Thylakoid protein phosphorylation plays a central role in the dynamic acclimation of the photosynthetic electron transfer chain to changing light conditions. This work identifies a protein phosphatase that is required for dephosphorylation of photosystem II subunits and counteracts the activity of the protein kinase STN8.
Abstract
Reversible protein phosphorylation plays a major role in the acclimation of the photosynthetic apparatus to changes in light. Two paralogous kinases phosphorylate subsets of thylakoid membrane proteins. STATE TRANSITION7 (STN7) phosphorylates LHCII, the light-harvesting antenna of photosystem II (PSII), to balance the activity of the two photosystems through state transitions. STN8, which is mainly involved in phosphorylation of PSII core subunits, influences folding of the thylakoid membranes and repair of PSII after photodamage. The rapid reversibility of these acclimatory responses requires the action of protein phosphatases. In a reverse genetic screen, we identified the chloroplast PP2C phosphatase, PHOTOSYSTEM II CORE PHOSPHATASE (PBCP), which is required for efficient dephosphorylation of PSII proteins. Its targets, identified by immunoblotting and mass spectrometry, largely coincide with those of the kinase STN8. The recombinant phosphatase is active in vitro on a synthetic substrate or on isolated thylakoids. Thylakoid folding is affected in the absence of PBCP, while its overexpression alters the kinetics of state transitions. PBCP and STN8 form an antagonistic kinase and phosphatase pair whose substrate specificity and physiological functions are distinct from those of STN7 and the counteracting phosphatase PROTEIN PHOSPHATASE1/THYLAKOID-ASSOCIATED PHOSPHATASE38, but their activities may overlap to some degree.
INTRODUCTION
The primary reactions of plant and algal photosynthesis occur in the thylakoid membranes of chloroplasts. These membranes harbor large multimeric pigment-protein complexes called photosystems that convert light energy into chemical energy with high efficiency, a process that ultimately fuels most of the biosphere. In the linear mode of electron transfer through the photosynthetic chain, photosystem II (PSII) and photosystem I (PSI) are connected in series through plastoquinone (PQ), the cytochrome b6f complex, and plastocyanin (Eberhard et al., 2008). The activities of PSII and PSI must be balanced for efficient electron transfer, which requires an appropriate redox poise of the intermediate electron carriers. Under low light, the activity of the two photosystems is tuned through a mechanism called state transitions (Allen, 1992; Wollman, 2001; Aro and Ohad, 2003; Rochaix, 2007; Lemeille and Rochaix, 2010; Minagawa, 2011). A mobile part of the light-harvesting complex II (LHCII) light-harvesting antenna is dynamically allocated to PSII or PSI depending on the redox state of the PQ pool. Binding of reduced plastoquinol to the Qo site of the b6f complex activates the thylakoid kinase STN7, which phosphorylates components of the LHCII antenna and causes their dissociation from PSII and at least in part their association with PSI (state 2) (Vener et al., 1997; Zito et al., 1999; Bellafiore et al., 2005; Bonardi et al., 2005; Minagawa, 2011; Tikkanen and Aro, 2012). This process is reversible; when the PQ pool is oxidized, dephosphorylation by the recently identified PROTEIN PHOSPHATASE1/THYLAKOID-ASSOCIATED PHOSPHATASE38 (PPH1/TAP38) phosphatase (Pribil et al., 2010; Shapiguzov et al., 2010) results in the association of the LHCII components with PSII (state 1). In the green alga Chlamydomonas reinhardtii, a large fraction of the antenna is implicated in state transitions, which are controlled by the protein kinase Stt7, the ortholog of STN7 (Depège et al., 2003). In the alga, transition to state 2 induces cyclic electron flow around PSI and favors the production of ATP (Finazzi et al., 2002).
The thylakoid membranes of plant chloroplasts form a continuous network of flattened membrane vesicles organized into regular stacks with many tightly appressed layers, called grana, interconnected by nonappressed stromal lamellae (Dekker and Boekema, 2005). The two photosystems are unevenly distributed in the thylakoid membranes. PSII is confined to grana, while PSI is enriched in stromal lamellae as well as in the margins and ends of grana stacks. State transitions involve changes in thylakoid membrane stacking that become strikingly apparent when de-enveloped chloroplasts are examined under the electron microscope (Chuartzman et al., 2008). In vivo, phosphorylation of thylakoid proteins such as the core subunits of PSII by the kinase STN8, a paralog of STN7, favors changes in membrane folding (Vainonen et al., 2005; Fristedt et al., 2009a). In vitro, stacking is strongly promoted by increasing cation concentrations, and this can counteract the effect of thylakoid protein phosphorylation (Barber, 1980, 1982; Fristedt et al., 2010).
PSII has the remarkable ability to extract electrons from water using energy from light. Photooxidation of the reaction center chlorophyll (P680+) creates one of the strongest oxidants in biology. This powerful photochemistry can cause collateral damage to the PSII complex itself, particularly to its D1 subunit (Edelman and Mattoo, 2008). Moreover, molecular oxygen can react with light-activated chlorophyll molecules or reduced cofactors of the electron transfer chain to produce highly reactive oxygen species, which can be damaging to the photosynthetic machinery and to other components of the chloroplast. Thus, photosynthetic organisms, which are subjected to large fluctuations in their light environment, must continuously adapt to maximize photosynthetic activity under low light and to minimize photooxidative damage under excess light (Eberhard et al., 2008; Li et al., 2009). Photooxidative damage occurs at all light intensities, but when the rate of damage exceeds the capacity for repair, photoinhibition is manifested as a decrease in photosynthetic activity and in the proportion of active PSII reaction centers (Edelman and Mattoo, 2008). Under conditions when the absorbed light excitation energy exceeds the capacity of the photosynthetic electron transfer chain or the metabolic demands of the cell, a number of different mechanisms intervene to downregulate light harvesting and alleviate photooxidative damage. Alternative routes of electron flow, which counteract the excessive reduction of electron carriers, include cyclic electron flow around PSI, the chlororespiratory pathway involving plastid terminal oxidase, as well as the Mehler reaction and the water-water cycle (Eberhard et al., 2008). Nonphotochemical quenching (NPQ) is induced in the light-harvesting antenna of PSII (LHCII) to dissipate excess captured light energy as heat (Li et al., 2009). The induction of NPQ limits the reduction of the PQ pool by decreasing the activity of PSII. Thus, in high light, NPQ takes over a major role instead of state transitions in maintaining the redox poise of the electron transfer chain (Tikkanen et al., 2011; Tikkanen and Aro, 2012). Whereas phosphorylation of LHCII by the STN7 kinase is inhibited under high light, most likely through the thioredoxin pathway (Rintamäki et al., 2000; Martinsuo et al., 2003), phosphorylation of the PSII core subunits is enhanced (Tikkanen et al., 2008, 2010).
The D1 subunit of PSII is the primary target of photooxidative damage, and an active cycle of repair functions to selectively replace the damaged subunit. Because PSII is mainly localized in the tightly appressed membranes of the thylakoid grana, the repair cycle requires its migration to membrane domains that are exposed to the stroma, allowing the selective degradation of the damaged D1 subunit and the synthesis, maturation, and assembly of a new subunit (Baena-González and Aro, 2002; Takahashi and Murata, 2008). This process is facilitated by the protein kinase STN8 (Tikkanen et al., 2008), which shows specificity toward proteins of the PSII core (Bonardi et al., 2005; Vainonen et al., 2005). The action of the STN8 kinase favors unfolding of thylakoid membranes and the ability of the degradation and repair machinery to access damaged D1 (Fristedt et al., 2009a).
The kinase STN7 and the phosphatase PPH1/TAP38 form a pair with opposing effects on phosphorylation of the LHCII antenna, an antagonism that allows the reversible regulation of state transitions to maintain the redox poise of the electron transfer chain. Similarly, it is expected that chloroplast phosphatase(s) will counterbalance the activity of the STN8 kinase. Here, we identify a protein phosphatase that is required for efficient dephosphorylation of several core subunits of PSII in vivo and show that the purified recombinant protein is active in vitro. Mutants lacking this phosphatase show changes in the folding of thylakoid membranes. In the mutants, state transitions are not affected, but they are altered in transgenic lines overexpressing the phosphatase.
RESULTS
Efficient Dephosphorylation of the PSII Core Requires the Protein Phosphatase PBCP
We designed a genetic screen for chloroplast phosphatases involved in dephosphorylation of thylakoid proteins in Arabidopsis thaliana (Shapiguzov et al., 2010). In brief, we first searched genomic databases for putative catalytic or regulatory subunits of protein phosphatases and then selected those that were predicted to be targeted to the chloroplast (Suba II; Heazlewood et al., 2007). They were ranked by the strength of these predictions and the degree of correlated coexpression of their mRNAs with those of the protein kinases STN7 and STN8 in the Genevestigator microarray database (Zimmermann et al., 2005). Homozygous mutants for these predicted phosphatases were obtained from the SALK collection (Alonso and Ecker, 2006). Seedlings were systematically screened for defective dephosphorylation of thylakoid proteins after a shift from white to far-red light, using a streamlined immunoblot assay with antiphosphothreonine antibodies (Rintamäki et al., 1997). The PPH1/TAP38 phosphatase previously identified in this screen is required for efficient dephosphorylation of LHCII antenna proteins and transition from state 2 to state 1 (Pribil et al., 2010; Shapiguzov et al., 2010).
