Abstract
Stem cell cytogenetic abnormalities constitute a roadblock to regenerative therapies. We investigated the possibility that reactive oxygen species (ROS) influence genomic stability in cardiac and embryonic stem cells. Karyotypic abnormalities in primary human cardiac stem cells were suppressed by culture in physiological (5%) oxygen, but addition of antioxidants to the medium unexpectedly increased aneuploidy. Intracellular ROS levels were moderately decreased in physiological oxygen, but dramatically decreased by the addition of high-dose antioxidants. Quantification of DNA damage in cardiac stem cells and in human embryonic stem cells revealed a biphasic dose-dependence: antioxidants suppressed DNA damage at low concentrations, but potentiated such damage at higher concentrations. High-dose antioxidants decreased cellular levels of the ATM (ataxia-telangiectasia mutated) and other DNA repair enzymes, providing a potential mechanistic basis for the observed effects. These results indicate that physiological levels of intracellular ROS are required to activate the DNA repair pathway for maintaining genomic stability in stem cells. The concept of an “oxidative optimum” for genomic stability has broad implications for stem cell biology and carcinogenesis.
Keywords: Reactive oxygen species, genomic stability, DNA repair, stem cells
Introduction
Chromosomal abnormalities are found in up to 50% of long-term cultured human embryonic stem (ES) cells [1,2]. In preliminary process development for an ongoing clinical trial (CADUCEUS; ClinicalTrials.gov Identifier NCT00893360), G-banding karyotype analysis revealed that ~30% of preliminary production runs of primary cardiosphere-derived cells (CDCs, a heart-derived mixed-cell population rich in cardiac stem cells) [3,4], yielded cells with chromosomal abnormalities (Table S1). Given that genomic alterations of stem cells may increase in vivo carcinogenesis and impair therapeutic potency [5,6], we included genetic screening as a product release criterion in CADUCEUS, while initiating process improvement efforts to minimize genomic alterations of stem cells during ex vivo expansion.
Stem cells are generally cultured in media equilibrated with 95% air and 5% CO2 (~20% O2), which is much higher than in the in vivo physiological microenvironment of the stem cell niche (~1–5% O2, depending on the tissue) [7]. Exposure of stem cells to a non-physiological hyperoxic state in culture may lead to oxidative stress, which is well-known to induce DNA damage and genomic instability [8,9]. Therefore, we tried to reduce the incidence of genomic alterations by culturing CDCs under physiological oxygen (5% O2) or by culture in a traditional 20% O2 incubator with the addition of antioxidants to routine culture media. In so doing, we discovered a complex bidirectional effect of intracellular ROS on genomic stability.
Materials and Methods
Long-term culture of human cardiosphere-derived cells (CDCs) under different conditions
Adult human cardiac stem cells were cultured as described previously [3,4], with minor modifications. Briefly, percutaneous septal endomyocardial heart tissue biopsies (about 10–20 μg) were obtained from patients during clinically-indicated procedures (to monitor heart transplant recipients for rejection [10]) after informed consent. Biopsies were minced into small fragments and digested with 0.2 mg/ml collagenase for 30 minutes. The digested tissue fragments were then equally moved to each of four 6-cm diameter culture dishes coated with 20 μg/ml fibronectin(BD Biosciences), and randomly selected to culture as “explants” in the following four conditions: 1) in a typical 20% O2 incubator (95% air/5% CO2); 2) in a 5% O2 “hypoxia incubator”; 3) in a typical 20% O2 incubator with the addition of 1000-fold diluted proprietary antioxidant supplement (Sigma-Aldrich, Catalogue Number: Sigma A1345, Antioxidant A) or 4) a homemade antioxidant cocktail consisting of 100 μM L-ascorbate, L-glutathione, and α-tocopherol acetate (Sigma-Aldrich, Antioxidant B). The cardiosphere and CDC amplification steps were performed under the same conditions in each group. IMDM basic medium (Gibco) supplemented with 10% FBS (Hyclone) and 20 mg/ml gentamycin was used for all cultures.
Karyotype analysis
Twice-passaged CDCs (with long-term culture for 1–2 months from the date of tissue biopsy) were seeded onto fibronectin-coated 25-cm2 tissue culture flask (104 cells/cm2). After ~24 hours of incubation, cells were treated with 0.1 μg/ml colcemid (Invitrogen) for 4 hours, then trypsinized, treated with hypotonic solution, and fixed. Metaphases were spread on microscope slides, and karyotype analysis was done by using standard G banding technique. The chromosomes were classified according to the International System for Human Cytogenetic Nomenclature [11]. At least 20 metaphases were analyzed per cell sample.
