Abstract
Background
Systematic reviews are increasingly informing policies in tuberculosis (TB) care and control. They may also be a source of questions for future research. As part of the process of developing the International Roadmap for TB Research, we did a systematic review of published systematic reviews on TB, to identify research priorities that are most frequently suggested in reviews.
Methodology/Principal Findings
We searched EMBASE, MEDLINE, Web of Science, and the Cochrane Library for systematic reviews and meta-analyses on any aspect of TB published between 2005 and 2010. One reviewer extracted data and a second reviewer independently extracted data from a random subset of included studies. In total, 137 systematic reviews, with 141 research questions, were included in this review. We used the UK Health Research Classification System (HRCS) to help us classify the research questions and priorities. The three most common research topics were in the area of detection, screening and diagnosis of TB (32.6%), development and evaluation of treatments and therapeutic interventions (23.4%), and TB aetiology and risk factors (19.9%). The research priorities determined were mainly focused on the discovery and evaluation of bacteriological TB tests and drug-resistant TB tests and immunological tests. Other important topics of future research were genetic susceptibility linked to TB and disease determinants attributed to HIV/TB. Evaluation of drug treatments for TB, drug-resistant TB and HIV/TB were also frequently proposed research topics.
Conclusions
Systematic reviews are a good source of key research priorities. Findings from our survey have informed the development of the International Roadmap for TB Research by the TB Research Movement.
Introduction
Tuberculosis (TB) continues to pose a major threat to global health [1], and research is a key component of the Global Plan to Stop TB2011-2015 [2]. Research is particularly critical for developing new tools and approaches needed for eliminating TB by 2050 [3]. Recognizing this, the Stop TB Partnership and the World Health Organization's (WHO) Stop TB Department have launched the TB Research Movement, with the aim of boosting TB research and accelerating progress in TB control towards international targets [4]. One of the main outputs of the TB Research Movement in 2011 was the publication of the International Roadmap for Tuberculosis Research [5] in October of 2011. This roadmap outlines all priority areas for investment in TB research and is intended to promote coordination and harmonization of scientific work on TB. Research priorities are identified in the areas of epidemiology; fundamental research; R&D of new diagnostics, drugs and vaccines; and operational and public health research. The ultimate goal is to reach all populations, including people with TB/HIV co-infection or MDR-TB and children, with new and better methods of prevention, diagnosis and treatment [5].
The process for developing this roadmap has been recently described by Lienhardt and colleagues [4]. Briefly, the research roadmap was developed through a priority ranking exercise conducted by a multidisciplinary group of 50 research experts, a multidisciplinary Delphi consultation, a series of systematic reviews and an open web-based survey [4].Among the systematic reviews that were commissioned, one was focused on all the TB research agendas that have been published from 1998 to 2010 [6]. As a next step, we were commissioned to review all the published systematic reviews and meta-analyses on TB (in all areas, including drugs, vaccines, diagnostics), to assess what research priorities have been identified in these reviews. The objectives of our systematic review were as follows: (1) to identify all systematic reviews and meta-analyses pertaining to any aspect of tuberculosis from 2005 to 2010, and (2) to assess, compile and rank the research priorities that were identified.
Methods
Searching
MEDLINE, EMBASE, Web of Science, and the Cochrane Library were searched for systematic reviews and meta-analyses on TB. The search strategy was developed in consultation with a medical librarian. The search was limited to systematic reviews and meta-analyses published between January 1, 2005 and July 1, 2010, in order to focus on contemporary TB literature and identify research priorities of greatest relevance to current TB control.
The search strategy included the following keywords and MeSH terms: [‘tuberculosis’ (explode) OR ‘Mycobacterium tuberculosis’(explode) OR ‘tuberculosis’.ti,ab. OR ‘tuberculos*’.tw] AND [‘meta analysis’ (explode) OR ‘meta analyses’.ti,ab OR ‘meta-analyses’.ti,ab OR ‘meta-analysis’.ti,ab OR ‘metanalys*’.ti,ab OR ‘systematic review’.tw]. The search was not limited to English and the last search was performed on August 18, 2010.
Selection
Studies were included if they focused on any aspect of tuberculosis. We included systematic reviews and meta-analyses published in English, French, Spanish, and Italian. The languages included were based on the skill set of our research team. We included systematic review and meta-analyses that had focused on tuberculosis or on a tuberculosis related topic (e.g. BCG), in the title or abstract. We considered a study to be a systematic review or meta-analysis if the authors identified the study as such, or if the title or abstract contained the words “systematic review” or “meta-analysis”. Moreover, studies were regarded as systematic reviews if the authors reported a systematic, explicit approach to identify, select, and synthesize the available evidence.
The first screening of the titles and abstracts obtained following the electronic search was done by one reviewer (IN). Subsequently, the same reviewer (IN) screened the full text articles, determined the eligibility, and decided on the final inclusion of studies in the systematic review. Further, a second reviewer (MP) independently searched, screened and identified studies for the inclusion in the review.
Data abstraction
We developed a data extraction form which was pilot-tested by two reviewers (IN and DL). The reviewers independently piloted the forms until there were no major disagreements in the data extraction process. One reviewer (IN) extracted the data from all the included studies and the second reviewer (DL) extracted data in duplicate for a random subset of 15% of the total number of included articles. Additionally, a third reviewer (LT) independently extracted data for all included studies on the study characteristics section of the data extraction form. Disagreements between the three reviewers were resolved by consensus.
Study characteristics
We extracted data from the text or online supplement of each included systematic review or meta-analysis. Information was collected on two main points: i) the main focus of the systematic review, and ii) questions and priorities identified for future research. The UK Health Research Classification System (HRCS) [7], developed by the UK Clinical Research Collaboration for the classification and analysis of all types of health research, was used to determine the focus of the included studies as well as the focus of the research questions/priorities. In particular, the HRCS Research Activity Codes [7] were used to assign a category for the main focus of the studies and the research questions/priorities.
The main focus of each included systematic review was determined by extracting keywords from the title and abstract and matching them with the criteria developed by the HRCS. The Codes were divided into eight major categories: (1) Underpinning research; (2) Aetiology; (3) Prevention of disease and conditions, and promotion of well-being; (4) Detection, screening and diagnosis; (5) Development of treatments and therapeutic interventions; (6) Evaluation of treatments and therapeutic interventions; (7) Management of diseases and conditions; and (8) Health and social care services research (see Table 1 for full description). These research categories were used in Tables 2 and 3, to provide an overarching framework for grouping TB research.
Table 1. Description of the Health Research Classification System.
