Abstract
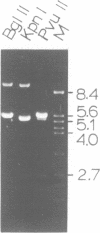
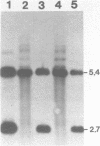
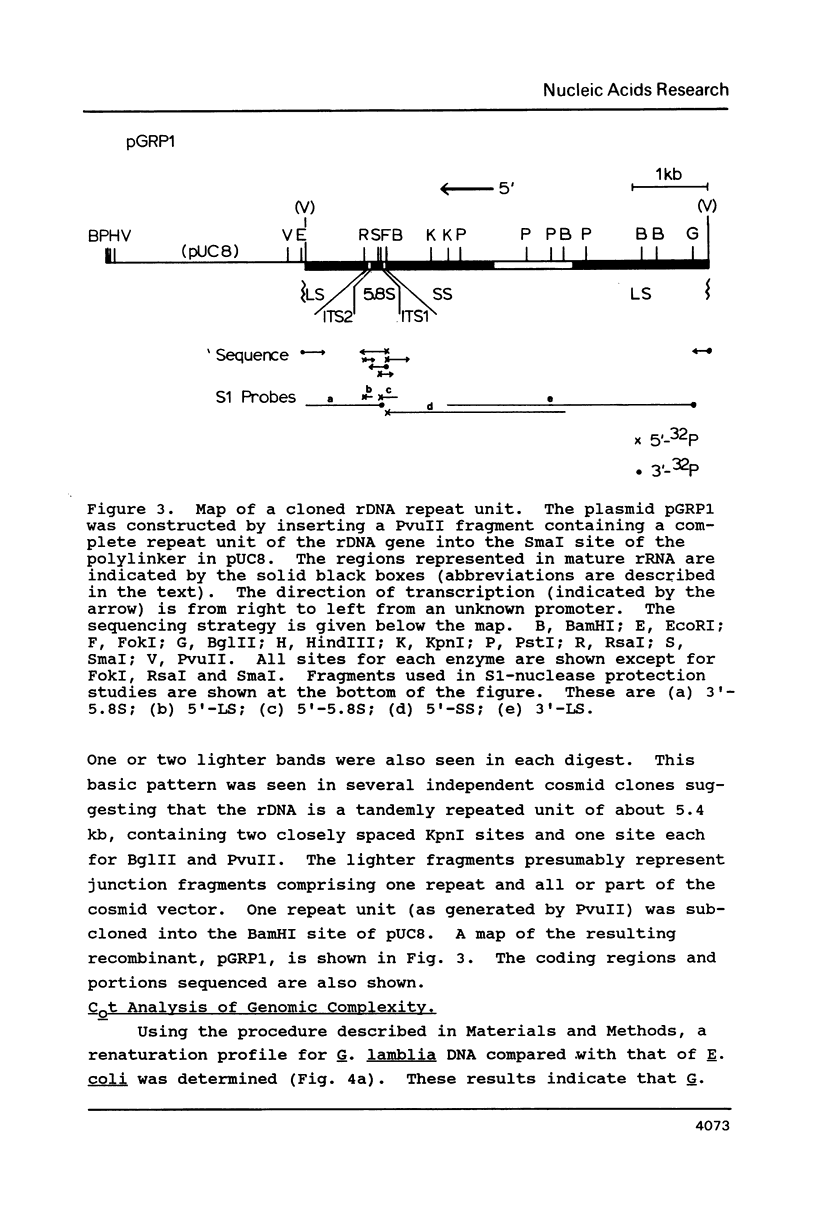
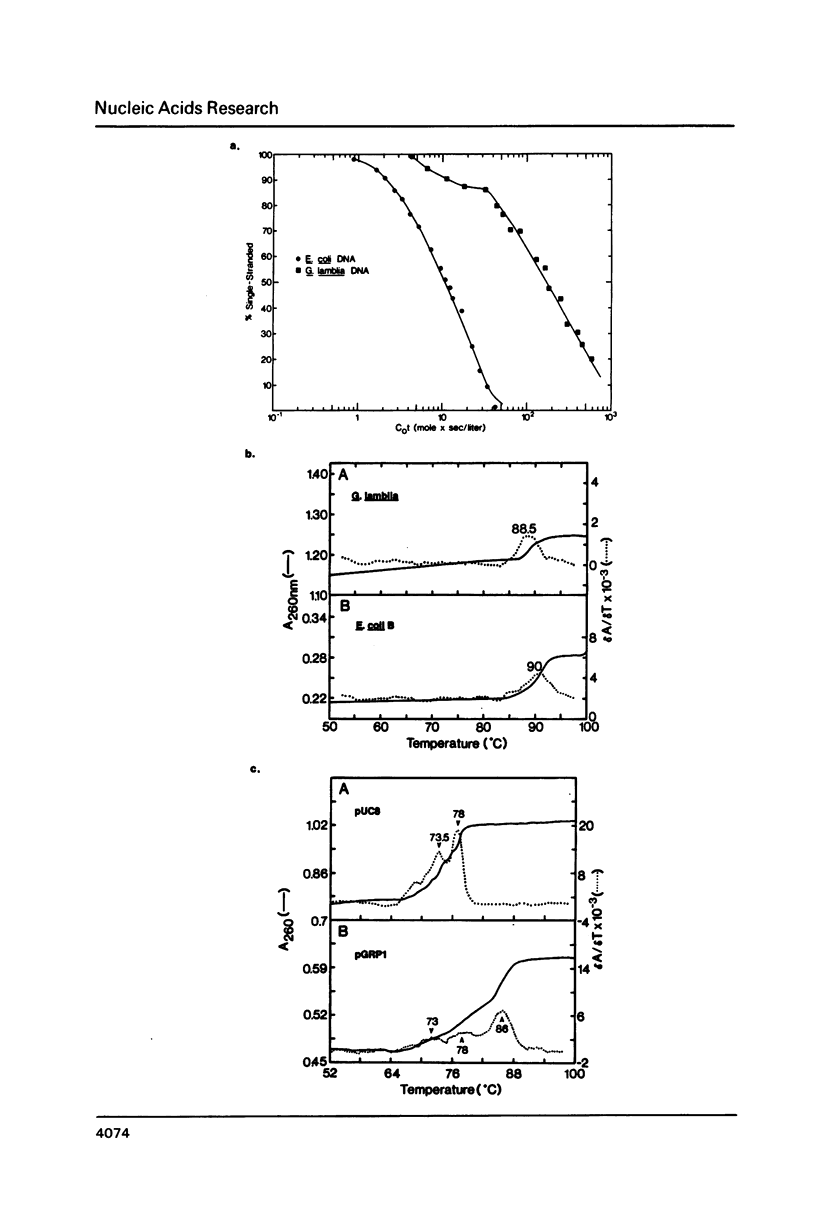
The ribosomal RNA (rRNA) genes of the protozoan parasite Giardia lamblia have been analyzed with respect to size, composition and copy number. They are found to be remarkable in several respects. First, the rRNAs themselves are the smallest yet reported for any eukaryotic organism. Second, the genes encoding them are found as an exceptionally small tandemly repeated unit of only 5.4 kilobase-pairs. Third, the genes are extraordinarily G:C rich, even in regions which are highly conserved between all other eukaryotic rRNA genes. Finally, by analogy to other organisms, the 5.8S RNA appears to lack about 15 nucleotides from its 3'-end, a region previously thought to be essential for 5.8S RNA function. We also provide the first estimates of the genomic complexity and total G:C content of this important protozoan pathogen.