Determination of ROS
To assay intracellular ROS levels ([ROS]), twice-passaged CDCs were seeded in 6- or 96-well plates coated with 20 μg/ml fibronectin, and continuously cultured under the abovementioned four different conditions. At about 90% confluence, cells were incubated with 10 μM 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) (Invitrogen) for 60 min to allow DCFH-DA to diffuse into cells. The DCF fluorescence intensity in cells cultured in 96-well plates is directly determined using SpectraMax M5 (Molecular Devices Corp.) with an excitation wavelength of 495 nm and an emission wavelength of 520 nm. Cells cultured in 6-well plates were trypsin-treated and fixed. The DCF fluorescence intensity in cells was analyzed using a FACS Calibur flow cytometer with CellQuest software (BD Biosciences).
To observe the changes of [ROS] during short-term exposure to different concentrations of antioxidants, catalase, and H2O2, CDCs were expanded by traditional conditions under 20% O2 as described above. Twice-passaged cells were seeded in 6- or 96-well plates coated with 20 μg/ml fibronectin. When about 70% confluent, cell cultures were supplemented with antioxidant A (100–1,000,000-fold dilution), homemade antioxidant cocktail “B” (0.1–1000 μM), catalase (0.1–1000 units/ml), or H2O2 (0.1–1000 μM), as indicated. After 24 hours of culture, the DCF fluorescence intensity in cells was measured using the same methods as described above.
Analysis of DNA damage in CDCs and ES cells
DNA damage in human CDCs and ES cells was evaluated by immunostaining for phosphorylation of histone H2AX on serine 139 (γ-H2AX), a marker of DNA double-strand breaks, after short-term culture with different concentrations of antioxidants, catalase, and H2O2. CDCs were expanded in conventional conditions under 20% O2 as described above. Twice-passaged CDCs and ES cells (passage #23, line CSES2 [12]) were studied. Cells were seeded in 96-well plates coated with 20 μg/ml fibronectin. When about 70% confluent, cell cultures were supplemented with antioxidant A (100–1,000,000-fold dilution), homemade antioxidant cocktail “B” (0.1–1000 μM), catalase (0.1–1000 units/ml), or H2O2 (0.1–1000 μM), as indicated. After 24 hours of culture, cells were fixed, permeabilized and stained with rabbit polyclonal antibody against γ-H2AX (phosphor S139, Abcam Inc.). After being washed, the cells were stained with a PE-conjugated secondary antibody and 4,6-diamidino-2-phenylindole (DAPI). Quantification of cells positive for γ-H2AX foci was performed by fluorescence microscopy (x40 magnification). Briefly, at least 5 images were captured from each culture condition from randomly-selected fields using Q-imaging (RETIGA EXi FAST, Canada) with the same exposure time. Cells with γ-H2AX foci in the nuclei were counted by a single observer blinded to treatment regimen, and the percentage of cells with γ-H2AX foci in each culture condition was used for statistical analysis.
To quantify DNA damage in long-term cultured CDCs under the abovementioned four different conditions, twice-passaged CDCs were seeded in 6- or 96-well plates coated with 20 μg/ml fibronectin, and continuously cultured for 24 hours. The analysis of γ-H2AX foci was done as described above.
Western blotting
To examine the protein levels of ATM and other DNA repair-related factors, total protein was purified from twice-passaged CDCs cultured under the abovementioned four different conditions, as described previously[3,4]. Harvested cells were homogenized in a lysis buffer containing a protease inhibitor mixture (Roche Applied Science) on ice. After centrifugation at 15,000 rpm for 10 min, the supernatant was collected for experiments. The equivalent of 30 μg of total protein was loaded onto 5% or 10% SDS-PAGE gels, and then transferred to PVDF membranes. After overnight blocking in 3% milk TBS-T, membranes were incubated with the following primary antibodies: 1:5000 dilution of rabbit anti-ATM polyclonal antibody, 1:1000 dilution of rabbit anti-ATR polyclonal antibody, 1:500 dilution of rabbit anti-Chk1 (phosphor S317) polyclonal antibody, 1:200 dilution of rabbit anti-Chk2 (phosphor T26) polyclonal antibody, 1:1000 dilution of mouse anti-Rad50 monoclonal antibody, 1:1000 dilution of mouse anti-Rad51 monoclonal antibody (all from Abcam Inc.) and 1:3000 dilution of rabbit anti-β-actin monoclonal antibody. The appropriate horseradish peroxidase-conjugated secondary antibodies were used, and then the blots were visualized by using SuperSignal West Femto maximum sensitivity substrate (Thermo Scientific)) and exposed to Gel Doc™ XR System (Bio-Rad Lab. Inc.). Quantitation for blots was done by Quantity One software, and expressions were normalized by β-actin.