| Research Activity Code (Description) and Subcategory |
| 1. Underpinning research: ( Research that underpins investigations into the cause, development, detection, treatment and management of diseases, conditions and ill health. ) |
| 1.1 Normal biological development and functioning (i.e. genes, molecular and biological pathways) |
| 1.2 Psychological and socioeconomic processes (i.e. health and well-being) |
| 1.3 Chemical and physical sciences (i.e. molecular modeling, chemical structures, bioengineering) |
| 1.4 Methodologies and measurements (i.e. statistical methods, mapping methodologies, biological/socioeconomic research methods) |
| 1.5 Resources and infrastructure (i.e. development/distribution of resources, cell lines, DNA banks, infrastructure to support research networks and centers) |
| 2. Aetiology: ( Identification of determinants that are involved in the cause, risk or development of diseases, conditions and ill health. ) |
| 2.1 Biological and endogenous factors (i.e. risk factors liked to ethnicity, age, gender, gene products) |
| 2.2 Factors relating to physical environment (i.e. physical agents, environmental surroundings, radiation and pollution) |
| 2.3 Psychological, social, and economic factors (i.e. individual or group behaviors and lifestyle) |
| 2.4 Surveillance and distribution |
| 2.5 Research design and methodologies-under aetiology category (i.e. development and evaluation of novel research designs, new epidemiological research measurements) |
| 2.6 Resources and infrastructure (under aetiology category) |
| 3. Prevention of disease and conditions, and promotion of well-being: ( Research aimed at the primary prevention of disease, conditions or ill health, or promotion of well-being. ) |
| 3.1 Primary prevention interventions to modify behaviors or promote well-being (i.e. risk behaviors associated with diet, tobacco use, alcohol, substance misuse) |
| 3.2 Interventions to alter physical and biological environmental risks (i.e. radiation, second hand smoke, physical and chemical agents) |
| 3.3 Nutrition and chemoprevention |
| 3.4 Vaccines |
| 3.5 Resources and infrastructure (prevention) |
| 4. Detection, screening and diagnosis: ( Discovery, development and evaluation of diagnostic, prognostic and predictive markers and technologies. ) |
| 4.1 Discovery and preclinical testing of markers and technologies |
| 4.2 Evaluation of markers and technologies |
| 4.3 Influences and impact of screening and factors affecting uptake (i.e. attitudes and beliefs such as culture and religious practices, issues relating to gender/age/ethnicity, genetic counseling) |
| 4.4 Population screening programmes (i.e. feasibility studies, evaluation of effectiveness, benefits and economic evaluation) |
| 4.5 Resources and infrastructure (detection) |
| 5. Development of treatments and therapeutic interventions: ( Discovery and development of therapeutic interventions and testing in model systems and preclinical settings. ) |
| 5.1 Identification and development of pharmaceuticals (i.e. drug screening, mechanism of action, pharmacogenetics) |
| 5.2 Discovery and development of cellular, tissue and gene therapies (i.e. gene therapy, stem cell therapy, development of delivery systems) |
| 5.3 Discovery and development of medical devices |
| 5.4 Development of surgical interventions |
| 5.5 Radiotherapy |
| 5.6 Resources and infrastructure (development of treatments) |
| 6. Evaluation of treatments and therapeutic interventions: ( Testing and evaluation of therapeutic interventions in clinical, community or applied settings. ) |
| 6.1 Pharmaceuticals (i.e. phase I, II, III and IV trials) |
| 6.2 Clinical application and evaluation of cellular, tissue and gene therapies |
| 6.3 Clinical and applied application and evaluation of surgical interventions |
| 6.4 Radiotherapy |
| 6.5 Resources and infrastructure (evaluation of treatments) |
| 7. Management of diseases and conditions: ( Research into individual care needs and management of disease, and conditions or ill health. ) |
| 7.1 Studies of patients and service user care needs |
| 7.2 Studies involving all issues related to palliative care and end of life care |
| 7.3 Management of diseases, ill health and conditions by health and social care professionals |
| 7.4 Resources and infrastructure (disease management) |
| 8. Health and social care services research: ( Research into the provision and delivery of health and social care services, health policy and studies of research design, measurements and methodologies. ) |
| 8.1 Examining the organization and provision of health and social care services and evaluating factors affecting the quality of care |
| 8.2 Economic evaluation of health and social care interventions and delivery |
| 8.3 Policy, ethics and research governance |
| 8.4 Development of research designs and novel methodologies for health care including treatment, management and health services research |
| 8.5 Resources and infrastructure (health services) |
Table 2. Focus of tuberculosis systematic reviews.
| Category and subdivision | TB research focus | Proportion (%) |
| Aetiology: 28 of N = 141 a (19.9%) | ||
| Biological and endogenous risk factors | Genetic susceptibility/gene targets; risk factors for MDR-TB; vitamin D receptor | 11/28 (39.3) |
| Factors relating to the physical environment | Travel risk and LTBI; nosocomial exposure to TB | 5/28 (17.9) |
| Socio-economic risk factors | Risk of TB transmission | 1/28 (3.6) |
| Surveillance and distribution | TB/HIV; MDRTB and HIV; diabetes and TB | 11/28 (39.3) |
| Prevention of disease and conditions, and promotion of well-being: 17 of N = 141 (12.1%) | ||
| Intervention to modify risk behaviours and lifestyles | Tobacco | 3/17 (17.6) |
| Alcohol | 2/17 (11.8) | |
| Diet/BMI | 1/17 (5.9) | |
| Interventions to alter physical and biological environment | Air pollutant | 1/17 (5.9) |
| Nutrition and chemoprevention | Isoniazid Preventive Therapy in MDRTB | 2/17 (11.8) |
| Vaccines | 5/17 (29.4) | |
| Detection, screening and diagnosis: 46 of N = 141 (32.6%) | ||
| Discovery and preclinical testing of technologies | Non-pulmonary TB | 1/46 (2.2) |
| Evaluation of markers and technologies | Bacteriological TB diagnostic tests | 17/46 (37.0) |
| Bacteriological MDRTB diagnostic tests | 9/46 (19.6) | |
| Immunological diagnostics | 9/46 (19.6) | |
| LTBI diagnostic tests | 3/46 (6.5) | |
| Population surveillance | Active case finding TB, TB/HIV | 3/46 (6.5) |
| TB screening | 1/46 (2.2) | |
| Development and evaluation of treatments and therapeutic interventions b : 33 of N = 141 (23.4%) | ||
| Pharmaceuticals | Drug-resistant TB | 10/33 (30.3) |
| TB treatment | 9/33 (27.3) | |
| LTBI treatment | 6/33 (18.2) | |
| TB/HIV treatment | 3/33 (9.1) | |
| Surgery | Spinal TB | 1/33 (3.0) |
| Radiotherapy | Laser therapy for TB | 1/33 (3.0) |
| Management of diseases/condition: 14 of N = 141 (9.9%) | ||
| Individual care needs, secondary disease prevention | TB treatment adherence; reminder systems for LTBI treatment | 7/14 (50.0) |
| Organization and delivery of programs, and factors affecting quality of care | DOT program evaluation; cost-benefit analysis of TB health services; evaluating quality of care | 7/14 (50.0) |
Denominator N = 141 represents the total number of research focuses identified by all the included reviews. In this case N is greater than the 137 number of included systematic reviews because some reviews had a research focus captured by more than one category.
Categories 5 and 6 of Table 1 were merged. Note: Cells do not equal 100% because the subdivisions “Other” in “Prevention of diseases and conditions”, “Detection, screening, and diagnosis”, and “Development and evaluation of treatments” were omitted. There was no SR on “Underpinning research” and “Health and social services research.”
Table 3. Summary of research priorities identified.
| Category and subdivision | TB research priority identified | Proportion (%) |
| Underpinning research: 6 of N = 191 c (3.1%) | ||
| Biological pathways and processes | Investigating pathways risk of infection and infection to disease; detecting mechanism TB influences lung cancer | 2/6 (33.3) |
| Methodologies and measurements | Implementing large scale studies for precise estimate of the epidemic status of TB/HIV co-infection.Defining immune reconstitution inflammatory syndrome; developing multicentric studies to form a criteria that differentiate pericardial TB | 4/6 (66.7) |
| Aetiology: 42 of N = 191 (22.0%) | ||
| Biological and endogenous risk factors | Detecting genetic susceptibility/gene targets/TB gene clusters; investigating biological risk factors HIV/TB and XDRTB; determining BMI and diet risk factors for TB | 15/42 (35.7) |
| Factors relating to the physical environment | Investigating travel risk for LTBI, and the risk of air travel and fuel combustion/air pollutants for TB | 7/42 (16.7) |
| Socio-economic risk factors | Investigating the risk of TB transmission in asylum seekers/refugees/immigrants | 2/42 (4.8) |
| Surveillance and distribution | Implementing large scale studies to better diagnose and survey TB/HIV co-morbidity and epidemic status | 1/42 (2.4) |
| Research design and methodologies | Designing well-powered studies to measure HIV status and drug resistance in TB patients; designing blinded, prospective studies to compare fluorescence to conventional microscopy, and sputum processing methods to direct smears in high and low HIV prevalence settings; innovating designs for studies on phage assays; creating reference standards and laboratory protocols for evaluating NAATs; improving methodology on quality appraisal | 17/42 (40.5) |
| Prevention of disease and conditions, and promotion of well-being 15 of N = 191 (7.9%) | ||
| Intervention to modify risk behaviours and lifestyles | Evaluating the association between tobacco/passive smoking/biomass fuel combustion and increase TB risk | 4/15 (26.7) |
| Investigating the association between alcohol use and TB risk | 1/15 (6.7) | |
| Investigating diet/BMI/diabetes mellitus as TB risk factors | 1/15 (6.7) | |
| Nutrition and chemoprevention | Assessing the effects of isoniazid preventive therapy and risk of monoresistance in TB | 1/15 (6.7) |
| Investigating the relationship between vitamin D supplements/nutritional supplements and TB | 2/15 (13.3) | |
| Vaccines | Developing vaccine candidates and protective markers | 6/15 (40.0) |
| Detection, screening and diagnosis: 50 of N = 191 (26.2%) | ||
| Discovery and preclinical testing of technologies | Developing bacteriological TB diagnostic tests | 5/50 (10.2) |
| Bacteriological MDRTB diagnostic tests | 5/50 (10.2) | |
| Discovering new immunological diagnostics | 3/50 (6.0) | |
| Developing LTBI diagnostic tests | 1/50 (2.0) | |
| Other | 1/50 (2.0) | |
| Evaluation of markers and technologies | Evaluating bacteriological TB diagnostic tests | 14/50 (28.0) |
| Evaluating bacteriological MDRTB diagnostic tests | 4/50 (8.0) | |
| Evaluating new immunological diagnostics | 6/50 (12.0) | |
| Evaluating new LTBI diagnostic tests | 1/50 (2.0) | |
| Other | 4/50 (8.0) | |
| Influences and impact | Assessing the contribution of diagnostic tests to health care systems; factors affecting uptake such as economic and social factors | 3/50 (6.0) |
| Population surveillance | Intensifying active case finding as a method to control TB | 2/50 (4.0) |
| Evaluating the usefulness of TB screening in health care workers | 1/50 (2.0) | |
| Development of treatment regimens and therapeutic interventions: 11 of N = 191 (5.8%) | ||
| Pharmaceuticals | Developing treatment for active TB | 3/11 (27.3) |
| Discovering treatment for drug-resistant TB | 3/11 (27.3) | |
| Developing TB/HIV treatment | 5/11 (45.4) | |
| Evaluation of treatments and therapeutic interventions: 37 of N = 191 (19.4%) | ||
| Pharmaceuticals | Evaluating treatments for TB | 8/37 (21.6) |
| Evaluating treatments for drug-resistant TB | 11/37 (29.7) | |
| Evaluating TB/HIV treatments | 12/37 (32.4) | |
| Other | 1/37 (2.7) | |
| Surgery | 2/37 (3.1) | |
| Other | 3/37 (8.1) | |
| Management of diseases/condition | ||
| Individual care needs, secondary disease prevention | Assessing TB treatment adherence; reminder systems for LTBI treatment | 13/191 (6.8) |
| Health and social care services research | ||
| Organization and delivery of programs, and factors affecting quality of care | Evaluating DOT program; cost-benefit analysis of TB health services; evaluating quality of care | 16/191 (8.4) |
| Other | 1/191 (0.5) | |
Denominator N = 191 represents the total number of research priorities identified by all the included studies.
