Almost a half century ago, waterfowl biologists noticed a strange phenomenon common in many duck populations: nests containing eggs laid by multiple females but tended by a single female or, for some nests, not tended at all (1–3). At the time, interest focused on the management implications and population consequences of the so-called “dump nests,” which were often less successful than typical nests. Two key papers published in the early 1980s (4, 5) dramatically shifted the focus of inquiry to understanding the adaptive basis of these puzzling nests, which we now consider as examples of conspecific brood parasitism (CBP). Conspecific parasitism is enigmatic from an evolutionary perspective, because it is not immediately clear why individuals should provide costly parental care to the offspring of strangers. By documenting CBP in over fifty species of birds, Yom Tov (4) showed that it was too common and taxonomically widespread to be dismissed merely as a reproductive error, and he outlined several adaptive hypotheses. Building on this work, Andersson (5) added a new theoretical perspective by considering brood parasitism in the context of game theory and alternative reproductive tactics (6). He also highlighted the importance of considering the behavior of the individuals (hosts) who receive the parasitic eggs. Clearly, a full understanding of CBP would require analysis of the fitness consequences to all participants in the game–hosts as well as parasites (7).
Because of the interest generated by these two papers, the list of birds known to exhibit CBP has nearly quadrupled in the last two decades (8–11), and the phenomenon has been discovered in a diversity of insect taxa as well (12, 13). The adaptive framework first developed by Yom Tov and Andersson has been applied and extended in about a dozen detailed behavioral and ecological avian studies (reviewed in ref. 14). These studies reveal considerable variation in the context of parasitism; in some cases, parasites are nesting females, in others they are non-nesting individuals (11, 15–19). Clearly, different costs, benefits, and trade-offs are at play, and no single adaptive explanation will likely account for all cases of CBP. A strong taxonomic pattern does emerge, however; CBP is disproportionately common in one group of birds, the waterfowl (order Anseriformes) (refs. 7–9; Figs. 1 and 2), in which CPB is known to occur in 76 of the 162 species (10). Moreover, whereas ducks, geese, and swans constitute only about 2% of avian species, at least 26% of known conspecific parasites are waterfowl (8, 20). This pattern did not escape Andersson's attention (5), and he wondered whether it was related to the unusual pattern of philopatry in this group. In waterfowl, unlike most birds, it is the females, not males, who return to their birth sites. If natal philopatry were sufficiently local, host and parasite females could be close relatives, raising the possibility that kin selection (21) might facilitate the evolution of brood parasitism. Kin selection would provide a compelling explanation for the enigma of why a female would accept eggs and raise chicks not her own. Now, sixteen years after Andersson first suggested this idea (5), a new paper by Andersson and Åhlund in this issue of PNAS (22) provides the first evidence that relatedness and kin selection may be an important component of parasitism in waterfowl.
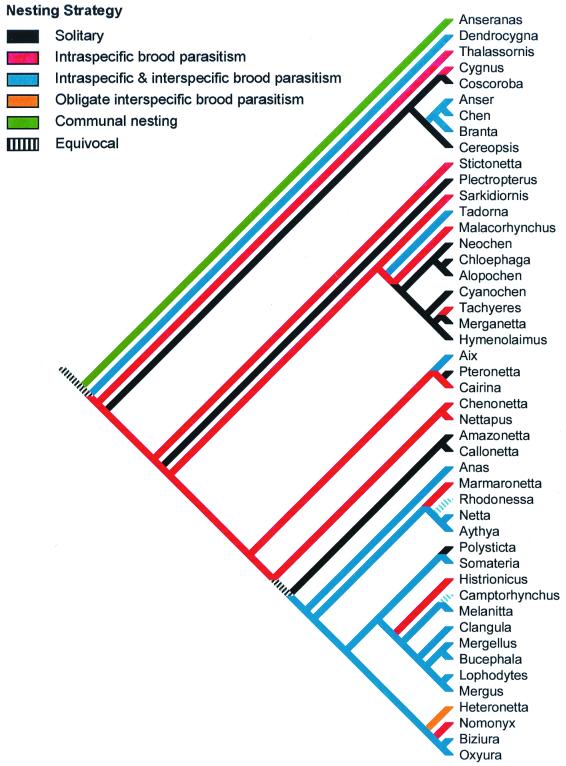
Figure 1.