To observe the expression of ATM and other DNA repair-related factors during short-term exposure to different concentrations of antioxidants, catalase, and H2O2, CDCs were expanded by traditional culture in 20% O2 as described above. Twice-passaged cells were seeded in 6-well plates coated with 20 μg/ml fibronectin. When about 70% confluent, cells were cultured in 20% O2 with the supplement of antioxidant A (100–1,000,000-fold dilution), homemade antioxidant cocktail “B” (0.1–1000 μM), catalase (0.1–1000 units/ml), or H2O2 (0.1–1000 μM), as indicated. After 24 hours of culture, cells were harvested and total protein was purified. The expression of ATM and other DNA repair-related factors was assessed by Western blotting as described above.
Measurement of the intracellular ATP level and mitochondrial transmembrane potential
The intracellular ATP level was measured by the luciferin-luciferase method using an ATP-determination kit (Invitrogen). Briefly, twice-passaged CDCs (2×105 cells/well) were seeded in 6-well plates coated with 20 μg/ml fibronectin, and continuously cultured under the abovementioned four different conditions for 24 hours. The cells were washed twice with ice-cold PBS and lysed in 200 μl lysis buffer with protease inhibitors. The lysates (20 μl normalized by protein content) were added to the reaction solution (200 μl) containing 0.5 μM luciferin, 1.25 μg/ml luciferase, and 1 μM DTT, and the bioluminescence was measured using a Monolight™ 3010 (Pharmingen).
To measure mitochondrial transmembrane potential, twice-passaged CDCs (2×105 cells/well) were seeded in 6-well plates coated with 20 μg/ml fibronectin, and continuously cultured under the abovementioned four different conditions for 24 hours. Cells were loaded with TMRE at 37°C for 30 minutes, and harvested by trypsinization. The fluorescence intensity in cells was analyzed using a FACS Calibur flow cytometer with CellQuest software (BD Biosciences).
Statistical analysis
All results are presented as mean ± SD. Statistical significance was determined using the 2-tailed chi-square test for karyotype data and ANOVA followed by Bonferroni post hoc test for other data (Dr. SPSS II). Differences were considered statistically significant when p<0.05.
Results
Genomic alterations decrease in physiological oxygen, but unexpectedly increase with antioxidant supplements
Source human heart biopsies (n=16) were divided and processed in parallel in the various culture conditions, facilitating direct comparisons. Confirming our initial karyotyping data (Table S1), CDCs grown under conventional conditions not infrequently included cells with genomic alterations (6 of 16 samples; Figure 1, Supporting information Table 2). The most common changes were trisomy 8 and Y chromosome loss (Supporting information Figure 1A, Supporting information Table 2). When CDCs were cultured in 5% O2, genomic alterations were detected in only 3 of 16 samples (Figure 1, Supporting information Table 2), and the changes were relatively innocuous [13]: one sample contained one cell with a balanced translocation, another had 8 cells with a derivative chromosome, and the third included one cell with loss of the Y chromosome (Figure 1, Supporting information Table 2). The reduction of the frequency of chromosomal abnormalities in CDCs cultured in 5% O2 (P=0.007 vs 20% O2 culture by chi-square test, Figure 1) indicates that physiological oxygen culture enhances the genomic stability of stem cells, consistent with previous findings [14,15].
Figure 1. Karyotyping data of human cardiosphere-derived cells by G-banding analysis after culture under different conditions.
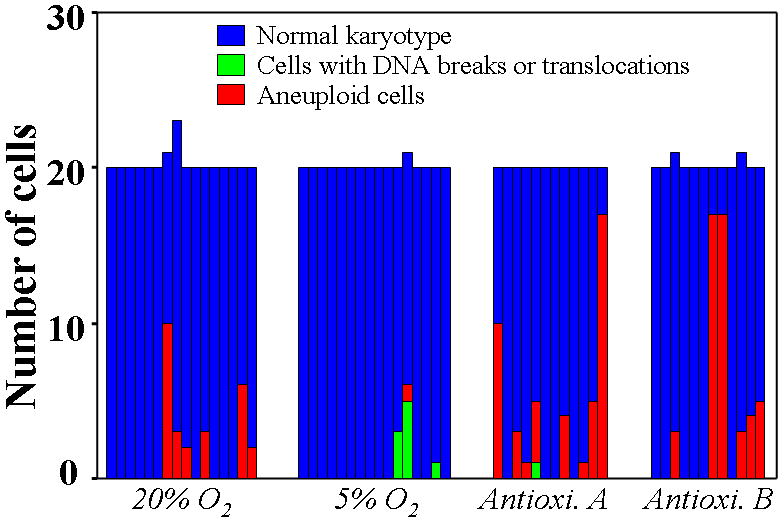
Each bar represents a histogram of one sample of stem cells; blue denotes cells with a normal karyotype. Compared with culture in traditional 20% O2 incubator (95% room air/5% CO2), the number of cells with DNA breaks or translocations (colored green) and losses or gains of chromosomes (red) was decreased by culture under 5% O2 (p=0.007), but increased by culture in traditional 20% O2 incubator with the addition of a commercial antioxidant supplement in 1000-fold dilution (Antioxi. A) or a homemade antioxidant cocktail at 100 μM (Antioxi. B) (p<0.001).
