Abstract
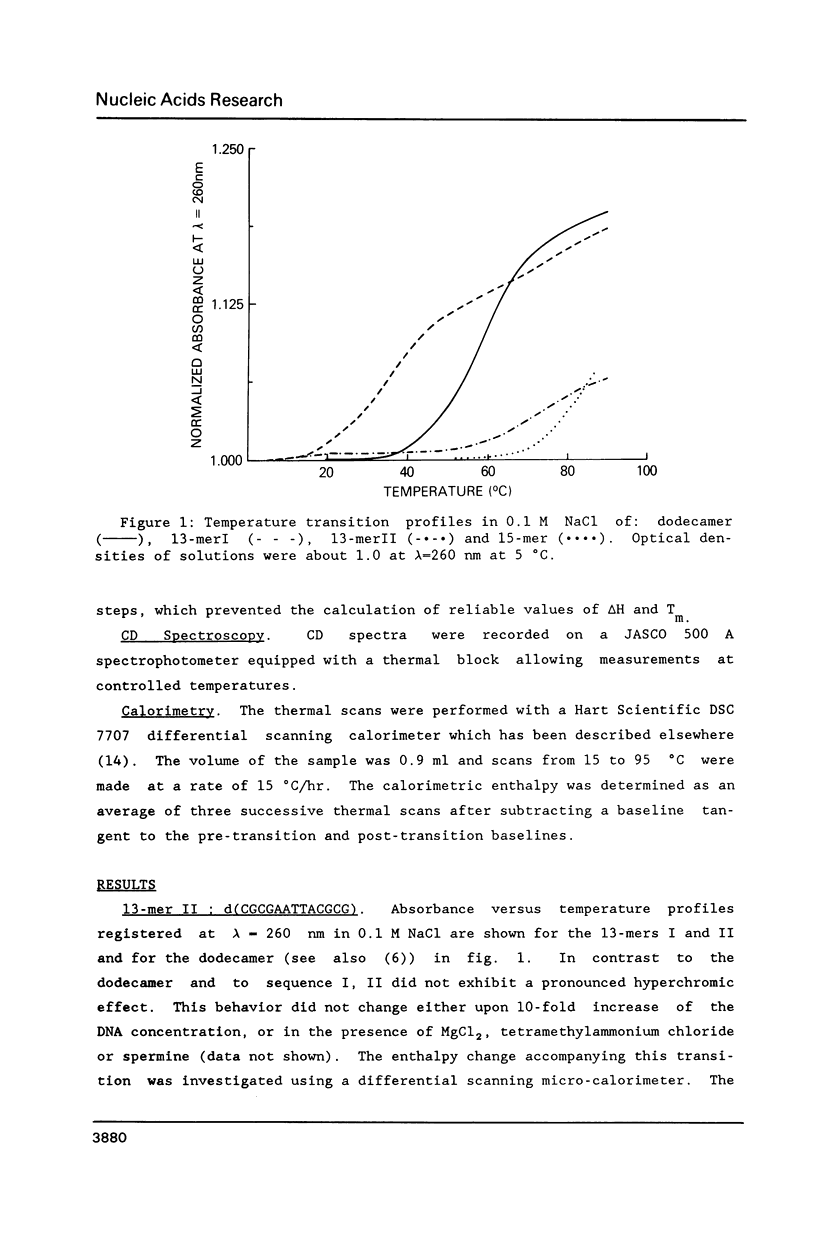
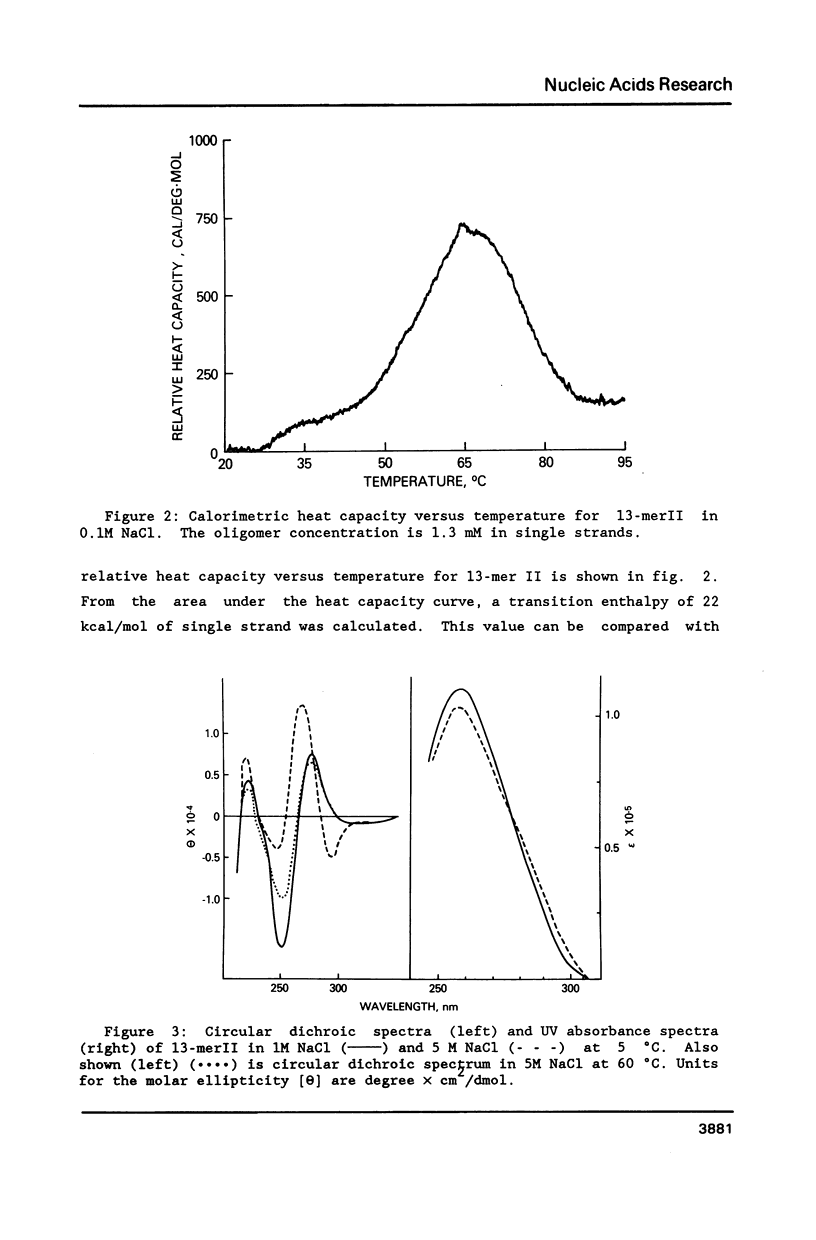
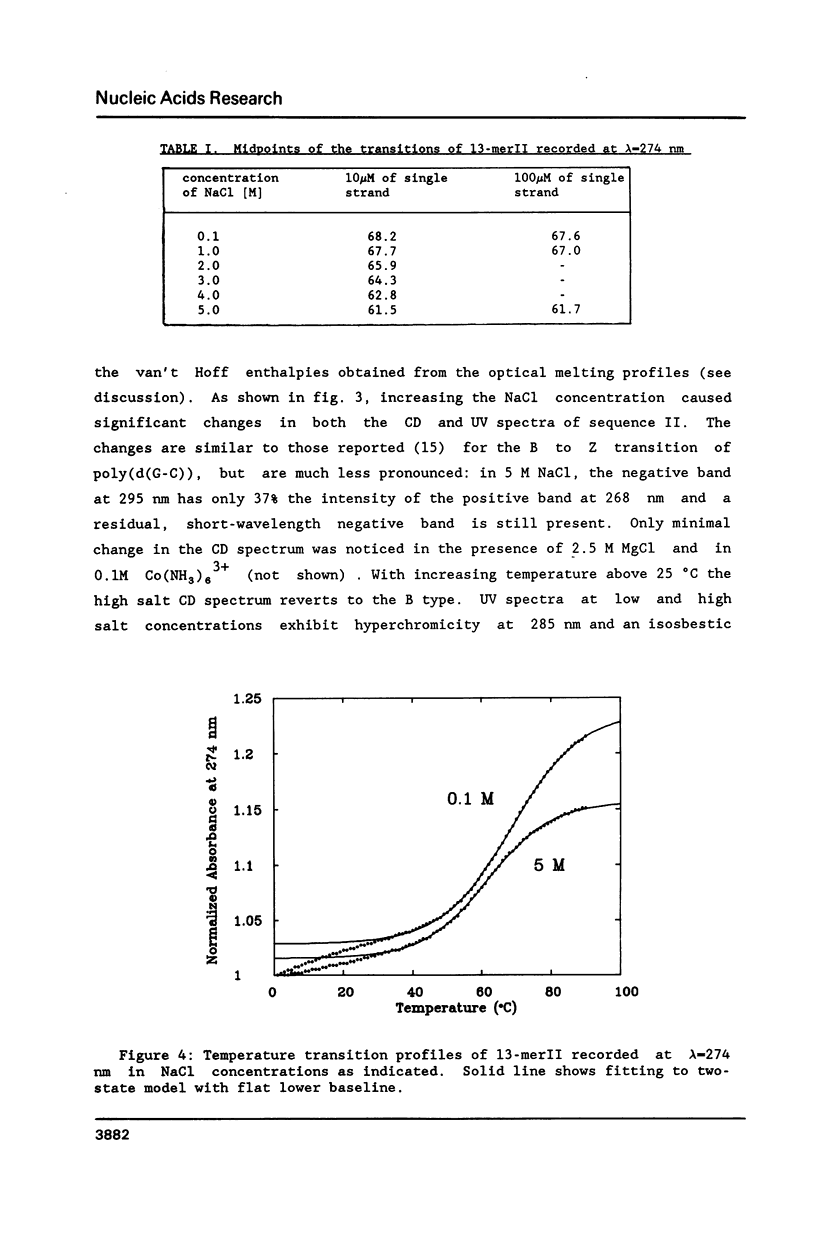
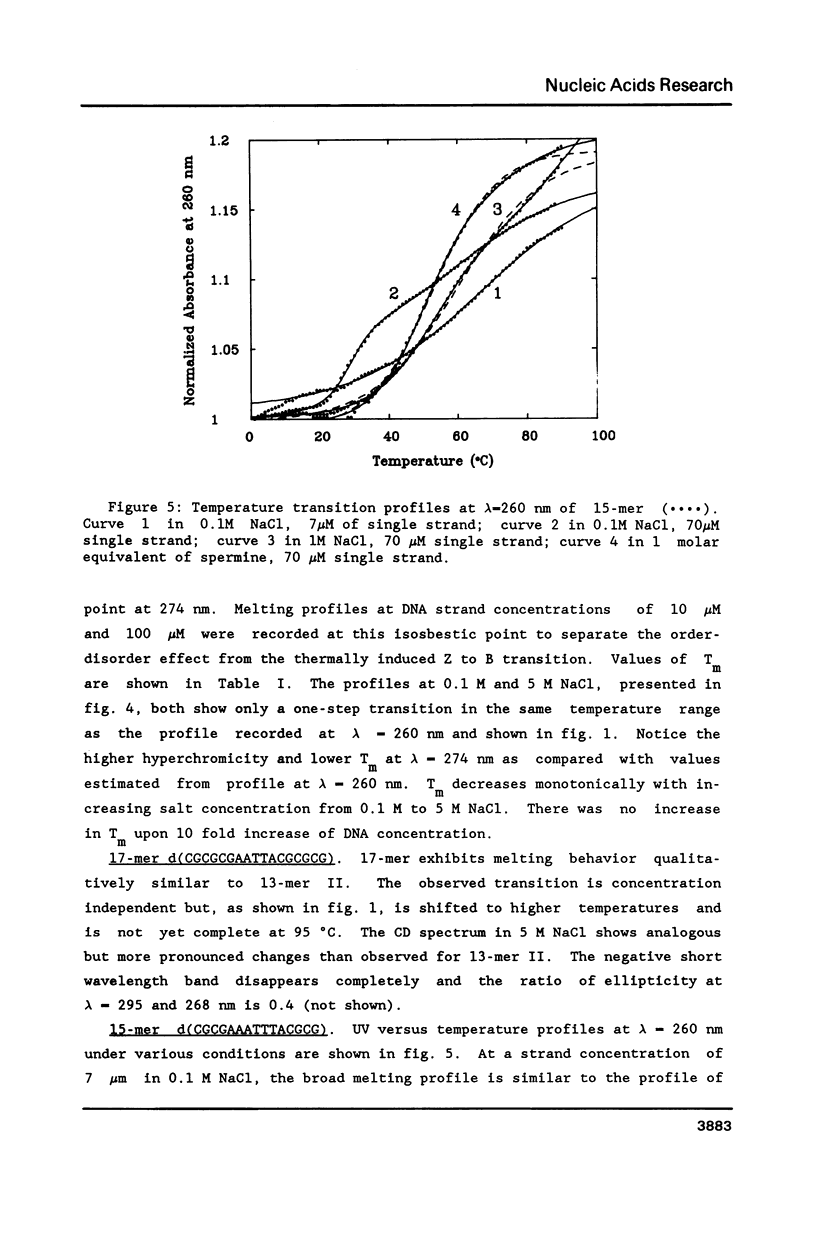
Conformational transitions for a series of imperfect palindromes related to the dodecamer d(CGCGAATTCGCG) have been investigated. These sequences are: two isomeric 13-mers - d(CGCAGAATTCGCG) (13-merI) and d(CGCGAATTACGCG) (13-merII), 17-mer d(CGCGCGAATTACGCGCG) and 15-mer d(CGCGAAATTTACGCG). Insertion of a single adenine nucleotide prevents these sequences from being self-complementary. Analysis of thermodynamic parameters derived from the melting profiles together with other data at higher concentrations (NMR and calorimetry) indicates that the insertion of the additional nucleotide which lacks a complement in the opposite strand does not change the enthalpy of the duplex formation, but does alter the number of stable nucleation configurations. The relative position of the insertion within the self-complementary sequence determines the equilibrium between the duplex form and the single-stranded hairpin loop. C-G segments separated by the insertion from the rest of the molecule can undergo an independent conformational transition at high salt concentration, probably to the Z form.
