This work examines the function of a maize heat-stable, less inhibitor–sensitive form of ADP-glucose pyrophosphorylase, which increases maize yield by increasing seed number. This work shows that this increase requires high temperature during early seed development and results from transgene function in maternal tissues to increase the probability that an ovary will produce a seed.
Abstract
The maize (Zea mays) shrunken-2 (Sh2) gene encodes the large subunit of the rate-limiting starch biosynthetic enzyme, ADP-glucose pyrophosphorylase. Expression of a transgenic form of the enzyme with enhanced heat stability and reduced phosphate inhibition increased maize yield up to 64%. The extent of the yield increase is dependent on temperatures during the first 4 d post pollination, and yield is increased if average daily high temperatures exceed 33°C. As found in wheat (Triticum aestivum) and rice (Oryza sativa), this transgene increases maize yield by increasing seed number. This result was surprising, since an entire series of historic observations at the whole-plant, enzyme, gene, and physiological levels pointed to Sh2 playing an important role only in the endosperm. Here, we present several lines of evidence that lead to the conclusion that the Sh2 transgene functions in maternal tissue to increase seed number and, in turn, yield. Furthermore, the transgene does not increase ovary number; rather, it increases the probability that a seed will develop. Surprisingly, the number of fully developed seeds is only ∼50% of the number of ovaries in wild-type maize. This suggests that increasing the frequency of seed development is a feasible agricultural target, especially under conditions of elevated temperatures.
INTRODUCTION
Starch makes up ∼70% of the cereal seed and, hence, is the major contributor to yield. The precursor of starch, ADP-Glc, is synthesized by the enzyme ADP-glucose pyrophosphorylase (AGPase) from Glc-1-P and ATP. Plant AGPases are heterotetramers composed of two identical small subunits and two identical large subunits. The enzyme is subject to allosteric control, and the effectors orthophosphate and 3-phosphoglycerate have received the most attention (reviewed in Ballicora et al., 2003; Hannah, 2007; Hannah and Greene, 2008; Hannah and James, 2008; Preiss, 2009; Keeling and Myers, 2010). We (Boehlein et al., 2010) showed that the maize (Zea mays) endosperm enzyme exhibits an ordered Theorell-Chance mechanism with ATP adding first and ADP-Glc releasing last.
The rate-limiting role that AGPase plays in starch synthesis is evident from a series of transgenic and genetic studies. This was first shown by Stark et al. (1992), who placed the allosterically enhanced Escherichia coli glgC-16 AGPase gene in potato (Solanum tuberosum) and increased tuber yield by 35%. A form of AGPase with diminished response to orthophosphate increased seed weight ∼12% in some maize lines (Giroux et al., 1996). Expression of a modified maize shrunken-2 (Sh2) gene encoding the large subunit of AGPase with enhanced heat stability and reduced orthophosphate inhibition (HS33/Rev6 Sh2) increased wheat (Triticum aestivum) yield 38% (Smidansky et al., 2002) and rice (Oryza sativa) yield 23% (Smidansky et al., 2003). An 11% increase in rice yield was also derived from the expression of another E. coli variant enzyme (Sakulsingharoj et al., 2004). Expression of E. coli glgC-16 in maize increased individual seed weight 13 to 25% (Wang et al., 2007), and overexpression of wild-type Sh2 and brittle-2 (Bt2; which encodes the endosperm small subunit of AGPase) increased individual maize seed weight 15% by increasing starch content (Li et al., 2011). Furthermore, addition of allosterically altered AGPases increased Arabidopsis thaliana leaf transitory starch turnover, improved its growth characteristics (Obana et al., 2006), and increased fresh weights of aerial parts of lettuce (Lactuca sativa) plants (Lee et al., 2009).
Surprisingly, the yield increase in wheat and rice by incorporation of the maize HS33/Rev6 Sh2 gene was due to an increase in seed number rather than an increase in individual seed weight. This result was surprising, since it was thought that Sh2 only had a physiologically important role in the endosperm and a mechanism by which this would increase seed number was not obvious. Here, we used maize genetics to decipher the cause of increased seed number. We show that HS33/Rev6 Sh2 does increase yield in maize by increasing seed number. Yield increases up to 64% were observed, depending on the temperature during the first 4 d following pollination. Genetic, physiological, and molecular data point to HS33/Rev6 Sh2 expression in maternal tissue, rather than in the seed, as the cause of enhanced seed number. HS33/Rev6 Sh2 enhances the probability of seed development rather than increasing the number of ovaries. Surprisingly, only about one-half of the wild-type Sh2 ovaries are represented as fully developed, mature kernels. Our data suggest that a significant fraction of the developing kernels, chosen at random, stop development and their contents disintegrate.
RESULTS
The HS33/Rev6 Sh2 Transgene Increases Maize Yield by Increasing Seed Number
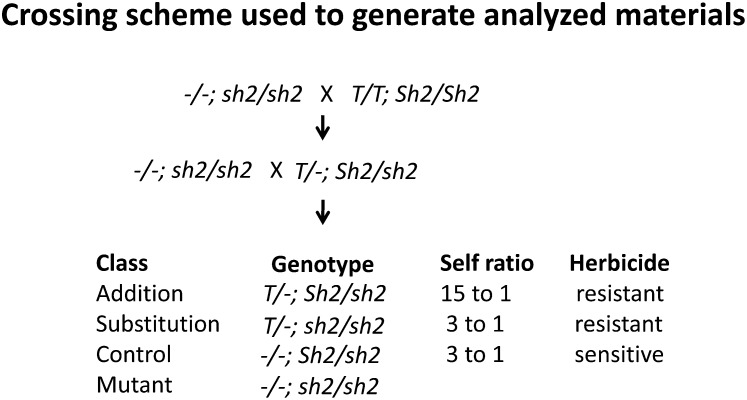
Maize backcross lines segregating for the endogenous functional Sh2 gene and for a Sh2 transgene containing the two changes described above (HS33 and Rev6) were synthesized and used for all analyses (Figure 1). A hybrid line homozygous for the transgene (termed T) and the endogenous functional allele, Sh2, was crossed to an inbred lacking Sh2 and T. Resulting progeny were backcrossed to the sh2/sh2 parent, resulting in backcross 1 families segregating three plump to one shrunken progeny. Plump seeds were composed of the three equally represented genotypes given in Figure 1. The three genotypes could be distinguished by the plump:shrunken kernel ratios seen upon selfing and tolerance to Basta herbicide applied to a small strip on one leaf 7 d post pollination.
Figure 1.
Crossing Scheme Used to Generate Segregating Materials for Analysis.
T refers to the HS33/Rev6 Sh2 transgene. The nontransgenic recurrent parent is a vigorous inbred homozygous for the loss-of-function sh2-R allele. Backcross 1 families segregate three plump to one shrunken. The plump class is composed of three equally represented genotypes as listed. The transgene also contains the BAR gene, allowing the identification of T-containing plants via application of the herbicide on a small portion of the leaf applied 7 d post pollination. T-containing plants containing or lacking the endogenous functional Sh2 allele are distinguished by the ratio of plump kernels in self-pollinations or by the ratios in crosses onto sh2/sh2 plants. Backcross 2 progeny were derived from crossing backcross 1 Addition plants to the recurrent parent.
Backcross 1 progeny from eight independent transformation events were grown, self-pollinated, and kernel number and weight were recorded. Total kernel weights from self-pollination of plants containing only the transgene (T/-; sh2/sh2; termed the Substitution line) or only the endogenous functional Sh2 allele (−/−; Sh2/sh2; termed the Control) are given in Table 1. An average increase in total kernel weight of 39% was observed in the Substitution lines. In agreement with the findings in wheat (Smidansky et al., 2002) and in rice (Smidansky et al., 2003), increased total kernel weight was not due to increased individual seed weight but rather to an increase in seed number.