In the HRCS, each of the eight major categories is further subdivided into five to nine subcategories with definitions for the type of research that belonged to that subcategory. For instance, “(1)Underpinning research” includes five subcategories: (1.1)studies of normal biological development and functioning, including gene, gene products, biological pathways, molecular and cellular structures, and development and characterization of model systems; (1.2) studies that do not address health directly but cover issues such as psychological and socioeconomic processes, individual or group characteristics and behaviours, and social and cultural beliefs; (1.3) research in chemical and physical sciences that may lead to the future development of diagnostic tools or treatments; (1.4) studies that target the development of novel methodologies and measurements including the development of statistical methods, and the development of mapping methodologies; and (1.5)research involving the development and/or distribution of resources for use by the research community, and infrastructure to support research networks. Using the main categories and the subdivisions within each category, we mapped the corresponding TB research areas found in the literature search (refer to Tables 1 and 2).
Quantitative data synthesis
Study characteristics were summarized using descriptive statistics. Measures such as total count, frequency, and proportion, were used to summarize data. Data analyses were performed using STATA Version 11.0.
Results
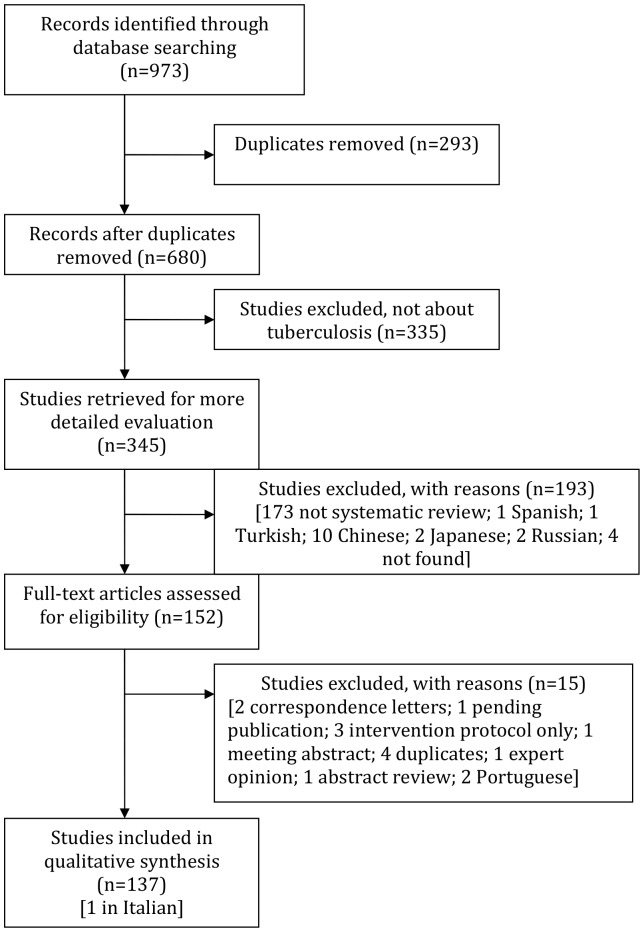
There were a total of 973 records identified through the electronic database search (Figure 1). The first screening of titles and abstracts was done on 680 records. Following the first screening process, 528 records were excluded. The reasons for exclusion are listed in Figure 1. The full text screening of articles was performed on 152 records. Overall, there were 137 systematic reviews included in our analysis [8]–[144].
Figure 1. Flow chart of the study selection process.
Characteristics of included TB systematic reviews
The 137 reviews were published in 61 different journals. The majority of reviews (39.4%) were published in journals with impact factors of five or less, and only six (4.3%) reviews were published in journals with a high impact factor (>15). However, a large proportion of the reviews (38.6%) were published in journals that did not have an impact factor. In addition, approximately 24% of the main authors were from the United States and 41% were from four other countries (China, UK, Canada, and Italy). The remaining 34.1% of authors were from 26 different countries.
Out of the 137 reviews, 131 (95.6%) self identified as a systematic review or meta-analysis, which means that they used the term “systematic review” or “meta-analysis” in the title or abstract. Approximately 91% (124) of all reviews were not Cochrane reviews. Among the 13 Cochrane reviews, 9 of them focused on “evaluation of treatments and therapeutic interventions”.
Half of the reviews (67 [48.9%]) reported having a funding source, whereas only 15 reviews (11.0%) reported not being funded and 55 reviews (40.1%) did not report funding status. Most of the reviews (109 [79.6%]) included less than 50 studies in their review and within those reviews, the majority had between 1,000 and 10,000 participants (34/109[31.2%]).
Focus of TB systematic reviews
The main focus of each review was determined using the HRCS as described in the Methods section. The classification categories were subdivided into major tuberculosis research areas as described in Table 2. The three most common review categories, in decreasing order, were “Detection, screening and diagnosis” with 46/141(32.6%) systematic reviews, “Development and evaluation of treatments and therapeutic interventions” with 33/141(23.4%) systematic reviews and “Aetiology” with 28/141(19.9%) systematic reviews.
Within the category of “Detection, screening and diagnosis”, 17/46 (37%) of the reviews focused on bacteriological diagnostics for active TB, such as improving processing methods of sputum smear microscopy, and assessing the use of nucleic acid amplification tests (NAATs). The two other most common TB research aims were bacteriological diagnostics for MDR-TB (9/46[20%]) and immunological diagnostics (9/46[20%]). More specifically, bacteriological diagnostics for MDR-TB included tests such as line-probe assays, bacteriophage based assays, and colorimetric redox assays. Immunological diagnostics were focused mainly on testing and evaluating interferon-gamma release assays (IGRAs).
In the category “Development and evaluation of treatments and therapeutic interventions”, 10/33 (30%) studies focused on drug resistant tuberculosis treatment, 9/33 (27.3%) studies on evaluating different regimen combinations for tuberculosis treatment, and 6/33(18.2%) on treatment of latent tuberculosis infection (LTBI).
In the category “Aetiology”, 11/28 (39.3%) systematic reviews focused on biological/genetic risk factors such as genetic susceptibility and gene targets,11/28 (39.3%) studies targeted surveillance and distribution of TB/HIV co-infection, MDRTB and HIV, and diabetes and TB, and 5/28(17.9%) focused on travel risk for LTBI and nosocomial TB exposure.
Research priorities
Out of 137 reviews, 103 (75%) identified at least one research question or a research priority. Of these, 48 (46.6%) identified only one research priority, 33 (32.0%) two research priorities, 7 (6.8%) three, 7 (6.8%) four, and 8 (7.8%) five research priorities. None of the reviews identified more than five research priorities.
Table 3 shows the summary of research priorities by category, subdivision, and TB-specific research priority. The three major categories of research priorities/questions were “Detection, screening and diagnosis” responsible for 50/191 (26.2%) of all the identified research priorities, “Aetiology” with 42/191 (22.0%), and “Evaluation of treatments and therapeutic interventions” with 37/191 (19.4%).
