Abstract
Rotating night shift work is associated with increased risk of breast cancer, likely via circadian disruption. We hypothesized that circadian pathway genes influence breast cancer risk, particularly in rotating night shift workers. We selected 178 common variants across 15 genes pertinent to the circadian system. Using a mixed candidate- and tag-single nucleotide polymorphism approach, we tested for associations between these variants and breast cancer risk in 1,825 women within the Nurses’ Health Study II cohort and investigated potential interactions between genotype and rotating shift-work in a subset of 1,318 women. Multiple-testing-adjusted p-values were obtained by permutation (n=10,000). None of the selected variants was significantly associated with breast cancer risk. However, when accounting for potential effect modification, rs23051560 (Ala394Thr) in the largest circadian gene, Neuronal PAS domain protein 2 (NPAS2) was most strongly associated with breast cancer risk (nominal test for interaction p-value=0.0005; 10,000-permutation-based main-effects p-value among women with <24 months of shift-work=0.003). The observed multiplicative association with breast cancer risk per minor allele (A) was 0.65 (95%CI=0.51–0.82) among women with <24 months of shift-work, and 1.19 (95%CI=0.93–1.54) with ≥24 months of shift-work. Women homozygous for the minor allele (AA) with ≥24 months of shift-work had a 2.83-times higher breast cancer risk compared to homozygous AA women with <24 months of shift-work (95%CI=1.47–5.56).
In smmary, common variation in circadian genes plays at most a small role in breast cancer risk among women of European ancestry. The impact of NPAS2 Ala394Thr in the presence of rotating shift-work requires further investigation.
Keywords: circadian genes, breast cancer, rotating shift work, night work
INTRODUCTION
The central pacemaker for the human circadian clock is located within the suprachiasmatic nuclei (SCN) of the hypothalamus. This clock follows an approximately 24-hour pattern, regulating, through the periodic transcription of genes, many physiological and behavioral functions including anticipation of the light-dark and sleep/wake cycle 1.
Repeated unnatural exposure to light and dark, as occurs with jet-lag and shift-work, disrupts the internal pacemaker, inhibiting synchronization of the circadian clock to the light-dark cycle 2, 3. This lack of synchronization with environmental queues may affect breast cancer risk, likely via hormonal disruption 4. In epidemiological studies including the NHS2 cohort, night workers have been consistently found to have an increased risk of breast cancer 5–13. The role of common variants in genes that regulate the circadian system (circadian genes) in these associations is currently unknown. We hypothesized that circadian genes influence breast cancer risk, particularly in groups that work rotating night shifts.
METHODS
Study Participants and Samples
Methods for The Nurses’ Health Study II (NHS2) have been previously described 14. Briefly, NHS2 was established in 1989 as a prospective cohort of 116,671 US registered female nurses 25–42 years of age at the time they completed their baseline questionnaire. Participants are followed biennially to update information on exposures and diagnoses. Between 1996 and 1999, blood samples were provided by 29,611 cancer-free members of the cohort ages 32–54 15. The study was approved by the Committee on the Use of Human Subjects in Research at Harvard School of Public Health and Brigham and Women’s Hospital.
Information about total number of years worked on rotating night shifts (defined as working at least 3 nights/month, in addition to days or evenings in that month) was queried at baseline in 1989, and regularly updated by follow-up questionnaire. Breast cancer cases were self-identified in the biennial questionnaire and confirmed by medical record review. Deaths were reported by next of kin or the postal service. The National Death Index was used to identify deaths among non-respondents. Breast cancer cases were matched to controls according to year of birth (±2 years), menopausal status at blood draw and at diagnosis of breast cancer (postmenopausal, premenopausal, unknown), timing of blood draw (day and month (±2 months), fasting status (<2, 2–4, 5–7, 8–11, ≥12 hours), luteal day, and self-declared ethnicity (African-American, Asian, Hispanic, Southern European/Scandinavian/Other Caucasian, and Other). We successfully genotyped 609 prevalent and incident breast cancer cases and 1,216 matched controls. Data on cumulative exposure to rotating shift-work prior to the last year of blood draw was available on a subset of these participants (438 incident cases and 880 matched controls). Breast cancer was classified as prevalent if reported between follow-up cycles 1989 and 1997 (i.e., before blood draw), and incident if reported between 1999 and 2005. We used both prevalent and incident cases in the main effects analysis as breast cancer diagnosis cannot alter an individuals’ genotype (all questionnaire data was used only prospectively). Analyses investigating interactions between clock genes and shift-work are restricted to incident cases with data on shift-work, as diagnosis with breast cancer may influence work patterns.