In contrast, karyotypic abnormalities were dramatically increased in frequency and severity when CDCs were cultured in 20% O2 with either of two antioxidant cocktails: a proprietary antioxidant supplement for cell culture (product A1345, Sigma-Aldrich, 1000-fold dilution; Antioxidant A), or a homemade antioxidant mixture [16] (L-ascorbate, L-glutathione, and α Tocopherol acetate, each 100 μM; Antioxidant B) (Figure 1, Supporting information Table 2). Among the 12 samples of CDCs cultured with Antioxidant A or B, 8 and 6 samples included 46 and 49 cells with genomic alterations, respectively (p<0.001 vs 20% O2 culture by chi-square test, Figure 1). Unlike conventional 20% O2 culture, where trisomy 8 and loss of Y predominated (Supporting information Table 1 and 2), the karyotypic abnormalities seen with antioxidants were numerous and varied (trisomy 2, 7, 8, 12, 18, and 20). Some of these, namely trisomy 7 (Supporting information Figure 1B) and trisomies 2 and 20 (Supporting information Figure 1C), have not been reported in previous stem cell studies. The effects of antioxidants on genomic stability appear not to reflect generalized toxicity, as CDCs proliferated normally without obvious morphologic abnormalities (Supporting information Figure 2). Furthermore, neither the intracellular ATP level nor mitochondrial transmembrane potential showed any obvious differences among culture conditions (Supporting information Figure 3). Indeed, the similarities in cell proliferative activity and intracellular ATP levels indicate that energy metabolism is not severely undermined in any of the long-term culture conditions.
Antioxidants decrease intracellular ROS monotonically, but DNA damage shows a biphasic response in stem cells
We next measured intracellular [ROS] in CDCs exposed for 24 hours to a wide range of antioxidant concentrations, with catalase (a pure ROS scavenger) and hydrogen peroxide (H2O2, a powerful oxidant) as controls. Catalase decreased, and H2O2 increased, [ROS] in a progressive dose-dependent manner (Figure 2C–D, Supporting information Figure 4C–D). Like catalase, antioxidants A and B both decreased [ROS] monotonically (Figure 2A–B, Supporting information Figure 4A–B). At the concentrations used to culture CDCs for karyotyping analysis (1000-fold dilution of antioxidant A and 100 μM antioxidant B, respectively), [ROS] was very low. Likewise, [ROS] was depressed in CDCs sent for karyotyping analysis after 1–2 months in 5% oxygen, and even more so in antioxidants, relative to routine culture conditions (Figure 3). Assuming that [ROS] in CDCs cultured under 5% O2 approximates the physiological intracellular concentration, long-term culture with antioxidants suppresses [ROS] to sub-physiological levels (Figures 2 and 3, Supporting information Figure 4).
Figure 2. Intracellular ROS levels in human cardiosphere-derived cells after 24 hours culture under 20% O2 with different concentrations of antioxidants, catalase, and H2O2.

The same cells were initially used and maintained in traditional 5% CO2/20% O2 culture condition. Intracellular ROS levels in cells cultured in 96-well plates were measured by the fluorescence intensity after staining with DCF-DA for 60 minutes using a multiple counter. The intracellular ROS levels were decreased with the addition of 100–1,000,000-fold diluted antioxidant supplement (A), 0.1–1000 μM homemade antioxidant cocktail (B), and 0.1–1000 unit/ml catalase (C), but increased with the addition of 0.1–1000 μM H2O2 (D), in a dose-dependent manner. The “0” in each group test the baseline level of ROS. Compared to baseline, the DCF fluorescence was decreased by antioxidants (A–C), but increased by oxidative stress stimulator of H2O2. Results are means and s.d. for six separated experiments by using different twice-passaged CDCs. a.u.: arbitrary units. * p<0.01, † p<0.05 vs the baseline levels.
Figure 3. Intracellular ROS concentration in human cardiosphere-derived cells with long-term culture under 20% O2, 20% O2, or under 20% O2 with added antioxidants.

