Abstract
The mechanisms underlying the modulation of both the malaria-specific immune response and the course of clinical malaria in the context of concomitant helminth infection are poorly understood. We used multiparameter flow cytometry to characterize the quality and the magnitude of malaria-specific T cell responses in filaria-infected and -uninfected individuals with concomitant asymptomatic Plasmodium falciparum malaria in Mali. In comparison with filarial-uninfected subjects, filarial infection was associated with higher ex vivo frequencies of CD4+ cells producing IL-4, IL-10, and IL-17A (p = 0.01, p = 0.001, and p = 0.03, respectively). In response to malaria Ag stimulation, however, filarial infection was associated with lower frequencies of CD4+ T cells producing IFN-γ, TNF-α, and IL-17A (p < 0.001, p = 0.04, and p = 0.04, respectively) and with higher frequencies of CD4+IL10+T cells (p = 0.0005). Importantly, filarial infection was associated with markedly lower frequencies of malaria Ag-specific Th1 (p < 0.0001), Th17 (p = 0.012), and “TNF-α” (p = 0.0008) cells, and a complete absence of malaria-specific multifunctional Th1 cells. Filarial infection was also associated with a marked increase in the frequency of malaria-specific adaptive regulatory T/Tr1 cells (p = 0.024), and the addition of neutralizing anti–IL-10 Ab augmented the amount of Th1-associated cytokine produced per cell. Thus, among malaria-infected individuals, concomitant filarial infection diminishes dramatically the frequencies of malaria-specific Th1 and Th17 T cells, and alters the quality and magnitude of malaria-specific T cell responses.
Despite their complexity, parasitic helminths are the most prevalent pathogens in resource-poor countries, especially in sub-Saharan Africa where soil- and vector-transmitted helminths affect ~500 million people (1). Although helminths often cause chronic infections that rarely result in mortality, they do induce a regulatory environment that can influence the efficacy of vaccines and can modulate the host immune response to other pathogens (2). Although early or acute infection with tissue-invasive helminth parasites, such as schistosomes or filariae, are associated with proinflammatory innate responses and with a nonpolarized or mixed type 1/type 2 adaptive responses, as these infections become patent (when egg laying occurs or microfilariae appear), type 1 responses are markedly downregulated, and there is an expansion of those cells mediating type 2 responses (3, 4). With more longstanding infection, the dominant parasite-specific immune response is one characterized by the induction of regulatory networks mediated in large part by IL-10–producing adaptive regulatory T cells (aTregs)/Tr1 cells and natural regulatory T cells (nTregs) (5, 6). These regulatory networks induced during chronic helminth infection (particularly schisto-some and filarial infections) have been implicated in both the modulation of parasite-specific immune responses and responses to nonparasite Ag, the latter through bystander effects (so-called spillover suppression) (7). Not only does the helminth-induced attenuation of the immune response extend to nonparasite soluble Ag, but also to responses to orally and parenterally administered vaccines (8–10), other infectious diseases (e.g., Helicobacter pylori, Mycobacterium tuberculosis, Plasmodium falciparum, and HIV) (11–14), and aeroallergens (15).
Of the diseases whose immune responses are modulated by the presence of concomitant helminth infection, malaria holds primacy as the infection affects >2 billion people worldwide resulting in >500 million clinical cases that lead to death in 1–2 million people annually (16, 17). Ninety percent of the clinical episodes and deaths occur in sub-Saharan Africa (16, 17). Sub-Saharan Africa bears not only the heaviest burden of malaria, but the region also has the greatest prevalence of the neglected tropical diseases, particularly soil- and vector-transmitted helminths (1). As in most tropical regions of the world, malaria and helminth infections in Africa often occur in the same host (18) with the outcome and/or control of one infection being influenced by opposing responses induced by the other (2, 19, 20). Despite the conflicting data with respect to clinical outcomes of malaria in helminth/malaria coinfection (13), it is clear that helminth infections [particularly those that are tissue invasive (e.g., filariae)] induce regulatory immune responses that modulate responses to a variety of pathogens, including malaria (21–23). Indeed, in a recent study in a malaria/filaria coendemic area of Mali, we showed that chronic filarial infection was associated with an IL-10–dependent diminution of malaria-specific production of IL-12p70, IFN-γ, and IP-10 (22), cytokines known to play an important role in mediating immunity to malaria (20, 24–28).
The advent of multiparameter flow cytometry and the development of polychromatic Abs for intracellular staining has led not only to the characterization of multifunctional Th cells and their association with disease outcomes, but also to the elucidation of the correlates of protection for diseases involving cellular immune responses (29, 30). Thus, in this study, we sought to use multiparameter flow cytometry to characterize the malaria-specific T cell compartments (effector and regulatory T cells [Tregs]) and to determine definitively the source(s) of IL-10 in the context of malaria/filaria coinfection. In so doing, we found that the presence of a patent filarial infection modulates the magnitude and quality of effector T cell responses to malaria Ag stimulation and does so through the expansion of both nTregs and aTregs. Moreover, we found that CD4+CD25−/low FOXP3− T cells were the major source of IL-10 that, in turn, was primarily responsible for the modulation of effector T cell responses.
Materials and Methods
Study population
The study was carried out in Tienéguébougou and Bougoudiana, two villages situated ~105 km northeast of Bamako, Mali, in a malaria-endemic area with seasonal transmission. The study (NCT00471666) was approved by the National Institute of Allergy and Infectious Diseases Institutional Review Board and the Ethical Committee of the University of Mali, and informed consent was obtained from all participants in the local language.
Detection of filarial infection
Before the start of the study, screening of adults in the villages showed the prevalence of circulating Wuchereria bancrofti filarial antigenemia (TropBio, Townsville, Australia) (31) to be 53% in Tienéguébougou and 36% in Bougoudiana. The prevalence rate of Mansonella perstans microfilariae by calibrated thick smear examination was 62% in Tienéguébougou and 63% in Bougoudiana. At the time of the study, stool examinations revealed only Hymenolepis nana in four individuals and Enterobius vermicularis in one individual. No other helminth eggs or larvae were detected, likely reflecting the administration of single-dose mebendazole and praziquantel 6 wk before the study given to treat potential confounding schistosome and intestinal helminth infections.
The filaria-positive group (Fil+) was defined as positive for circulating filarial Ag and/or having either W. bancrofti or M. perstans microfilariae on examination of nighttime blood smears. The filaria-negative group (Fil−) had neither circulating Ag for W. bancrofti nor microfilariae of either W. bancrofti or M. perstans.
Detection of asymptomatic malaria
Malaria parasites were detected by microscopy using routine Giemsa-stained thick and thin blood smears. Slides were read in two different laboratories at the Malaria Research and Training Center Bamako by two well-trained biologists. Discordant results were reconciled by a third expert microscopist. In addition to parasitemia, the level of circulating P. falciparum histidine-rich protein 2 (PfHRP2) was determined using a commercially available PfHRP2 ELISA kit (Standard Diagnostics, Kyonggido, Korea) using a modified protocol as previously described (32). Recombinant PfHRP2 used to set a standard curve was a gift from Dr. D. Sullivan (Johns Hopkins Malaria Institute, Baltimore, MD). Asymptomatic malaria was defined as positive for either P. falciparum trophozoites by microscopy or positive for PfHRP2 ELISA and no symptoms. For this study, 28 volunteers between 11 and 18 y of age with asymptomatic malaria (13 Fil− and 15 Fil+) were enrolled before the start of the malaria transmission season.
Whole-blood culture
Heparinized blood was collected from study subjects in the villages and transported at ambient temperature to the laboratory in Bamako for processing. Whole blood (1 ml) was used for leukocyte counts and differentials using an automated cell counter (Beckman-Coulter). The remaining blood samples were diluted 1:1 in RPMI 1640 supplemented with penicillin/streptomycin (100 U/100 µg/ml), l-glutamine (2 mM), and HEPES (10 mM) (all from Invitrogen, San Diego, CA). P. falciparum schizont lysate (PfSL) was prepared as described previously (22) and used at 104 infected RBCs/ml final concentration. One milliliter of diluted blood samples was left unstimulated, with the rest being stimulated PfSL or purified protein derivative ([PPD] Statens Serum Institut, Copenhagen, Denmark) or Staphylococcus aureus enterotoxin B ([SEB] Toxin Technologies, Sarasota, FL) for 24 h in a CO2 incubator at 37°C with 5% CO2. Separate blood samples stimulated with PfSL were also cultured in the presence of 20 µg/ml neutralizing anti-human IL-10 or an isotype-matched control Ab (R&D Systems, Minneapolis, MN). Brefeldin A (20 µg/ml final concentration; Sigma-Aldrich, St. Louis, MO) was added to the samples after 12 h of incubation.
