Summary
Von Hippel-Lindau (VHL) disease is a highly penetrant autosomal dominant systemic malignancy that gives rise to cystic and highly vascularized tumors in a constellation of organs. Patients with VHL disease commonly present with hemangioblastomas in the central nervous system and the eye while other manifestations include pheochromocytoma, clear cell renal cell carcinoma, endolymphatic sac tumors of the middle ear, pancreatic cystadenomas, epididymal and broad ligament cystadenomas. Animal models inactivating the VHL gene product in various organ tissues have been constructed over the past 15 years to parse its HIF-associated mechanisms and its link to tumorigenesis. These models, despite advancing our understanding the molecular role of VHL, are by and large unable to recapitulate the more common features of human VHL disease. Up to date, no model exists that develop retinal hemangioblastomas, the most common clinical manifestation. The purpose of this review is: (1) to discuss the need for an ocular VHL model, (2) to review the animal models that recapitulate clinical VHL disease and (3) to propose potential mechanisms of tumorigenesis for the development of ocular VHL.
Keywords: von Hippel-Lindau, Retinal hemangioblastoma, Animal model, Tumorigenesis, Eye
Introduction
Von Hippel-Lindau (VHL) is an autosomal dominant disease that results in a constellation of cysts or highly vascularized tumors. It has an incidence of approximately 1 in 36,000 live births and has a mean age of onset at 26 years (Lonser et al., 2003). VHL is an age-dependent and highly penetrant disease with the more common manifestations being retinal hemangioblastomas, cerebellar and spinal hemangioblastomas, clear cell renal cell carcinomas (CCRCC), pheochromocytomas, endolymphatic sac tumors of the middle ear, neuroendocrine tumors of the pancreas and cystadenomas in the pancreas, broad ligament, and epididymis (Maher et al., 1990; Lonser et al., 2003). Less common finding involve liver and pulmonary hemangiomas (McGrath et al., 1992; Takahashi et al., 2006)
The VHL gene is found on the 3p25 chromosome (Seizinger et al., 1988). The earliest known mechanism for the pathogenesis of VHL disease is explained by Knudson’s 2 hit hypothesis- one allele of VHL is usually an inherited mutant copy while the second one is acquired (Knudson, 1971). This is typically accomplished through somatic mutation or inactivation by hypermethylation. Familial VHL is categorized into two types, I and II, and is based the presence of pheochromocytoma. Type I have a low risk of developing pheochromocytoma. Type II, on the other hand, has high risk for developing pheochromocytoma. Type II is further subdivided into IIA, IIB, and IIC based on risk of developing clear cell renal cell carcinoma in addition to pheochromocytoma. Patients in IIA have low risk, IIB have high risk and IIC have no risk (Zbar et al., 1996).
The gene product, VHL protein (pVHL), is a part of an E3 ubiquitin ligase complex that ubiquitylates the hypoxia inducible factor α (HIFα) subunit leading to rapid degradation. This ubiquitin complex is comprised of several other proteins namely cullin2, elongin B, elongin C, NEDD8 and Rbx1 (Sufan et al., 2004). HIF is a transcription factor can activate various angiogeneic factors such as erythropoietin (EPO) and vascular endothelial growth factor (VEGF). HIF’s α subunit is oxygen sensitive and is hydroxylated by prolyl-4-hydroxylase domain proteins. In the presence of oxygen, iron and ascorbate the reaction occurs and HIF is able to bind to pVHL for degradation. Conversely, in hypoxic conditions, it is not hydroxylated and is unable to bind pVHL leading to its stabilization. There are hundreds of HIF target genes involved in: angiogenesis, tumor formation, response to therapy, survival, invasion, and metabolism. A succinct yet comprehensive review on HIF, including the things just mentioned, has recently been written elsewhere (Semenza, 2011). pVHL plays a role in HIF-independent pathways as well. It is involved in microtubule stability, ECM deposition and maintenance, senescence, neuronal apoptosis, cell survival and apoptosis. These functions are explained in another recent review (Li and Kim, 2011).
This review briefly describes the animal models for VHL disease. It discusses the need for an animal model that characterizes retinal hemangioblastoma, the mechanisms for tumorigenesis and a possible strategy to accomplish this in the eye.
VHL disease animal models
With the need to better characterize the role of pVHL plays in the disease it is named after, new techniques for developing sophisticated animal models could not come at a better time (Gu et al., 1994; Feil et al., 1996). The first VHL animal model lead to an aberrant placental development that was lethal to the embryo. The mice perished in utero at E10.5 and E12.5 (Gnarra et al., 1997). However, the use of VHL conditional knockouts in mice has proven to be an effective approach to delineate the role of VHL in individual organ systems.
Eye
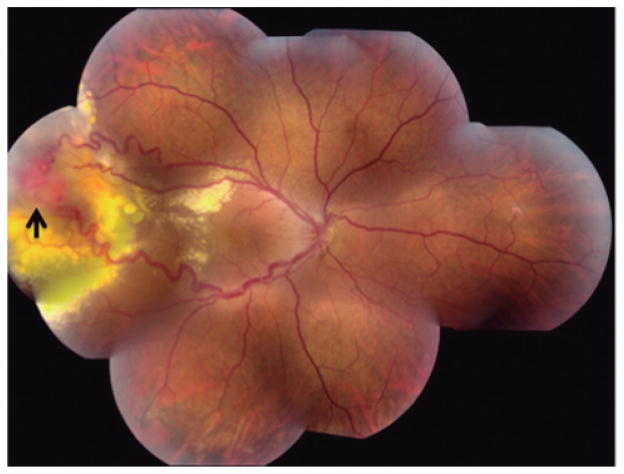
VHL-associated retinal hemangioblastomas occurs in over 60% of patients (Welch, 1970; Singh et al., 2001b) and is the first manifestation of the disease in 43% of patients (Maher et al., 1990). Visual symptoms can occur as early as in the later teens and bilaterality is common (Singh et al., 2001a; Wong et al., 2008). The most common clinical finding of these retinal hemangioblastomas is a highly vascularized tumor in the superiotemporal region of the retina (Singh et al., 2001a; Dollfus et al., 2002) (Fig. 1). Complications of the disease usually entail exudative or tractional effects surrounding the tumor (Kreusel et al., 2006). In a recent cross sectional study, vision loss in 335 patients with VHL-associated retinal hemangioblastomas more likely occurred when the lesions were in the juxtapapillary region, it was also a function of the patients age, and lastly it was related to the number and size of tumors in the periphery (Wong et al., 2008). This study also showed that although bilaterallity is common, the rate of bilateral visual impairment is less common due to the asymmetric disease burden. However, the tumor can still lead to blindness and the rate of significant morbidity in one eye remains high. In addition, there is currently no effective treatment for these severe cases.
Fig. 1.
Fundus photograph of a patient with retinal hemangioblastoma (arrow) in the temporal periphery of the right eye. Two dilated vessels emanate from the tumor. Marked yellow exudation surrounds the tumor and extends into the posterior pole. (Courtesy of Emily Y. Chew, M.D.).
Retinal hemangioblastomas are generally treated with cryotherapy or laser photocoagulation and patients enjoy a 72% and 74% success rate respectively (Singh et al., 2002). A study showed that patients with smaller lesions (less than 1.5 mm) were more likely to remain stable. Those that progressed in this group were well controlled with cryotherapy or photocoagulation (Singh et al., 2002). On the other hand, larger tumors have been shown to be non-responsive to medical treatment- a retrospective study of three patients showed that for lesions between 7–9 mm, surgical resection improved visual acuity or kept it the same (Liang et al., 2007; Schlesinger et al., 2007). The understanding of pVHL as described above has led to the exploration of targeting vasculogenic entities such as VEGF and platelet derived growth factor (PDGF) (Rosenblatt and Azar, 2006). However the efficacy of agents in this class such as SU5416, bevacizumab, sunitinib, ranibizumab and pegaptanib are uncertain (Aiello et al., 2002; Girmens et al., 2003; Madhusudan et al., 2004; Rosenblatt and Azar, 2006; von Buelow et al., 2007).