Using the same screen, we identified a different phosphatase that is required for efficient dephosphorylation of PSII core subunits. In moderate white light, many thylakoid proteins arephosphorylated by the kinases STN7 and STN8, including components of the light-harvesting complex LHCII and of PSII, as well as other thylakoid proteins, such as THYLAKOID SOLUBLE PROTEIN9 and CALCIUM SENSING (CaS) (Vener, 2007; Vainonen et al., 2008; Fristedt et al., 2009b; Reiland et al., 2011). Upon exposure to far-red light, which favors PSI excitation, the PQ pool is oxidized, a transition to state 1 is induced, and several thylakoid proteins are dephosphorylated. In the wild type, strong dephosphorylation of the core subunits of PSII and of the LHCII antenna is apparent within 20 min in immunoblots with antiphosphothreonine antibodies (from Zymed; Figure 1A). By contrast, phosphorylation of the PSII subunits CP43, D2, and D1 is partly retained even after 40 min in the mutant affecting PBCP (for photosystem II core phosphatase; At2g30170), while the dephosphorylation of the LHCII antenna proceeds as efficiently as in the wild type. It is interesting to contrast the specificity of the pbcp-1 phenotype with that of pph1-3, where dephosphorylation of the PSII subunits proceeds normally while the LHCII antenna remains phosphorylated (Figure 1A). Thus, the two phosphatases seem to have complementary roles: PBCP is primarily required for efficient dephosphorylation of the PSII core, while PPH1/TAP38 is required for efficient dephosphorylation of the LHCII antenna.
Figure 1.
PSII Dephosphorylation Is Deficient in pbcp Mutants.
(A) Wild-type (WT), pph1-3, and pbcp-1 seedlings were grown in white light for 10 d and then transferred to far-red light (FR) for 20 or 40 min. Total protein extracts were analyzed by immunoblotting with antiphosphothreonine antibodies from Zymed and then with antiactin antibodies as a loading control.
(B) Wild-type, pbcp-1, and pbcp-2 seedlings were treated and analyzed as in (A) with antiphosphothreonine antibodies (Cell Signaling). The relative intensities of the phosphoprotein signals differ with different sources of antiphosphothreonine antibodies (cf. to [A], where the antibody from Zymed was used).
(C) Wild-type, pbcp-1, and rescued (resc; pbcp-1;35S:PBCP:HA) seedlings were treated and analyzed as in (A) with antiphosphothreonine antibodies from Zymed. Anti-PBCP serum was also used to monitor the level of expression of the transgenic protein in the rescued line. Its slower migration is due to the presence of the triple HA epitope, but the nature of the doublet band that is observed is not known.
(D) Wild-type, pbcp-1, and pbcpOE overexpressor seedlings were analyzed as in (C). Minor bands that comigrate with PBCP:HA in the wild-type and pbcp-1 samples (FR) are due to artifactual spillover of the samples from the adjacent lanes.
Mass Spectrometry Analysis of PBCP-Dependent Thylakoid Protein Dephosphorylation
To measure the phosphorylation of thylakoid proteins specifically and quantitatively, we used two methods based on HPLC coupled to electrospray ionization mass spectrometry (LC-MS). Thylakoid membranes were isolated from the leaves of mutant and wild-type plants that had been exposed to blue light or far-red light for 30 min. The surface-exposed peptides were then released by extensive proteolytic shaving with trypsin. In the first method of analysis, the peptide samples from the wild type and the mutant were differentially labeled by esterification of carboxylic groups with hydrogen- or deuterium-containing methanol, respectively (Vener et al., 2001; Ficarro et al., 2002; Shapiguzov et al., 2010). A 1:1 mixture of these two preparations was subjected to immobilized metal ion affinity chromatography (IMAC) to retain the phosphorylated peptide methyl esters. These were then subjected to LC-MS, which allowed simultaneous measurements of light- and heavy-isotope labeled phosphopeptide pairs and, thus, the determination of the ratio of phosphorylation of specific peptides in the mutant and the wild type (Table 1). In the second method of analysis, the surface-exposed peptides released by trypsin were directly analyzed by high-resolution LC-MS. Both the phosphorylated and dephosphorylated forms of peptides from the core subunits of PSII were identified and quantified as described previously (Fristedt et al., 2010). The values for phosphorylation of individual peptides were corrected for the difference in the signal intensities of the phosphorylated and dephosphorylated forms, and the percentage of phosphorylation of each peptide was calculated (Table 2).
Table 1. Ratios of Phosphopeptides in pbcp-1 versus Wild-Type Leaves.
|
pbcp-1/Wild Type Ratio |
||||
| Protein Name | Identified Phosphopeptide | At Gene Identifier | Blue | Far Red |
| D1 | Ac-tAILER | AtCg00020 | 1.4 ± 0.1** | 2.2 ± 0.2** |
| D2 | Ac-tIALGK | AtCg00270 | 1.3 ± 0.1** | 2.0 ± 0.2** |
| PsbH | AtQTVEDSSR | AtCg00710 | 1.3 ± 0.5 | 2.0 ± 0.9 |
| PsbH | AtQtVEDSSR | AtCg00710 | 1.6 ± 0.5 | 1.5 ± 0.5 |
| LHCB 1.1 | Ac-RKtVAKPK | At1g29910 | 0.7 ± 0.1** | 0.9 ± 0.2 |
| LHCB 1.2 | At1g29920 | |||
| LHCB 1.3 | At1g29930 | |||
| LHCB 1.5 | At2g34420 | |||
| LHCB 2.1 | Ac-RRtVK | At2g05100 | 0.8 ± 0.2 | 0.8 ± 0.1 |
| LHCB 2.2 | At2g05070 | |||
| LHCB 2.4 | At3g27690 | |||
| CaS | SGtKFLPSSD | At5g23060 | 1.0 ± 0.3 | 1.1 ± 0.3 |
Some members of the LHCB family share a common tryptic peptide as shown; phosphorylation cannot be ascribed to a specific gene product. “Ac” denotes N-terminal acetylation and “t” represents P-Thr. Results are from four IMAC experiments with differential stable isotope labeling. Asterisks indicate a statistically significant difference at P < 0.01 in the Student’s t test.
Table 2. In Vivo Phosphorylation Stoichiometry (Percentage of Phosphorylation) for the PSII Core Proteins from Wild-Type and pbcp-1 Leaves.
| Blue Light |
Far-Red Light |
|||||
| Protein | Wild Type | pbcp-1 | t Test | Wild Type | pbcp-1 | t Test |
| P-D1 | 42 ± 4 | 60 ± 6 | ** | 20 ± 3 | 45 ± 4 | ** |
| P-D2 | 42 ± 7 | 56 ± 9 | * | 17 ± 4 | 32 ± 2 | ** |
| P-PsbH | 35 ± 6 | 40 ± 2 | 20 ± 3 | 42 ± 3 | ** | |
| PP-PsbH | 23 ± 9 | 37 ± 4 | ** | 12 ± 6 | 19 ± 5 | |
A single asterisk and a double asterisk represent statistically significant differences between the pbcp-1 mutant and the wild type at P < 0.05 and P < 0.01, respectively, in the Student’s t test.
Strikingly, after exposure to far-red light, the ratio of phosphorylation of the D1 and D2 polypeptides of PSII in the pbcp-1 mutant versus the wild type was approximately two (Table 1), in agreement with the results of immunoblotting. In the wild type, the percentage of phosphorylation of D1 and D2 was twofold lower after far-red light treatment compared with blue light (Table 2). By contrast, in the pbcp-1 mutant phosphorylation decreased only slightly compared with the decrease in the wild type. We also noted that in blue light D1 and D2 were phosphorylated to a higher extent in the mutant than in the wild type (Tables 1 and 2).
We could also detect and quantify two phosphorylated forms of PsbH. After far-red treatment, the isoform of PsbH that is monophosphorylated at Thr-2 was over twofold more abundant in the mutant than in the wild type, reflecting its higher degree of phosphorylation in the pbcp-1 mutant (Table 2). The form that is diphosphorylated at Thr-2 and Thr-4 showed a different behavior. It was present in similar proportions in the mutant and the wild type after far-red treatment, even though in the mutant it may have been slightly overphosphorylated in blue light (Table 2). The proportion of diphosphorylated PsbH in the wild type and in pbcp-1 diminished to a similar extent in far-red compared with blue, suggesting that PBCP is not required for dephosphorylation of P-Thr at position 4.
After far-red treatment, there was no significant difference between the mutant and the wild type in the level of phosphorylation of the LHCII antenna proteins Lhcb1 and Lhcb2 (Table 1), in agreement with the results obtained by immunoblotting with antiphosphothreonine antibodies (Figure 1A). Under blue light, the mutant showed reduced levels of Lhcb1 phosphorylation compared with the wild type. One interpretation of this observation is that compensatory changes in the activity of other phosphatases could occur when PBCP is deficient. The phosphorylation levels of the CaS protein, a presumptive target of STN8, were also unaffected in the mutant (Vainonen et al., 2008). Phosphopeptides from CP43 were not detected in this mass spectrometry (MS) analysis; conversely, PsbH was not analyzed by immunoblotting. Taken together, the data from the two methods indicate that PBCP is required for efficient dephosphorylation of the core subunits of PSII (CP43, D1, D2, and PsbH-Thr2) but not of the LHCII proteins, such as Lhcb1 and Lhcb2.
PBCP Belongs to the Family of PP2C Phosphatases
We obtained two alleles of PBCP: pbcp-1 carries a SALK T-DNA insertion in the fourth exon and pbcp-2 contains a WiscDs-Lox insert in the 5′ untranslated region (see Supplemental Figure 1 online) (Alonso and Ecker, 2006; Woody et al., 2007). Both homozygous mutants show similar delays in dephosphorylation of the core proteins of PSII (Figure 1B). In this panel, an antiphosphothreonine antibody from a different commercial source (Cell Signaling) was used for comparison with Figure 1A (antibody from Zymed). Different antibodies have distinct recognition specificities, as demonstrated by contrasting the relative signal intensities of the main phosphoproteins detected in Figures 1A and 1B (Vainonen et al., 2005). In particular, the antibodies from Zymed detect phosphorylation of the PSII subunits more efficiently; conversely, those from Cell Signaling recognize LHCII more strongly.