The diversity of nesting behaviors observed in the waterfowl make it an ideal group for comparative studies of avian breeding systems, including CBP. The phylogeny shown here for Anseriforme genera closely follows Livezey (36), whereas data for nesting strategies are from refs. 7 and 10. Nesting strategies were optimized on the phylogenetic tree by using an acctran algorithm (37).
Figure 2.
Broods of mixed maternity, like this one, are common in goldeneye ducks and arise either through CBP or amalgamation of broods after hatching. The ducklings with different cheek colors were color marked at different nest boxes and thus originated from the nests of different females (photograph by B. Lyon).
Studies of kin selection require analysis of relatedness, often a challenging endeavor. Andersson and Åhlund's study is noteworthy not only for their interesting empirical results, but also for the novel technique they developed to assess kinship. Field ecologists have increasingly turned to the use of molecular methods to assess parentage and relatedness in natural populations (23), resulting in a veritable revolution in behavioral ecology and sociobiology (24). However, despite the power of these techniques, they are not without limitations. For example, most require samples of DNA from all individuals of interest. In many cases, however, obtaining DNA from offspring is hampered by events such as nest failure before hatching or, more critically, by the tactics and countertactics of the parents themselves. For example, parasitic eggs often suffer reduced hatching success because they are laid too late in the host's breeding cycle or are neglected or destroyed by the hosts (25, 26). Thus, researchers are often left with an incomplete sample of individuals in a study population, resulting in a potentially biased assessment of parentage and kinship.
Andersson and Åhlund's (22) technique of protein fingerprinting provides an elegant solution to these problems. With this method, a small amount of egg albumin is removed through a hole drilled in the eggshell, which is then resealed. The hatching success of the eggs is unaffected (27). Isoelectric focusing in immobilized pH gradients is then used to obtain a pattern of variable albumin bands, much like that seen in a DNA minisatellite fingerprint. Analysis of a population of female common goldeneyes (Bucephala clangula) in Sweden revealed that these “protein fingerprints” yield unique banding patterns for each female, presumably because albumin contains over a dozen proteins, many of which have been demonstrated to be genetically polymorphic (27). Moreover, because albumin is secreted by cells in the oviduct during egg formation, the resulting fingerprint is entirely of female origin. Given the high variability and maternal uniqueness of the band patterns, protein fingerprinting will likely prove to be an ideal method to assess maternity of offspring and kinship of the female parents in cases of CBP. It could also be a boon to studies of interspecific brood parasitism, where there is interest in tracing patterns of host specialization and fecundities of individual females (14).
Armed with this new technique, Andersson and Åhlund (22) examined patterns of relatedness in their Swedish goldeneye population, specifically with respect to brood parasitism. Statistical analyses of protein bandsharing among females revealed that hosts and primary parasites (those laying the most parasitic eggs) were indeed often related, with an estimate of average relatedness similar to that observed between first cousins (22). Although this pattern of relatedness suggests that parasites recognize kin and preferentially lay eggs in the nests of relatives, it is also possible that relatives use the same nest simply by chance, because of extreme natal philopatry and high female survival. Andersson and Åhlund evaluated these two possibilities. In support of the kin discrimination hypothesis, they found that protein bandsharing coefficients among hosts and parasites were higher in nests where parasites laid a large number of eggs (i.e., parasites either preferentially laid eggs or were permitted to lay eggs in nests of more closely related hosts). In contrast, protein bandsharing coefficients were lower in parasitized nests that failed (an indication of potential conflict between less related host and parasite). Moreover, the researchers calculated the probability that a female who parasitizes an active nest by chance would have a nestmate as a host, given the patterns of philopatry observed, and found that this probability was very low (here nestmate refers to both the parent and all offspring at the focal female's birth nest). Thus, Andersson and Åhlund's results support the idea not only that parasites are more likely to be related to hosts, but also that some mechanism of kin recognition is involved. How would parasites find the nests of relatives? Andersson and Åhlund suggest that relatives associate together on breeding lakes and visit nests jointly. In support of this idea, pairs of nestmates were observed more often together on lakes than expected by chance, and remained together for significantly longer than non-nestmate pairs.
Andersson and Åhlund's observations provide evidence that relatedness may facilitate the evolution of brood parasitism in waterfowl, as Andersson (5) originally predicted. The next critical step will be to determine whether kin selection is actually operating. Not all interactions between kin are cooperative (28, 29), and not all theory predicts that relatedness facilitates CBP. For example, a new model by Zink (30), based on reproductive skew theory (31), predicts that relatedness between the host and parasite can actually decrease the likelihood of CBP, opposite to Andersson's (5) prediction. How can two models, apparently asking the same question, lead to opposite predictions?