With regards to refining treatment through improved pre-clinical diagnoses and disease surveillance, there have been recent genotype-phenotype correlations with respect to prognoses (Wong et al., 2007). In this study it was shown that complete deletions of pVHL was associated with better visual acuity and decreased incidence of retinal hemangioblastomas compared to missense and truncating mutations of VHL. In a more recent cross-sectional study of the same but expanded group of 412 VHL patients, it was shown that missense mutations of the VHL subunit is associated with increased incidence of ocular disease and higher associations with juxtapapillary lesions (Mettu et al., 2010).
To date, there is still no suitable model to study hemangioblastomas of the CNS and the retina. A zebrafish model has been developed that expresses retinal neovascularization by vascular leakage, retinal edema and detachment (van Rooijen et al., 2010). Studies have shown increased VEGF and CXCR4A in the CNS in these zebrafish. Indeed, this model manifests certain aspects of age related macular degeneration, diabetic retinopathy and some cases of VHL (Chew, 2005). However the zebrafish does not develop hemangioblastomas, van Rooijen and colleagues were able use this zebrafish model to demonstrate inhibition of angiogenesis through the administration of VEGF receptor tyrosine kinase inhibition, namely sunitinib and 676475.
Dysregulation of hypoxic factors in retinal astrocytes can contribute to pathological angiogenesis. GFAPcre/VHLf/f astrocyte conditional knockout mice showed that HIF-2α driven overexpression of VEGF led to severe hypervascularity in the retina starting from P7 until adulthood localized in the superficial vascular plexus (Weidemann et al., 2010).
In short, though there are mouse models that target VHL in the eye, none are able to develop retinal hemangioblastomas to serve as a viable model to study. Preclinical animal studies are a necessity for the advancement of targeted therapies as they afford the ability to observe the tumor’s interaction with the microenvironment. In the case of VHL, it is understood that the VHL inactivated stromal cells (tumor cells) act on the surrounding endothelial cells (Chan et al., 1999; Vortmeyer et al., 2003) (Fig. 2). An ocular orthotopic xenograft model may also prove useful for studying efficacy, side effects and toxicities of targeted therapies (Fig. 3). Studies in CCRCC xenografts shed light on the potential efficacy of PDGF/VEGF receptor inhibitors, mTOR inhibitors and others (Alleman et al., 2004; Clark, 2009; Hammers et al., 2010; Duignan et al., 2011; Karam et al., 2011; Nagaprashantha et al., 2011). In addition, drugs like topotecan and digoxin can effectively block HIF synthesis at low concentrations (Melillo, 2007; Zhang et al., 2008). It would be worthwhile to test such therapeutics for ocular VHL as well. A genetic mouse model would be useful to study the disease pathogenesis of ocular VHL and may prove to be a superior model of clinical VHL disease.
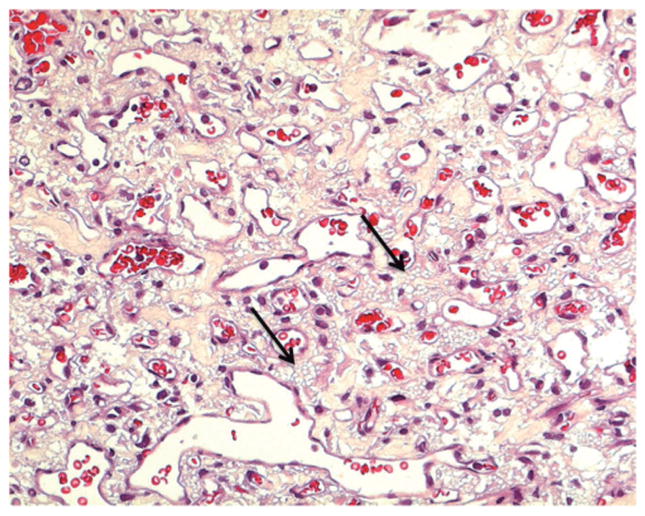
Fig. 2.
Photomicrograph retinal hemangioblastoma. Many vacuolated “foamy” stromal cells (arrows) reside among the thin capillary-like channels. Hematoxylin & eosin. x 400
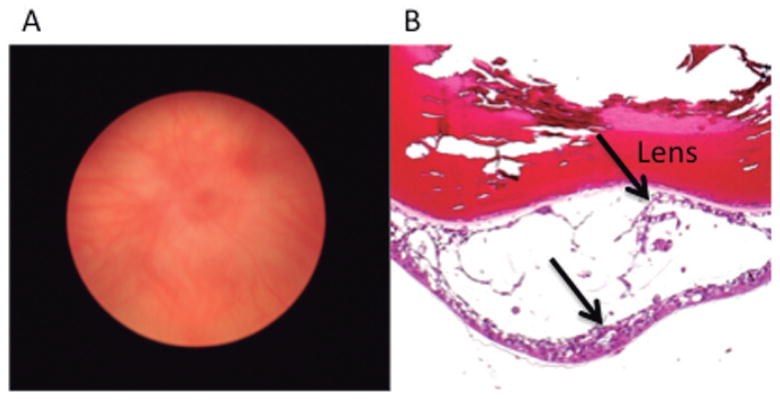
Fig. 3.
Orthotopic xenograft model for ocular VHL. A. Macroscopic photograph of a SCID mouse 2 weeks following intraocular injection of UMRC-6 cells, a human clear cell renal cell carcinoma cell line, shows intraocular tumor cells obscured the view of the fundus. B. Photomicrograph of this eye illustrates UMRC-6 cells (arrows) growing behind the lens and forms cystic structure. Hematoxylin & eosin. x 200
Kidney
CCRCC is a malignancy of the kidney that arises sporadically or hereditarily through VHL inactivation. Rankin and her colleagues were the first group to successfully create a model that generated renal microcysts and macrocysts with similar morphological and molecular features found in VHL-associated renal disease (Rankin et al., 2006). Using the Cre-loxp system, they deleted VHL expression from the proximal tubule using the phosphoenolpyruvate carboxy kinase (PEPCK)-promoter to drive Cre expression. This was sufficient enough to generate cystic lesions with benign clear cell morphology, an indicative preneoplastic characteristic. Further, to test which element downstream VHL had a greater contribution to the phenotype, the group created a double knockout of this model by knocking out its heterodimer partner Arnt (also called HIFβ) or HIF1α. Interestingly, VHL/HIF1α dKO was unable to prevent cyst formation but rather the VHL/HIF2α dKO and VHL/HIFβ dKO models were able to stop cyst formation. Furthermore, it is thought that pVHL’s role in ciliary maintenance contributes to cyst formation. In the background of pVHL inactivation, the additional inhibition of GSK3β, through overexpression of the PI2K signaling, causes loss of primary cilia (Thoma et al., 2007a,b). The group did not report any progression of these cysts into CCRCC in these mice. Molecular mechanisms and appropriate genetic backgrounds have yet to be elucidated.
Other tissue specific models shed light on associations with CCRCC. Glomerulus specific deletions of VHL have led to the development of rapidly progressive glomerulonephritis (Ding et al., 2006), a known association with CCRCC (Kagan et al., 1993). In addition, it has been shown that VHL is crucial in maintenance of the ultrastructure and barrier functions of the glomerular basement membrane as VHL deficiency results in proteinuria, ectopic collagen deposition and membrane thickening (Steenhard et al., 2010).
