Abstract
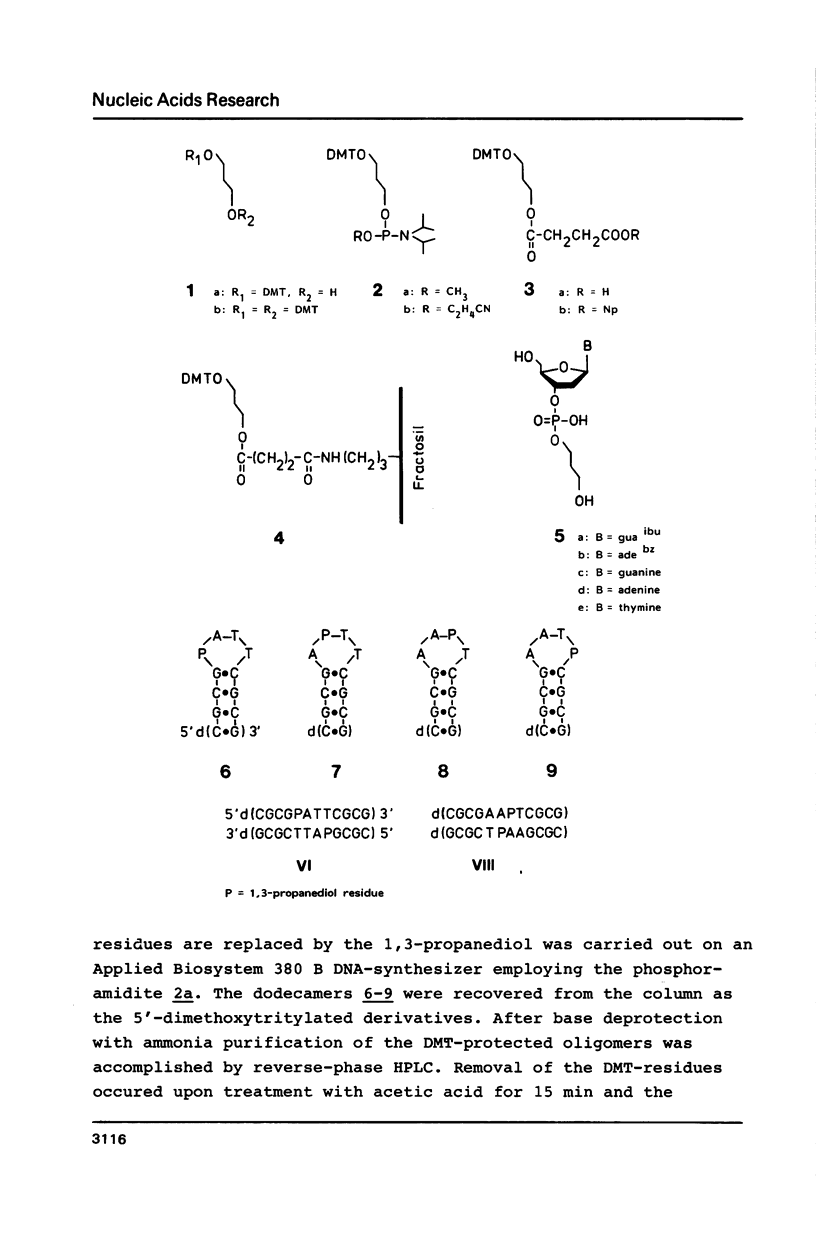
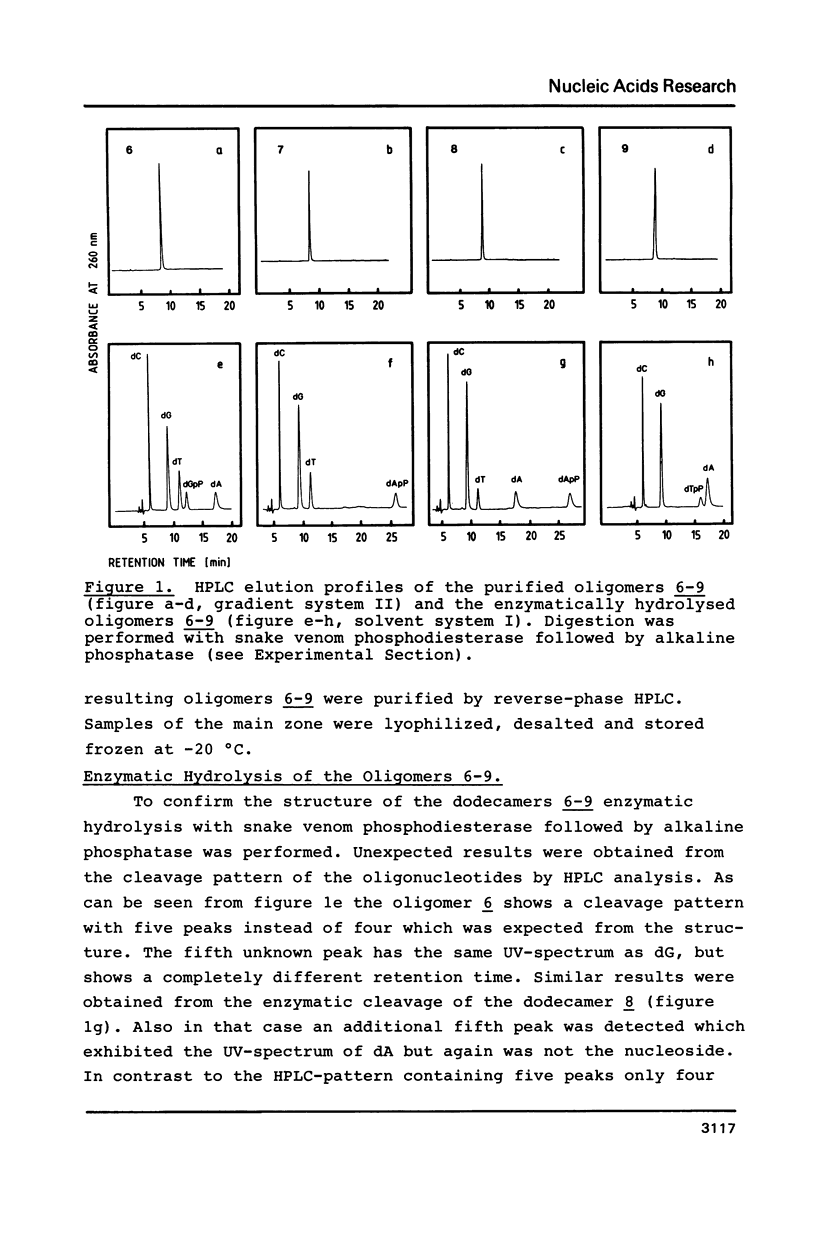
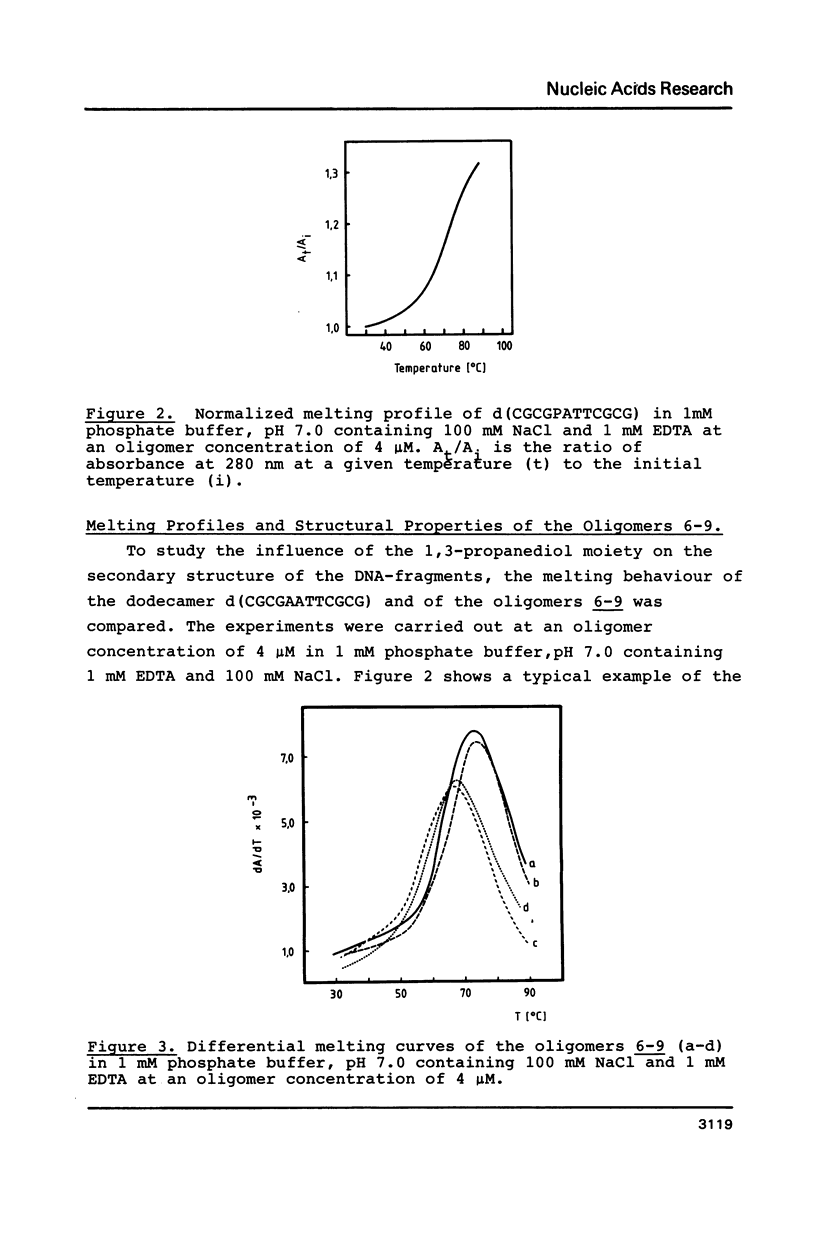
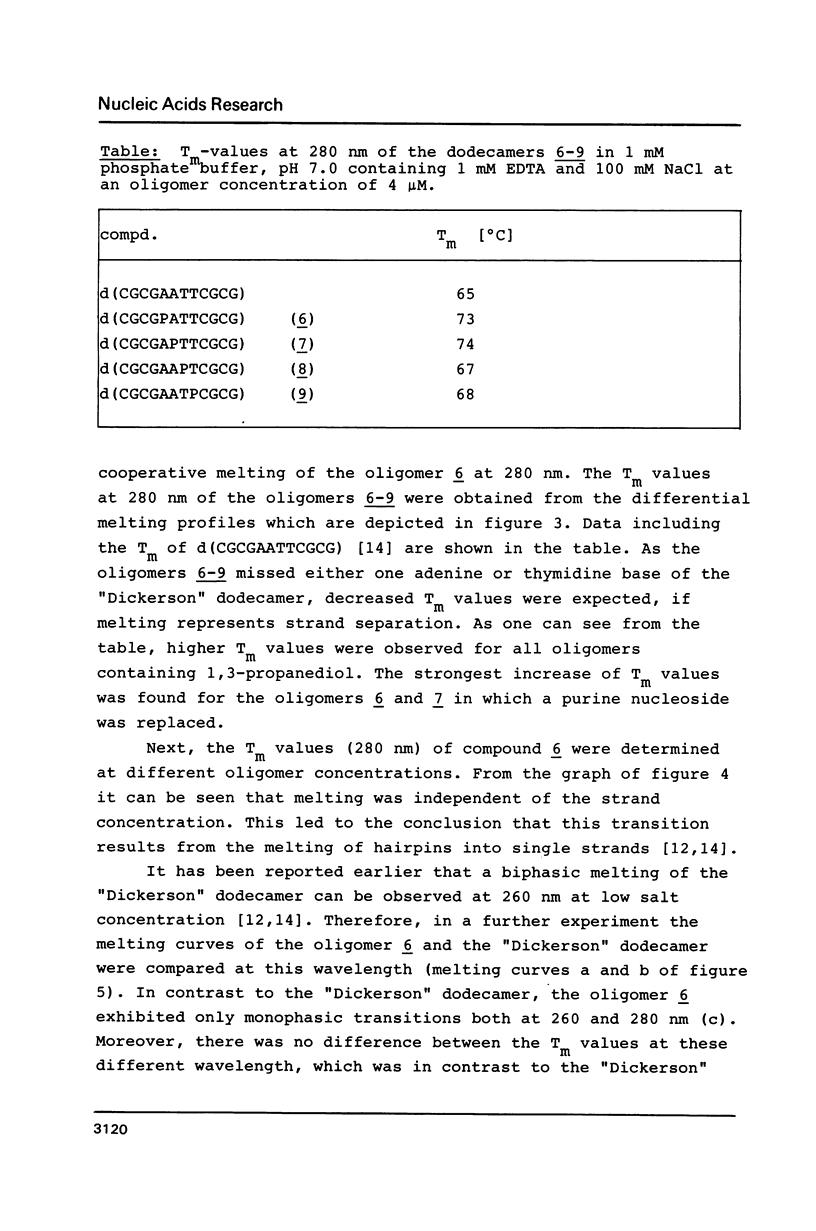