We used Agrobacterium tumefaciens to transform the homozygous pbcp-1 mutant with a wild-type copy of the PBCP cDNA tagged with a triple-haemagglutinin (HA) epitope and placed under the control of the cauliflower mosaic virus 35S promoter. Among the pbcp-1;35S:PBCP:HA transformed lines, one was selected in which PBCP accumulates to levels only slightly higher than the wild type (dubbed “rescued”). As shown in Figure 1C, rapid dephosphorylation of the PSII core subunits, induced by a transfer from white to far-red light, was restored to a large extent in the rescued line. Taken together, these data show that the defect in dephosphorylation of the PSII core is indeed caused by a mutation in PBCP. A second pbcp-1;35S:PBCP:HA line was also obtained, in which PBCP is strongly overexpressed (pbcpOE; Figure 1D). In this line, dephosphorylation of the core subunits of PSII was more rapid than in the wild type. Unexpectedly, the LHCII antenna was also somewhat less phosphorylated compared with the wild type in white light or after 20 min of far-red treatment. Since phosphorylation of LHCII is not elevated in the pbcp mutants, it is not clear whether dephosphorylation of LHCII in pbcpOE indicates that the antenna is a target of PBCP or whether overexpression induces a loss of specificity and a promiscuous activity of the phosphatase toward LHCII.
The PBCP polypeptide deduced from the cDNA sequence has 298 residues, a molecular mass of 32 kD, and is predicted to carry an N-terminal transit peptide for chloroplast targeting by the algorithms TargetP (Emanuelsson et al., 2000), Wolfpsort (Horton et al., 2007), and Multiloc (Höglund et al., 2006). The PBCP protein is not predicted to contain any transmembrane domains. The mature protein belongs to the family of PP2C protein phosphatases, which comprises ∼80 members in plants (Kerk et al., 2008; Xue et al., 2008) and includes the PPH1/TAP38 phosphatase. PBCP and its orthologs belong to a phylogenetic clade that extends from monocots and dicots to the lycopod Selaginella moellendorffii, the moss Physcomitrella patens, and the green algae C. reinhardtii and Volvox carteri (see Supplemental Figure 2, Supplemental References 1, and Supplemental Data Set 1 online). This phylogenetic analysis thus shows that PBCP has been conserved throughout the evolution of plants (Viridiplantae). PBCP shares only ∼16% sequence identity with PPH1/TAP38 and is more closely related to other PP2C phosphatases, such as At4G33500, which is part of a neighboring phylogenetic clade. However, insertion mutants of the latter did not show any detectable phenotype in a dephosphorylation assay with far-red light similar to those presented in Figure 1.
A polyclonal antiserum raised against PBCP recognizes a polypeptide of ∼32 kD in the wild type (Figures 1C and 1D; see Supplemental Figure 1C online). This is approximately the predicted molecular mass of the mature PBCP protein after removal of the putative transit peptide. The protein is not detectable in pbcp-1 and pbcp-2 (see Supplemental Figure 1C online), indicating that both are strong or null alleles and confirming that the 32-kD band recognized by the antiserum is indeed the PBCP protein. A slightly retarded migration of PBCP, which can be ascribed to the presence of the triple-HA tag, is observed in the rescued mutant and in the pbcpOE line (Figures 1C and 1D).
PBCP Is a Chloroplast Protein
The deduced amino acid sequence of PBCP carries a predicted N-terminal transit peptide for chloroplast targeting. Indeed, D. Leister and coworkers (Schliebner et al., 2008) observed that the fluorescent protein dsRed fused to the transit peptide of PBCP (At2g30170) clearly localizes to the chloroplasts in transfected Arabidopsis protoplasts. This protein was also identified in purified chloroplast fractions by MS (Zybailov et al., 2008). To confirm the localization of PBCP, we prepared chloroplasts from rosette leaves and further fractionated them by centrifugation into a stromal supernatant and a membrane pellet. These fractions were analyzed by immunoblotting using antiserum against PBCP, and the quality of the fractionation was assessed with antisera against the thylakoid protein D1, the stromal enzyme phosphoribulokinase (PRK), and the cytoskeletal protein actin (Figure 2). The PBCP protein was largely found in the chloroplast stromal fraction, and its distribution closely paralleled the stromal marker PRK. A minor amount of PBCP was also observed in the membrane fraction.
Figure 2.
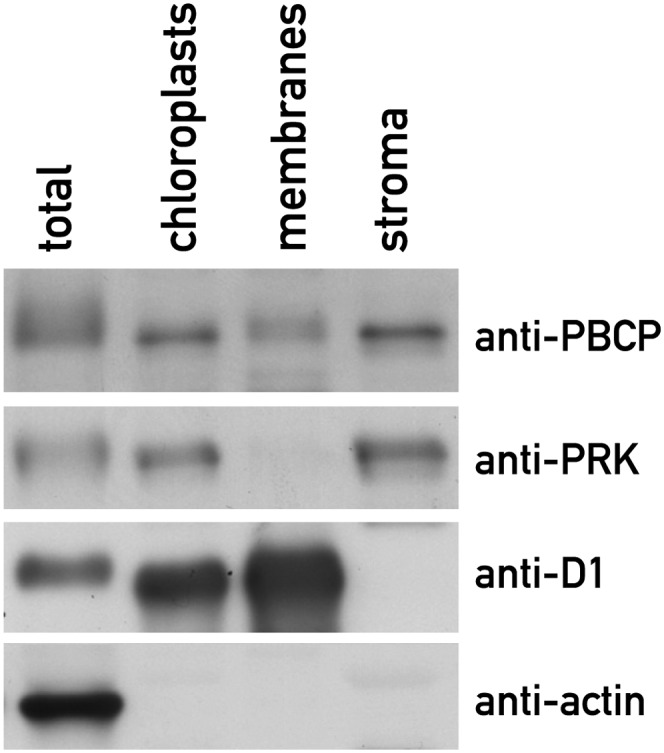
PBCP Is a Chloroplast Protein.
Chloroplasts from wild-type plants were isolated and further fractionated into a membrane pellet and a stromal supernatant. Equal amounts of protein from each fraction (10 µg) were analyzed by immunoblotting with antibodies against PBCP, the stromal protein PRK, the thylakoid membrane protein D1 of PSII, and the cytoskeletal protein actin.
PBCP Dephosphorylates Subunits of PSII in Vitro
As described above, PBCP belongs to the family of PP2C phosphatases, and thylakoid protein dephosphorylation is affected in the pbcp mutants in planta. To confirm that PBCP indeed has phosphatase activity and to gain insight regarding whether its action on the core subunits of PSII is direct, we purified recombinant PBCP (rPBCP) and assayed its activity in vitro. The mature form of PBCP, without the predicted transit peptide but with a C-terminal poly-His tag, was expressed in Escherichia coli. The enzyme was purified under denaturing conditions by Ni-affinity chromatography, allowed to refold by dialysis into nondenaturing conditions, and then further purified by size exclusion chromatography (Figure 3A). A His-tagged bacterial maltose binding protein (MBP) was expressed and purified in parallel as a negative control. The phosphatase activity of rPBCP was first demonstrated with the chromogenic substrate, para-nitrophenyl phosphate (pNPP) (Figure 3B). Consistent with some similar phosphatases of the PP2C class, the activity of rPBCP was much higher with Mn2+ than with Mg2+ as a cofactor in the assay (Pullen et al., 2004; Wehenkel et al., 2007), and the activity was strongly inhibited by the addition of EDTA as a chelator. Surprisingly, the activity was almost unaffected by the addition of 10 mM NaF, a general inhibitor of thylakoid protein phosphatases (Bennett, 1980; Hammer et al., 1995; Vener et al., 1999). Addition of the reducing agent DTT stimulated the activity, raising the possibility that the phosphatase could be regulated by the redox state of its Cys residues. To test the activity of rPBCP on its natural substrates, thylakoid membranes were purified from the pbcp-1 mutant and exposed to white light for 15 min in the presence of ATP to maximize thylakoid protein phosphorylation. The membranes were then incubated in the dark in the presence of purified rPBCP, using as controls the buffer or the purified MBP protein. Dephosphorylation was monitored by immunoblotting with antiphosphothreonine antibodies. After 5 min of incubation, the dephosphorylation activity of rPBCP in the presence of Mn2+ was apparent as a decrease in the phosphorylation of CP43, D1, and D2 (Figure 3C, lane 5 versus lane 1). Phosphorylation decreased by 40 to 50% for D1 and CP43, and ∼70% for D2. The activity was strongly inhibited by the addition of EDTA (lane 7). It was much lower in the presence of Mg2+ and was not significantly inhibited by NaF (see Supplemental Figure 3 online). The addition of DTT did not have a detectable effect on the activity (Figure 3C, lane 6), but the sensitivity of the assay may be insufficient to detect the effect observed with pNPP as a substrate. Some dephosphorylation of the LHCII proteins was apparent in this in vitro assay, similar to what was observed in vivo for the pbcpOE overexpressor.
Figure 3.
Recombinant PBCP Has Phosphatase Activity in Vitro.
(A) SDS-PAGE of purified rPBCP loaded under reducing and denaturing conditions. The sizes of the markers in lane M are indicated on the left (kD).