A. representative histograms show that the intracellular ROS was lower in cells cultured under 5% O2 than under 20% O2. Intracellular ROS was decreased to very low levels by the addition of 1000-fold diluted antioxidant supplement (Antioxi. A) or 100 μM homemade antioxidant cocktail (Antioxi. B). B. DCF fluorescence intensity measured by a multiple counter also showed a significant decrease of intracellular ROS levels in cells cultured under 5% O2, which was decreased even further by the addition of antioxidants. C. Representative images of γ-H2AX foci in CDCs (arrows) cultured for long-term under different conditions. D. Compared to traditional 20% O2 culture, quantitative data showed that γ-H2AX foci in CDCs was significantly decreased in 5% O2 culture, but increased by the supplement with antioxidants. * p<0.01 vs other groups, † p<0.01 vs 5% O2 and 20% O2.
The concept of “reductive stress” (an extreme suppression of ROS) has recently been proposed to underlie a form of cardiomyopathy due to protein aggregation [17]. To identify whether an excessive decrease of [ROS] likewise induces DNA damage and genomic instability, we quantified γ-H2AX foci (Supporting information Figure 5) [18], a marker of DNA double-strand breaks, in CDCs and human ES cells. Oxidative stress induced by H2O2 increased DNA damage dose-dependently (Figure 4D). Here, however, the effects were biphasic: the percentage of CDCs with γ-H2AX foci was decreased at low antioxidant concentrations, but increased at higher doses (Figure 4A–B). A similar result was observed with increasing concentrations of catalase (Figure 4C). The biphasic response is not limited to CDCs: human ES cells exhibited a similar pattern (red crosses with dotted lines, Figure 4A–C), although the overall percentages of ES cells with γ-H2AX foci were lower than in adult CDCs. Interestingly, the number of γ-H2AX foci was minimal at modest concentrations of antioxidants (10,000-fold dilution of antioxidant A, 10 μM of antioxidant B, and 10 units/ml of catalase; Figure 4A–C), the same concentrations that drive [ROS] to “physiological” levels. These results motivate the concept of an “oxidative optimum”, a narrow range of [ROS] within which stem cells maintain optimal genomic stability.
Figure 4. DNA damage in human cardiosphere-derived cells and human embryonic stem cells after 24 hours culture under 20% O2 with antioxidants, catalase, and H2O2.

DNA damage was evaluated by the formation of γ-H2AX foci, a marker of DNA double-strand breaks, in cells using immunostaining analysis. A. The percentages of CDCs with γ-H2AX foci (bar graph) were decreased at low doses (10,000–100,000-fold dilution), but increased by high doses (100–1000-fold dilution) of antioxidant supplement (Antioxidant A). Similar results were also observed in ES cells (red crosses and line). B. The percentages of CDCs with γ-H2AX foci (bar graph) were decreased at low doses (1–20 μM), but conversely increased by high doses (100–1000 μM) of homemade antioxidant cocktail (Antioxidant B). Similar results were also observed in human ES cells (red crosses and line). C. The percentages of CDCS (bar graph) and ES cells (red crosses and line) with γ-H2AX foci were decreased at low doses (1–10 units/ml), but increased at high doses (100–1000 units/ml) of catalase. D. The percentages of CDCs (bar graph) and ES cells (red crosses and line) with γ-H2AX foci were increased by H2O2, in a dose-dependent manner. Results are means and s.d. for six separate experiments using different twice-passaged CDCs.
In twice-passaged CDCs cultured under the abovementioned four different long-term conditions, γ-H2AX foci were significantly decreased in 5% O2 culture, but increased by supplementation with antioxidants, when compared to traditional 20% O2 culture (Figure 3C–D).
Extreme suppression of intracellular ROS down-regulates ATM and other DNA repair factors
A clue as to the underlying mechanism arises from recent work on the protein kinase ATM (ataxia-telangiectasia mutated), which figures prominently in DNA repair. Intracellular ROS enhances the expression of ATM [19], which phosphorylates a host of downstream targets in response to DNA double-strand breaks, inducing cell cycle arrest and inhibiting apoptosis [20–22]. We wondered whether excessive suppression of [ROS] might down-regulate ATM, thereby favoring genomic instability. ATM protein levels were indeed decreased at high concentrations of antioxidants A and B (≥1000-fold dilution, and ≥100 μM, respectively), or catalase (≥100 units/ml) (Figure 5A–C). Various other DNA repair factors, including ATR and downstream factors Rad50, Rad51, Chk1, and Chk2, were also decreased by antioxidants (Supporting information Figure 6 and 7), at the same concentrations that extremely suppress [ROS] (Figures 2 and 3, Supporting information Figure 4). The expression levels of ATM and other DNA repair-related factors were down-regulated only when ROS levels were decreased to “sub-physiological” levels at very high concentrations of antioxidants. We interpret this finding as follows: low concentrations of antioxidants drive ROS levels to an optimal “physiological” range, which reduces oxidative stress-induced DNA damage without impairing the DNA repair system. Given that ATM protein levels fell within 24 hours of exposure to antioxidants, we speculate that physiological [ROS] is critical for stabilizing ATM and other DNA repair-related protein kinases, consistent with the notion that reductive stress induces intracellular protein aggregation [17]. However, more work is required to distinguish this possibility from alternatives such as transcriptional down-regulation. While ATM and related DNA repair factors may underlie the genomic instability seen with high antioxidants, the levels of these proteins do not change when CDCs are grown in low antioxidant concentrations or physiological oxygen (Figure 6). Conventional mechanisms of ROS-mediated DNA injury are likely to underlie the salutary effects of modest ROS suppression [23]. The balance of DNA injury during oxidative stress, and faulty DNA repair in reductive stress, provides a rationale for the biphasic relationship between antioxidant concentrations and DNA damage.