Genotyping
We tagged nine genes with established key roles in circadian regulation [ARNTL (aryl hydrocarbon receptor nuclear translocator-like; a.k.a. Bmal1), CLOCK (hClock), CRY1 (Cryptochrome1), CRY2 (Cryptochrome2), CSNK1A1 (Casein kinase 1є), NPAS2 (Neuronal PAS domain protein 2), PER1 (Period1), PER2 (Period2), and PER3 (Period3)], as well as six genes for their role in the metabolism of a key marker of the circadian system, melatonin [AANAT (arylalkylamine N-acetyltransferase), NAT1 (arylamine N-acetyltransferase 1), NAT2 (arylamine N-acetyltransferase 2), TPH1 (tryptophan hydroxylase 1), and TPH2 (tryptophan hydroxylase 2), and SLC6A4 (solute carrier family 6)] 16. We also included 2 candidate SNPs identified from the literature [rs2305160 (Ala394Thr) in NPAS2 and rs1801260 in CLOCK].
NPAS2 and CLOCK share significant sequence homology allowing them to heterodimerize with ARNTL and serve as a transcriptional enhancer in the positive circadian feedback loop regulating circadian rhythm. Ala394Thr, in NPAS2, was included as prior studies had observed an association between this variant and cancer, specifically with breast cancer, risk 17 and prognosis 18 and with risk of non-Hodgkins lymphoma 19. Rs1801260 in Clock, was included based on its association with sleep and activity patterns 20.
We further included 11 SNPs identified from a breast cancer genome association scan in the Nurses’ Health Study (NHS CGEMS) 21, where a one-time assessment of shift work exposure allowed us to screen for SNP*shift work interactions. A detailed description of the study methods can be found in the Supplementary Appendix. In total, we assessed 178 SNPs.
Statistical Analyses
We used unconditional logistic regression to estimate the main effects of each SNP (modeled additively by minor allele count) on breast cancer susceptibility. We defined shift-work, as having worked either less than 2 years or 2+ years of rotating shift-work prior to 1999. We added this binary term and the product of this term with the minor allele count into the above main effects model to test for interaction. P-values are reported for both the independent effect of the interaction term and the joint effect of genotype and the interaction term 22. All analyses were restricted to women of self-reported European ancestry and adjusted for age at blood draw and menopausal status.
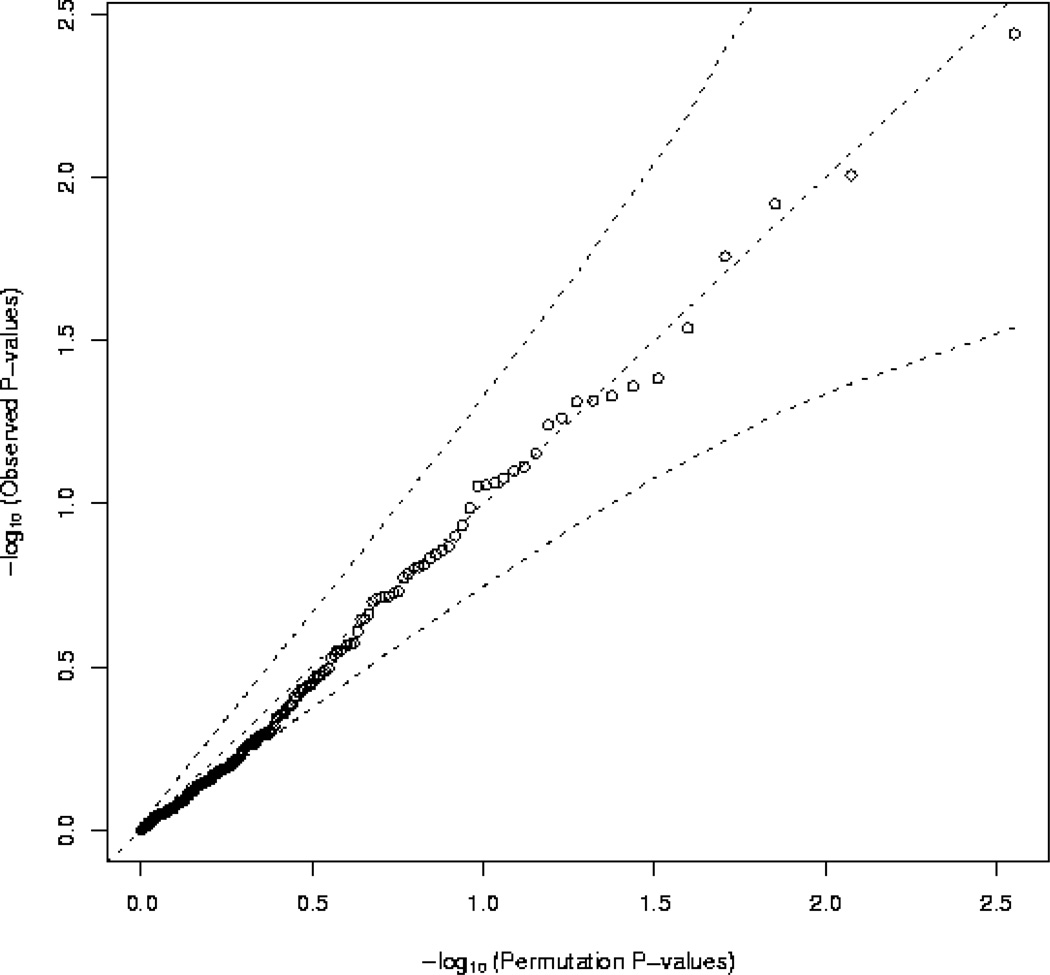
Multiple-testing adjusted p-values were obtained for main effect and stratum specific analyses using the max(T) permutation procedure implemented in PLINK 23 within covariate clusters defined by menopausal status (pre-/post-/ unkown) at baseline and blood draw, and age quartile using 10,000 permutations. A q-q-plot (Figure) was generated to plot the observed p-values from the main effects analysis, against the permutation obtained expected distribution of p-values under the null hypothesis that none of the tested markers is associated with breast cancer risk.