In the most common category, “Detection, screening and diagnosis”, the top research priority was the evaluation of bacteriological TB diagnostic tests in 14/50 (28.0%) reviews. Other frequently cited TB research priorities were: evaluation of immunological TB diagnostic tests (6/50 [12.0%]); discovery and development of new TB diagnostic tests (5/50 [10.2%]); and development of new bacteriological MDR-TB diagnostics (5/50 [10.2%]). Two priorities had almost equal importance and were highly prevalent in TB literature. The main priority in that category was to investigate the detection, screening and diagnosis of drug-resistant TB and MDR-TB. Studies called for the need to develop studies that detect resistance from smear positive specimens, determine the accuracy of colorimetric methods, line-probe assays, phage-based assays for rapid screening and nitrate reductase assay (NRA), and find the clinical usefulness of rapid diagnosis of rifampicin-resistant TB. Another frequency priority was to address unresolved research questions on interferon-gamma release assays (IGRAs), discover new antigens with immunodiagnostic potential, and test IGRAs in various populations and settings to establish test reproducibility. Evaluating sputum processing methods and smear microscopy, assessing nucleic acid amplification tests (NAATs), and evaluating tests for extrapulmonary TB (e.g. adenosine deaminase for pleural TB) were commonly cited priorities.
Within the “Aetiology” category, the main TB research priorities were: development of new research methods; better study designs or statistical tools for studying drug resistant TB, MDR-TB, links between HIV and MDR-TB; comparison of diagnostic tests (17/42 [40.5%]); identification of biological and genetic risk factors (15/42 [35.7%]); and evaluation of the role of risk factors such as tobacco and air pollutants (7/42 [16.7%]). The most frequent priority was to examine gene and gene products in relation to TB disease and susceptibility to disease. Key genes such as vitamin D receptor polymorphisms, IL10 gene, and drug-metabolizing enzyme (DME) gene polymorphisms were commonly mentioned for future research. The second most frequent research priority on TB/HIV included recommendations to conduct studies investigating XDR-TB and HIV co-infection, identifying a comprehensive definition of IRIS (immune reconstitution inflammatory syndrome), and investigating sputum processing methods with direct smears in settings with high and low HIV prevalence.
The category “Evaluation of treatments and therapeutic interventions” was the third most frequent. It focused on TB/HIV drug treatments (12/37 [32.4%]), drug-resistant TB treatments (11/37 [29.7%]), new TB drugs and active tuberculosis regimens (8/37[21.6%]). Implementing studies that evaluate new treatments and therapeutic interventions for drug-resistant TB, MDR-TB, and XDR-TB, was a prominent research priority. Such studies would need to examine methods to improve treatment outcomes for patients with XDR TB such as using later-generation fluoroquinolones, discovering methods to tailor treatment regimens for various forms of TB drug resistance, and investigating the use of quality-controlled laboratory testing for all first and second-line drugs that define XDR-TB. Another frequently cited priority was designing trials to evaluate the optimal duration of TB treatment, the influence of level of immunosuppression on effectiveness of TB drugs, and the combination of anti-TB chemoprophylaxis with antiretroviral therapy.
Discussion
Systematic reviews and meta-analyses are widely acknowledged as a key component of the policy and guideline development process [145]. A large number of systematic reviews have been published in the area of TB diagnostics [146], and these are increasingly being used for developing guidelines [147]. To this end, the Grading of Recommendations Assessment, Development and Evaluation (GRADE) tool has increasingly been adopted by policy makers and guideline developers to provide an explicit, comprehensive and transparent process for moving from evidence to recommendations [145].
Systematic reviews often conclude by making suggestions for the direction of future research, and thus could be a good source for identifying the most important questions for TB research. Our survey collected descriptive information from all eligible systematic reviews and meta-analyses that were subsequently used to generate a list of research priorities in TB which were used for developing the International Roadmap for Tuberculosis Research [5].
Our systematic search showed that a fairly high number of systematic reviews were published on TB during the period of 2005 to 2010. The findings of our review need to be interpreted along with a recent systematic review by Rylance and colleagues [6] on 33 articles with research agendas on TB. These authors found that the top priority areas for research were new TB drug development (28 articles), diagnosis and diagnostic tests (27), epidemiology (20), health services research (16), basic research (13), and vaccine development and use (13).
In our review of 137 TB systematic reviews, the top three categories for the focus of the research priorities/questions were “Detection, screening and diagnosis” “Aetiology” and “Evaluation of treatments and therapeutic interventions.” TB diagnosis and treatment were among the most important research priorities in both reviews. One possible reason of why TB diagnosis research ranked high on our list could be that our review focused on years 2005 to 2010, a period when major advances have been made in TB diagnostics, especially with IGRAs becoming a very popular subject of research [148]. Also, this time period saw the introduction of several WHO policies on TB diagnostics. Further, the emphasis on new tools in the Global Plan to Stop TB 2006–2015 [149], along with the creation of product development partnerships such as the Foundation for Innovative New Diagnostics (FIND), AERAS, and Global Alliance for TB Drug Development, may have inspired research on new diagnostics and drugs.
The research priorities determined were mainly focused on the discovery and evaluation of bacteriological TB tests, drug-resistant TB tests and immunological tests, with special focus on IGRA tests. Also, tests for extra-pulmonary TB came up as a frequently cited priority in the Detection of TB category. Other important topics of future research were genetic susceptibility to TB and disease determinants attributed to HIV/TB. Evaluation of drug treatments for TB, drug-resistant TB and HIV/TB were also frequently proposed research topics. Many articles cited the need for improved and tailored treatment methods for MDR-TB and XDR-TB.
Although several systematic reviews identified areas for further research, the questions themselves were often framed in a generic way, rather than in a highly focused manner with specific recommendation for action. Future TB systematic reviews will need to be more focused, and propose highly specific, answerable questions that are amenable to well-designed research studies.
Our study has several limitations. Due to the poor overall quality of reporting of the systematic reviews, the findings may not be representative of the general output from the TB research community [150]. The inclusion of eligible studies was limited by the fact that we only reviewed articles in three other languages besides English. We were also unable to search ‘grey’ literature, contact authors, or hand search journals. The review also did not include any unpublished literature. Due to its overarching and generic nature, the Health Research Classification System categories were at times non-specific and difficult to match with specific areas of TB research. Furthermore, it was difficult to classify research priorities into narrow subdivisions since some research priorities could qualify for more than one subdivision. By categorizing research priorities into larger, predefined categories, we lost detailed information on individual research priorities. To remedy this, we condensed each priority and extracted the topic words from it. The topic words were then grouped together to form the summary of repeated priorities/questions and calculate the frequency.
There has been a lot of recent attention and focus on childhood TB, but because our search was last performed in 2010, our analysis may have missed research priorities in this important area.
In summary, our systematic review of published systematic reviews on TB helped identify several key priorities for future TB research. This exercise was useful to describe the landscape of TB research and the overarching TB research themes arising from systematic reviews and meta-analyses conducted over the last 5 years. Their scope is, however, limited, since systematic reviews themselves are influenced by current hot topics or new technologies. They are nevertheless useful in indicating research priorities on areas that receive high attention, either due to recent scientific developments or increasing questions surrounding advancement of knowledge in these very areas. They bring useful additional arguments and information to the broader, deeper and more rigorously conducted process of international research agenda development.
Funding Statement
This work was supported in part by the Stop TB Partnership and World Health Organization. Dr Christian Lienhardt from the Stop TB Partnership and WHO provided input in study design and interpretation and revised the manuscript for intellectual content. Additional funding was provided by the European-Developing Countries Clinical Trials Programme (EDCTP; TB-NEAT grant). EDCTP had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization (2011) Global tuberculosis control 2011. Geneva: World Health Organization. 1–242 p. [Google Scholar]
- 2.World Health Organization (2010) Global Plan to Stop Tuberculosis 2011–2015. Geneva: World Health Organization. 1–242 p. [Google Scholar]
- 3. Chaisson RE, Harrington M (2009) How research can help control tuberculosis. Int J Tuberc Lung Dis 13: 558–568. [PubMed] [Google Scholar]
- 4. Lienhardt C, Espinal M, Pai M, Maher D, Raviglione MC (2011) What Research Is Needed to Stop TB? Introducing the TB Research Movement. PLoS Med 8: e1001135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stop TB Partnership & World Health Organization (2011) An international roadmap for tuberculosis research: towards a world free of tuberculosis. Geneva: World Health Organization. [Google Scholar]
- 6. Rylance J, Pai M, Lienhardt C, Garner P (2010) Priorities for tuberculosis research: a systematic review. Lancet Infect Dis 10: 886–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.UK Clinical Research Collaboration (2011) Health Research Classification System (HRCS). London, UK: UKCRC.