Full text
PDF


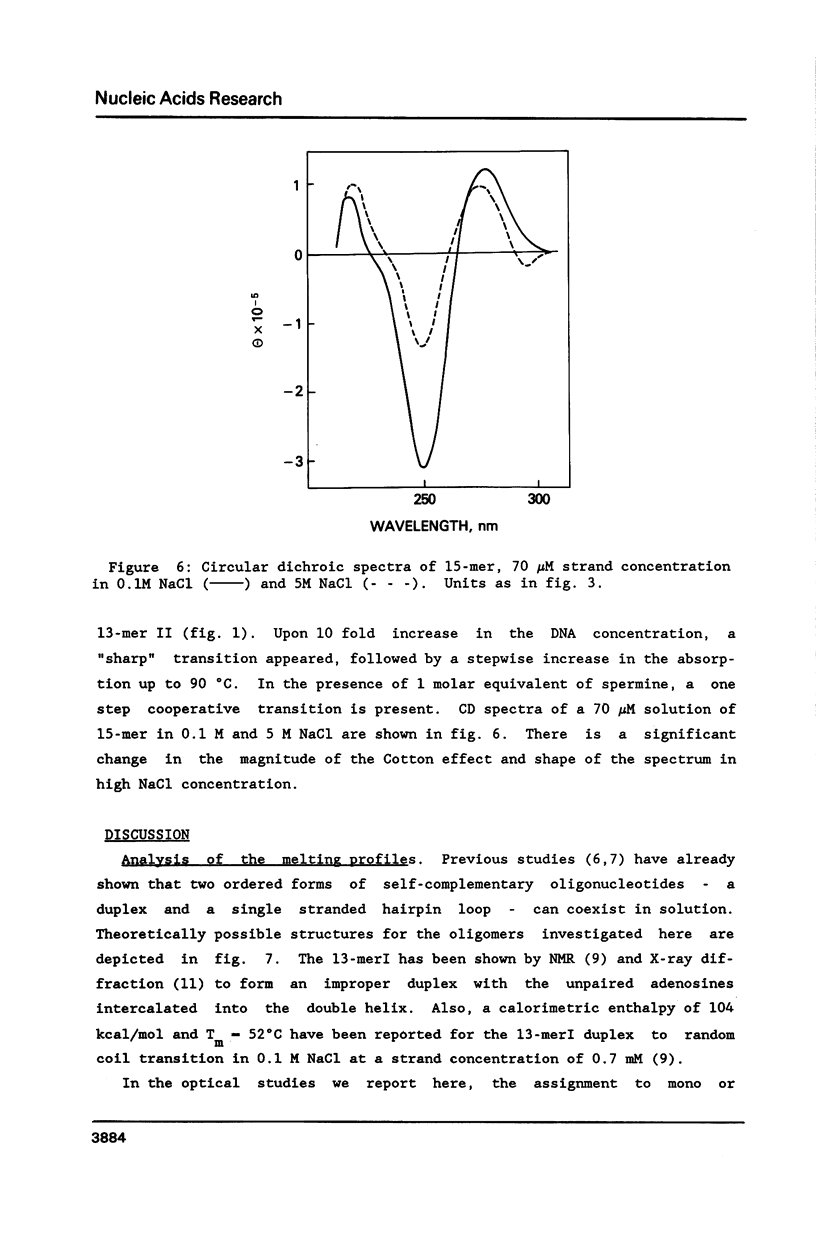

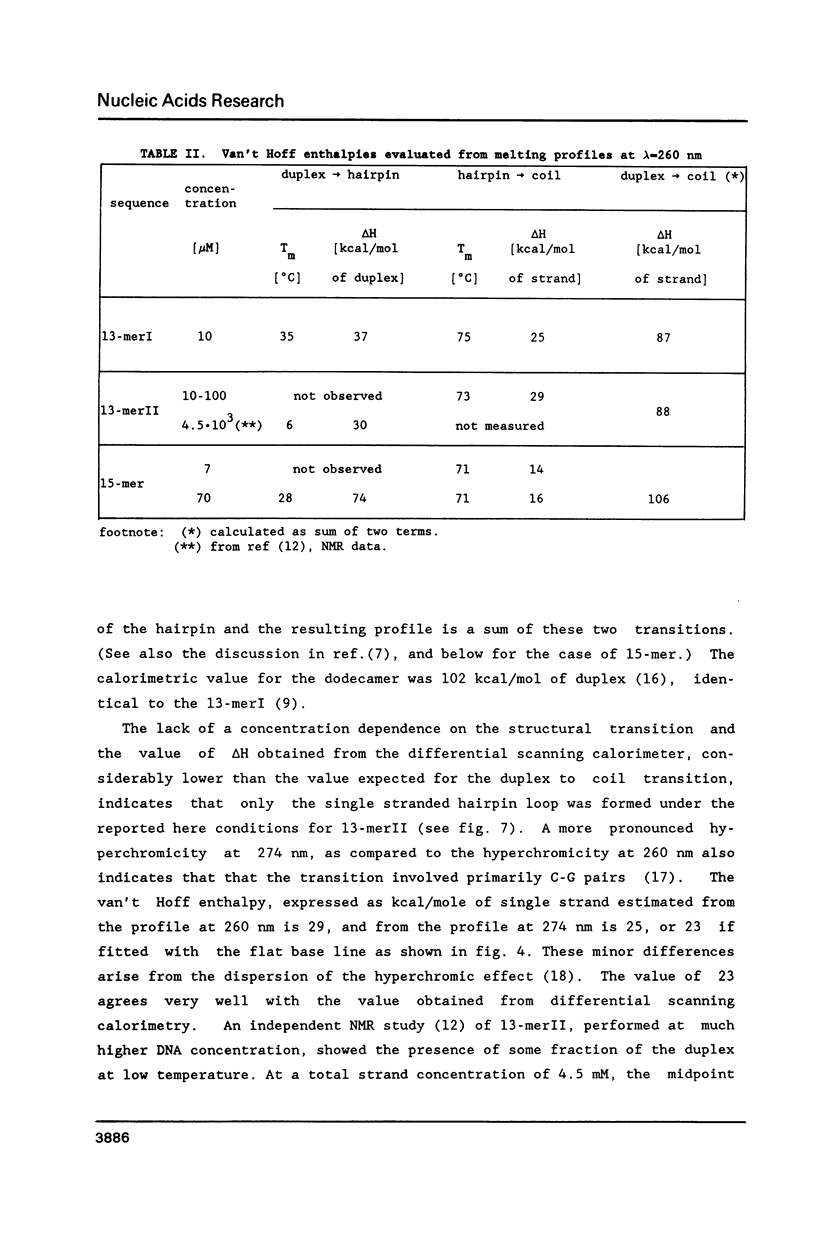




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albergo D. D., Marky L. A., Breslauer K. J., Turner D. H. Thermodynamics of (dG--dC)3 double-helix formation in water and deuterium oxide. Biochemistry. 1981 Mar 17;20(6):1409–1413. doi: 10.1021/bi00509a001. [DOI] [PubMed] [Google Scholar]
- Dickson R. C., Abelson J., Barnes W. M., Reznikoff W. S. Genetic regulation: the Lac control region. Science. 1975 Jan 10;187(4171):27–35. doi: 10.1126/science.1088926. [DOI] [PubMed] [Google Scholar]
- FELSENFELD G., SANDEEN G. The dispersion of the hyperchromic effect in thermally induced transitions of nucleic acids. J Mol Biol. 1962 Dec;5:587–610. doi: 10.1016/s0022-2836(62)80088-6. [DOI] [PubMed] [Google Scholar]
- Germann M. W., Schoenwaelder K. H., van de Sande J. H. Right- and left-handed (Z) helical conformations of the hairpin d(C-G)5T4(C-G)5 monomer and dimer. Biochemistry. 1985 Oct 8;24(21):5698–5702. doi: 10.1021/bi00342a002. [DOI] [PubMed] [Google Scholar]
- Haasnoot C. A., de Bruin S. H., Berendsen R. G., Janssen H. G., Binnendijk T. J., Hilbers C. W., van der Marel G. A., van Boom J. H. Structure, kinetics and thermodynamics of DNA hairpin fragments in solution. J Biomol Struct Dyn. 1983 Oct;1(1):115–129. doi: 10.1080/07391102.1983.10507429. [DOI] [PubMed] [Google Scholar]
- Marky L. A., Blumenfeld K. S., Kozlowski S., Breslauer K. J. Salt-dependent conformational transitions in the self-complementary deoxydodecanucleotide d(CGCAATTCGCG): evidence for hairpin formation. Biopolymers. 1983 Apr;22(4):1247–1257. doi: 10.1002/bip.360220416. [DOI] [PubMed] [Google Scholar]
