mixture was calcined in air at 1,173 K for 1 h and then at 1,373 K for 10 h in an alumina crucible. SrTiO3∶Rh photocatalyst electrodes were prepared by coating the paste composed of 20 mg of SrTiO3∶Rh photocatalyst powders, 20 μL of acetylacetone (Kanto Chemical; 99.5%), and 40 μL of distilled water on an indium tin oxide (ITO) transparent electrode and then calcining at 573 K for 2 h in air (25).
Characterization.
The crystal form of obtained BiVO4 thin film was confirmed by X-ray diffraction (Rigaku; miniFlex). Diffuse reflectance spectra were obtained using a UV-vis-NIR spectrometer (Jasco; Ubest-570) and were converted from reflection to absorbance by the Kubelka-Munk method, if necessary. The formation process of the BiVO4 thin film from the precursor solution were examined using a thermobalance (Ulvac; TGD-9600) for thermogravimetric–differential thermal analyses (TG-DTA) and an X-ray photoelectron spectrometer (Jeol; JPS-9010MC) with a Mg Kα (1253.6 eV) target. Obtained XPS spectra were corrected by C1s of adventitious carbon (284.6 eV). The morphology of obtained BiVO4 thin films was observed by scanning electron microscopy (Jeol; JSM-6700F).
Photoelectrochemical Measurement.
Photoelectrochemical properties were evaluated using a potentiostat (Hokuto Denko; HZ-5000 or HSV-100) with a three-electrode H-type cell divided into working and counter electrode compartments by Nafion 117 (DuPont). The BiVO4 thin film electrode, a Pt electrode, and a saturated Ag/AgCl electrode (DKK TOA; HS-205C) were used as working, counter, and reference electrodes, respectively. A SrTiO3∶Rh electrode was also employed as a photocathode (25). 0.1 mol L-1 of an aqueous K2SO4 (Kanto Chemical; 99.0%) solution was used as an electrolyte. An aqueous phosphate buffer solution [0.025 mol L-1 of KH2PO4 (Kanto Chemical; 99.6%) +0.025 mol L-1 of Na2HPO4 (Kanto Chemical; 99.5%)] was employed to adjust the pH to 6.9, if necessary.
Photoelectrochemical water splitting was carried out in an Ar gas flow system at 10 mL min-1 of a flow rate. The amounts of evolved gases were determined with gas chromatography (Shimadzu; MS-5A column, Ar carrier, TCD). The light source was a 300 W Xe-lamp (PerkinElmer; Cermax- PE300BF). Wavelength of the incident light was controlled by cut-off filters (HOYA), an NIR-absorbing filter (Sigma Koki; CCF-50S-500C), and a Plano convex lens (Sigma Koki; SLSQ- 60-150P). The working electrode was irradiated with light from an FTO side. The electrolytes in both compartments were bubbled with N2 or Ar before measurements for removal of dissolved O2. A 300 W Xe-lamp (Ashahi Spectra; MAX-301) with band-pass filters was employed for IPCE measurement. The number of incident photons was measured using a photodiode head (OPHIRA; PD300-UV) and a power monitor (NOVA). IPCE was calculated according to Eq. 1.
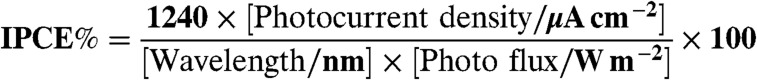 |
[1] |
A solar simulator (Peccell Technologies; PEC-L11, 100 mW cm-2) was employed for solar energy conversion determined by Eq. 2.
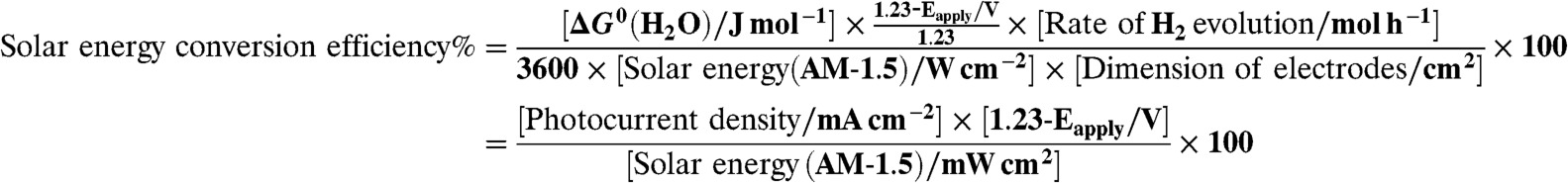 |
[2] |
Eapply represents of the applied external bias between working and Pt counter electrodes.
Supplementary Material
ACKNOWLEDGMENTS.
This work was supported by Toyota Motor Corporation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1204623109/-/DCSupplemental.
