Abstract
Microglia, which contribute substantially to the tumor mass of glioblastoma, have been shown to play an important role in glioma growth and invasion. While a large number of experimental studies on functional attributes of microglia in glioma provide evidence for their tumor-supporting roles, there also exist hints in support of their anti-tumor properties. Microglial activities during glioma progression seem multifaceted. They have been attributed to the receptors expressed on the microglia surface, to glioma-derived molecules that have an effect on microglia, and to the molecules released by microglia in response to their environment under glioma control, which can have autocrine effects. In this paper, the microglia and glioma literature is reviewed. We provide a synopsis of the molecular profile of microglia under the influence of glioma in order to help establish a rational basis for their potential therapeutic use. The ability of microglia precursors to cross the blood–brain barrier makes them an attractive target for the development of novel cell-based treatments of malignant glioma.
Keywords: alternatively activated macrophage, glioma-infiltrating microglia/macrophages, immunosuppression, synthetic biology, tumor-associated macrophages
Malignant gliomas are among the most common brain tumors. They are characterized by morphological and genetic complexity, and they infiltrate diffusely into normal brain parenchyma.1 This feature renders all current therapeutic strategies ineffective, and patients show significant mortality, with a life expectancy of only 14 months on average from the time of diagnosis in the case of glioblastoma multiforme (GBM) despite multimodal therapy.2–5 Furthermore, conventional strategies are limited by nonspecific damage to surrounding normal brain tissue.6 A wide range of other therapeutic strategies, including immunotherapy and gene therapy, have been tried or are under evaluation. However, until today none of these has proven effective. As a result, there is a pressing need for better therapies that enable precise targeting of glioma cells while sparing the neighboring normal tissue.
Within a glioma, microglia/macrophages make up the largest population of tumor-infiltrating cells, contributing at least one third of the total tumor mass.7–10 These glioma-infiltrating microglia/macrophages (the macrophage phenotype may predominate) are present in both intact glioma tissue and necrotic areas,8 and their density in gliomas is positively correlated with glioma grade and invasiveness.11,12 There is compelling evidence that microglial cells are involved in creating a microenvironment that favors glioma growth.11–16 Specifically, glioma invasion12,17–19 and the establishment of an immunosuppressive milieu20,21 are facilitated by the presence of intratumoral microglia. However, the precise molecular mechanisms underlying these phenomena have remained unclear. Elucidation of the molecular profile of microglia, including the receptors they express and the molecules they release in response to the microenvironment created by glioma, will not only help to unravel the role of microglia in glioma, but also lead to the design of more effective treatment options for these fatal tumors. In this review, we detail the known molecular mechanisms that govern microglia–glioma interactions.
Glioma and Microglia: Worse than a Symbiosis
The presence of microglia in brain tumors was first reported by Rio-Hortega and Asua in 1921,22 and Penfield in 192523 provided the first detailed description of “microglia and the process of phagocytosis in gliomas.” However, the true extent of microglial infiltration in both animal and human glioma was not widely appreciated until recently.9,10,24–29 The initial actions of glioma-infiltrating microglia, as the resident macrophages of the CNS, would be expected to be migration to the tumor site for rescue and display of properties similar to peripheral macrophages, such as phagocytosis, antigen presentation, and release of cytokines/chemokines as well as cytotoxins.30–33 However, such glioma-cytotoxic effects of microglia have been observed in vitro only.34,35 Most in vivo studies report that microglial cells actually promote glioma cell migration and growth.10,16 Interestingly, a recent study by Voisin et al. found that microglia were in an activated state with phagocytic activity within the first 3 h of co-culture with C6 glioma cells.36 However, these microglia lost their phagocytic properties when in longer contact with glioma cells, ie., after 6 h of co-culture.36 This and other experimental work16 have provided evidence in support of the view that the defense and immune functions of microglia are suppressed and controlled by the glioma. Other studies even suggest that in case of glioma, microglia acquire a distinct phenotype that is induced by the tumor cells and is different from the inflammatory phenotype.11,12,37 Microglia under the influence of glioma do not release pro-inflammatory cytokines that could help fight tumors; instead they exhibit upregulation of metalloprotease-II, which facilitates tumor invasion.38 Moreover, they have been found to secrete matrix metalloproteinase (MMP)–9,39 epidermal growth factor (EGF),40 and vascular endothelial growth factor (VEGF),41,42 which are all known to promote tumor proliferation. In addition, microglia in glioma release interleukin (IL)-10, which helps to create an immunosuppressive microenvironment.7,20,21,43 In turn, receptors expressed by glioma-infiltrating microglia including EGF receptor (EGFR) and Met, enable them to receive signals from the tumor, via EGF and hepatocyte growth factor/scatter factor (HGF/SF), which further increases glioma invasion and migration. Gliomas also promote the recruitment and proliferation of microglia by releasing monocyte chemoattractant proteins 1 and 3 (MCP-1 and MCP-3), granulocyte macrophage colony-stimulating factor (GM-CSF), and granulocyte colony-stimulating factor (G-CSF).44–46 Even the armament of microglia is tactically employed by glioma cells to facilitate their survival, growth, and spread.47 Therefore, the close physical association of microglia and tumor cells in a glioma seems to suggest a symbiotic relationship, but it is actually highly skewed to the advantage of the glioma. As a result, microglia that infiltrate a glioma lose their defense and immune functions.
Sources of Microglia in Glioma
Developmental Aspects
The origin of microglia and whether and how they renew in healthy adult brain have been controversial for more than a century. A recent experimental study by Ginhoux et al.48 appears to have brought previous long-running disputes to an end. Based on the expression pattern of the runt-related transcription factor 1 (Runx-1), Ginhoux and colleagues used a genetic pulse-labeling strategy to identify yolk sac macrophages between embryonic days 6.5 and 10 and found that adult microglia arise from yolk sac macrophages present between embryonic days 7.25 and 7.5 and enter the embryo right after vascularization at day 8, before primitive or definitive hematopoiesis starts in the embryo. These experiments establish that during development, microglia originate from a single population of extra-embryonic yolk sac macrophages, which are distinct with respect to the postnatal hematopoietic origin of other tissue macrophages and the fact that their numbers in the adult brain are maintained independently of circulating hematopoietic cells. Also in contrast to monocytes, microglia seem to depend during development on growth factor receptor colony-stimulating factor (CSF)–1R and its recently identified ligand, IL-34,49 rather than CSF-1, in keeping with the presumed distinct ontogeny of microglia.48
Origin of Tumor-Associated Macrophages and Microglia
Despite the fact that the vast majority of microglia in the healthy adult brain have to be considered yolk sac–derived, there remains the burning question as to whether microglia in the diseased brain can develop at least in part from other sources, such as bone marrow. This is of great interest because there may be functional differences between microglia from different sources and, consequently, different treatment targets once cellular and genetic therapies for glioma have become realities. The idea of a possible “on-demand influx” of bone marrow–derived microglia precursors into the diseased brain is in keeping with the observation that microglia express several properties usually seen in stem cells and bone marrow progenitors. Both in vivo and in vitro studies on microglia that proliferate under pathological conditions demonstrate that microglia in diseased CNS harbor at least one phenotypic marker of bone marrow myeloid progenitors: CD34, the stem and progenitor cell antigen.50,51 Moreover, a varying degree of recruitment of bone marrow–derived precursors that colonize the CNS and transform into ramified microglia under different pathological conditions has been observed, in experimental models of stroke,52 brain ischemia,53,54 multiple sclerosis,55 Parkinson's disease,56 Alzheimer's disease,57 and others.58–60 Notably, such microglia engraftment originating from bone marrow precursors in the adult diseased brain not necessarily associated with disruption of the blood–brain barrier (BBB) occurs only under defined host conditions. The use of irradiation chimeras for assessing microglia turnover has been questioned because irradiation itself can damage the BBB and augment or even cause cell infiltration.61 A recent study by Alshakweer et al. has provided evidence in support of the view that bone marrow–derived microglia give rise to tumor-infiltrating microglia/macrophages in pilocytic astrocytoma in the absence of irradiation.62 This finding is matched by experimental results demonstrating that both bone marrow–derived macrophages and resident microglia invade glioma.63 Thus, microglial cells populating glioma indeed originate from at least 2 sources: intrinsic parenchymal microglia and microglia freshly derived from their bone marrow precursors in the blood.8 It should be noted that polarization properties may specifically distinguish the 2 populations of cells.64
Whether tumor stem cells in glioma can give rise to intratumoral microglia/macrophages potentially representing a third source is unknown at present.65 Microglia in nondiseased CNS should be referred to as resident or ramified microglia rather than resting66 microglial cells.
Glioma: Taking Control of the Microglia
Glioma-Derived Factors Contributing to the Establishment of an Immunosuppressive Tumor Environment
Transforming growth factor–β.— TGF-β is the best-characterized soluble immunosuppressive cytokine secreted by gliomas. TGF-β protein exists in at least 3 different isoforms in humans: TGF-β1, TGF-β2, and TGF-β3. These isoforms utilize different cell surface mechanisms to elicit distinct intracellular responses.67 TGF-β isoforms 1–3 are differentially expressed in glioma cell cultures68,69 and human glioma tissues,70 with TGF-β2 as the most abundant isoform upregulated in GBM.69,71 TGF-β2 has been implicated in glioma-associated immunosuppression,71 whereas TGF-β1 has been shown to act as a stimulator of glioma cell motility.72 Significantly, TGF-β inhibits development and activation of antigen-presenting cells, including microglia, represses natural killer (NK) cells, and prevents the activation and differentiation of cytotoxic T cells.73–76 The mechanism underlying TGF-β–evoked immunosuppression may include the downregulation of major histocompatibility complex (MHC) class II antigen expression on microglial cells,77 as well as deactivation of microglia as phagocytic cells.78 It is thus believed that glioma-derived TGF-β induces glioma-infiltrating microglia to shift to a distinct tumor supportive phenotype.79 Recently, Crane and colleagues80 reported that TGF-β downregulates the activating receptor NKG2D, which is expressed by NK cells and CD8+ T cells and has a role in the specific killing of transformed cells in glioma patients. In addition to its immunosuppressive function, glioma-derived TGF-β promotes tumor cell migration and invasion by inducing MMP expression, suppressing expression of the tissue inhibitor of metalloproteinase,81–83 assisting neovascularization of tumor tissue by TGF-β–mediated expression of angiogenic factors, such as VEGF and fibroblast growth factors (FGFs),84–86 and stimulating tumor cell proliferation by means of increasing expression of EGFR.87 A recent study supported the invasion-promoting role of TGF-β by showing that glioma-initiating cells (glioma stem cells) pretreated with TGF-β signaling inhibitor were less aggressive and showed less lethal potency in an intracranial transplantation assay.88 This finding was confirmed by an in vivo study using neutralizing TGF-β antibody that was shown to reduce invasion of the glioma cells into the adjacent normal brain.89
Prostaglandin E2.— PGE2 is a small lipid-soluble molecule that is produced by both immune-competent cells and tumor cells. Glioma cells have been shown to release significant amounts of PGE2 in vitro and in vivo, compared with PGE2 synthesis in normal brain.90–92 Importantly, elevated levels of PGE2 in glioma were found to downregulate the activity of lymphokine-activated killer (LAK) cells93 and the surface expression of MHC class II, human leukocyte antigen (HLA)–DR, on antigen presenting cells such as microglia and dendritic cells.94,95 Moreover, the increased production of PGE2 by glioma is also associated with suppression of T-cell activation and proliferation.96,97 Regulatory T cells are induced by PGE2.98 In sum, PGE2 plays an important role in the generation of an immunosuppressive milieu in glioma. Furthermore, PGE2 promotes glioma cell proliferation via a signaling pathway involving activation of protein kinase A.99,100 With regard to the cellular source of PGE2 in glioma, microglia have been found to produce PGE2 when co-cultured with glioma cells or conditioned glioma medium, strongly suggesting that microglia contribute to local immunosuppression by glioma.101 PGE2 biosynthesis is regulated by inducible membrane-associated PGE2 synthase cyclooxygenase-2 (COX-2) and microsomal PGE synthase (mPGES)–1.102,103 Abnormal expression of COX-2 and mPGES-1 has been detected in human glioma,100,104 and conditioned glioma medium was found to enhance the expression of COX-2 and mPGES-1 in microglial cells.101 Thus, the mechanism underlying the elevated level of PGE2 in glioma could be related to the increased production of COX-2 and mPGES-1 in microglia, although the exact mechanism has remained obscure.
Fas ligand.— FasL, or CD95L, is a 42-kDa transmembrane protein that belongs to the TNF family. When bound to its receptor, Fas (CD95/APO-1), FasL initiates an intracellular signaling cascade that leads to the induction of apoptosis in Fas-expressing cells. Fas–FasL ligation has been suggested to be one of the main pathways mediating programmed cell death in a variety of cell types.43 FasL has been detected on the surface of human glioblastoma cells and was also found to be expressed by rat glioma cell lines 9L, F98, and C6.105 Both microglia and activated T cells express Fas and thus may be susceptible to a death signal delivered by functionally active FasL expressed on astrocytoma cells.106–108 In line with this, Jansen and colleagues105 recently reported that FasL was responsible for the death of T lymphocytes when co-cultured with glioma cells in vitro. These authors also demonstrated that downregulation of FasL expression in glioma cells enhances tumor infiltration of T cells and inhibits tumor growth in vivo.105 Thus, it is now recognized that FasL expressed in tumors contributes to local immunosuppression and evasion of immune surveillance by inducing apoptosis of Fas-expressing T cells. In contrast, there is so far no evidence that glioma-derived FasL induces the apoptosis of microglial cells despite the presence of Fas on microglia.106 In addition, FasL also functions in the regulation of glioma motility and invasion. Blocking of Fas signaling has been found to impair MMP-2 activity, resulting in a reduction of glioma invasiveness and motility.109 Moreover, Choi and colleagues110 found that Fas ligation induces expression of intercellular adhesion molecule 1 (ICAM-1) in human astrocytoma cells and postulated that FasL may induce angiogenesis in glioma. Glioma cancer stem cells (gCSCs) were found to be resistant to Fas-induced apoptosis, suggesting that gCSCs are an important factor of resistance to chemotherapy.111 Therefore, targeting endogenous FasL in glial malignancies could enhance the efficacy of immune-based treatment strategies.