Table 1. Number and Weight of Plump and Shrunken Seeds from Self-Pollination of Backcross 1 Plants Containing or Lacking the Designated Transgenic Event.
| Event | Parental Genotype | Wt plmp | No. plmp | Wt sh | No. sh | Total Weight | Weight Ratio | Total Seeds | Seed No. Ratio |
|---|---|---|---|---|---|---|---|---|---|
| 97 | Substitution | 61.70** | 293** | 11.86** | 96** | 73.56** | 1.84 | 389** | 1.72 |
| Control | 32.29 | 161 | 7.77 | 65 | 40.07 | 227 | |||
| 73 | Substitution | 55.10** | 260** | 10.41** | 95** | 65.51** | 2.05 | 355** | 2.06 |
| Control | 26.07 | 125 | 5.91 | 48 | 31.98 | 173 | |||
| 133 | Substitution | 71.00* | 300 | 17.00* | 97 | 88.00* | 1.53 | 396 | 1.30 |
| Control | 47.90 | 227 | 10.38 | 78 | 58.29 | 305 | |||
| 183 | Substitution | 67.75* | 317 | 14.12* | 121* | 81.9* | 1.31 | 439 | 1.23 |
| Control | 51.69 | 260 | 10.80 | 96 | 62.49 | 356 | |||
| 114 | Substitution | 42.42 | 190 | 8.25 | 73 | 50.67 | 1.20 | 263 | 1.30 |
| Control | 34.45 | 147 | 7.36 | 55 | 41.81 | 201 | |||
| 286 | Substitution | 36.34 | 153 | 8.75 | 69 | 45.09 | 1.05 | 222 | 1.00 |
| Control | 34.42 | 157 | 8.43 | 64 | 42.85 | 221 | |||
| 25 | Substitution | 47.59 | 219 | 9.53 | 71 | 57.12 | 1.10 | 290 | 1.09 |
| Control | 42.36 | 192 | 9.38 | 75 | 51.74 | 267 | |||
| 76 | Substitution | 55.30* | 244 | 11.12 | 90 | 66.43 | 1.25 | 334 | 1.22 |
| Control | 42.83 | 198 | 10.19 | 76 | 53.02 | 275 | |||
| Average | Substitution | 55.40** | 250** | 11.60** | 90** | 67.01** | 1.39 | 340** | 1.23 |
| Control | 39.49 | 186 | 8.81 | 70 | 48.31 | 276 |
Plants having the transgene were of the genotype T/-; sh2/sh2 (Substitution; Figure 1), and plants lacking the transgene were −/−; Sh2/sh2 (Control). Wt plmp and Wt sh refer to the total weight, in grams, of nonmutant or plump kernels and mutant or shrunken kernels, respectively. Plants were grown in Citra, Florida, in the spring of 2004. Significant differences at the 5% (*) and 1% (**) levels are noted. Averages and ratios of all events were calculated from all individual data. The population size was 169 ears and 52,591 kernels.
Surprisingly, there was a highly significant 29% increase in the number of shrunken seeds on plants containing the transgene compared with the nontransgenic Control plants. Since the shrunken seeds are genetically identical (−/−; sh2/sh2) in the two families, it was expected that their numbers would be equal if increased seed number is controlled only by the genotype of the seeds.
Increased seed number was also observed in the Addition family, containing both the transgene and the endogenous Sh2 allele (Table 2). Plants containing both the transgene and Sh2 produced 64% more seeds compared with the nontransgenic control containing only Sh2. While comparison of total seed weight of the genotypes T/-; Sh2/sh2 and −/−; Sh2/sh2 is uninformative, since the former class segregates 15 plump to 1 shrunken while the latter class segregates 3 plump to 1 shrunken, comparison of individual seed weights is useful. Importantly, incorporation of the transgene does not decrease individual plump seed weight. There is a reduction in the seed weight of the shrunken class, but this decrease does not occur when the transgene is substituted for the endogenous allele (cf. Substitution with Control). Perhaps the reduction in individual shrunken seed weight in selfed progeny of T/-; Sh2/sh2 plants, compared with T/-; sh2/sh2 and −/−; Sh2/sh2 plants, is due to an enhanced competition for photosynthate from the additional plump seeds developing on Addition line ears.
Table 2. Total Seed Number from Self-Pollination of Backcross 1 Plants Containing or Lacking the Designated Transgenic Event and Containing or Lacking the Endogenous Functional Sh2 Allele.
| Event | Parental Genotype | Weight, Individual Plump Seed | Weight, Individual Shrunken Seed | Total Seed Number | Ratio |
|---|---|---|---|---|---|
| 97 | Addition | 0.202 | 0.113 | 397** | 1.75 |
| Substitution | 0.210 | 0.123 | 389** | 1.72 | |
| Control | 0.200 | 0.118 | 227 | ||
| 73 | Addition | 0.197 | 0.108 | 351** | 2.03 |
| Substitution | 0.212 | 0.109 | 355** | 2.06 | |
| Control | 0.208 | 0.124 | 173 | ||
| 133 | Addition | 0.192 | 0.110* | 481** | 1.58 |
| Substitution | 0.237 | 0.175 | 396 | 1.30 | |
| Control | 0.211 | 0.133 | 305 | ||
| 183 | Addition | 0.211 | 0.118 | 408 | 1.14 |
| Substitution | 0.213 | 0.116 | 439 | 1.23 | |
| Control | 0.199 | 0.112 | 356 | ||
| 114 | Addition | 0.228 | 0.123 | 403** | 2.13 |
| Substitution | 0.223 | 0.114 | 263 | 1.21 | |
| Control | 0.235 | 0.134 | 202 | ||
| 286 | Addition | 0.221 | 0.101** | 368 | 1.61 |
| Substitution | 0.237 | 0.127 | 222 | 1.00 | |
| Control | 0.219 | 0.132 | 221 | ||
| 25 | Addition | 0.221 | 0.111 | 340 | 1.27 |
| Substitution | 0.217 | 0.133 | 290 | 1.09 | |
| Control | 0.221 | 0.125 | 267 | ||
| 76 | Addition | 0.232 | 0.112* | 430 | 1.56 |
| Substitution | 0.227 | 0.123 | 334 | 1.22 | |
| Control | 0.216 | 0.134 | 275 | ||
| Average | Addition | 0.213 | 0.112** | 400** | 1.64 |
| Substitution | 0.222 | 0.128 | 340** | 1.23 | |
| Control | 0.217 | 0.127 | 276 |
The three genotypic classes are defined in Figure 1. Plants were grown in Citra, Florida, in the spring of 2004. Total seed numbers marked with asterisks designate significant differences at the 5% (*) or 1% (**) level from the cognate nontransgene control. The ratio is the seed number of each of the two transgene-containing genotypes divided by the seed number of the nontransgenic control. Weights of individual seeds are in grams. Averages and ratios of all events were calculated from all individual data. The population size was 269 ears and 92,213 kernels.
Backcross 2 families with the same genotypes as above were examined the following growing season (Table 3). Increases in seed number were again noted (last column). The extent of increase, however, was not as great as that seen in the preceding generation. Whereas there was a 64% increase in seed number conditioned by the addition of the Sh2 transgene in the first generation, there was only a 26% increase in the second generation.
Table 3. Total Seed Number from Self-Pollination of Backcross 2 Plants Containing or Lacking the Designated Transgenic Event and Containing or Lacking the Endogenous Functional Sh2 Allele.