Flow cytometry
For flow cytometry, cells were prepared and analyzed exactly as described previously (5). In brief, cells from stimulated whole blood fixed after lysing the RBCs were stored at 280°C in PBS-10% DMSO and transported to the United States in liquid nitrogen. After thawing the cells, they were washed in PBS, permeabilized, and blocked as described previously. The cells were then stained with the Ab panels for effector and regulatory cells (Supplemental Table I). Samples were acquired on a BD LSRII (BD Pharmingen) and analyzed using FlowJo (Tree Star, Ashland, OR). Fluorescence minus one staining was used to set up gates for positive events (Supplemental Fig. 1).
Statistical analyses
Both medians and geometric means (GMs) were used as a measure of central tendency. The Mann–Whitney U and Wilcoxon signed rank tests were used for paired and unpaired analyses, respectively; the Spearman rank test was used for correlations; and p values were corrected for multiple comparisons using the Holm’s correction. All analyses were performed using Prism V5.0 (GraphPad Software, San Diego, CA).
Results
Study population
Patients used for this study were a subset of a cohort of Fil+ and Fil− subjects followed for a clinical trial in malaria/filaria coendemic region. The study was carried out in two Malian villages where malaria (P. falciparum) and filarial infections are coendemic. Two filarial parasites species are present in these villages, W. bancrofti and M. perstans. Malaria in these regions is caused mainly by P. falciparum. Twenty-eight individuals all with asymptomatic P. falciparum malaria were enrolled in the study before the malaria transmission season. As shown in Table I, 13 individuals were Fil− and 15 individuals were Fil+. At the time of the study, all individuals had asymptomatic malaria as determined by the presence of P. falciparum trophozoites on thick blood smear or PfHRP2 Ag ELISA. There were no differences between the groups in terms of demographics or measured hematologic parameters. As shown in Table I, there were no differences in the concentration of PfHRP2, the number of trophozoites, the hemoglobin levels, or the total number of WBCs between the two groups.
Table I.
Study population
| Infection Status |
|||
|---|---|---|---|
| Fil−/Mal+ (n = 13) | Fil+/Mal+ (n = 15) | p Value | |
| Age GM, y (range) | 14.47 (11–17) | 13.85 (11–18) | NS |
| Female/male sex, n | 1/12 | 4/11 | |
| Wb cAg level GM, U/ml (range) | 0 | 460.8 (36.18–32000) | N/A |
| Microfilaremia GM, mf/ml (range) | 0 | 31.79 (17.0–238.0) | N/A |
| Median Pftz/ml (range) | 92.3 (0.0–727.3) | 214.3 (0.0–1017) | NS |
| Median PfHRP2, µg/ml (range) | 3.44 (0.0–25.58) | 0.36 (0.0–8.36) | NS |
| WBC × 103/ml GM (range) | 6.75 (3.5–9.3) | 7.46 (4.9–11.8) | NS |
| Hb GM, g/dl (range) | 11.5 (9.4–13.20) | 11.9 (9.9–14.8) | NS |
cAg, circulating Ag; Hb, hemoglobin; mf, microfilaria; N/A, not applicable; Pf, P. falciparum; Pftz, Pf trophozoites; Wb, W. bancrofti.
Filarial infection is associated with lower frequencies of PPD- and malaria-specific proinflammatory cytokine-producing CD4+ cells
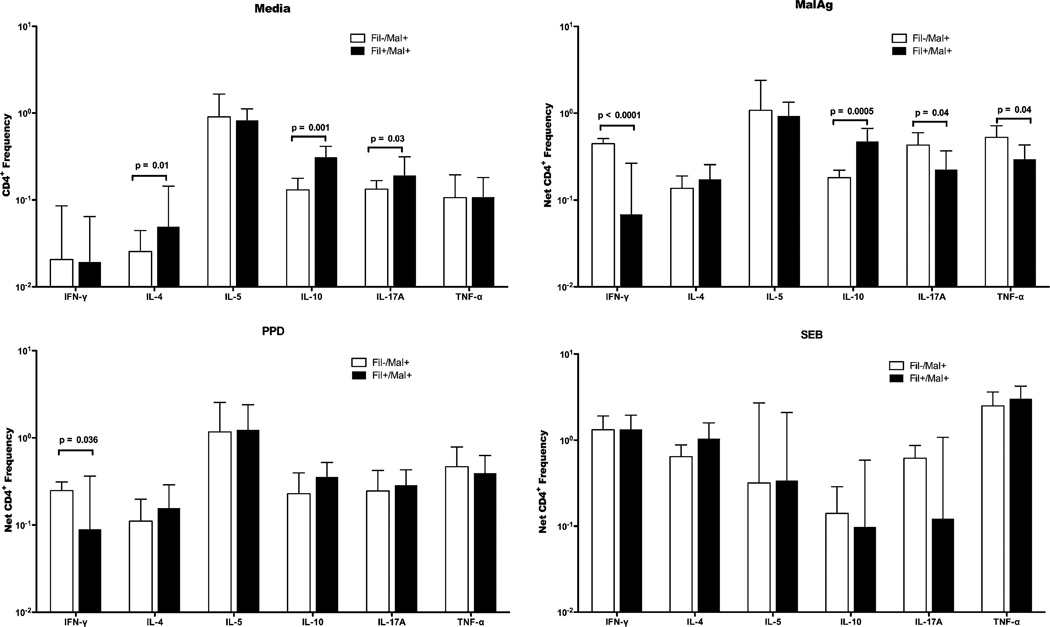
As shown in Fig. 1, coinfected subjects spontaneously had higher frequencies of CD4+ T cells producing IL-17A, IL-10, and IL-4 in concert with what we have previously reported (5). The net frequencies of PPD and malaria Ag-specific cytokine-producing CD4+ cells (after subtraction of the frequencies of spontaneous production) were determined and compared between the Fil− and Fil+ groups (Fig. 1). The frequencies of malaria Ag-driven CD4+ cells producing IFN-γ (median [range]: 0.48 [0.31–0.56] versus 0.13 [0.00–0.16]; p < 0.0001), IL-17A (0.43 [0.12–0.86] versus 0.23 [0.02–0.88]; p = 0.04), and TNF-α (0.53 [0.27–1.36] versus 0.28 [0.14–1.30]; p = 0.04) were significantly higher in the Fil−/Mal+ group compared with the Fil+/Mal+ group. In contrast, the frequency of malaria Ag-driven CD4+ cells producing IL-10 (0.47 [0.08–1.58] versus 0.18 [0.1–0.32]; p = 0.0005) was significantly higher in the Fil+/Mal+ group compared with the Fil−/Mal− group (Fig. 1). There were no differences in the frequencies of cytokine-producing CD4+ cells between the two groups in response to PPD or SEB stimulation.
FIGURE 1.
Filarial infection is associated with lower frequencies of malaria-specific IFN-γ–, IL-17A–, and TNF-α–producing but higher frequency of IL-10–producing CD4+ cells and lower frequency of PPD-specific IFN-γ–producing CD4+ T cells. Flow cytometry was used to determine the frequency of cytokine-producing CD4+ T cells in unstimulated, PfSL-, PPD-, or SEB-stimulated whole blood. Graphs show the GM frequencies of CD4+ cells producing cytokines at homeostasis (media, top left panel) and in response to malaria Ag (PfSL, top right panel), PPD (bottom left panel) or SEB (bottom right panel). Data are expressed as the GM (+ 95% confidence limits) frequencies in 13 Fil−Mal+ patients (open bars) and 15 Fil+Mal+ (closed bars) individuals.