Role of Signal Transducer and Activator of Transcription Protein 3 in Mediating Immunosuppressive Effects in Glioma
Signal transducer and activator of transcription protein (STAT)3 is a member of a transcription factor family, which is encoded by the STAT3 gene in humans. STAT3 activation causes expression of genes that play important roles in mediating the signals of cytokines and growth factors involved in cell growth, proliferation, differentiation, and apoptosis.112,113 STAT3 was first implicated in glioblastoma pathology through studies demonstrating that STAT3 is constitutively activated in GBM cell lines as well as human GBM tissue.114–117 STAT3 activation has been found to reduce the expression of MHC class II molecules, CD80, and CD86 in hematopoietic cells and glioma-infiltrating microglia.118 In addition, STAT3 activation has been implicated in inhibiting the T-cell response against glioma.119 When STAT3 activation was inhibited, gCSC-induced IL-10 production in glioma-infiltrating microglia was reduced, and gCSC-dependent inhibition of phagocytosis by glioma-infiltrating microglia was also reversed.79 STAT3 thus appears to be a key mediator of immunosuppression in glioma. Apart from its role in immune evasion, STAT3 also contributes to gliomagenesis and progression. STAT3 activation is required for glioma growth120 and for proliferation and maintenance of gCSCs.121 Inhibition of STAT3 has been shown to suppress proliferation and growth and to induce apoptosis in glioblastoma cells both in vitro122 and in vivo.123 Moreover, inhibition of STAT3 has also been found to enhance radiosensitivity of glioma.124
Many factors found in glioma, including IL-10, IL-6, EGF, and FGF, are activators of STAT3. IL-6 was found to induce STAT3 activation in glioma cell lines125 and in mouse models.126,127 Furthermore, oncostatin M, a member of the IL-6 cytokine family, increases the STAT3-depedent expression and activation of VEGF and MMP-9 in human astrocytoma cell lines.128,129 It is thus reasonable to postulate that IL-6 derived from glioma cells functions in tumor invasion and angiogenesis, at least in part through its activation of STAT3. There is also evidence to support a role for EGFR in glioblastoma-associated STAT3 activation. EGFR is amplified in approximately 50% of GBMs, and in about 50% of these amplified cases the GBMs express EGFRvIII, a mutant EGFR that lacks a portion of the extracellular ligand-binding domain. EGFRvIII was shown to be persistently autophosphorylated at low levels,130 resulting in inefficient EGF signal attenuation and persistent activation of downstream kinase pathways, including those involving STAT3, Ras/MAPK, and Akt.131 Moreover, as a downstream transcriptional target of FGF receptor, STAT3 can be activated by FGF via the FGF receptor.132 Interestingly, STAT3 target molecules, such as IL-10, can also activate STAT3 in various cells. Therefore, a feed-forward mechanism appears to account for the constitutive activation of STAT3 in glioma cells and glioma-infiltrating microglia so that these STAT3-regulated molecules and STAT3-regulating molecules continue to accumulate. However, gene mutations of STAT3 have not been detected in glioblastoma. Aberrant STAT3 activity may also be caused by dysregulation of upstream tyrosine kinases or loss of negative feedback.133 Therefore, in spite of the fact that multiple cytokines and growth factors may contribute independently to glioblastoma pathology, substantial data gathered from cell lines, rodent models, and patient samples support a role for STAT3 as a critical “molecular hub” that links multiple pathways involved in glioblastoma pathology. 133
Polarization of Microglia (M2)
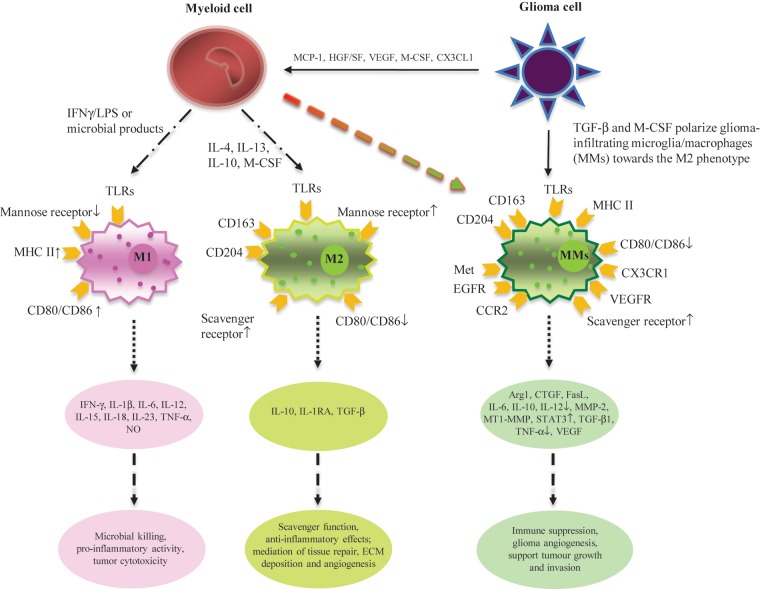
Heterogeneity of macrophage activation provides a basis for the conceptual classification of macrophages into 2 polarized functional categories: M1 (classically activated macrophages) and M2 (alternatively activated macrophages). As illustrated in Fig. 1, these 2 types of macrophage functional states differ in terms of activating signals, cytokine/chemokine production, receptor expression, and biological effects. Originally, macrophages were found to be activated by exposure to microbial products, such as lipopolysaccharides, along with exposure to interferon gamma (IFNγ). Activated type 1 polarized macrophages are characterized by preferential production of high levels of pro-inflammatory cytokines, such as IL-12, and oxidative metabolites, such as nitric oxide (NO),134 and by displaying elevated expression levels of MHC class II and co-stimulatory molecules CD80 and CD86.135 Thus, these macrophages, which are also referred to as classically activated macrophages, exhibit potent microbicidal/tumoricidal activities but also cause damage to healthy tissue as a side effect.136 The first hint to the existence of alternatively activated macrophages came in the early 1990s, when Gordon and colleagues137 treated macrophages with IL-4 and IL-13 in a study examining the regulation of mannose receptor expression on elicited macrophages. They observed signs of macrophage activation; however, these macrophages exhibited diverse biological properties that were clearly distinct from those of their classically activated counterparts. These M2 cells typically produce anti-inflammatory cytokines, such as IL-10, and show a lower level of NO production. Furthermore, although they still upregulate the expression of MHC class II molecules, they are not efficient at antigen presentation. In contrast, M2 macrophages have been found to promote tissue remodeling, angiogenesis, and repair and act to suppress tissue-destructive immune reactions.138 The key determinant in polarizing macrophages is the microenvironment in which they dwell, and the latter selectively induces specific M1 or M2 functional programs.136
Fig. 1.
Microglia in glioma are polarized. M1 (classically activated macrophages) and M2 (alternatively activated macrophages) differ with respect to activating signals, receptor expression, cytokine/chemokine production, and biological behavior. When mononuclear/phagocytic cells are stimulated by IFNγ, lipopolysaccharides, and other microbial products, they differentiate into the M1 phenotype. Microbial products are recognized by PRRs on the surface of M1, such as TLRs, and stimulate the production of pro-inflammatory cytokines as well as the expression of receptors that are involved in antigen presentation. When mononuclear/phagocytic cells are activated by IL-4, IL-13, IL-10, and M-CSF, they differentiate into the M2 phenotype. Tumor-derived molecules, such as TGF-β and M-CSF, can polarize glioma-infiltrating microglia/microphages (MMs) toward the M2 phenotype and accordingly stimulate the production of anti-inflammatory molecules. Some other glioma-derived molecules, such as MCP-1 and VEGF, can recruit myeloid cells into the tumor site.
Polarization of glioma-infiltrating microglia toward the M2 phenotype has been well documented both in vitro and in vivo. Rodrigues et al.139 reported that peripheral blood mononuclear cells (PBMCs) exposed to glioma, which were obtained from either normal donors or glioma patients, acquire immunosuppressive properties. This includes reduced expression of CD14 (but not of CD11b); increased expression of immunosuppressive IL-10, TGF-β, and B7-H1; decreased phagocytic capacity; and the increased ability to induce apoptosis in activated lymphocytes when monocytes were exposed to glioma cells in vitro. In addition, Wu and colleagues79 have demonstrated that gCSC-conditioned medium polarizes glioma-infiltrating microglia toward an M2 phenotype. These M2-polarized microglia in glioma exhibited reduced phagocytic activity and secretion of immunosuppressive cytokines, such as IL-10 and TGF-β, and were less capable of stimulating T-cell proliferation. This is in line with previous findings by Hussain et al.140 that glioma-infiltrating microglia do not secrete the pro-inflammatory cytokines IL-1β and TNF-α that play a critical role in the development of effective innate immune responses nor are capable of mediating an anti-tumor adaptive immune response.37 It is believed that glioma-derived molecules such as TGF-β, macrophage (M)-CSF, IL-10, and IL-4 induce the shift of glioma-infiltrating microglia toward the M2 phenotype.141,142 This view is supported by the following in vivo studies. Using a mouse model, Gabrusiewicz et al.63 recently demonstrated that glioma-infiltrating microglia acquire the M2 phenotype in vivo, as evidenced by upregulation of IL-10 and GM-CSF, as well as the increased expression of genes characteristic for the alternative and pro-invasive phenotype of glioma-infiltrating microglia, arginase 1 (Arg-1), membrane type 1 metalloprotease (MT1-MMP), and chemokine (C-X-C motif) ligand 14 (CXCL14). Furthermore, M-CSF expression in glioma cells was found to correlate with the expression of CD163 in glioma-infiltrating microglia in vivo, supporting the role of glioma cells in microglia polarization.143 Specifically, expression of CD163 and CD204, both of which are considered M2 macrophage markers, by microglia/macrophages infiltrating glioma was significantly higher in grade IV glioma when compared with World Health Organization grades II and III glioma, indicating that polarization of glioma-infiltrating microglia toward the M2 phenotype correlates with a more malignant histologic grade.143 Taken together, glioma-derived factors are able to “educate” glioma-infiltrating microglia to acquire the M2 phenotype and thus create a favorable microenvironment for glioma growth.
Molecules Promoting the Proliferation of Microglia
M-CSF, also known as CSF-1, is a growth factor that was found to be expressed in mice and human glioma tissues as well as GBM cell lines.40,144 The M-CSF receptor (R), encoded by the c-fms proto-oncogene, is the main receptor for M-CSF145 and is expressed on microglial cells146 and possibly on neurons.147 Increased M-CSFR expression in activated microglia has been reported following mouse facial nerve axotomy146,148 and cerebral ischemia147 and in a transgenic mouse model of Alzheimer's disease.149 Furthermore, M-CSFR overexpression was found to result in microglial cell proliferation and increased expression of inducible NO synthase, pro-inflammatory cytokine IL-1α, macrophage inflammatory protein (MIP) 1α, IL-6, and M-CSF itself.150 A recent study employing the rat facial nerve axotomy model reported that the expression of M-CSF in microglia was upregulated, which triggered increased expression of M-CSFR in microglia.151 This autocrine loop between M-CSF and M-CSFR in microglia apparently underlies the proliferation of microglia in this model.151 Despite the fact that there is so far no evidence for the expression of M-CSFR on glioma-infiltrating microglia, the production of M-CSF by glioma cells and the presence of M-CSFR on activated microglia151 raise the possibility of a paracrine loop that could promote intratumoral proliferation of microglia in glioma. Papavasiliou et al.152 demonstrated the existence of a paracrine loop in medulloblastoma, reporting that the tumor cells expressed M-CSF but not M-CSFR, and microglia treated with media conditioned by serum-free medulloblastoma significantly increased their proliferation in vitro. In addition, M-CSF and M-CSFR paracrine communication between GBM and microglia has been confirmed by a recent in vivo study, which further indicated that blockade of M-CSFR signaling reduces the number of glioma-infiltrating microglia and thus GBM invasion.40 It is noteworthy that expression of the M-CSF gene may result in 2 different isoforms of the M-CSF protein, ie., the secreted form (sM-CSF) and the membrane-bound form (mM-CSF), which elicit different effects on glioma-infiltrating microglia. Glioma-derived sM-CSF promotes tumor invasion by stimulating the proliferation and recruitment of glioma-infiltrating microglia.144 In contrast, expression of mM-CSF by tumor cells elicits anti-glioma activity in microglia, stimulating direct tumor cell killing and antigen processing.153–155 Disruption of internal potassium ion homeostasis in mM-CSF–expressing glioma cells through channel activation of functional big potassium (BK) may represent an alternative mechanism underlying the mM-CSF–mediated anti-glioma activity of microglia.156 Furthermore, GBM-derived sM-CSF induces polarization of glioma-infiltrating microglia toward the immunosuppressive M2 phenotype, which increases proliferation and migration of glioma cells.143 This finding was further corroborated by a recent study on gCSCs, which are demonstrated to also produce sM-CSF that can drive polarization of microglia toward the M2 phenotype.79
Molecules Controlling the Recruitment/Migration of Microglia
Chemokine (C-C motif) ligand 2.— CCL2, introduced earlier in this paper as MCP-1, is a protein belonging to the CC chemokine family, encoded by the CCL2 gene. By binding to chemokine (C-C motif) receptor 2 (CCR2), MCP-1 induces the migration of monocytes, memory T cells, and dendritic cells to sites of tissue injury, infection, and inflammation. MCP-1 has been found to be produced by glioma cells,45,157,158 and its expression is positively correlated with microglial infiltration of human gliomas.159 This is in keeping with the results of an in vivo study by Platten et al.,158 which provided direct experimental evidence that MCP-1 expression promotes the recruitment of microglial cells to the site of glioma. MCP-1 is therefore considered a critical chemoattractant for glioma-infiltrating microglial cells. Furthermore, MCP-1 expression is correlated with the grade of malignancy,160 and MCP-1–expressing gliomas appear more anaplastic and vascularized than control tumors.158 These studies suggest that the expression of MCP-1 by glioma cells not only induces the recruitment of microglial cells to the site of glioma, but also promotes tumor growth and neo-angiogenesis as a consequence of local infiltration of microglia. Interestingly, while causing migration and proliferation of microglia, MCP-1 does not appear to directly activate an inflammatory response in microglia,161 and the authors concluded that other factors may be necessary to cause the changes that result in the neuronal damage commonly observed in situations where MCP-1 levels are elevated. MCP-1 may stimulate CCR2-bearing microglia to produce and secrete IL-6, which in turn acts on glioma cells to promote their invasiveness.162
Hepatocyte growth factor/scatter factor.— HGF/SF is a protein functioning through its exclusive receptor, tyrosine kinase c-Met. In the case of GBM, HGF/SF and c-Met are expressed by both tumor cells and microglial cells.163–165 Glioma-derived HGF/SF is able to chemotactically attract isolated microglial cells in vitro,163 which suggests that it has a role in microglia chemotaxis in glioma in vivo. In addition, glioma-derived HGF/SF and c-Met have emerged as crucial determinants of glioma growth and angiogenesis. The expression levels of HGF/SF and c-Met in glioma cells increase with the grade of malignancy.164,165 This autocrine HGF/SF–c-Met signaling loop in glioma has been demonstrated to be associated with tumor invasion and proliferation both in vitro164,166–168 and in vivo.169 Furthermore, HGF/SF has been shown to be a potent angiogenic molecule.167,170 The angiogenic activity of HGF/SF is mediated either through direct actions on brain tumor endothelial cells (including stimulation of cell migration, proliferation, protease production, invasion, and organization into capillary-like tubes167) or through induction of VEGF expression and secretion in malignant glioma cells.171 Hypoxia can induce c-Met expression in glioma cells,172 whereas gangliosides and TGF-β have been found to stimulate the production of HGF/SF in human glioma cells.173 Radiation enhances HGF/SF secretion, resulting in increased angiogenic potential of the tumor, which may be a factor in the radioresistance of glioma.174 Accordingly, Buchanan and colleagues175 recently reported that modulation of Met signaling with anti-HGF monoclonal antibody–AMG102 is able to increase the radiosensitivity of glioma cells, raising the possibility of a novel radiation sensitizing strategy. Inhibition of c-Met further enhances the chemosensitivity of glioma cell lines.176
Vascular endothelial growth factor.— VEGF is a signaling protein that is important for stimulating vasculogenesis and angiogenesis. Glioma cells constitutively secrete large amounts of VEGF,42,177 and the level of VEGF production in glioma increases with the degree of tumor malignancy.178,179 Thus, VEGF is considered to play a pivotal role in glioma growth. One possible mechanism underlying the VEGF glioma-promoting function is its chemotactic effect on microglial cells. VEGF has been found to induce the migration and proliferation of microglial cells that express VEGFR-1 in vitro.180 Thus, it is likely that glioma-derived VEGF at least in part accounts for infiltration of microglia into glioma. Significantly, VEGF also contributes to the recruitment of bone marrow–derived cells.181 Kerber et al.15 reported that inducing VEGF overexpression in glioma tissues leads to a substantial infiltration of bone marrow–derived microglia/macrophages in vivo, and there was a 3.1-fold increase in infiltration compared with tumors created by implantation of wild-type glioma cells.
One well-known mechanism underlying the VEGF glioma-promoting function is the major role VEGF plays in angiogenesis in glioma. Endothelial cells in the vicinity of a glioma coexpress both VEGFR-1 and VEGFR-2, which serve a paracrine-signaling loop with glioma-derived VEGF that stimulates endothelial cell migration and proliferation and consequently new blood vessel formation.181,182 This finding confirms the results of a previous study on primary brain tumors, which demonstrated that VEGF expression was correlated with vascularization in gliomas.178 Glioma cancer stem cells have been proposed to be critical in sustaining tumor progression due to their capacity for self-renewal and their proliferative potential. Folkins and colleagues183 found that tumors high in gCSCs express increased levels of VEGF and exhibit increased microvessel density and blood perfusion, as well as increased mobilization and recruitment of bone marrow–derived endothelial progenitor cells into the tumor. Blockade of the VEGF–VEGFR pathway alone or in combination with cytokines such as IL-6 has been reported to inhibit tumor growth.184
Abnormal energy metabolism is one feature of glioma. Glioma cells require a higher rate of glucose and glutamine uptake and metabolism than normal to maintain their survival and growth.185–188 Significantly, human glioma cells secrete glutamate,189,190 and high glutamate secretion conveys a growth advantage.191 Therefore, glutamate release is thought to contribute to glioma spread as well as excitotoxicity.191 Glucose and glutamine are also important fuels for microglia, macrophages, and other cells of the immune system.192,193 Intriguingly, glutamate has been found to induce directed chemotaxis of microglia.194 Both radiation and chemotherapy can induce necrosis and inflammation, which will increase tissue glutamate levels.195,196
Microglia Supporting Glioma Growth
Microglia Listening to Glioma
Receptors involved in microglia chemotaxis and proliferation.—The exact mechanisms underlying the recruitment of microglial cells into a glioma are still not entirely clear, but chemotactic factors released by tumor cells likely play a pivotal role. Several such factors have been identified, with MCP-1 turning out to be the most powerful. MCP-1 is constitutively produced by glioma cells as evidenced by both in vitro and in vivo studies,45,157 whereas the specific MCP-1 receptor, CCR2, is expressed on glioma-infiltrating microglia/macrophages.197,198 Importantly, an in vitro study by Platten et al.158 provided direct experimental evidence that MCP-1 expression recruits microglial cells to the site of glioma. Thus, MCP-1 locally produced by glioma cells binds to CCR2 receptors expressed on the surface of microglial cells, and MCP-1/CCR2 binding facilitates recruitment of resident microglia into the site of glioma. In contrast, Okada and colleagues44 recently claimed that it is tumor-derived MCP-3, not MCP-1, that facilitates the infiltration of microglia into glioma tissues. However, MCP-1 binds to CCR2 exclusively, whereas MCP-3 binds to CCR1, CCR2, and CCR3.199 The study did not report which receptor(s) respond to MCP-3 production in glioma.