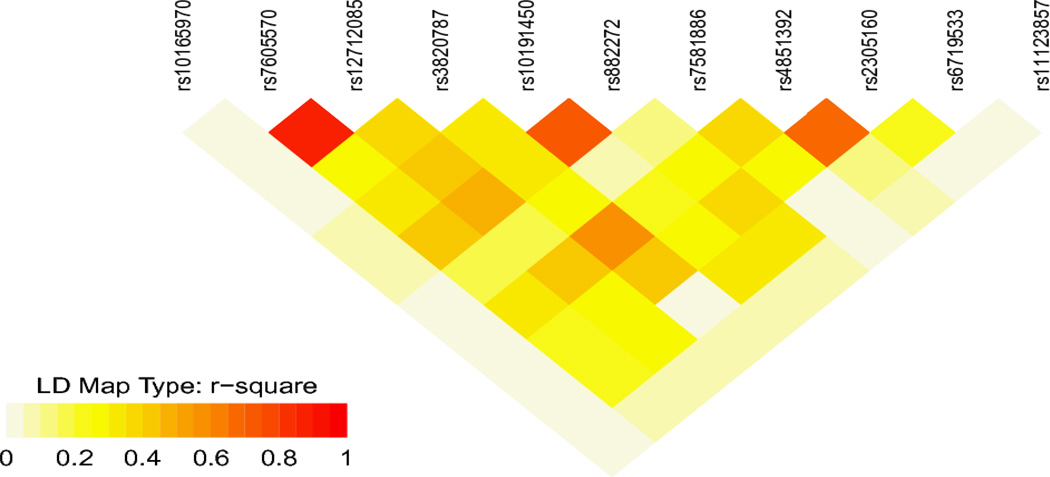
We used PLINK to estimate linkage disequilibrium (r2) among the controls, between SNPs within NPAS2 with any p-value <0.05. These values were graphically presented in a heatmap (Figure 2) using the snp.plotter package in R 24. All analyses were performed in PLINK or R. All reported p-values are two-sided.
Figure 2.
Heatmap illustrating the r2 linkage disequilibrium estimates for all SNPs on NPAS2 with a p-value <0.05 in any of the main effect or interaction analyses.
RESULTS
Population
Participants ranged between 32–52 years of age (mean, 45 years) at blood collection (Table 1). Roughly one third of all participants were postmenopausal. Differences between cases and controls, overall, were relatively small. Cases, on average, had worked more months of shiftwork than controls (41.5 vs. 37.7 months).
Table 1.
Characteristics at blood collection of 609 incident and prevalent breast cancer cases and 1,216 matched controls from NHS2.
| Cases (n=609) | Controls (n= 1,216) |
p-value* | |
|---|---|---|---|
| Age (years), mean(SD) | 46.0 (4.4) | 45.9 (4.3) | Matching factor |
| Parity in 1995,a mean(SD) | 1.7 (1.2) | 1.9 (1.2) | 0.002 |
| Height (in), mean (SD) | 65.2 (2.5) | 64.9 (2.5) | 0.006 |
| BMI (kg/m2), mean(SD) | 25.9 (5.4) | 26.2 (6.0) | 0.17 |
| Cumulative Rotating Shift-work by the 1999 questionnaire (months), mean(SD) |
41.5 (55.8) | 37.7 (48.4) | 0.14 |
| Family history of breast cancer (%) | 17.2 | 10.4 | <0.0001 |
| History of benign breast disease (%) | 26.3 | 17.9 | <0.0001 |
| Age at menarche ≥14 (%) | 14.3 | 17.5 | 0.62 |
| Menopausal status Pre menopausal (%) Post menopausal (%) Unknown (%) |
61.3 28.2 10.5 |
60.6 30.3 9.1 |
Matching factor |
Among parous women only
Implicitly adjusted for matching factors using conditional logistic regression
Main Effects
Figure 1 illustrates that the observed p-values corresponding to the main effects analysis of the association between individual variants in the genes that regulate circadian rhythm and breast cancer susceptibility are consistent with the distribution of p-values that would be expected by chance under the null hypothesis of no association (all observed p-values fall within the 95% confidence bounds established by the permuted data; probability of observing data as or more extreme: p-value=0.20).