| Event | Parental Genotype | First Planting Seed No. | Ratio | Second Planting Seed No. | Ratio | Third Planting Seed No. | Ratio | Average | Ratio |
|---|---|---|---|---|---|---|---|---|---|
| 97 | Addition | 301* | 1.23 | 302 | 1.08 | 407 | 1.71 | 337** | 1.31 |
| Substitution | 338* | 1.39 | 267 | 0.96 | 390 | 1.64 | 335** | 1.31 | |
| Control | 243 | 279 | 237 | 257 | |||||
| 73 | Addition | 340** | 1.61 | 303* | 1.51 | 457 | 1.26 | 344* | 1.26 |
| Substitution | 276 | 1.31 | 355 | 1.77 | 359 | 0.99 | 324 | 1.18 | |
| Control | 211 | 200 | 362 | 274 | |||||
| 133 | Addition | 375 | 0.93 | 503* | 1.53 | 450 | 0.80 | 469 | 1.24 |
| Substitution | 360 | 0.89 | 521* | 1.59 | 462 | 0.82 | 419 | 1.11 | |
| Control | 408 | 329 | 562 | 377 | |||||
| 183 | Addition | 414 | 1.14 | 450 | 1.20 | 414* | 1.44 | 425** | 1.25 |
| Substitution | 313 | 0.86 | 377 | 1.00 | 346 | 1.20 | 344 | 1.01 | |
| Control | 364 | 376 | 287 | 340 | |||||
| 114 | Addition | 314 | 0.98 | 416 | 0.97 | 411* | 1.11 | 382 | 1.09 |
| Substitution | 327 | 1.02 | 373 | 0.87 | 352 | 0.95 | 354 | 1.01 | |
| Control | 321 | 428 | 371 | 352 | |||||
| 286 | Addition | 328 | 1.15 | 402* | 1.35 | 466** | 1.45 | 385** | 1.30 |
| Substitution | 273 | 0.95 | 429** | 1.43 | 397* | 1.23 | 379** | 1.28 | |
| Control | 286 | 299 | 323 | 297 | |||||
| 25 | Addition | 255 | 1.15 | 413** | 1.48 | 389 | 1.37 | 360** | 1.15 |
| Substitution | 290 | 1.31 | 381* | 1.37 | 425 | 1.50 | 334** | 1.31 | |
| Control | 222 | 278 | 284 | 241 | |||||
| 76 | Addition | 263 | 1.04 | 364 | 1.26 | 438* | 1.32 | 351* | 1.23 |
| Substitution | 259 | 1.02 | 341 | 1.18 | 383 | 1.15 | 338* | 1.19 | |
| Control | 252 | 290 | 332 | 284 | |||||
| Average | Addition | 330**c | 1.19 | 380**b | 1.24 | 426**a | 1.31 | 378** | 1.26 |
| Substitution | 304b | 1.10 | 368**a | 1.20 | 381**a | 1.17 | 350** | 1.17 | |
| Control | 276b | 307a,b | 325a | 299 |
The three genotypes are defined in Figure 1. Plants were grown in Citra, Florida, in the spring of 2005. Plantings were done weekly over 3 weeks and are labeled first, second, and third planting. Total seed numbers marked with asterisks designate significant differences at the 5% (*) or 1% (**) level from the cognate nontransgene control of the same planting date. The ratio is the seed number of each of the two transgene-containing genotypes divided by the seed number of the nontransgenic control that contains one copy of the functional Sh2 allele. Letters refer to significant (5%) differences distinguishing the three planting dates. Averages and ratios of all events were calculated from all individual data. The total population was 498 ears and 170,448 kernels.
Increased Seed Number Is Dependent on Temperatures during Early Kernel Development
To facilitate crossing to other lines, these backcross 2 families were planted three times over a 14-d period and seed number data for each planting date were determined (Table 3, middle columns). Significant differences in seed number were noted across planting dates, pointing to environmental factors affecting the extent of increase in seed number conditioned by the transgene. In assessing the cause of this difference, we noted that plants were irrigated two to three times per week; hence, water stress occurring at different times in the life cycle cannot account for these differences. Also, the experimental design incorporated two individual 4.6-m (15 plants) rows for each genotype planted serpentine style across 16 rows of 91 m, with the three planting dates interspersed. Since plants of the different planting dates were intermixed within the experiment, local pockets of differing soil fertility would not account for these differences.
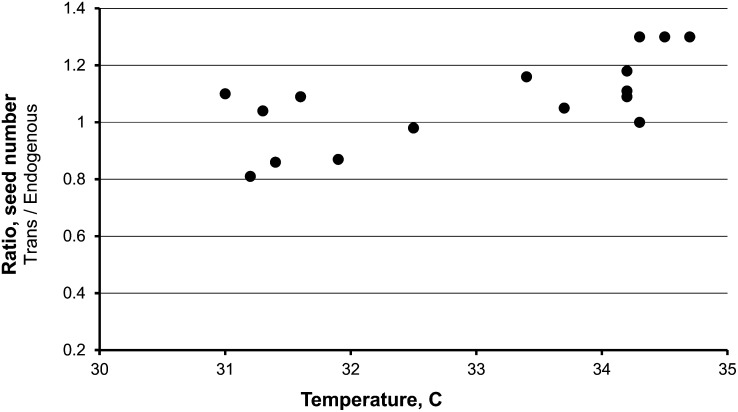
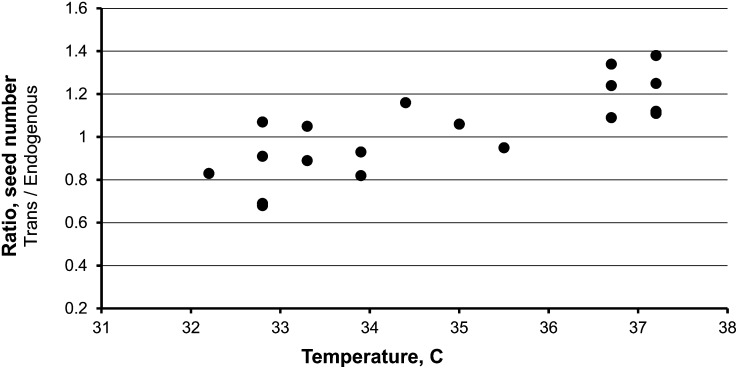
A relationship between increased seed number and field temperatures was found. Starting in 2006, the first date of pollination of each ear was recorded and the difference in seed number conditioned by the transgene was measured across all events. Correlations were examined for both daily high and low temperatures versus different times before or after pollination. The only consistent difference found involved high temperatures early in seed development. Plotted in Figure 2 is the increase in seed number caused by the transgene versus the average daily high temperature for the first 4 d post pollination. The greatest reproducible increases in seed number occurred only when temperatures exceeded 33°C. Analogous data from the 2008 planting are given in Figure 3. Again, reproducible increases in seed number occurred only at the higher temperatures. Consistent with this interpretation, only 2 d of the ∼24 d of pollinations in 2007 were above 33°C, and no transgene-induced increase in seed number was detected. While pollination dates were not recorded in 2004, the year of the greatest increase, pollinations were made in the last week of May and daily high temperatures ranged from 34 to 37°C during this period. We conclude that temperatures exceeding ∼33°C early in seed development are required for HS33/Rev6 Sh2–induced seed increase. Data for the graphs in Figures 2 and 3 are presented in Supplemental Table 1 online. Interestingly, whereas seed number is strongly positively correlated with average daily high temperatures for transgene-containing plants, no positive (or negative) correlation was noted for plants lacking the transgene.
Figure 2.
Seed Number of Backcross 3 Plants Containing the Sh2 Transgene Divided by the Seed Number of Sibling Plants Lacking the Transgene Plotted Versus the Average Daily High Temperature for the First 4 d Post Pollination.
Plants were grown in Citra, Florida, in 2006. In total, 761 ears and 194,916 kernels were manually scored.
Figure 3.
Seed Number of Backcross 3 Plants Containing the Sh2 Transgene Divided by the Seed Number of Sibling Plants Lacking the Transgene Plotted Versus the Average Daily High Temperature for the First 4 d Post Pollination.
Plants were grown in Citra, Florida, in 2008. In total, 737 ears and 124,267 kernels were manually scored.
The HS33/Rev6 Sh2 Transgene Increases Yield in Commercial Maize
The HS33/Rev6 Sh2 transgene increased yield in a commercial F1 maize hybrid (Table 4). Three independent transformation events of this gene were placed into a commercial hybrid and were grown in Citra, Florida, in 2004, 2006, and 2007 under high-plant-density conditions and compared with their isogenic controls lacking the transgene. (The 2004 experiment was lost to two hurricanes.)
Table 4. Transgene-Induced Maize Yield Increase.
| Event | 2005 | 2007 |
|---|---|---|
| 38 | 2134 | 2134 (26%) |
| 114 | 2134 | 1820 (22%) |
| 286 | 2260 | 3829 (47%) |
Increases are listed the in kg/hectare. The percentage increase in 2007 is also shown. Hybrid is Mycogen 2784.
Transgene-induced yield increases occurred in both 2006 and 2007. It should be noted that the flowering time for the hybrid material in 2007 was approximately 1 month later than that of the experimental material grown that year. Averaged across the six experiments of the 2 years, an increase of 2385 ± 721 kg/hectare was observed. However, repeated yield trials over multiple years and multiple locations throughout the Midwest failed to detect a reproducible yield increase. Average temperatures during flowering throughout the Midwest did not exceed 33°C during the years of the yield trials.