The absolute numbers of cytokine-producing CD4+ T cells were also calculated and compared between the two groups (Supplemental Table II). The absolute numbers of CD4+ cells producing IFN-γ (median [range]: 1767.0 [322.0–5023] versus 481.4 [0.0–631.49]; p = 0.0013) and TNF-α (2064.0 [234.5–7458.0] versus 1055 [48.26–4072.8]; p = 0.03) in response to PfSL stimulation were significantly higher in Fil− compared with Fil+ and reflected the difference seen using relative frequencies. Not surprisingly, the absolute number of IL-10–producing CD4+ T cells (884.3 [92.38–2929.0] versus 2261.0 [37.12–11239.0]; p = 0.04) was significantly higher in the Fil+ compared with Fil− group (Supplemental Table II). There were no differences in the absolute numbers of cytokine-producing CD4+ cells between the two groups in response to SEB stimulation (Supplemental Table II).
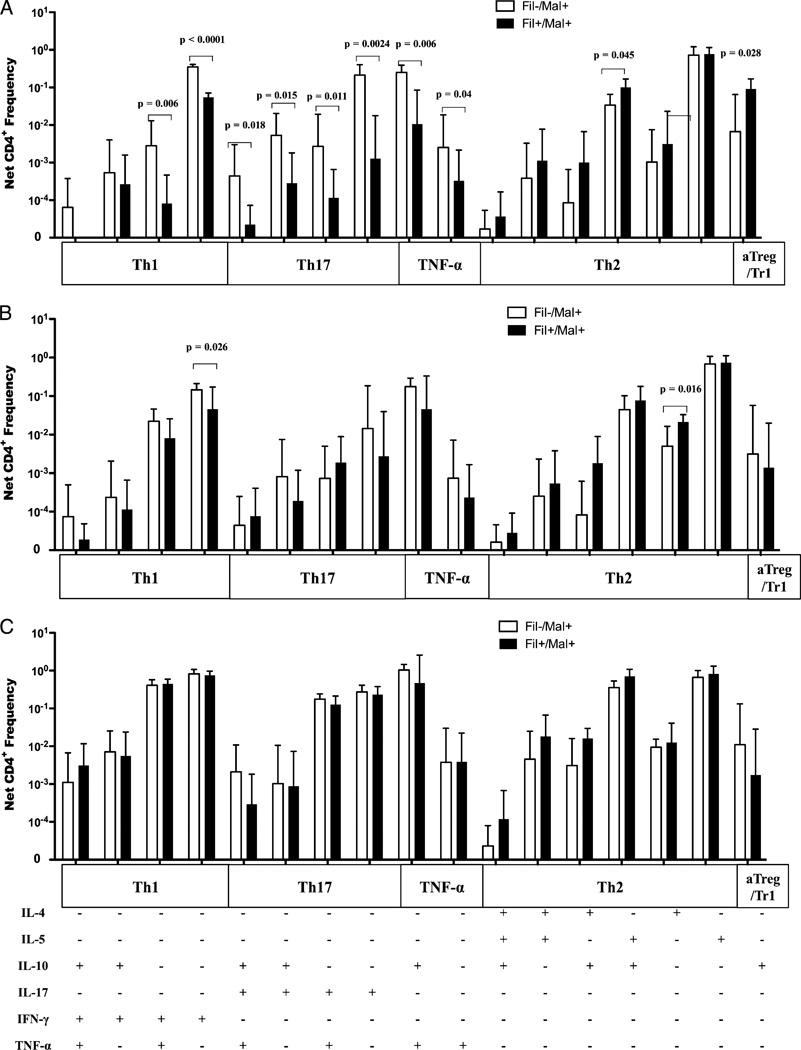
Frequency of Ag-specific multiple cytokine-producing CD4+ T cells
In the context of coinfection with two parasites that tend to polarize the immune response toward different effector CD4+ Th subsets, we used multiparameter flow cytometry to characterize the Th subsets induced by malaria Ag, PPD, or SEB stimulation in Fil+/Mal+ and Fil−/Mal+ individuals. The frequencies of malaria-specific CD4+ cells producing only IFN-γ, IL-17A, or TNF-α were significantly higher in the Fil−/Mal+ compared with the Fil+/Mal+ group (p < 0.0001, p = 0.0024, and p = 0.04, respectively; Fig. 2A). In contrast, the frequency of CD4+ cells producing IL-10 alone (p = 0.028) was significantly higher in the Fil+/Mal+ compared with the Fil−/Mal+ group. In addition, the frequencies of CD4+ cells coexpressing IFN-γ/TNF-α, IL-17A/TNF-α, 17A/IL-10, IL-10/TNF-α, or 17A/IL-10/TNF-α were significantly higher in the Fil−/Mal+ group compared with the Fil+/Mal+ group (p = 0.006, p = 0.011, p = 0.015, p = 0.006, and p = 0.018; Fig. 2A). However, the frequencies of CD4+ cells producing IL-4/IL-10 were significantly higher in Fil+/Mal+ compared with Fil−/Mal+ groups (p = 0.04). These differences were specific to malaria Ag because only the frequencies of CD4+ cytokine-producing cells in response to PPD (Fig. 2B) or SEB (Fig. 2C) were not different (with the exception of CD4+ IFN-γ+ cell in response to PPD; p = 0.026) between the two groups.
FIGURE 2.
Coinfection is associated with lower frequencies of malaria-specific IFN-γ/TNF-α, IL-17A/IL-10/TNF-α multiple cytokine-producing CD4+ T cells, lower frequencies of malaria and PPD-specific IFN-γ single cytokine-producing CD4+ T cells. The frequencies of multiple cytokine-producing CD4+ T cells were determined with Boolean gating, and the net frequencies of cytokine-producing CD4+ T cells from malaria Ag- (A), PPD- (B), or SEB-stimulated (C) cultures are shown. The bars and error bars represent the GM + 95% confidence limit.
Multifunctional T cells in response to Ag stimulation
The ability to measure the simultaneous expression of multiple cytokines allowed us to categorize the cytokine-producing CD4+ T cells into functional subsets (Th1, Th2, Th17. “TNF-α,” and aTreg/Tr1; Supplemental Table II). Because both Th1 and Th17 cells produce TNF-α and IL-10, CD4 T cells producing TNF-α either alone or together with IL-10 were arbitrarily classified as “TNF-α”. As shown in Fig. 3, in response to malaria Ag stimulation, the frequencies of malaria-specific Th1. “TNF-α,” and Th17 cells were significantly higher in the Fil−/Mal+ compared with the Fil+/Mal+ groups (median [range]: 0.39 [0.26–0.53] versus 0.09 [0.05–0.12], p < 0.0001; 0.25 [0.05–0.77] versus 0.17 [0.00–0.21], p = 0.0008; and 0.38 [0.17–0.71] versus 0.017 [0.07–0.17], p = 0.012, respectively). In contrast, the frequency of aTreg/Tr1 cells was significantly higher in the Fil+/Mal+ compared with the Fil−/Mal+ group (0.08 [0.01–0.57] versus 0.03 [0.00–0.19]; p = 0.024), with no differences seen in the frequencies of malaria-specific Th2 cells between the groups (Fig. 3A). In response to PPD, only the frequency of Th1 CD4+ T cells was significantly higher in the Fil−/Mal+ compared with the Fil+/Mal+ groups (0.23 [0.05–0.41] versus 0.11 [0.01–0.24]; p = 0.002; Fig. 3B). In response to SEB, the frequencies of all of these subsets were comparable between the two groups except for that of Th2 cells, which were higher in the Fil+/Mal+ compared with the Fil−/Mal+ groups (0.89 [0.13–4.00] versus 0.48 [0.01–1.71]; p = 0.03; Fig. 3C).
FIGURE 3.
Filarial infection is associated with a lower frequency of malaria-specific Th1 and Th17 and PPD-specific Th1-associated CD4+ cells, the absence of malaria-specific multifunctional Th1 cells. Cytokine-producing CD4 T cells were grouped into T cell subsets categories based on the cytokine they produced. The net frequencies of CD4+ Th cell subsets were compared between Fil−Mal+ and Fil+Mal+ subjects in response to malaria Ag (A), PPD (B), and SEB (C). The proportion of subjects with CD4+ Th1 cells producing three, two, or one cytokine in response to malaria Ag (D), PPD (E), and SEB (F) stimulation are shown on the pie charts.