Figure 1.
Q-Q-Plot corresponding to the main effects analysis of 178 common variants in genes that regulate circadian rhythm with respect to breast cancer susceptibility, adjusted for matching factors. The distribution of expected p-values was estimated based on 10,000 permutations. The dashed lines illustrate the permutation based 95% confidence bounds.
P-values (both uncorrected and multiple-testing-adjusted) and odds ratios (where appropriate) for the main effects, interaction, and stratified analyses are presented in Supplementary Table 1. The main effects analysis yielded no more statistically significant associations than would be expected by chance; the minimum multiple-testing-adjusted p-value was 0.41.
Shift Work Interactions
Ala394Thr was the marker most strongly associated with breast cancer using the joint test incorporating both main and interaction effects (nominal P=0.0005). Ala394Thr and rs4851392 were the only markers, when stratifying by shift-work exposure, to have a multiple-testing-adjusted p-value < 0.05. When restricting to individuals with <2 years of rotating shift-work both SNPs shared a permutation corrected p-value=0.003 (p-value=0.009 following further multiple testing correction using the Bonferroni method to account for the assessment of stratum specific effects); they shared a p-value>0.999 when restricting to 2+ years of rotating shift-work. The estimated r2 between Ala394Thr and rs4851392 is 0.68 among the controls (Figure 2).
The observed odds ratio for breast cancer among women with the Ala/Thr genotype relative to women with the common Ala/Ala genotype was 0.58 (95% CI: 0.42–0.81) among individuals with <2 years of cumulative rotating shift-work, and 0.76 (95% CI: 0.53–1.09) among individuals with 2+ years of cumulative rotating shift-work. Per genotype odds ratios according to strata of shift-work exposure are presented in Table 2. Changing the referent group (Table 3), we estimate women with the Ala/Thr genotype with 2+ years of cumulative rotating shift-work to have an odds of breast cancer 1.31 times greater than that of women of the same genotype exposed to <2 years of cumulative rotating shift-work (95% CI: 0.91–1.88). Women with the Thr/Thr genotype and 2+ years of cumulative rotating shift-work were observed to have an odds of breast cancer 2.83 times greater than that of women of the same genotype exposed to <2 years of cumulative rotating shift-work (95% CI: 1.47–5.56).
Table 2.
Odds ratios (and 95% confidence intervals) comparing the odds of breast cancer among women with NPAS2 Ala394Thr genotype Ala/Ala and less than 24 months of rotating shift-work, to women with the variant Ala/Thr or Thr/Thr genotypes, and to women with 24 or more months of rotating shift-work experience.
| Months of shift-work | Ala/Ala | Ala/Thr | Thr/Thr |
|---|---|---|---|
| <24 | 1 ncase=126, ncontrol=194 |
0.58 (0.42–0.81) ncase=97, ncontrol=255 |
0.47 (0.27–0.79) ncase=22, ncontrol=71 |
| ≥24 | 0.80 (0.56–1.13) ncase=81, ncontrol=157 |
0.76 (0.53–1.09) ncase=76, ncontrol=155 |
1.33 (0.79–2.22) ncase=34, ncontrol=40 |
Table 3.
Odds ratios (and 95% confidence intervals) comparing the odds of breast cancer among women with less than 24 months of rotating shift-work to women with 24 or more months of rotating shift-work experience, within strata of NPAS2 Ala394Thr genotype.