(B) In vitro spectrophotometric assay of PBCP activity. The dephosphorylation of pNPP was measured by the increase in absorption at 405 nm as a function of time in 1-mL reaction volumes containing 5 µg recombinant PBCP, 300 mM NaCl, 50 mM HEPES, pH 8.0, 10 mM pNPP, and the additives indicated. Error bars represent the sd of triplicate measurements.
(C) Thylakoid membranes (containing 0.16 µg chlorophyll) isolated from the pbcp-1 mutant, exposed to white light for 15 min, and then incubated for 5 min in the dark in the presence of purified recombinant PBCP (5 µg), or with protein buffer or recombinant MBP as controls, in a final reaction volume of 100 μL containing 5 mM MnCl2. The effects of DTT (10 mM) and EDTA (5 mM) were tested as indicated. The samples were analyzed by immunoblotting with antiphosphothreonine antibodies from Zymed.
Role of PBCP in Thylakoid Membrane Organization
In the stn8 mutant and the stn7 stn8 double mutant, which are deficient in thylakoid protein phosphorylation, the membranes tend to form extended grana stacks (Fristedt et al., 2009a), suggesting that thylakoid protein phosphorylation affects membrane folding and grana size. We examined the ultrastructure of the thylakoid membranes in leaf chloroplasts of the pbcp mutants, the pbcpOE overexpressor, and the wild type by transmission electron microscopy. We compared 11-d-old seedlings treated with blue light for 3 h to favor thylakoid protein phosphorylation and further treated for 1.5 h with far-red light to promote dephosphorylation (Figure 4). To avoid any differences due to cell type–specific differentiation of the plastids, we always selected chloroplasts in the subepidermal cell layer on the adaxial side, the presumptive palisade mesophyll cells of these developing leaves. There was a striking difference between the wild type and the pbcp mutants, which showed many regions where only a few membranes were appressed and, thus, a concomitant reduction in the number of layers in the grana stacks (Figures 4A to 4D). Thylakoid membrane folding in the pbcpOE was similar to the wild type (Figures 4E and 4F). To represent these differences quantitatively, we measured the total length of thylakoid membranes that were found in single stromal lamellae or in grana stacks with different number of layers. The resulting histogram (Figure 4G) shows that in the wild type the broad distribution extended to stacks with 10 or more layers, while in the mutants the distribution was clearly displaced toward fewer layers and showed only very few grana stacks with eight layers or more. Thus, the activities of the PBCP phosphatase and of the STN8 kinase seem to affect the folding of the thylakoid membrane in opposing ways. The distribution in the pbcpOE was similar to the wild type, as might be expected after prolonged far-red treatment when thylakoid protein phosphorylation in the wild type and the overexpressor reaches comparable low levels (Figure 1).
Figure 4.
Thylakoid Folding Is Altered in pbcp Mutants.
Electron micrographs of thin sections from leaves of 11-d-old seedlings exposed to blue light for 3 h and then shifted to far-red light for 1.5 h to induce dephosphorylation of the PSII core subunits. (B), (D), and (F) show larger magnifications of (A), (C), and (E), respectively. WT, the wild type. Bars = 500 nm.
(A) and (B) The wild type.
(C) and (D) pbcp-2.
(E) and (F) pbcpOE.
(G) The length of thylakoid membranes in stromal lamella and in grana stacks with different numbers of layers were measured in electron micrographs of the wild type (n = 30), the pbcp-1 mutant (n = 22), the pbcp-2 mutant (n = 12), and the pbcpOE overexpressor (n = 9). The histograms show the length of membranes in domains with the indicated numbers of layers (one layer for stromal lamellae and two or more layers in grana stacks) as a fraction of the total length of membranes measured.
State Transitions in the pbcp Mutants and pbcpOE Overexpressor
In the different pbcp-1 transgenic lines transformed with the 35S:PBCP:HA construct, PBCP was expressed at different levels (Figures 1C and 1D). It was thus possible to compare different lines forming an allelic series with increasing amounts of PBCP, from the pbcp mutants devoid of PBCP, through the wild type or the rescued line with slightly elevated levels of PBCP, to the pbcpOE overexpressor with strongly increased accumulation of the phosphatase. We observed a consistent trend in the levels of PSII phosphorylation and the kinetics of dephosphorylation. In the pbcp-1 mutant exposed to white or blue light, phosphorylation of the thylakoid proteins was somewhat elevated compared with the wild type (Figure 1, Tables 1 and 2), and dephosphorylation upon transfer to far-red light was strongly retarded. The rescued line showed normal phosphorylation patterns in white light and restored dephosphorylation upon transfer to far-red light (Figure 1C). In pbcpOE, we observed slightly reduced phosphorylation in white light and rapid dephosphorylation upon transfer to far-red light (Figure 1D). This is consistent with the presumed antagonistic effects of the STN8 kinase and the PBCP phosphatase in white or blue light, where the kinase is active, and the role of PBCP in dephosphorylation in far-red light, where the kinase is thought to be downregulated. However, these observations are also compatible with the possibility that the activity of PBCP is regulated.
State transitions, which involve phosphorylation of the LHCII antennae and their dynamic allocation to PSII or PSI, are regulated by the STN7 kinase and the PPH1/TAP38 phosphatase. Blue or white light induces a transition to state 2 through the activation of the STN7 kinase and the association of phosphorylated LHCII with PSI. This is reversed in far-red light, which induces dephosphorylation of the LHCII antennae under the action of the PPH1/TAP38 phosphatase and allocation of LHCII to PSII. While the STN8 kinase has substrate specificity distinct from STN7, the activities of the two kinases may partly overlap. This is suggested by the residual phosphorylation of LHCII and PSII subunits, which is observed in the single mutants of the kinases, and is more extensively abolished in the double mutant stn7 stn8 (Bonardi et al., 2005; Tikkanen et al., 2008). It was therefore of interest to investigate whether the PBCP phosphatase mutants were affected in state transitions, which were measured by monitoring chlorophyll fluorescence in two sets of experiments, either at room temperature or at 77K.
At room temperature, chlorophyll fluorescence emanates almost exclusively from PSII and its light-harvesting antennae. Under white light, which favors state 2, part of the LHCII antenna is dissociated from PSII so the maximal fluorescence Fm2 induced by a saturating flash is lower than the maximal fluorescence Fm1 in far-red light, which induces state 1 and the association of LHCII with PSII (Figure 5A). The state transition parameter, qT = (Fm1 − Fm2) × 100/Fm1 was approximately +3 in the wild-type seedlings but decreased to a negative value of −2 in the stn7 mutant and was also strongly affected in the pph1-3 mutant, both of which were used as controls that are known to affect LHCII phosphorylation and state transitions (Figure 5B). qT was comparable to the wild type in the stn8 mutant and in the rescued line, as well as in the pbcp-1 and pbcp-2 mutants. Thus, state transitions are not significantly affected in the absence of PBCP. Interestingly, qT was strongly reduced in the pbcpOE overexpressor line, an indication that state transitions may be affected by the increased activity of PBCP. This was also apparent when the kinetics of state transitions were monitored by following the fluorescence parameter Fs, which is influenced by the redox state of the PQ pool. When the far-red light is turned off, Fs increases abruptly, reflecting the overreduction of the PQ pool, and then Fs gradually decreases as transition to state 2 allows reoxidation of the pool (Figure 5C). Compared with the wild type, this decrease was blocked in the stn7 mutant, was strongly retarded in the pph1-3 mutant, and was not affected in the stn8, pbcp-1, and pbcp-2 lines. The kinetics of the Fs decrease in the transition from state 1 to state 2 were strikingly slower in the pbcpOE overexpressor, suggesting that state transitions are affected by the increased activity of the phosphatase. A small effect was also apparent in the rescued line, which has slightly elevated levels of PBCP (Figure 1C).
Figure 5.
State Transitions in the pbcp Mutants and pbcpOE Overexpressor.
(A) The kinetics of state transitions were analyzed by continuously monitoring chlorophyll fluorescence with a FluorCam (PhotoSystems Instruments). After a preincubation of 30 min in darkness, actinic light (represented below as a white bar) was turned on for 17 min to induce state 2 before maximal fluorescence (Fm2) was measured with a saturating flash (arrow). Far-red light was then turned on for 17 min (represented below as a red bar) to induce state 1, and maximal fluorescence (Fm1) was again measured with a saturating flash (arrow) before the far-red light was turned off to return to state 2. The curves (normalized to Fo) are the average of 26 seedlings of each genotype from two experiments. Line breaks indicate that the Fm peaks are out of scale. WT, the wild type.
(B) The state transition parameter qT was calculated from the experiments in (A) (qT= (Fm1 − Fm2) × 100/Fm1). The bars represent the mean and sd of 26 seedlings of each genotype from two experiments. The asterisks indicate P < 0.001 in Student’s t test.
(C) Chlorophyll fluorescence was normalized for the value of Fs immediately after turning the far-red light off to allow comparisons of the kinetics of the transition to state 2.
State transitions can also be monitored by measuring the fluorescence emission spectra of chlorophyll at 77K. Emission at 685 nm can be ascribed to PSII and emission at 732 nm to PSI. As the LHCII antenna is reallocated by state transitions, changes in the cross section of the two photosystems are reflected by changes in the relative size of the respective fluorescence emission peaks (see Supplemental Figure 4 online). When seedlings were transferred from far-red light to blue light, the amplitude of the transition to state 2 was reduced in the pbcpOE line compared with the wild type, but not as strongly as in the stn7 mutant. Conversely, when the seedlings were returned to far-red light, the transition to state 1 was more pronounced in the pbcpOE line than in the wild type. Consistent with the qT and Fs measurements at room temperature, this indicates that overexpression of PBCP affects state transition. These results can be correlated with the observation that the LHCII proteins are less phosphorylated in pbcpOE compared with the wild type (Figure 1D).