References
- 1.Fujishima A, Honda K. Electrochemical photolysis of water at a semiconductor electrode. Nature. 1972;238:37–38. doi: 10.1038/238037a0. [DOI] [PubMed] [Google Scholar]
- 2.Kudo A, Miseki Y. Heterogeneous photocatalyst materials for water splitting. Chem Soc Rev. 2009;38:253–278. doi: 10.1039/b800489g. [DOI] [PubMed] [Google Scholar]
- 3.Yoneyama H, Sakamoto H, Tamura H. Photo-electrochemical cell with production of hydrogen and oxygen by a cell reaction. Electrochim Acta. 1975;20:341–345. [Google Scholar]
- 4.Nozic AJ. p-n photoelectrolysis cells. Appl Phys Lett. 1976;29:150–153. [Google Scholar]
- 5.Watanabe T, Fujishima A, Honda K. Photoelectrochemical reactions at SrTiO3 single-crystal electrode. Bull Chem Soc Jpn. 1976;49:355–358. [Google Scholar]
- 6.Wrighton MS, et al. Strontium-titanate photoelectrodes-efficient photoassisted electrolysis of water at zero applied potential. J Am Chem Soc. 1976;98:2774–2779. [Google Scholar]
- 7.Kennedy JH, Frese KW. Photooxidation of water at barium-titanate electrodes. J Electrochem Soc. 1976;123:1683–1686. [Google Scholar]
- 8.Ellis AB, Kaiser SW, Wrighton MS. Semiconducting potassium tantalate electrodes—Photoassistance agents for efficient electrolysis of water. J Phys Chem. 1976;80:1325–1328. [Google Scholar]
- 9.Lindgren T, et al. Aqueous photoelectrochemistry of hematite nanorod array. Sol Energy Mater Sol Cells. 2002;71:231–243. [Google Scholar]
- 10.Sivula K, Le Formal F, Grätzel M. Solar water splitting: progress using hematite (alpha-Fe2O3) photoelectrodes. Chem Sus Chem. 2011;4:432–449. doi: 10.1002/cssc.201000416. [DOI] [PubMed] [Google Scholar]
- 11.Duret A, Grätzel M. Visible light-induced water oxidation on mesoscopic alpha-Fe2O3 films made by ultrasonic spray pyrolysis. J Phys Chem B. 2005;109:17184–17191. doi: 10.1021/jp044127c. [DOI] [PubMed] [Google Scholar]
- 12.Spichigerulmann M, Augustynski J. Aging effects in N-type semiconducting WO3 films. J Appl Phys. 1983;54:6061–6064. [Google Scholar]
- 13.Alexander BD, Kulesza PJ, Rutkowska L, Solarska R, Augustynski J. Metal oxide photoanodes for solar hydrogen production. J Mater Chem. 2008;18:2298–2303. [Google Scholar]
- 14.Miseki Y, Kusama H, Sugihara H, Sayama K. Cs-modified WO3 photocatalyst showing efficient solar energy conversion for O2 production and Fe (III) ion reduction under visible light. J Phys Chem Lett. 2010;1:1196–1200. [Google Scholar]
- 15.Sayama K, et al. Photoelectrochemical decomposition of water on nanocrystalline BiVO4 film electrodes under visible light. Chem Commun. 2003:2908–2909. doi: 10.1039/b310428a. [DOI] [PubMed] [Google Scholar]
- 16.Sayama K, et al. Photoelectrochemical decomposition of water into H2 and O2 on porous BiVO4 thin-film electrodes under visible light and significant effect of Ag ion treatment. J Phys Chem B. 2006;110:11352–11360. doi: 10.1021/jp057539+. [DOI] [PubMed] [Google Scholar]
- 17.Long MC, Beranek R, Cai WM, Kisch H. Hybrid semiconductor electrodes for light-driven photoelectrochemical switches. Electrochim Acta. 2008;53:4621–4626. [Google Scholar]
- 18.Long MC, Cai WM, Kisch H. Visible light induced photoelectrochemical properties of n-BiVO4 and n-BiVO4/p-CO3O4. J Phys Chem C. 2008;112:548–554. [Google Scholar]
- 19.Iwase A, Kudo A. Photoelectrochemical water splitting using visible-light-responsive BiVO4 fine particles prepared in an aqueous acetic acid solution. J Mater Chem. 2010;20:7536–7542. [Google Scholar]
- 20.Ye H, Lee J, Jang JS, Bard AJ. Rapid screening of BiVO4-based photocatalysts by scanning electrochemical microscopy (SECM) and studies of their photoelectrochemical properties. J Phys Chem C. 2010;114:13322–13328. [Google Scholar]
- 21.Berglund SP, Flaherty DW, Hahn NT, Bard AJ, Mullins CB. Photoelectrochemical oxidation of water using nanostructured BiVO4 films. J Phys Chem C. 2011;115:3794–3802. [Google Scholar]