- 8. Abba K, Sudarsanam TD, Grobler L, Volmink J (2008) Nutritional supplements for people being treated for active tuberculosis. Cochrane Database of Systematic Reviews CD006086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abubakar I (2010) Tuberculosis and air travel: a systematic review and analysis of policy. The Lancet Infectious Diseases 10: 176–183. [DOI] [PubMed] [Google Scholar]
- 10. Agarwal R, Srinivas R, Aggarwal AN (2008) Parenchymal pseudotumoral tuberculosis: Case series and systematic review of literature. Respiratory Medicine 102: 382–389. [DOI] [PubMed] [Google Scholar]
- 11. Akolo C, Adetifa I, Shepperd S, Volmink J (2010) Treatment of latent tuberculosis infection in HIV infected persons. Cochrane database of systematic reviews (Online) CD000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arshad S, Bavan L, Gajari K, Paget SNJ, Baussano I (2010) Active screening at entry for tuberculosis among new immigrants: A systematic review and meta-analysis. European Respiratory Journal 35: 1336–1345. [DOI] [PubMed] [Google Scholar]
- 13. Balcells ME, Thomas SL, Godfrey-Faussett P, Grant AD (2006) Isoniazid preventive therapy and risk for resistant tuberculosis. Emerging Infectious Diseases 12: 744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bates MN, Khalakdina A, Pai M, Chang L, Lessa F, et al. (2007) Risk of tuberculosis from exposure to tobacco smoke: A systematic review and meta-analysis. Archives of Internal Medicine 167: 335–342. [DOI] [PubMed] [Google Scholar]
- 15. Bliven EE, Podewils LJ (2009) The role of chronic hepatitis in isoniazid hepatotoxicity during treatment for latent tuberculosis infection. International Journal of Tuberculosis and Lung Disease 13: 1054–1060. [PubMed] [Google Scholar]
- 16. Brasil PEAAd, Braga JU (2008) Meta-analysis of factors related to health services that predict treatment default by tuberculosis patients. Cadernos de Saude Publica 24 Suppl 4s485–502. [DOI] [PubMed] [Google Scholar]
- 17. Bwanga F, Hoffner S, Haile M, Joloba ML (2009) Direct susceptibility testing for multi drug resistant tuberculosis: A meta-analysis. BMC Infectious Diseases 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cattamanchi A, Davis JL, Pai M, Huang L, Hopewell PC, et al. (2010) Does Bleach Processing Increase the Accuracy of Sputum Smear Microscopy for Diagnosing Pulmonary Tuberculosis? Journal of Clinical Microbiology 48: 2433–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chang KC, Leung CC (2010) Systematic review of interferon-gamma release assays in tuberculosis: Focus on likelihood ratios. Thorax 65: 271–276. [DOI] [PubMed] [Google Scholar]
- 20. Chang KC, Leung CC, Yew WW, Chan SL, Tam CM (2006) Dosing schedules of 6-month regimens and relapse for pulmonary tuberculosis. American Journal of Respiratory and Critical Care Medicine 174: 1153–1158. [DOI] [PubMed] [Google Scholar]
- 21. Chang KC, Leung ECC, Leung CC (2009) Interferon-gamma release assays in childhood tuberculosis: A systematic review. Hong Kong Journal of Paediatrics 14: 86–95. [Google Scholar]
- 22. Chang KC, Yew WW, Chan RC (2010) Rapid assays for fluoroquinolone resistance in Mycobacterium tuberculosis: a systematic review and meta-analysis. J Antimicrob Chemother 65: 1551–1561. [DOI] [PubMed] [Google Scholar]
- 23. Chang KC, Yew WW, Zhang Y (2009) A systematic review of rapid drug susceptibility tests for multidrug-resistant tuberculosis using rifampin resistance as a surrogate. Expert Opinion on Medical Diagnostics 3: 99–122. [DOI] [PubMed] [Google Scholar]
- 24. Clark RC, Mytton J (2007) Estimating infectious disease in UK asylum seekers and refugees: a systematic review of prevalence studies. Journal of Public Health 29: 420–428. [DOI] [PubMed] [Google Scholar]
- 25. Cox HS, Morrow M, Deutschmann PW (2008) Long term efficacy of DOTS regimens for tuberculosis: Systematic review. Bmj 336: 484–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Daley P, Thomas S, Pai M (2007) Nucleic acid amplification tests for the diagnosis of tuberculous lymphadenitis: A systematic review. International Journal of Tuberculosis and Lung Disease 11: 1166–1176. [PubMed] [Google Scholar]
- 27. Davies G, Cerri S, Richeldi L (2007) Rifabutin for treating pulmonary tuberculosis. Cochrane Database of Systematic Reviews CD005159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Diel R, Loddenkemper R, Nienhaus A (2010) Evidence-based comparison of commercial interferon-gamma release assays for detecting active TB: a metaanalysis. Chest 137: 952–968. [DOI] [PubMed] [Google Scholar]
- 29. Dinnes J, Deeks J, Kunst H, Gibson A, Cummins E, et al. (2007) A systematic review of rapid diagnostic tests for the detection of tuberculosis infection. Health Technology Assessment 11: 1–178. [DOI] [PubMed] [Google Scholar]
- 30. Ena J, Valls V (2005) Short-course therapy with rifampin plus isoniazid, compared with standard therapy with isoniazid, for latent tuberculosis infection: A meta-analysis. Clinical Infectious Diseases 40: 670–676. [DOI] [PubMed] [Google Scholar]
- 31. Engel ME, Matchaba PT, Volmink J (2007) Corticosteroids for tuberculous pleurisy. Cochrane Database of Systematic Reviews CD001876. [DOI] [PubMed] [Google Scholar]
- 32. European Concerted Action on New Generation Genetic M, Techniques for the E, Control of T (2006) Beijing/W genotype Mycobacterium tuberculosis and drug resistance. Emerging Infectious Diseases 12: 736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Farhat M, Greenaway C, Pai M, Menzies D (2006) False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? International Journal of Tuberculosis & Lung Disease 10: 1192–1204. [PubMed] [Google Scholar]
- 34. Faustini A, Hall AJ, Perucci CA (2005) Tuberculosis treatment outcomes in Europe: A systematic review. European Respiratory Journal 26: 503–510. [DOI] [PubMed] [Google Scholar]
- 35. Faustini A, Hall AJ, Perucci CA (2006) Risk factors for multidrug resistant tuberculosis in Europe: A systematic review. Thorax 61: 158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Flores LL, Pai M, Colford JM Jr, Riley LW (2005) In-house nucleic acid amplification tests for the detection of Mycobacterium tuberculosis in sputum specimens: meta-analysis and meta-regression. BMC Microbiology 5: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fok A, Numata Y, Schulzer M, FitzGerald MJ (2008) Risk factors for clustering of tuberculosis cases: A systematic review of population-based molecular epidemiology studies. International Journal of Tuberculosis and Lung Disease 12: 480–492. [PubMed] [Google Scholar]
- 38. Fontela PS, Pai NP, Schiller I, Dendukuri N, Ramsay A, et al. (2009) Quality and reporting of diagnostic accuracy studies in TB, HIV and malaria: Evaluation using QUADAS and STARD standards. PLoS ONE 4 (11): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fraser A, Paul M, Attamna A, Leibovici L (2006) Drugs for preventing tuberculosis in people at risk of multiple-drug-resistant pulmonary tuberculosis. Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fraser A, Paul M, Attamna A, Leibovici L (2006) Treatment of latent tuberculosis in persons at risk for multidrug-resistant tuberculosis: Systematic review. International Journal of Tuberculosis and Lung Disease 10: 19–23. [PubMed] [Google Scholar]
- 41. Freeman RJ, Mancuso J, Keep L (2008) A systematic review and meta-analysis of tuberculosis infection risk in deployed military personnel and long-term civilian travelers. American Journal of Tropical Medicine and Hygiene 79: 711. [Google Scholar]
- 42. Freeman RJ, Mancuso JD, Riddle MS, Keep LW (2010) Systematic Review and Meta-Analysis of TST Conversion Risk in Deployed Military and Long-Term Civilian Travelers. Journal of Travel Medicine 17: 233–242. [DOI] [PubMed] [Google Scholar]
- 43. Gao L, Tao Y, Zhang L, Jin Q (2010) Vitamin D receptor genetic polymorphisms and tuberculosis: Updated systematic review and meta-analysis. International Journal of Tuberculosis and Lung Disease 14: 15–23. [PubMed] [Google Scholar]