The use of polychromatic Abs in intracellular cytokine staining has led to the characterization of multifunctional Th cells and their association with disease outcomes (29, 30, 33). Thus, we calculated the percentage of patients in whom multifunctional T cells could be induced by malaria Ag, PPD, or SEB stimulation in the Fil− and Fil+ groups. As shown in Fig. 3D the presence of filarial infection was associated with the complete absence of multifunctional Th1 cells (Th1 triple producers, p = 0.003) and a lower percentage (compared with Fil−) of patients with multifunctional and single Th17 cells (p = 0.016 and p = 0.007, respectively; data not shown) in response to malaria Ag stimulation. In contrast, there were no differences in the proportion of patients who produced multifunctional Th cells in response to PPD and SEB between the two groups (Fig. 3E, 3D respectively).
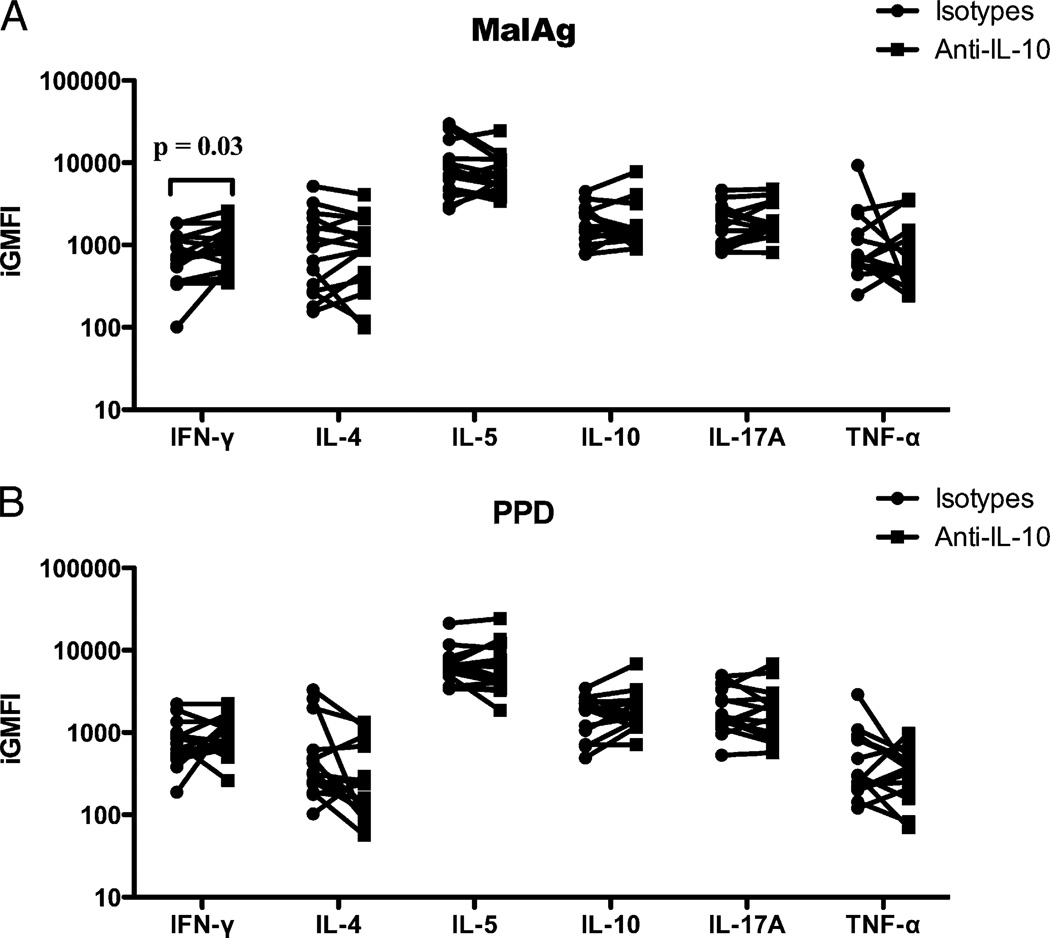
Effect of anti–IL-10 blockade on the frequency of malaria Ag-induced cytokine-producing cells
Several studies have shown that blocking IL-10 can reverse the modulated Th1-type cytokine secretion by cells from Fil+ subjects (22, 34). Thus, we examined the effect of neutralizing anti–IL-10 Ab on Ag-specific cytokine production based on the integrated GM fluorescence intensity (GMFI), a parameter that encompasses cell number (frequency of cytokine-producing cells) and the quantity of the cytokine produced (GMFI). When the integrated GMFI of each cytokine produced in the presence or absence (isotype control) of neutralizing IL-10 Ab in malaria Ag-stimulated whole blood from Fil+/Mal+ and Fil−/Mal+ patients was calculated, we found that, in response to malaria Ag stimulation, the production of IFN-γ (GM [range]: 1296 [847.8–2978] versus 524.7 [47.1–1868]; p = 0.03) was significantly increased in the presence of neutralizing anti–IL-10 Ab (Fig. 4A). However, the addition of neutralizing anti–IL-10 to PPD-stimulated cultures did not increase cytokine production in CD4+ T cells (Fig. 4B).
FIGURE 4.
Addition of neutralizing anti–IL-10 Ab significantly increased the relative amount of IFN-γ in response to malaria Ag stimulation in filarial/malaria coinfected subjects. Integrated GMFI (iGMFI) showing the relative amount of cytokine produced in response to malaria Ag (A) or to PPD (B) stimulation in the presence of anti–IL-10 (squares) or isotype control (circles) Abs. Each pair of dots represents an individual patient’s cells.
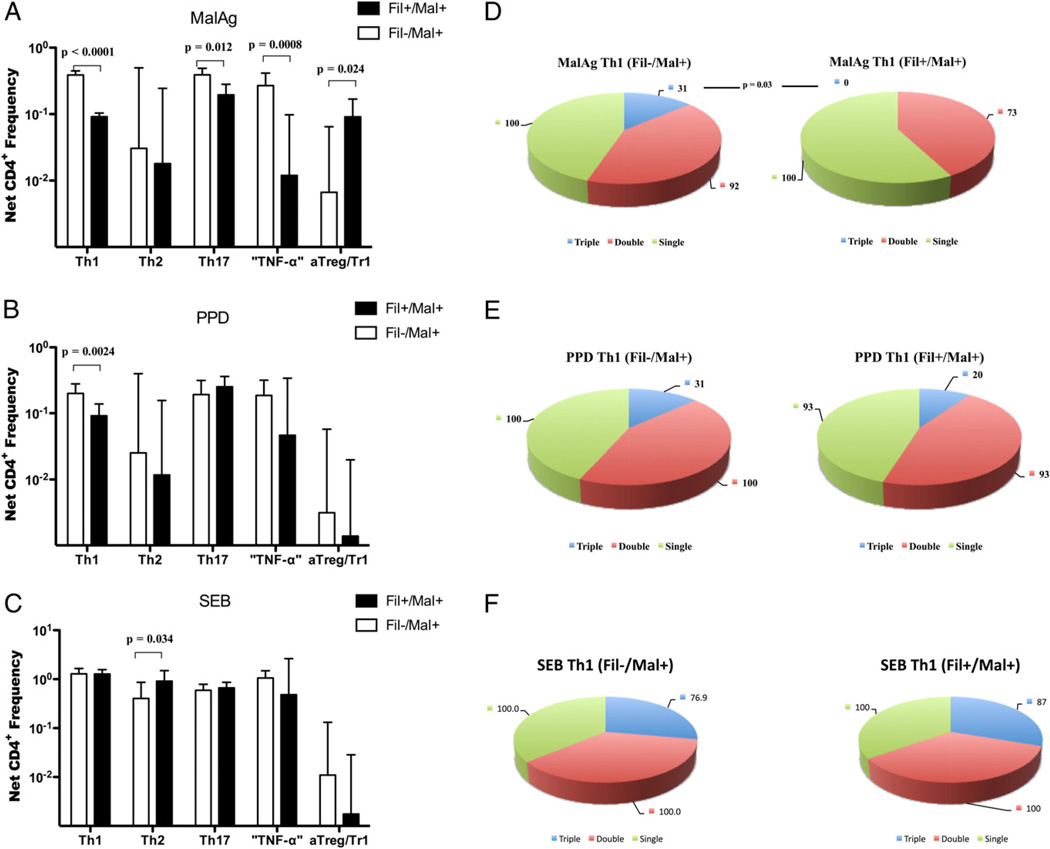
Frequency of nTreg and the sources of IL-10 in response to malaria Ag stimulation
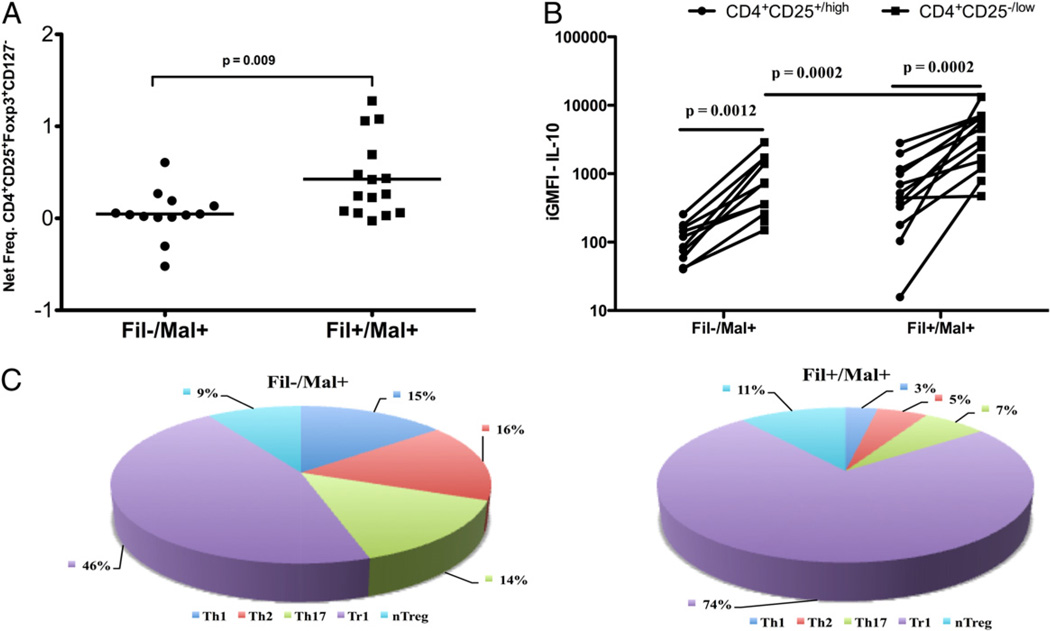
The frequency of nTreg induced by PfSL was next examined and shown to be significantly increased in the Fil+/Mal+ group compared with the Fil−/Mal+ group (median [range]: 0.26 [0.0–1.28] versus 0.04 [0.0–0.61]; p = 0.009; Fig. 5A).