| Months of shift-work | Ala/Ala | Ala/Thr | Thr/Thr |
|---|---|---|---|
| <24 | 1 ncase=126, ncontrol=194 |
1 ncase=97, ncontrol=255 |
1 ncase=22, ncontrol=71 |
| ≥24 | 0.80 (0.56–1.13) ncase=81, ncontrol=157 |
1.31 (0.91–1.88) ncase=76, ncontrol=155 |
2.83 (1.47–5.56) ncase=34, ncontrol=40 |
DISCUSSION
To our knowledge, we are the first to investigate gene-environment interactions with rotating shift work to advance our understanding of the elevated breast cancer risk consistently observed among shift workers. With its large proportion of shift workers and prospective assessment of shift work exposure, the NHS2 is uniquely positioned worldwide to conduct such a study. After accounting for multiple testing, in the present study, no marginal effect of circadian genes on breast cancer risk was apparent. However, when stratifying by shift work status, we found that among women with little to no exposure to rotating night work (i.e., <2 years), NPAS2 Ala394Thr variant Thr genotypes (Ala/Thr and Thr/Thr) were associated with significantly lower risk of breast cancer, compared to the Ala/Ala genotype. By contrast, women with the Thr/Thr genotype who had worked 2+ years of rotating night shifts had a nearly 3-fold higher risk of breast cancer compared to women of the same genotype with less than 2 years of rotating shift work exposure. In the NHS2 cohort, 13% of all women carry the Thr/Thr genotype.
Previous studies in Caucasian populations have reported interesting associations with NPAS2 Ala394Thr genotype. Similar to our results, a recent study by Zhu et al. of the association between three SNPs within NPAS2 and breast cancer risk found the Ala/Thr genotype to be associated with a reduced breast cancer risk relative to the most common Ala/Ala genotype (OR = 0.61, 95% CI: 0.46–0.81, p-value = 0.001) and no effect of Thr/Thr on risk.17 Further, they found the Thr/Thr genotype associated with poorer disease-free survival among breast cancer cases.18 Prior studies by this and one other group had observed an inverse association between the Thr/Thr genotype and non-Hodgkin’s lymphoma 19 as well as prostate cancer.25 Evidence from these studies is not strong, but collectively implicates a potential effect of the NPAS2 variant on cancer risk.
NPAS2 and cells with RNA interference-mediated knockdown of NPAS2, in vitro failed to demonstrate G1-G2 cell cycle delay in response to a mutagen, and demonstrated significant down regulation of genes important to DNA repair, binding, and cell cycle regulation26. Ala394Thr is described by web-based functional analysis tool, FASTSNP, as having a moderate risk of affecting a disease phenotype; it yields a conservative change in protein structure, and may further influence alternative splicing via disruption of an exonic splicing enhancer or silencer binding site 27. It is not clear whether the change in protein structure is adequate to influence dimerization of NPAS2 with ARNTL or the functional ability of the heterodimer. In addition to circadian gene regulation, the NPAS2/ARNTL heterodimer inhibits c-Myc transcription, preventing genomic instability and the accumulation of damaged cells 28.
Using data from the readily available Nurses’ Health Study (NHS) CGEMS breast cancer study 21 we conducted comparable analyses among postmenopausal women of self-declared European ancestry (see Supplementary Table 2) for each of the 19 SNPs having a p-value less than 0.05 in NHS2. We did not find any of these SNPs including Ala394Thr, to be marginally associated with breast cancer risk, regardless of rotating night work status.
When stratifying by menopausal status, Zhu et al. observed the effect of the Ala394Thr variant to be stronger among premenopausal women than postmenopausal women (OR = 0.44, 95% CI: 0.25–0.77, p-value = 0.004 vs. OR = 0.65, 95% CI: 0.46–0.91, p-value = 0.014). Given the small proportion of postmenopausal women in NHS2 (only 27 postmenopausal cases and 60 controls in our sample were exposed to 2+ years of shift-work) we are underpowered to assess whether the Ala394Thr-shift-work effect that we have observed is modified by menopausal status. However, this may explain the lack of association in the NHS-CGEMS breast cancer study and suggests that younger women with less the common Thr genotype may be particularly susceptible to the effects of shift work on breast cancer risk.
Interestingly, in a recent study by Kovanen et al. NPAS2 Ala394Thr carriers of the Thr genotype (Ala/Thr and Thr/Thr individuals) were observed to have less seasonal variation in sleep length, social activity, mood, weight, appetite, and energy level (as measured by a Global Seasonality Scores; a metric used to diagnose seasonal affective disorder) 29. This could imply a more rigid circadian system of Thr carriers, making them especially sensitive to circadian disruption. The Thr carriers in our study who had little to no shift work exposure had a significantly lower risk of breast cancer relative to women with the Ala/Ala genotype; however, if they were exposed to 2+ years of shift work, they appeared to be at particularly high risk of developing breast cancer.
Asian populations and African populations have lower frequencies of Thr while European populations have higher Thr frequencies. If the apparent interaction is causal we would expect the effect of shift work to appear weaker in a population with a lower prevalence of the Thr genotype at Ala394Thr. The Shanghai Women’s Health Study, a prospective cohort of women representing urban communities of Shanghai, China, observed no association between lifetime history of shift work and breast cancer risk 30 A study of Shanghai men observed that 5% of men in the study were Thr/Thr at Ala394Thr 31 therefore, interaction between Ala394Thr and shift work may contribute to the lack of replication.