- 22.Sayama K, et al. Effect of carbonate ions on the photooxidation of water over porous BiVO4 film photoelectrode under visible light. Chem Lett. 2010;39:17–19. [Google Scholar]
- 23.Ng YH, Iwase A, Kudo A, Amal R. Reducing graphene oxide on a visible-light BiVO4 photocatalyst for an enhanced photoelectrochemical water splitting. J Phys Chem Lett. 2010;1:2607–2612. [Google Scholar]
- 24.Ye H, Park HS, Bard AJ. Screening of electrocatalysts for photoelectrochemical water oxidation on W-doped BiVO4 photocatalysts by scanning electrochemical microscopy. J Phys Chem C. 2011;115:12464–12470. [Google Scholar]
- 25.Iwashina K, Kudo A. Rh-doped SrTiO3 photocatalyst electrode showing cathodic photocurrent for water splitting under visible-ight irradiation. J Am Chem Soc. 2011;133:13272–13275. doi: 10.1021/ja2050315. [DOI] [PubMed] [Google Scholar]
- 26.Abe R, Higashi M, Domen K. Facile fabrication of an efficient oxynitride TaON photoanode for overall water splitting into H2 and O2 under visible light irradiation. J Am Chem Soc. 2010;132:11828–11829. doi: 10.1021/ja1016552. [DOI] [PubMed] [Google Scholar]
- 27.Higashi M, Domen K, Abe R. Fabrication of efficient TaON and Ta3N5 photoanodes for water splitting under visible light irradiation. Energy Environ Sci. 2011;4:4138–4147. [Google Scholar]
- 28.Ishikawa A, Takata T, Kondo JN, Hara M, Domen K. Electrochemical behavior of thin Ta3N5 semiconductor film. J Phys Chem B. 2004;108:11049–11053. [Google Scholar]
- 29.Maeda K, Higashi M, Siritanaratkul B, Abe R, Domen K. SrNbO2N as a water-splitting photoanode with a wide visible-light absorption band. J Am Chem Soc. 2011;133:12334–12337. doi: 10.1021/ja203391w. [DOI] [PubMed] [Google Scholar]
- 30.Cong YQ, et al. Tantalum cobalt nitride photocatalysts for water oxidation under visible light. Chem Mater. 2012;24:579–586. [Google Scholar]
- 31.Lim AR, Choh SH, Jang MS. Prominent ferroelastic domain walls in BiVO4 crystal. J Phys: Condens Matter. 1995;7:7309–7323. [Google Scholar]
- 32.Bierlein JD, Sleight AW. Ferroelasticity in BiVO4. Solid State Commun. 1975;16:69–70. [Google Scholar]
- 33.Hirota K, Komatsu G, Yamashita M, Takemura H, Yamaguchi O. Formation, characterization and sintering of alkoxy-derived bismuth vanadate. Mater Res Bull. 1992;27:823–830. [Google Scholar]
- 34.Tokunaga S, Kato H, Kudo A. Selective preparation of monoclinic and tetragonal BiVO4 with scheelite structure and their photocatalytic properties. Chem Mater. 2001;13:4624–4628. [Google Scholar]
- 35.Kudo A, Omori K, Kato H. A novel aqueous process for preparation of crystal form-controlled and highly crystalline BiVO4 powder from layered vanadates at room temperature and its photocatalytic and photophysical properties. J Am Chem Soc. 1999;121:11459–11467. [Google Scholar]
- 36.Iwase A, Kato H, Kudo A. A simple preparation method of visible-light-driven BiVO4 photocatalysts from oxide starting materials (Bi2O3 and V2O5) and their photocatalytic activities. J Sol Energ Eng. 2010;132:021106. [Google Scholar]
- 37.Kanan MW, Nocera DG. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+ Science. 2008;321:1072–1075. doi: 10.1126/science.1162018. [DOI] [PubMed] [Google Scholar]
- 38.Pijpers JJH, Winkler MT, Surendranath Y, Buonassisi T, Nocera DG. Light-induced water oxidation at silicon electrodes functionalized with a cobalt oxygen-evolving catalyst. Proc Natl Acad Sci USA. 2011;108:10056–10061. doi: 10.1073/pnas.1106545108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barroso M, et al. The role of cobalt phosphate in enhancing the photocatalytic activity of alpha-Fe2O3 toward water oxidation. J Am Chem Soc. 2011;133:14868–14871. doi: 10.1021/ja205325v. [DOI] [PubMed] [Google Scholar]