Pancreas
Patients with VHL may present with pancreatic cysts and neuroendocrine tumors (Lubensky et al., 1998; Mohr et al., 2000). Mice models, with the exception of one (Shen et al., 2009) showed none of the characteristic pancreatic cysts and neuroendocrine tumors that are seen in patients with VHL (Cantley et al., 2009; Puri et al., 2009; Choi et al., 2011). Shen and colleagues produced a model using the insulin promoter factor 1 (Pdx) promoter to drive Cre expression. Survivors secondary to incomplete penetrance expressed highly vascularized cysts and microcystic adenomas by knocking out VHL expression in the entire pancreas. With regards to the other models, possible explanations include that the cysts and tumors do not arise from the Cre transgenes the model was made from. Another possibility is that other events are required to take place before these masses arise. Also, there is a lack of overexpression of HIF2α in pancreatic cells (Wiesener et al., 2003; Cantley et al., 2009). HIF is a crucial player for tumor formation in other VHL associated tumors (Raval et al., 2005).
Reproductive tract
Benign cysts of the broad ligament of the uterus in females and of the epididymis in males are not uncommon in those with VHL disease. Pten, a tumor suppressor involved in the regulation of PI2K-AKT-mTOR pathway (Maehama and Dixon, 1998; Cantley and Neel, 1999; Burgering and Medema, 2003; Vivanco et al., 2007) is absent and HIF1α is elevated in human clear cell cystadenoma tissue (Glasker et al., 2006). Ksp1.3-Cre mice were crossed with Vhlhfl/fl mice and Ptenfl/fl mice. Ksp-cadherin is a unique, tissue-specific member of the cadherin family of cell adhesion molecules that is expressed in tubular epithelial cells in the kidney and developing genitourinary tract. The engineered mice were then intercrossed to generate a VHL and Pten double deficient mouse model specific for the epithelium of the genital tract. These mice were able to recapitulate clear cell cystadenoma of the genital tract in both male and females (Frew et al., 2008). Mice with a single knockout displayed only a mild phenotype. Interestingly, squamous metaplasia was also observed which is not associated with VHL.
Liver
Hepatohemangiomas are an extremely rare VHL disease phenotype with only a few documented case reports (McGrath et al., 1992; Takahashi et al., 2006). A mouse model that acutely inactivated VHL in utero resulted in embryonic lethality with necrosis to the liver and vasculature defects (Hong et al., 2006). One mouse model that conditionally inactivated VHL in hepatocytes led to hepatic hemangiomas (Haase et al., 2001). Similarly, another model that conditionally deactivated VHL in a mosaic pattern in multiple organs showed hepatic hemangiomas as well as angiectasias in the pancreas, heart, lung, and kidney (Ma et al., 2003). In both models, the liver pathology was reported histologically similar to CNS hemangioblastomas. For liver hemangiomas, as seen in other organs, the pathogenesis of these hemangiomas appears to be HIF2α dependent and HIF1α independent (Rankin et al., 2005, 2008). HIF also seems to play a role in liver lipid metabolism, EPO production, iron homeostasis, and mitochondrial respiration (Peyssonnaux et al., 2007; Rankin et al., 2007, 2009; Kucejova et al., 2011).
Summary of murine VHL models
Current animal models can partially capture VHL disease of a particular organ (Table 1). However, much work is still needed to develop one that can progress to recapitulate VHL disease. Other manifestations of VHL disease such as endolymphatic sac tumors of the middle ear, pheochromocytoma, cerebellar and cervical hemangioblastomas are not seen in any of the aforementioned models. In addition, non-VHL disease associated phenotypes may also arise from mutated VHL protein product. Chuvash polycythemia is an exceedingly rare condition unrelated to the classical VHL disease. It arises from point mutations, R200W and H191D, in the VHL gene. This gives rise to polycythemia but none are at risk for any cancer (Ang et al., 2002; Pastore et al., 2003a,b; Bento et al., 2005; Perrotta et al., 2006). This disease has been successfully modeled in rodents (Hickey et al., 2007). Finally, the role of pVHL has been explored in areas not associated with VHL disease such as the heart, chondrocytes, integument, gastronintestinal tract, immune system, mammary glands and host defense (Cramer et al., 2003; Biju et al., 2004; Karhausen et al., 2004; Pfander et al., 2004; Neumann et al., 2005; Peyssonnaux et al., 2005; Boutin et al., 2008; Lei et al., 2008; Seagroves et al., 2010).
Table 1.
Available animal models for VHL disease.
| Reference | Organ | Cell/Cre controller | Animal | Disease phenotype |
|---|---|---|---|---|
| van Rooijen et al., 2010 | Eye | - | zebrafish | neovascularization, no hemangioblastoma |
| Rankin et al., 2006 | Kidney | proximal tubule/PEPCK | mouse | cysts, no CCRCC |
| Shen et al., 2009 | Pancreas | whole pancreas/Pdx | mouse | cysts, microcystadenomas |
| Frew et al., 2008 | Genitourinary tract | tubular epithelial cells/Ksp1.3 | mouse | cysts |
| Haase et al., 2001; Ma et al., 2003 | Liver | - | mouse | cavernous hemangioblastomas |
Perspective on VHL tumorigenesis and potential strategies for designing models that may develop ocular VHL-associated tumors
It is possible that VHL mutation alone may not sufficient to develop VHL-associated neoplasms. The mutations can be altering the less-understood HIF-independent pathways (Champion et al., 2008) or other oncogenes or tumor suppressor genes (Chan et al., 2002). It has been shown that the loss of VHL gene does not promote tumor growth in primary cells (Mack et al., 2003) while it has been shown in renal cell carcinoma (RCC) cell lines, the loss of VHL gene promotes growth and the re-introduction of wild type VHL gene suppresses tumor growth (Gnarra et al., 1996; Iliopoulos et al., 1996). Additional mutations in the RCC could be responsible for this growth advantage in the setting of VHL deficiency. Studies on cytogenetic patterns of chromosomal loss and gain in human CCRCC tumor tissue samples have been done. The cytogenetic profiles were from various stages of tumor progression show that −3p, +5q, −14q, +7 and −8p are the most frequent alterations. The 3p loss is often associated with 5q gain due to unbalanced translocations and this is usually followed by continual deletions on 3p secondary to genome instability (Pei et al., 2010; Zhang et al., 2010; Bhat Singh and Amare Kadam, 2012).
pVHL’s contribution to tumorigenesis may be due to a parallel dysregulation of its HIF-independent functions. A well-known clinical example of this hypothesis is that patients with Class IIC VHL develop pheochromocytoma yet they have a functioning VHL-HIF axis (Hoffman et al., 2001). Pheochromocytoma-associated VHL disease results from an accumulation of JUNB, which is an inhibitor of the pro-apoptotic molecule JUN. JUN is implicated in modulating excessive growth of sympathetic neurons and may be protective from the formation of pheochromocytoma (Lee et al., 2005). Another example involves renal and genital tract cyst formation in VHL disease. Studies have shown that tubular epithelial cells that encase these cysts have lost pVHL function. Loss of pVHL can lead to microtubule instability and subsequent defective ciliary function and cyst formation (Thoma et al., 2007a, b). Research in CCRCC shows that deficient pVHL prevents β-catenin for degredation leading to dysregulation of Wnt signaling and subsequent contribution to tumorigenesis (Linehan et al., 2009). Finally, pVHL has recently been shown to have a novel tumor suppressor function. It marks Skp2 for degradation in an E3 ubiquitin ligase independent manner resulting in increased p27/kip1, which results in S-phase arrest and prevents cell proliferation in the context of DNA damage. In pVHL deficient RCC tissue, Skp2 levels are pathologically elevated with low p27/kip1 (Roe et al., 2011).