The paradox dissolves if we consider the ecological context of parasitism. The predictions of the two models (5, 30) differ because they explore very different ecological contexts of brood parasitism. Andersson's model (5) focused on host behavior and examined the inclusive fitness gained when a host allows a relative to lay her eggs in the host's nest instead of nesting on her own. If the mortality risks associated with nesting are high, the host gains inclusive fitness from the increased survival of her relative. Zink's (30) model differed in two important ways: he focused on the decisions of parasites, not hosts, and his parasites could have their own nests. He evaluated the conditions when it benefited these nesting females to lay some of their eggs parasitically. Zink's assumption that parasitism is very costly to hosts is particularly critical to the prediction that relatedness decreases the evolution of CBP because this prediction reverses if CBP has minimal costs or is beneficial to hosts (30). When parasitism is costly to hosts, relatedness decreases the net benefit of parasitism to the parasite because it may harm her indirect fitness more than it enhances her direct fitness. The differences between these two models emphasize that relatedness is only half of the equation for kin selection—the evolution of cooperative behavior also depends on the magnitude of the costs and benefits to the participants, and on what other reproductive options are available to them (21). We now know that these factors often differ dramatically among species, even within the waterfowl.
Given the range of predictions about how relatedness affects the evolution of CBP, we should not be surprised that relatedness shows diverse patterns with CBP. For example, recent studies of moorhens (32) and lacebugs (33) also found a link between relatedness and brood parasitism. In contrast, parasitic female wood ducks (Aix sponsa) have been shown to actively avoid parasitizing close kin (20), whereas our own studies of CPB in the Barrow's goldeneye (B. islandica) in British Columbia revealed no evidence of relatedness between hosts and parasites based on DNA fingerprinting analyses (J.McA.E. and R. Fernando, unpublished data). These observations suggest that kin selection may vary among species both in importance and in the way in which it shapes the behavior of hosts and parasites.
Irrespective of how widespread kin selection turns out to be as a mechanism facilitating brood parasitism in waterfowl and other taxa, Andersson and Åhlund's findings (22) are exciting because they blur the distinction between what we perceive as competitive vs. cooperative breeding systems. Brood parasitism has been viewed traditionally as an interaction that squarely pits the interests of the “parasite” against those of the host, leading perhaps to escalating coevolutionary arms races between them. If, however, parasites and hosts are related, the argument reverses and what appeared to have been a parasitic exchange becomes, instead, a cooperative one not dissimilar from other well-known cooperative breeding systems (34). It is intriguing, then, to speculate that relatively small changes in (i) the costs and benefits of parasitism to both hosts and parasites, (ii) the constraints on solitary breeding, and (iii) the opportunities for interacting with relatives, facilitated by high natal philopatry, could generate a continuum of breeding systems from purely parasitic to communal or cooperative. Other authors have noted previously a possible connection between brood parasitism and cooperative breeding (30, 34, 35), but it may be only now, equipped with techniques such as protein fingerprinting and aided by the integrative framework offered by reproductive skew theory (31), that we will be able to predict precisely what sort of breeding system should arise under different ecological contexts.
Waterfowl may be an ideal group for developing and testing such predictions, and it is perhaps no accident that Andersson and Åhlund chose this group for study. Aside from high levels of female natal philopatry and frequent CBP, waterfowl exhibit a broad spectrum of breeding systems (Fig. 1), ranging from solitary nesting, CBP, interspecific brood parasitism (including the only known obligate brood parasite with precocial young Heteronetta atricapilla), and communal nesting, in which two or three females mate with a single male and lay eggs and tend young cooperatively in a single nest (Anseranas semipalmata). Moreover, these interactions are not limited to the nest site; several species also amalgamate broods after hatching (ref. 7; Fig. 2). The plasticity in these reproductive behaviors along the waterfowl lineage (Fig. 1) suggests that waterfowl breeding systems are not phylogenetically constrained, but rather vary in response to ecological conditions and opportunities (10, 17). The challenge now will be to obtain the necessary data on relatedness, ecological constraints, and the costs and benefits of these many behavioral alternatives. CBP—the strange phenomenon noted by waterfowl biologists half a century ago—may well turn out to be a fascinating and important link in the evolution of avian breeding systems.
Acknowledgments
We thank Ada Fowler and Elena Berg for comments on the manuscript. We are especially grateful to Kerstin Wasson, whose many editorial suggestions on several drafts of the manuscript greatly improved the paper.