Figure 5. ATM protein levels in human cardiosphere-derived cells after 24 hours culture under 20% O2 with antioxidants, catalase, and H2O2.

A. The protein levels of ATM in CDCs were decreased at high doses (≤1000-fold dilution), but not at low doses (≥10,000-fold dilution) of antioxidant supplement (Antioxidant A). B. ATM in CDCs decreases at ≥100 μM, but not at ≤10 μM, of homemade antioxidant cocktail (Antioxidant B). C. Catalase also decreases ATM protein level in CDCs at ≥100 units/ml, but not at ≤10 units/ml. D. The expression of ATM in CDCs was slightly increased at a high dose (≥100 μM) of H2O2. Quantitative data are means and s.d. for four separate experiments using different twice-passaged CDCs.
Figure 6. Expressions of DNA repair-related factors in human cardiosphere-derived cells with 1–2 months long-term culture under different conditions.

A. Compared with cells cultured under traditional 20% O2, ATM expression did not obviously change in cells cultured under 5% O2, but was significantly decreased by the addition of 1000-fold diluted antioxidant supplement (Antioxi. A) or 100 μM homemade antioxidant cocktail (Antioxi. B). B. Compared with cells cultured under traditional 20% O2, the expression of ATR, Rad50, Rad51, Chk 1, and Chk2 did not obviously change in cells cultured under 5% O2. However, many of these factors were decreased in cells cultured under traditional 20% O2 with the addition of 1000-fold diluted antioxidant supplement (Antioxi. A) or 100 μM homemade antioxidant cocktail (Antioxi. B).
Discussion
We have discovered a biphasic relationship between intracellular ROS levels and genomic stability in human cardiac and ES cells: modest ROS suppression by culture in physiological oxygen (5%) decreases karyotypic abnormalities, but profound ROS suppression by antioxidant supplements paradoxically enhances genomic alterations. Figure 7 depicts schematically our interpretation of the results. Oxidative stress is well-known to induce DNA damage, accounting for the high frequency of karyotypic abnormalities in 20% O2 culture (Figure. 7, right panels). On the other hand, excessive suppression of ROS to sub-physiological levels down-regulates DNA repair pathways, thereby contributing to genomic instability (Figure. 7, left panels). Our results suggest a new concept that optimal “physiological” levels of ROS are required for activation of DNA repair pathways to maintain genomic stability in stem cells (Figure 7, center panels). The notion that excessive inhibition of ROS production leads to defective DNA repair rationalizes the increased frequency of karyotypic abnormalities seen with high antioxidants. Such a mechanism may also help explain the polymorphic nature of the antioxidant-related genomic alterations, insofar as generalized inhibition of DNA repair pathways may favor random, rather than systematic, chromosomal changes.
Figure 7. Schematic of our hypothesis regarding the links between intracellular ROS levels and genomic stability.

Left panels: Excessive suppression of ROS undermines DNA repair pathways in a novel manifestation of reductive stress. Center panels: Optimal ROS levels maintain competent DNA repair and minimize DNA damage. Right panels: Excessive ROS levels lead to DNA damage by conventional oxidative injury.
While cytogenetic abnormalities in stem cells have been acknowledged to occur with some frequency [1,2,24], little attention has been focused on the problem. Our findings highlight the magnitude of this complication even for primary adult stem cells, where cytogenetic analysis has not been performed routinely. However, it is possible that cytogenetic abnormalities may have occurred more frequently in the immunosuppressed heart transplant patient population studied here, which was chosen for preclinical product development and validation. Due to the immunosuppression, post-transplant patients are at risk for developing lymphomas, some of which include clonal chromosomal abnormalities in blood or lymph cells [25]. Therefore, we might expect CDCs derived from post-transplant patients to exhibit chromosomal abnormalities at a higher frequency than the non-immunosuppressed study population targeted in CADUCEUS, a conjecture consistent with our observation that only one of 10 clinical-production CADUCEUS study samples to date has been abnormal by cytogenetic screening (unpublished). In any case, our results point to specific interventions (culture in physiological oxygen or low-dose antioxidants) which may well obviate the concerns once and for all.