It is also important to consider the origin from which VHL associated tumors arise. Since the VHL-associated tumor cells are actually the stromal component of the mass (Chan et al., 1999; Vortmeyer et al., 2003), they may arise from the arrested hemangioblast progenitor cells (Wilkinson et al., 1990; Vortmeyer et al., 1997; Huber et al., 2004; Chan et al., 2005). It has been shown, in CNS and retinal hemangioblastomas, that they express cell markers of mesoderm-derived hemangioblasts and hematopoietic stem cells. These cells have been shown to differentiate into either endothelial or hematopoietic cells when cultured under the appropriate conditions (Park et al., 2007). Interestingly, it appears that HIF2α maintains cell pluripotency on the OCT4 transcription factor (Chan et al., 2005; Covello et al., 2006). Other factors may also be required. Whatever the cause is, tumor development from HIF2α-expressing arrested hemangioblasts seems associated with acquired expression of HIF1α, brachyury and other hemangioblast developmental markers (Shively et al., 2011).
Creating an animal model using knock-in missense mutations in targeted areas of the VHL gene rather than using whole deletions may confer an ocular VHL phenotype. Common VHL point mutations like R167Q in embryonic stem (ES) cells, and more generally, mutations in the alpha domain, causes impairment of elongin C binding to VHL which has been shown to impair HIF2α regulation and intriguingly has a growth advantage over VHL deleted ES cells (Bonicalzi et al., 2001; Lee et al., 2009). In VHL deficient tumors, blocking HIF2α suppresses tumor progression and reintroduction of degradation resistant HIF2α causes tumor progression (Kondo et al., 2003; Zimmer et al., 2004). It is also possible that there are unidentified neighboring genes in chromosome 3p that may be tumorigeneic. Deletions may provide a measure of protection from tumor formation whereas point mutations would not affect these genes. Brk1 maps near the VHL gene and it functions as a regulator of the actin cytoskeleton. Loss of this gene is protective against tumors and causes defects in migration in RCC and other tumors (Escobar et al., 2010). To reiterate from before, the study reported by Wong and colleagues has showed a distinct genotype-phenotype correlation in VHL patients with retinal hemangioblastomas. Those with complete deletions have better visual acuity and decreased incidence of retinal hemangioblastomas. Patients with missense mutations had higher incidence of ocular disease. The most frequent mutation was found at codon 167 (Wong et al., 2007; Mettu et al., 2010). Retinal hemangioblastomas begin to occur in much younger VHL patients and so it is within the realm of possibility that its pathogenesis may very well not be as complicated as CCRCC’s. A missense mutation may be adequate to produce an ocular VHL phenotype but it is always possible that a background of additional engineered mutations and/or microenvironment factors are also needed.
VHL is a complicated systemic disease that has important functions for the entire body mainly through HIF-dependent and HIF-independent pathways. Parsing out each pathway that is tumorigenic for the eye remains a challenge. However, it is a necessary task in order to understand the disease and to treat patients with VHL-associated retinal hemangioblastoma.
Acknowledgments
This work was supported by the NIH intramural research program.
References
- Aiello LP, George DJ, Cahill MT, Wong JS, Cavallerano J, Hannah AL, Kaelin WG., Jr Rapid and durable recovery of visual function in a patient with von hippel-lindau syndrome after systemic therapy with vascular endothelial growth factor receptor inhibitor su5416. Ophthalmology. 2002;109:1745–1751. doi: 10.1016/s0161-6420(02)01159-4. [DOI] [PubMed] [Google Scholar]
- Alleman WG, Tabios RL, Chandramouli GV, Aprelikova ON, Torres-Cabala C, Mendoza A, Rogers C, Sopko NA, Linehan WM, Vasselli JR. The in vitro and in vivo effects of re-expressing methylated von Hippel-Lindau tumor suppressor gene in clear cell renal carcinoma with 5-aza-2′-deoxycytidine. Clin Cancer Res. 2004;10:7011–7021. doi: 10.1158/1078-0432.CCR-04-0516. [DOI] [PubMed] [Google Scholar]
- Ang SO, Chen H, Hirota K, Gordeuk VR, Jelinek J, Guan Y, Liu E, Sergueeva AI, Miasnikova GY, Mole D, Maxwell PH, Stockton DW, Semenza GL, Prchal JT. Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nat Genet. 2002;32:614–621. doi: 10.1038/ng1019. [DOI] [PubMed] [Google Scholar]
- Bento MC, Chang KT, Guan Y, Liu E, Caldas G, Gatti RA, Prchal JT. Congenital polycythemia with homozygous and heterozygous mutations of von Hippel-Lindau gene: five new Caucasian patients. Haematologica. 2005;90:128–129. [PubMed] [Google Scholar]
- Bhat Singh R, Amare Kadam PS. Investigation of tumor suppressor genes apart from VHL on 3p by deletion mapping in sporadic clear cell renal cell carcinoma (cRCC) Urol Oncol. 2012 doi: 10.1016/j.urolonc.2011.08.012. (in press) [DOI] [PubMed] [Google Scholar]
- Biju MP, Neumann AK, Bensinger SJ, Johnson RS, Turka LA, Haase VH. Vhlh gene deletion induces Hif-1-mediated cell death in thymocytes. Mol Cell Biol. 2004;24:9038–9047. doi: 10.1128/MCB.24.20.9038-9047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonicalzi ME, Groulx I, de Paulsen N, Lee S. Role of exon 2-encoded beta -domain of the von Hippel-Lindau tumor suppressor protein. J Biol Chem. 2001;276:1407–1416. doi: 10.1074/jbc.M008295200. [DOI] [PubMed] [Google Scholar]
- Boutin AT, Weidemann A, Fu Z, Mesropian L, Gradin K, Jamora C, Wiesener M, Eckardt KU, Koch CJ, Ellies LG, Haddad G, Haase VH, Simon MC, Poellinger L, Powell FL, Johnson RS. Epidermal sensing of oxygen is essential for systemic hypoxic response. Cell. 2008;133:223–234. doi: 10.1016/j.cell.2008.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgering BM, Medema RH. Decisions on life and death: FOXO Forkhead transcription factors are in command when PKB/Akt is off duty. J Leukoc Biol. 2003;73:689–701. doi: 10.1189/jlb.1202629. [DOI] [PubMed] [Google Scholar]
- Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA. 1999;96:4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley J, Selman C, Shukla D, Abramov AY, Forstreuter F, Esteban MA, Claret M, Lingard SJ, Clements M, Harten SK, Asare-Anane H, Batterham RL, Herrera PL, Persaud SJ, Duchen MR, Maxwell PH, Withers DJ. Deletion of the von Hippel-Lindau gene in pancreatic beta cells impairs glucose homeostasis in mice. J Clin Invest. 2009;119:125–135. doi: 10.1172/JCI26934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion KJ, Guinea M, Dammai V, Hsu T. Endothelial function of von Hippel-Lindau tumor suppressor gene: control of fibroblast growth factor receptor signaling. Cancer Res. 2008;68:4649–4657. doi: 10.1158/0008-5472.CAN-07-6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CC, Vortmeyer AO, Chew EY, Green WR, Matteson DM, Shen DF, Linehan WM, Lubensky IA, Zhuang Z. VHL gene deletion and enhanced VEGF gene expression detected in the stromal cells of retinal angioma. Arch Ophthalmol. 1999;117:625–630. doi: 10.1001/archopht.117.5.625. [DOI] [PubMed] [Google Scholar]