Footnotes
See companion article on page 13188.
References
- 1.Leopold A S. Condor. 1951;53:209–220. [Google Scholar]
- 2.Jones R E, Leopold A S. J Wildl Manage. 1967;31:221–228. [Google Scholar]
- 3.McCamant R E, Bolen E G. J Wildl Manage. 1979;43:936–943. [Google Scholar]
- 4.Yom Tov Y. Biol Rev. 1980;55:93–108. [Google Scholar]
- 5.Andersson M. In: Producers and Scroungers. Barnard C J, editor. London: Croom Helm; 1984. pp. 195–228. [Google Scholar]
- 6.Maynard Smith J. Evolution and the Theory of Games. Cambridge, U.K.: Cambridge Univ. Press; 1982. [Google Scholar]
- 7.Eadie J M, Kehoe F P, Nudds T D. Can J Zool. 1988;66:1709–1721. [Google Scholar]
- 8.Eadie J, Sherman P, Semel B. In: Behavioral Ecology and Conservation Biology. Caro T, editor. Oxford: Oxford Univ. Press; 1998. pp. 306–340. [Google Scholar]
- 9.Rohwer F C, Freeman S. Can J Zool. 1989;67:239–253. [Google Scholar]
- 10.Beauchamp G. Auk. 1997;114:11–21. [Google Scholar]
- 11.Lyon B E. Anim Behav. 1993;46:911–928. [Google Scholar]
- 12.Field J. Biol Rev. 1992;67:79–126. [Google Scholar]
- 13.Tallamy D W, Horton L A. Anim Behav. 1990;39:352–359. [Google Scholar]
- 14.Davies N B. Cuckoos, Cowbirds and other Cheats. London: Poyser; 2000. [Google Scholar]
- 15.Evans P G H. Anim Behav. 1988;36:1282–1294. [Google Scholar]
- 16.Lank D B, Cooch E G, Rockwell R F, Cooke F. J Anim Ecol. 1989;58:29–45. [Google Scholar]
- 17.Eadie J M. Acta XX Congr Int Ornithol. 1991;2:1031–1040. [Google Scholar]
- 18.Brown C, Brown M B. Nature (London) 1988;331:66–68. [Google Scholar]
- 19.Jackson W M. Behav Ecol Sociobiol. 1993;32:119–126. [Google Scholar]
- 20.Semel, B. & Sherman, P. (2000) Anim. Behav., in press.
- 21.Hamilton W D. J Theor Biol. 1964;7:1–52. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- 22.Andersson M, Åhlund M. Proc Natl Acad Sci USA. 2000;97:13188–13193. doi: 10.1073/pnas.220137897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughes C. Ecology. 1998;79:383–399. [Google Scholar]
- 24.Weatherhead P J, Montgomerie R D. Trends Ecol Evol. 1991;6:173–174. [Google Scholar]
- 25.Lyon B E. Behav Ecol Sociobiol. 1993;33:87–100. [Google Scholar]
- 26.McRae S B. Anim Behav. 1995;49:1073–1088. [Google Scholar]
- 27.Andersson, M. & Åhlund, M. (2000) Ecology, in press.
- 28.Trivers R L. Am Zool. 1974;14:249–264. [Google Scholar]
- 29.Mock D W, Parker G A. The Evolution of Sibling Rivalry. Oxford: Oxford Univ. Press; 1997. [Google Scholar]
- 30.Zink A G. Am Nat. 2000;155:395–405. doi: 10.1086/303325. [DOI] [PubMed] [Google Scholar]
- 31.Vehrencamp S L. Am Zool. 1983;23:327–335. [Google Scholar]
- 32.McRae S B, Burke T. Behav Ecol Sociobiol. 1996;38:115–129. [Google Scholar]
- 33.Loeb M L G, Diener L M, Pfennig D W. Anim Behav. 2000;59:379–383. doi: 10.1006/anbe.1999.1328. [DOI] [PubMed] [Google Scholar]
- 34.Brown J L. Helping and Communal Breeding in Birds. Princeton: Princeton Univ. Press; 1987. [Google Scholar]
- 35.Vehrencamp S L. Behav Ecol. 2000;11:334–344. [Google Scholar]
- 36.Livezey B C. Auk. 1986;103:737–754. [Google Scholar]
- 37.Maddison W P, Maddison D R. MacClade: Analysis of Phlylogeny and Character Evolution. Sunderland, MA: Sinauer; 1992. [Google Scholar]