1,3-Propanediol was protected with one dimethoxytrityl residue and converted into the methoxy- and cyanoethoxyphosphoramidites 2a and 2b, respectively. Solid-phase oligonucleotide synthesis, employing the phosphoramidite 2a resulted in the dodecamers d(CGCGAATTCGCG) (6-9), in which dA or dT residues were replaced by 1,3-propanediol. These oligomers showed a high tendency to form hairpins. Their phosphodiester bonds between the 3'-position of a nucleoside and the propanediol moiety was not cleaved by snake venom phosphodiesterase.
Full text
PDF



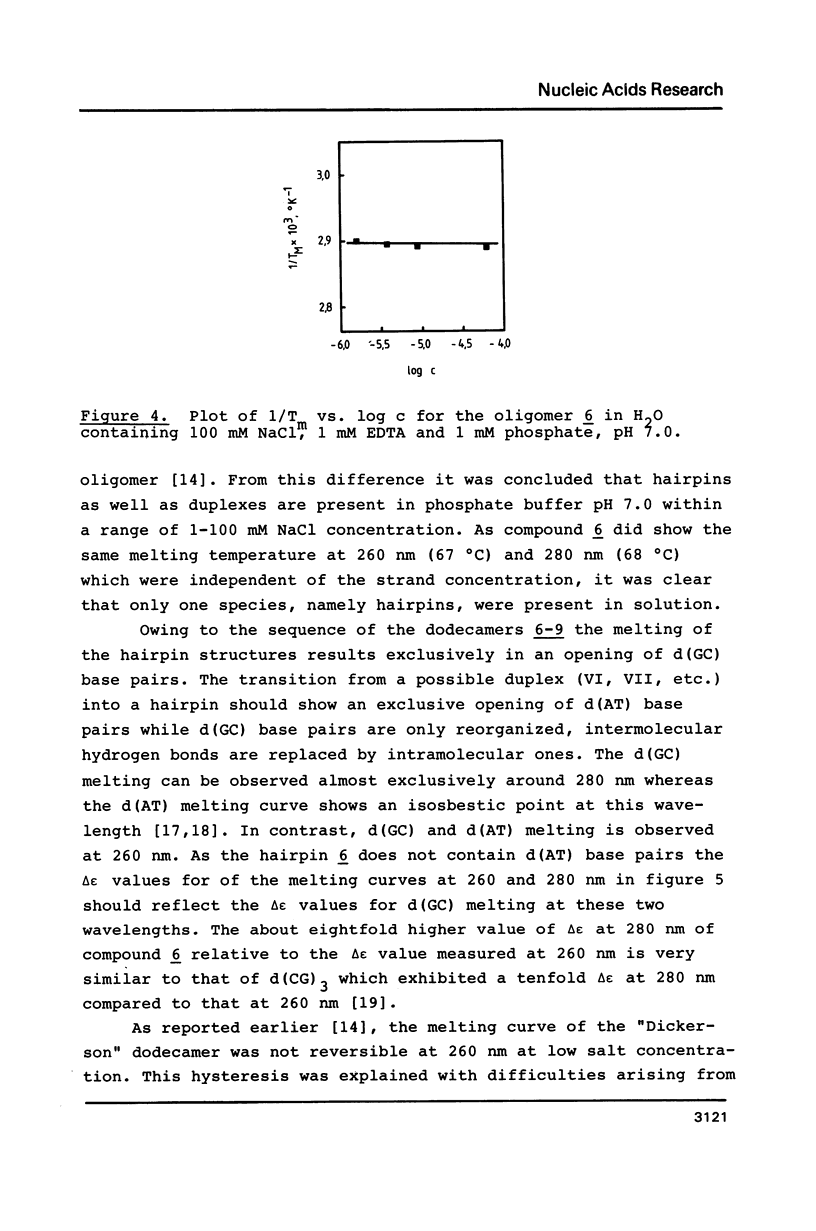







Selected References
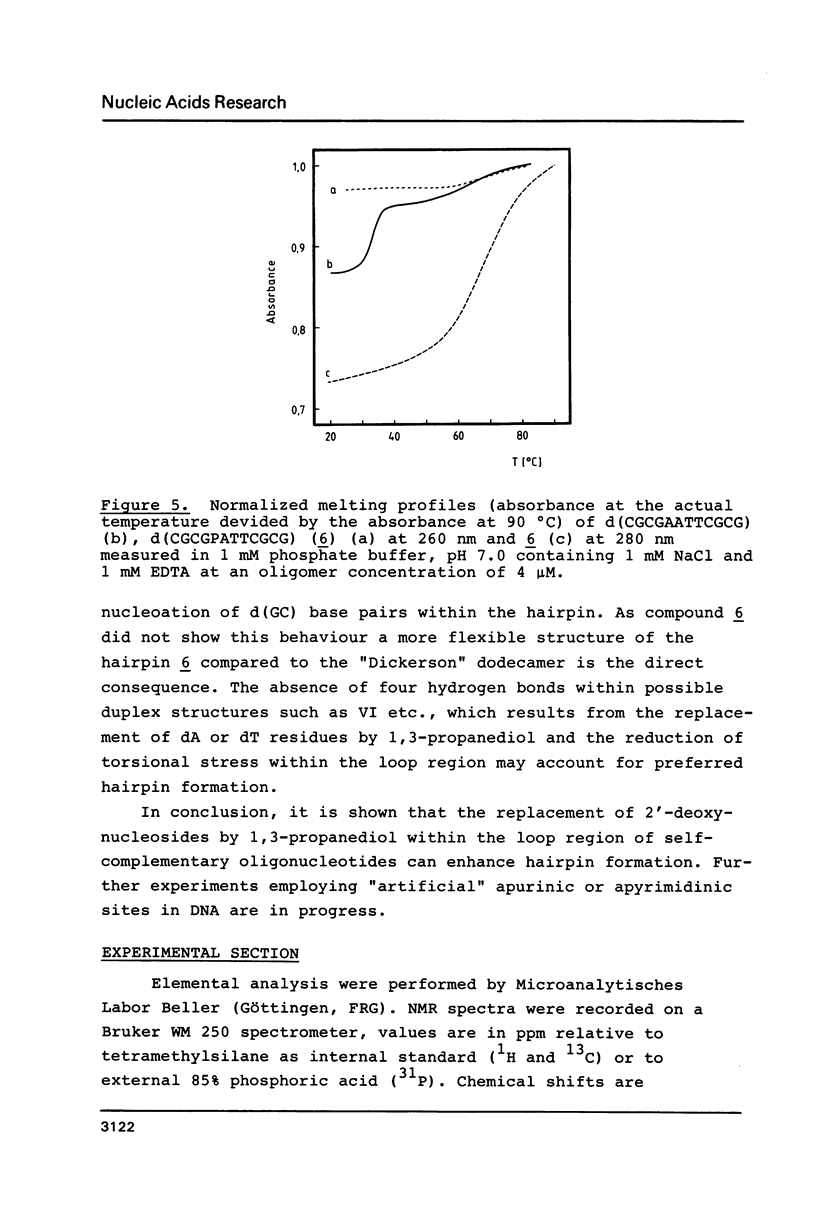
These references are in PubMed. This may not be the complete list of references from this article.