DISCUSSION
While PPH1/TAP38 was shown to be necessary for dephosphorylation of LHCII subunits (LHCB 1 and LHCB 2) (Pribil et al., 2010; Shapiguzov et al., 2010), here, we demonstrate that PBCP is required for efficient dephosphorylation of the core subunits CP43, D1, D2, and PsbH of PSII. The substrate specificities of the two phosphatases are similar to those of the two kinases, STN7 and STN8 (Bellafiore et al., 2005; Bonardi et al., 2005; Vainonen et al., 2005; Fristedt and Vener, 2011; Reiland et al., 2011). The PBCP phosphatase and the STN8 kinase thus seem to form an antagonistic pair, whose reciprocal activities on phosphorylation of PSII core subunits could allow a balanced acclimatory response. This is similar to the pair formed by the STN7 kinase and the PPH1/TAP38 phosphatase, which have opposing effects on phosphorylation of the LHCII antenna to regulate state transitions and maintain a balanced redox state of the electron transfer chain.
Although the two phosphatases appear to have distinct specificities, overexpression of PBCP perturbed state transitions and the phosphorylation of LHCII, which are the major roles ascribed to PPH1/TAP38. The converse effects were not obvious in the pbcp mutants, which did not show elevated levels of LHCII phosphorylation in white or blue light (Figure 1, Table 1) or changes in the kinetics of state transitions (Figure 5; see Supplemental Figure 4 online). Thus caution should be used in interpreting the phenotype of the pbcpOE line, which is a dominant gain-of-function mutant, since overexpression of PBCP might cause a loss of its normal specificity. Nevertheless, it is interesting to consider three more possible interpretations, which are not entirely mutually exclusive, of these observations. One is that PBCP may have some overlapping activity toward LHCII, which only becomes apparent upon overexpression, and that its specificity may not be entirely restricted to the PSII core. A similar situation seems to prevail for the two kinases, whose substrate specificity appears to overlap since the residual phosphorylation that is apparent in the single kinase mutants, stn7 or stn8, is abolished in the double mutant stn7 stn8 (Tikkanen et al., 2008; Fristedt et al., 2009a). An alternative interpretation is that PBCP may have functional interactions with other members of a regulatory network, for example, PPH1 or STN7, and influence their activity directly or indirectly. A different interpretation could be that phosphorylation of the core subunits influences the mobility of proteins within the thylakoid membrane, which in turn may influence the rate of state transitions. Because of steric hindrance, PSI and ATP synthase are excluded from the PSII-enriched appressed membranes of grana stacks. This segregation is thought to minimize spillover of energy between the photosystems (Dekker and Boekema, 2005). Changes in protein phosphorylation may affect the function of the photosynthetic electron transfer chain by changing the distribution and interactions of the photosynthetic complexes and in particular may influence state transitions by limiting the transfer of LHCII between PSI and PSII. Phosphorylation by the STN7 and STN8 kinases was shown to influence the mobility of thylakoid proteins within the membrane (Goral et al., 2010).
Phosphorylation of PsbH reveals another aspect of the antagonism between STN8 and PBCP. There are two phosphorylation sites at the N terminus of PsbH, at Thr-2 and Thr-4. We find that in the pbcp mutant, it is Thr-2 that retains phosphorylation in far-red light, whereas Thr-4 is dephosphorylated as in the wild type. However, MS analysis of phosphorylation in the stn8 mutant has shown that the STN8 kinase is responsible for phosphorylation at Thr-4 and that this requires prior phosphorylation at Thr-2 (Vainonen et al., 2005; Fristedt and Vener, 2011). Thus, in the case of PsbH, antagonism of PBCP and STN8 might involve the dephosphorylation of Thr-2 by PBCP, which in turn would prevent phosphorylation by STN8 at Thr-4. Little is known on the physiological role of phosphorylation at the individual Thr residues in PsbH or in D2 and CP43. In C. reinhardtii, a mutation of PsbH that prevents phosphorylation of Thr-2 by replacing it with Ala had no apparent phenotypic consequence (O’Connor et al., 1998). Mutation of the D2 subunit changing Thr-2 to Ala allowed normal photosynthetic activity, but mutations to Asp or Glu, which mimic phosphorylation, strongly affected PSII accumulation (Fleischmann and Rochaix, 1999).
What then may be the function of STN8 and PBCP in light acclimation? In the stn8 mutant, phosphorylation of PSII is reduced and the size of the thylakoid membrane grana increases. Conversely, we observed in the pbcp mutants that phosphorylation of PSII is retained upon exposure to far-red light and that the proportion of large grana stacks decreases. Furthermore, STN8 may facilitate the repair cycle of PSII by promoting unfolding of the membranes and allowing easier access of membrane proteases such as FtsH and cotranslational integration of D1 (Tikkanen et al., 2008; Fristedt et al., 2009a). However, our attempts to determine whether photoinhibition and D1 turnover are affected in the pbcp mutants did not give consistent results.
The rPBCP purified from E. coli showed phosphatase activity in vitro using the artificial substrate pNPP. Since rPBCP promoted dephosphorylation of the core subunits of PSII in isolated thylakoids, it seems likely that these are its direct substrates. However, we cannot entirely exclude the possibility that PBCP is only indirectly required for dephosphorylation of PSII. As with some other PP2C phosphatases, PBCP is Mn2+ dependent and has negligible activity with Mg2+ as the metal cofactor (Pullen et al., 2004; Wehenkel et al., 2007). The activity of the phosphatase is strongly inhibited by the addition of EDTA. Surprisingly NaF, which is a general inhibitor of thylakoid phosphatases (Bennett, 1980; Vener et al., 1999), has only a relatively minor effect on PBCP activity in vitro, both when assayed with pNPP or with thylakoid membranes. This observation is puzzling because in pumpkin (Cucurbita maxima) leaves NaF could prevent dephosphorylation of the PSII core subunits and retard degradation of D1 (Rintamäki et al., 1996). It will thus be interesting to determine whether in Arabidopsis the PBCP phosphatase is directly involved in the repair cycle of D1 or whether another phosphatase is involved.
The PP2C family of protein phosphatases comprises ∼80 members in Arabidopsis or rice (Oryza sativa; Xue et al., 2008). The number is much larger than in animals, and this expansion in plants suggests that PP2C phosphatases have acquired distinct substrate specificities or novel functions (Kerk et al., 2008). Indeed, our data indicate that for the dephosphorylation of thylakoid membrane proteins, PPH1/TAP38 and PBCP have different substrate preferences. The presence of different phosphatases is one possible explanation for the various kinetic classes that were discerned in assays of endogenous phosphatase activity with isolated thylakoid membranes from pea (Pisum sativum) chloroplasts (Silverstein et al., 1993). The strong preference of PBCP for Mn2+ and its insensitivity to NaF distinguish it from both the stromal and the membrane phosphatases active on LHCII phosphopeptides, which were previously purified from pea chloroplasts (Hammer et al., 1995, 1997). It is expected that further phosphatases involved in regulating thylakoid membrane structure and function remain to be identified.
METHODS
Plant Material
Arabidopsis thaliana mutant lines in Columbia-0 background were obtained from the European Arabidopsis Stock Center (pbcp-1 is SALK_127920.31.10.N, and pbcp-2 is WiscDsLox_359F02). They were genotyped as described in Supplemental Figure 1 online. The stn7 (Bellafiore et al., 2005), stn8 (Vainonen et al., 2005; Fristedt et al., 2009a), and pph1-3 mutants (Shapiguzov et al., 2010) were described.
To rescue the pbcp-1 mutant with the wild-type cDNA, the complete coding sequence of PBCP (At2g30170), excluding the stop codon, was amplified by PCR from a cDNA clone (pda00938) obtained from the Riken Institute (Japan). The forward primer (5′-ATCTCGAGCGAGAGAGCAAGCCATTGCAG-3′) and reverse primer (5′-GTCGACTGAGCTAACAACTTTGGCAACG-3′) included XhoI and SalI sites, respectively. The PCR product was inserted in the XhoI and SalI sites of pCF399 under the control of the cauliflower mosaic virus 35S promoter and the NOS terminator with a triple HA epitope tag at the C terminus (Lorrain et al., 2008). Plants of Arabidopsis were transformed by floral dipping using Agrobacterium tumefaciens GV3101 strain. T1, T2, and T3 progeny seedlings were selected on Murashige and Skoog medium supplemented with 1.5% Suc and 10 µg mL−1 Basta (Pentanal).
Dephosphorylation in Vivo
For the genetic screen, seedlings were grown for 10 d on Murashige and Skoog agar in a growth chamber (Percival CU36L5) in long days (16 h light and 8 h dark) at 22°C under fluorescent white light (50 to 60 µE m−2 s−1). Dephosphorylation was monitored as described (Shapiguzov et al., 2010) by placing seedlings under far-red light-emitting diodes (LEDs; L735; Epitex) for the designated time. Seedlings were snap-frozen in liquid nitrogen and ground twice for 10 s with glass beads in a triturator (Silamat S5; Ivoclar Vivadent). Protein extracts were prepared by incubating in lysis buffer containing 100 mM Tris-HCl, pH 7.7, 2% SDS, 50 mM NaF, and 2× Protease Inhibitor Mixture (Sigma-Aldrich) for 30 min at 37°C. The samples were separated by Tris-Gly SDS-PAGE in 12% acrylamide gels supplemented with 6 M urea, which allowed of better separation of the D1 and D2 phosphoproteins. Immunoblotting was performed using antiphosphothreonine antibodies (Zymed or Cell Signaling) or antiactin as a control (Sigma-Aldrich).