The HS33/Rev6 Sh2 Transgene Appears to Function in Maternal Tissue to Increase Seed Number
The results presented in Table 1 show that the number of mutant (sh2/sh2) seeds on selfed ears from Substitution ears (T/-;sh2/sh2) was higher than that observed on Control ears. This pattern was seen in subsequent generations as well (see Supplemental Data Sets 1 and 2 online). This result is unexpected if the HS33/Rev6 Sh2 transgene functions only in the endosperm to increase seed number. Because of this, we considered two alternative explanations. (1) The HS33/Rev6 Sh2 transgene functions in a maternal tissue to enhance seed number, and the genotype of the seeds is irrelevant in terms of enhanced seed number. (2) The HS33/Rev6 Sh2 transgene functions only in the endosperm and, because of enhanced rates of starch synthesis in transgene-containing kernels, the sink strength of the developing ear is enhanced; the development of additional mutant seeds is a consequence of enhanced ear sink strength caused by the development of additional plump seeds. Data relevant to these alternative hypotheses are presented in Table 5. Sibling plants of the three genotypes were crossed as male onto the sh2/sh2 recurrent parent. Self-pollination of these plants gave rise to a transgene-induced increase in seed number (Table 3), but no increase in seed number or seed weight was observed when these plants were crossed as male onto a sh2 line. (Self-pollination and crossing were done with individual plants on the same set of 2 d.) This result is consistent with the idea that the HS33/Rev6 Sh2 transgene functions in maternal tissue to enhance seed number. The simplest explanation is that the genotype of the maternal parent rather than the genotype of the seeds causes the increased seed number.
Table 5. Total Seed Number and Seed Weight from Crossing as Male Backcross 2 Plants Containing or Lacking the Designated Transgenic Event and Containing or Lacking the Endogenous Functional Sh2 Allele onto the sh2/sh2 Recurrent Inbred.
| Event | Paternal Genotype | Seed No. | Ratio, Seed No. | Seed Weight | Ratio, Seed Weight |
|---|---|---|---|---|---|
| 97 | Addition | 241 | 0.85 | ||
| Substitution | 275 | 0.97 | 36.60 | 1.20 | |
| Control | 282 | 30.42 | |||
| 73 | Addition | 267 | 0.87 | ||
| Substitution | 212 | 0.69 | 31.98 | 0.71 | |
| Control | 308 | 45.01 | |||
| 133 | Addition | 216 | 0.97 | ||
| Substitution | 300 | 1.35 | 37.23 | 1.21 | |
| Control | 221 | 30.75 | |||
| 183 | Addition | 289 | 1.02 | ||
| Substitution | 219 | 0.77 | 31.65 | 0.88 | |
| Control | 282 | 36.04 | |||
| 114 | Addition | 245 | 0.92 | ||
| Substitution | 243 | 0.91 | 33.26 | 0.88 | |
| Control | 266 | 37.94 | |||
| 286 | Addition | 229 | 0.78 | ||
| Substitution | 259 | 0.88 | 41.45 | 1.04 | |
| Control | 293 | 39.99 | |||
| 25 | Addition | 311 | 1.19 | ||
| Substitution | 246 | 0.94 | 39.79 | 1.01 | |
| Control | 262 | 39.39 | |||
| 76 | Addition | 261 | 1.17 | ||
| Substitution | 302 | 1.35 | 44.60 | ||
| Control | 224 | 35.75 | |||
| Average | Addition | 245 | 0.90 | ||
| Substitution | 263 | 0.97 | 38.96 | 0.98 | |
| Control | 272 | 39.55 |
Results from the self-pollination of these plants are given in Table 3. The three genotypes are listed. Plants were grown in Citra, Florida, in the spring of 2005, and the data are averaged over the three planting dates. The ratio is the seed number or weight of each of the two transgene-containing genotypes divided by the seed number or weight of the nontransgenic control that contains one copy of the functional Sh2 allele. Averages and ratios of all events were calculated from all individual data. The total population was 286 ears and 74,314 kernels.
Increased Seed Number Is Not Due Simply to a Sh2 Dosage Effect in the Maternal Parent
In the tests described above, self-pollination of plants containing both the transgene and the endogenous gene (Addition lines) generally yielded more kernels than those containing only the transgene or the endogenous gene. If enhanced seed number reflects the genotype of the maternal parent, we considered the possibility that the superior performance of the Addition line is due simply to a dosage effect. These plants contain two copies of a functional Sh2 gene, whereas the Control and Substitution lines contain only one copy. If a dosage effect exists, then plants completely lacking a functional Sh2 allele should produce fewer kernels compared with sibling plants containing one or two functional copies of the gene.
Seed numbers derived from self-pollination of sibling plants having two copies (Addition), one copy (Substitution and Control), or no copy (Mutant) of a functional Sh2 allele are given in Table 6 for six transgenic events. Plants lacking a functional Sh2 allele in maternal tissue did not have fewer kernels compared with plants containing one copy of the endogenous functional allele. We conclude that the enhanced seed number produced by Addition lines is not due to a dosage effect.
Table 6. Total Seed Number and Seed Weight from Self-Pollination of Backcross 3 Plants Containing or Lacking the Designated Transgenic Event and Containing or Lacking the Endogenous Functional Sh2 Allele.
| Event | Parental Genotype | Seed No. | Ratio, Seed No. | Seed Weight | Ratio, Seed Weight |
|---|---|---|---|---|---|
| 97 | Addition | 344** | 1.32 | ||
| Substitution | 319 | 1.23 | 43.37 | 1.07 | |
| Control | 260 | 40.50 | |||
| Mutant | 294 | 1.13 | |||
| 73 | Addition | 348** | 1.28 | ||
| Substitution | 289 | 1.06 | 50.31 | 1.09 | |
| Control | 273 | 45.98 | |||
| Mutant | 290 | 1.06 | |||
| 133 | Addition | 335 | 1.03 | ||
| Substitution | 352 | 1.08 | 47.89 | 1.09 | |
| Control | 326 | 43.85 | |||
| Mutant | 318 | 0.98 | |||
| 114 | Addition | 332 | 1.12 | ||
| Substitution | 315 | 1.06 | 45.89 | 1.03 | |
| Control | 297 | 44.71 | |||
| Mutant | 275 | 0.92 | |||
| 286 | Addition | 341** | 1.30 | ||
| Substitution | 332** | 1.27 | 48.20* | 1.18 | |
| Control | 262 | 40.75 | |||
| Mutant | 294 | 1.12 | |||
| 25 | Addition | 349* | 1.20 | ||
| Substitution | 317 | 1.09 | 49.76 | 1.11 | |
| Control | 290 | 44.85 | |||
| Mutant | 307 | 1.06 | |||
| Ave | Addition | 341** | 1.18 | ||
| Substitution | 319** | 1.10 | 48.08** | 1.09 | |
| Control | 289 | 44.07 | |||
| Mutant | 294 | 1.02 |
The four genotypes are defined in Figure 1. Plants were grown in Citra, Florida, in the spring of 2006. Total seed numbers and seed weight marked with asterisks designate significant differences at the 5% (*) or 1% (**) level from the cognate nontransgene control. The ratio is the seed number or seed weight of each of the two transgene-containing genotypes as well as the genotype lacking any functional Sh2 alleles divided by the seed number or seed weight of the nontransgenic control containing the functional endogenous Sh2 allele. Averages and ratios of all events were calculated from all individual data. In total, 761 ears and 194,916 kernels were manually scored.
