Abstract
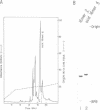
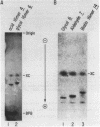
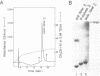
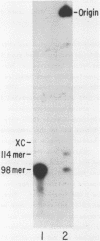
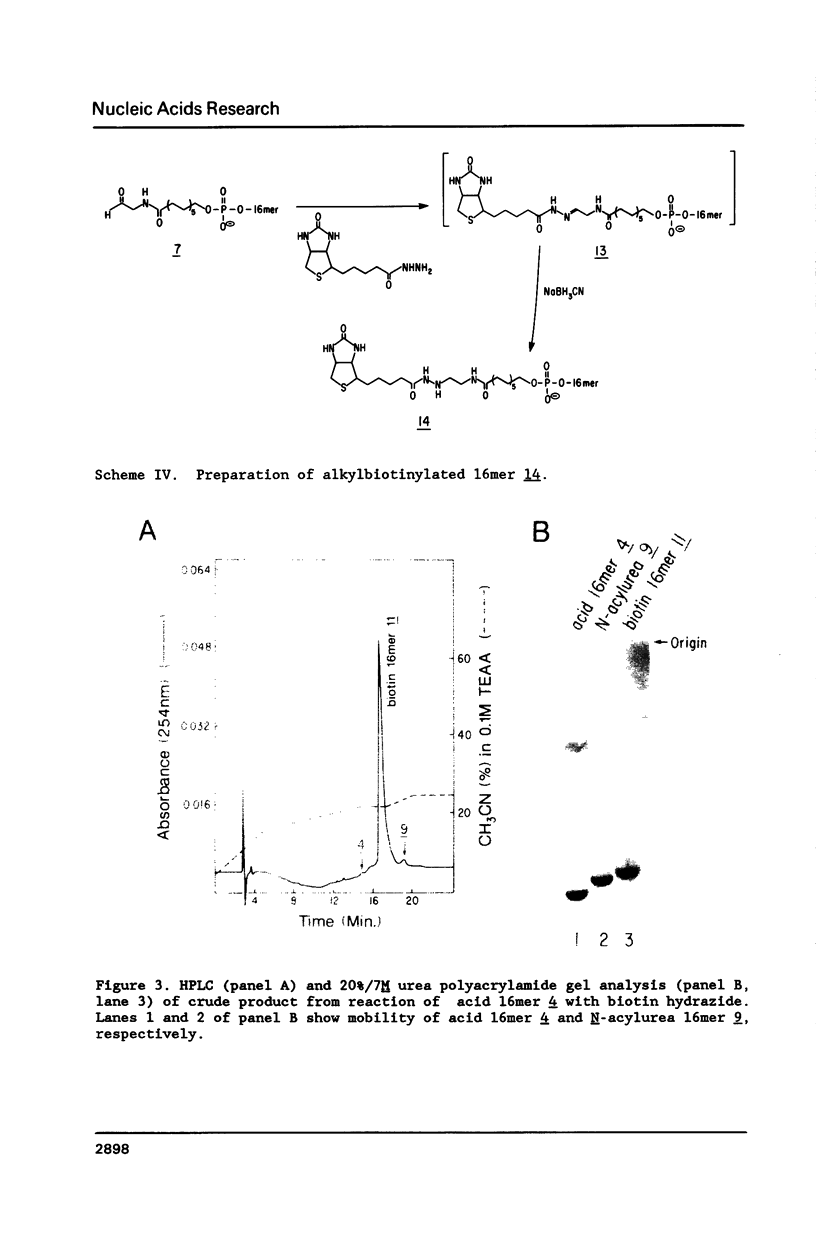
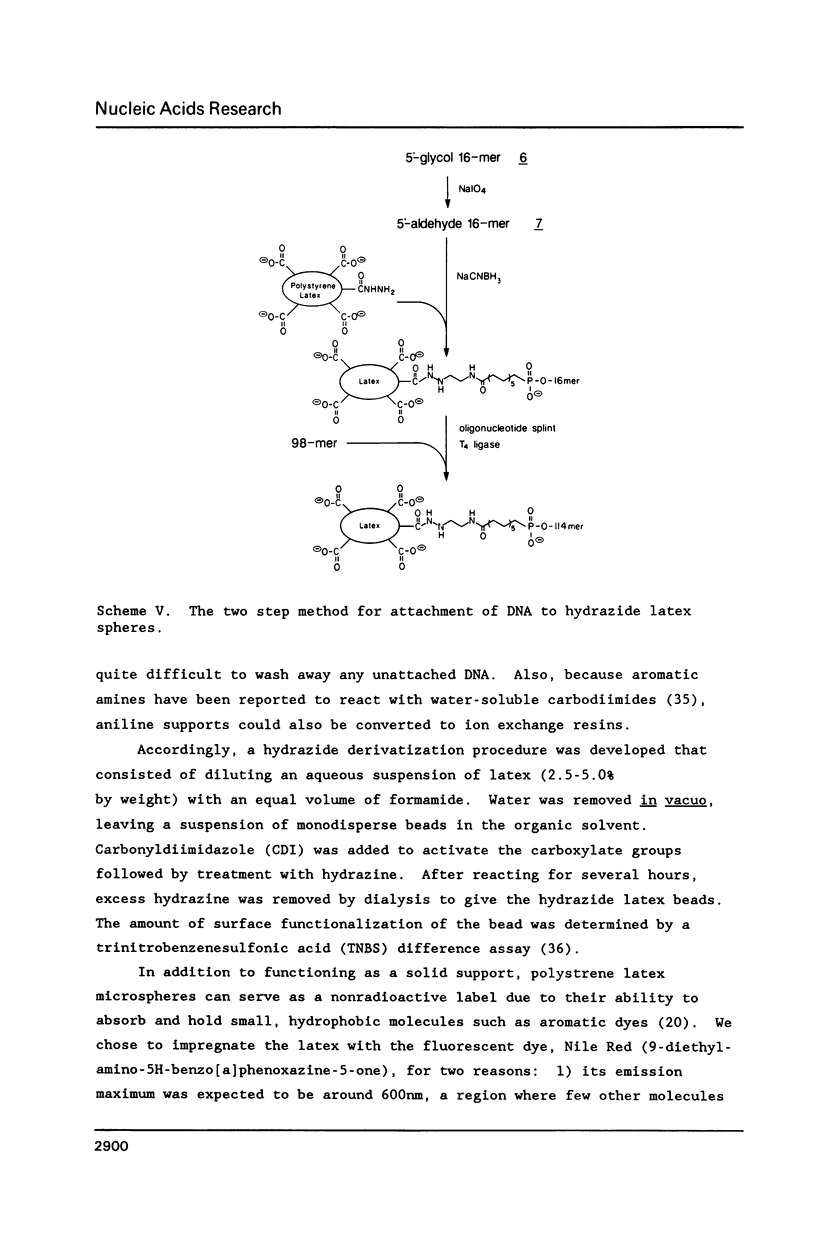
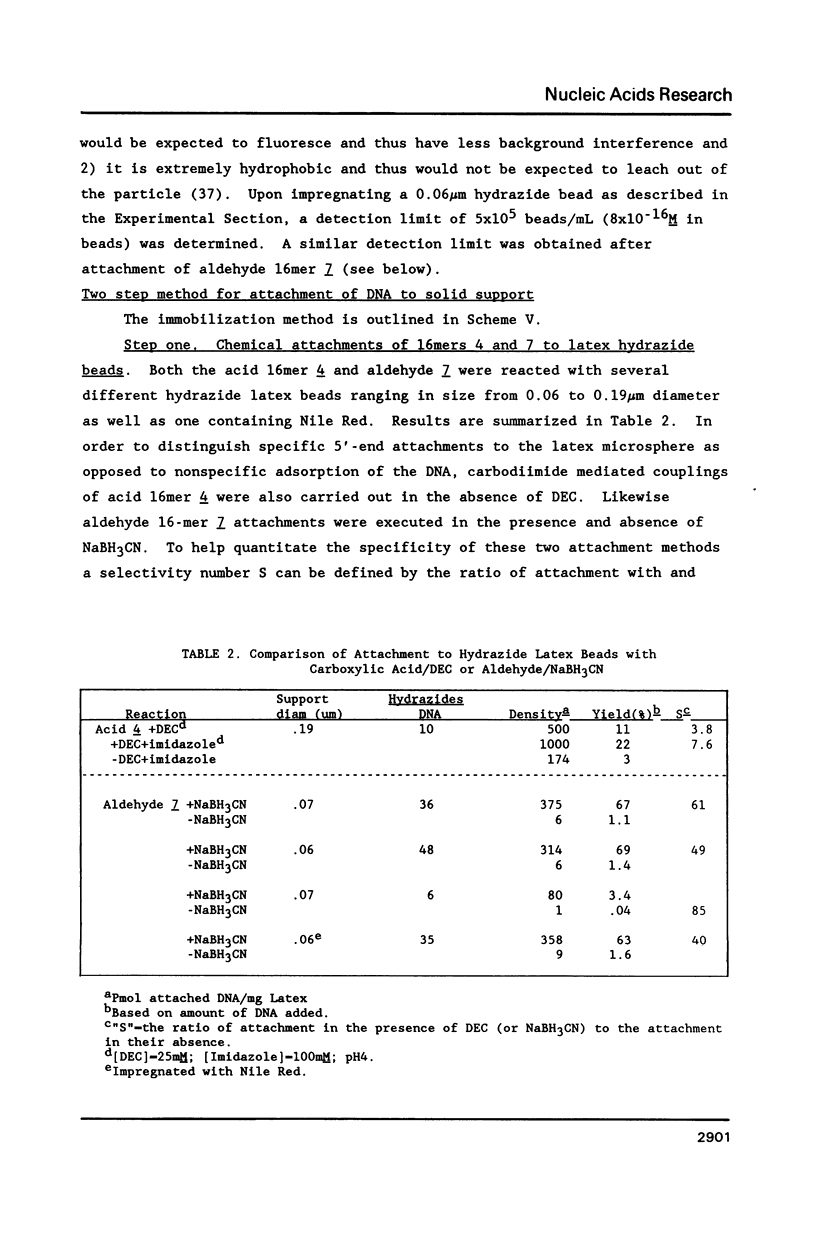
A general method for the immobilization of DNA through its 5'-end has been developed. A synthetic oligonucleotide, modified at its 5'-end with an aldehyde or carboxylic acid, was attached to latex microspheres containing hydrazide residues. Using T4 polynucleotide ligase and an oligonucleotide splint, a single stranded 98mer was efficiently joined to the immobilized synthetic fragment. After impregnation of the latex microspheres with the fluorescent dye, Nile Red and attachment of an aldehyde 16mer, 5 X 10(5) bead-DNA conjugates could be detected with a conventional fluorimeter.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal S., Christodoulou C., Gait M. J. Efficient methods for attaching non-radioactive labels to the 5' ends of synthetic oligodeoxyribonucleotides. Nucleic Acids Res. 1986 Aug 11;14(15):6227–6245. doi: 10.1093/nar/14.15.6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni G., Presentini R., Neri P. A simple method for the estimation of amino groups on insoluble matrix beads. Anal Biochem. 1983 Feb 15;129(1):60–63. doi: 10.1016/0003-2697(83)90052-0. [DOI] [PubMed] [Google Scholar]
- Arndt-Jovin D. J., Jovin T. M., Bähr W., Frischauf A. M., Marquardt M. Covalent attachment of DNA to agarose. Improved synthesis and use in affinity chromatography. Eur J Biochem. 1975 Jun;54(2):411–418. doi: 10.1111/j.1432-1033.1975.tb04151.x. [DOI] [PubMed] [Google Scholar]