FIGURE 5.
Despite the higher frequency of nTregs cells in filaria-infected subjects, IL-10 is mainly produced by CD4+CD25−/low T cells, with Tr1 cells as the major producer after malaria Ag stimulation. The net frequencies of CD4+CD25+FOXP3+CD127− cells are shown (A) with each dot representing a single patient and the horizontal line representing the GM for the group in response to PfSL stimulation (A). B, The integrated GMFI (iGMFI) of IL-10 produced by either CD4+CD25+/high or CD4+CD25−/low for each individual. C, The contribution of each malaria-specific CD4+ T cell subset to the relative amount of IL-10 produced in response to PfSL stimulation in Fil−Mal+ (left pie chart) and Fil+Mal+ (right pie chart).
Because IL-10 has long been implicated in immune modulation in both malaria and human lymphatic filariasis (35, 36), we sought to identify more definitively the sources of T cell-derived IL-10 in the context of coinfection. To this end, we compared the amount of IL-10 produced by effector cells (CD4+CD25−/lowFOXP3−) and Tregs (CD4+CD25highFOXP3+) within the same subject between Fil− and Fil+ groups in response to malaria Ag stimulation (Fig. 5B). Within a given individual, the amount of malaria-specific IL-10 produced by effector cells (CD4+CD25−/low) was significantly higher than that of IL-10 produced by nTregs (CD4+CD25+/high) in either the Fil− and Fil+ groups (p = 0.0012 and p = 0.0002, respectively). Aggregated group data showed the amount of IL-10 produced by CD4+ effector cells was significantly greater in the Fil+/Mal+ compared with the Fil−/Mal+ groups (p = 0.0002).
Because IL-10 has been shown to be produced by all CD4+ helper and regulatory subsets, we next examined the contribution of each of the major CD4+ cell subset to the CD4-derived IL-10 pool induced by malaria Ag. As shown in Fig. 5C irrespective of filarial status, the aTreg/Tr1 cells provided the overwhelmingly largest contribution to IL-10 pool. The contribution of other IL-10–producing CD4+ subsets differed to a small degree as well between the two groups. Thus, in the context of coinfection, aTreg/Tr1 cells are the predominant producer of IL-10, with nTreg contributing little to the IL-10 pool in Fil+ subjects.
Discussion
Tissue-invasive helminth parasites (such as schistosomes or the filariae) cause chronic infections that induce an immune response dominated by modulating cytokines (such as IL-10 and TGF-β) (35) and regulatory cell populations (36, 37). This immunomodulating environment not only regulates the immune response to specific parasite Ags but also to nonparasite Ags through bystander or “spillover” suppression (7). Although the mechanisms underlying this suppression are still not completely understood, it is thought that IL-10 plays the major role (22, 34). The IL-10–dominated environment induced by chronic helminth infection has been shown previously to modulate the responses to vaccination (8, 9, 12), allergen skin tests (15), and nonhelminth pathogens, including H. pylori (11), M. tuberculosis (38, 39), P. falciparum (21, 22, 40), and HIV (14).
Although helminth infections modulate the responses to many pathogens, their effect on the immune responses to pathogens that induce type 1 immune responses such as malaria and tuberculosis may have the greatest impact because the regions with the greatest prevalence of helminth infections are also where malaria and tuberculosis are most deadly (17, 41). Interestingly, protection to disease associated with these pathogens is mediated in large part by type 1 (Th1)-associated cytokines (IFN-γ, IL-2, and TNF-α) (42–44).
In helminth/malaria coendemic areas, infection with either schistosomiasis or the filarial parasite, W. bancrofti has been shown to downregulate the production of malaria-specific IFN-γ in vitro, most likely through the actions of IL-10 (22). These data do not preclude the involvement of other regulatory mechanisms induced by helminth parasites, however. In fact, in a recent study in Indonesia, depletion of CD4+CD25+ Tregs was shown to reverse a downregulated malaria- and bacillus Calmette-Guérin–specific T cell proliferative response, as well as IFN-γ production (23).
IFN-γ and other Th1-associated cytokines play a pivotal role in immunity to malaria (26, 45). Although many cell types can produce IFN-γ, CD4+ Th1 cells are considered the major source of this cytokine in malaria (46, 47) though the presence of concomitant systemic helminth infection has been shown to impair the production of IFN-γ (23, 34).
Using multicolor flow cytometry, we were able to define broadly the different subsets of Th cells in helminth-infected and –uninfected subjects with concomitant asymptomatic malaria infection and, in so doing, found that filarial infections were associated with lower frequencies of malaria-specific Th1, Th17, and “TNF-α” CD4+ subsets. This suggests that the presence of a patent filarial infection prevents expansion of these important effector T cell subsets. Although the role of Th17 cells in malaria remains to be delineated, Th17 cells and their associated cytokines have been implicated in mediating some of the pathologic consequences in autoimmune diseases (48) and schistosomiasis-associated liver pathology (49). Furthermore, in a filarial/M. tuberculosis coinfection study in India, filarial infection was associated with reduced production of M. tuberculosis-specific Th1- and Th17-associated cytokines (IFN-γ, IL-12, IL-17, and IL-23) (38).
The “TNF-α” subset, defined as malaria Ag-driven CD4+ T cells producing TNF-α either alone or concurrently with IL-10, was also significantly diminished in filarial-infected individuals. The low production of TNF-α in Fil+ individuals may have direct consequences on the severity of and/or protection against malaria. Although exuberant production of TNF-α and other proinflammatory cytokines, such as IL-6 and IL-1b, have been associated with severe malaria, high plasma levels of TNF-α have also been associated with rapid malaria parasite clearance and cure (50, 51). Although TNF-α is produced by almost all cell types, CD4+ T cells have been shown to be an important source of this cytokine during malaria (52).