Sh2 Expression in Nonseed Tissue
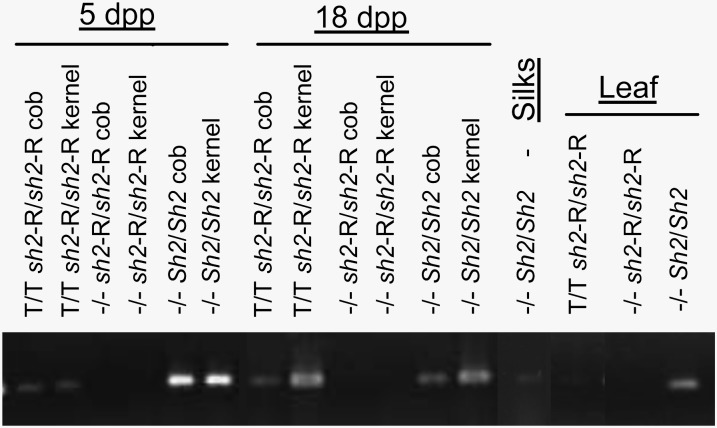
Sh2 expression at the RNA and protein/enzyme levels was monitored in various maize tissues, and transcript detection via RT-PCR in various maize tissues is shown in Figure 4. Three genotypes of sibling plants were monitored: those containing only the HS33/Rev6 Sh2 transgene, those containing only the functional Sh2 allele, and those containing only the mutant sh2-R allele. This mutant contains a large insertion and does not produce a detectable transcript or protein (Giroux and Hannah, 1994). It is readily apparent that the Sh2 transcript encoded by both the endogenous gene and the transgene is detectable in tissues other than just the endosperm (Figure 4). The transcript was found in leaf, cob, and silk tissue as well as in the developing kernel. We then monitored these tissues for the SH2 protein and Sh2-encoded AGPase activity.
Figure 4.
Sh2 Transcripts in Various Maize Tissues.
Tissues were harvested from sibling plants containing only the HS33/Rev6 Sh2 transgene, only the functional Sh2 allele, or lacking a transcriptionally active Sh2 allele and were monitored via PCR for Sh2 transcript. The mutant sh2 allele, sh2-R, contains a large insertion giving rise to no detectable transcript. Silk and leaf tissue were harvested at flowering.
Because AGPase assays of total protein preparations would detect all isoforms of AGPase, we employed a monoclonal antibody that recognizes only the Sh2-encoded AGPase activity as well as the SH2 protein. The antigen/antibody complex retains enzyme activity. The monoclonal antibody was bound to wells, crude protein extracts were placed into the wells to allow binding of SH2, and nonbound material was then washed from the wells. Using this specific assay, we could only detect SH2 protein and the Sh2-encoded AGPase activity in developing kernels 11 d post pollination and later. The SH2 protein and the Sh2-encoded AGPase activity were not detected in the nonseed tissues monitored in Figure 4.
Increased Seed Number Occurs Primarily by Increasing the Length of Kernel Rows
Ear scoring included a determination of the number of rows of kernels on each ear and the number of seeds per row. Shown in Table 7 are correlations of row number and row length with total seed number for materials grown in 2006 and 2008. Data from other years (see Supplemental Data Set 2 online) are consistent with those shown. These data show that total seed number is greater than 90% correlated with the length of rows. There is also a positive correlation of row number and total seed number, and differences in row number conditioned by the transgene are sometimes significant at the 1% or 5% level (see Supplemental Data Sets 1 and 2 online).
Table 7. Correlations between Total Number of Kernels and Row Length and Row Number.
| Year | Genotype | Row Length | Row No. |
|---|---|---|---|
| 2006 | Addition | 0.921 | 0.554 |
| Substitution | 0.925 | 0.535 | |
| Control | 0.936 | 0.641 | |
| Mutant | 0.914 | 0.399 | |
| Total | 0.927 | 0.539 | |
| 2008 | Addition | 0.962 | 0.652 |
| Substitution | 0.951 | 0.563 | |
| Control | 0.956 | 0.388 |
The HS33/Rev6 Sh2 Transgene Increases the Probability That an Ovary Will Give Rise to a Viable Mature Seed
We asked whether the HS33/Rev6 Sh2 transgene functions to increase the number of ovaries or whether it functions by increasing the probability that an ovary will give rise to a fully developed seed (Table 8). Ears from backcross 2 families segregating for the 114 or the 133 HS33/Rev6 Sh2 transgene events were covered with conventional ear bags or caps to block pollination, harvested 2 to 3 d past the time of normal pollination, and ovaries were counted. Other plants from these families were self-pollinated and the resulting kernels were counted. The experiment was replicated over time, giving rise to different pollination dates. Only material pollinated on June 2 experienced temperatures above 34°C during the first 4 d of kernel development. Nevertheless, these data show that while there is no increase in ovary number conditioned by the transgene, total kernel number does increase. Therefore, we concluded that the transgene functions primarily to enhance the probability of kernel development.
Table 8. Ovary and Seed Number of Sibling Backcross 2 Plants Containing or Lacking the Designated Transgenic Event.
| Event | Average Pollination Date | Transgene | Ovaries per Ear | Ratio | Seeds per Ear | Ratio |
|---|---|---|---|---|---|---|
| 114 | June 2 | + | 570 | 0.91 | 355* | 1.14 |
| − | 624 | 311 | ||||
| 114 | June 11 | + | 572 | 1.01 | 287 | 1.04 |
| − | 564 | 275 | ||||
| 133 | June 2 | + | 622 | 0.94 | 399 | 1.02 |
| − | 660 | 393 | ||||
| 133 | June 17 | + | 594 | 1.13 | 251 | 1.02 |
| − | 528 | 244 | ||||
| Average | Average | + | 589 | 1.00 | 327** | 1.07 |
| − | 589 | 303 |
Seeds were derived from self-pollination. Plants were grown in Citra, Florida, in the spring of 2008. Total seed numbers marked with asterisks designate significant differences at the 5% (*) or 1% (**) level from the cognate nontransgene control. The ratio is the seed or ovary number of each of the transgene-containing genotypes divided by the seed or ovary number of the nontransgenic control containing the functional endogenous Sh2 allele. Ovaries were counted on 61 transgene-containing and 34 control ears. Seeds were counted on 45 transgene-containing and 29 control ears. Averages and ratios of all events were calculated from all individual data.
Surprisingly, the Number of Ovaries Giving Rise to a Seed Is Only ∼50%
Perusal of the data in Table 8 shows that only 56% of the transgene-containing ovaries and only 51% of the control ovaries gave rise to mature seeds. We measured the percentage of ovaries that gave rise to a seed in a commercially used hybrid to determine whether the low percentage we observed in Table 8 is common to maize or whether this was due to the fact that our stocks are fairly inbred and, accordingly, lack vigor.
Ovary and seed number through development was measured in a commercially used hybrid (Table 9). Consistent with the percentages noted with our experimental, inbred materials, only 57% (386 of 675) of the ovaries gave rise to well-developed kernels. Kernel development was monitored at 7 and 12 d post pollination. Kernels enlarging in size were clearly evident at these stages as well as tissues indistinguishable from ovaries (labeled as “other” in Table 9). Whether these represent unfertilized ovaries or early blocks in seed development is unknown. Note that the total number of developing and nondeveloping seeds or ovaries decreases over time. Examination of mature ears following kernel removal revealed no evidence for underdeveloped or poorly developed kernels.
Table 9. Ovary and Seed Number per Ear of the Mycogen Hybrid 2784 as a Function of Development.
| Days Post Pollination | No. of Rows | Ovaries, Developing Seeds, and Nondeveloping, Mature Seeds | Developing Seeds | Other |
|---|---|---|---|---|
| 0 (44) | 16.5 | 675a | ||
| 7 (11) | 16.2 | 607b | 527 | 79 |
| 12 (25) | 16.1 | 511c | 418 | 93 |
| Mature (225) | 16.4 | 386d |
Ears were harvested before pollination to determine ovary number, at 7 and 12 d post pollination to determine the number of developing kernels, and at maturity to measure the number of fully developed mature kernels. Numbers in parentheses refers to the number of ears scored. Significant differences are designated by letters.
DISCUSSION
The HS33/Rev6 Sh2 Transgene Can Increase Seed Yield up to 64% by Increasing Seed Number
We show that the HS33/Rev6 Sh2 transgene can increase maize seed yield up to 64%, to our knowledge the greatest yield increase conditioned by any AGPase transgene reported so far (Giroux et al., 1996; Smidansky et al., 2002, 2003; Sakulsingharoj et al., 2004; Obana et al., 2006; Wang et al., 2007; Lee et al., 2009; Li et al., 2011). The significance of this is especially evident when one considers that maize yields have increased ∼1% per year via conventional breeding programs (Duvick, 2001). This effect on yield increase also occurred in a commercially viable hybrid. The yield increase was environmentally dependent, and average high temperatures during the first 4 d post pollination must reach ∼33°C for the increase. We initially focused on field temperatures, since one of the amino acid changes in the transgene (HS33) was selected because it enhances the heat stability of AGPase by strengthening subunit interactions (Greene and Hannah, 1998a, 1998b). We conclude that the HS33/Rev6 Sh2 transgene has agricultural value in hot environments and likely will become more important as global growth temperatures rise.