Full text
PDF

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bates P. F., Swift R. A. Double cos site vectors: simplified cosmid cloning. Gene. 1983 Dec;26(2-3):137–146. doi: 10.1016/0378-1119(83)90183-x. [DOI] [PubMed] [Google Scholar]
- Bernards A., Van der Ploeg L. H., Frasch A. C., Borst P., Boothroyd J. C., Coleman S., Cross G. A. Activation of trypanosome surface glycoprotein genes involves a duplication-transposition leading to an altered 3' end. Cell. 1981 Dec;27(3 Pt 2):497–505. doi: 10.1016/0092-8674(81)90391-3. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boseley P. G., Tuyns A., Birnstiel M. L. Mapping of the Xenopus laevis 5.8S rDNA by restriction and DNA sequencing. Nucleic Acids Res. 1978 Apr;5(4):1121–1137. doi: 10.1093/nar/5.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimacombe R., Stiege W. Structure and function of ribosomal RNA. Biochem J. 1985 Jul 1;229(1):1–17. doi: 10.1042/bj2290001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Graham D. E., Neufeld B. R. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974;29:363–418. doi: 10.1016/0076-6879(74)29033-5. [DOI] [PubMed] [Google Scholar]
- Dorfman D. M., Lenardo M. J., Reddy L. V., Van der Ploeg L. H., Donelson J. E. The 5.8S ribosomal RNA gene of Trypanosoma brucei: structural and transcriptional studies. Nucleic Acids Res. 1985 May 24;13(10):3533–3549. doi: 10.1093/nar/13.10.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall L. M., Maden B. E. Nucleotide sequence through the 18S-28S intergene region of a vertebrate ribosomal transcription unit. Nucleic Acids Res. 1980 Dec 20;8(24):5993–6005. doi: 10.1093/nar/8.24.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Borst P., van den Burg J., Weissmann C., Cross G. A. The isolation of plasmids containing DNA complementary to messenger RNA for variant surface glycoproteins of Trypanosoma brucei. Gene. 1980 Mar;8(4):391–417. doi: 10.1016/0378-1119(80)90043-8. [DOI] [PubMed] [Google Scholar]
- Mandal R. K. The organization and transcription of eukaryotic ribosomal RNA genes. Prog Nucleic Acid Res Mol Biol. 1984;31:115–160. doi: 10.1016/s0079-6603(08)60376-1. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Salim M., Maden B. E. Nucleotide sequence of Xenopus laevis 18S ribosomal RNA inferred from gene sequence. Nature. 1981 May 21;291(5812):205–208. doi: 10.1038/291205a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Schnare M. N., Spencer D. F., Gray M. W. Primary structures of four novel small ribosomal RNAs from Crithidia fasciculata. Can J Biochem Cell Biol. 1983 Jan;61(1):38–45. doi: 10.1139/o83-006. [DOI] [PubMed] [Google Scholar]
- Sogin M. L., Elwood H. J., Gunderson J. H. Evolutionary diversity of eukaryotic small-subunit rRNA genes. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1383–1387. doi: 10.1073/pnas.83.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subrahmanyam C. S., Cassidy B., Busch H., Rothblum L. I. Nucleotide sequence of the region between the 18S rRNA sequence and the 28S rRNA sequence of rat ribosomal DNA. Nucleic Acids Res. 1982 Jun 25;10(12):3667–3680. doi: 10.1093/nar/10.12.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn J. C., Sperbeck S. J., Ramsey W. J., Lawrence C. B. A universal model for the secondary structure of 5.8S ribosomal RNA molecules, their contact sites with 28S ribosomal RNAs, and their prokaryotic equivalent. Nucleic Acids Res. 1984 Oct 11;12(19):7479–7502. doi: 10.1093/nar/12.19.7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Vossbrinck C. R., Woese C. R. Eukaryotic ribosomes that lack a 5.8S RNA. Nature. 1986 Mar 20;320(6059):287–288. doi: 10.1038/320287a0. [DOI] [PubMed] [Google Scholar]
- Wang A. L., Wang C. C. A linear double-stranded RNA in Trichomonas vaginalis. J Biol Chem. 1985 Mar 25;260(6):3697–3702. [PubMed] [Google Scholar]
- Ware V. C., Tague B. W., Clark C. G., Gourse R. L., Brand R. C., Gerbi S. A. Sequence analysis of 28S ribosomal DNA from the amphibian Xenopus laevis. Nucleic Acids Res. 1983 Nov 25;11(22):7795–7817. doi: 10.1093/nar/11.22.7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T. C., Rudenko G., Borst P. Three small RNAs within the 10 kb trypanosome rRNA transcription unit are analogous to domain VII of other eukaryotic 28S rRNAs. Nucleic Acids Res. 1986 Dec 9;14(23):9471–9489. doi: 10.1093/nar/14.23.9471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. W., Clayton D. A. DNA primase of human mitochondria is associated with structural RNA that is essential for enzymatic activity. Cell. 1986 Jun 20;45(6):817–825. doi: 10.1016/0092-8674(86)90556-8. [DOI] [PubMed] [Google Scholar]