Analyses of in Vivo Protein Phosphorylation by MS
Thylakoids for MS analyses were isolated from 15-d-old seedlings grown on Murashige and Skoog agar plates. The plants were dark-adapted for 1 h and then placed under far-red or blue LEDs for 30 min to induce state 1 or state 2, respectively. Thylakoid isolation was performed in the dark (under green light) in the cold room as described (Fristedt et al., 2009a). For trypsin treatment thylakoids were resuspended in 25 mM NH4HCO3 and 10 mM NaF at a concentration of 2 mg mL−1 of chlorophyll and incubated with sequencing-grade modified trypsin (Promega) for 3 h at room temperature. Peptide extracts were cleared by ultracentrifugation, aliquoted, and dried in a SpeedVac.
For relative quantitative MS studies, the collected peptides from trypsin-treated wild-type and pbcp-1 mutant thylakoids were subjected to esterification by either d0-methyl methanol or d3-methyl methanol (Sigma-Aldrich) prior to enrichment of phosphopeptides by IMAC. Both esterification of peptides and the subsequent phosphopeptide enrichment by IMAC were done as described before (Shapiguzov et al., 2010). The esterified samples were mixed 1:1, subjected to IMAC, and analyzed by nano-liquid chromatography electrospray ionization MS, which allowed simultaneous measurements of intensities for light and heavy isotope–labeled phosphopeptide pairs and quantitative comparison of the phosphorylation differences in the mutant and wild-type proteins. The reverse labeling of peptides from the wild type and pbcp-1 was performed as an internal control. For analysis of the peptides, an online nano-flow HPLC system (EASY-nLC; Bruker Daltonics) was used and MS analysis with the HCTultra PTM discovery system (Bruker Daltonics) was performed using the same settings as before (Shapiguzov et al., 2010). The automated, online, tandem MS analyses were made using alternating collision-induced dissociation/electron transfer dissociation fragmentation of peptide ions.
Label-free characterization of the phosphorylation status for the PSII core proteins was done by LC-MS analyses of the peptides obtained from the proteolytic treatment of wild-type and pbcp-1 thylakoids without further enrichment, as described (Fristedt et al., 2010). LC-MS separation and detection of the phosphorylated peptides and their nonphosphorylated counterparts was used for determination of the phosphorylation stoichiometry for PSII proteins. The level of phosphorylation for the PSII core proteins was calculated from the ratios of phosphorylated to nonphosphorylated peptide intensities in each LC-MS chromatogram using normalization for the difference in the signal intensities of the phosphorylated and dephosphorylated peptide ions from the D1, D2, and PbsH proteins as determined (Fristedt et al., 2010).
Purification of Recombinant PBCP
The cDNA sequence of the predicted mature PBCP protein was cloned into pET28a (Invitrogen) to include a C-terminal 6x-His tag and expressed in Escherichia coli strain BL21. Following cell lysis and centrifugation at 7600g, the pellet containing recombinant PBCP was solubilized in 6 M guanidine with phosphate buffer at pH 8.0. Cell debris was pelleted at 23,000g, and the denatured proteins were loaded onto Ni-nitrilotriacetic acid (Ni-NTA) resin (Qiagen). The Ni-NTA was washed with 4 M urea containing 35 mM imidazole, and rPBCP was eluted with 2 M urea containing 300 mM imidazole and 200 mM NaCl. EDTA (10 mM) and 20 mM 2-mercaptoethanol were added to the eluate. Denatured PBCP was refolded by dialyzing the eluate with 300 mM NaCl and 50 mM HEPES, pH 8.0. Following dialysis, the rPBCP eluate was centrifuged at 23,000g and further purified by size exclusion chromatography on a preparative grade Superdex-200 column (GE Healthcare) with a mobile phase of 300 mM NaCl and 50 mM HEPES, pH 8.0. Recombinant PBCP eluted at the expected globular particle size of ∼30 kD and was concentrated to stocks of 1 mg mL−1. As a source of antigen to raise a rabbit anti-PBCP serum, the recombinant protein was purified by Ni-NTA chromatography as recommended by the manufacturer (Qiagen) followed by preparative SDS-PAGE.
Activity Assays of Recombinant PBCP
Recombinant PBCP phosphatase activity was assayed in vitro by monitoring the dephosphorylation of pNPP. The reaction rate was calculated by the increase in absorbance at 405 nm as a function of time across the linear portion of the curve. The reaction mixture contained 300 mM NaCl, 50 mM HEPES, pH 8.0, 5 mM MnCl2 or MgCl2, 10 mM pNPP, and 5 µg mL−1 PBCP (∼170 nM). Stocks of pNPP, NaF, DTT, and EDTA were prepared in aqueous solution containing 300 mM NaCl, 50 mM HEPES, pH 8.0, and 5 mM MnCl2 or MgCl2.
Plants for thylakoid extraction were cultivated for 3 weeks on soil in growth chambers at 24°C under white light (100 µE) in short days (8 h light, 16 h dark). Thylakoids were prepared as described (Fristedt et al., 2009a) without addition of NaF, washed in a buffer containing 25 mM Tricine-NaOH, pH 7.8, 100 mM sorbitol, 10 mM KCl, and either 5 mM MgCl2 or 5 mM MnCl2. Thylakoids (containing 0.17 mg mL−1 chlorophyll) were supplemented with ATP to a concentration of 0.4 mM and incubated in white light (100 μE m−2·s−1) for 15 min. Recombinant PBCP, MBP, or buffer was added to the thylakoids and, where indicated, supplemented with 10 mM DTT or 50 mM NaF and incubated in the dark for the designated times before the reaction was stopped by the addition of the 4× gel-loading lysis buffer (10% SDS, 40% Suc, 1 mM EDTA, 50 mM Tris-HCl, pH 6.8, and 20% β-mercaptoethanol). Thylakoid protein phosphorylation was analyzed by immunoblotting with antiphosphothreonine antibodies and quantified by comparison to a dilution series of an untreated thylakoid sample.
Isolation of Intact Chloroplasts and Fractionation
Intact chloroplasts were isolated from mature plants grown on soil in long days (12 h light, 12 h dark). The procedure was performed on ice. The leaves were ground in 20 mM HEPES-KOH, pH 8.0, 5 mM EDTA, and 330 mM sorbitol with a Polytron mixer (Kinematica). The homogenate was filtered through two layers of Miracloth (Calbiochem) and centrifuged at 1000g at 4°C for 5 min. The chloroplast pellet was resuspended in 0.5 mL of the same buffer, and intact chloroplasts were isolated on a Percoll gradient as described (Aronsson and Jarvis, 2002). Washed intact chloroplasts were lysed by hypo-osmotic shock in 10 mM Tricine-NaOH, pH 7.8, 5 mM MgCl2, and 10 mM NaF. The insoluble fraction containing thylakoid membranes was pelleted by centrifugation (21,000g for 10 min). The soluble fraction enriched in chloroplast stromal proteins was cleared by an additional centrifugation step (21,000g for 30 min). The samples were treated with 10% trichloroacetic acid to precipitate protein.
Transmission Electron Microscopy
Seedlings were grown on Murashige and Skoog agar plates (16 h light, 8 h dark, 50 to 60 µE m−2 s−1) for 11 d. Four hours after the onset of light, they were exposed for 3 h to blue LEDs and then for 1.5 h to far-red LEDs (L470 and L735; Epitex). Cuttings from the first pair of leaves, taken in the middle of the lamina, were immediately immersed in 2.5% glutaraldehyde in 100 mM cacodylate sodium buffer, pH 7.0, containing 0.01% Tween 20. The leaf pieces were fixed overnight at 4°C under agitation. They were then rinsed sequentially (30 min/step) in cacodylate sodium buffer containing (1) 0.1% Tween, (2) 0.05% Tween, and (3) three times in cacodylate alone. The samples were postfixed in 1.5% OsO4 for 2 h, washed three times in cacodylate buffer, then once in milliQ water, and further fixed in 1% aqueous uranyl acetate. Sample preparation was performed at 4°C. After washing in distilled water, the tissue was dehydrated in a graded ethanol series and embedded in Epon 812. Ultrathin cross sections (85 nm thick) were cut and stained with 2.5% uranyl acetate and then Reynolds lead citrate. The sections were viewed in a FEI Tecnai G2 Sphera transmission electron microscope at 120 kV. Care was taken to always select plastids located in adaxial subepidermal cells (presumptive palisade parenchyma cells). The lengths of membranes from electron micrographs were measured using ImageJ software (http://rsbweb.nih.gov/ij/). Data presented in Figure 4G are from 30 images of the wild type and 22 of pbcp-1 (from five independent experiments), 12 of pbcp-2 (two experiments), and nine of pbcpOE (one experiment).