- Blake R. D., Lefoley S. G. Spectral analysis of high resolution direct-derivative melting curves of DNA for instantaneous and total base composition. Biochim Biophys Acta. 1978 Apr 27;518(2):233–246. doi: 10.1016/0005-2787(78)90180-6. [DOI] [PubMed] [Google Scholar]
- Dickerson R. E., Drew H. R. Structure of a B-DNA dodecamer. II. Influence of base sequence on helix structure. J Mol Biol. 1981 Jul 15;149(4):761–786. doi: 10.1016/0022-2836(81)90357-0. [DOI] [PubMed] [Google Scholar]
- FELSENFELD G., SANDEEN G. The dispersion of the hyperchromic effect in thermally induced transitions of nucleic acids. J Mol Biol. 1962 Dec;5:587–610. doi: 10.1016/s0022-2836(62)80088-6. [DOI] [PubMed] [Google Scholar]
- Laskowski M., Sr Purification and properties of venom phosphodiesterase. Methods Enzymol. 1980;65(1):276–284. doi: 10.1016/s0076-6879(80)65037-x. [DOI] [PubMed] [Google Scholar]
- Lindahl T. Uracil-DNA glycosylase from Escherichia coli. Methods Enzymol. 1980;65(1):284–290. doi: 10.1016/s0076-6879(80)65038-1. [DOI] [PubMed] [Google Scholar]
- Marky L. A., Blumenfeld K. S., Kozlowski S., Breslauer K. J. Salt-dependent conformational transitions in the self-complementary deoxydodecanucleotide d(CGCAATTCGCG): evidence for hairpin formation. Biopolymers. 1983 Apr;22(4):1247–1257. doi: 10.1002/bip.360220416. [DOI] [PubMed] [Google Scholar]
- Nielsen J., Taagaard M., Marugg J. E., van Boom J. H., Dahl O. Application of 2-cyanoethyl N,N,N',N'-tetraisopropylphosphorodiamidite for in situ preparation of deoxyribonucleoside phosphoramidites and their use in polymer-supported synthesis of oligodeoxyribonucleotides. Nucleic Acids Res. 1986 Sep 25;14(18):7391–7403. doi: 10.1093/nar/14.18.7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochet S., Huynh-Dinh T., Neumann J. M., Tran-Dinh S., Adam S., Taboury J., Taillandier E., Igolen J. NMR, CD and IR spectroscopies of a tridecanucleotide containing a no-base residue: coexistence of B and Z conformations. Nucleic Acids Res. 1986 Jan 24;14(2):1107–1126. doi: 10.1093/nar/14.2.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riazuddin S. Purification and properties of 3-methyladenine-DNA glycosylase from Escherichia coli. Methods Enzymol. 1980;65(1):290–295. doi: 10.1016/s0076-6879(80)65039-3. [DOI] [PubMed] [Google Scholar]
- Sinha N. D., Biernat J., McManus J., Köster H. Polymer support oligonucleotide synthesis XVIII: use of beta-cyanoethyl-N,N-dialkylamino-/N-morpholino phosphoramidite of deoxynucleosides for the synthesis of DNA fragments simplifying deprotection and isolation of the final product. Nucleic Acids Res. 1984 Jun 11;12(11):4539–4557. doi: 10.1093/nar/12.11.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing R., Drew H., Takano T., Broka C., Tanaka S., Itakura K., Dickerson R. E. Crystal structure analysis of a complete turn of B-DNA. Nature. 1980 Oct 23;287(5784):755–758. doi: 10.1038/287755a0. [DOI] [PubMed] [Google Scholar]
- Xodo L. E., Manzini G., Quadrifoglio F., van der Marel G. A., van Boom J. H. Thermodynamic behaviour of the heptadecadeoxynucleotide d(CGCGCGTTTTTCGCGCG) forming B and Z hairpins in aqueous solution. Nucleic Acids Res. 1986 Jul 11;14(13):5389–5398. doi: 10.1093/nar/14.13.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]