- Chan CC, Chew EY, Shen D, Hackett J, Zhuang Z. Expression of stem cells markers in ocular hemangioblastoma associated with von Hippel-Lindau (VHL) disease. Mol Vis. 2005;11:697–704. [PMC free article] [PubMed] [Google Scholar]
- Chan DA, Sutphin PD, Denko NC, Giaccia AJ. Role of prolyl hydroxylation in oncogenically stabilized hypoxia-inducible factor-1alpha. J Biol Chem. 2002;277:40112–40117. doi: 10.1074/jbc.M206922200. [DOI] [PubMed] [Google Scholar]
- Chew EY. Ocular manifestations of von Hippel-Lindau disease: clinical and genetic investigations. Trans Am Ophthalmol Soc. 2005;103:495–511. [PMC free article] [PubMed] [Google Scholar]
- Choi D, Cai EP, Schroer SA, Wang L, Woo M. Vhl is required for normal pancreatic beta cell function and the maintenance of beta cell mass with age in mice. Lab Invest. 2011;91:527–538. doi: 10.1038/labinvest.2010.207. [DOI] [PubMed] [Google Scholar]
- Clark PE. The role of VHL in clear-cell renal cell carcinoma and its relation to targeted therapy. Kidney Int. 2009;76:939–945. doi: 10.1038/ki.2009.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu CJ, Labosky PA, Simon MC, Keith B. HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20:557–570. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding M, Cui S, Li C, Jothy S, Haase V, Steer BM, Marsden PA, Pippin J, Shankland S, Rastaldi MP, Cohen CD, Kretzler M, Quaggin SE. Loss of the tumor suppressor Vhlh leads to upregulation of Cxcr4 and rapidly progressive glomerulonephritis in mice. Nat Med. 2006;12:1081–1087. doi: 10.1038/nm1460. [DOI] [PubMed] [Google Scholar]
- Dollfus H, Massin P, Taupin P, Nemeth C, Amara S, Giraud S, Beroud C, Dureau P, Gaudric A, Landais P, Richard S. Retinal hemangioblastoma in von Hippel-Lindau disease: a clinical and molecular study. Invest Ophthalmol Vis Sci. 2002;43:3067–3074. [PubMed] [Google Scholar]
- Duignan IJ, Corcoran E, Pennello A, Plym MJ, Amatulli M, Claros N, Iacolina M, Youssoufian H, Witte L, Samakoglu S, Schwartz J, Surguladze D, Tonra JR. Pleiotropic stromal effects of vascular endothelial growth factor receptor 2 antibody therapy in renal cell carcinoma models. Neoplasia. 2011;13:49–59. doi: 10.1593/neo.101162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar B, de Carcer G, Fernandez-Miranda G, Cascon A, Bravo-Cordero JJ, Montoya MC, Robledo M, Canamero M, Malumbres M. Brick1 is an essential regulator of actin cytoskeleton required for embryonic development and cell transformation. Cancer Res. 2010;70:9349–9359. doi: 10.1158/0008-5472.CAN-09-4491. [DOI] [PubMed] [Google Scholar]
- Feil R, Brocard J, Mascrez B, LeMeur M, Metzger D, Chambon P. Ligand-activated site-specific recombination in mice. Proc Natl Acad Sci USA. 1996;93:10887–10890. doi: 10.1073/pnas.93.20.10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frew IJ, Minola A, Georgiev S, Hitz M, Moch H, Richard S, Vortmeyer AO, Krek W. Combined VHLH and PTEN mutation causes genital tract cystadenoma and squamous metaplasia. Mol Cell Biol. 2008;28:4536–4548. doi: 10.1128/MCB.02132-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girmens JF, Erginay A, Massin P, Scigalla P, Gaudric A, Richard S. Treatment of von Hippel-Lindau retinal hemangioblastoma by the vascular endothelial growth factor receptor inhibitor SU5416 is more effective for associated macular edema than for hemangioblastomas. Am J Ophthalmol. 2003;136:194–196. doi: 10.1016/s0002-9394(03)00101-6. [DOI] [PubMed] [Google Scholar]
- Glasker S, Tran MG, Shively SB, Ikejiri B, Lonser RR, Maxwell PH, Zhuang Z, Oldfield EH, Vortmeyer AO. Epididymal cystadenomas and epithelial tumourlets: effects of VHL deficiency on the human epididymis. J Pathol. 2006;210:32–41. doi: 10.1002/path.2029. [DOI] [PubMed] [Google Scholar]
- Gnarra JR, Duan DR, Weng Y, Humphrey JS, Chen DY, Lee S, Pause A, Dudley CF, Latif F, Kuzmin I, Schmidt L, Duh FM, Stackhouse T, Chen F, Kishida T, Wei MH, Lerman MI, Zbar B, Klausner RD, Linehan WM. Molecular cloning of the von Hippel-Lindau tumor suppressor gene and its role in renal carcinoma. Biochim Biophys Acta. 1996;1242:201–210. doi: 10.1016/0304-419x(95)00012-5. [DOI] [PubMed] [Google Scholar]
- Gnarra JR, Ward JM, Porter FD, Wagner JR, Devor DE, Grinberg A, Emmert-Buck MR, Westphal H, Klausner RD, Linehan WM. Defective placental vasculogenesis causes embryonic lethality in VHL-deficient mice. Proc Natl Acad Sci USA. 1997;94:9102–9107. doi: 10.1073/pnas.94.17.9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Marth JD, Orban PC, Mossmann H, Rajewsky K. Deletion of a DNA polymerase beta gene segment in T cells using cell type-specific gene targeting. Science. 1994;265:103–106. doi: 10.1126/science.8016642. [DOI] [PubMed] [Google Scholar]
- Haase VH, Glickman JN, Socolovsky M, Jaenisch R. Vascular tumors in livers with targeted inactivation of the von Hippel-Lindau tumor suppressor. Proc Natl Acad Sci USA. 2001;98:1583–1588. doi: 10.1073/pnas.98.4.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammers HJ, Verheul HM, Salumbides B, Sharma R, Rudek M, Jaspers J, Shah P, Ellis L, Shen L, Paesante S, Dykema K, Furge K, Teh BT, Netto G, Pili R. Reversible epithelial to mesenchymal transition and acquired resistance to sunitinib in patients with renal cell carcinoma: evidence from a xenograft study. Mol Cancer Ther. 2010;9:1525–1535. doi: 10.1158/1535-7163.MCT-09-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey MM, Lam JC, Bezman NA, Rathmell WK, Simon MC. von Hippel-Lindau mutation in mice recapitulates Chuvash polycythemia via hypoxia-inducible factor-2alpha signaling and splenic erythropoiesis. J Clin Invest. 2007;117:3879–3889. doi: 10.1172/JCI32614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MA, Ohh M, Yang H, Klco JM, Ivan M, Kaelin WG., Jr von Hippel-Lindau protein mutants linked to type 2C VHL disease preserve the ability to downregulate HIF. Hum Mol Genet. 2001;10:1019–1027. doi: 10.1093/hmg/10.10.1019. [DOI] [PubMed] [Google Scholar]
- Hong SB, Furihata M, Baba M, Zbar B, Schmidt LS. Vascular defects and liver damage by the acute inactivation of the VHL gene during mouse embryogenesis. Lab Invest. 2006;86:664–675. doi: 10.1038/labinvest.3700431. [DOI] [PubMed] [Google Scholar]
- Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432:625–630. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- Iliopoulos O, Levy AP, Jiang C, Kaelin WGJr, Goldberg MA. Negative regulation of hypoxia-inducible genes by the von Hippel-Lindau protein. Proc Natl Acad Sci USA. 1996;93:10595–10599. doi: 10.1073/pnas.93.20.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan A, Sinay-Trieman L, Czernobilsky B, Barzilai N, Bar-Khayim Y. Is the association between crescentic glomerulonephritis and renal cell carcinoma coincidental? Nephron. 1993;65:642–643. doi: 10.1159/000187581. [DOI] [PubMed] [Google Scholar]
- Karam JA, Zhang XY, Tamboli P, Margulis V, Wang H, Abel EJ, Culp SH, Wood CG. Development and characterization of clinically relevant tumor models from patients with renal cell carcinoma. Eur Urol. 2011;59:619–628. doi: 10.1016/j.eururo.2010.11.043. [DOI] [PubMed] [Google Scholar]
- Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest. 2004;114:1098–1106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson AG., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci USA. 1971;68:820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo K, Kim WY, Lechpammer M, Kaelin WG., Jr Inhibition of HIF2alpha is sufficient to suppress pVHL-defective tumor growth. PLoS Biol. 2003;1:E83. doi: 10.1371/journal.pbio.0000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreusel KM, Bechrakis NE, Krause L, Neumann HP, Foerster MH. Retinal angiomatosis in von Hippel-Lindau disease: a longitudinal ophthalmologic study. Ophthalmology. 2006;113:1418–1424. doi: 10.1016/j.ophtha.2006.02.059. [DOI] [PubMed] [Google Scholar]
- Kucejova B, Sunny NE, Nguyen AD, Hallac R, Fu X, Pena-Llopis S, Mason RP, Deberardinis RJ, Xie XJ, Debose-Boyd R, Kodibagkar VD, Burgess SC, Brugarolas J. Uncoupling hypoxia signaling from oxygen sensing in the liver results in hypoketotic hypoglycemic death. Oncogene. 2011;30:2147–2160. doi: 10.1038/onc.2010.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CM, Hickey MM, Sanford CA, McGuire CG, Cowey CL, Simon MC, Rathmell WK. VHL Type 2B gene mutation moderates HIF dosage in vitro and in vivo. Oncogene. 2009;28:1694–1705. doi: 10.1038/onc.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Nakamura E, Yang H, Wei W, Linggi MS, Sajan MP, Farese RV, Freeman RS, Carter BD, Kaelin WGJr, Schlisio S. Neuronal apoptosis linked to EglN3 prolyl hydroxylase and familial pheochromocytoma genes: developmental culling and cancer. Cancer Cell. 2005;8:155–167. doi: 10.1016/j.ccr.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Lei L, Mason S, Liu D, Huang Y, Marks C, Hickey R, Jovin IS, Pypaert M, Johnson RS, Giordano FJ. Hypoxia-inducible factor-dependent degeneration, failure, and malignant transformation of the heart in the absence of the von Hippel-Lindau protein. Mol Cell Biol. 2008;28:3790–3803. doi: 10.1128/MCB.01580-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Kim WY. Two sides to every story: the HIF-dependent and HIF-independent functions of pVHL. J Cell Mol Med. 2011;15:187–195. doi: 10.1111/j.1582-4934.2010.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Shen D, Huang Y, Yin C, Bojanowski CM, Zhuang Z, Chan CC. Molecular pathology and CXCR4 expression in surgically excised retinal hemangioblastomas associated with von Hippel-Lindau disease. Ophthalmology. 2007;114:147–156. doi: 10.1016/j.ophtha.2006.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan WM, Rubin JS, Bottaro DP. VHL loss of function and its impact on oncogenic signaling networks in clear cell renal cell carcinoma. Int J Biochem Cell Biol. 2009;41:753–756. doi: 10.1016/j.biocel.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonser RR, Glenn GM, Walther M, Chew EY, Libutti SK, Linehan WM, Oldfield EH. von Hippel-Lindau disease. Lancet. 2003;361:2059–2067. doi: 10.1016/S0140-6736(03)13643-4. [DOI] [PubMed] [Google Scholar]
- Lubensky IA, Pack S, Ault D, Vortmeyer AO, Libutti SK, Choyke PL, Walther MM, Linehan WM, Zhuang Z. Multiple neuroendocrine tumors of the pancreas in von Hippel-Lindau disease patients: histopathological and molecular genetic analysis. Am J Pathol. 1998;153:223–231. doi: 10.1016/S0002-9440(10)65563-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Tessarollo L, Hong SB, Baba M, Southon E, Back TC, Spence S, Lobe CG, Sharma N, Maher GW, Pack S, Vortmeyer AO, Guo C, Zbar B, Schmidt LS. Hepatic vascular tumors, angiectasis in multiple organs, and impaired spermatogenesis in mice with conditional inactivation of the VHL gene. Cancer Res. 2003;63:5320–5328. [PubMed] [Google Scholar]
- Mack FA, Rathmell WK, Arsham AM, Gnarra J, Keith B, Simon MC. Loss of pVHL is sufficient to cause HIF dysregulation in primary cells but does not promote tumor growth. Cancer Cell. 2003;3:75–88. doi: 10.1016/s1535-6108(02)00240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhusudan S, Deplanque G, Braybrooke JP, Cattell E, Taylor M, Price P, Tsaloumas MD, Moore N, Huson SM, Adams C, Frith P, Scigalla P, Harris AL. Antiangiogenic therapy for von Hippel-Lindau disease. JAMA. 2004;291:943–944. doi: 10.1001/jama.291.8.943. [DOI] [PubMed] [Google Scholar]
- Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- Maher ER, Yates JR, Harries R, Benjamin C, Harris R, Moore AT, Ferguson-Smith MA. Clinical features and natural history of von Hippel-Lindau disease. Q J Med. 1990;77:1151–1163. doi: 10.1093/qjmed/77.2.1151. [DOI] [PubMed] [Google Scholar]
- McGrath FP, Gibney RG, Morris DC, Owen DA, Erb SR. Case report: multiple hepatic and pulmonary haemangio-blastomas--a new manifestation of von Hippel-Lindau disease. Clin Radiol. 1992;45:37–39. doi: 10.1016/s0009-9260(05)81467-9. [DOI] [PubMed] [Google Scholar]
- Melillo G. Targeting hypoxia cell signaling for cancer therapy. Cancer Metastasis Rev. 2007;26:341–352. doi: 10.1007/s10555-007-9059-x. [DOI] [PubMed] [Google Scholar]