Processes relevant to carcinogenesis such as DNA repair and cellular proliferation are known to have circadian variation 32. Common variation in genes that regulate circadian rhythms may not have a detectable effect unless the rhythmicity of the circadian clock is adequately challenged in the study population. Our decision to use 2+ years of shift-work as the exposure group was made to ensure adequate numbers of subjects in each genotype-by exposure stratum. However, previously, in the NHS2, significant effects of shift work were only detectable when comparing women having worked 20+ years of rotating shift-work to women who had reported never working a year or more of rotating shifts (RR = 1.79, CI: 1.06–3.01) 5. By contrast, the relative risk of breast cancer for women with 2+ years of rotating night work was 0.99 (95% CI, 0.88–1.14), when compared to women without any night work in this cohort (unpublished data). Overall, it remains unclear whether actual duration of shift work rather than timing of shift work (i.e., longer durations could be reflective of exposures earlier in life) is more important in these associations, but it is conceivable that our 2-year threshold selection may have attenuated the apparent interaction effect, particularly among women who work longer durations of night work.
In conclusion, we found evidence that a coding SNP in NPAS2 (Ala394Thr; rs2305160) may modify the effects of shift work on breast cancer risk. Further study is needed in large populations to replicate and clarify the nature of this finding and investigate this effect in populations of different ancestry and with sufficient power to stratify by menopausal status and varying levels of shift work exposure. This interaction may explain the lack of association between shift work and breast cancer risk observed in a recent study in China.
Novelty and Impact Statements.
In this, to our knowledge, first investigation of breast cancer risk considering gene-environment interactions with rotating shift work, we found that women with the Thr/Thr genotype of NPAS2 Ala394Thr and 2+ years of rotating night shifts had a 3-fold higher risk of breast cancer compared to women of the same genotype with less than 2 years of rotating shift work exposure.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by National Institutes of Health (NIH) grant CA114534 (PI Schernhammer). The Broad Institute Center for Genotyping and Analysis is supported by grant U54 RR020278 from the National Center for Research Resources. Data collection for the NHS was supported by NIH grant 5P01CA087969-12. Genevieve Monsees was supported by NIH grant CA114534 for SNP selection and Public Health Service grant T32-ES016645-01 and NIH National Cancer Institute grant CI T32-CA09168 for different phases of the analysis and manuscript preparation. The authors thank Parveen Bhatti for discussions, Constance Chen and Carolyn Guo for assistance preparing and analyzing data from NHS, Aidan Crook and Yan Liu for programming insight, and Daniel Mirel, Hardeep Ranu, Carolyn Guo, Megan Rice, and Fangyi Gu for help preparing the NHS2 data. In addition, they would like to thank the participants and staff of the NHS, for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. Finally they thank the participants of the NHS and the NHS2 for their continued dedication.
APPENDIX
SNP Selection
Tagging SNPs were chosen from HapMap (build36) using Tagger within Haploview 33 for all fifteen genes (ARNTL, CLOCK, CRY1, CRY2, CSNK1A1, NPAS2, PER1, PER2, PER3, AANAT, NAT1, NAT2, TPH1, TPH2, SLC6A4) and the 20 Kb upstream and 10Kb downstream regions. Eleven SNPs were “forced” in based on suggestive evidence for associations with breast cancer risk in the Nurses’ Health Study (CGEMS) study (nominal p<0.01) 21. Two candidate SNPs from the literature (rs2305160 in NPAS2 17 and rs1801260 in Clock 20) were also selected.
Genotyping method
High throughput genotyping was performed at the Massachusetts Institute of Technology Broad Institute Center for Genotyping and Analysis using the Illumina Golden Gate with Bead Express (Vera Code) technology. The applied protocol was highly multiplexed and utilized barcoded microwell plates and robust automation systems. 186 SNPs were retained after the design phase. 177 (95.16%) of the SNPs had a genotyping success rate ≥95%. 159 (85.48%) of the SNPs had a genotyping success rate ≥99%. Following genotyping, 8 of the 186 SNPs and 51 of the 2208 samples (inclusive of quality control samples) had a call rate below 90% and were excluded from analysis. Each of the remaining 178 SNPs had a Hardy Weinberg Equilibrium p-value ≥0.0001 and MAF≥0.05 in the controls in our population.
188 blinded quality control samples were included from 40 individuals; between two and four replicate samples were included for each of these individuals. The largest estimated error rate for the 178 SNPs was 1.8%. 117 SNPs (65.7%) were concordant over all replicates. 165 (92.7%) had an error rate <1%.