Met. Met is a transmembrane tyrosine kinase receptor encoded by the proto-oncogene MET. Badie et al.163 detected Met immuoreactivity in BV-2 murine microglia, and this is in keeping with what had been reported by Yamada et al.,200 who described the expression of Met in microglia, predominantly in the white matter of human brain tissue. A more recent study also confirmed the expression of Met in human glioma–associated microglia.165 As discussed earlier, the cognate ligand of c-Met, HGF/SF, is expressed by glioma cells.163–165 Badie et al.163 have demonstrated that glioma-derived HGF/SF is able to induce the migration of microglial cells in vitro, suggesting the existence of a paracrine loop of HGF/SF–Met signaling that plays a role in microglia chemotaxis in glioma in vivo. The existence of paracrine HGF/SF effects is supported by the results of an in vitro study that analyzed the effects of a panel of chemokines on glioma motility, demonstrating that HGF/SF was the most potent chemotactic factor for 3 glioblastoma cell lines.201 Interestingly, microglia have also been reported to produce HGF/SF.163,200,202,203 These findings raise the possibility of an autocrine motility loop in microglia, which HGF/SF–Met underlie.
Epidermal Growth Factor Receptor. EGFR is a 170-kDa transmembrane protein characterized by its ligand-dependent tyrosine kinase activity.204 Activation of EGFR results in increased tumor cell proliferation and angiogenesis and decreased apoptosis and is associated with invasiveness of the tumor. Ilschner and colleagues205 have described the transient appearance of K+ outward currents in murine microglial cells that were induced by EGF, confirming the presence of EGFR on microglia.206 Nolte et al.207 from the same group demonstrated the expression of EGFR on microglial cells in vitro and further showed a dose-dependent effect of EGF on microglial motility and chemotaxis. Interestingly, glioma cells, especially in GBM, also express EGFR,208,209 usually as a result of EGFR gene amplification.210,211 A recent study demonstrated that EGFR signaling in GBM is necessary for microglial stimulation of GBM invasion.40 This indicates the existence of an EGF–EGFR paracrine loop linking GBM cells and microglia.
VEGF signals via 2 tyrosine kinase receptors, VEGFR1 (Fms-like tyrosine kinase 1 [FLT-1]) and VEGFR-2 (kinase insert domain receptor [KDR]/fetal liver kinase 1 [FLK-1]).182 Both receptors are expressed on endothelial cells,212 while only VEGFR-1 is found on cells of the monocyte/macrophage lineage.213 Forstreuter et al.180 reported that both rat microglial cells and mouse BV-2 microglia cell lines express VEGFR-1, but not VEGFR-2. Using in vitro assays, Forstreuter and colleagues in the same study further demonstrated that VEGF increases the chemotaxis and proliferation of microglial cells. Thus, apart from CCR2, Met, and EGFR, VEGFR-1 may be another candidate receptor involved in microglia chemotaxis.
Receptors involved in tumor immunity.— Many cytokines and cytokine receptors are expressed by microglia in the immunosuppressive microenvironment of glioma, and the binding of the respective cytokines to their receptors plays a key role in tumor immunity. Chemokine receptors represent a subclass of cytokine receptors that are expressed on the surface of microglia. They have been observed to mediate an efficient cross talk between glioma-infiltrating microglia and glioma cells.
Chemokine (C-X3-C Motif) Ligand 1. CX3CL1 is one of the most highly expressed chemokines in the CNS. It can be expressed as a membrane-bound form mediating cell–cell adhesion or as a soluble form sustaining chemotaxis.214 Human glioma cells express both forms and, significantly, according to Sciume and colleagues,215 the tumor cells also express the cognate receptor for CXC3CL1, CX3C chemokine receptor 1 (CX3CR1), on their surface. These authors further reported that disruption of CX3CR1/CX3CL1 interaction by means of an anti-CX3CL1 neutralizing antibody enhances glioma cell invasion, indicating that CX3CL1 inhibits glioma invasion.215 In contrast, in a study on the expression and function of CX3CR1/CX3CL1 in human glioma, Held-Feindt et al.216 demonstrated that CX3CR1 (also termed RBS11 or V28) was exclusively expressed in glioma-infiltrating microglia/macrophages, whereas its ligand CX3CL1 was expressed solely in glioma cells. The latter results are in agreement with previous observations on the expression of CX3CR1 by microglial cells in a murine glioma model,217 as well as human glioma.218 In addition, Held-Feindt and colleagues found that glioma-derived CX3CL1 not only promotes recruitment of human glioma-infiltrating microglia/macrophages, but also enhances expression of MMP2, -9, and -14 in these cells. This finding is significant because the enhanced expression of MMPs might favor adhesion and migration not only of glioma-infiltrating microglia but also of glioma cells.11 Taken together, CXC3CL1 may act in an autocrine as well as paracrine fashion to promote the adhesion and chemotaxis of CX3CR1-expressing glioma and microglial cells during tumor growth.
Receptors involved in antigen presentation.— Antigen presentation is crucial for the generation of a specific anti-tumor response by the adaptive immune system. This process requires physical interaction between the T-cell receptor and immunogenic peptides presented via MHC class II molecules on the cytoplasmic membrane of antigen-presenting cells. A productive dialogue between microglia and T cells to result in T-cell proliferation requires a second signal, which is the simultaneous expression of co-stimulatory molecules, such as CD80 (B7-1) and CD86 (B7-2), on the surface of the antigen-presenting cells. The expression of MHC molecules has been described on microglia in both human and experimental glioma.219–221 However, the expression of MHC class II by microglia is reduced in high-grade glioma.221 Therefore, it appears that microglia in glioma are deficient to helper and cytotoxic T cells in a proper antigen-presentation capacity. Using a cytotoxicity assay based on fluorescence-activated cell sorting, Flugel and colleagues222 demonstrated that the ability of microglia was severely compromised to present C6 glioma antigen or stimulate C6-primed T cells. Furthermore, the expression of MHC class II and co-stimulatory B7 molecules in rodent glioma correlates directly with tumor immunogenicity and the extent of lymphocyte infiltration into tumors.223 Schartner and colleagues224 have reported downregulation of MHC class II and CD80 on the surface of microglia in glioma-bearing rats, which is consistent with a study that showed microglia isolated from human GBM exhibit downregulation of HLA-DR expression and suppression of CD80 induction by lipopolysaccharides.225 Similarly, there was a lack of expression of the co-stimulatory molecules CD86 and CD80 in glioma-infiltrating microglia.37 In contrast, the expression of MHC class II and B7 co-stimulatory molecules recovered after microglia were isolated from tumors and cultured for a short period of time in the absence of glioma cells.223 These studies suggest that antigen-presenting functions of microglia are torpedoed by glioma cells.
Receptors involved in phagocytosis and cytotoxicity.— Cytotoxic and phagocytic functions of microglia are considered critical for anti-glioma responses. These defense functions require microglia to upregulate the expression of complement (CR1, CR3, CR4) and Fc-gamma receptors (CD64, CD32, CD16). Both enhance phagocytic capacity by binding to complement components and immunoglobulin fragments, respectively.226,227 The expression of complement receptors, namely CR3 (CD11b/CD18), on the surface of microglia was found throughout glioma, and at an increased density along the tumor's periphery.228 However, no evidence of glioma killing by CR3+ microglia was seen.228 Furthermore, a significant positive correlation was found between the number of CR3+ cells and the proliferation rate of human glioma.229 Taken together, these studies suggest that glioma-infiltrating microglia are in an activated state, yet exhibit tumor-supportive rather than tumor-phagocytic functions.8
Activation of phagocytes, including microglia, is the most common function of Fc receptors. Microglial cells are potent effector cells in antibody-dependent cell-mediated cytotoxicity in vitro,35 which involves Fc receptor expression on the surface of microglia that stimulates microglial cells to release cytotoxic molecules that kill antibody-covered target cells.35 Morimura and colleagues229 have demonstrated abundant concomitant expression of Fc receptors (CD64, CD32, CD16) and CR3 on the surface of microglia in human glioma, which was found to be more intense in the area of tumor necrosis. Interestingly, Fc-gamma receptors 1 and 2 (CD64 and CD32) were detected mainly on microglia inside the tumor and to a much lower extent on microglia of peritumoral tissue, indicating that the expression of Fc-gamma receptors depends on the state of activation of microglia in glioma. These studies provide evidence that myeloid cells in human gliomas are equipped with the respective surface receptors for antibody- and complement-mediated cytotoxic actions. However, they are quite obviously not effective in real life.
Pattern Recognition Receptors. PRRs are proteins expressed by cells of the innate immune system, which allow the detection of pathogen-associated molecular patterns (PAMPs) associated with microbes or cellular stress. Toll-like receptors (TLRs) are the main family of PRRs that are necessary for the induction of an adaptive immune response to PAMPs or tumor cells through the activation and maturation of macrophages and dendritic cells.230–232 The interaction between TLRs and their specific PAMPs induces signaling by NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells), activation of the mitogen-activated protein kinase (MAPK) pathway, and consequently the secretion of pro-inflammatory cytokines, such as TNF-α, IFN, and IL-12, all of which can help to limit tumor growth.232 Microglia have been reported to be the predominant TLR-expressing cell type in the CNS,233 and microglia freshly isolated from human glioma tissue also express substantial levels of TLRs (TLR2, TLR3, and especially TLR4).37 This expression can be accompanied by expression of CD14 in glioma-infiltrating microglia.37 CD14 is otherwise expressed mainly by macrophages and acts as a co-receptor of TLR4 in the detection of bacterial lipopolysaccharides.234 Yet, these TLR-expressing glioma-infiltrating microglia do not express cytokines that would be reflective of tumoricidal activity.37 In contrast, microglia have been found to facilitate glioma cell invasion via TLRs.10 Glioma-released factors stimulate the expression and activity of MT1-MMP via TLRs and their downstream molecules MyD88 and p38 MAPK in microglia, and MT1-MMP–expressing microglia in turn promote glioma expansion by degrading the extracellular matrix.10 Independently, the high-mobility group box 1 protein (HMGB1), an alarmin protein released from dying glioma cells, has recently been identified as an endogenous ligand for TLR2.235 Curtin et al.235 further demonstrated that dying glioma-derived HMGB1 acts as a TLR2 agonist, induces endogenous TLR2 signaling, and initiates a CD8+ T cell-dependent anti-GBM immune response. Furthermore, a recent in vitro study reported that human microglia isolated from brain tumors exerted tumor-suppressing activities if they were pretreated with polyinosinic-polycytidylic acid.236 These studies suggest that the tumor-supporting function of glioma-infiltrating microglia could be overridden by tumor-suppressing activities if they were activated via TLR agonists.
Scavenger Receptors. These receptors are thought to participate in the removal of many foreign substances and waste materials in the living body, and they also play a role in modulating phagocytic activity of microglia. Macrophage scavenger receptor 1 (MSR1 or CD204), a class A scavenger receptor, is a protein encoded by the MSR1 gene in human. CD204 was found to be expressed in high-grade gliomas, and tumor culture supernatants from glioma cell line T98G induced its upregulation in human macrophages in vitro.143 Increased MSR1 expression was also seen in ovarian cancers,237 and targeted depletion of CD204-positive macrophages blocked ovarian tumor progression and ascites accumulation, as demonstrated in a murine model of ovarian cancer.238 It is postulated that CD204 signaling in tumor-associated microglia cells may negatively regulate microglia activation. Therefore, CD204 may be one of the more promising targets for the immunotherapy of some cancers, including ovarian cancer and glioma.
Receptors potentially exerting an anti-tumorigenic function.— Adenosine is an endogenous ubiquitous biological mediator that regulates many physiological processes. The extracellular concentration of adenosine increases in response to cellular stress and tissue damage. Adenosine signals through 4 known adenosine receptor subtypes: A1AR, A2AAR, A2BAR, and A3AR, each featuring a different tissue distribution, ligand affinity, and signal transduction mechanism. A1AR is found throughout the brain239 and is expressed mainly on microglia/macrophages as well as neurons.240 In glioma, expression of A1AR is high in glioma-infiltrating microglia, whereas its expression in tumor cells is minimal in both human GBM and rodent glioma.241 Synowitz et al.241 showed that A1AR-mediated inhibition of glioma growth depends on the presence of microglial cells because the size of the glioma was increased in microglia-depleted brain even when A1AR was activated by the specific agonist. Furthermore, when comparing A1AR−/− mice with their A1AR+/+ littermate controls, the tumor volume was also significantly larger, as expected, and simultaneously the number of glioma-infiltrating microglia was significantly higher in A1AR−/− mice than in controls.241 The same study further demonstrated that activation of A1AR markedly decreases the activity of glioma-induced MMP-2 in microglia, suggesting that the inhibitory effect of adenosine on glioma invasion is at least in part due to decreased extracellular protease activity. This finding complements results of an earlier study in a model of multiple sclerosis, which revealed that A1AR activation on microglia/macrophages interferes with the production of MMPs such as MMP-12 and of cytokines such as IL-1β.242 In summary, A1AR inhibition of glioma growth represents an interesting novel mechanism whereby microglia can modulate the biological behavior of a glioma.
Molecules Derived from Glioma-Infiltrating Microglia
Molecules contributing to the immune-suppressive milieu in glioma.—Interleukin 10. Originally termed cytokine synthesis inhibitory factor (CSIF), IL-10 is a 17- to 21-kDa cytokine with a broad spectrum of immunosuppressive activities, including inhibition of antigen presentation, ie., antigen-specific T-cell proliferation,243 and of inflammatory cytokine synthesis by infiltrating monocytes/macrophages.244 Increased IL-10 mRNA expression has been reported in human glioblastomas in vivo compared with low-grade tumors, but only weak expression was seen in human glioblastoma cell lines.245 Using in situ hybridization analyses of native tissue samples of glioblastomas, Huettner et al.20 demonstrated that both microglia and astroglia might contribute to IL-10 gene expression in vivo. Subsequent studies using primary cultures from human glioma specimens indicated that the IL-10 gene and its protein product are expressed by glioma-infiltrating microglia, and to a much lesser extent by the glioma cells.21 IL-10 secreted by glioma-infiltrating microglia in the immunosuppressive microenvironment of glioma not only promotes glioma cell proliferation, but also enhances the ability of glioma cells to migrate in vitro.20 Thus, glioma-infiltrating microglia contribute to the maintenance of an immunosuppressive microenvironment in glioma by producing and secreting immunosuppressive IL-10.7,43 The expression of IL-10 in glioma-infiltrating microglia is regulated by upstream stimulating factor 1 (USF-1)246 and STAT3.247 Inhibition of USF-1 derived from glioma-infiltrating microglia results in upregulation of IL-10 in the glioma-infiltrating microglia,246 as does increased STAT3 activity in the murine microglia cell line N9.247
In addition to its expression by glioma cells105 (see earlier section), FasL has been found to be expressed by glioma-infiltrating microglia43,106 and activated T cells in GBM.248 Using flow cytometry analysis, Badie and colleagues43,106 showed that glioma infiltrating microglia are the major cellular source of FasL in murine glioma, and nearly every microglia cell expressed FasL on its membrane. This study thus suggests that microglia, and not the T lymphocytes or tumor cells, are responsible for the increased expression of FasL in glioma. Considering the fact that microglia can induce apoptosis of activated T cells in vitro249 and that apoptotic T cells in GBM express Fas,250 it is postulated that glioma-infiltrating microglia may contribute to the local immunosuppressive microenvironment in glioma by mediating T-cell apoptosis via the Fas–FasL pathway. This is further evidenced by the significant increase in the number of tumor-infiltrated lymphocytes after an injection of anti-FasL neutralizing antibody into intracranial murine G26 glioma.43 However, in contrast to Badie's findings in murine glioma, Hussain et al.37 determined that human glioma-infiltrating microglia did not express FasL, or expressed it at only very low levels, indicating that apoptosis mediated by Fas–microglial FasL may not be the predominant mechanism of immune evasion in human tumors. Rat glioma cell–derived FasL is also able to mediate the death of T lymphocytes when T cells are co-cultured with glioma cells.105 Furthermore, Jansen et al.105 demonstrated that downregulation of FasL expression in glioma cells following the application of short hairpin RNA can enhance T-cell infiltration in glioma and thus inhibit tumor growth. Moreover, T-cell apoptosis in GBM was even found to be mediated by ligation of Fas with FasL on the same T cell.248 In spite of the uncertainty surrounding the main cellular source of FasL in glioma, Fas–FasL interaction is likely to contribute to the immunosuppressive microenvironment of glioma. Further investigations are therefore needed to elucidate the main cellular source of FasL in glioma in vivo.