- Mettu P, Agron E, Samtani S, Chew EY, Wong WT. Genotype-phenotype correlation in ocular von Hippel-Lindau (VHL) disease: the effect of missense mutation position on ocular VHL phenotype. Invest Ophthalmol Vis Sci. 2010;51:4464–4470. doi: 10.1167/iovs.10-5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr VH, Vortmeyer AO, Zhuang Z, Libutti SK, Walther MM, Choyke PL, Zbar B, Linehan WM, Lubensky IA. Histopathology and molecular genetics of multiple cysts and microcystic (serous) adenomas of the pancreas in von Hippel-Lindau patients. Am J Pathol. 2000;157:1615–1621. doi: 10.1016/S0002-9440(10)64799-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaprashantha LD, Vatsyayan R, Singhal J, Lelsani P, Prokai L, Awasthi S, Singhal SS. 2′-hydroxyflavanone inhibits proliferation, tumor vascularization and promotes normal differentiation in VHL-mutant renal cell carcinoma. Carcinogenesis. 2011;32:568–575. doi: 10.1093/carcin/bgr021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann AK, Yang J, Biju MP, Joseph SK, Johnson RS, Haase VH, Freedman BD, Turka LA. Hypoxia inducible factor 1 alpha regulates T cell receptor signal transduction. Proc Natl Acad Sci USA. 2005;102:17071–17076. doi: 10.1073/pnas.0506070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DM, Zhuang Z, Chen L, Szerlip N, Maric I, Li J, Sohn T, Kim SH, Lubensky IA, Vortmeyer AO, Rodgers GP, Oldfield EH, Lonser RR. von Hippel-Lindau disease-associated hemangioblastomas are derived from embryologic multipotent cells. PLoS Med. 2007;4:e60. doi: 10.1371/journal.pmed.0040060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastore YD, Jedlickova K, Guan Y, Liu E, Fahner J, Hasle H, Prchal JF, Prchal JT. Mutations of von Hippel-Lindau tumor-suppressor gene and congenital polycythemia. Am J Hum Genet. 2003a;73:412–419. doi: 10.1086/377108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastore YD, Jelinek J, Ang S, Guan Y, Liu E, Jedlickova K, Krishnamurti L, Prchal JT. Mutations in the VHL gene in sporadic apparently congenital polycythemia. Blood. 2003b;101:1591–1595. doi: 10.1182/blood-2002-06-1843. [DOI] [PubMed] [Google Scholar]
- Pei J, Feder MM, Al-Saleem T, Liu Z, Liu A, Hudes GR, Uzzo RG, Testa JR. Combined classical cytogenetics and microarray-based genomic copy number analysis reveal frequent 3;5 rearrangements in clear cell renal cell carcinoma. Genes Chromosomes Cancer. 2010;49:610–619. doi: 10.1002/gcc.20771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotta S, Nobili B, Ferraro M, Migliaccio C, Borriello A, Cucciolla V, Martinelli V, Rossi F, Punzo F, Cirillo P, Parisi G, Zappia V, Rotoli B, Della Ragione F. Von Hippel-Lindau-dependent polycythemia is endemic on the island of Ischia: identification of a novel cluster. Blood. 2006;107:514–519. doi: 10.1182/blood-2005-06-2422. [DOI] [PubMed] [Google Scholar]
- Peyssonnaux C, Datta V, Cramer T, Doedens A, Theodorakis EA, Gallo RL, Hurtado-Ziola N, Nizet V, Johnson RS. HIF-1alpha expression regulates the bactericidal capacity of phagocytes. J Clin Invest. 2005;115:1806–1815. doi: 10.1172/JCI23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyssonnaux C, Zinkernagel AS, Schuepbach RA, Rankin E, Vaulont S, Haase VH, Nizet V, Johnson RS. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs) J Clin Invest. 2007;117:1926–1932. doi: 10.1172/JCI31370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfander D, Kobayashi T, Knight MC, Zelzer E, Chan DA, Olsen BR, Giaccia AJ, Johnson RS, Haase VH, Schipani E. Deletion of Vhlh in chondrocytes reduces cell proliferation and increases matrix deposition during growth plate development. Development. 2004;131:2497–2508. doi: 10.1242/dev.01138. [DOI] [PubMed] [Google Scholar]
- Puri S, Cano DA, Hebrok M. A role for von Hippel-Lindau protein in pancreatic beta-cell function. Diabetes. 2009;58:433–441. doi: 10.2337/db08-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin EB, Higgins DF, Walisser JA, Johnson RS, Bradfield CA, Haase VH. Inactivation of the arylhydrocarbon receptor nuclear translocator (Arnt) suppresses von Hippel-Lindau disease-associated vascular tumors in mice. Mol Cell Biol. 2005;25:3163–3172. doi: 10.1128/MCB.25.8.3163-3172.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin EB, Tomaszewski JE, Haase VH. Renal cyst development in mice with conditional inactivation of the von Hippel-Lindau tumor suppressor. Cancer Res. 2006;66:2576–2583. doi: 10.1158/0008-5472.CAN-05-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin EB, Biju MP, Liu Q, Unger TL, Rha J, Johnson RS, Simon MC, Keith B, Haase VH. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest. 2007;117:1068–1077. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin EB, Rha J, Unger TL, Wu CH, Shutt HP, Johnson RS, Simon MC, Keith B, Haase VH. Hypoxia-inducible factor-2 regulates vascular tumorigenesis in mice. Oncogene. 2008;27:5354–5358. doi: 10.1038/onc.2008.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin EB, Rha J, Selak MA, Unger TL, Keith B, Liu Q, Haase VH. Hypoxia-inducible factor 2 regulates hepatic lipid metabolism. Mol Cell Biol. 2009;29:4527–4538. doi: 10.1128/MCB.00200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raval RR, Lau KW, Tran MG, Sowter HM, Mandriota SJ, Li JL, Pugh CW, Maxwell PH, Harris AL, Ratcliffe PJ. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol Cell Biol. 2005;25:5675–5686. doi: 10.1128/MCB.25.13.5675-5686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe JS, Kim HR, Hwang IY, Cho EJ, Youn HD. von Hippel-Lindau protein promotes Skp2 destabilization on DNA damage. Oncogene. 2011;30:3127–3138. doi: 10.1038/onc.2011.40. [DOI] [PubMed] [Google Scholar]
- Rosenblatt MI, Azar DT. Anti-angiogenic therapy: Prospects for treatment of ocular tumors. Semin Ophthalmol. 2006;21:151–160. doi: 10.1080/08820530500350787. [DOI] [PubMed] [Google Scholar]
- Schlesinger T, Appukuttan B, Hwang T, Atchaneeyakasul LO, Chan CC, Zhuang Z, Stout JT, Wilson DJ. Internal en bloc resection and genetic analysis of retinal capillary hemangioblastoma. Arch Ophthalmol. 2007;125:1189–1193. doi: 10.1001/archopht.125.9.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seagroves TN, Peacock DL, Liao D, Schwab LP, Krueger R, Handorf CR, Haase VH, Johnson RS. VHL deletion impairs mammary alveologenesis but is not sufficient for mammary tumorigenesis. Am J Pathol. 2010;176:2269–2282. doi: 10.2353/ajpath.2010.090310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seizinger BR, Rouleau GA, Ozelius LJ, Lane AH, Farmer GE, Lamiell JM, Haines J, Yuen JW, Collins D, Majoor-Krakauer D, Bonner T, Mathew C, Rubenstein A, Halperin J, McConkie-Rosell A, Green JS, Trofatter JA, Ponder BA, Eierman L, Bowmer MI, Schimke R, Oostra B, Aronin N, Smith DI, Drabkin H, Waziri MH, Hobbs WJ, Martuza RL, Conneally PM, Hsia YE, Gusella JF. Von Hippel-Lindau disease maps to the region of chromosome 3 associated with renal cell carcinoma. Nature. 1988;332:268–269. doi: 10.1038/332268a0. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011;365:537–547. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- Shen HC, Adem A, Ylaya K, Wilson A, He M, Lorang D, Hewitt SM, Pechhold K, Harlan DM, Lubensky IA, Schmidt LS, Linehan WM, Libutti SK. Deciphering von Hippel-Lindau (VHL/Vhl)-associated pancreatic manifestations by inactivating Vhl in specific pancreatic cell populations. PLoS One. 2009;4:e4897. doi: 10.1371/journal.pone.0004897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively SB, Falke EA, Li J, Tran MG, Thompson ER, Maxwell PH, Roessler E, Oldfield EH, Lonser RR, Vortmeyer AO. Developmentally arrested structures preceding cerebellar tumors in von Hippel-Lindau disease. Mod Pathol. 2011;24:1023–1030. doi: 10.1038/modpathol.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AD, Nouri M, Shields CL, Shields JA, Smith AF. Retinal capillary hemangioma: a comparison of sporadic cases and cases associated with von Hippel-Lindau disease. Ophthalmology. 2001a;108:1907–1911. doi: 10.1016/s0161-6420(01)00758-8. [DOI] [PubMed] [Google Scholar]