Assessment of shift work
In the baseline questionnaire, participants were asked to indicate “the total number of years during which [they] worked rotating night shifts (at least 3 nights/month in addition to days or evenings worked in that month)” from the following pre-specified responses: Never, 1–2 years, 3–5, 6–9, 10–14, 15–19, and 20 years or more. Questionnaires issued in 1991, 1993, and 1997 repeated the question in terms of months of exposure since the previous questionnaire. No questions pertaining to shift-work were asked on the 1995 or 1999 questionnaires. Participants were asked to recall their 1993–1995, 1995–1997, and 1997–1999 exposures on the 2001 questionnaire.
Individuals who did not estimate their cumulative exposure to rotating shift-work on the baseline questionnaire or who had reported prevalent breast cancer prior to or when they provided a blood sample were excluded from the interaction analysis. Participants were assigned the midpoint of the range they had reported (in months of rotating shift-work) for each exposure interval. If a response was missing for a given interval the previous response was carried forward.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Fu L, Lee CC. The circadian clock: pacemaker and tumour suppressor. Nat Rev Cancer. 2003;3:350–361. doi: 10.1038/nrc1072. [DOI] [PubMed] [Google Scholar]
- 2.Folkard S, Monk TH, Lobban MC. Short and long-term adjustment of circadian rhythms in 'permanent' night nurses. Ergonomics. 1978;21:785–799. doi: 10.1080/00140137808931782. [DOI] [PubMed] [Google Scholar]
- 3.Patkai P, Akerstedt T, Pettersson K. Field studies of shiftwork: I. Temporal patterns in psychophysiological activation in permanent night workers. Ergonomics. 1977;20:611–619. doi: 10.1080/00140137708931672. [DOI] [PubMed] [Google Scholar]
- 4.Stevens RG. Working against our endogenous circadian clock: Breast cancer and electric lighting in the modern world. Mutat Res. 2009;680:106–108. doi: 10.1016/j.mrgentox.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Schernhammer ES, Hankinson SE, Colditz GA, Laden F, Kroenke CH, Willett WW. Night work and risk of breast cancer in women participating in the Nurses' Health Study II. Epidemiology. 2005 in press. [Google Scholar]
- 6.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Colditz GA. Rotating night shifts and risk of breast cancer in women participating in the Nurses' Health Study. J Natl Cancer Inst. 2001;93:1563–1568. doi: 10.1093/jnci/93.20.1563. [DOI] [PubMed] [Google Scholar]
- 7.Whelan EA. Cancer incidence in airline cabin crew. Occup Environ Med. 2003;60:805–806. doi: 10.1136/oem.60.11.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pukkala E, Auvinen A, Wahlberg G. Incidence of cancer among Finnish airline cabin attendants. BMJ. 1995;311:649–652. doi: 10.1136/bmj.311.7006.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rafnsson V, Sulem P, Tulinius H, Hrafnkelsson J. Breast cancer risk in airline cabin attendants: a nested case-control study in Iceland. Occup Environ Med. 2003;60:807–809. doi: 10.1136/oem.60.11.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tynes T, Hannevik M, Andersen A, Vistnes AI, Haldorsen T. Incidence of breast cancer in Norwegian female radio and telegraph operators. Cancer Causes Control. 1996;7:197–204. doi: 10.1007/BF00051295. [DOI] [PubMed] [Google Scholar]
- 11.Hansen J. Increased breast cancer risk among women who work predominantly at night. Epidemiology. 2001;12:74–77. doi: 10.1097/00001648-200101000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst. 2001;93:1557–1562. doi: 10.1093/jnci/93.20.1557. [DOI] [PubMed] [Google Scholar]
- 13.Megdal SP, Kroenke CH, Laden F, Pukkala E, Schernhammer ES. Night work and breast cancer risk: a systematic review and meta-analysis. Eur J Cancer. 2005;41:2023–2032. doi: 10.1016/j.ejca.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Rockhill B, Willett WC, Hunter DJ, Manson JE, Hankinson SE, Spiegelman D, Colditz GA. Physical activity and breast cancer risk in a cohort of young women. J Natl Cancer Inst. 1998;90:1155–1160. doi: 10.1093/jnci/90.15.1155. [DOI] [PubMed] [Google Scholar]