As was the case in wheat (Smidansky et al., 2002) and rice (Smidansky et al., 2003), the yield increase in maize conditioned by HS33/Rev6 Sh2 is due to an increased seed number rather than increased individual seed weight. This result was not anticipated, since many earlier observations point to Sh2 functioning only in the endosperm; hence, it was difficult to envisage how enhanced synthesis of ADP-Glc in the seed endosperm would increase seed number. We used biochemical, physiological, and primarily genetic analyses to address this question and conclude that this paradox is resolved by proposing that HS33/Rev6 Sh2 functions in tissues other than the seeds and enhances the probability of seed development. This hypothesis is suggested in spite of the many observations at the biochemical, molecular, physiological, and whole-plant levels that point to physiologically significant Sh2 function only in the endosperm.
The HS33/Rev6 Sh2 Transgene Functions in Maternal Tissue to Increase Seed Number
Historically, Sh2 was thought to have a physiologically important role only in the endosperm. At the whole-organism level, loss of Sh2 function via knockout mutation conditions an easily scorable phenotype only in the endosperm. While poor seedling vigor is associated with a loss of Sh2 function, we (Parera et al., 1996) used a chromosome in which the Sh2 portion of chromosome 3 was translocated to a centromere-containing portion of a B chromosome to create nonconcordant seeds lacking Sh2 function in the endosperm but having Sh2 function in the embryo. These seeds exhibited the same loss of vigor seen in concordant sh2 seeds, leading to the conclusion that poor vigor is a consequence of the mutant endosperm.
Multiple maize genes encoding AGPase large subunits have been described at the enzyme, transcript, and DNA levels. At the enzyme level, AGPase activity is not reduced in the embryo (Hannah and Nelson, 1976) or leaf (Fuchs, 1977; Fuchs and Smith, 1977) by the loss of Sh2 function. Genes other than Sh2 that encode AGPase large subunit genes have been cloned and sequenced from maize embryo and leaf cDNA libraries (Giroux and Hannah, 1994; Giroux et al., 1994, 1995), and Georgelis et al. (2007) summarized evidence for the presence of at least three genes in the maize genome that encode the large subunit of AGPase.
Physiological and subcellular localization studies point to Sh2 function only in the endosperm. It is clear (reviewed in Hannah, 2007; Hannah and Greene, 2008; Keeling and Myers, 2010) that the major form of AGPase is located in the cytosol in all cereal endosperms examined, whereas the enzyme is in the plastid in the potato tuber and the spinach (Spinacia oleracea) leaf. Moreover, the movement of ADP-Glc into the cereal plastid occurs via the action of a transport protein encoded by the Bt1 locus (Cao et al., 1995; Shannon et al., 1996). Beckles et al. (2001) surveyed several tissues and concluded that AGPase is in the cytosol only in cereal endosperms. Consistent with only a cytosolic function for Sh2 is the fact that the translated SH2 protein, as well as its small subunit-pairing partner BT2, lacks a transit peptide (Giroux and Hannah, 1994).
Despite the observations that Sh2 functions only in the endosperm of the seeds, the finding of increased seed number in wheat and rice led us initially to reexamine tissue-specific Sh2 function in maize. We could do this precisely by exploiting a loss-of-function sh2 allele as well as the seed number–increasing HS33/Rev6 Sh2 form of this gene in these studies.
If Sh2 functions only in the endosperm, then the number of shrunken seeds derived from self-pollination of sibling plants of the genotypes −/−;Sh2/sh2 and T/-; sh2/sh2 should be identical, since both classes lack Sh2 function. The data presented here show that the number of mutant seeds is greater from plants containing the transgene. And if Sh2 functions only in the endosperm, then seed number should increase when the HS33/Rev6 Sh2 transgene is transmitted to the endosperm through the male gamete. Our data clearly show no increase in seed number when the gene is male transmitted, but interpretation of this observation is complicated by two factors: dosage effects and gene imprinting. The endosperm is triploid, with two copies coming from the female parent, and AGPase shows a Sh2 dosage effect at the enzyme level (Hannah and Nelson, 1976). As first shown in maize (Kermicle, 1970), the mode of sexual transmission affects the level of expression of some genes in a host of organisms. Nevertheless, we know that the HS33/Rev6 Sh2 transgene functions when male transmitted, as evidenced by the 3:1 ratios derived from self-pollination of T/-; sh2/sh2 plants. The simplest interpretation of all extant observations is that the HS33/Rev6 Sh2 transgene functions in tissues other than the seeds to enhance seed number.
While expression level is not evidence for the importance of gene function, we nevertheless monitored Sh2 expression in nonendosperm tissues. RNA transcripts from both the functional endogenous gene and the transgene were detected in leaves, silks, and cob tissue; however, an assay we developed to quickly purify the SH2 protein and its cognate AGPase failed to detect the presence of the enzyme or protein. Perhaps our assay lacked the necessary sensitivity, or perhaps the relevant maternal tissue was not assayed.
Interestingly, the greatest increase in seed number occurs when the maternal parent contains both the endogenous gene and the transgene (Addition lines). This is not simply a dosage effect, since seed number does not decrease when the dosage of a functional gene in the maternal parent decreases from one to zero. Rather, the presence of both the endogenous gene and the transgene, per se, is required. AGPase is a heterotetramer containing two copies of the SH2 protein. Hence, hybrid enzyme molecules containing the two different forms of the large subunit could only be formed in the superior Addition lines, suggesting that the two large subunits act in a nonadditive, synergistic fashion to affect AGPase activity.
The HS33/Rev6 Sh2 Transgene Increases the Probability of Successful Seed Development
Our observations point to the HS33/Rev6 Sh2 transgene increasing the probability of successful seed development (versus an increase in ovary number) and show that environmental conditions during the first 4 d following pollination are critical in this regard. If the HS33/Rev6 Sh2 transgene functioned only during this stage of development, we would expect an increased number of seeds per row rather than an increased row number, since the number of rows is determined at an earlier stage in development. In agreement, total seed number and number of seeds per row showed a greater than 90% correlation. However, row number and total seed number are positively correlated, and Addition lines sometimes had significantly more rows compared with sibling Substitution and Control lines. These data point to Sh2 function occurring before fertilization as well; indeed, Sh2 transcripts from the endogenous Sh2 gene were detected in early-developing cobs (A. Eveland and D. Jackson, personal communication). All extant data point to the HS33/Rev6 Sh2 transgene functioning at various times during the maize life cycle.
Only One-Half of the Ovaries in Wild-Type Maize Give Rise to a Fully Developed Kernel
Perhaps of most significance, we show that only about one-half of the ovaries on a maize ear gave rise to fully developed seeds under our typical growing conditions. Enhancing this percentage, then, is an important agricultural target. Developmental studies of ears from vigorous hybrid plants revealed that while the number of developing kernels decreases over time, there was no evidence for unpollinated ovaries or poorly developed seeds. Apparently, ovaries not fated for development disintegrate. Other observations point to random seed development fate. Large-scale maize mutagenic projects (Neuffer and Sheridan, 1980; McCarty et al., 2005) routinely identified recessive mutations that block seed development. These mutants give rise to kernel phenotypes termed empty pericarp, etched, defective kernel, and the like, depending on the timing of developmental arrest. Seeds exhibiting the mutant phenotype are clearly visible on self-pollinated ears and can occur in the expected Mendelian 3:1 ratio. In other words, even though these mutants do not produce a fully developed kernel, they are not preferentially targeted for abortion during ear development. Hence, it appears that seed developmental fate is random. The HS33/Rev6 Sh2 gene, then, in a hot environment increases the probability that an ovary is fated for development. Since only ∼50% of the ovaries produce seeds, we suggest that this is an important target for other transgenic and conventional maize improvement programs.
Finally, it is intriguing that the reduced potential for starch synthesis in maize ovaries at the time of pollination leads to abortion (Zinselmeier et al., 1999; McLaughlin and Boyer, 2004). This suggests that the Sh2 transgene could function in a source tissue to enhance the amount of sugars flowing to the developing ovary and seeds or possibly function in the ovary itself, thereby blocking abortion.