Molecules contributing to glioma angiogenesis.— Neo-angiogenesis is very conspicuous in glioblastoma, and has been widely recognized as a key process in the malignant progression of glioma.251,252 The process is thought to be driven largely by VEGF, which is a signaling protein functioning via its 2 receptors, VEGFR-1 and VEGFR-2. These 2 receptors are co-expressed on endothelial cells. Consequently, it is a widely held view that VEGF interacts with its receptors in vascular endothelium in a paracrine fashion and that it plays a major role in the regulation of tumor neo-angiogenesis.177,253,254 In addition to glioma cells, microglial cells also secrete VEGF.41,42 It is therefore speculated that microglial cells play a role in tumor progression by supporting angiogenesis as well.19,255
Molecules contributing to glioma growth and invasion.— TGF-β1 protein is produced by microglia under certain pathological conditions, such as experimental allergic neuritis,256 and in traumatically injured brain.257 TGF-β signals through TGF-β type II receptor (TβRII), which recruits and activates TGF-β type I receptor (TβRI).18 Using in situ hybridization, Kiefer and colleagues258 revealed that both activated microglial cells and glioma cells produce TGF-β1 in the vicinity of glioma in vivo. A recent study by Wesolowska et al.18 confirmed that cultured microglial cells secrete TGF-β1, and their exposure to glioma cells strongly enhances secretion of TGF-β1. In addition, the study also demonstrated that the invasion-promoting activity of microglia was lost in glioma cells exhibiting downregulated TβRII, indicating that the promotion of glioma invasion of microglia is mediated by TGF-β.18 Furthermore, expression of TGF-β may upregulate the expression of its receptors, TβRI and TβRII, both of which show increased expression in gliomas compared with normal tissues, and their antagonists are able to inhibit human glioma cell line proliferation and motility.69,259,260 Thus, additional paracrine loops seem to exist that skew the microglial cell–glioma relationship in favor of the tumor, in that glioma-derived TGF-β exerts immunosuppressive effects in and around the tumor that render the glioma-infiltrating microglia ineffective, whereas microglia-derived TGF-β promotes tumor growth and invasion by stimulating the upregulation of its cognate receptors, TβRI and TβRII, on glioma cells.
Degradation of the extracellular matrix by proteolytic enzymes, such as membrane-bound and secreted MMPs, is a key mechanism utilized by invading glioma.39 MMP-2, also known as gelatinase A, was found to play an especially important role in this process. Expression of the MMP-2 gene in human glioma tissues is upregulated when compared with normal brain and is dramatically increased in GBM.261–263 Immunostaining of human glioma biopsies confirmed upregulation of MMP-2 and identified tumor cells as the cellular source of MMP-2.262 The activity of this enzyme has been directly correlated to glioma invasiveness and the survival rate of tumor-bearing mice.11,264 Both microglial cells and glioma cells produce MMP-2, as demonstrated both in vitro11,263 and in human glioma tissue.265 Notably, MMP-2 released by glioma cells is in an inactive soluble form (pro-MMP-2), is found especially at the actively invasive tumor zone, and needs to be cleaved to become activated.265 The extracellular activator of MMP-2, MT1-MMP, is required for this cleavage.39,266 However, glioma cells themselves cannot produce MT1-MMP but have to rely on microglial cells, which are the major, though not exclusive, cellular source of MT1-MMP in glioma.10 In normal brain, MT1-MMP expression in microglia is low and detectable only in white matter.266 In the context of glioma, MT1-MMP expression has been found to be upregulated in human, mouse, and rat glioma-infiltrating microglia, and its immunoreactivity was particularly pronounced when the microglia were in close contact with glioma cells.10 A recent in vitro study demonstrated that attenuating microglial MT1-MMP expression using minocycline reduces glioma growth and invasion.267 Moreover, deletion of the TLR adapter protein, MyD88, prevented overexpression of MT1-MMP, which had been induced by glioma-conditioned medium (GCM) in microglia, suggesting that the expression of MT1-MMP in glioma is regulated by TLR signaling.10 Furthermore, TLR-mediated MT1-MMP expression was dependent on p38 MAPK, which resides downstream of MyD88.10 Among the major candidate mechanisms underlying glioma invasion facilitated by microglia appear to be the production of heat-shock proteins, HMGB1, and hyluronan.268–270 These glioma-derived molecules can activate microglial TLR signaling (via MyD88) and its downstream p38 MAPK pathway and thereby trigger the upregulation of MT1-MMP in microglia. MT1-MMP expressed in microglia is then thought to convert glioma-derived pro-MMP-2 into MMP-2. MMP-2 in turn promotes glioma cell invasion and tumor expansion.10 Thus, microglia can serve as activators for the degradation of extracellular matrix, which is key for glioma invasion.
Connective Tissue Growth Factor. CTGF, also known as CCN2, is a cysteine-rich, matrix-associated, heparin-binding protein encoded by an immediate early gene, which is associated with drug resistance in GBM.271 CTGF has been found to be implicated in cancer progression272,273 when binding the cell surface protein beta 1 integrin (ITGB1),274 tyrosine kinase receptor type A (TrkA), and co-receptor p75NTR.275 Edwards and colleagues276 have shown that a CTGF-rich microenvironment increases the invasiveness of malignant gliomas. Halliday and Holland277 have pointed out that parallels exist between brain tumors and brain injury with regard to CTGF expression and emphasize that CTGF is expressed by microglia under pathological conditions. Thus, microglia come into the spotlight again because expression levels of CTGF are prognostic of tumor progression as well as survival of patients with glioma.278
Interleukin-6. IL-6 is a protein of 185 amino acids encoded by the IL6 gene in humans. It has been reported that GBMs display a significantly higher level of IL-6 expression than normal brain tissue.279 In addition, IL-6 expression has been correlated with glioma growth invasiveness.280,281 Glioma cells as well as microglia have been reported to secrete IL-6,47,162,282,283 and both express IL-6 receptors.94,284 Glioma-derived IL-6, working together with other tumor-secreted factors, such as TGFβ and PGE2, polarize glioma-infiltrating microglia toward the M2 phenotype,47,282 and microglia-derived IL-6 has been reported to induce glioma cell migration and invasiveness.162,283 Notably, IL-6 has been confirmed as a growth factor for glioma stem cells.285 The mechanism by which IL-6 promotes the invasiveness of glioma cells is unclear. IL-6 has been found to increase the production of MMP-2 by glioma cells, suggesting that it may exert its tumor-promoting role via MMP-2.281 Furthermore, it has been hypothesized that activated microglial cells synthesize IL-6 following NF-κB activation, which in turn may stimulate transcription factors STAT3 and NF-κB in glioma cells that initiate specific pathways of glioma progression such as angiogenesis, migration, and apoptosis inhibition.94,286
STAT3 activation of glioma-infiltrating microglia.— STAT3 activity has been shown to be higher in glioma-infiltrating microglia compared with microglia from normal animals.247 Activation of the STAT3 pathway was found to increase expression of IL-10 and IL-6 in glioma-infiltrating microglia.247 STAT3 blockage stimulates the immunological activation of glioma-infiltrating microglia, as evidenced by their increased expression of co-stimulatory molecules CD80 and CD86.119 Furthermore, in vivo STAT3 inhibition in murine glioma-infiltrating microglia was shown to reduce expression of immunosuppressive cytokines, such as IL-10 and IL-6, while it stimulates production of pro-inflammatory TNF-α.247
Activation of STAT3 in glioma-infiltrating microglia can be induced by means of several immunosuppressive factors that are highly expressed in gliomas, such as IL-10, and VEGF.287 Interestingly, these soluble factors can also activate STAT3 in hematopoietic cells in the tumor microenvironment.118 In addition, Zhang et al.288 recently reported that S100B, which is constitutively expressed by glioma cells, might be yet another factor that induces the activation of STAT3 in glioma-infiltrating microglia through interaction with RAGE, the receptor, for advanced glycation end product, in glioma-infiltrating microglia both in vitro and in vivo. Therefore, it appears that glioma-derived factors polarize glioma-infiltrating microglia toward the immunosuppressive M2 phenotype through STAT3 activation, which upregulates expression of immunosuppressive factors by glioma-infiltrating microglia while limiting their expression of co-stimulatory molecules.
Conclusions and Future Directions
GBM is the most common and aggressive primary tumor of the brain and has one of the worst 5-year survival rates among all human cancers.289 Microglial cells and macrophages have been found to populate malignant glioma in large numbers, contributing one third or more to their actual tumor mass. Representing the immune effector cells of the CNS, microglia have been found to phagocytose glioma cells in vitro but their behavior is quite different in vivo, where they act more like nodding sycophants of tumor cells. As illustrated in Fig. 2, experimental studies have demonstrated that microglia express a variety of receptors on their surface, which are able to receive signals from glioma. In response, microglia under the influence of glioma release several classes of molecules that foster glioma growth and progression. There is clearly more than one self-amplifying autocrine and paracrine loop, which can spiral out of control and the tumor progresses. However, we now also know of a number of potential molecular targets in tumor-associated microglia that could be used to alter the fatal course of glioma. A significant portion of the microglia found in glioma appear to be derived from bone marrow precursors, and it may become possible to enable these bone marrow–derived microglia to track down glioma cells using the same genes that stem cells employ for sniffing out glioma in the brain.290 Consequently, the great attraction of glioma for microglia and the principal ability of microglia precursors to cross the BBB make them attractive targets for the development of a truly novel therapeutic approach. Emerging technologies291 may allow us to genetically enhance (reprogram) microglia65 so that they are protected and able to turn against the glioma.
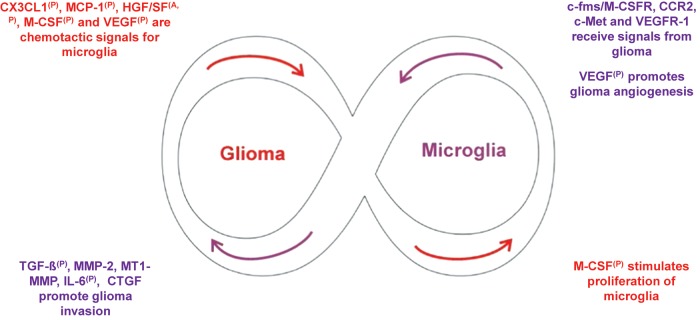
Fig. 2.
Glioma-microglia synergies drive a self-amplifying process that spirals out of control. Glioma and microglial cells have a symbiotic relationship that becomes highly skewed in favor of the glioma. The immunosuppressive microenvironment created by molecules such as TGF-β, FasL, and IL-10 polarizes glioma-infiltrating microglia toward the M2 phenotype (cf. Fig. 1). Glioma (red) produces chemotactic factors, such as MCP-1, resulting in the recruitment of microglia. Glioma further promotes the proliferation of microglia. In turn, microglia (purple) promote glioma angiogenesis as well as glioma cell invasion. The cross talk between glioma and microglia is governed by multiple paracrine loops formed by glioma and microglia released molecules and their receptors, as indicated by superscript (P) in the figure. In addition, some of the molecules act in an autocrine fashion, as indicated by the superscript (A) and regulate either glioma or microglia behavior.
Funding
Support came from The Brain and Mind Research Institute.
Acknowledgments
We thank The Brain and Mind Research Institute for funding support.
Conflict of interest statement. None declared.
References
- 1.Holland EC. Glioblastoma multiforme: the terminator. Proc Natl Acad Sci USA. 2000;97(12):6242–6244. doi: 10.1073/pnas.97.12.6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takakura K, Abe H, Tanaka R, et al. Effects of ACNU and radiotherapy on malignant glioma. J Neurosurg. 1986;64(1):53–57. doi: 10.3171/jns.1986.64.1.0053. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro WR, Green SB, Burger PC, et al. A randomized comparison of intra-arterial versus intravenous BCNU, with or without intravenous 5-fluorouracil, for newly diagnosed patients with malignant glioma. J Neurosurg. 1992;76(5):772–781. doi: 10.3171/jns.1992.76.5.0772. [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5.Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60(3):166–193. doi: 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imperato JP, Paleologos NA, Vick NA. Effects of treatment on long-term survivors with malignant astrocytomas. Ann Neurol. 1990;28(6):818–822. doi: 10.1002/ana.410280614. [DOI] [PubMed] [Google Scholar]
- 7.Graeber MB, Scheithauer BW, Kreutzberg GW. Microglia in brain tumors. Glia. 2002;40(2):252–259. doi: 10.1002/glia.10147. [DOI] [PubMed] [Google Scholar]
- 8.Badie B, Schartner J. Role of microglia in glioma biology. Microsc Res Tech. 2001;54(2):106–113. doi: 10.1002/jemt.1125. [DOI] [PubMed] [Google Scholar]
- 9.Ribot E, Bouzier-Sore A-K, Bouchaud V, et al. Microglia used as vehicles for both inducible thymidine kinase gene therapy and MRI contrast agents for glioma therapy. Cancer Gene Ther. 2007;14(8):724–737. doi: 10.1038/sj.cgt.7701060. [DOI] [PubMed] [Google Scholar]
- 10.Markovic DS, Vinnakota K, Chirasani S, et al. Gliomas induce and exploit microglial MT1-MMP expression for tumor expansion. Proc Natl Acad Sci USA. 2009;106(30):12530–12535. doi: 10.1073/pnas.0804273106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grasbon-Frodl EMFA, Wolz P, Klinkert WEF, Kreutzberg GW, Graeber MB. Untersuchungen zur Funktion von Mikroglia in Hirntumoren: Förderung des Wachstums von C6-Gliomzellen in vitro. Jahrestagung der Neuroonkologischen Arbeitsgemeinschaft der Deutschen Gesellschaft für Neurochirurgie in Dresden. 1998 6–7 November 1998. [Google Scholar]
- 12.Markovic DS, Glass R, Synowitz M, Rooijen Nv, Kettenmann H. Microglia stimulate the invasiveness of glioma cells by increasing the activity of metalloprotease-2. J Neuropathol Exp Neurol. 2005;64(9):754–762. doi: 10.1097/01.jnen.0000178445.33972.a9. [DOI] [PubMed] [Google Scholar]
- 13.Sliwa M, Markovic D, Gabrusiewicz K, et al. The invasion promoting effect of microglia on glioblastoma cells is inhibited by cyclosporin A. Brain. 2007;130(Pt 2):476–489. doi: 10.1093/brain/awl263. [DOI] [PubMed] [Google Scholar]
- 14.Watters JJ, Schartner JM, Badie B. Microglia function in brain tumors. J Neurosci Res. 2005;81(3):447–455. doi: 10.1002/jnr.20485. [DOI] [PubMed] [Google Scholar]
- 15.Kerber M, Reiss Y, Wickersheim A, et al. Flt-1 signaling in macrophages promotes glioma growth in vivo. Cancer Res. 2008;68(18):7342–7351. doi: 10.1158/0008-5472.CAN-07-6241. [DOI] [PubMed] [Google Scholar]
- 16.Zhai H, Heppner FL, Tsirka SE. Microglia/macrophages promote glioma progression. Glia. 2011;59(3):472–485. doi: 10.1002/glia.21117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daginakatte GC, Gutmann DH. Neurofibromatosis-1 (Nf1) heterozygous brain microglia elaborate paracrine factors that promote Nf1-deficient astrocyte and glioma growth. Hum Mol Genet. 2007;16(9):1098–1112. doi: 10.1093/hmg/ddm059. [DOI] [PubMed] [Google Scholar]
- 18.Wesolowska A, Kwiatkowska A, Slomnicki L, et al. Microglia-derived TGF-beta as an important regulator of glioblastoma invasion—an inhibition of TGF-beta-dependent effects by shRNA against human TGF-beta type II receptor. Oncogene. 2008;27(7):918–930. doi: 10.1038/sj.onc.1210683. [DOI] [PubMed] [Google Scholar]