- 44. Gao L, Zhou F, Li XW, Jin Q (2010) HIV/TB Co-Infection in Mainland China: A Meta-Analysis. Plos One 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gao XF, Li J, Yang ZW, Li YP (2009) Rifapentine vs. rifampicin for the treatment of pulmonary tuberculosis: A systematic review. International Journal of Tuberculosis and Lung Disease 13: 810–819. [PubMed] [Google Scholar]
- 46. Gao XF, Wang L, Liu GJ, Wen J, Sun X, et al. (2006) Rifampicin plus pyrazinamide versus isoniazid for treating latent tuberculosis infection: A meta-analysis. International Journal of Tuberculosis and Lung Disease 10: 1080–1090. [PubMed] [Google Scholar]
- 47. Gray DM, Zar H, Cotton M (2009) Impact of tuberculosis preventive therapy on tuberculosis and mortality in HIV-infected children. Cochrane Database of Systematic Reviews (1) [DOI] [PubMed] [Google Scholar]
- 48. Greco S, Rulli M, Girardi E, Piersimoni C, Saltini C (2009) Diagnostic accuracy of in-house PCR for pulmonary tuberculosis in smear-positive patients: Meta-analysis and metaregression. Journal of Clinical Microbiology 47: 569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Greveson K (2009) Can ELISpot replace the tuberculin skin test for latent tuberculosis? (Provisional abstract). British Journal of Nursing pp 1248–1254. [DOI] [PubMed] [Google Scholar]
- 50. Guo N, Marra F, Marra CA (2009) Measuring health-related quality of life in tuberculosis: A systematic review. Health and Quality of Life Outcomes 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Han T (2006) Effectiveness of standard short-course chemotherapy for treating tuberculosis and the impact of drug resistance on its outcome (Structured abstract). International Journal of Evidence-Based Healthcare pp 101–117. [DOI] [PubMed] [Google Scholar]
- 52. Hirsch-Moverman Y, Daftary A, Franks J, Colson PW (2008) Adherence to treatment for latent tuberculosis infection: Systematic review of studies in the US and Canada. International Journal of Tuberculosis and Lung Disease 12: 1235–1254. [PubMed] [Google Scholar]
- 53. Holty JEC, Gould MK, Meinke L, Keeffe EB, Ruoss SJ (2009) Tuberculosis in liver transplant recipients: A systematic review and meta-analysis of individual patient data. Liver Transplantation 15: 894–906. [DOI] [PubMed] [Google Scholar]
- 54. Horne DJ, Royce SE, Gooze L, Narita M, Hopewell PC, et al. (2010) Sputum monitoring during tuberculosis treatment for predicting outcome: systematic review and meta-analysis. The Lancet Infectious Diseases 10: 387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Houben R, Glynn JR (2009) A systematic review and meta-analysis of molecular epidemiological studies of tuberculosis: development of a new tool to aid interpretation. Tropical Medicine & International Health 14: 892–909. [DOI] [PubMed] [Google Scholar]
- 56. Houben R, Glynn JR (2009) Systematic review and analysis of population-based molecular epidemiological studies. International Journal of Tuberculosis and Lung Disease 13: 275–275. [PubMed] [Google Scholar]
- 57. Jacobson KR, Tierney DB, Jeon CY, Mitnick CD, Murray MB (2010) Treatment outcomes among patients with extensively drug-resistant tuberculosis: Systematic review and meta-analysis. Clinical Infectious Diseases 51: 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jeon CY, Murray MB (2008) Diabetes mellitus increases the risk of active tuberculosis: A systematic review of 13 observational studies. PLoS Medicine 5: 1091–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jiang J, Shi HZ, Liang QL, Qin SM, Qin XJ (2007) Diagnostic value of interferone-gamma in tuberculous pleurisy: A metaanalysis. Chest 131: 1133–1141. [DOI] [PubMed] [Google Scholar]
- 60. Johnston JC, Shahidi NC, Sadatsafavi M, Fitzgerald JM (2009) Treatment outcomes of multidrug-resistant tuberculosis: A systematic review and meta-analysis. PLoS ONE 4 (9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Joshi R, Reingold AL, Menzies D, Pai M (2006) Tuberculosis among health-care workers in low- and middle-income countries: A systematic review. PLoS Medicine 3: 2376–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jutte PC, Van Loenhout-Rooyackers JH (2006) Routine surgery in addition to chemotherapy for treating spinal tuberculosis. Cochrane Database of Systematic Reviews CD004532. [DOI] [PubMed] [Google Scholar]
- 63. Kakisi OK, Kechagia AS, Kakisis IK, Rafailidis PI, Falagas ME (2010) Tuberculosis of the oral cavity: a systematic review. European Journal of Oral Sciences 118: 103–109. [DOI] [PubMed] [Google Scholar]
- 64. Kalantri S, Pai M, Pascopella L, Riley L, Reingold A (2005) Bacteriophage- based tests for the detection of Mycobacterium tuberculosis in clinical specimens: A systematic review and meta-analysis. BMC Infectious Diseases 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kangovi S, Mukherjee J, Bohmer R, Fitzmaurice G (2009) A classification and meta-analysis of community-based directly observed therapy programs for tuberculosis treatment in developing countries. Journal of Community Health 34: 506–513. [DOI] [PubMed] [Google Scholar]
- 66. Kettaneh A, Seng L, Tiev KP, Toledano C, Fabre B, et al. (2006) Human leukocyte antigens and susceptibility to tuberculosis: A meta-analysis of case-control studies. International Journal of Tuberculosis and Lung Disease 10: 717–725. [PubMed] [Google Scholar]
- 67. Khan FA, Minion J, Pai M, Royce S, Burman W, et al. (2010) Treatment of active tuberculosis in HIV-coinfected patients: a systematic review and meta-analysis. Clinical Infectious Diseases 50: 1288–1299. [DOI] [PubMed] [Google Scholar]
- 68. Klinkenberg E, Manissero D, Semenza JC, Verver S (2009) Migrant tuberculosis screening in the EU/EEA: yield, coverage and limitations. European Respiratory Journal 34: 1180–1189. [DOI] [PubMed] [Google Scholar]
- 69. Kranzer K, Houben R, Glynn JR, Bekker LG, Wood R, et al. (2010) Yield of HIV-associated tuberculosis during intensified case finding in resource-limited settings: a systematic review and meta-analysis. Lancet Infectious Diseases 10: 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kruk ME, Schwalbe NR, Aguiar CA (2008) Timing of default from tuberculosis treatment: A systematic review. Tropical Medicine and International Health 13: 703–712. [DOI] [PubMed] [Google Scholar]
- 71. Leone S, Nicastri E, Giglio S, Narciso P, Ippolito G, et al. (2010) Immune reconstitution inflammatory syndrome associated with Mycobacterium tuberculosis infection: a systematic review. International Journal of Infectious Diseases 14: e283–e291. [DOI] [PubMed] [Google Scholar]
- 72. Lew W, Pai M, Oxlade O, Martin D, Menzies D (2008) Initial drug resistance and tuberculosis treatment outcomes: Systematic review and meta-analysis. Annals of Internal Medicine 149: 123–134. [DOI] [PubMed] [Google Scholar]
- 73. Lewis SJ, Baker I, Smith GD (2005) Meta-analysis of vitamin D receptor polymorphisms and pulmonary tuberculosis risk. International Journal of Tuberculosis and Lung Disease 9: 1174–1177. [PubMed] [Google Scholar]
- 74. Li HT, Zhang TT, Zhou YQ, Huang QH, Huang J (2006) SLC11A1 (formerly NRAMP1) gene polymorphisms and tuberculosis susceptibility: A meta-analysis. International Journal of Tuberculosis and Lung Disease 10: 3–12. [PubMed] [Google Scholar]
- 75. Liang HY, Li XL, Yu XS, Guan P, Yin ZH, et al. (2009) Facts and fiction of the relationship between preexisting tuberculosis and lung cancer risk: A systematic review. International Journal of Cancer 125: 2936–2944. [DOI] [PubMed] [Google Scholar]
- 76. Liang QL, Shi HZ, Wang K, Qjn SM, Qin XJ (2008) Diagnostic accuracy of adenosine deaminase in tuberculous pleurisy: A meta-analysis. Respiratory Medicine 102: 744–754. [DOI] [PubMed] [Google Scholar]
- 77. Lin HH, Ezzati M, Murray M (2007) Tobacco smoke, indoor air pollution and tuberculosis: A systematic review and meta-analysis. PLoS Medicine 4: 0173–0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lin MY, Lin SJ, Chan LC, Lu YC (2010) Impact of food and antacids on the pharmacokinetics of anti-tuberculosis drugs: Systematic review and meta-analysis. International Journal of Tuberculosis and Lung Disease 14: 806–818. [PubMed] [Google Scholar]