- Nadeau J. G., Gilham P. T. Anomalous hairpin formation in an oligodeoxyribonucleotide. Nucleic Acids Res. 1985 Nov 25;13(22):8259–8274. doi: 10.1093/nar/13.22.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. J., Kozlowski S. A., Marky L. A., Broka C., Rice J. A., Itakura K., Breslauer K. J. Premelting and melting transitions in the d(CGCGAATTCGCG) self-complementary duplex in solution. Biochemistry. 1982 Feb 2;21(3):428–436. doi: 10.1021/bi00532a002. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Kozlowski S. A., Marky L. A., Rice J. A., Broka C., Itakura K., Breslauer K. J. Extra adenosine stacks into the self-complementary d(CGCAGAATTCGCG) duplex in solution. Biochemistry. 1982 Feb 2;21(3):445–451. doi: 10.1021/bi00532a004. [DOI] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Pörschke D., Eigen M. Co-operative non-enzymic base recognition. 3. Kinetics of the helix-coil transition of the oligoribouridylic--oligoriboadenylic acid system and of oligoriboadenylic acid alone at acidic pH. J Mol Biol. 1971 Dec 14;62(2):361–381. doi: 10.1016/0022-2836(71)90433-5. [DOI] [PubMed] [Google Scholar]
- Ripley L. S. Model for the participation of quasi-palindromic DNA sequences in frameshift mutation. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4128–4132. doi: 10.1073/pnas.79.13.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. J. Directory of restriction endonucleases. Methods Enzymol. 1979;68:27–41. doi: 10.1016/0076-6879(79)68004-7. [DOI] [PubMed] [Google Scholar]