Free radicals are popularly viewed as harmful by-products of cell metabolism, and antioxidant dietary supplements are touted to be beneficial in the prevention of cancer and a host of other diseases. However, a large, long-term clinical trial showed no beneficial effects of antioxidant supplementation for the prevention of cardiovascular disease [26], and meta-analyses of randomized trials have hinted that some antioxidant supplements actually increase the risk of death [27,28]. In fact, ROS are recognized as key players in intracellular signaling [29,30], and they are crucial for early hematopoietic development and the formation of immune cells [31]. Furthermore, augmented expression and activities of antioxidative enzymes have been pinpointed as causes of reductive stress and protein aggregation cardiomyopathy, although [ROS] was not measured [17]. Here, we have demonstrated that extreme suppression of ROS by high-dose antioxidants increases DNA damage and genomic instability, which is associated with down-regulation of DNA repair-related protein kinases. Therefore, ROS likely plays dual roles in the genomic stability of stem cells (and, quite possibly, of cells in general): a physiological level of ROS is required for effective DNA repair, but high ROS induces DNA damage.
The implications for developmental biology and carcinogenesis have not escaped our notice. Along these lines, studies in p53 knockout mice have revealed that in utero exposure to low-dose vitamin E decreases carcinogenesis, while the opposite is true at high doses [32,33]. Perhaps not fortuitously, plasma concentrations of vitamin C and E in humans normally fall slightly above the “optimal” range for genomic stability defined here, but can reach the danger zone with oral supplementation [34,35]. Our new findings provide a mechanistic basis for the paradoxical enhancement of carcinogenesis by high-dose antioxidants, and serve as a caution against excessive inhibition of oxidative signaling (either in cell culture or in vivo).
Supplementary Material
Acknowledgments
We thank Drs. Linda Marbán and Rhona Schreck for helpful discussions, Dr. Juan-Carlos Biancotti for providing human ES cells, Dr. Clive Svendsen for comments on the manuscript, and Christiane Houde for technical assistance. This work was supported by NIH (R01HL083109 to E.M.).
Footnotes
Author Contributions:
Tao-Sheng Li: Conception and design, Manuscript writing, Collection and/or assembly of data, Data analysis and interpretation.
Eduardo Marbán: Conception and design, Manuscript writing, Financial support, Final approval of manuscript.
Disclosure of Potential Conflicts of Interest: E.M. is a founder and holds equity in Capricor, Inc. Capricor is developing products in the stem cell field, but provided no support for the present studies. T-S. L. has no financial interests to disclose.
References
- 1.Maitra A, Arking DE, Shivapurkar N, et al. Genomic alterations in cultured human embryonic stem cells. Nat Genet. 2005;37:1099–1103. doi: 10.1038/ng1631. [DOI] [PubMed] [Google Scholar]
- 2.Baker DE, Harrison NJ, Maltby E, et al. Adaptation to culture of human embryonic stem cells and oncogenesis in vivo. Nat Biotechnol. 2007;25:207–215. doi: 10.1038/nbt1285. [DOI] [PubMed] [Google Scholar]
- 3.Smith RR, Barile L, Cho HC, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 4.Davis DR, Zhang Y, Smith RR, et al. Validation of the cardiosphere method to culture cardiac progenitor cells from myocardial tissue. PLoS One. 2009;4:e7195. doi: 10.1371/journal.pone.0007195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubio D, Garcia-Castro J, Martín MC, et al. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035–3039. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- 6.Furlani D, Li W, Pittermann E, et al. A transformed cell population derived from cultured mesenchymal stem cells has no functional effect after transplantation into the injured heart. Cell Transplant. 2009;18:319–331. doi: 10.3727/096368909788534906. [DOI] [PubMed] [Google Scholar]
- 7.Ivanovic Z. Hypoxia or in situ normoxia: The stem cell paradigm. J Cell Physiol. 2009;219:271–275. doi: 10.1002/jcp.21690. [DOI] [PubMed] [Google Scholar]
- 8.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Gent DC, Hoeijmakers JH, Kanaar R. Chromosomal stability and the DNA double-stranded break connection. Nat Rev Genet. 2001;2:196–206. doi: 10.1038/35056049. [DOI] [PubMed] [Google Scholar]
- 10.Shaffer LG, Slovak ML, Campbell LJ. An international system for human cytogenetic nomenclature. 1. S Karger AG; 2009. [Google Scholar]