- Bünemann H. Immobilization of denatured DNA to macroporous supports: II. Steric and kinetic parameters of heterogeneous hybridization reactions. Nucleic Acids Res. 1982 Nov 25;10(22):7181–7196. doi: 10.1093/nar/10.22.7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bünemann H., Westhoff P., Herrmann R. G. Immobilization of denatured DNA to macroporous supports: I. Efficiency of different coupling procedures. Nucleic Acids Res. 1982 Nov 25;10(22):7163–7180. doi: 10.1093/nar/10.22.7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chollet A., Kawashima E. H. Biotin-labeled synthetic oligodeoxyribonucleotides: chemical synthesis and uses as hybridization probes. Nucleic Acids Res. 1985 Mar 11;13(5):1529–1541. doi: 10.1093/nar/13.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu B. C., Wahl G. M., Orgel L. E. Derivatization of unprotected polynucleotides. Nucleic Acids Res. 1983 Sep 24;11(18):6513–6529. doi: 10.1093/nar/11.18.6513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici L., Campagnari F., de Rooij J. F., van Boom J. H. Preparation of polydeoxynucleotides linked to a solid support by coupling CNBr-activated cellulose with 5'-NH2-terminated oligo and poly(pdT)'s. Nucleic Acids Res. 1979 Jan;6(1):247–258. doi: 10.1093/nar/6.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici L., Campagnari F., de Rooij J. F., van Boom J. H. Preparation of polydeoxynucleotides linked to a solid support by coupling CNBr-activated cellulose with 5'-NH2-terminated oligo and poly(pdT)'s. Nucleic Acids Res. 1979 Jan;6(1):247–258. doi: 10.1093/nar/6.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly B. A., Rider P. Chemical synthesis of oligonucleotides containing a free sulphydryl group and subsequent attachment of thiol specific probes. Nucleic Acids Res. 1985 Jun 25;13(12):4485–4502. doi: 10.1093/nar/13.12.