- 19.Kaur G, Han SJ, Yang I, Crane C. Microglia and central nervous system immunity. Neurosurg Clin N Am. 2010;21(1):43–51. doi: 10.1016/j.nec.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Huettner C, Czub S, Kerkau S, Roggendorf W, Tonn JC. Interleukin 10 is expressed in human gliomas in vivo and increases glioma cell proliferation and motility in vitro. Anticancer Res. 1997;17(5):3217–3224. [PubMed] [Google Scholar]
- 21.Wagner S, Czub S, Greif M, et al. Microglial/macrophage expression of interleukin 10 in human glioblastomas. Int J Cancer. 1999;82(1):12–16. doi: 10.1002/(sici)1097-0215(19990702)82:1<12::aid-ijc3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 22.Rio-Hortega P, del AFd. Sobre la fagocytosis en los tumores y en otros procesos patologicos. Arch Cardiol Hematol. 1921;2:161–220. [Google Scholar]
- 23.Penfield W. Microglia and the process of phagocytosis in gliomas. Am J Pathol. 1925;1:77–97. [PMC free article] [PubMed] [Google Scholar]
- 24.Morantz RA, Wood GW, Foster M, Clark M, Gollahon K. Macrophages in experimental and human brain tumors. Part 1: Studies of the macrophage content of experimental rat brain tumors of varying immunogenicity. J Neurosurg. 1979;50(3):298–304. doi: 10.3171/jns.1979.50.3.0298. [DOI] [PubMed] [Google Scholar]
- 25.Morantz RA, Wood GW, Foster M, Clark M, Gollahon K. Macrophages in experimental and human brain tumors. Part 2: studies of the macrophage content of human brain tumors. J Neurosurg. 1979;50(3):305–311. doi: 10.3171/jns.1979.50.3.0305. [DOI] [PubMed] [Google Scholar]
- 26.Shinonaga M, Chang CC, Suzuki N, Sato M, Kuwabara T. Immunohistological evaluation of macrophage infiltrates in brain tumors. Correlation with peritumoral edema. J Neurosurg. 1988;68(2):259–265. doi: 10.3171/jns.1988.68.2.0259. [DOI] [PubMed] [Google Scholar]
- 27.Wierzba-Bobrowicz T, Kuchna I, Matyja E. Reaction of microglial cells in human astrocytomas (preliminary report) Folia Neuropathol. 1994;32(4):251–252. [PubMed] [Google Scholar]
- 28.Roggendorf W, Strupp S, Paulus W. Distribution and characterization of microglia/macrophages in human brain tumors. Acta Neuropathol. 1996;92(3):288–293. doi: 10.1007/s004010050520. [DOI] [PubMed] [Google Scholar]
- 29.Badie B, Schartner JM. Flow cytometric characterization of tumor-associated macrophages in experimental gliomas. Neurosurgery. 2000;46(4):957–961. doi: 10.1097/00006123-200004000-00035. discussion 961–2. [DOI] [PubMed] [Google Scholar]
- 30.Aloisi F. Immune function of microglia. Glia. 2001;36(2):165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- 31.Beyer M, Gimsa U, Eyupoglu IY, Hailer NP, Nitsch R. Phagocytosis of neuronal or glial debris by microglial cells: upregulation of MHC class II expression and multinuclear giant cell formation in vitro. Glia. 2000;31(3):262–266. doi: 10.1002/1098-1136(200009)31:3<262::aid-glia70>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 32.Flaris NA, Densmore TL, Molleston MC, Hickey WF. Characterization of microglia and macrophages in the central nervous system of rats: definition of the differential expression of molecules using standard and novel monoclonal antibodies in normal CNS and in four models of parenchymal reaction. Glia. 1993;7(1):34–40. doi: 10.1002/glia.440070108. [DOI] [PubMed] [Google Scholar]
- 33.Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40(2):140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- 34.Frei K, Siepl C, Groscurth P, Bodmer S, Schwerdel C, Fontana A. Antigen presentation and tumor cytotoxicity by interferon-gamma-treated microglial cells. Eur J Immunol. 1987;17(9):1271–1278. doi: 10.1002/eji.1830170909. [DOI] [PubMed] [Google Scholar]
- 35.Sutter A, Hekmat A, Luckenbach GA. Antibody-mediated tumor cytotoxicity of microglia. Pathobiology. 1991;59(4):254–258. doi: 10.1159/000163657. [DOI] [PubMed] [Google Scholar]
- 36.Voisin P, Bouchaud V, Merle M, et al. Microglia in close vicinity of glioma cells: correlation between phenotype and metabolic alterations. Front Neuroenergetics. 2010;2:131. doi: 10.3389/fnene.2010.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro Oncol. 2006;8(3):261–279. doi: 10.1215/15228517-2006-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanisch U-K, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10(11):1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 39.Rao JS. Molecular mechanisms of glioma invasiveness: the role of proteases. Nat Rev Cancer. 2003;3(7):489–501. doi: 10.1038/nrc1121. [DOI] [PubMed] [Google Scholar]
- 40.Coniglio SJ, Eugenin E, Dobrenis K, et al. Microglial stimulation of glioblastoma invasion involves EGFR and CSF-1R signaling. Mol Med. 2012 doi: 10.2119/molmed.2011.00217. doi:10.2119/molmed.2011.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lafuente JV, Adan B, Alkiza K, Garibi JM, Rossi M, Cruz-Sanchez FF. Expression of vascular endothelial growth factor (VEGF) and platelet-derived growth factor receptor-beta (PDGFR-beta) in human gliomas. J Mol Neurosci. 1999;13(1–2):177–185. doi: 10.1385/JMN:13:1-2:177. [DOI] [PubMed] [Google Scholar]
- 42.Tsai JC, Goldman CK, Gillespie GY. Vascular endothelial growth factor in human glioma cell lines: induced secretion by EGF, PDGF-BB, and bFGF. J Neurosurg. 1995;82(5):864–873. doi: 10.3171/jns.1995.82.5.0864. [DOI] [PubMed] [Google Scholar]
- 43.Badie B, Schartner J, Prabakaran S, Paul J, Vorpahl J. Expression of Fas ligand by microglia: possible role in glioma immune evasion. J Neuroimmunol. 2001;120(1–2):19–24. doi: 10.1016/s0165-5728(01)00361-7. [DOI] [PubMed] [Google Scholar]
- 44.Okada M, Saio M, Kito Y, et al. Tumor-associated macrophage/microglia infiltration in human gliomas is correlated with MCP-3, but not MCP-1. Int J Oncol. 2009;34(6):1621–1627. doi: 10.3892/ijo_00000292. [DOI] [PubMed] [Google Scholar]
- 45.Prat E, Baron P, Meda L, et al. The human astrocytoma cell line U373MG produces monocyte chemotactic protein (MCP)-1 upon stimulation with beta-amyloid protein. Neurosci Lett. 2000;283(3):177–180. doi: 10.1016/s0304-3940(00)00966-6. [DOI] [PubMed] [Google Scholar]
- 46.Tweardy DJ, Mott PL, Glazer EW. Monokine modulation of human astroglial cell production of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor. I. Effects of IL-1 alpha and IL-beta. J Immunol. 1990;144(6):2233–2241. [PubMed] [Google Scholar]
- 47.Ghosh A, Chaudhuri S. Microglial action in glioma: a boon turns bane. Immunol Lett. 2010;131(1):3–9. doi: 10.1016/j.imlet.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 48.Ginhoux F, Greter M, Leboeuf M, et al. Fate Mapping Analysis Reveals That Adult Microglia Derive from Primitive Macrophage. Sci Express. 2010;330(6005):841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin H, Lee E, Hestir K, et al. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science. 2008;320(5877):807–811. doi: 10.1126/science.1154370. [DOI] [PubMed] [Google Scholar]
- 50.Davoust N, Vuaillat C, Cavillon G, et al. Bone marrow CD34+/B220+ progenitors target the inflamed brain and display in vitro differentiation potential toward microglia. FASEB J. 2006;20(12):2081–2092. doi: 10.1096/fj.05-5593com. [DOI] [PubMed] [Google Scholar]
- 51.Ladeby R, Wirenfeldt M, Dalmau I, et al. Proliferating resident microglia express the stem cell antigen CD34 in response to acute neural injury. Glia. 2005;50(2):121–131. doi: 10.1002/glia.20159. [DOI] [PubMed] [Google Scholar]
- 52.Clausen BH, Lambertsen KL, Babcock AA, Holm TH, Dagnaes-Hansen F, Finsen B. Interleukin-1beta and tumor necrosis factor-alpha are expressed by different subsets of microglia and macrophages after ischemic stroke in mice. J Neuroinflammation. 2008;5:46. doi: 10.1186/1742-2094-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lambertsen KL, Clausen BH, Babcock AA, et al. Microglia protect neurons against ischemia by synthesis of tumor necrosis factor. J Neurosci. 2009;29(5):1319–1330. doi: 10.1523/JNEUROSCI.5505-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Priller J, Flugel A, Wehner T, et al. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat Med. 2001;7(12):1356–1361. doi: 10.1038/nm1201-1356. [DOI] [PubMed] [Google Scholar]
- 55.Flugel A, Bradl M, Kreutzberg GW, Graeber MB. Transformation of donor-derived bone marrow precursors into host microglia during autoimmune CNS inflammation and during the retrograde response to axotomy. J Neurosci Res. 2001;66(1):74–82. doi: 10.1002/jnr.1198. [DOI] [PubMed] [Google Scholar]
- 56.Rodriguez M, Alvarez-Erviti L, Blesa FJ, et al. Bone-marrow-derived cell differentiation into microglia: a study in a progressive mouse model of Parkinson's disease. Neurobiol Dis. 2007;28(3):316–325. doi: 10.1016/j.nbd.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 57.Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer's disease. Neuron. 2006;49(4):489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 58.Cartier N, Hacein-Bey-Abina S, Bartholomae CC, et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326(5954):818–823. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- 59.Mildner A, Schmidt H, Nitsche M, et al. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10(12):1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- 60.Prinz M, Mildner A. Microglia in the CNS: immigrants from another world. Glia. 2011;59(2):177–187. doi: 10.1002/glia.21104. [DOI] [PubMed] [Google Scholar]
- 61.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10(12):1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 62.AlShakweer W, Alwelaie Y, Mankung AM, Graeber MB. Bone marrow-derived microglia in pilocytic astrocytoma. Front Biosci (Elite Ed) 2011;3:371–379. doi: 10.2741/e252. [DOI] [PubMed] [Google Scholar]
- 63.Gabrusiewicz K, Ellert-Miklaszewska A, Lipko M, Sielska M, Frankowska M, Kaminska B. Characteristics of the alternative phenotype of microglia/macrophages and its modulation in experimental gliomas. PLoS One. 2011;6(8):e23902. doi: 10.1371/journal.pone.0023902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Durafourt BA, Moore CS, Zammit DA, et al. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia. 2012;60(5):717–727. doi: 10.1002/glia.22298. [DOI] [PubMed] [Google Scholar]
- 65.Graeber MB, Fluegel A, Moran LB, Scheithauer BW. Immune biology of microglia in gliomas. In: Hagel C, Stavrou DK, editors. Immnue Biology of Brain Tumours. Muenchen/Orlando: Dustri-Verlag Dr. Karl Feistle; 2010. pp. 69–80. ISBN 978–3–87185–401–9. [Google Scholar]
- 66.Graeber MB. Changing face of microglia. Science. 2010;330(6005):783–788. doi: 10.1126/science.1190929. [DOI] [PubMed] [Google Scholar]
- 67.Platten M, Wick W, Wild-Bode C, Aulwurm S, Dichgans J, Weller M. Transforming growth factors beta(1) (TGF-beta(1)) and TGF-beta(2) promote glioma cell migration via Up-regulation of alpha(V)beta(3) integrin expression. Biochem Biophys Res Commun. 2000;268(2):607–611. doi: 10.1006/bbrc.2000.2176. [DOI] [PubMed] [Google Scholar]
- 68.Constam DB, Philipp J, Malipiero UV, ten Dijke P, Schachner M, Fontana A. Differential expression of transforming growth factor-beta 1, -beta 2, and -beta 3 by glioblastoma cells, astrocytes, and microglia. J Immunol. 1992;148(5):1404–1410. [PubMed] [Google Scholar]
- 69.Kjellman C, Olofsson SP, Hansson O, et al. Expression of TGF-beta isoforms, TGF-beta receptors, and SMAD molecules at different stages of human glioma. Int J Cancer. 2000;89(3):251–258. doi: 10.1002/1097-0215(20000520)89:3<251::aid-ijc7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 70.Samuels V, Barrett JM, Bockman S, Pantazis CG, Allen MB., Jr. Immunocytochemical study of transforming growth factor expression in benign and malignant gliomas. Am J Pathol. 1989;134(4):894–902. [PMC free article] [PubMed] [Google Scholar]
- 71.Bodmer S, Strommer K, Frei K, et al. Immunosuppression and transforming growth factor-beta in glioblastoma. Preferential production of transforming growth factor-beta 2. J Immunol. 1989;143(10):3222–3229. [PubMed] [Google Scholar]
- 72.Merzak A, McCrea S, Koocheckpour S, Pilkington GJ. Control of human glioma cell growth, migration and invasion in vitro by transforming growth factor beta 1. Br J Cancer. 1994;70(2):199–203. doi: 10.1038/bjc.1994.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Espevik T, Figari IS, Shalaby MR, et al. Inhibition of cytokine production by cyclosporin A and transforming growth factor beta. J Exp Med. 1987;166(2):571–576. doi: 10.1084/jem.166.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ranges GE, Figari IS, Espevik T, Palladino MA., Jr. Inhibition of cytotoxic T cell development by transforming growth factor beta and reversal by recombinant tumor necrosis factor alpha. J Exp Med. 1987;166(4):991–998. doi: 10.1084/jem.166.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tsunawaki S, Sporn M, Ding A, Nathan C. Deactivation of macrophages by transforming growth factor-beta. Nature. 1988;334(6179):260–262. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]
- 76.Weller M, Fontana A. The failure of current immunotherapy for malignant glioma. Tumor-derived TGF-beta, T-cell apoptosis, and the immune privilege of the brain. Brain Res Brain Res Rev. 1995;21(2):128–151. doi: 10.1016/0165-0173(95)00010-0. [DOI] [PubMed] [Google Scholar]
- 77.Black KL, Chen K, Becker DP, Merrill JE. Inflammatory leukocytes associated with increased immunosuppression by glioblastoma. J Neurosurg. 1992;77(1):120–126. doi: 10.3171/jns.1992.77.1.0120. [DOI] [PubMed] [Google Scholar]
- 78.Suzumura A, Sawada M, Yamamoto H, Marunouchi T. Transforming growth factor-beta suppresses activation and proliferation of microglia in vitro. J Immunol. 1993;151(4):2150–2158. [PubMed] [Google Scholar]
- 79.Wu A, Wei J, Kong LY, et al. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro Oncol. 2010;12(11):1113–1125. doi: 10.1093/neuonc/noq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Crane CA, Han SJ, Barry JJ, Ahn BJ, Lanier LL, Parsa AT. TGF-beta downregulates the activating receptor NKG2D on NK cells and CD8+ T cells in glioma patients. Neuro-Oncol. 2010;12(1):7–13. doi: 10.1093/neuonc/nop009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baumann F, Leukel P, Doerfelt A, et al. Lactate promotes glioma migration by TGF-beta2-dependent regulation of matrix metalloproteinase-2. Neuro-Oncol. 2009;11(4):368–380. doi: 10.1215/15228517-2008-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nakao A, Imamura T, Souchelnytskyi S, et al. TGF-beta receptor-mediated signalling through Smad2, Smad3 and Smad4. EMBO J. 1997;16(17):5353–5362. doi: 10.1093/emboj/16.17.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wick W, Platten M, Weller M. Glioma cell invasion: regulation of metalloproteinase activity by TGF-beta. J Neuro-Oncol. 2001;53(2):177–185. doi: 10.1023/a:1012209518843. [DOI] [PubMed] [Google Scholar]
- 84.Pertovaara L, Kaipainen A, Mustonen T, et al. Vascular endothelial growth factor is induced in response to transforming growth factor-beta in fibroblastic and epithelial cells. J Biol Chem. 1994;269(9):6271–6274. [PubMed] [Google Scholar]
- 85.Akhurst RJ, Derynck R. TGF-beta signaling in cancer—a double-edged sword. Trends Cell Biol. 2001;11(11):S44–S51. doi: 10.1016/s0962-8924(01)02130-4. [DOI] [PubMed] [Google Scholar]
- 86.Koochekpour S, Merzak A, Pilkington GJ. Vascular endothelial growth factor production is stimulated by gangliosides and TGF-beta isoforms in human glioma cells in vitro. Cancer Lett. 1996;102(1–2):209–215. doi: 10.1016/0304-3835(96)04161-4. [DOI] [PubMed] [Google Scholar]
- 87.Lu Y, Jiang F, Zheng X, et al. TGF-beta1 promotes motility and invasiveness of glioma cells through activation of ADAM17. Oncol Rep. 2011;25(5):1329–1335. doi: 10.3892/or.2011.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ikushima H, Todo T, Ino Y, Takahashi M, Miyazawa K, Miyazono K. Autocrine TGF-beta signaling maintains tumorigenicity of glioma-initiating cells through Sry-related HMG-box factors. Cell Stem Cell. 2009;5(5):504–514. doi: 10.1016/j.stem.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 89.Hulper P, Schulz-Schaeffer W, Dullin C, et al. Tumor localization of an anti-TGF-beta antibody and its effects on gliomas. Int J Oncol. 2011;38(1):51–59. [PubMed] [Google Scholar]