- 11.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–1720. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 12.Lavon N, Narwani K, Golan-Lev T, et al. Derivation of euploid human embryonic stem cells from aneuploid embryos. Stem Cells. 2008;26:1874–1882. doi: 10.1634/stemcells.2008-0156. [DOI] [PubMed] [Google Scholar]
- 13.Lobo I. Nature Education. 2008. Chromosome abnormalities and cancer cytogenetics. [Google Scholar]
- 14.Wang F, Thirumangalathu S, Loeken MR. Establishment of new mouse embryonic stem cell lines is improved by physiological glucose and oxygen. Cloning Stem Cells. 2006;8:108–116. doi: 10.1089/clo.2006.8.108. [DOI] [PubMed] [Google Scholar]
- 15.Fehrer C, Brunauer R, Laschober G, et al. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell. 2007;6:745–757. doi: 10.1111/j.1474-9726.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- 16.Hainsworth AH, Bhuiyan N, Green AR. The nitrone disodium 2,4-sulphophenyl-N-tert-butylnitrone is without cytoprotective effect on sodium nitroprusside-induced cell death in N1E-115 neuroblastoma cells in vitro. J Cereb Blood Flow Metab. 2008;28:24–28. doi: 10.1038/sj.jcbfm.9600517. [DOI] [PubMed] [Google Scholar]
- 17.Rajasekaran NS, Connell P, Christians ES, et al. Human alpha B-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice. Cell. 2007;130:427–39. doi: 10.1016/j.cell.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossi DJ, Bryder D, Seita J, et al. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- 19.Qin K, Zhao L, Ash RD, McDonough WF, Zhao RY. ATM-mediated transcriptional elevation of prion in response to copper-induced oxidative stress. J Biol Chem. 2009;284:4582–4593. doi: 10.1074/jbc.M808410200. [DOI] [PubMed] [Google Scholar]
- 20.Falck J, Coates J, Jackson SP. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature. 2005;434:605–611. doi: 10.1038/nature03442. [DOI] [PubMed] [Google Scholar]
- 21.Bredemeyer AL, Sharma GG, Huang CY, et al. ATM stabilizes DNA double-strand-break complexes during V(D)J recombination. Nature. 2006;442:466–470. doi: 10.1038/nature04866. [DOI] [PubMed] [Google Scholar]
- 22.Bao S, Tibbetts RS, Brumbaugh KM, et al. ATR/ATM-mediated phosphorylation of human Rad17 is required for genotoxic stress responses. Nature. 2001;411:969–974. doi: 10.1038/35082110. [DOI] [PubMed] [Google Scholar]
- 23.Seifried HE, Anderson DE, Fisher EI, Milner JA. A review of the interaction among dietary antioxidants and reactive oxygen species. J Nutr Biochem. 2007;18:567–579. doi: 10.1016/j.jnutbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Sareen D, McMillan E, Ebert AD, et al. Chromosome 7 and 19 trisomy in cultured human neural progenitor cells. PLoS One. 2009;4:e7630. doi: 10.1371/journal.pone.0007630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Djokic M, Le Beau MM, Swinnen LJ, et al. Post-transplant lymphoproliferative disorder subtypes correlate with different recurring chromosomal abnormalities. Genes Chromosomes Cancer. 2006;45:313–318. doi: 10.1002/gcc.20287. [DOI] [PubMed] [Google Scholar]
- 26.Sesso HD, Buring JE, Christen WG, et al. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2008;300:2123–2133. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 28.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, et al. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 29.Foreman J, Demidchik V, Bothwell JH, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- 30.Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Owusu-Ansah E, Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009;461:537–541. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen CS, Squire JA, Wells PG. Reduced tumorigenesis in p53 knockout mice exposed in utero to low-dose vitamin E. Cancer. 2009;115:1563–1575. doi: 10.1002/cncr.24130. [DOI] [PubMed] [Google Scholar]
- 33.Chen CS, Wells PG. Enhanced tumorigenesis in p53 knockout mice exposed in utero to high-dose vitamin E. Carcinogenesis. 2006;27:1358–1368. doi: 10.1093/carcin/bgi325. [DOI] [PubMed] [Google Scholar]
- 34.Levine M, Conry-Cantilena C, Wang Y, et al. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci USA. 1996;93:3704–3709. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh U, Otvos J, Dasgupta A, et al. High-dose alpha-tocopherol therapy does not affect HDL subfractions in patients with coronary artery disease on statin therapy. Clin Chem. 2007;53:525–528. doi: 10.1373/clinchem.2006.078865. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