Furthermore, despite the paucity of flow cytometry-based studies in malaria, previous studies using four-color flow cytometry have found that the frequency of CD8+ and CD4+ T cells expressing either IFN-γ or IL-2 alone or coexpressing IL-2 and IFN-γ increased with age (53), and that the frequency of CD3+ cells producing and TNF-α or IFN-γ alone or in combination were associated with resistance to P. falciparum reinfection and protection against severe disease (54, 55).
A major finding in this study was the association of filarial infection with the absence of malaria-specific multifunctional Th1 cells and a lower proportion of malaria-specific Th17 single-, double-, and triple-cytokine–producing cells. Although the functional significance of these multifunctional CD4+ T cells in malaria remains to be elucidated among malaria-endemic populations, their importance in vaccine models in humans and animals has been elucidated. In fact, in a vaccine study with malaria-naive volunteers, Roestenberg and colleagues (56) showed that immunized volunteers were protected against peripheral blood parasitemia and induced polyfunctional memory Th1 cells. In animal models, vaccination of mice with an adenoviral vector coding for a liver stage Ag induced malaria-specific polyfunctional CD8+ cells and protection in a model of malaria (53, 57). Moreover, vaccination of either nonhuman primates or C57BL/6 mice with P. falciparum circumsporozoite protein with polyinosinic:polycytidylic acid (as an adjuvant) induced malaria-specific Abs and multifunctional CD4 T cells in both animal models (58). In fact, multifunctional CD4 T cells have been shown to be correlated with successful vaccination (33, 59, 60) and protection in disease involving cellular immune responses in HIV, hepatitis C virus, and tuberculosis (61–63). Because natural immunity to malaria is complex and involves both cell-mediated and Ab responses, the ability to detect multifunctional T cells during malaria is of utmost importance in assessing the quality of T cell response induced by natural infection and ultimately to measure the outcome of vaccine regimens (64).
In this study, we also found that filarial infection was associated with higher frequencies of nTreg and aTreg/Tr1 cells, the latter being the major source of IL-10. We have previously reported that filarial infection was associated with increased in vitro secretion of IL-10 that was responsible for the downregulation of malaria-specific proinflammatory cytokines (22). These data have parallels in a murine model of malaria in which IL-10 (capable of modulating malaria-specific proinflammatory responses) were produced by CD4+ FOXP3−CD25− cells (65). In this study, we investigated the impact of filarial infection on the malaria-specific T cell response in young infants with relatively shorter experience with chronic filarial infection compared with adults, or with no pathology. Although the immune interaction between filarial infection and malaria-specific immune response may be different in adults with patent filarial infection or with pathology, we recently showed that chronic filarial infection even in infants was associated with increased frequencies of nTregs and aTregs/Tr1 cells (5).
Our data demonstrate that in filarial/malaria coinfection, the presence of filarial infection clearly modulates both the magnitude and the quality of T cell responses to malaria Ag. This quantitative deficiency in malaria-specific Th1, Th17, and TNF-α CD4+ cells and the complete absence of multifunctional Th1 cells associated with filarial infection suggest that concomitant filarial infection may lead to a profound inability to mount a response important for providing protection from severe malaria and for dampening the success of malaria vaccination programs in filaria-endemic regions of the world, including sub-Saharan Africa.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Program of the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Abbreviations used in this article
- aTreg
adaptive regulatory T cell
- Fil−
filarialnegative (uninfected)
- Fil+
filaria-positive (infected)
- GM
geometric mean
- GMFI
geometric mean fluorescence intensity
- nTreg
natural regulatory T cell
- PfHRP2
Plasmodium falciparum histidine-rich protein 2
- PfSL
P. falciparum schizont lysate
- PPD
purified protein derivative
- SEB
Staphylococcus aureus enterotoxin B
- Treg
regulatory T cell
Footnotes
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Hotez PJ, Kamath A. Neglected tropical diseases in sub-saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl. Trop. Dis. 2009;3:e412. doi: 10.1371/journal.pntd.0000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor MD, Allen JE. Helminth parasites—masters of regulation. Immunol. Rev. 2004;201:89–116. doi: 10.1111/j.0105-2896.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- 3.Babu S, Blauvelt CP, Nutman TB. Filarial parasites induce NK cell activation, type 1 and type 2 cytokine secretion, and subsequent apoptotic cell death. J. Immunol. 2007;179:2445–2456. doi: 10.4049/jimmunol.179.4.2445. [DOI] [PubMed] [Google Scholar]
- 4.Fallon PG, Smith P, Dunne DW. Type 1 and type 2 cytokine-producing mouse CD4+ and CD8+ T cells in acute Schistosoma mansoni infection. Eur. J. Immunol. 1998;28:1408–1416. doi: 10.1002/(SICI)1521-4141(199804)28:04<1408::AID-IMMU1408>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 5.Metenou S, Dembele B, Konate S, Dolo H, Coulibaly SY, Coulibaly YI, Diallo AA, Soumaoro L, Coulibaly ME, Sanogo D, et al. At homeostasis filarial infections have expanded adaptive T regulatory but not classical Th2 cells. J. Immunol. 2010;184:5375–5382. doi: 10.4049/jimmunol.0904067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Figueiredo CA, Barreto ML, Rodrigues LC, Cooper PJ, Silva NB, Amorim LD, Alcantara-Neves NM. Chronic intestinal helminth infections are associated with immune hyporesponsiveness and induction of a regulatory network. Infect. Immun. 2010;78:3160–3167. doi: 10.1128/IAI.01228-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Riet E, Hartgers FC, Yazdanbakhsh M. Chronic helminth infections induce immunomodulation: consequences and mechanisms. Immunobiology. 2007;212:475–490. doi: 10.1016/j.imbio.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Cooper PJ, Chico M, Sandoval C, Espinel I, Guevara A, Levine MM, Griffin GE, Nutman TB. Human infection with Ascaris lumbricoides is associated with suppression of the interleukin-2 response to recombinant cholera toxin B subunit following vaccination with the live oral cholera vaccine CVD 103-HgR. Infect. Immun. 2001;69:1574–1580. doi: 10.1128/IAI.69.3.1574-1580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elias D, Wolday D, Akuffo H, Petros B, Bronner U, Britton S. Effect of deworming on human T cell responses to mycobacterial antigens in helminth-exposed individuals before and after bacille Calmette-Guérin (BCG) vaccination. Clin. Exp. Immunol. 2001;123:219–225. doi: 10.1046/j.1365-2249.2001.01446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nookala S, Srinivasan S, Kaliraj P, Narayanan RB, Nutman TB. Impairment of tetanus-specific cellular and humoral responses following tetanus vaccination in human lymphatic filariasis. Infect. Immun. 2004;72:2598–2604. doi: 10.1128/IAI.72.5.2598-2604.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du Y, Agnew A, Ye XP, Robinson PA, Forman D, Crabtree JE. Helicobacter pylori and Schistosoma japonicum co-infection in a Chinese population: helminth infection alters humoral responses to H pylori and serum pepsinogen I/II ratio. Microbes Infect. 2006;8:52–60. doi: 10.1016/j.micinf.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 12.Elias D, Britton S, Aseffa A, Engers H, Akuffo H. Poor immunogenicity of BCG in helminth infected population is associated with increased in vitro TGF-beta production. Vaccine. 2008;26:3897–3902. doi: 10.1016/j.vaccine.2008.04.083. [DOI] [PubMed] [Google Scholar]