- 40.McDonald KJ, Choi KS. Photodeposition of co-based oxygen evolution catalysts on alpha-Fe2O3 photoanodes. Chem Mater. 2011;23:1686–1693. [Google Scholar]
- 41.Zhong DK, Cornuz M, Sivula K, Graetzel M, Gamelin DR. Photo-assisted electrodeposition of cobalt-phosphate (Co-Pi) catalyst on hematite photoanodes for solar water oxidation. Energy Environ Sci. 2011;4:1759–1764. [Google Scholar]
- 42.Seabold JA, Choi KS. Effect of a cobalt-based oxygen evolution catalyst on the stability and the selectivity of photo-oxidation reactions of a WO3 photoanode. Chem Mater. 2011;23:1105–1112. [Google Scholar]
- 43.Jeon TH, Choi W, Park H. Cobalt-phosphate complexes catalyze the photoelectrochemical water oxidation of BiVO4 electrodes. Phys Chem Chem Phys. 2011;13:21392–21401. doi: 10.1039/c1cp23135a. [DOI] [PubMed] [Google Scholar]
- 44.Wang DE, et al. Photocatalytic water oxidation on BiVO4 with the electrocatalyst as an oxidation cocatalyst: essential relations between electrocatalyst and photocatalyst. J Phys Chem C. 2012;116:5082–5089. [Google Scholar]
- 45.Pilli SK, et al. Cobalt-phosphate (Co-Pi) catalyst modified Mo-doped BiVO4 photoelectrodes for solar water oxidation. Energy Environ Sci. 2011;4:5028–5034. [Google Scholar]
- 46.Zhong DK, Choi S, Gamelin DR. Near-complete suppression of surface recombination in solar photoelectrolysis by “Co-Pi” catalyst-modified W∶BiVO4. J Am Chem Soc. 2011;133:18370–18377. doi: 10.1021/ja207348x. [DOI] [PubMed] [Google Scholar]
- 47.Seabold JA, Choi KS. Efficient and stable photo-oxidation of water by a bismuth vanadate photoanode coupled with an iron oxyhydroxide oxygen evolution catalyst. J Am Chem Soc. 2012;134:2186–2192. doi: 10.1021/ja209001d. [DOI] [PubMed] [Google Scholar]
- 48.Schuhl Y, et al. Study of mixed-oxide catalysts containing bismuth, vanadium and antimony-preparation, phase-composition, spectroscopic characterization and catalytic-oxidation of propene. J Chem Soc, Faraday Trans 1. 1983;79:2055–2069. [Google Scholar]
- 49.Nefedov VI, Firsov MN, Shaplygin IS. Electronic-structures of MRhO2, MRh2O4, RhMO4 and Rh2MO6 on the basis of X-Ray spectroscopy and esca data. J Electron Spectrosc Relat Phenom. 1982;26:65–78. [Google Scholar]
- 50.Barbaray B, Contour JP, Mouvier G. Effects of nitrogen-dioxide and water-vapor on oxidation of sulfur-dioxide over V2O5 particles. Environ Sci Technol. 1978;12:1294–1297. [Google Scholar]
- 51.Blass PM, Zhou XL, White JM. Coadsorption and reaction of water and potassium on Ag(111) J Phys Chem. 1990;94:3054–3062. [Google Scholar]
- 52.Stec WJ, Vanwazer JR, Proctor WG, Morgan WE. Inner-orbital photoelectron spectroscopy of several pairs of similar phosphorus-compounds. J Inorg Nucl Chem. 1972;34:1100–1104. [Google Scholar]
-
53.Mezentzeff P, Lifshitz Y, Rabalais JW. Compositional and chemical modifications of V2O5 and NaVO3 induced by
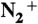 bombardment. Nucl Instrum Methods Phys Res, Sect B. 1990;44:296–301. [Google Scholar]
bombardment. Nucl Instrum Methods Phys Res, Sect B. 1990;44:296–301. [Google Scholar] - 54.Aduru S, Contarini S, Rabalais JW. Electron-stimulated, X-Ray-stimulated, and ion-stimulated decomposition of nitrate salts. J Phys Chem. 1986;90:1683–1688. [Google Scholar]
- 55.Musin RN, Lin MC. Novel bimolecular reactions between NH3 and HNO3 in the gas phase. J Phys Chem A. 1998;102:1808–1814. [Google Scholar]
- 56.Pourbaix M. Atlas of Electrochemical Equilibria in Aqueous Solutions. 2nd Ed. Houston, TX: National Association of Corrosion Engineers International Cebelcor; 1974. p. 533. [Google Scholar]
- 57.Pourbaix M. Atlas of Electrochemical Equilibria in Aqueous Solutions. 2nd Ed. Houston, TX: National Association of Corrosion Engineers International Cebelcor; 1974. p. 234. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.