METHODS
HS33/Rev6 Sh2 Transgenic Events
The gene contained the two changes termed HS33 (Greene and Hannah, 1998b) and Rev6 (Giroux et al., 1996). As detailed by Smidansky et al. (2002), the construct contained the Sh2 promoter, the Sh2 first intron, and the Sh2 terminator. The construct also contained the Bar gene for Basta resistance. Transgenics were made by WISKERS (Wang et al., 1995) or Agrobacterium tumefaciens–mediated transformation (Ishida et al., 2007). A total of eight transgenic events were chosen for further characterization based on low copy number, level of expression, and genetic stability from 142 primary transgenic events.
Plant Growth and Seed Production
All field operations were done at the University of Florida Institute of Food and Agricultural Sciences Plant Science Research and Education Unit in Citra, Florida. Archived weather data are available at http://fawn.ifas.ufl.edu/data/reports/. Maize (Zea mays) plants were watered two to three times per week with overhead irrigation or by rainfall and were sprayed two to three times per week with pesticides. Experimental materials were grown in several 4.6-m-long rows planted serpentine style in 91-m blocks. Plant density was ∼35,880 plants/hectare. Materials were arranged by transgenic event, and the different planting dates were interspersed within the event.
Controlled pollinations followed conventional protocols except that the plants were pollinated on 2 successive days to maximize seed set. At least 7 d following the last pollination in a family, a narrow band of a leaf was painted with diluted Basta herbicide. The leaves were scored for resistance or susceptibility to the herbicide after 5 to 7 d. Following mechanical drying, row number, total number of wild-type and mutant kernels, and their weights were recorded for each ear. Statistical differences were measured by Student’s t test using Excel 2007.
Yield trials involved harvesting grain from replicated, four-row plots of an identical isogenic hybrid with and without the various HS33/Rev6 transgenic events. The trial was designed as a split plot to separate HS33/Rev6 transgenic hybrids from nontransgenic checks in order to minimize pollen flow between blocks. Border rows of HS33/Rev6 transgenic and nontransgenic hybrids were also used to buffer their respective blocks from outside pollen flow. Plants were open pollinated, and the two middle rows were harvested. Plant density was ∼64,580 plants/hectare. Yields were calculated and adjusted to 15.5% moisture.
RNA Isolation and RT-PCR
Total RNA was isolated using Trizol (Invitrogen) and the manufacturer’s protocol from the following maize tissues: leaves, silks, kernels at 5 and 18 d post pollination, and the cob area surrounding the kernels. The genotypes of the plants that gave rise to the leaves, kernels, and cobs were (1) HS33/Rev6 Sh2/HS33/Rev6 Sh2; sh2-R/sh2-R, (2) −/−;Sh2/Sh2, and (3) −/−;sh2-R/sh2-R. The latter two genotypes lack the transgene. The silks were from a wild-type (−/−;Sh2/Sh2) plant. Two methods were used for end-point RT-PCR. In one method, first-strand cDNA synthesis was performed using the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen) followed by PCR using Invitrogen’s Taq DNA polymerase. The second method used the ProtoScript II RT-PCR kit (New England Biolabs). This kit includes the ingredients for both first-strand synthesis and PCR amplification. Approximately 1 μg of total RNA served as the starting material. An oligo(dT) primer was used for first-strand synthesis in both methods, and both methods yielded the same results. The Sh2 gene-specific primers used for PCR were 5′-CCCCGAGCTCACTATATGACAGACCCATCGTTGATGG-3′, a reverse primer that annealed to the stop codon and 23 bases 5′, and 5′-CCCGATGCTTGCCTCCGACGC-3′, a forward primer that annealed bases 1139 to 1159 from the start site. The 424-bp product was visualized on 1.2% agarose gels.
AGPase Assays and SH2 Protein Detection
Protein gel blots were used to detect the large subunit of the maize endosperm AGPase and were visualized utilizing colorimetric and chemiluminescent techniques. First, a conventional colorimetric protein gel blot was developed using a secondary antibody–linked alkaline phosphatase. Briefly, 100 μg of total extract from the particular tissue was extracted in 5% glycerol extraction buffer (0.1 M Hepes, pH 7.5, 5 mM MgCl2, 10 mM DTT, 10 mM KPi, 5 mM EDTA, and 5% glycerol). Material was pulverized with a mortar and pestle on dry ice and then centrifuged following the addition of buffer. Supernatants were quantified, and 100 μg of total protein was loaded onto each SDS gel. Crude recombinant maize AGPase expressed in Escherichia coli (Boehlein et al., 2010) was used as a positive control (35 μg of crude supernatant per lane). Detection limits using this technique visualized ∼50 ng of protein. Chemiluminescence detection using the ECL Protein Gel Blotting kit (Amersham Life Science; catalog No. RPN2108) was also used according to the manufacturer’s instructions. Here, the detection limit was ∼10 ng.
Bound AGPase activity was assayed as follows. Briefly, the maize extract supernatants were divided into aliquots onto a 96-well plate that had been coated overnight with the SH2-specific antibody (a gift from Dow AgroSciences) at a concentration of 2.1 μg/mL (1:1000 dilution in 1× PBS), 200 μL per well. The plate was incubated on ice for 45 to 60 min to capture AGPase from the kernel extract. Plates were then rinsed four times with 200 μL of 5% glycerol extraction buffer and blotted dry. AGPase reaction buffer (100 mM Hepes, pH 7.5, 0.4 μg/mL BSA, 5 mM MgCl2, 2.0 mM ATP, 2.0 mM Glc-1-P, and 20 mM 3-phosphoglycerate) at 200 μL was added to each well and incubated at 37°C for 4 h. Reactions were stopped by removal of the assay mix from the microtiter plate. Reactions were developed by standard procedures (Boehlein et al., 2010). As judged from assays of purified recombinant maize endosperm AGPase, this assay can detect 5 ng of enzyme.
Accession Numbers
Sequence data from this article can be found in the GenBank database under the following accession numbers: m81603 for Sh2 and AF334959 for Bt2.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table 1. Total Seed Number from Self-Pollination of Transgene-Containing and Transgene-Lacking Plants and Average High Temperatures for the First 4 d after Pollination for 2006 and 2008.
Supplemental Data Set 1. Row Number and Total Number and Weight of Plump and Shrunken Seeds from Self-Pollination of Backcross 2 Plants Containing or Lacking the Designated Transgenic Event and Containing or Lacking the Endogenous Functional Sh2 Allele.
Supplemental Data Set 2. Pollination Date, Row Number, and Total Number and Weight of Plump and Shrunken Seeds from Self-Pollination of Backcross 3 Plants Containing or Lacking the Designated Transgenic Event and Containing or Lacking the Endogenous Functional Sh2 Allele.
Acknowledgments
We appreciate the very useful comments of two anonymous reviewers, and we thank the many inmate volunteers from the Marion County Florida Correctional Facility for assisting in field operations. We thank David Jackson and Andrea Eveland for sharing unpublished data. This work was supported by the National Science Foundation (Grants IBM 0444031 and IOS 0815104 to L.C.H.), the U.S. Department of Agriculture Competitive Grants Program (Grants 2006-35100-17220 and 2008-35318-18649 to L.C.H.), and the National Institute of Food and Agriculture (Grant 2010-04228 to L.C.H.).
AUTHOR CONTRIBUTIONS
L.C.H. designed the research, performed research, analyzed data, and wrote the article; B.F. performed research; J.B. designed the research, performed research, and analyzed data; J.R.S. performed research; S.B. performed research; J.D.S. designed the research; R.B. analyzed data; N.G. performed research; T.G. designed the research, performed research, and analyzed data.