- 13.Hartgers FC, Yazdanbakhsh M. Co-infection of helminths and malaria: modulation of the immune responses to malaria. Parasite Immunol. 2006;28:497–506. doi: 10.1111/j.1365-3024.2006.00901.x. [DOI] [PubMed] [Google Scholar]
- 14.Walson JL, Otieno PA, Mbuchi M, Richardson BA, Lohman-Payne B, Macharia SW, Overbaugh J, Berkley J, Sanders EJ, Chung MH, John-Stewart GC. Albendazole treatment of HIV-1 and helminth co-infection: a randomized, double-blind, placebo-controlled trial. AIDS. 2008;22:1601–1609. doi: 10.1097/QAD.0b013e32830a502e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fallon PG, Mangan NE. Suppression of TH2-type allergic reactions by helminth infection. Nat. Rev. Immunol. 2007;7:220–230. doi: 10.1038/nri2039. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. World Malaria Report 2008. Geneva, Switzerland: World Health Organization; 2008. [Google Scholar]
- 17.World Health Organization. World Malaria Report 2009. Geneva, Switzerland: World Health Organization; 2009. [Google Scholar]
- 18.Muturi EJ, Jacob BG, Kim CH, Mbogo CM, Novak RJ. Are coinfections of malaria and filariasis of any epidemiological significance? Parasitol. Res. 2008;102:175–181. doi: 10.1007/s00436-007-0779-1. [DOI] [PubMed] [Google Scholar]
- 19.Cruz Cubas AB, Ballet JJ, Gentilini M, Monjour L. [Cell-mediated immunity and protection against blood stages of Plasmodium falciparum] Presse Med. 1993;22:1967–1973. [PubMed] [Google Scholar]
- 20.McCall MB, Hopman J, Daou M, Maiga B, Dara V, Ploemen I, Nganou-Makamdop K, Niangaly A, Tolo Y, Arama C, et al. Early interferon-gamma response against Plasmodium falciparum correlates with interethnic differences in susceptibility to parasitemia between sympatric Fulani and Dogon in Mali. J. Infect. Dis. 2010;201:142–152. doi: 10.1086/648596. [DOI] [PubMed] [Google Scholar]
- 21.Hartgers FC, Obeng BB, Kruize YC, Dijkhuis A, McCall M, Sauerwein RW, Luty AJ, Boakye DA, Yazdanbakhsh M. Responses to malarial antigens are altered in helminth-infected children. J. Infect. Dis. 2009;199:1528–1535. doi: 10.1086/598687. [DOI] [PubMed] [Google Scholar]
- 22.Metenou S, Dembélé B, Konate S, Dolo H, Coulibaly SY, Coulibaly YI, Diallo AA, Soumaoro L, Coulibaly ME, Sanogo D, et al. Patent filarial infection modulates malaria-specific type 1 cytokine responses in an IL-10-dependent manner in a filaria/malaria-coinfected population. J. Immunol. 2009;183:916–924. doi: 10.4049/jimmunol.0900257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wammes LJ, Hamid F, Wiria AE, de Gier B, Sartono E, Maizels RM, Luty AJ, Fillié Y, Brice GT, Supali T, et al. Regulatory T cells in human geohelminth infection suppress immune responses to BCG and Plasmodium falciparum. Eur. J. Immunol. 2010;40:437–442. doi: 10.1002/eji.200939699. [DOI] [PubMed] [Google Scholar]
- 24.Good MF, Doolan DL. Immune effector mechanisms in malaria. Curr. Opin. Immunol. 1999;11:412–419. doi: 10.1016/S0952-7915(99)80069-7. [DOI] [PubMed] [Google Scholar]
- 25.Gramzinski RA, Doolan DL, Sedegah M, Davis HL, Krieg AM, Hoffman SL. Interleukin-12- and gamma interferon-dependent protection against malaria conferred by CpG oligodeoxynucleotide in mice. Infect. Immun. 2001;69:1643–1649. doi: 10.1128/IAI.69.3.1643-1649.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCall MB, Sauerwein RW. Interferon-γ—central mediator of protective immune responses against the pre-erythrocytic and blood stage of malaria. J. Leukoc. Biol. 2010;88:1131–1143. doi: 10.1189/jlb.0310137. [DOI] [PubMed] [Google Scholar]
- 27.Su Z, Stevenson MM. IL-12 is required for antibody-mediated protective immunity against blood-stage Plasmodium chabaudi AS malaria infection in mice. J. Immunol. 2002;168:1348–1355. doi: 10.4049/jimmunol.168.3.1348. [DOI] [PubMed] [Google Scholar]
- 28.Torre D. Early production of gamma-interferon in clinical malaria: role of interleukin-18 and interleukin-12. Clin. Infect. Dis. 2009;48:1481–1482. doi: 10.1086/598508. [DOI] [PubMed] [Google Scholar]
- 29.Bolton DL, Roederer M. Flow cytometry and the future of vaccine development. Expert Rev. Vaccines. 2009;8:779–789. doi: 10.1586/erv.09.41. [DOI] [PubMed] [Google Scholar]
- 30.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat. Rev. Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 31.Rocha A, Addiss D, Ribeiro ME, Norões J, Baliza M, Medeiros Z, Dreyer G. Evaluation of the Og4C3 ELISA in Wuchereria bancrofti infection: infected persons with undetectable or ultra-low microfilarial densities. Trop. Med. Int. Health. 1996;1:859–864. doi: 10.1111/j.1365-3156.1996.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 32.Kifude CM, Rajasekariah HG, Sullivan DJ, Jr, Stewart VA, Angov E, Martin SK, Diggs CL, Waitumbi JN. Enzyme-linked immunosorbent assay for detection of Plasmodium falciparum histidine-rich protein 2 in blood, plasma, and serum. Clin. Vaccine Immunol. 2008;15:1012–1018. doi: 10.1128/CVI.00385-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 34.Mahanty S, Ravichandran M, Raman U, Jayaraman K, Kumaraswami V, Nutman TB. Regulation of parasite antigen-driven immune responses by interleukin-10 (IL-10) and IL-12 in lymphatic filariasis. Infect. Immun. 1997;65:1742–1747. doi: 10.1128/iai.65.5.1742-1747.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doetze A, Satoguina J, Burchard G, Rau T, Löliger C, Fleischer B, Hoerauf A. Antigen-specific cellular hyporesponsiveness in a chronic human helminth infection is mediated by T(h)3/T(r)1-type cytokines IL-10 and transforming growth factor-beta but not by a T(h)1 to T(h)2 shift. Int. Immunol. 2000;12:623–630. doi: 10.1093/intimm/12.5.623. [DOI] [PubMed] [Google Scholar]
- 36.Satoguina J, Mempel M, Larbi J, Badusche M, Löliger C, Adjei O, Gachelin G, Fleischer B, Hoerauf A. Antigen-specific T regulatory-1 cells are associated with immunosuppression in a chronic helminth infection (onchocerciasis) Microbes Infect. 2002;4:1291–1300. doi: 10.1016/s1286-4579(02)00014-x. [DOI] [PubMed] [Google Scholar]
- 37.Taylor MD, Harris A, Babayan SA, Bain O, Culshaw A, Allen JE, Maizels RM. CTLA-4 and CD4+ CD25+ regulatory T cells inhibit protective immunity to filarial parasites in vivo. J. Immunol. 2007;179:4626–4634. doi: 10.4049/jimmunol.179.7.4626. [DOI] [PubMed] [Google Scholar]
- 38.Babu S, Bhat SQ, Kumar NP, Jayantasri S, Rukmani S, Kumaran P, Gopi PG, Kolappan C, Kumaraswami V, Nutman TB. Human type 1 and 17 responses in latent tuberculosis are modulated by coincident filarial infection through cytotoxic T lymphocyte antigen-4 and programmed death-1. J. Infect. Dis. 2009;200:288–298. doi: 10.1086/599797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Resende Co T, Hirsch CS, Toossi Z, Dietze R, Ribeiro-Rodrigues R. Intestinal helminth co-infection has a negative impact on both anti-Mycobacterium tuberculosis immunity and clinical response to tuberculosis therapy. Clin. Exp. Immunol. 2007;147:45–52. doi: 10.1111/j.1365-2249.2006.03247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hartgers FC, Obeng BB, Boakye D, Yazdanbakhsh M. Immune responses during helminth-malaria co-infection: a pilot study in Ghanaian school children. Parasitology. 2008;135:855–860. doi: 10.1017/S0031182008000401. [DOI] [PubMed] [Google Scholar]
- 41.WHO. Tuberculosis. Tuberculosis. Fact sheet No. 104. 2010 Available at: http://www.who.int/mediacentre/factsheets/fs104/en/.