- 79. Ling DI, Flores LL, Riley LW, Pai M (2008) Commercial nucleic-acid amplification tests for diagnosis of pulmonary tuberculosis in respiratory specimens: meta-analysis and meta-regression. PLoS ONE [Electronic Resource] 3: e1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ling DI, Zwerling AA, Pai M (2008) GenoType MTBDR assays for the diagnosis of multidrug-resistant tuberculosis: A meta-analysis. European Respiratory Journal 32: 1165–1174. [DOI] [PubMed] [Google Scholar]
- 81. Liu Q, Abba K, Alejandria MM, Balanag VM, Berba RP, et al. (2008) Reminder systems and late patient tracers in the diagnosis and management of tuberculosis. Cochrane Database of Systematic Reviews (4) [DOI] [PubMed] [Google Scholar]
- 82. Liu Q, Garner P, Wang Y, Huang B, Smith H (2008) Drugs and herbs given to prevent hepatotoxicity of tuberculosis therapy: Systematic review of ingredients and evaluation studies. BMC Public Health 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lonnroth K, Williams BG, Cegielski P, Dye C (2010) A consistent log-linear relationship between tuberculosis incidence and body mass index. International Journal of Epidemiology 39: 149–155. [DOI] [PubMed] [Google Scholar]
- 84. Lonnroth K, Williams BG, Stadlin S, Jaramillo E, Dye C (2008) Alcohol use as a risk factor for tuberculosis - A systematic review. BMC Public Health 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lutge Elizabeth E, Knight Stephen E, Volmink J (2009) Incentives for improving patient adherence to anti-tuberculosis treatment. Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd. [Google Scholar]
- 86. Martin A, Panaiotov S, Portaels F, Hoffner S, Palomino JC, et al. (2008) The nitrate reductase assay for the rapid detection of isoniazid and rifampicin resistance in Mycobacterium tuberculosis: A systematic review and meta-analysis. Journal of Antimicrobial Chemotherapy 62: 56–64. [DOI] [PubMed] [Google Scholar]
- 87. Martin A, Portaels F, Palomino JC (2007) Colorimetric redox-indicator methods for the rapid detection of multidrug resistance in Mycobacterium tuberculosis: A systematic review and meta-analysis. Journal of Antimicrobial Chemotherapy 59: 175–183. [DOI] [PubMed] [Google Scholar]
- 88. Mase SR, Ramsay A, Ng V, Henry M, Hopewell RC, et al. (2007) Yield of serial sputum specimen examinations in the diagnosis of pulmonary tuberculosis: a systematic review. International Journal of Tuberculosis and Lung Disease 11: 485–495. [PubMed] [Google Scholar]
- 89. Menon PR, Lodha R, Sivanandan S, Kabra SK (2010) Intermittent or daily short course chemotherapy for tuberculosis in children: Meta-analysis of randomized controlled trials. Indian Pediatrics 47: 67–73. [DOI] [PubMed] [Google Scholar]
- 90. Menzies D, Benedetti A, Paydar A, Martin I, Royce S, et al. (2009) Effect of duration and intermittency of rifampin on tuberculosis treatment outcomes: A systematic review and meta-analysis. PLoS Medicine 6 (9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Menzies D, Benedetti A, Paydar A, Royce S, Pai M, et al. (2009) Standardized treatment of active tuberculosis in patients with previous treatment and/or with mono-resistance to isoniazid: A systematic review and meta-analysis. PLoS Medicine 6 (9): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Menzies D, Pai M, Comstock G (2007) Meta-analysis: New tests for the diagnosis of latent tuberculosis infection: Areas of uncertainty and recommendations for research. Annals of Internal Medicine 146: 340–354. [DOI] [PubMed] [Google Scholar]
- 93. Millership SE, Anderson C, Cummins AJ, Bracebridge S, Abubakar I (2009) The risk to infants from nosocomial exposure to tuberculosis. Pediatric Infectious Disease Journal 28: 915–916. [DOI] [PubMed] [Google Scholar]
- 94. Minion J, Pai M (2010) Bacteriophage assays for rifampicin resistance detection in Mycobacterium tuberculosis: updated meta-analysis. Int J Tuberc Lung Dis 14: 941–951. [PubMed] [Google Scholar]
- 95. Morgan M, Kalantri S, Flores L, Pai M (2005) A commercial line probe assay for the rapid detection of rifampicin resistance in Mycobacterium tuberculosis: a systematic review and meta-analysis. BMC Infectious Diseases 5: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Morisson P, Neves DD (2008) Evaluation of adenosine deaminase in the diagnosis of pleural tuberculosis: a Brazilian meta-analysis. Jornal Brasileiro De Pneumologia: Publicacao Oficial Da Sociedade Brasileira De Pneumologia E Tisilogia 34: 217–224. [DOI] [PubMed] [Google Scholar]
- 97. Morrison J, Pai M, Hopewell PC (2008) Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: a systematic review and meta-analysis. The Lancet Infectious Diseases 8: 359–368. [DOI] [PubMed] [Google Scholar]
- 98. Munro SA, Lewin SA, Smith HJ, Engel ME, Fretheim A, et al. (2007) Patient adherence to tuberculosis treatment: A systematic review of qualitative research. PLoS Medicine 4: 1230–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Murphy DJ, Brown JR (2007) Identification of gene targets against dormant phase Mycobacterium tuberculosis infections. BMC Infectious Diseases 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Nnoaham KE, Clarke A (2008) Low serum vitamin D levels and tuberculosis: A systematic review and meta-analysis. International Journal of Epidemiology 29: 113–119. [DOI] [PubMed] [Google Scholar]
- 102. Noyes J, Popay J (2007) Directly observed therapy and tuberculosis: how can a systematic review of qualitative research contribute to improving services? A qualitative meta-synthesis. Journal of Advanced Nursing 57: 227–243. [DOI] [PubMed] [Google Scholar]
- 103. Obihara CC, Bollen CW, Beyers N, Kimpen JLL (2007) Mycobacterial infection and atopy in childhood: A systematic review. Pediatric Allergy and Immunology 18: 551–559. [DOI] [PubMed] [Google Scholar]
- 104. Orenstein EW, Basu S, Shah NS, Andrews JR, Friedland GH, et al. (2009) Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. The Lancet Infectious Diseases 9: 153–161. [DOI] [PubMed] [Google Scholar]
- 105. Pacheco AG, Cardoso CC, Moraes MO (2008) IFNG +874T/A, IL10 -1082G/A and TNF -308G/A polymorphisms in association with tuberculosis susceptibility: A meta-analysis study. Human Genetics 123: 477–484. [DOI] [PubMed] [Google Scholar]
- 106. Pai M, Kalantri S, Pascopella L, Riley LW, Reingold AL (2005) Bacteriophage-based assays for the rapid detection of rifampicin resistance in Mycobacterium tuberculosis: A meta-analysis. Journal of Infection 51: 175–187. [DOI] [PubMed] [Google Scholar]
- 107. Pai M, Zwerling A, Menzies D (2008) Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: An update. Annals of Internal Medicine 149: 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Pasipanodya JG, Gumbo T (2010) Clinical and Toxicodynamic Evidence that High-Dose Pyrazinamide Is Not More Hepatotoxic than the Low Doses Currently Used. Antimicrobial Agents and Chemotherapy 54: 2847–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Payne B, Bellamy R, Payne B, Bellamy R (2007) Tuberculosis in people with HIV. Clinical Evidence [PubMed] [Google Scholar]
- 110. Pereira SM, Dantas OMS, Ximenes R, Barreto ML (2007) [BCG vaccine against tuberculosis: its protective effect and vaccination policies]. Revista de Saude Publica 41 Suppl 159–66. [DOI] [PubMed] [Google Scholar]
- 111. Piersimoni C, Olivieri A, Benacchio L, Scarparo C (2006) Current perspectives on drug susceptibility testing of Mycobacterium tuberculosis complex: the automated nonradiometric systems. Journal of Clinical Microbiology 44: 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Placidi D, Tonozzi B, Alessio L, Porru S (2007) [Tuberculin skin test (TST) survey among healthcare workers (HCWs) in hospital: a systematic review of the literature]. Giornale Italiano di Medicina del Lavoro Ed Ergonomia 29: 409–411. [PubMed] [Google Scholar]
- 113. Prasad K, Singh MB (2008) Corticosteroids for managing tuberculous meningitis. Cochrane Database of Systematic Reviews (1) [DOI] [PubMed] [Google Scholar]