- Roy S., Weinstein S., Borah B., Nickol J., Appella E., Sussman J. L., Miller M., Shindo H., Cohen J. S. Mechanism of oligonucleotide loop formation in solution. Biochemistry. 1986 Nov 18;25(23):7417–7423. doi: 10.1021/bi00371a025. [DOI] [PubMed] [Google Scholar]
- Saper M. A., Eldar H., Mizuuchi K., Nickol J., Appella E., Sussman J. L. Crystallization of a DNA tridecamer d(C-G-C-A-G-A-A-T-T-C-G-C-G). J Mol Biol. 1986 Mar 5;188(1):111–113. doi: 10.1016/0022-2836(86)90487-0. [DOI] [PubMed] [Google Scholar]
- Sinden R. R., Broyles S. S., Pettijohn D. E. Perfect palindromic lac operator DNA sequence exists as a stable cruciform structure in supercoiled DNA in vitro but not in vivo. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1797–1801. doi: 10.1073/pnas.80.7.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers M. F., Byrd R. A., Gallo K. A., Samson C. J., Zon G., Egan W. Nuclear magnetic resonance and circular dichroism studies of a duplex--single-stranded hairpin loop equilibrium for the oligodeoxyribonucleotide sequence d(CGCGATTCGCG). Nucleic Acids Res. 1985 Sep 11;13(17):6375–6386. doi: 10.1093/nar/13.17.6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemmer D. E., Chou S. H., Hare D. R., Reid B. R. Duplex-hairpin transitions in DNA: NMR studies on CGCGTATACGCG. Nucleic Acids Res. 1985 May 24;13(10):3755–3772. doi: 10.1093/nar/13.10.3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing R., Drew H., Takano T., Broka C., Tanaka S., Itakura K., Dickerson R. E. Crystal structure analysis of a complete turn of B-DNA. Nature. 1980 Oct 23;287(5784):755–758. doi: 10.1038/287755a0. [DOI] [PubMed] [Google Scholar]