- 90.Castelli MG, Chiabrando C, Fanelli R, et al. Prostaglandin and thromboxane synthesis by human intracranial tumors. Cancer Res. 1989;49(6):1505–1508. [PubMed] [Google Scholar]
- 91.Cooper C, Jones HG, Weller RO, Walker V. Production of prostaglandins and thromboxane by isolated cells from intracranial tumours. J Neurol Neurosurg Psychiatry. 1984;47(6):579–584. doi: 10.1136/jnnp.47.6.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Loh JK, Hwang SL, Lieu AS, Huang TY, Howng SL. The alteration of prostaglandin E2 levels in patients with brain tumors before and after tumor removal. J Neurooncol. 2002;57(2):147–150. doi: 10.1023/a:1015782809966. [DOI] [PubMed] [Google Scholar]
- 93.Kuppner MC, Sawamura Y, Hamou MF, de Tribolet N. Influence of PGE2- and cAMP-modulating agents on human glioblastoma cell killing by interleukin-2-activated lymphocytes. J Neurosurg. 1990;72(4):619–625. doi: 10.3171/jns.1990.72.4.0619. [DOI] [PubMed] [Google Scholar]
- 94.Conti A, Gulì C, La Torre D, Tomasello C, Angileri F, Aguennouz M. Role of Inflammation and Oxidative Stress Mediators in Gliomas. Cancers. 2010;2(2):693–712. doi: 10.3390/cancers2020693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Taniguchi Y, Ono K, Yoshida S, Tanaka R. Antigen-presenting capability of glial cells under glioma-harboring conditions and the effect of glioma-derived factors on antigen presentation. J Neuroimmunol. 2000;111(1–2):177–185. doi: 10.1016/s0165-5728(00)00361-1. [DOI] [PubMed] [Google Scholar]
- 96.Roszman TL, Brooks WH, Elliott LH. Inhibition of lymphocyte responsiveness by a glial tumor cell-derived suppressive factor. J Neurosurg. 1987;67(6):874–879. doi: 10.3171/jns.1987.67.6.0874. [DOI] [PubMed] [Google Scholar]
- 97.Wojtowicz-Praga S. Reversal of tumor-induced immunosuppression: a new approach to cancer therapy. J Immunother. 1997;20(3):165–177. [PubMed] [Google Scholar]
- 98.Akasaki Y, Liu G, Chung NH, Ehtesham M, Black KL, Yu JS. Induction of a CD4+ T regulatory type 1 response by cyclooxygenase-2-overexpressing glioma. J Immunol. 2004;173(7):4352–4359. doi: 10.4049/jimmunol.173.7.4352. [DOI] [PubMed] [Google Scholar]
- 99.Bidwell P, Joh K, Leaver HA, Rizzo MT. Prostaglandin E2 activates cAMP response element-binding protein in glioma cells via a signaling pathway involving PKA-dependent inhibition of ERK. Prostaglandins Other Lipid Mediat. 2010;91(1–2):18–29. doi: 10.1016/j.prostaglandins.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 100.Payner T, Leaver HA, Knapp B, et al. Microsomal prostaglandin E synthase-1 regulates human glioma cell growth via prostaglandin E(2)-dependent activation of type II protein kinase A. Mol Cancer Ther. 2006;5(7):1817–1826. doi: 10.1158/1535-7163.MCT-05-0548. [DOI] [PubMed] [Google Scholar]
- 101.Nakano Y, Kuroda E, Kito T, et al. Induction of prostaglandin E2 synthesis and microsomal prostaglandin E synthase-1 expression in murine microglia by glioma-derived soluble factors. Laboratory investigation. J Neurosurg. 2008;108(2):311–319. doi: 10.3171/JNS/2008/108/2/0311. [DOI] [PubMed] [Google Scholar]
- 102.Murakami M, Naraba H, Tanioka T, et al. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J Biol Chem. 2000;275(42):32783–32792. doi: 10.1074/jbc.M003505200. [DOI] [PubMed] [Google Scholar]
- 103.Williams CS, DuBois RN. Prostaglandin endoperoxide synthase: why two isoforms? Am J Physiol. 1996;270(3 Pt 1):G393–G400. doi: 10.1152/ajpgi.1996.270.3.G393. [DOI] [PubMed] [Google Scholar]
- 104.Joki T, Heese O, Nikas DC, et al. Expression of cyclooxygenase 2 (COX-2) in human glioma and in vitro inhibition by a specific COX-2 inhibitor, NS-398. Cancer Res. 2000;60(17):4926–4931. [PubMed] [Google Scholar]
- 105.Jansen T, Tyler B, Mankowski JL, et al. FasL gene knock-down therapy enhances the antiglioma immune response. Neuro-Oncol. 2010;12(5):482–489. doi: 10.1093/neuonc/nop052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Badie B, Schartner J, Vorpahl J, Preston K. Interferon-gamma induces apoptosis and augments the expression of Fas and Fas ligand by microglia in vitro. Exp Neurol. 2000;162(2):290–296. doi: 10.1006/exnr.1999.7345. [DOI] [PubMed] [Google Scholar]
- 107.Klas C, Debatin KM, Jonker RR, Krammer PH. Activation interferes with the APO-1 pathway in mature human T cells. Int Immunol. 1993;5(6):625–630. doi: 10.1093/intimm/5.6.625. [DOI] [PubMed] [Google Scholar]
- 108.Miyawaki T, Uehara T, Nibu R, et al. Differential expression of apoptosis-related Fas antigen on lymphocyte subpopulations in human peripheral blood. J Immunol. 1992;149(11):3753–3758. [PubMed] [Google Scholar]
- 109.Wisniewski P, Ellert-Miklaszewska A, Kwiatkowska A, Kaminska B. Non-apoptotic Fas signaling regulates invasiveness of glioma cells and modulates MMP-2 activity via NFkappaB-TIMP-2 pathway. Cell Signal. 2010;22(2):212–220. doi: 10.1016/j.cellsig.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 110.Choi K, Benveniste EN, Choi C. Induction of intercellular adhesion molecule-1 by Fas ligation: proinflammatory roles of Fas in human astroglioma cells. Neurosci Lett. 2003;352(1):21–24. doi: 10.1016/j.neulet.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 111.Bertrand J, Begaud-Grimaud G, Bessette B, Verdier M, Battu S, Jauberteau MO. Cancer stem cells from human glioma cell line are resistant to Fas-induced apoptosis. Int J Oncol. 2009;34(3):717–727. doi: 10.3892/ijo_00000198. [DOI] [PubMed] [Google Scholar]
- 112.Germain D, Frank DA. Targeting the cytoplasmic and nuclear functions of signal transducers and activators of transcription 3 for cancer therapy. Clin Cancer Res. 2007;13(19):5665–5669. doi: 10.1158/1078-0432.CCR-06-2491. [DOI] [PubMed] [Google Scholar]
- 113.Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19(21):2548–2556. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- 114.Lo HW, Cao X, Zhu H, Ali-Osman F. Constitutively activated STAT3 frequently coexpresses with epidermal growth factor receptor in high-grade gliomas and targeting STAT3 sensitizes them to Iressa and alkylators. Clin Cancer Res. 2008;14(19):6042–6054. doi: 10.1158/1078-0432.CCR-07-4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mizoguchi M, Betensky RA, Batchelor TT, Bernay DC, Louis DN, Nutt CL. Activation of STAT3, MAPK, and AKT in malignant astrocytic gliomas: correlation with EGFR status, tumor grade, and survival. J Neuropathol Exp Neurol. 2006;65(12):1181–1188. doi: 10.1097/01.jnen.0000248549.14962.b2. [DOI] [PubMed] [Google Scholar]
- 116.Rahaman SO, Harbor PC, Chernova O, Barnett GH, Vogelbaum MA, Haque SJ. Inhibition of constitutively active Stat3 suppresses proliferation and induces apoptosis in glioblastoma multiforme cells. Oncogene. 2002;21(55):8404–8413. doi: 10.1038/sj.onc.1206047. [DOI] [PubMed] [Google Scholar]
- 117.Schaefer LK, Ren Z, Fuller GN, Schaefer TS. Constitutive activation of Stat3alpha in brain tumors: localization to tumor endothelial cells and activation by the endothelial tyrosine kinase receptor (VEGFR-2) Oncogene. 2002;21(13):2058–2065. doi: 10.1038/sj.onc.1205263. [DOI] [PubMed] [Google Scholar]
- 118.Kortylewski M, Kujawski M, Wang T, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11(12):1314–1321. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- 119.Hussain SF, Kong LY, Jordan J, et al. A novel small molecule inhibitor of signal transducers and activators of transcription 3 reverses immune tolerance in malignant glioma patients. Cancer Res. 2007;67(20):9630–9636. doi: 10.1158/0008-5472.CAN-07-1243. [DOI] [PubMed] [Google Scholar]
- 120.Dasgupta A, Raychaudhuri B, Haqqi T, et al. Stat3 activation is required for the growth of U87 cell-derived tumours in mice. Eur J Cancer. 2009;45(4):677–684. doi: 10.1016/j.ejca.2008.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sherry MM, Reeves A, Wu JK, Cochran BH. STAT3 is required for proliferation and maintenance of multipotency in glioblastoma stem cells. Stem Cells. 2009;27(10):2383–2392. doi: 10.1002/stem.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen F, Xu Y, Luo Y, et al. Down-regulation of Stat3 decreases invasion activity and induces apoptosis of human glioma cells. J Mol Neurosci. 2010;40(3):353–359. doi: 10.1007/s12031-009-9323-3. [DOI] [PubMed] [Google Scholar]
- 123.Iwamaru A, Szymanski S, Iwado E, et al. A novel inhibitor of the STAT3 pathway induces apoptosis in malignant glioma cells both in vitro and in vivo. Oncogene. 2007;26(17):2435–2444. doi: 10.1038/sj.onc.1210031. [DOI] [PubMed] [Google Scholar]
- 124.Gao L, Li F, Dong B, et al. Inhibition of STAT3 and ErbB2 suppresses tumor growth, enhances radiosensitivity, and induces mitochondria-dependent apoptosis in glioma cells. Int J Radiat Oncol Biol Phys. 2010;77(4):1223–1231. doi: 10.1016/j.ijrobp.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 125.Schaefer LK, Menter DG, Schaefer TS. Activation of stat3 and stat1 DNA binding and transcriptional activity in human brain tumour cell lines by gp130 cytokines. Cell Signal. 2000;12(3):143–151. doi: 10.1016/s0898-6568(99)00077-7. [DOI] [PubMed] [Google Scholar]
- 126.Loeffler S, Fayard B, Weis J, Weissenberger J. Interleukin-6 induces transcriptional activation of vascular endothelial growth factor (VEGF) in astrocytes in vivo and regulates VEGF promoter activity in glioblastoma cells via direct interaction between STAT3 and Sp1. Int J Cancer. 2005;115(2):202–213. doi: 10.1002/ijc.20871. [DOI] [PubMed] [Google Scholar]
- 127.Weissenberger J, Loeffler S, Kappeler A, et al. IL-6 is required for glioma development in a mouse model. Oncogene. 2004;23(19):3308–3316. doi: 10.1038/sj.onc.1207455. [DOI] [PubMed] [Google Scholar]
- 128.Chen SH, Gillespie GY, Benveniste EN. Divergent effects of oncostatin M on astroglioma cells: influence on cell proliferation, invasion, and expression of matrix metalloproteinases. Glia. 2006;53(2):191–200. doi: 10.1002/glia.20264. [DOI] [PubMed] [Google Scholar]
- 129.Repovic P, Fears CY, Gladson CL, Benveniste EN. Oncostatin-M induction of vascular endothelial growth factor expression in astroglioma cells. Oncogene. 2003;22(50):8117–8124. doi: 10.1038/sj.onc.1206922. [DOI] [PubMed] [Google Scholar]
- 130.Maher EA, Furnari FB, Bachoo RM, et al. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15(11):1311–1333. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- 131.Rao RD, James CD. Altered molecular pathways in gliomas: an overview of clinically relevant issues. Semin Oncol. 2004;31(5):595–604. doi: 10.1053/j.seminoncol.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 132.Deo DD, Axelrad TW, Robert EG, Marcheselli V, Bazan NG, Hunt JD. Phosphorylation of STAT-3 in response to basic fibroblast growth factor occurs through a mechanism involving platelet-activating factor, JAK-2, and Src in human umbilical vein endothelial cells. Evidence for a dual kinase mechanism. J Biol Chem. 2002;277(24):21237–21245. doi: 10.1074/jbc.M110955200. [DOI] [PubMed] [Google Scholar]
- 133.Brantley EC, Benveniste EN. Signal transducer and activator of transcription-3: a molecular hub for signaling pathways in gliomas. Mol Cancer Res. 2008;6(5):675–684. doi: 10.1158/1541-7786.MCR-07-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 135.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 136.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 137.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176(1):287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 139.Rodrigues JC, Gonzalez GC, Zhang L, et al. Normal human monocytes exposed to glioma cells acquire myeloid-derived suppressor cell-like properties. Neuro-Oncol. 2010;12(4):351–365. doi: 10.1093/neuonc/nop023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hussain SF, Yang D, Suki D, Grimm E, Heimberger AB. Innate immune functions of microglia isolated from human glioma patients. J Transl Med. 2006;4:15. doi: 10.1186/1479-5876-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4(1):71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 142.Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42(6):717–727. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 143.Komohara Y, Ohnishi K, Kuratsu J, Takeya M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol. 2008;216(1):15–24. doi: 10.1002/path.2370. [DOI] [PubMed] [Google Scholar]
- 144.Alterman RL, Stanley ER. Colony stimulating factor-1 expression in human glioma. Mol Chem Neuropathol. 1994;21(2–3):177–188. doi: 10.1007/BF02815350. [DOI] [PubMed] [Google Scholar]
- 145.Bourette RP, Rohrschneider LR. Early events in M-CSF receptor signaling. Growth Factors. 2000;17(3):155–166. doi: 10.3109/08977190009001065. [DOI] [PubMed] [Google Scholar]
- 146.Raivich G, Gehrmann J, Kreutzberg GW. Increase of macrophage colony-stimulating factor and granulocyte-macrophage colony-stimulating factor receptors in the regenerating rat facial nucleus. J Neurosci Res. 1991;30(4):682–686. doi: 10.1002/jnr.490300412. [DOI] [PubMed] [Google Scholar]
- 147.Wang Y, Berezovska O, Fedoroff S. Expression of colony stimulating factor-1 receptor (CSF-1R) by CNS neurons in mice. J Neurosci Res. 1999;57(5):616–632. [PubMed] [Google Scholar]
- 148.Raivich G, Haas S, Werner A, Klein MA, Kloss C, Kreutzberg GW. Regulation of MCSF receptors on microglia in the normal and injured mouse central nervous system: a quantitative immunofluorescence study using confocal laser microscopy. J Comp Neurol. 1998;395(3):342. doi: 10.1002/(sici)1096-9861(19980808)395:3<342::aid-cne6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 149.Murphy GM, Jr., Zhao F, Yang L, Cordell B. Expression of macrophage colony-stimulating factor receptor is increased in the AbetaPP(V717F) transgenic mouse model of Alzheimer's disease. Am J Pathol. 2000;157(3):895–904. doi: 10.1016/s0002-9440(10)64603-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mitrasinovic OM, Perez GV, Zhao F, Lee YL, Poon C, Murphy GM., Jr. Overexpression of macrophage colony-stimulating factor receptor on microglial cells induces an inflammatory response. J Biol Chem. 2001;276(32):30142–30149. doi: 10.1074/jbc.M104265200. [DOI] [PubMed] [Google Scholar]
- 151.Yamamoto S, Nakajima K, Kohsaka S. Macrophage-colony stimulating factor as an inducer of microglial proliferation in axotomized rat facial nucleus. J Neurochem. 2010;115(4):1057–1067. doi: 10.1111/j.1471-4159.2010.06996.x. [DOI] [PubMed] [Google Scholar]
- 152.Papavasiliou AK, Mehler MF, Mabie PC, et al. Paracrine regulation of colony-stimulating factor-1 in medulloblastoma: implications for pathogenesis and therapeutic interventions. Neurosurgery. 1997;41(4):916–923. doi: 10.1097/00006123-199710000-00028. [DOI] [PubMed] [Google Scholar]
- 153.Graf MR, Jadus MR, Hiserodt JC, Wepsic HT, Granger GA. Development of systemic immunity to glioblastoma multiforme using tumor cells genetically engineered to express the membrane-associated isoform of macrophage colony-stimulating factor. J Immunol. 1999;163(10):5544–5551. [PubMed] [Google Scholar]
- 154.Jadus MR, Chen Y, Boldaji MT, et al. Human U251MG glioma cells expressing the membrane form of macrophage colony-stimulating factor (mM-CSF) are killed by human monocytes in vitro and are rejected within immunodeficient mice via paraptosis that is associated with increased expression of three different heat shock proteins. Cancer Gene Ther. 2003;10(5):411–420. doi: 10.1038/sj.cgt.7700583. [DOI] [PubMed] [Google Scholar]
- 155.Jadus MR, Williams CC, Avina MD, et al. Macrophages kill T9 glioma tumor cells bearing the membrane isoform of macrophage colony stimulating factor through a phagocytosis-dependent pathway. J Immunol. 1998;160(1):361–368. [PubMed] [Google Scholar]
- 156.Hoa NT, Zhang JG, Delgado CL, et al. Human monocytes kill M-CSF-expressing glioma cells by BK channel activation. Lab Invest. 2007;87(2):115–129. doi: 10.1038/labinvest.3700506. [DOI] [PubMed] [Google Scholar]