- 42.Baird JK, Jones TR, Danudirgo EW, Annis BA, Bangs MJ, Basri, Purnomo H, Masbar S. Age-dependent acquired protection against Plasmodium falciparum in people having two years exposure to hyperendemic malaria. Am. J. Trop. Med. Hyg. 1991;45:65–76. doi: 10.4269/ajtmh.1991.45.65. [DOI] [PubMed] [Google Scholar]
- 43.Leoratti FM, Durlacher RR, Lacerda MV, Alecrim MG, Ferreira AW, Sanchez MC, Moraes SL. Pattern of humoral immune response to Plasmodium falciparum blood stages in individuals presenting different clinical expressions of malaria. Malar. J. 2008;7:186. doi: 10.1186/1475-2875-7-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robinson LJ, D’Ombrain MC, Stanisic DI, Taraika J, Bernard N, Richards JS, Beeson JG, Tavul L, Michon P, Mueller I, Schofield L. Cellular tumor necrosis factor, gamma interferon, and interleukin-6 responses as correlates of immunity and risk of clinical Plasmodium falciparum malaria in children from Papua New Guinea. Infect. Immun. 2009;77:3033–3043. doi: 10.1128/IAI.00211-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.D’Ombrain MC, Robinson LJ, Stanisic DI, Taraika J, Bernard N, Michon P, Mueller I, Schofield L. Association of early interferon-gamma production with immunity to clinical malaria: a longitudinal study among Papua New Guinean children. Clin. Infect. Dis. 2008;47:1380–1387. doi: 10.1086/592971. [DOI] [PubMed] [Google Scholar]
- 46.Horowitz A, Newman KC, Evans JH, Korbel DS, Davis DM, Riley EM. Cross-talk between T cells and NK cells generates rapid effector responses to Plasmodium falciparum-infected erythrocytes. J. Immunol. 2010;184:6043–6052. doi: 10.4049/jimmunol.1000106. [DOI] [PubMed] [Google Scholar]
- 47.Othoro C, Moore JM, Wannemuehler KA, Moses S, Lal A, Otieno J, Nahlen B, Slutsker L, Shi YP. Elevated gamma interferon-producing NK cells, CD45RO memory-like T cells, and CD4 T cells are associated with protection against malaria infection in pregnancy. Infect. Immun. 2008;76:1678–1685. doi: 10.1128/IAI.01420-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fouser LA, Wright JF, Dunussi-Joannopoulos K, Collins M. Th17 cytokines and their emerging roles in inflammation and autoimmunity. Immunol. Rev. 2008;226:87–102. doi: 10.1111/j.1600-065X.2008.00712.x. [DOI] [PubMed] [Google Scholar]
- 49.Rutitzky LI, Stadecker MJ. CD4 T cells producing pro-inflammatory interleukin-17 mediate high pathology in schistosomiasis. Mem. Inst. Oswaldo Cruz. 2006;101(Suppl 1):327–330. doi: 10.1590/s0074-02762006000900052. [DOI] [PubMed] [Google Scholar]
- 50.Kern P, Hemmer CJ, Van Damme J, Gruss HJ, Dietrich M. Elevated tumor necrosis factor alpha and interleukin-6 serum levels as markers for complicated Plasmodium falciparum malaria. Am. J. Med. 1989;87:139–143. doi: 10.1016/s0002-9343(89)80688-6. [DOI] [PubMed] [Google Scholar]
- 51.Kremsner PG, Winkler S, Brandts C, Wildling E, Jenne L, Graninger W, Prada J, Bienzle U, Juillard P, Grau GE. Prediction of accelerated cure in Plasmodium falciparum malaria by the elevated capacity of tumor necrosis factor production. Am. J. Trop. Med. Hyg. 1995;53:532–538. doi: 10.4269/ajtmh.1995.53.532. [DOI] [PubMed] [Google Scholar]
- 52.Kemp K, Akanmori BD, Adabayeri V, Goka BQ, Kurtzhals JA, Behr C, Hviid L. Cytokine production and apoptosis among T cells from patients under treatment for Plasmodium falciparum malaria. Clin. Exp. Immunol. 2002;127:151–157. doi: 10.1046/j.1365-2249.2002.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Capone S, Reyes-Sandoval A, Naddeo M, Siani L, Ammendola V, Rollier CS, Nicosia A, Colloca S, Cortese R, Folgori A, Hill AV. Immune responses against a liver-stage malaria antigen induced by simian adenoviral vector AdCh63 and MVA prime-boost immunisation in non-human primates. Vaccine. 2010;29:256–265. doi: 10.1016/j.vaccine.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 54.Ramharter M, Willheim M, Winkler H, Wahl K, Lagler H, Graninger W, Winkler S. Cytokine profile of Plasmodium falciparum-specific T cells in non-immune malaria patients. Parasite Immunol. 2003;25:211–219. doi: 10.1046/j.1365-3024.2003.00628.x. [DOI] [PubMed] [Google Scholar]
- 55.Ramharter M, Winkler H, Kremsner PG, Adegnika AA, Willheim M, Winkler S. Age-dependency of Plasmodium falciparum-specific and nonspecific T cell cytokine responses in individuals from a malaria-endemic area. Eur. Cytokine Netw. 2005;16:135–143. [PubMed] [Google Scholar]
- 56.Roestenberg M, McCall M, Hopman J, Wiersma J, Luty AJ, van Gemert GJ, van de Vegte-Bolmer M, van Schaijk B, Teelen K, Arens T, et al. Protection against a malaria challenge by sporozoite inoculation. N. Engl. J. Med. 2009;361:468–477. doi: 10.1056/NEJMoa0805832. [DOI] [PubMed] [Google Scholar]
- 57.Reyes-Sandoval A, Berthoud T, Alder N, Siani L, Gilbert SC, Nicosia A, Colloca S, Cortese R, Hill AV. Prime-boost immunization with adenoviral and modified vaccinia virus Ankara vectors enhances the durability and polyfunctionality of protective malaria CD8+ T-cell responses. Infect. Immun. 2010;78:145–153. doi: 10.1128/IAI.00740-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tewari K, Flynn BJ, Boscardin SB, Kastenmueller K, Salazar AM, Anderson CA, Soundarapandian V, Ahumada A, Keler T, Hoffman SL, et al. Poly(I:C) is an effective adjuvant for antibody and multi-functional CD4+ T cell responses to Plasmodium falciparum circumsporozoite protein (CSP) and αDEC-CSP in non human primates. Vaccine. 2010;28:7256–7266. doi: 10.1016/j.vaccine.2010.08.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aagaard C, Hoang TT, Izzo A, Billeskov R, Troudt J, Arnett K, Keyser A, Elvang T, Andersen P, Dietrich J. Protection and polyfunctional T cells induced by Ag85B-TB10.4/IC31 against Mycobacterium tuberculosis is highly dependent on the antigen dose. PLoS ONE. 2009;4:e5930. doi: 10.1371/journal.pone.0005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aagaard CS, Hoang TT, Vingsbo-Lundberg C, Dietrich J, Andersen P. Quality and vaccine efficacy of CD4+ T cell responses directed to dominant and subdominant epitopes in ESAT-6 from Mycobacterium tuberculosis. J. Immunol. 2009;183:2659–2668. doi: 10.4049/jimmunol.0900947. [DOI] [PubMed] [Google Scholar]
- 61.Ciuffreda D, Comte D, Cavassini M, Giostra E, Bühler L, Perruchoud M, Heim MH, Battegay M, Genné D, Mulhaupt B, et al. Polyfunctional HCV-specific T-cell responses are associated with effective control of HCV replication. Eur. J. Immunol. 2008;38:2665–2677. doi: 10.1002/eji.200838336. [DOI] [PubMed] [Google Scholar]
- 62.Duvall MG, Precopio ML, Ambrozak DA, Jaye A, McMichael AJ, Whittle HC, Roederer M, Rowland-Jones SL, Koup RA. Polyfunctional T cell responses are a hallmark of HIV-2 infection. Eur. J. Immunol. 2008;38:350–363. doi: 10.1002/eji.200737768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Forbes EK, Sander C, Ronan EO, McShane H, Hill AV, Beverley PC, Tchilian EZ. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J. Immunol. 2008;181:4955–4964. doi: 10.4049/jimmunol.181.7.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reyes-Sandoval A, Pearson FE, Todryk S, Ewer K. Potency assays for novel T-cell-inducing vaccines against malaria. Curr. Opin. Mol. Ther. 2009;11:72–80. [PubMed] [Google Scholar]
- 65.Couper KN, Blount DG, Wilson MS, Hafalla JC, Belkaid Y, Kamanaka M, Flavell RA, de Souza JB, Riley EM. IL-10 from CD4CD25Foxp3CD127 adaptive regulatory T cells modulates parasite clearance and pathology during malaria infection. PLoS Pathog. 2008;4:e1000004. doi: 10.1371/journal.ppat.1000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.