Glossary
- AGPase
ADP-glucose pyrophosphorylase
References
- Ballicora M.A., Iglesias A.A., Preiss J. (2003). ADP-glucose pyrophosphorylase, a regulatory enzyme for bacterial glycogen synthesis. Microbiol. Mol. Biol. Rev. 67: 213–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckles D.M., Smith A.M., ap Rees T. (2001). A cytosolic ADP-glucose pyrophosphorylase is a feature of graminaceous endosperms, but not of other starch-storing organs. Plant Physiol. 125: 818–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehlein S.K., Shaw J.R., Stewart J.D., Hannah L.C. (2010). Studies of the kinetic mechanism of maize endosperm ADP-glucose pyrophosphorylase uncovered complex regulatory properties. Plant Physiol. 152: 1056–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H., Sullivan T., Boyer C., Shannon J. (1995). BT1, a structural gene for the major 39–44 kDa amyloplast membrane polypeptides. Physiol. Plant. 95: 176–186 [Google Scholar]
- Duvick D.N. (2001). Biotechnology in the 1930s: The development of hybrid maize. Nat. Rev. Genet. 2: 69–74 [DOI] [PubMed] [Google Scholar]
- Fuchs, R.L. (1977). Purification and Characterization of ADP-Glucose Pyrophosphorylase A from Maize Endosperm. (College Station, TX: Texas A&M University). [Google Scholar]
- Fuchs R., Smith J. (1977). Purification and characterization of ADP-glucose pyrophosphorylase-B from Zea mays. Genetics 86: S21–S22 [Google Scholar]
- Georgelis N., Braun E.L., Shaw J.R., Hannah L.C. (2007). The two AGPase subunits evolve at different rates in angiosperms, yet they are equally sensitive to activity-altering amino acid changes when expressed in bacteria. Plant Cell 19: 1458–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroux M., Smith-White B., Gilmore V., Hannah L.C., Preiss J. (1995). The large subunit of the embryo isoform of ADP glucose pyrophosphorylase from maize. Plant Physiol. 108: 1333–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroux M.J., Boyer C., Feix G., Hannah L.C. (1994). Coordinated transcriptional regulation of storage product genes in the maize endosperm. Plant Physiol. 106: 713–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroux M.J., Hannah L.C. (1994). ADP-glucose pyrophosphorylase in shrunken-2 and brittle-2 mutants of maize. Mol. Gen. Genet. 243: 400–408 [DOI] [PubMed] [Google Scholar]
- Giroux M.J., Shaw J., Barry G., Cobb B.G., Greene T., Okita T., Hannah L.C. (1996). A single mutation that increases maize seed weight. Proc. Natl. Acad. Sci. USA 93: 5824–5829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene T.W., Hannah L.C. (1998a). Enhanced stability of maize endosperm ADP-glucose pyrophosphorylase is gained through mutants that alter subunit interactions. Proc. Natl. Acad. Sci. USA 95: 13342–13347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene T.W., Hannah L.C. (1998b). Maize endosperm ADP-glucose pyrophosphorylase SHRUNKEN2 and BRITTLE2 subunit interactions. Plant Cell 10: 1295–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah, L. (2007). Starch formation in the maize endosperm. In Endosperm, Developmental and Molecular Biology, O.-A. Olsen, ed (Berlin: Springer Books), pp. 179–194. [Google Scholar]
- Hannah, L., and Greene, T. (2008). The complexities of starch biosynthesis in cereal endosperms. In Biotechnology in Agriculture and Forestry, Vol. 63: Molecular Genetic Approaches to Maize Improvement, B. Larkins and A. Kriz, eds (Berlin: Springer), pp. 287–298. [Google Scholar]
- Hannah L.C., James M. (2008). The complexities of starch biosynthesis in cereal endosperms. Curr. Opin. Biotechnol. 19: 160–165 [DOI] [PubMed] [Google Scholar]
- Hannah L.C., Nelson O.E., Jr (1976). Characterization of ADP-glucose pyrophosphorylase from shrunken-2 and brittle-2 mutants of maize. Biochem. Genet. 14: 547–560 [DOI] [PubMed] [Google Scholar]
- Ishida Y., Hiei Y., Komari T. (2007). Agrobacterium-mediated transformation of maize. Nat. Protoc. 2: 1614–1621 [DOI] [PubMed] [Google Scholar]
- Keeling P.L., Myers A.M. (2010). Biochemistry and genetics of starch synthesis. Annu Rev Food Sci Technol 1: 271–303 [DOI] [PubMed] [Google Scholar]
- Kermicle J.L. (1970). Dependence of the R-mottled aleurone phenotype in maize on mode of sexual transmission. Genetics 66: 69–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Ryu T., Kim S., Okita T., Kim D. (2009). Kinetic and regulatory properties of plant ADP-glucose pyrophosphorylase genetically modified by heterologous expression of potato upreg mutants in vitro and in vivo. Plant Cell Tissue Organ Cult. 96: 161–170 [Google Scholar]
- Li N., Zhang S., Zhao Y., Li B., Zhang J. (2011). Over-expression of AGPase genes enhances seed weight and starch content in transgenic maize. Planta 233: 241–250 [DOI] [PubMed] [Google Scholar]
- McCarty D.R., et al. (2005). Steady-state transposon mutagenesis in inbred maize. Plant J. 44: 52–61 [DOI] [PubMed] [Google Scholar]
- McLaughlin J.E., Boyer J.S. (2004). Glucose localization in maize ovaries when kernel number decreases at low water potential and sucrose is fed to the stems. Ann. Bot. (Lond.) 94: 75–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuffer M.G., Sheridan W.F. (1980). Defective kernel mutants of maize. I. Genetic and lethality studies. Genetics 95: 929–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obana Y., Omoto D., Kato C., Matsumoto K., Nagai Y., Kavakli I., Hamada S., Edwards G., Okita T., Matsui H., Ito H. (2006). Enhanced turnover of transitory starch by expression of up-regulated ADP-glucose pyrophosphorylases in Arabidopsis thaliana. Plant Sci. 170: 1–11 [Google Scholar]
- Parera C., Cantliffe D., McCarty D., Hannah L. (1996). Improving vigor in shrunken-2 corn seedlings. J. Am. Soc. Hortic. Sci. 121: 1069–1075 [Google Scholar]
- Preiss, J. (2009). Biochemistry and molecular biology of starch biosynthesis. In Starch: Chemistry and Technology, 3rd ed. R.L. Whistler and J. BeMiller, eds (Oxford, UK: Elsevier), pp. 83–147. [Google Scholar]
- Sakulsingharoj C., Choi S., Hwang S., Edwards G., Bork J., Meyer C., Preiss J., Okita T. (2004). Engineering starch biosynthesis for increasing rice seed weight: The role of the cytoplasmic ADP-glucose pyrophosphorylase. Plant Sci. 167: 1323–1333 [Google Scholar]
- Shannon J.C., Pien F.M., Liu K.C. (1996). Nucleotides and nucleotide sugars in developing maize endosperms: Synthesis of ADP-glucose in brittle-1. Plant Physiol. 110: 835–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smidansky E.D., Clancy M., Meyer F.D., Lanning S.P., Blake N.K., Talbert L.E., Giroux M.J. (2002). Enhanced ADP-glucose pyrophosphorylase activity in wheat endosperm increases seed yield. Proc. Natl. Acad. Sci. USA 99: 1724–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smidansky E.D., Martin J.M., Hannah L.C., Fischer A.M., Giroux M.J. (2003). Seed yield and plant biomass increases in rice are conferred by deregulation of endosperm ADP-glucose pyrophosphorylase. Planta 216: 656–664 [DOI] [PubMed] [Google Scholar]
- Stark D.M., Timmerman K.P., Barry G.F., Preiss J., Kishore G.M. (1992). Regulation of the amount of starch in plant tissues by ADP glucose pyrophosphorylase. Science 258: 287–292 [DOI] [PubMed] [Google Scholar]
- Wang Z., Chen X., Wang J., Liu T., Liu Y., Zhao L., Wang G. (2007). Increasing maize seed weight by enhancing the cytoplasmic ADP-glucose pyrophosphorylase activity in transgenic plants. Plant Cell Tissue Organ Cult. 88: 83–92 [Google Scholar]
- Wang K., Drayton P., Frame B., Dunwell J., Thompson J. (1995). Whisker-mediated plant transformation: An alternative technology. In Vitro Cell. Dev. Biol. Plant 31: 101–104 [Google Scholar]
- Zinselmeier C., Jeong B.-R., Boyer J.S. (1999). Starch and the control of kernel number in maize at low water potentials. Plant Physiol. 121: 25–36 [DOI] [PMC free article] [PubMed] [Google Scholar]