- 157.Kielian T, van Rooijen N, Hickey WF. MCP-1 expression in CNS-1 astrocytoma cells: implications for macrophage infiltration into tumors in vivo. J Neurooncol. 2002;56(1):1–12. doi: 10.1023/a:1014495613455. [DOI] [PubMed] [Google Scholar]
- 158.Platten M, Kretz A, Naumann U, et al. Monocyte chemoattractant protein-1 increases microglial infiltration and aggressiveness of gliomas. Ann Neurol. 2003;54(3):388–392. doi: 10.1002/ana.10679. [DOI] [PubMed] [Google Scholar]
- 159.Leung SY, Wong MP, Chung LP, Chan AS, Yuen ST. Monocyte chemoattractant protein-1 expression and macrophage infiltration in gliomas. Acta Neuropathol. 1997;93(5):518–527. doi: 10.1007/s004010050647. [DOI] [PubMed] [Google Scholar]
- 160.Kuratsu J, Yoshizato K, Yoshimura T, Leonard EJ, Takeshima H, Ushio Y. Quantitative study of monocyte chemoattractant protein-1 (MCP-1) in cerebrospinal fluid and cyst fluid from patients with malignant glioma. J Natl Cancer Inst. 1993;85(22):1836–1839. doi: 10.1093/jnci/85.22.1836. [DOI] [PubMed] [Google Scholar]
- 161.Hinojosa AE, Garcia-Bueno B, Leza JC, Madrigal JL. CCL2/MCP-1 modulation of microglial activation and proliferation. J Neuroinflammation. 2011;8(1):77. doi: 10.1186/1742-2094-8-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang J, Sarkar S, Cua R, Zhou Y, Hader W, Yong VW. A dialog between glioma and microglia that promotes tumor invasiveness through the CCL2/CCR2/interleukin-6 axis. Carcinogenesis. 2012;33(2):312–319. doi: 10.1093/carcin/bgr289. [DOI] [PubMed] [Google Scholar]
- 163.Badie B, Schartner J, Klaver J, Vorpahl J. In vitro modulation of microglia motility by glioma cells is mediated by hepatocyte growth factor/scatter factor. Neurosurgery. 1999;44(5):1077–1082. doi: 10.1097/00006123-199905000-00075. discussion 1082–3. [DOI] [PubMed] [Google Scholar]
- 164.Koochekpour S, Jeffers M, Rulong S, et al. Met and hepatocyte growth factor/scatter factor expression in human gliomas. Cancer Res. 1997;57(23):5391–5398. [PubMed] [Google Scholar]
- 165.Kunkel P, Muller S, Schirmacher P, et al. Expression and localization of scatter factor/hepatocyte growth factor in human astrocytomas. Neuro-Oncol. 2001;3(2):82–88. doi: 10.1093/neuonc/3.2.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Laterra J, Rosen E, Nam M, Ranganathan S, Fielding K, Johnston P. Scatter factor/hepatocyte growth factor expression enhances human glioblastoma tumorigenicity and growth. Biochem Biophys Res Commun. 1997;235(3):743–747. doi: 10.1006/bbrc.1997.6853. [DOI] [PubMed] [Google Scholar]
- 167.Rosen EM, Laterra J, Joseph A, et al. Scatter factor expression and regulation in human glial tumors. Int J Cancer. 1996;67(2):248–255. doi: 10.1002/(SICI)1097-0215(19960717)67:2<248::AID-IJC16>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 168.Tu H, Zhou Z, Liang Q, et al. CXCR4 and SDF-1 production are stimulated by hepatocyte growth factor and promote glioma cell invasion. Onkologie. 2009;32(6):331–336. doi: 10.1159/000216352. [DOI] [PubMed] [Google Scholar]
- 169.Laterra J, Nam M, Rosen E, et al. Scatter factor/hepatocyte growth factor gene transfer enhances glioma growth and angiogenesis in vivo. Lab Invest. 1997;76(4):565–577. [PubMed] [Google Scholar]
- 170.Moriyama T, Kataoka H, Kawano H, et al. Comparative analysis of expression of hepatocyte growth factor and its receptor, c-met, in gliomas, meningiomas and schwannomas in humans. Cancer Lett. 1998;124(2):149–155. doi: 10.1016/s0304-3835(97)00469-2. [DOI] [PubMed] [Google Scholar]
- 171.Moriyama T, Kataoka H, Hamasuna R, et al. Up-regulation of vascular endothelial growth factor induced by hepatocyte growth factor/scatter factor stimulation in human glioma cells. Biochem Biophys Res Commun. 1998;249(1):73–77. doi: 10.1006/bbrc.1998.9078. [DOI] [PubMed] [Google Scholar]
- 172.Eckerich C, Zapf S, Fillbrandt R, Loges S, Westphal M, Lamszus K. Hypoxia can induce c-Met expression in glioma cells and enhance SF/HGF-induced cell migration. Int J Cancer. 2007;121(2):276–283. doi: 10.1002/ijc.22679. [DOI] [PubMed] [Google Scholar]
- 173.Chu SH, Ma YB, Zhang H, et al. Hepatocyte growth factor production is stimulated by gangliosides and TGF-beta isoforms in human glioma cells. J Neurooncol. 2007;85(1):33–38. doi: 10.1007/s11060-007-9387-2. [DOI] [PubMed] [Google Scholar]
- 174.Sheng-Hua C, Yan-Bin M, Zhi-An Z, et al. Radiation-enhanced hepatocyte growth factor secretion in malignant glioma cell lines. Surg Neurol. 2007;68(6):610–613. doi: 10.1016/j.surneu.2006.12.050. discussion 613-4. [DOI] [PubMed] [Google Scholar]
- 175.Buchanan IM, Scott T, Tandle AT, et al. Radiosensitization of glioma cells by modulation of Met signalling with the hepatocyte growth factor neutralizing antibody, AMG102. J Cell Mol Med. 2011;15(9):1999–2006. doi: 10.1111/j.1582-4934.2010.01122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lou X, Zhou Q, Yin Y, Zhou C, Shen Y. Inhibition of the met receptor tyrosine kinase signaling enhances the chemosensitivity of glioma cell lines to CDDP through activation of p38 MAPK pathway. Mol Cancer Ther. 2009;8(5):1126–1136. doi: 10.1158/1535-7163.MCT-08-0904. [DOI] [PubMed] [Google Scholar]
- 177.Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359(6398):845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- 178.Chaudhry IH, O'Donovan DG, Brenchley PE, Reid H, Roberts IS. Vascular endothelial growth factor expression correlates with tumour grade and vascularity in gliomas. Histopathology. 2001;39(4):409–415. doi: 10.1046/j.1365-2559.2001.01230.x. [DOI] [PubMed] [Google Scholar]
- 179.Schmidt NO, Westphal M, Hagel C, et al. Levels of vascular endothelial growth factor, hepatocyte growth factor/scatter factor and basic fibroblast growth factor in human gliomas and their relation to angiogenesis. Int J Cancer. 1999;84(1):10–18. doi: 10.1002/(sici)1097-0215(19990219)84:1<10::aid-ijc3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 180.Forstreuter F, Lucius R, Mentlein R. Vascular endothelial growth factor induces chemotaxis and proliferation of microglial cells. J Neuroimmunol. 2002;132(1–2):93–98. doi: 10.1016/s0165-5728(02)00315-6. [DOI] [PubMed] [Google Scholar]
- 181.Iwamoto FM, Fine HA. Bevacizumab for malignant gliomas. Arch Neurol. 2010;67(3):285–288. doi: 10.1001/archneurol.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Shibuya M. Vascular endothelial growth factor-dependent and -independent regulation of angiogenesis. BMB Rep. 2008;41(4):278–286. doi: 10.5483/bmbrep.2008.41.4.278. [DOI] [PubMed] [Google Scholar]
- 183.Folkins C, Shaked Y, Man S, et al. Glioma tumor stem-like cells promote tumor angiogenesis and vasculogenesis via vascular endothelial growth factor and stromal-derived factor 1. Cancer Res. 2009;69(18):7243–7251. doi: 10.1158/0008-5472.CAN-09-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Saidi A, Hagedorn M, Allain N, et al. Combined targeting of interleukin-6 and vascular endothelial growth factor potently inhibits glioma growth and invasiveness. Int J Cancer. 2009;125(5):1054–1064. doi: 10.1002/ijc.24380. [DOI] [PubMed] [Google Scholar]
- 185.Seyfried TN, Kiebish MA, Marsh J, Shelton LM, Huysentruyt LC, Mukherjee P. Metabolic management of brain cancer. Biochim Biophys Acta. 2011;1807(6):577–594. doi: 10.1016/j.bbabio.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 186.Seyfried TN, Shelton LM. Cancer as a metabolic disease. Nutr Metab (Lond) 2010;7:7. doi: 10.1186/1743-7075-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Shelton LM, Huysentruyt LC, Seyfried TN. Glutamine targeting inhibits systemic metastasis in the VM-M3 murine tumor model. Int J Cancer. 2010;127(10):2478–2485. doi: 10.1002/ijc.25431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yang C, Sudderth J, Dang T, Bachoo RM, McDonald JG, DeBerardinis RJ. Glioblastoma cells require glutamate dehydrogenase to survive impairments of glucose metabolism or Akt signaling. Cancer Res. 2009;69(20):7986–7993. doi: 10.1158/0008-5472.CAN-09-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Behrens PF, Langemann H, Strohschein R, Draeger J, Hennig J. Extracellular glutamate and other metabolites in and around RG2 rat glioma: an intracerebral microdialysis study. J Neurooncol. 2000;47(1):11–22. doi: 10.1023/a:1006426917654. [DOI] [PubMed] [Google Scholar]
- 190.Ye ZC, Sontheimer H. Glioma cells release excitotoxic concentrations of glutamate. Cancer Res. 1999;59(17):4383–4391. [PubMed] [Google Scholar]
- 191.Takano T, Lin JH, Arcuino G, Gao Q, Yang J, Nedergaard M. Glutamate release promotes growth of malignant gliomas. Nat Med. 2001;7(9):1010–1015. doi: 10.1038/nm0901-1010. [DOI] [PubMed] [Google Scholar]
- 192.Lewis C, Murdoch C. Macrophage responses to hypoxia: implications for tumor progression and anti-cancer therapies. Am J Pathol. 2005;167(3):627–635. doi: 10.1016/S0002-9440(10)62038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Newsholme P. Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? J Nutr. 2001;131(9 Suppl):2515S–2522S. doi: 10.1093/jn/131.9.2515S. discussion 2523S-4S. [DOI] [PubMed] [Google Scholar]
- 194.Liu GJ, Nagarajah R, Banati RB, Bennett MR. Glutamate induces directed chemotaxis of microglia. Eur J Neurosci. 2009;29(6):1108–1118. doi: 10.1111/j.1460-9568.2009.06659.x. [DOI] [PubMed] [Google Scholar]
- 195.Kallenberg K, Bock HC, Helms G, et al. Untreated glioblastoma multiforme: increased myo-inositol and glutamine levels in the contralateral cerebral hemisphere at proton MR spectroscopy. Radiology. 2009;253(3):805–812. doi: 10.1148/radiol.2533071654. [DOI] [PubMed] [Google Scholar]
- 196.Lee WH, Sonntag WE, Mitschelen M, Yan H, Lee YW. Irradiation induces regionally specific alterations in pro-inflammatory environments in rat brain. Int J Radiat Biol. 2010;86(2):132–144. doi: 10.3109/09553000903419346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Galasso JM, Stegman LD, Blaivas M, Harrison JK, Ross BD, Silverstein FS. Experimental gliosarcoma induces chemokine receptor expression in rat brain. Exp Neurol. 2000;161(1):85–95. doi: 10.1006/exnr.1999.7249. [DOI] [PubMed] [Google Scholar]
- 198.Zhang J, Zhou Y, Yong V. Wee. Monocyte chemoattactant protein-1 (MCP-1) expression and functional role in glioma. Paper presented at: AACR Meeting Abstracts; 2005. 2005. [Google Scholar]
- 199.Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- 200.Yamada T, Tsubouchi H, Daikuhara Y, et al. Immunohistochemistry with antibodies to hepatocyte growth factor and its receptor protein (c-MET) in human brain tissues. Brain Res. 1994;637(1–2):308–312. doi: 10.1016/0006-8993(94)91250-5. [DOI] [PubMed] [Google Scholar]
- 201.Brockmann MA, Ulbricht U, Gruner K, Fillbrandt R, Westphal M, Lamszus K. Glioblastoma and cerebral microvascular endothelial cell migration in response to tumor-associated growth factors. Neurosurgery. 2003;52(6):1391–1399. doi: 10.1227/01.neu.0000064806.87785.ab. discussion 1399. [DOI] [PubMed] [Google Scholar]
- 202.Di Renzo MF, Bertolotto A, Olivero M, et al. Selective expression of the Met/HGF receptor in human central nervous system microglia. Oncogene. 1993;8(1):219–222. [PubMed] [Google Scholar]
- 203.Yamagata T, Muroya K, Mukasa T, et al. Hepatocyte growth factor specifically expressed in microglia activated Ras in the neurons, similar to the action of neurotrophic factors. Biochem Biophys Res Commun. 1995;210(1):231–237. doi: 10.1006/bbrc.1995.1651. [DOI] [PubMed] [Google Scholar]
- 204.Pandiella A, Beguinot L, Vicentini LM, Meldolesi J. Transmembrane signalling at the epidermal growth factor receptor. Trends Pharmacol Sci. 1989;10(10):411–414. doi: 10.1016/0165-6147(89)90190-9. [DOI] [PubMed] [Google Scholar]
- 205.Ilschner S, Nolte C, Kettenmann H. Complement factor C5a and epidermal growth factor trigger the activation of outward potassium currents in cultured murine microglia. Neuroscience. 1996;73(4):1109–1120. doi: 10.1016/0306-4522(96)00107-8. [DOI] [PubMed] [Google Scholar]
- 206.Schlote W. Fig. 45. In: Streit WJ, Graeber MB, editors. Microglia: A Pictorial. Stuttgart: Gustav Fischer; 1996. pp. 84–85. [Google Scholar]
- 207.Nolte C, Kirchhoff F, Kettenmann H. Epidermal growth factor is a motility factor for microglial cells in vitro: evidence for EGF receptor expression. Eur J Neurosci. 1997;9(8):1690–1698. doi: 10.1111/j.1460-9568.1997.tb01526.x. [DOI] [PubMed] [Google Scholar]
- 208.Shinojima N, Tada K, Shiraishi S, et al. Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res. 2003;63(20):6962–6970. [PubMed] [Google Scholar]
- 209.Torp SH, Helseth E, Dalen A, Unsgaard G. Epidermal growth factor receptor expression in human gliomas. Cancer Immunol Immunother. 1991;33(1):61–64. doi: 10.1007/BF01742530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wong AJ, Bigner SH, Bigner DD, Kinzler KW, Hamilton SR, Vogelstein B. Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc Natl Acad Sci U S A. 1987;84(19):6899–6903. doi: 10.1073/pnas.84.19.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Libermann TA, Nusbaum HR, Razon N, et al. Amplification and overexpression of the EGF receptor gene in primary human glioblastomas. J Cell Sci Suppl. 1985;3:161–172. doi: 10.1242/jcs.1985.supplement_3.16. [DOI] [PubMed] [Google Scholar]
- 212.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13(1):9–22. [PubMed] [Google Scholar]
- 213.Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87(8):3336–3343. [PubMed] [Google Scholar]
- 214.Bazan JF, Bacon KB, Hardiman G, et al. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385(6617):640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 215.Sciume G, Soriani A, Piccoli M, Frati L, Santoni A, Bernardini G. CX3CR1/CX3CL1 axis negatively controls glioma cell invasion and is modulated by transforming growth factor-beta1. Neuro Oncol. 2010;12(7):701–710. doi: 10.1093/neuonc/nop076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Held-Feindt J, Hattermann K, Müerköster SS, et al. CX3CR1 promotes recruitment of human glioma-infiltrating microglia/macrophages (GIMs) Exp Cell Res. 2010;316(9):1553–1566. doi: 10.1016/j.yexcr.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 217.Liu C, Luo D, Streit WJ, Harrison JK. CX3CL1 and CX3CR1 in the GL261 murine model of glioma: CX3CR1 deficiency does not impact tumor growth or infiltration of microglia and lymphocytes. J Neuroimmunol. 2008;198(1–2):98–105. doi: 10.1016/j.jneuroim.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Rodero M, Marie Y, Coudert M, et al. Polymorphism in the microglial cell-mobilizing CX3CR1 gene is associated with survival in patients with glioblastoma. J Clin Oncol. 2008;26(36):5957–5964. doi: 10.1200/JCO.2008.17.2833. [DOI] [PubMed] [Google Scholar]
- 219.Graeber MB, Bise K, Mehraein P. CR3/43, a marker for activated human microglia: application to diagnostic neuropathology. Neuropathol Appl Neurobiol. 1994;20(4):406–408. doi: 10.1111/j.1365-2990.1994.tb00987.x. [DOI] [PubMed] [Google Scholar]
- 220.Proescholdt MA, Merrill MJ, Ikejiri B, et al. Site-specific immune response to implanted gliomas. J Neurosurg. 2001;95(6):1012–1019. doi: 10.3171/jns.2001.95.6.1012. [DOI] [PubMed] [Google Scholar]
- 221.Tran CT, Wolz P, Egensperger R, et al. Differential expression of MHC class II molecules by microglia and neoplastic astroglia: relevance for the escape of astrocytoma cells from immune surveillance. Neuropathol Appl Neurobiol. 1998;24(4):293–301. doi: 10.1046/j.1365-2990.1998.00120.x. [DOI] [PubMed] [Google Scholar]
- 222.Flügel A, Labeur MS, Grasbon-Frodl EM, Kreutzberg GW, Graeber MB. Microglia only weakly present glioma antigen to cytotoxic T cells. Int J Dev Neurosci. 1999;17(5–6):547–556. doi: 10.1016/s0736-5748(99)00020-9. [DOI] [PubMed] [Google Scholar]
- 223.Badie B, Bartley B, Schartner J. Differential expression of MHC class II and B7 costimulatory molecules by microglia in rodent gliomas. J Neuroimmunol. 2002;133(1–2):39–45. doi: 10.1016/s0165-5728(02)00350-8. [DOI] [PubMed] [Google Scholar]