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosstick R., McLaughlin L. W., Eckstein F. Fluorescent labelling of tRNA and oligodeoxynucleotides using T4 RNA ligase. Nucleic Acids Res. 1984 Feb 24;12(4):1791–1810. doi: 10.1093/nar/12.4.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz H. J., Belagaje R., Brown E. L., Fritz R. H., Jones R. A., Lees R. G., Khorana H. G. High-pressure liquid chromatography in polynucleotide synthesis. Biochemistry. 1978 Apr 4;17(7):1257–1267. doi: 10.1021/bi00600a020. [DOI] [PubMed] [Google Scholar]
- Gilham P. T. The synthesis of celluloses containing covalently bound nucleotides, polynucleotides, and nucleic acids. Biochemistry. 1968 Aug;7(8):2809–2813. doi: 10.1021/bi00848a016. [DOI] [PubMed] [Google Scholar]
- Greenspan P., Mayer E. P., Fowler S. D. Nile red: a selective fluorescent stain for intracellular lipid droplets. J Cell Biol. 1985 Mar;100(3):965–973. doi: 10.1083/jcb.100.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovin T. M., Kornberg A. Polynucleotide celluloses as solid state primers and templates for polymerases. J Biol Chem. 1968 Jan 25;243(2):250–259. [PubMed] [Google Scholar]
- Kempe T., Sundquist W. I., Chow F., Hu S. L. Chemical and enzymatic biotin-labeling of oligodeoxyribonucleotides. Nucleic Acids Res. 1985 Jan 11;13(1):45–57. doi: 10.1093/nar/13.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyzosiak W. J., Biernat J., Ciesiołka J., Górnicki P., Wiewiórowski M. Chemical modification of N6-(N-threonylcarbonyl) adenosine. Part II. Condensation of the carboxyl group with amines. Nucleic Acids Res. 1979 Nov 24;7(6):1663–1674. doi: 10.1093/nar/7.6.