- 15.Tworoger SS, Sluss P, Hankinson SE. Association between plasma prolactin concentrations and risk of breast cancer among predominately premenopausal women. Cancer Res. 2006;66:2476–2482. doi: 10.1158/0008-5472.CAN-05-3369. [DOI] [PubMed] [Google Scholar]
- 16.Wurtman RJ, Anton-Tay F. The mammalian pineal as a neuroendocrine transducer. Recent Prog Horm Res. 1969;25:493–522. doi: 10.1016/b978-0-12-571125-8.50014-4. [DOI] [PubMed] [Google Scholar]
- 17.Zhu Y, Stevens RG, Leaderer D, Hoffman A, Holford T, Zhang Y, Brown HN, Zheng T. Non-synonymous polymorphisms in the circadian gene NPAS2 and breast cancer risk. Breast Cancer Res Treat. 2008;107:421–425. doi: 10.1007/s10549-007-9565-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yi C, Mu L, de la Longrais IA, Sochirca O, Arisio R, Yu H, Hoffman AE, Zhu Y, Katsaro D. The circadian gene NPAS2 is a novel prognostic biomarker for breast cancer. Breast Cancer Res Treat. 2010;120:663–669. doi: 10.1007/s10549-009-0484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu Y, Leaderer D, Guss C, Brown HN, Zhang Y, Boyle P, Stevens RG, Hoffman A, Qin Q, Han X, Zheng T. Ala394Thr polymorphism in the clock gene NPAS2: a circadian modifier for the risk of non-Hodgkin's lymphoma. Int J Cancer. 2007;120:432–435. doi: 10.1002/ijc.22321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benedetti F, Dallaspezia S, Fulgosi MC, Lorenzi C, Serretti A, Barbini B, Colombo C, Smeraldi E. Actimetric evidence that CLOCK 3111 T/C SNP influences sleep and activity patterns in patients affected by bipolar depression. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:631–635. doi: 10.1002/ajmg.b.30475. [DOI] [PubMed] [Google Scholar]
- 21.Hunter DJ, Kraft P, Jacobs KB, Cox DG, Yeager M, Hankinson SE, Wacholder S, Wang Z, Welch R, Hutchinson A, Wang J, Yu K, et al. A genome-wide association study identifies alleles in FGFR2 associated with risk of sporadic postmenopausal breast cancer. Nat Genet. 2007;39:870–874. doi: 10.1038/ng2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraft P, Yen YC, Stram DO, Morrison J, Gauderman WJ. Exploiting gene-environment interaction to detect genetic associations. Hum Hered. 2007;63:111–119. doi: 10.1159/000099183. [DOI] [PubMed] [Google Scholar]
- 23.PLINK. Whole genome data analysis toolset [Google Scholar]
- 24.The R Project for Statistical Computing [Google Scholar]
- 25.Chu LW, Zhu Y, Yu K, Zheng T, Yu H, Zhang Y, Sesterhenn I, Chokkalingam AP, Danforth KN, Shen MC, Stanczyk FZ, Gao YT, et al. Variants in circadian genes and prostate cancer risk: a population-based study in China. Prostate Cancer Prostatic Dis. 2008;11:342–348. doi: 10.1038/sj.pcan.4501024. [DOI] [PubMed] [Google Scholar]
- 26.Hoffman AE, Zheng T, Ba Y, Zhu Y. The circadian gene NPAS2, a putative tumor suppressor, is involved in DNA damage response. Mol Cancer Res. 2008;6:1461–1468. doi: 10.1158/1541-7786.MCR-07-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan HY, Chiou JJ, Tseng WH, Liu CH, Liu CK, Lin YJ, Wang HH, Yao A, Chen YT, Hsu CN. FASTSNP: an always up-to-date and extendable service for SNP function analysis and prioritization. Nucleic Acids Res. 2006;34:W635–W641. doi: 10.1093/nar/gkl236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41–50. doi: 10.1016/s0092-8674(02)00961-3. [DOI] [PubMed] [Google Scholar]
- 29.Kovanen L, Saarikoski ST, Aromaa A, Lonnqvist J, Partonen T. ARNTL (BMAL1) and NPAS2 gene variants contribute to fertility and seasonality. PLoS One. 5:e10007. doi: 10.1371/journal.pone.0010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pronk A, Ji BT, Shu XO, Xue S, Yang G, Li HL, Rothman N, Gao YT, Zheng W, Chow WH. Night-shift work and breast cancer risk in a cohort of Chinese women. Am J Epidemiol. 171:953–959. doi: 10.1093/aje/kwq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chu LW, Zhu Y, Yu K, Zheng T, Chokkalingam AP, Stanczyk FZ, Gao YT, Hsing AW. Correlation between circadian gene variants and serum levels of sex steroids and insulin-like growth factor-I. Cancer Epidemiol Biomarkers Prev. 2008;17:3268–3273. doi: 10.1158/1055-9965.EPI-08-0073. [DOI] [PubMed] [Google Scholar]
- 32.Kang TH, Reardon JT, Kemp M, Sancar A. Circadian oscillation of nucleotide excision repair in mammalian brain. Proc Natl Acad Sci U S A. 2009;106:2864–2867. doi: 10.1073/pnas.0812638106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.