- 114. Rajagopala S, Agarwal R (2008) Tubercular Mastitis in Men: Case Report and Systematic Review. American Journal of Medicine 121: 539–544. [DOI] [PubMed] [Google Scholar]
- 115. Rehm J, Samokhvalov AV, Neuman MG, Room R, Parry C, et al. (2009) The association between alcohol use, alcohol use disorders and tuberculosis (TB). A systematic review. BMC Public Health 9: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Riquelme A, Calvo M, Salech F, Valderrama S, Pattillo A, et al. (2006) Value of adenosine deaminase (ADA) in ascitic fluid for the diagnosis of tuberculous peritonitis - A meta-analysis. Journal of Clinical Gastroenterology 40: 705–710. [DOI] [PubMed] [Google Scholar]
- 117. Sanai FM, Bzeizi KI (2005) Systematic review: Tuberculous peritonitis - Presenting features, diagnostic strategies and treatment. Alimentary Pharmacology and Therapeutics 22: 685–700. [DOI] [PubMed] [Google Scholar]
- 118. Setia MS, Steinmaus C, Ho CS, Rutherford GW (2006) The role of BCG in prevention of leprosy: a meta-analysis. Lancet Infectious Diseases 6: 162–170. [DOI] [PubMed] [Google Scholar]
- 119. Slama K, Chiang CY, Enarson DA, Hassmiller K, Fanning A, et al. (2007) Tobacco and tuberculosis: A qualitative systematic review and meta-analysis. International Journal of Tuberculosis and Lung Disease 11: 1049–1061. [PubMed] [Google Scholar]
- 120. Slama K, Chiang CY, Hinderaker SG, Bruce N, Vedal S, et al. (2010) Indoor solid fuel combustion and tuberculosis: Is there an association? International Journal of Tuberculosis and Lung Disease 14: 6–14. [PubMed] [Google Scholar]
- 121. Sotgiu G, Ferrara G, Matteelli A, Richardson MD, Centis R, et al. (2009) Epidemiology and clinical management of XDR-TB: A systematic review by TBNET. European Respiratory Journal 33: 871–881. [DOI] [PubMed] [Google Scholar]
- 122. Sreeramareddy CT, Panduru KV, Menten J, Van den Ende J (2009) Time delays in diagnosis of pulmonary tuberculosis: A systematic review of literature. BMC Infectious Diseases 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Steingart K, Henry M, Ng V, Hopewell PC, Ramsay A, et al. (2006) Fluorescence versus conventional sputum smear microscopy for tuberculosis: a systematic review. Lancet Infectious Diseases 6: 570–581. [DOI] [PubMed] [Google Scholar]
- 124. Steingart KR, Dendukuri N, Henry M, Schiller I, Nahid P, et al. (2009) Performance of purified antigens for serodiagnosis of pulmonary tuberculosis: A meta-analysis. Clinical and Vaccine Immunology 16: 260–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Steingart KR, Henry M, Laal S, Hopewell PC, Ramsay A, et al. (2007) A systematic review of commercial serological antibody detection tests for the diagnosis of extrapulmonary tuberculosis. Postgraduate Medical Journal 83: 705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Steingart KR, Henry M, Laal S, Hopewell PC, Ramsay A, et al. (2007) Commercial serological antibody detection tests for the diagnosis of pulmonary tuberculosis: A systematic review. PLoS Medicine 4: 1041–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Steingart KR, Ng V, Henry M, Hopewell PC, Ramsay A, et al. (2006) Sputum processing methods to improve the sensitivity of smear microscopy for tuberculosis: a systematic review. Lancet Infectious Diseases 6: 664–674. [DOI] [PubMed] [Google Scholar]
- 128. Storla DG, Yimer S, Bjune GA (2008) A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Suchindran S, Brouwer ES, Van Rie A (2009) Is HIV infection a risk factor for multi-drug resistant tuberculosis? A systematic review. PLoS ONE 4 (5): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Sun F, Chen Y, Xiang Y, Zhan S (2008) Drug-metabolising enzyme polymorphisms and predisposition to anti-tuberculosis drug-induced liver injury: A meta-analysis. International Journal of Tuberculosis and Lung Disease 12: 994–1002. [PubMed] [Google Scholar]
- 131. Trunz BB, Fine P, Dye C (2006) Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet 367: 1173–1180. [DOI] [PubMed] [Google Scholar]
- 132. Tuon FF, Higashino HR, Lopes MIBF, Litvoc MN, Atomiya AN, et al. (2010) Adenosine deaminase and tuberculous meningitisA systematic review with meta-analysis. Scandinavian Journal of Infectious Diseases 42: 198–207. [DOI] [PubMed] [Google Scholar]
- 133. Tuon FF, Litvoc MN, Lopes M (2006) Adenosine deaminase and tuberculous pericarditis - A systematic review with meta-analysis. Acta Tropica 99: 67–74. [DOI] [PubMed] [Google Scholar]
- 134. van Zyl-Smit RN, Zwerling A, Dheda K, Pai M (2009) Within-subject variability of interferon-g assay results for tuberculosis and boosting effect of tuberculin skin testing: a systematic review. PLoS ONE 4: e8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Vassall A, Compernolle P (2006) Estimating the resource needs of scaling-up HIV/AIDS and tuberculosis interventions in sub-Saharan Africa: A systematic review for national policy makers and planners. Health Policy 79: 1–15. [DOI] [PubMed] [Google Scholar]
- 136.Vlassov Vasiliy V, Reze Andrey G (2006) Low level laser therapy for treating tuberculosis. Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Volmink J, Garner P (2006) Directly observed therapy for treating tuberculosis. Cochrane Database of Systematic Reviews CD003343. [DOI] [PubMed] [Google Scholar]
- 138. Wisnivesky JP, Serebrisky D, Moore C, Sacks HS, Iannuzzi MC, et al. (2005) Validity of clinical prediction rules for isolating inpatients with suspected tuberculosis: A systematic review. Journal of General Internal Medicine 20: 947–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Yang XY, Chen QF, Cui XH, Yu Y, Li YP (2010) Mycobacterium vaccae vaccine to prevent tuberculosis in high risk people: A meta-analysis. Journal of Infection 60: 320–330. [DOI] [PubMed] [Google Scholar]
- 140. Ziakas PD, Mylonakis E (2009) 4 months of rifampin compared with 9 months of isoniazid for the management of latent tuberculosis infection: A meta-analysis and cost-effectiveness study that focuses on compliance and liver toxicity. Clinical Infectious Diseases 49: 1883–1889. [DOI] [PubMed] [Google Scholar]
- 141. Ziganshina LE, Garner P, Ziganshina LE, Garner P (2009) Tuberculosis (HIV-negative people). Clinical Evidence [PMC free article] [PubMed] [Google Scholar]
- 142. Ziganshina LE, Squire SB (2008) Fluoroquinolones for treating tuberculosis. Cochrane Database of Systematic Reviews CD004795. [DOI] [PubMed] [Google Scholar]
- 143. Ziganshina LE, Vizel AA, Squire SB (2005) Fluoroquinolones for treating tuberculosis. Cochrane Database of Systematic Reviews CD004795. [DOI] [PubMed] [Google Scholar]
- 144. Zodpey SP (2007) Protective effect of bacillus Calmette Guerin (BCG) vaccine in the prevention of leprosy: A meta-analysis. Indian Journal of Dermatology, Venereology and Leprology 73: 86–93. [DOI] [PubMed] [Google Scholar]
- 145. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, et al. (2008) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Bmj 336: 924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Pai M, Ramsay A, O'Brien R (2008) Evidence-based tuberculosis diagnosis. PLoS Med 5: e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Pai M, Minion J, Steingart K, Ramsay A (2010) New and improved tuberculosis diagnostics: evidence, policy, practice, and impact. Curr Opin Pulm Med 16: 271–284. [DOI] [PubMed] [Google Scholar]
- 148. Wallis RS, Pai M, Menzies D, Doherty TM, Walzl G, et al. (2010) Biomarkers and diagnostics for tuberculosis: progress, needs, and translation into practice. Lancet 375: 1920–1937. [DOI] [PubMed] [Google Scholar]
- 149.Stop TB Partnership (2006) The Global Plan to Stop TB, 2006–2015: Actions for life: towards a world free of tuberculosis. Geneva: World Health Organization (WHO/HTM/STB/2006.35). [PubMed]
- 150. Nicolau I, Ling D, Tian L, Lienhardt C, Pai M (2010) Epidemiology, quality and reporting of tuberculosis systematic reviews: an analysis using AMSTAR and PRISMA - Unpublished report submitted to the TB Research Movement and the Stop TB Partnership. McGill University [Google Scholar]