Chlorophyll Fluorescence Analysis
Room temperature chlorophyll fluorescence was monitored with a FluorCam 800MF kinetic imaging fluorimeter (PhotoSystems Instruments) essentially as described (Willig et al., 2011). Seedlings grown on Murashige and Skoog agar for 15 d were dark-adapted for 30 min. White actinic light (8%) was then switched on for 17 min to induce state 2. Far-red light (100%) was then turned on against the background of continuing white light for 17 min to stimulate transition from state 2 to state 1. Finally, far-red light was turned off to initiate the reverse transition. Measuring flashes continued throughout the protocol to record Fs. Saturating light flashes were applied at the end of the dark, at the end of the 17-min white, and at the end of the 17-min white plus far-red light periods to measure Fm, Fm2, and Fm1, respectively. The fluorescence signal was averaged for 26 seedlings. Chlorophyll fluorescence at 77K was measured on seedlings essentially as described (Shapiguzov et al., 2010). Seedlings were frozen in liquid nitrogen, ground, and resuspended in ice-cold buffer containing 50 mM HEPES-KOH, pH 7.5, 100 mM sorbitol, 10 mM MgCl2, and 10 mM NaF. The samples were filtered and frozen in liquid nitrogen, and chlorophyll fluorescence at 77K was recorded on a Jasco FP-750 spectrofluorometer. Excitation was at 480 nm (slit width 5 nm) and emission was recorded in the 600- to 800-nm range (slit width of 5 nm). Curves were normalized for the PSII peak at 685 nm.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under accession numbers AT2G30170.1 and AY063046.1.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Two Mutant Alleles of PBCP.
Supplemental Figure 2. Phylogenetic Analysis of PBCP Homologs.
Supplemental Figure 3. PBCP Activity in Vitro in the Presence of Mg2+ or NaF.
Supplemental Figure 4. Analysis of State Transitions by Chlorophyll Fluorescence at 77K.
Supplemental Data Set 1. Text File of Alignment Used for Phylogenetic Analysis in Supplemental Figure 2.
Supplemental References 1. Supplemental References for Supplemental Figure 2.
Acknowledgments
We thank Sam Zeeman for advice on devising the genetic screen, Elisabet Gas for help with chloroplast isolation, Natalia Cabanas Fiscowich for contributing to the chlorophyll fluorescence measurements, Christian Fankhauser for advice and a gift of pCF399, and Nicolas Roggli for preparing the figures. This work was supported by SystemsX.ch (RTD Plant Growth in a Changing Environment), grants from the Swiss National Foundation (3100AO-117712 and 31003A_133089/1), the FP7 Marie-Curie Initial Training Network (ITN) COSI (ITN 2008 GA 215-174), an EMBO postdoctoral fellowship (to G.F.), and a grant from the Swedish Research Council (2008-5490).
AUTHOR CONTRIBUTIONS
M.G.-C., J.-D.R., A.V.V., I.S., A.S, G.F., B.I., and M.C. designed the research. I.S., A.S., G.F., B.I., and M.C. performed research. M.G.-C., J.-D.R., A.V.V., I.S., A.S., G.F., B.I., and M.C. analyzed data. M.G.-C. and J.-D.R. wrote the article.
Glossary
- PSII
photosystem II
- PSI
photosystem I
- PQ
plastoquinone
- LHCII
light-harvesting complex II
- NPQ
nonphotochemical quenching
- LC-MS
HPLC coupled to electrospray ionization mass spectrometry
- IMAC
immobilized metal ion affinity chromatography
- HA
to be defined
- pNPP
para-nitrophenyl phosphate
- LED
light-emitting diode
- MS
mass spectrometry
- Ni-NTA
to be defined
References
- Allen J.F. (1992). Protein phosphorylation in regulation of photosynthesis. Biochim. Biophys. Acta 1098: 275–335 [DOI] [PubMed] [Google Scholar]
- Alonso J.M., Ecker J.R. (2006). Moving forward in reverse: Genetic technologies to enable genome-wide phenomic screens in Arabidopsis. Nat. Rev. Genet. 7: 524–536 [DOI] [PubMed] [Google Scholar]
- Aro E.M., Ohad I. (2003). Redox regulation of thylakoid protein phosphorylation. Antioxid. Redox Signal. 5: 55–67 [DOI] [PubMed] [Google Scholar]
- Aronsson H., Jarvis P. (2002). A simple method for isolating import-competent Arabidopsis chloroplasts. FEBS Lett. 529: 215–220 [DOI] [PubMed] [Google Scholar]
- Baena-González E., Aro E.M. (2002). Biogenesis, assembly and turnover of photosystem II units. Philos. Trans. R. Soc. Lond. B Biol. Sci. 357: 1451–1459, discussion 1459–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber J. (1980). An explanation for the relationship between salt-induced thylakoid stacking and the chlorophyll fluorescence changes associated with changes in spillover of energy from photosystem II to photosystem I. FEBS Lett. 118: 1–10 [Google Scholar]
- Barber J. (1982). Influence of surface charges on thylakoid structure and function. Annu. Rev. Plant Physiol. 33: 261–295 [Google Scholar]
- Bellafiore S., Barneche F., Peltier G., Rochaix J.D. (2005). State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature 433: 892–895 [DOI] [PubMed] [Google Scholar]
- Bennett J. (1980). Chloroplast phosphoproteins. Evidence for a thylakoid-bound phosphoprotein phosphatase. Eur. J. Biochem. 104: 85–89 [DOI] [PubMed] [Google Scholar]
- Bonardi V., Pesaresi P., Becker T., Schleiff E., Wagner R., Pfannschmidt T., Jahns P., Leister D. (2005). Photosystem II core phosphorylation and photosynthetic acclimation require two different protein kinases. Nature 437: 1179–1182 [DOI] [PubMed] [Google Scholar]
- Chuartzman S.G., Nevo R., Shimoni E., Charuvi D., Kiss V., Ohad I., Brumfeld V., Reich Z. (2008). Thylakoid membrane remodeling during state transitions in Arabidopsis. Plant Cell 20: 1029–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker J.P., Boekema E.J. (2005). Supramolecular organization of thylakoid membrane proteins in green plants. Biochim. Biophys. Acta 1706: 12–39 [DOI] [PubMed] [Google Scholar]
- Depège N., Bellafiore S., Rochaix J.D. (2003). Role of chloroplast protein kinase Stt7 in LHCII phosphorylation and state transition in Chlamydomonas. Science 299: 1572–1575 [DOI] [PubMed] [Google Scholar]
- Eberhard S., Finazzi G., Wollman F.A. (2008). The dynamics of photosynthesis. Annu. Rev. Genet. 42: 463–515 [DOI] [PubMed] [Google Scholar]
- Edelman M., Mattoo A.K. (2008). D1-protein dynamics in photosystem II: The lingering enigma. Photosynth. Res. 98: 609–620 [DOI] [PubMed] [Google Scholar]
- Emanuelsson O., Nielsen H., Brunak S., von Heijne G. (2000). Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J. Mol. Biol. 300: 1005–1016 [DOI] [PubMed] [Google Scholar]
- Ficarro S.B., McCleland M.L., Stukenberg P.T., Burke D.J., Ross M.M., Shabanowitz J., Hunt D.F., White F.M. (2002). Phosphoproteome analysis by mass spectrometry and its application to Saccharomyces cerevisiae. Nat. Biotechnol. 20: 301–305 [DOI] [PubMed] [Google Scholar]
- Finazzi G., Rappaport F., Furia A., Fleischmann M., Rochaix J.D., Zito F., Forti G. (2002). Involvement of state transitions in the switch between linear and cyclic electron flow in Chlamydomonas reinhardtii. EMBO Rep. 3: 280–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann M.M., Rochaix J.D. (1999). Characterization of mutants with alterations of the phosphorylation site in the D2 photosystem II polypeptide of Chlamydomonas reinhardtii. Plant Physiol. 119: 1557–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fristedt R., Carlberg I., Zygadlo A., Piippo M., Nurmi M., Aro E.M., Scheller H.V., Vener A.V. (2009b). Intrinsically unstructured phosphoprotein TSP9 regulates light harvesting in Arabidopsis thaliana. Biochemistry 48: 499–509 [DOI] [PubMed] [Google Scholar]
- Fristedt R., Granath P., Vener A.V. (2010). A protein phosphorylation threshold for functional stacking of plant photosynthetic membranes. PLoS ONE 5: e10963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fristedt R., Vener A.V. (2011). High light induced disassembly of photosystem II supercomplexes in Arabidopsis requires STN7-dependent phosphorylation of CP29. PLoS ONE 6: e24565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fristedt R., Willig A., Granath P., Crèvecoeur M., Rochaix J.D., Vener A.V. (2009a). Phosphorylation of photosystem II controls functional macroscopic folding of photosynthetic membranes in Arabidopsis. Plant Cell 21: 3950–3964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goral T.K., Johnson M.P., Brain A.P., Kirchhoff H., Ruban A.V., Mullineaux C.W. (2010). Visualizing the mobility and distribution of chlorophyll proteins in higher plant thylakoid membranes: Effects of photoinhibition and protein phosphorylation. Plant J. 62: 948–959 [DOI] [PubMed] [Google Scholar]
- Hammer M.F., Gautam S., Osterman J.C., Markwell J. (1995). Assessing modulation of stromal and thylakoid light-harvesting complex-II phosphatase activities with phosphopeptide substrates. Photosynth. Res. 44: 107–115 [DOI] [PubMed] [Google Scholar]
- Hammer M.F., Markwell J., Sarath G. (1997). Purification of a protein phosphatase from chloroplast stroma capable of dephosphorylating the light-harvesting complex-II. Plant Physiol. 113: 227–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heazlewood J.L., Verboom R.E., Tonti-Filippini J., Small I., Millar A.H. (2007). SUBA: The Arabidopsis Subcellular Database. Nucleic Acids Res. 35 (Database issue): D213–D218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglund A., Dönnes P., Blum T., Adolph H.W., Kohlbacher O. (2006). MultiLoc: Prediction of protein subcellular localization using N-terminal targeting sequences, sequence motifs and amino acid composition. Bioinformatics 22: 1158–1165 [DOI] [PubMed] [Google Scholar]