- 224.Schartner JM, Hagar AR, Van Handel M, Zhang L, Nadkarni N, Badie B. Impaired capacity for upregulation of MHC class II in tumor-associated microglia. Glia. 2005;51(4):279–285. doi: 10.1002/glia.20201. [DOI] [PubMed] [Google Scholar]
- 225.Kostianovsky AM, Maier LM, Anderson RC, Bruce JN, Anderson DE. Astrocytic regulation of human monocytic/microglial activation. J Immunol. 2008;181(8):5425–5432. doi: 10.4049/jimmunol.181.8.5425. [DOI] [PubMed] [Google Scholar]
- 226.Barnum SR. Inhibition of complement as a therapeutic approach in inflammatory central nervous system (CNS) disease. Mol Med. 1999;5(9):569–582. [PMC free article] [PubMed] [Google Scholar]
- 227.Peress NS, Fleit HB, Perillo E, Kuljis R, Pezzullo C. Identification of Fc gamma RI, II and III on normal human brain ramified microglia and on microglia in senile plaques in Alzheimer's disease. J Neuroimmunol. 1993;48(1):71–79. doi: 10.1016/0165-5728(93)90060-c. [DOI] [PubMed] [Google Scholar]
- 228.Morioka T, Baba T, Black KL, Streit WJ. Immunophenotypic analysis of infiltrating leukocytes and microglia in an experimental rat glioma. Acta Neuropathol. 1992;83(6):590–597. doi: 10.1007/BF00299407. [DOI] [PubMed] [Google Scholar]
- 229.Morimura T, Neuchrist C, Kitz K, et al. Monocyte subpopulations in human gliomas: expression of Fc and complement receptors and correlation with tumor proliferation. Acta Neuropathol. 1990;80(3):287–294. doi: 10.1007/BF00294647. [DOI] [PubMed] [Google Scholar]
- 230.Hou B, Reizis B, DeFranco AL. Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms. Immunity. 2008;29(2):272–282. doi: 10.1016/j.immuni.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5(10):987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 232.Mantovani A, Sica A, Allavena P, Garlanda C, Locati M. Tumor-associated macrophages and the related myeloid-derived suppressor cells as a paradigm of the diversity of macrophage activation. Hum Immunol. 2009;70(5):325–330. doi: 10.1016/j.humimm.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 233.Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173(6):3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- 234.Kitchens RL. Role of CD14 in cellular recognition of bacterial lipopolysaccharides. Chem Immunol. 2000;74:61–82. doi: 10.1159/000058750. [DOI] [PubMed] [Google Scholar]
- 235.Curtin JF, Liu N, Candolfi M, et al. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med. 2009;6(1):e10. doi: 10.1371/journal.pmed.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kees T, Lohr J, Noack J, et al. Microglia isolated from patients with glioma gain antitumor activities on poly (I:C) stimulation. Neuro-Oncol. 2012;14(1):64–78. doi: 10.1093/neuonc/nor182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Hagemann T, Wilson J, Burke F, et al. Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. J Immunol. 2006;176(8):5023–5032. doi: 10.4049/jimmunol.176.8.5023. [DOI] [PubMed] [Google Scholar]
- 238.Bak SP, Walters JJ, Takeya M, Conejo-Garcia JR, Berwin BL. Scavenger receptor-A-targeted leukocyte depletion inhibits peritoneal ovarian tumor progression. Cancer Res. 2007;67(10):4783–4789. doi: 10.1158/0008-5472.CAN-06-4410. [DOI] [PubMed] [Google Scholar]
- 239.Dunwiddie TV. The physiological role of adenosine in the central nervous system. Int Rev Neurobiol. 1985;27:63–139. doi: 10.1016/s0074-7742(08)60556-5. [DOI] [PubMed] [Google Scholar]
- 240.Johnston JB, Silva C, Gonzalez G, et al. Diminished adenosine A1 receptor expression on macrophages in brain and blood of patients with multiple sclerosis. Ann Neurol. 2001;49(5):650–658. [PubMed] [Google Scholar]
- 241.Synowitz M, Glass R, Farber K, et al. A1 adenosine receptors in microglia control glioblastoma-host interaction. Cancer Res. 2006;66(17):8550–8557. doi: 10.1158/0008-5472.CAN-06-0365. [DOI] [PubMed] [Google Scholar]
- 242.Tsutsui S, Schnermann J, Noorbakhsh F, et al. A1 adenosine receptor upregulation and activation attenuates neuroinflammation and demyelination in a model of multiple sclerosis. J Neurosci. 2004;24(6):1521–1529. doi: 10.1523/JNEUROSCI.4271-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.de Waal Malefyt R, Haanen J, Spits H, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174(4):915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Frei K, Lins H, Schwerdel C, Fontana A. Antigen presentation in the central nervous system. The inhibitory effect of IL-10 on MHC class II expression and production of cytokines depends on the inducing signals and the type of cell analyzed. J Immunol. 1994;152(6):2720–2728. [PubMed] [Google Scholar]
- 245.Huettner C, Paulus W, Roggendorf W. Messenger RNA expression of the immunosuppressive cytokine IL-10 in human gliomas. Am J Pathol. 1995;146(2):317–322. [PMC free article] [PubMed] [Google Scholar]
- 246.Zhang L, Handel MV, Schartner JM, et al. Regulation of IL-10 expression by upstream stimulating factor (USF-1) in glioma-associated microglia. J Neuroimmunol. 2007;184(1–2):188–197. doi: 10.1016/j.jneuroim.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 247.Zhang L, Alizadeh D, Van Handel M, Kortylewski M, Yu H, Badie B. Stat3 inhibition activates tumor macrophages and abrogates glioma growth in mice. Glia. 2009;57(13):1458–1467. doi: 10.1002/glia.20863. [DOI] [PubMed] [Google Scholar]
- 248.Walker DG, Chuah T, Rist MJ, Pender MP. T-cell apoptosis in human glioblastoma multiforme: implications for immunotherapy. J Neuroimmunol. 2006;175(1–2):59–68. doi: 10.1016/j.jneuroim.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 249.Ford AL, Foulcher E, Lemckert FA, Sedgwick JD. Microglia induce CD4 T lymphocyte final effector function and death. J Exp Med. 1996;184(5):1737–1745. doi: 10.1084/jem.184.5.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Didenko VV, Ngo HN, Minchew C, Baskin DS. Apoptosis of T lymphocytes invading glioblastomas multiforme: a possible tumor defense mechanism. J Neurosurg. 2002;96(3):580–584. doi: 10.3171/jns.2002.96.3.0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Bergers G. Bone marrow-derived cells in GBM neovascularization. In: Meir EGV, editor. CNS Cancer : Models, Markers, Prognostic Factors, Targets, and Therapeutic Approaches. New York: Humana Press; 2009. pp. 749–773. [Google Scholar]
- 252.Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8(8):610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 253.Fischer I, Gagner JP, Law M, Newcomb EW, Zagzag D. Angiogenesis in gliomas: biology and molecular pathophysiology. Brain Pathol. 2005;15(4):297–310. doi: 10.1111/j.1750-3639.2005.tb00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359(6398):843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 255.Yang I, Han SJ, Kaur G, Crane C, Parsa AT. The role of microglia in central nervous system immunity and glioma immunology. J Clin Neurosci. 2010;17(1):6–10. doi: 10.1016/j.jocn.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Kiefer R, Gold R, Gehrmann J, Lindholm D, Wekerle H, Kreutzberg GW. Transforming growth factor beta expression in reactive spinal cord microglia and meningeal inflammatory cells during experimental allergic neuritis. J Neurosci Res. 1993;36(4):391–398. doi: 10.1002/jnr.490360405. [DOI] [PubMed] [Google Scholar]
- 257.Lindholm D, Castren E, Kiefer R, Zafra F, Thoenen H. Transforming growth factor-beta 1 in the rat brain: increase after injury and inhibition of astrocyte proliferation. J Cell Biol. 1992;117(2):395–400. doi: 10.1083/jcb.117.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Kiefer R, Supler ML, Toyka KV, Streit WJ. In situ detection of transforming growth factor-beta mRNA in experimental rat glioma and reactive glial cells. Neurosci Lett. 1994;166(2):161–164. doi: 10.1016/0304-3940(94)90475-8. [DOI] [PubMed] [Google Scholar]
- 259.DaCosta Byfield S, Major C, Laping NJ, Roberts AB. SB-505124 is a selective inhibitor of transforming growth factor-beta type I receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2004;65(3):744–752. doi: 10.1124/mol.65.3.744. [DOI] [PubMed] [Google Scholar]
- 260.Hjelmeland MD, Hjelmeland AB, Sathornsumetee S, et al. SB-431542, a small molecule transforming growth factor-beta-receptor antagonist, inhibits human glioma cell line proliferation and motility. Mol Cancer Ther. 2004;3(6):737–745. [PubMed] [Google Scholar]
- 261.Forsyth PA, Wong H, Laing TD, et al. Gelatinase-A (MMP-2), gelatinase-B (MMP-9) and membrane type matrix metalloproteinase-1 (MT1-MMP) are involved in different aspects of the pathophysiology of malignant gliomas. Br J Cancer. 1999;79(11–12):1828–1835. doi: 10.1038/sj.bjc.6990291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Lampert K, Machein U, Machein MR, Conca W, Peter HH, Volk B. Expression of matrix metalloproteinases and their tissue inhibitors in human brain tumors. Am J Pathol. 1998;153(2):429–437. doi: 10.1016/S0002-9440(10)65586-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Yamamoto M, Mohanam S, Sawaya R, et al. Differential expression of membrane-type matrix metalloproteinase and its correlation with gelatinase A activation in human malignant brain tumors in vivo and in vitro. Cancer Res. 1996;56(2):384–392. [PubMed] [Google Scholar]
- 264.Du R, Petritsch C, Lu K, et al. Matrix metalloproteinase-2 regulates vascular patterning and growth affecting tumor cell survival and invasion in GBM. Neuro-Oncol. 2008;10(3):254–264. doi: 10.1215/15228517-2008-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Guo P, Imanishi Y, Cackowski FC, et al. Up-regulation of angiopoietin-2, matrix metalloprotease-2, membrane type 1 metalloprotease, and laminin 5 gamma 2 correlates with the invasiveness of human glioma. Am J Pathol. 2005;166(3):877–890. doi: 10.1016/s0002-9440(10)62308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Yamada T, Yoshiyama Y, Sato H, Seiki M, Shinagawa A, Takahashi M. White matter microglia produce membrane-type matrix metalloprotease, an activator of gelatinase A, in human brain tissues. Acta Neuropathol. 1995;90(5):421–424. doi: 10.1007/BF00294800. [DOI] [PubMed] [Google Scholar]
- 267.Markovic DS, Vinnakota K, van Rooijen N, et al. Minocycline reduces glioma expansion and invasion by attenuating microglial MT1-MMP expression. Brain Behav Immun. 2011;25(4):624–628. doi: 10.1016/j.bbi.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 268.Bassi R, Giussani P, Anelli V, et al. HMGB1 as an autocrine stimulus in human T98G glioblastoma cells: role in cell growth and migration. J Neurooncol. 2008;87(1):23–33. doi: 10.1007/s11060-007-9488-y. [DOI] [PubMed] [Google Scholar]
- 269.Gately CL, Muul LM, Greenwood MA, et al. In vitro studies on the cell-mediated immune response to human brain tumors. II. Leukocyte-induced coats of glycosaminoglycan increase the resistance of glioma cells to cellular immune attack. J Immunol. 1984;133(6):3387–3395. [PubMed] [Google Scholar]
- 270.Guzhova I, Kislyakova K, Moskaliova O, et al. In vitro studies show that Hsp70 can be released by glia and that exogenous Hsp70 can enhance neuronal stress tolerance. Brain Res. 2001;914(1–2):66–73. doi: 10.1016/s0006-8993(01)02774-3. [DOI] [PubMed] [Google Scholar]
- 271.Yin D, Chen W, O'Kelly J, et al. Connective tissue growth factor associated with oncogenic activities and drug resistance in glioblastoma multiforme. Intl J Cancer. 2010;127(10):2257–2267. doi: 10.1002/ijc.25257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5(10):816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 273.Tacconelli A, Farina AR, Cappabianca L, et al. TrkA alternative splicing: a regulated tumor-promoting switch in human neuroblastoma. Cancer Cell. 2004;6(4):347–360. doi: 10.1016/j.ccr.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 274.Gao R, Brigstock DR. Activation of nuclear factor kappa B (NF-kappaB) by connective tissue growth factor (CCN2) is involved in sustaining the survival of primary rat hepatic stellate cells. Cell Commun Signal. 2005;3:14. doi: 10.1186/1478-811X-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Wahab NA, Weston BS, Mason RM. Connective tissue growth factor CCN2 interacts with and activates the tyrosine kinase receptor TrkA. J Am Soc Nephrol. 2005;16(2):340–351. doi: 10.1681/ASN.2003100905. [DOI] [PubMed] [Google Scholar]
- 276.Edwards LA, Woolard K, Son MJ, et al. Effect of brain- and tumor-derived connective tissue growth factor on glioma invasion. J Natl Cancer Inst. 2011;103(15):1162–1178. doi: 10.1093/jnci/djr224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Halliday JJ, Holland EC. Connective tissue growth factor and the parallels between brain injury and brain tumors. J Natl Cancer Inst. 2011;103(15):1141–1143. doi: 10.1093/jnci/djr261. [DOI] [PubMed] [Google Scholar]
- 278.Xie D, Yin D, Wang HJ, et al. Levels of expression of CYR61 and CTGF are prognostic for tumor progression and survival of individuals with gliomas. Clin Cancer Res. 2004;10(6):2072–2081. doi: 10.1158/1078-0432.ccr-0659-03. [DOI] [PubMed] [Google Scholar]
- 279.Choi C, Gillespie GY, Van Wagoner NJ, Benveniste EN. Fas engagement increases expression of interleukin-6 in human glioma cells. J Neurooncol. 2002;56(1):13–19. doi: 10.1023/a:1014467626314. [DOI] [PubMed] [Google Scholar]
- 280.Goswami S, Gupta A, Sharma SK. Interleukin-6-mediated autocrine growth promotion in human glioblastoma multiforme cell line U87MG. J Neurochem. 1998;71(5):1837–1845. doi: 10.1046/j.1471-4159.1998.71051837.x. [DOI] [PubMed] [Google Scholar]
- 281.Li R, Li G, Deng L, et al. IL-6 augments the invasiveness of U87MG human glioblastoma multiforme cells via up-regulation of MMP-2 and fascin-1. Oncol Rep. 2010;23(6):1553–1559. doi: 10.3892/or_00000795. [DOI] [PubMed] [Google Scholar]
- 282.Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H. The brain tumor microenvironment. Glia. 2011;59(8):1169–1180. doi: 10.1002/glia.21136. [DOI] [PubMed] [Google Scholar]
- 283.Yeh WL, Lu DY, Liou HC, Fu WM. A forward loop between glioma and microglia: Glioma-derived extracellular matrix-activated microglia secrete IL-18 to enhance the migration of glioma cells. J Cell Physiol. 2011;227(2):558–568. doi: 10.1002/jcp.22746. [DOI] [PubMed] [Google Scholar]
- 284.Sawada M, Suzumura A, Yamamoto H, Marunouchi T. Activation and proliferation of the isolated microglia by colony stimulating factor-1 and possible involvement of protein kinase C. Brain Res. 1990;509(1):119–124. doi: 10.1016/0006-8993(90)90317-5. [DOI] [PubMed] [Google Scholar]
- 285.Wang H, Lathia JD, Wu Q, et al. Targeting interleukin 6 signaling suppresses glioma stem cell survival and tumor growth. Stem Cells. 2009;27(10):2393–2404. doi: 10.1002/stem.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Karin M. NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol. 2009;1(5):a000141. doi: 10.1101/cshperspect.a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Wang T, Niu G, Kortylewski M, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10(1):48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- 288.Zhang L, Liu W, Alizadeh D, et al. S100B attenuates microglia activation in gliomas: possible role of STAT3 pathway. Glia. 2011;59(3):486–498. doi: 10.1002/glia.21118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Ben-Hamo R, Efroni S. Gene-expression and network-based analysis reveals a novel role for hsa-mir-9 and drug control over the p38 network in Glioblastoma Multiforme progression. Genome Med. 2011;3(11):77. doi: 10.1186/gm293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Jurvansuu J, Zhao Y, Leung DS, et al. Transmembrane protein 18 enhances the tropism of neural stem cells for glioma cells. Cancer Res. 2008;68(12):4614–4622. doi: 10.1158/0008-5472.CAN-07-5291. [DOI] [PubMed] [Google Scholar]
- 291.Urnov FD, Miller JC, Lee YL, et al. Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature. 2005;435(7042):646–651. doi: 10.1038/nature03556. [DOI] [PubMed] [Google Scholar]