- Singh AD, Shields CL, Shields JA. von Hippel-Lindau disease. Surv Ophthalmol. 2001b;46:117–142. doi: 10.1016/s0039-6257(01)00245-4. [DOI] [PubMed] [Google Scholar]
- Singh AD, Nouri M, Shields CL, Shields JA, Perez N. Treatment of retinal capillary hemangioma. Ophthalmology. 2002;109:1799–1806. doi: 10.1016/s0161-6420(02)01177-6. [DOI] [PubMed] [Google Scholar]
- Steenhard BM, Isom K, Stroganova L, St John PL, Zelenchuk A, Freeburg PB, Holzman LB, Abrahamson DR. Deletion of von Hippel-Lindau in glomerular podocytes results in glomerular basement membrane thickening, ectopic subepithelial deposition of collagen {alpha}1{alpha}2{alpha}1(IV), expression of neuroglobin, and proteinuria. Am J Pathol. 2010;177:84–96. doi: 10.2353/ajpath.2010.090767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sufan RI, Jewett MA, Ohh M. The role of von Hippel-Lindau tumor suppressor protein and hypoxia in renal clear cell carcinoma. Am J Physiol Renal Physiol. 2004;287:F1–6. doi: 10.1152/ajprenal.00424.2003. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Iida K, Okimura Y, Takahashi Y, Naito J, Nishikawa S, Kadowaki S, Iguchi G, Kaji H, Chihara K. A novel mutation in the von Hippel-Lindau tumor suppressor gene identified in a Japanese family with pheochromocytoma and hepatic hemangioma. Intern Med. 2006;45:265–269. doi: 10.2169/internalmedicine.45.1547. [DOI] [PubMed] [Google Scholar]
- Thoma CR, Frew IJ, Hoerner CR, Montani M, Moch H, Krek W. pVHL and GSK3beta are components of a primary cilium-maintenance signalling network. Nat Cell Biol. 2007a;9:588–595. doi: 10.1038/ncb1579. [DOI] [PubMed] [Google Scholar]
- Thoma CR, Frew IJ, Krek W. The VHL tumor suppressor: riding tandem with GSK3beta in primary cilium maintenance. Cell Cycle. 2007b;6:1809–1813. doi: 10.4161/cc.6.15.4518. [DOI] [PubMed] [Google Scholar]
- van Rooijen E, Voest EE, Logister I, Bussmann J, Korving J, van Eeden FJ, Giles RH, Schulte-Merker S. von Hippel-Lindau tumor suppressor mutants faithfully model pathological hypoxia-driven angiogenesis and vascular retinopathies in zebrafish. Dis Model Mech. 2010;3:343–353. doi: 10.1242/dmm.004036. [DOI] [PubMed] [Google Scholar]
- Vivanco I, Palaskas N, Tran C, Finn SP, Getz G, Kennedy NJ, Jiao J, Rose J, Xie W, Loda M, Golub T, Mellinghoff IK, Davis RJ, Wu H, Sawyers CL. Identification of the JNK signaling pathway as a functional target of the tumor suppressor PTEN. Cancer Cell. 2007;11:555–569. doi: 10.1016/j.ccr.2007.04.021. [DOI] [PubMed] [Google Scholar]
- von Buelow M, Pape S, Hoerauf H. Systemic bevacizumab treatment of a juxtapapillary retinal haemangioma. Acta Ophthalmol Scand. 2007;85:114–116. doi: 10.1111/j.1600-0420.2006.00825.x. [DOI] [PubMed] [Google Scholar]
- Vortmeyer AO, Gnarra JR, Emmert-Buck MR, Katz D, Linehan WM, Oldfield EH, Zhuang Z. von Hippel-Lindau gene deletion detected in the stromal cell component of a cerebellar hemangioblastoma associated with von Hippel-Lindau disease. Hum Pathol. 1997;28:540–543. doi: 10.1016/s0046-8177(97)90075-7. [DOI] [PubMed] [Google Scholar]
- Vortmeyer AO, Frank S, Jeong SY, Yuan K, Ikejiri B, Lee YS, Bhowmick D, Lonser RR, Smith R, Rodgers G, Oldfield EH, Zhuang Z. Developmental arrest of angioblastic lineage initiates tumorigenesis in von Hippel-Lindau disease. Cancer Res. 2003;63:7051–7055. [PubMed] [Google Scholar]
- Weidemann A, Krohne TU, Aguilar E, Kurihara T, Takeda N, Dorrell MI, Simon MC, Haase VH, Friedlander M, Johnson RS. Astrocyte hypoxic response is essential for pathological but not developmental angiogenesis of the retina. Glia. 2010;58:1177–1185. doi: 10.1002/glia.20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch RB. Von Hippel-Lindau disease: the recognition and treatment of early angiomatosis retinae and the use of cryosurgery as an adjunct to therapy. Trans Am Ophthalmol Soc. 1970;68:367–424. [PMC free article] [PubMed] [Google Scholar]
- Wiesener MS, Jurgensen JS, Rosenberger C, Scholze CK, Horstrup JH, Warnecke C, Mandriota S, Bechmann I, Frei UA, Pugh CW, Ratcliffe PJ, Bachmann S, Maxwell PH, Eckardt KU. Widespread hypoxia-inducible expression of HIF-2alpha in distinct cell populations of different organs. FASEB J. 2003;17:271–273. doi: 10.1096/fj.02-0445fje. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Bhatt S, Herrmann BG. Expression pattern of the mouse T gene and its role in mesoderm formation. Nature. 1990;343:657–659. doi: 10.1038/343657a0. [DOI] [PubMed] [Google Scholar]
- Wong WT, Agron E, Coleman HR, Reed GF, Csaky K, Peterson J, Glenn G, Linehan WM, Albert P, Chew EY. Genotype-phenotype correlation in von Hippel-Lindau disease with retinal angiomatosis. Arch Ophthalmol. 2007;125:239–245. doi: 10.1001/archopht.125.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WT, Agron E, Coleman HR, Tran T, Reed GF, Csaky K, Chew EY. Clinical characterization of retinal capillary hemangioblastomas in a large population of patients with von Hippel-Lindau disease. Ophthalmology. 2008;115:181–188. doi: 10.1016/j.ophtha.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbar B, Kishida T, Chen F, Schmidt L, Maher ER, Richards FM, Crossey PA, Webster AR, Affara NA, Ferguson-Smith MA, Brauch H, Glavac D, Neumann HP, Tisherman S, Mulvihill JJ, Gross DJ, Shuin T, Whaley J, Seizinger B, Kley N, Olschwang S, Boisson C, Richard S, Lips CH, Linehan WM, Lerman M. Germline mutations in the Von Hippel-Lindau disease (VHL) gene in families from North America, Europe, and Japan. Hum Mutat. 1996;8:348–357. doi: 10.1002/(SICI)1098-1004(1996)8:4<348::AID-HUMU8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Zhang H, Qian DZ, Tan YS, Lee K, Gao P, Ren YR, Rey S, Hammers H, Chang D, Pili R, Dang CV, Liu JO, Semenza GL. Digoxin and other cardiac glycosides inhibit HIF-1alpha synthesis and block tumor growth. Proc Natl Acad Sci USA. 2008;105:19579–19586. doi: 10.1073/pnas.0809763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Wondergem B, Dykema K. A Comprehensive Study of Progressive Cytogenetic Alterations in Clear Cell Renal Cell Carcinoma and a New Model for ccRCC Tumorigenesis and Progression. Adv Bioinformatics. 2010;2010:428325. doi: 10.1155/2010/428325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer M, Doucette D, Siddiqui N, Iliopoulos O. Inhibition of hypoxia-inducible factor is sufficient for growth suppression of VHL−/− tumors. Mol Cancer Res. 2004;2:89–95. [PubMed] [Google Scholar]