- Horton P., Park K.J., Obayashi T., Fujita N., Harada H., Adams-Collier C.J., Nakai K. (2007). WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 35 (Web Server issue): W585–W587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerk D., Templeton G., Moorhead G.B. (2008). Evolutionary radiation pattern of novel protein phosphatases revealed by analysis of protein data from the completely sequenced genomes of humans, green algae, and higher plants. Plant Physiol. 146: 351–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemeille S., Rochaix J.D. (2010). State transitions at the crossroad of thylakoid signalling pathways. Photosynth. Res. 106: 33–46 [DOI] [PubMed] [Google Scholar]
- Li Z., Wakao S., Fischer B.B., Niyogi K.K. (2009). Sensing and responding to excess light. Annu. Rev. Plant Biol. 60: 239–260 [DOI] [PubMed] [Google Scholar]
- Lorrain S., Allen T., Duek P.D., Whitelam G.C., Fankhauser C. (2008). Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J. 53: 312–323 [DOI] [PubMed] [Google Scholar]
- Martinsuo P., Pursiheimo S., Aro E.M., Rintamäki E. (2003). Dithiol oxidant and disulfide reductant dynamically regulate the phosphorylation of light-harvesting complex II proteins in thylakoid membranes. Plant Physiol. 133: 37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa J. (2011). State transitions—The molecular remodeling of photosynthetic supercomplexes that controls energy flow in the chloroplast. Biochim. Biophys. Acta 1807: 897–905 [DOI] [PubMed] [Google Scholar]
- O’Connor H.E., Ruffle S.V., Cain A.J., Deak Z., Vass I., Nugent J.H., Purton S. (1998). The 9-kDa phosphoprotein of photosystem II. Generation and characterisation of Chlamydomonas mutants lacking PSII-H and a site-directed mutant lacking the phosphorylation site. Biochim. Biophys. Acta 1364: 63–72 [DOI] [PubMed] [Google Scholar]
- Pribil M., Pesaresi P., Hertle A., Barbato R., Leister D. (2010). Role of plastid protein phosphatase TAP38 in LHCII dephosphorylation and thylakoid electron flow. PLoS Biol. 8: e1000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen K.E., Ng H.L., Sung P.Y., Good M.C., Smith S.M., Alber T. (2004). An alternate conformation and a third metal in PstP/Ppp, the M. tuberculosis PP2C-Family Ser/Thr protein phosphatase. Structure 12: 1947–1954 [DOI] [PubMed] [Google Scholar]
- Reiland S., Finazzi G., Endler A., Willig A., Baerenfaller K., Grossmann J., Gerrits B., Rutishauser D., Gruissem W., Rochaix J.D., Baginsky S. (2011). Comparative phosphoproteome profiling reveals a function of the STN8 kinase in fine-tuning of cyclic electron flow (CEF). Proc. Natl. Acad. Sci. USA 108: 12955–12960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rintamäki E., Kettunen R., Aro E.M. (1996). Differential D1 dephosphorylation in functional and photodamaged photosystem II centers. Dephosphorylation is a prerequisite for degradation of damaged D1. J. Biol. Chem. 271: 14870–14875 [DOI] [PubMed] [Google Scholar]
- Rintamäki E., Martinsuo P., Pursiheimo S., Aro E.M. (2000). Cooperative regulation of light-harvesting complex II phosphorylation via the plastoquinol and ferredoxin-thioredoxin system in chloroplasts. Proc. Natl. Acad. Sci. USA 97: 11644–11649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rintamäki E., Salonen M., Suoranta U.M., Carlberg I., Andersson B., Aro E.M. (1997). Phosphorylation of light-harvesting complex II and photosystem II core proteins shows different irradiance-dependent regulation in vivo. Application of phosphothreonine antibodies to analysis of thylakoid phosphoproteins. J. Biol. Chem. 272: 30476–30482 [DOI] [PubMed] [Google Scholar]
- Rochaix J.D. (2007). Role of thylakoid protein kinases in photosynthetic acclimation. FEBS Lett. 581: 2768–2775 [DOI] [PubMed] [Google Scholar]
- Schliebner I., Pribil M., Zühlke J., Dietzmann A., Leister D. (2008). A survey of chloroplast protein kinases and phosphatases in Arabidopsis thaliana. Curr. Genomics 9: 184–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiguzov A., Ingelsson B., Samol I., Andres C., Kessler F., Rochaix J.D., Vener A.V., Goldschmidt-Clermont M. (2010). The PPH1 phosphatase is specifically involved in LHCII dephosphorylation and state transitions in Arabidopsis. Proc. Natl. Acad. Sci. USA 107: 4782–4787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein T., Cheng L., Allen J.F. (1993). Chloroplast thylakoid protein phosphatase reactions are redox-independent and kinetically heterogeneous. FEBS Lett. 334: 101–105 [DOI] [PubMed] [Google Scholar]
- Takahashi S., Murata N. (2008). How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 13: 178–182 [DOI] [PubMed] [Google Scholar]
- Tikkanen M., Aro E.M. (2012). Thylakoid protein phosphorylation in dynamic regulation of photosystem II in higher plants. Biochim. Biophys. Acta 1817: 232–238 [DOI] [PubMed] [Google Scholar]
- Tikkanen M., Grieco M., Aro E.M. (2011). Novel insights into plant light-harvesting complex II phosphorylation and ‘state transitions’. Trends Plant Sci. 16: 126–131 [DOI] [PubMed] [Google Scholar]
- Tikkanen M., Grieco M., Kangasjärvi S., Aro E.M. (2010). Thylakoid protein phosphorylation in higher plant chloroplasts optimizes electron transfer under fluctuating light. Plant Physiol. 152: 723–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikkanen M., Nurmi M., Kangasjärvi S., Aro E.M. (2008). Core protein phosphorylation facilitates the repair of photodamaged photosystem II at high light. Biochim. Biophys. Acta 1777: 1432–1437 [DOI] [PubMed] [Google Scholar]
- Vainonen J.P., Hansson M., Vener A.V. (2005). STN8 protein kinase in Arabidopsis thaliana is specific in phosphorylation of photosystem II core proteins. J. Biol. Chem. 280: 33679–33686 [DOI] [PubMed] [Google Scholar]
- Vainonen J.P., Sakuragi Y., Stael S., Tikkanen M., Allahverdiyeva Y., Paakkarinen V., Aro E., Suorsa M., Scheller H.V., Vener A.V., Aro E.M. (2008). Light regulation of CaS, a novel phosphoprotein in the thylakoid membrane of Arabidopsis thaliana. FEBS J. 275: 1767–1777 [DOI] [PubMed] [Google Scholar]
- Vener A.V. (2007). Environmentally modulated phosphorylation and dynamics of proteins in photosynthetic membranes. Biochim. Biophys. Acta 1767: 449–457 [DOI] [PubMed] [Google Scholar]
- Vener A.V., Harms A., Sussman M.R., Vierstra R.D. (2001). Mass spectrometric resolution of reversible protein phosphorylation in photosynthetic membranes of Arabidopsis thaliana. J. Biol. Chem. 276: 6959–6966 [DOI] [PubMed] [Google Scholar]
- Vener A.V., van Kan P.J, Rich P.R., Ohad I., Andersson B. (1997). Plastoquinol at the quinol oxidation site of reduced cytochrome bf mediates signal transduction between light and protein phosphorylation: Thylakoid protein kinase deactivation by a single-turnover flash. Proc. Natl. Acad. Sci. USA 94: 1585–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vener A.V., Rokka A., Fulgosi H., Andersson B., Herrmann R.G. (1999). A cyclophilin-regulated PP2A-like protein phosphatase in thylakoid membranes of plant chloroplasts. Biochemistry 38: 14955–14965 [DOI] [PubMed] [Google Scholar]
- Wehenkel A., Bellinzoni M., Schaeffer F., Villarino A., Alzari P.M. (2007). Structural and binding studies of the three-metal center in two mycobacterial PPM Ser/Thr protein phosphatases. J. Mol. Biol. 374: 890–898 [DOI] [PubMed] [Google Scholar]
- Willig A., Shapiguzov A., Goldschmidt-Clermont M., Rochaix J.D. (2011). The phosphorylation status of the chloroplast protein kinase STN7 of Arabidopsis affects its turnover. Plant Physiol. 157: 2102–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollman F.A. (2001). State transitions reveal the dynamics and flexibility of the photosynthetic apparatus. EMBO J. 20: 3623–3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody S.T., Austin-Phillips S., Amasino R.M., Krysan P.J. (2007). The WiscDsLox T-DNA collection: An Arabidopsis community resource generated by using an improved high-throughput T-DNA sequencing pipeline. J. Plant Res. 120: 157–165 [DOI] [PubMed] [Google Scholar]
- Xue T., Wang D., Zhang S., Ehlting J., Ni F., Jakab S., Zheng C., Zhong Y. (2008). Genome-wide and expression analysis of protein phosphatase 2C in rice and Arabidopsis. BMC Genomics 9: 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P., Hennig L., Gruissem W. (2005). Gene-expression analysis and network discovery using Genevestigator. Trends Plant Sci. 10: 407–409 [DOI] [PubMed] [Google Scholar]
- Zito F., Finazzi G., Delosme R., Nitschke W., Picot D., Wollman F.A. (1999). The Qo site of cytochrome b6f complexes controls the activation of the LHCII kinase. EMBO J. 18: 2961–2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zybailov B., Rutschow H., Friso G., Rudella A., Emanuelsson O., Sun Q., van Wijk K.J. (2008). Sorting signals, N-terminal modifications and abundance of the chloroplast proteome. PLoS One 3: e1994. [DOI] [PMC free article] [PubMed] [Google Scholar]