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdale J. A., Malcolm A. D. A rapid method of gene detection using DNA bound to Sephacryl. Gene. 1985;36(3):201–210. doi: 10.1016/0378-1119(85)90175-1. [DOI] [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Noyes B. E., Stark G. R. Nucleic acid hybridization using DNA covalently coupled to cellulose. Cell. 1975 Jul;5(3):301–310. doi: 10.1016/0092-8674(75)90105-1. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Seed B. Diazotizable arylamine cellulose papers for the coupling and hybridization of nucleic acids. Nucleic Acids Res. 1982 Mar 11;10(5):1799–1810. doi: 10.1093/nar/10.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sene C., Girot P., Boschetti E., Plassat J. L., Bloch J., Egly J. M. Purification of polyadenylated mRNA on three oligo(dT)-substituted gels: a comparative study. J Chromatogr. 1982 Oct 29;248(3):441–445. doi: 10.1016/s0021-9673(00)85054-2. [DOI] [PubMed] [Google Scholar]
- Siddell S. G. RNA hybridization to DNA coupled with cyanogen-bromide-activated sephadex. The purification of polyoma messenger RNA. Eur J Biochem. 1978 Dec;92(2):621–629. doi: 10.1111/j.1432-1033.1978.tb12785.x. [DOI] [PubMed] [Google Scholar]
- Smith L. M., Fung S., Hunkapiller M. W., Hunkapiller T. J., Hood L. E. The synthesis of oligonucleotides containing an aliphatic amino group at the 5' terminus: synthesis of fluorescent DNA primers for use in DNA sequence analysis. Nucleic Acids Res. 1985 Apr 11;13(7):2399–2412. doi: 10.1093/nar/13.7.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tu C. P., Cohen S. N. 3'-end labeling of DNA with [alpha-32P]cordycepin-5'-triphosphate. Gene. 1980 Jul;10(2):177–183. doi: 10.1016/0378-1119(80)90135-3. [DOI] [PubMed] [Google Scholar]